Abstract
Skeletal muscle mass, function, and repair capacity all progressively decline with aging, restricting mobility, voluntary function, and quality of life. Skeletal muscle repair is facilitated by a population of dedicated muscle stem cells (MuSCs), also known as satellite cells, that reside in anatomically defined niches within muscle tissues. In adult tissues, MuSCs are retained in a quiescent state until they are primed to regenerate damaged muscle through cycles of self-renewal divisions. With aging, muscle tissue homeostasis is progressively disrupted and the ability of MuSCs to repair injured muscle markedly declines. Until recently, this decline has been largely attributed to extrinsic age-related alterations in the microenvironment to which MuSCs are exposed. However, as highlighted in this Perspective, recent reports show that MuSCs also progressively undergo cell-intrinsic alterations that profoundly affect stem cell regenerative function with aging. A more comprehensive understanding of the interplay of stem cell–intrinsic and extrinsic factors will set the stage for improving cell therapies capable of restoring tissue homeostasis and enhancing muscle repair in the aged.
In 1865, Claude Bernard first termed the “Milieu intérieur,” later called homeostasis by Walter Bradford Cannon, as the key process by which the stability of an organism's internal environment is maintained, irrespective of the varying external influences it encounters. Within tissues, homeostasis is a dynamic process governed by multicellular communication that is necessary to adapt and maintain function in fluctuating circumstances1. In the context of skeletal muscle tissue, homeostatic interactions between MuSCs, other resident cells, and the tissue microenvironment govern adult skeletal muscle growth during normal development. We propose that tissue homeostasis is fundamental to proper muscle regeneration in response to damage and is regulated by a delicate balance of temporally coordinated cellular interactions and effectors, and molecular feedback circuits in which MuSCs have a central role.
Throughout adulthood, MuSCs, which are generally characterized by expression of the myogenic transcription factor Pax7 (ref. 2), are retained in a mitotically and metabolically quiescent state3,4. MuSCs, often referred to as satellite cells, are located in a protected membrane-enclosed niche between the basal lamina and plasma membrane of the mature contractile multinucleated myofiber. In response to myofiber damage, cytokines and growth factors in the tissue milieu transiently activate MuSCs. Subsequently, MuSCs undergo multiple rounds of self-renewing divisions that are essential to their function in regeneration, as demonstrated by transplantation, genetic ablation, and lineage tracing experiments5–12. In healthy muscle tissues, feedback mechanisms ensure that asymmetric self-renewing divisions yield sufficient numbers of fusion-competent muscle progenitor cells that contribute to myofiber repair, and uncommitted stem cells that remain in the satellite cell position in a quiescent state and serve as a MuSC reservoir13–16. This homeostatic relationship ensures that the successive regenerative demands that occur throughout adulthood can be met.
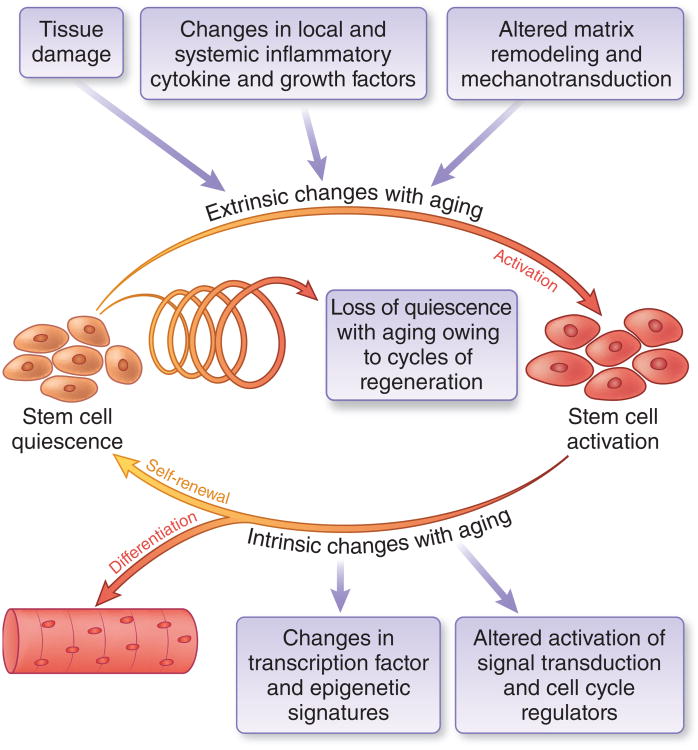
During aging, there is a striking decline in muscle regenerative function. This Perspective focuses on the central role of MuSCs in this process (Fig. 1). In adult muscles, MuSCs are essential for efficient repair of tissue damage. When MuSCs are conditionally ablated, even in aged mice, muscle repair is defective17. The regenerative function of MuSCs is regulated by their interaction with components of their extrinsic tissue microenvironment or ‘niche,’ including systemic proteins and localized structural and soluble factors that affect cell cycle and transcriptional regulation18 and alter muscle biomechanical properties and contractile forces19,20. These extrinsic factors derive from the myofiber itself, from immune cells, from fibrogenic and adipogenic cells within muscle tissue, and from the circulation. In parallel, cell-intrinsic alterations in signal transduction, cell cycle regulators, transcription factor profiles and epigenetic signatures are propagated through self-renewing divisions and accumulate in aged MuSCs.
Figure 1.
The role of MuSCS in tissue homeostasis with aging. In adult muscles, MuSCs are maintained in quiescence23. During muscle regeneration, MuSCs are transiently activated and self-renew to produce more stem cells and differentiated progeny, maintaining tissue homeostasis and repair capacity. Repair is initiated by tissue damage followed by a two-phase inflammatory response involving secretion of pro- and antiinflammatory cytokines66. ‘Extrinsic’ microenvironmental factors governing this process are provided by neighboring cells and from systemic sources23. Cytokines trigger the production of matrix degrading enzymes leading to extracellular matrix remodeling. These extrinsic stimuli converge to trigger ntrinsic changes in MuSCs signaling, cell cycle, and transcriptional networks that regulate self-renewal and differentiation18. With aging, progressive cycles of damage and repair lead to a loss of homeostasis, resulting in depletion of quiescent MuSCs with self-renewal capacity, owing to changes in both extrinsic and instrinic factors, resulting in impaired muscle regeneration.
Recent elucidation of cell-intrinsic alterations have been enabled by technological advances including improved methods of MuSC purification6,9,10,21–24, generation of new transgenic mouse models for MuSC lineage tracing5,25–27 and deletion17,28, more sensitive assessments of regenerative function by bioluminescence imaging10,29, and generation of bioengineered niches that support MuSC function in long-term culture29–31. With these insights, we posit a new model of defective skeletal muscle repair during aging, which places the MuSC itself at the center of the progressive changes that disrupt muscle tissue homeostasis to limit stem cell self-renewal and regenerative function. We propose that during aging, a shift in the balance of extrinsic influences in conjunction with accumulated heritable intrinsic changes within MuSCs alters muscle tissue homeostasis, so that ultimately the needs for regeneration cannot be met.
Extrinsic influences on muscle stem cell function with aging
Evidence from parabiotic mice
Grafting experiments, in which portions of intact muscle tissues are heterochronically transplanted between young and aged mice, provided the first indication that the chronological age of the muscle microenvironment exerts a profound influence on muscle regenerative capacity32. Aged muscle tissues grafted into muscles of young rats exhibit increased muscle mass and contractile strength, and the converse is true for young grafts into aged hosts. Not only is the function of grafted muscle cells defective, but angiogenesis and innervation of the grafted muscle are also markedly impaired in aged hosts33.
Experiments using a heterochronic parabiosis model rekindled strong interest in the influence of the microenvironment on regeneration34,35. When young (2–6 months) and aged (18–26 months) mice are conjoined and their circulatory systems are shared, the muscles of aged mice in such heterochronic parabiotic pairs exhibit a more robust regenerative response to local injury than do aged mice in isochronic pairs34,35. Conversely, regeneration of muscles in young mice is impaired when exposed to an aged circulation34. A caveat of these experiments is that young and aged mice in parabionts are stressed by their forced connection, and the observed systemic changes occur in the complex milieu of chronic inflammation36. Heterochronic parabiosis experiments first suggested that factors are present in the circulation of young mice capable of rejuvenating muscle repair in aged mice.
Localized and systemic factors
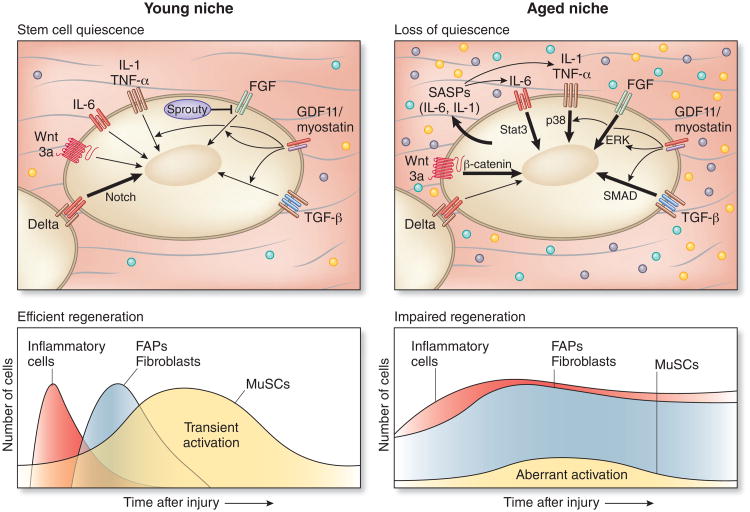
In aged mice, regenerative defects have been pinpointed as arising from signaling pathways governed by microenvironmental factors that are dysregulated in aging (Fig. 2). In response to damage, MuSCs in the niches of young muscles are exposed to a sequence of localized and systemic cytokines, growth factors, and hormones derived from immune cells, fibro-adipogenic progenitors (FAPs), fibroblasts, and myofibers. Transient, temporally controlled exposure of MuSCs to these cell types and factors facilitates regeneration and re-establishes a quiescent MuSC reservoir to meet future regenerative needs. By contrast, MuSCs in aged niches are exposed to dysregulated signals that lead to a loss of MuSC quiescence and impair MuSC function in regeneration. For example, the Notch and Wnt pathways, which have crucial roles in muscle development and are transiently activated in the regenerative process in healthy adult muscles37, are aberrantly regulated in aged muscles. Aged muscles exhibit decreased expression of the Notch ligand Delta-1 on the myofiber plasma membrane38, which normally operates to activate Notch-mediated genes that are critical for MuSC activation and self-renewal39,40. MuSCs and their myogenic progeny are exposed to increased levels of circulating Wnt3a (wingless-type MMTV integration site family, member 3A) in aged mice34. Wnt3a stimulates β-catenin signaling, which induces undesirable fibrogenic differentiation of muscle stem and progenitor cells34, and also antagonizes Notch signaling37. Together, these dysregulated signals impair MuSC-mediated myofiber repair and stem cell self-renewal in aged tissues and lead to the generation of fibroblasts34. In addition, in young adult mice, the hormone oxytocin promotes muscle regeneration, whereas in aged mice this hormone declines41. Targeting these cell-extrinsic changes in aged mice, either through parabiosis and exposure to factors in young serum or, more directly, via ectopic activation of Notch signaling, inhibition of Wnt3a-β-catenin signaling, or oxytocin supplementation, partially restores MuSC-mediated muscle regeneration34,37,38,41.
Figure 2.
Extrinsic and intrinsic regulators of MuSC function are altered in aging. In young muscles, MuSCs are transiently activated after tissue damage by released inflammatory cytokines and growth factor signaling coordinated by immune cells, fibroblasts and FAPs. These factors dynamically stimulate MuSC regulatory pathways, including Delta-Notch, governing cell cycle and transcription control of MuSC fate, resulting in temporary activation and self-renewal, with homeostasis reached upon completion of myofiber repair through re-induction of stem cell quiescence, in part via activation of Sprouty, an inhibitor of FGF signaling23,37. The thickness of the arrow indicates strength of activation of the pathway in young and aged MuSCs. In aged muscles, self-renewal signals (for example, Delta38) diminish and inflammatory and fibrogenic signals are elevated and prolonged34,65, resulting in aberrant MuSC activation and loss of quiescence13,29,56. These changes with aging are commensurate with the onset of aberrant activation in the β-catenin, Stat3, FGF, p38α/β MAPK, and SMAD signaling pathways. By elderly ages, a subset of aged MuSCs become senescent29,56.
Recent studies have turned the spotlight on GDF11, a putative systemic rejuvenation factor and transforming growth factor beta (TGF-β) superfamily member. Notably, results from two different groups on the function of GDF11 seem to be diametrically opposed42,43. One study42 reported that circulating GDF11 levels decline with aging44 and that systemic administration of recombinant GDF11 to aged mice enhances MuSC regenerative capacity after injury. In contrast, a second study reported GDF11 levels do not decrease with aging and that GDF11 supplementation in injured aged mice interferes with muscle repair43. Mature GDF11 shares 90% amino acid homology45 with myostatin (GDF8), another TGF-β superfamily member, which has a well-documented role in decreasing muscle mass and interfering with muscle repair. GDF11 and myostatin activate the same signaling pathways (SMAD2/3, p38 MAPK, and ERK) via the type IIB activin receptor (ActRIIB)46. Importantly, there are monomeric (12.5 kDa) and dimeric (25 kDa) forms of both myostatin and GDF11, which were not distinguished using the cross-reacting antibodies in these studies42,43. The first study42 focused on the monomeric forms of GDF11 and myostatin and showed that they cumulatively decrease in abundance in serum with aging42,44. The second study43 confirmed this observation, but also examined the active and more abundant dimeric forms and showed that they cumulatively increase with aging. This dimeric form was not assessed in the original study44.
An important caveat to these reports is that the regulation of the TGF-β superfamily of proteins, including the systemically delivered recombinant GDF11, can be complicated by time and dose-dependent effects, due in part to attenuation by binding proteins and inhibitors such as follistatin47 and growth differentiation factor (GDF)-associated serum proteins-1 and 2 (GASPs)48. Both follistatin and GASPs inhibit the activities of GDF11 and myostatin; accordingly, follistatin (Fst)-heterozygous47 and Gasp1-null or Gasp2-null48 mice have smaller muscles and increased GDF11 and myostatin bioactivity. This is consistent with the phenotype of myostatin (Mstn)-null mice, which exhibit a massive increase in muscle mass49, in agreement with the causative role of a natural MSTN mutation in the remarkable ‘Belgian Blue’ double-muscled cattle50. Intriguingly, adult Gdf11-null mice have no muscle defects51. Further experimentation to address the effect of GDF11 dosage and duration on regeneration is warranted, because transient and persistent regulators often have beneficial or deleterious consequences, respectively, especially in the course of aging (Fig. 2). More studies such as conditional Gdf11 loss- or gain-of-function experiments, postnatally and in specific tissues, are required to determine whether GDF11 ameliorates MuSC regenerative function in aging.
Intrinsic changes in muscle stem cells with aging
Defining muscle stem cells
Decades ago, studies of myogenic progenitors by numerous investigators indicated that their proliferative capacity declines with increasing age of the donor, in agreement with recent findings that FACS-purified MuSCs show cell-intrinsic changes during aging. In mouse aging, a decline in MuSC regenerative capacity is evident well before a decline in MuSC numbers is apparent. Indeed MuSC numbers do not decrease appreciably until a highly advanced ‘geriatric’ age (>26 months) as determined by scoring of MuSCs in their anatomically defined niche in muscle tissue sections52,53. However, these stem cells have undergone extensive self-renewal divisions during the course of development and regeneration. Studies demonstrating that intrinsic changes lead to impaired MuSC function in regeneration with aging have largely been enabled by advances in the isolation of MuSCs to high purity by flow cytometry.
The development of transgenic reporter mice, such as the Pax3-GFP reporter mouse9 and later the Pax7-ZsGreen5 and Pax7-GFP14 mouse models, have been instrumental to the FACS isolation of MuSCs, as expression of the transcription factors Pax7 and Pax3 are hallmarks of adult MuSCs. Indeed, a study using the Pax3-GFP reporter mice revealed that transplanted FACS-isolated MuSCs home to the classic ‘satellite cell niche’9, and hence constitute the satellite cells first identified by electron microscopy in 1961 (ref. 22). Further advances in MuSC isolation have revealed that this Pax7-expressing population can be enriched with high efficiency from any donor mouse strain by FACS with various combinations of antibodies to transmembrane proteins including CD34, α7-integrin, β1-integrin, syndecan-4, VCAM-1, and CXCR4 (refs. 6,9,10,21–24). The understanding of the dynamics of MuSC function over time in MuSC transplantation studies has been greatly facilitated by the use of a highly sensitive non-invasive bioluminescence imaging (BLI) technology10,29. By using BLI, single MuSCs isolated from transgenic mice that ubiquitously express a luciferase transgene were shown to give rise to thousands of progeny, differentiate into myofibers, and self-renew upon re-injury10. These studies showed that MuSCs meet the definition of a tissue-specific stem cell as established by the hematopoiesis field54.
Intrinsic alterations in aged muscle stem cells
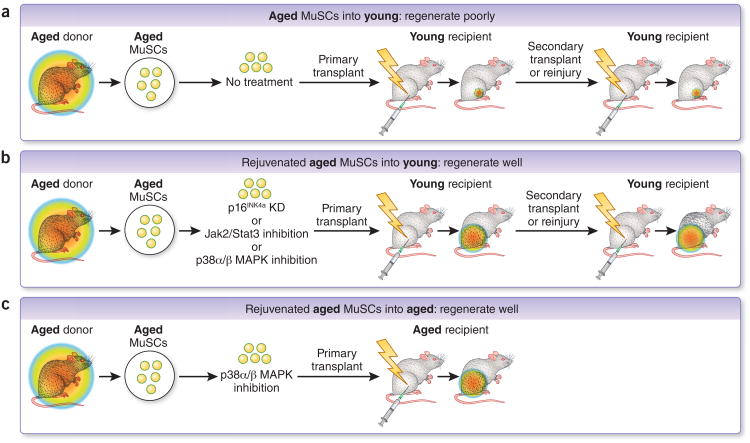
The prospective isolation methods described above enabled recent findings demonstrating that MuSCs from aged tissues accumulate intrinsic changes that lead to a reduction in self-renewal and repair capacity, relative to young tissues, when placed in equivalent in vivo repair settings22,25,29,55,56 (Fig. 3). MuSCs from young (typically 2–6 months), aged (18–26 months) or geriatric (26–32 months) mice were isolated and their self-renewal capacities compared either by stem cell re-isolation after the completion of repair in primary recipients or by serial transplantation into secondary recipients. MuSCs from older donors exhibited marked deficits both in self-renewal and expansion and in their contributions to mature myofiber formation, the two hallmarks of stem cell function25,29,55,56. These findings have been demonstrated in multiple transplantation models, in which MuSCs from aged mice are injected into young uninjured or injured recipient muscles that were either wild-type or immunocompromised. Remarkably, stem cells derived from primary transplants of aged MuSCs remained severely defective in their ability to engraft and repair damaged muscle tissues in secondary young recipients (Fig. 3), demonstrating that prolonged exposure to a young muscle tissue environment after transplantation is not sufficient to rejuvenate the aged MuSC population29,56. These results are in contrast with those obtained by parabiosis and suggest that irrespective of extrinsic regulators there is a cell-autonomous ‘memory’ for the aged or rejuvenated MuSC state.
Figure 3.
Evidence in support of intrinsic ‘memory’ in aged and rejuvenated MuSC populations. (a) Rainbow colored circles represent the ability to regenerate. A heritable cell-intrinsic defect in regenerative capacity of aged MuSCs is evident upon serial transplantation into young recipients. Regenerative capacity remains poor even in a young tissue microenvironment25,29,55,56. (b) After treatment with various ex vivo ‘rejuvenating’ treatments perturbing p16Ink4a, Jak2-Stat3, or p38α/β MAPK activity and transplantation into a young muscle microenvironment25,29,56, aged MuSC cells are capable of efficient regeneration even in serial transplantation. Factors intrinsic and extrinsic (i.e., in the recipient microenvironment) to the rejuvenated aged MuSCs may corroborate to enhance regenerative capacity in this setting25,29,56. (c) Rejuvenation of aged muscle stem cells after ex vivo exposure to soft hydrogel substrate and a chemical p38α/β MAPK inhibitor co-treatment leads to robust transplantation even in an aged tissue microenvironment, leading to enhanced muscle strength29.
The cell-intrinsic dysfunction of the aged MuSC population arises from alterations in a combination of multiple signal transduction cascades, which converge on a shared set of cell cycle kinases and myogenic differentiation factors. These changes include aberrant and cell-autonomous activation of the stress-associated p38α/β mitogen-activated protein kinase (MAPK) signaling axis, the growth factor-stimulated FGFR–Sprouty1 signaling axis, the cytokine-stimulated Jak2–Stat3 signaling axis, and p16Ink4a (also known as Cdkn2a)–Rb cell cycle inhibitors25,27,29,55,56 (Fig. 2). Depending on the ligand, p38α/β MAPK and Jak2–Stat3 signaling axes can negatively regulate MuSC self-renewal by restricting cell cycle progression and promoting myogenic differentiation through activation of commitment genes such as Myod1. Furthermore, aberrant p38α/β activity suppresses Pax7 expression through repressive chromatin modifications57. Moreover, the MuSC reservoir is progressively depleted by disruption of the balance of MuSC asymmetric self-renewal, leading to stem cell divisions in which both daughter cells become committed7,58. A substantial fraction of MuSCs from aged and geriatric mice display a premature cellular senescence phenotype, caused by the aging-associated derepression of the cell cycle inhibitor p16Ink4a. Subsequently, Rb/E2F target genes are repressed56 and the resulting senescent cells are characterized by p16Ink4a and p21Cip1 expression and cell cycle arrest. Cumulatively, these cell autonomous alterations limit MuSC self-renewal and capacity for regeneration25,29,55,56.
In contrast to their essential contributions to muscle repair, the role of MuSCs in the pathogenesis of sarcopenia, an aging-associated muscle wasting disorder, is less clear. Conditional ablation of Pax7-expressing MuSCs from aged muscles neither accelerates nor slows muscle wasting17,28. However, these findings are based on Pax7CreER-DTA models which do not completely ablate the MuSC population. The remaining MuSCs may be capable of extensive self-renewal or they may be senescent and negatively impact muscle function. Indeed, sub-populations of senescent MuSCs marked by expression of p16Ink4a contribute to sarcopenic wasting and repair dysfunction characteristic of aged muscles in the accelerated-aging BubR1 hypomorphic mouse model59,60. Conditional ablation of this p16Ink4a-expressing MuSC population and other senescent cells ameliorates muscle wasting, leading to improved performance in treadmill exercise tests60. These findings suggest that altering the function or phenotype of MuSCs in aged muscle tissue will enhance muscle repair29 and may also ameliorate sarcopenia61.
Counteracting intrinsic aging
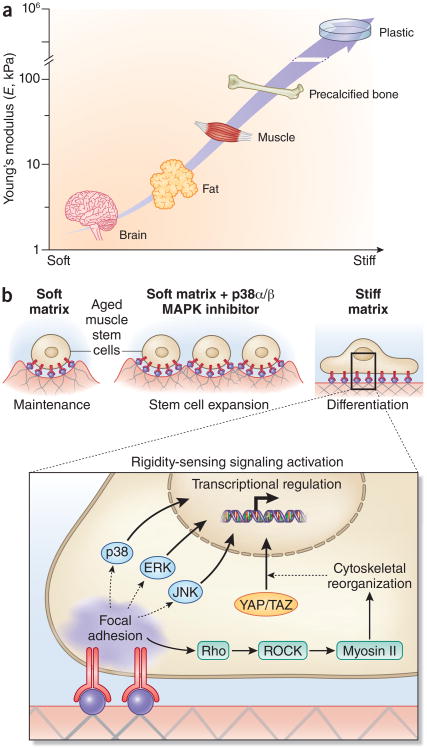
Rejuvenation of the regenerative capacity of aged and geriatric MuSC populations can be achieved by biochemical or genetic inhibition of aberrantly regulated intrinsic MuSC signal transduction pathways, including the p38α/β MAPK, and Jak2–Stat3 signaling axes25,29,55,56. Notably lentivirally-mediated genetic knockdown of p16Ink4a (officially known as Cdkn2a) (ref. 56) or transient biochemical inhibition of p38α/β MAPK29 ex vivo halts progression toward senescence and facilitates rejuvenation of the aged MuSC population in mouse. In particular, the rejuvenation of MuSCs ex vivo can be achieved by growing MuSCs on a soft hydrogel substrate with muscle-like rigidity (Figs. 3 and 4) and transient treatment with a chemical inhibitor of p38α/β MAPK 29. Thus, a combination of biophysical and biochemical cues is required to rejuvenate the aged MuSC population, suggesting that a synergistic interplay of extrinsic and intrinsic factors is necessary.
Figure 4.
Biophysical regulation of muscle stem cells in aging. (a) Tissue elasticity, the biophysical property of tissues and cell culture materials most commonly associated with effects on cell proliferation and differentiation, both in stem cells from skeletal muscle and other tissues, is typically characterized by a Young's modulus (E). The Young's modulus describes the elastic deformability of a material, and is proportional to the amount of force (stress) required to achieve a given deformation (strain). Characteristic tissue elasticities include brain and mammary glands (E = ∼1 kPa), adipose (∼5 kPa), skeletal muscle (∼10−30 kPa, and precalcified bone (∼50 kPa)102,113 Tissue culture substrates such as polystyrene and glass have typical Young's moduli of ∼106 kPa (ref. 114). In aging and muscular dystrophy, skeletal muscles become progressively fibrotic, resulting in increased rigidity92,93. (b) For muscle stem cells from aged mice, a soft 12-kPa matrix (mimicking the rigidity of healthy skeletal muscle31) supports muscle stem cell self-renewal expansion in synergy with targeted inhibition of the p38α/β MAPK signaling pathway29. A soft matrix alone supports aged MuSC maintenance whereas a stiff (106 kPa) matrix induces the differentiation of aged MuSCs. Cells sense the rigidity of their surrounding substrate through a variety of adhesion-dependent signaling pathways, which result in cellular deformations (for example, elongation) and transcriptional responses. Across many cell types, substrate rigidity is sensed by integrin clustering and focal adhesion stabilization, which yields prolonged activation of both canonica MAPK pathways (p38, ERK, JNK) and Rho/Rho-associated kinase (ROCK) signaling and subsequent myosin II–mediated cytoskeletal reorganization, leading to YAP/TAZ transcription factor translocalization115. Together, these and other adhesion-activated signaling pathways confer sensitivity to matrix stiffness in numerous cell types.
MuSCS do not simultaneously change with age. Instead, with aging, a shift toward a more heterogeneous, less regenerative MuSC population is observed. This results from a progressive increase in the subset of MuSCs that is dysfunctional in regeneration with age. Determining the features that characterize functional MuSCs at any point in time and isolating that functional subset remains a challenge. Even in young mice, MuSCs differ and subsets of MuSC populations have enhanced potential for self-renewal14,62–64. Aged MuSC populations are characterized by an increased proportion with reduced clonal proliferation capacity, altered profiles of transcription factor expression evident in single-cell RT-PCR and microarray assays, elevated phosphorylated p38α/β MAPK, increased expression of the senescence marker p16Ink4a, and elevated cytokines and cytokine receptors13,14,16. Single-cell assays can resolve differences within heterogeneous populations. For example, single-cell tracking by time-lapse microscopy of clonal division kinetics revealed that only a subset of aged MuSCs respond to chemical inhibition of p38α/β MAPK, not the entire population29. These findings suggest that rejuvenation of the aged MuSC population results from an expansion of a subset of residual highly regenerative cells rather than a conversion of dysfunctional cells to a functional cell state. Thus, rejuvenation strategies appear to overcome the prevalence of dysfunctional MuSCs within the aging population by augmenting a still-youthful subset. As a result, there is a shift in the aged MuSC population toward MuSCs with enhanced function, capable of restoring muscle homeostasis, and improving regeneration.
The role of inflammation in aged muscle regeneration
Upon injury to young adult muscle, in contrast to injury to aged muscles65, there is a temporally regulated acute and transient immune response that is critical to muscle regeneration66 (Fig. 2). Inflammatory cells, including neutrophils, eosinophils and macrophages, are recruited to the site of damage. The macrophages induce a pro-inflammatory phase that in muscle entails secretion of cytokines such as IL-6, TNF-α and IFN-γ, which promote myogenic cell proliferation. This phase is followed by an anti-inflammatory phase during which macrophages secrete TGF-β, IGF-1 and IL-10, which facilitate myogenic differentiation55,67,68. Evidence for this biphasic immune response includes experiments using a transgenic CD11b–diphtheria toxin receptor mouse model, in which the kinetics of monocyte and macrophage elimination can be temporally controlled68. Accordingly, MuSCs in a young niche are exposed to a regulated sequence of inflammatory cytokines. These studies showed that the timing of inflammatory responses can have profound effects on the kinetics and extent of regeneration69, findings confirmed by experiments using antibodies to block inflammatory cytokines70,71.
Although these inflammatory phases are normally transient, when these phases are not temporally coordinated, damage persists and regeneration is impaired65. The exposure of MuSCs in an aged niche with a progressively altered cytokine composition has deleterious effects on muscle tissue regeneration. Recent studies point to a role for the IL-6–Jak2–Stat3 signaling axis in muscle regeneration25,27. Indeed, in geriatric mice (24–28 months), IL-6 signaling has been shown to be elevated after injury, leading to muscle stem cell exhaustion and consequent muscle atrophy56. Thus, although a transient inflammatory response is beneficial to regeneration, the persistent inflammation that is characteristic of chronic muscle disease impedes regeneration65.
Several studies of MuSC function in muscle regeneration following injury indicate that MuSCs from aged mice (18–22 months) have stem cell–intrinsic defects25,29,55,56 (Fig. 3) that accrue before the depletion of MuSC numbers evident in geriatric mice13,56,72. These studies suggest that inflammatory cytokines that normally regulate muscle injury responses contribute to stem cell dysregulation and exhaustion in aged tissues. Indeed, one of the signaling pathways that is progressively disrupted in aged MuSCs is p38α/β MAPK, which is known to be downstream of TNF-α and IL-1 (refs. 27,56,57). Together, these findings show that intrinsic changes progressively accumulate in MuSCs during aging, which affect their regenerative function25,29,55,56. Moreover, these changes are not altered by transplantation into a youthful or aged microenvironment and are heritably transmitted from the stem cells to their progeny29,56.
The impact of cellular interactions on muscle regeneration in aging
Muscle tissue comprises multiple cell types in addition to myofibers and stem cells. These cells can interact with each other to secrete potent combinations of trophic factors, cytokines and extracellular matrix (ECM) components that exert cell-autonomous (autocrine) effects or act on neighboring cells (paracrine effects). For example, paracrine signals released by neighboring cells in aged muscles are known to affect intrinsic mediators of muscle stem cell quiescence (Figs. 1 and 2). Notably, recent reports demonstrate that Sprouty1, a negative regulator of receptor tyrosine kinase signaling, attenuates FGF2–FGFR signaling13. As a result, MuSCs with elevated Sprouty1 levels remain quiescent. However, during aging, Sprouty1 expression dramatically declines in MuSCs, leading to a reduction in quiescent cells and increased activation. This effect is further exacerbated by an increase in FGF-2 secretion by the muscle fiber itself in aged muscle tissues. Aged and geriatric MuSCs secrete IL-18 and IL-6, respectively, which act in an autocrine manner to induce proliferation of the MuSC population25,56. As a result, in the course of aging, MuSCs shift from a quiescent phenotype to an over-activated phenotype characterized by persistent p38α/β MAPK activity that eventually leads to premature differentiation senescence and stem cell exhaustion25,29,55,56.
Senescent MuSCs
Senescent cells within muscle tissues have a profound effect on the regenerative capacity of MuSCs. In aged muscle, senescent cells accumulate that are alive, but cease to divide. Senescent cells produce a suite of cytokines, growth factors, and proteases, known as the senescence-associated secretory phenotype (SASP)73. Transient secretion of SASP components, such as platelet-derived growth factor A (PDGF-A) has been shown to be beneficial for tissue repair when produced by fibroblasts and endothelial cells in an acute response to skin damage74. This finding is consistent with the attenuated muscle tissue aging observed when the release of SASP-associated cytokines is reduced upon conditional ablation of senescent cells in a mouse model of accelerated aging60. By contrast, persistent SASP pathway activation has deleterious effects. For example, chronic p16Ink4a expression in geriatric mice leads to an accumulation of senescent MuSCs and impaired regeneration, which can be circumvented by conditional ablation of the senescent cells56. These results suggest that in muscle, the transient expression of SASP components, such as inflammatory cytokines, can augment regeneration, whereas persistent expression of SASP components impedes repair (Fig. 2).
Mesenchymal stem cells, fibro-adipogenic progenitors, and fibroblasts
Mesenchymal stem cells (MSCs), which include the fibro-adipogenic progenitors (FAPs)75,76 have also been shown to impact MuSC function. MSCs secrete a number of Wnt ligands, and FAPs are known to secrete IL-6 (refs. 75,77). Under physiological conditions, FAPs remain quiescent but rapidly and transiently expand in response to acute muscle damage to facilitate myogenesis75 (Fig. 2). In part, the expansion of resident FAPs is mediated by the infiltration of eosinophils that secrete IL-4, which activates FAP proliferation78. Additionally, upon damage, macrophages infiltrate muscle tissue and mediate FAP clearance by inducing apoptosis via TNF-α secretion79. Similarly, fibroblasts have been shown to be required transiently during muscle regeneration. Genetic ablation of fibroblasts in muscle connective tissue via TCF4-mediated diphtheria toxin expression promotes MuSC differentiation, leading to depletion of stem cell niches, which impairs muscle regeneration80. Thus, when fibroblasts are appropriately regulated, they are beneficial to regeneration, but when in excess, as seen in aged tissues, they are detrimental. Notably, when damage is chronic, as in muscles of mdx mice, the FAP population persists and differentiates into fibro-adipocytes presumably due to an increase in TGF-β1 (ref. 79). We postulate that upon injury in aged mice, a type 2 innate immune response is suppressed, leading to reduced IL-4 and IL-13 signaling which together with TGF-β1 further promotes FAPs to differentiate into fibro-adipocytes. Accordingly, in chronic injury, accumulation of non-myogenic cell populations such as FAPs and fibroblasts disrupts muscle tissue homeostasis and impedes muscle regeneration 79,81,82. The dynamic regulation of cell types, cytokines and growth factors is key to the maintenance of the stem cell niche, which impacts stem cell function. A shift in this delicate balance of temporally coordinated cell types and regulators with aging could lead to MuSC malfunction and defective regeneration.
The effect of biophysical changes on muscle regeneration during aging
The status of the ECM affects mechanotransduction, which regulates mechanical stress, cell shape, architecture and signaling. During muscle development, growth, and regeneration, there is a dynamic balance of remodeling enzymes such as matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which control the integrity of the ECM83. In aging, this balance is disrupted by a persistent inflammatory response that increases fibrosis, leading to altered composition of the muscle tissue ECM and increased tissue rigidity. For example, IL-6–mediated induction of MMP9 (ref. 84) converts the matrix-tethered latent TGF-β complex to an active form 85. The active TGF-β subsequently stimulates matrix deposition in several cell types within muscle tissue, including myofibroblasts, leading to increased matrix rigidity86.
During aging, fibrotic and adipogenic cells increase in muscle and the extracellular matrix composition of myofibers is altered, affecting the biochemical and biophysical microenvironment of muscle stem cells87. The normally laminin-rich myofiber ECM shifts toward a composition enhanced in collagen IV (refs. 88,89), but with reduced collagen VI levels90. These alterations are commensurate with changes in the ability of the myofiber ECM to retain glycoproteins such as osteopontin, which bind heparan sulfate and other proteoglycans91. Furthermore, such protein composition changes could account for biomechanical changes to the myofiber basal lamina and muscle epimysium that have been shown to accompany aging. These changes lead to a more rigid tissue microenvironment92,93. In studies using biomimetic niches, substrates with an elastic stiffness of young adult skeletal muscle (∼12 kPa Young's modulus, the characteristic elastic rigidity of a material), which is five orders of magnitude softer than traditonal tissue culture plastic), promote MuSC self-renewal29,31 and myoblast differentiation94,95 (Fig. 4). In contrast, much softer or more rigid substrates result in a loss of MuSCs owing to decreased survival or increased myogenic commitment31. These results demonstrate that the composition and mechanics of the MuSC niche are altered with aging, and that these alterations can impede MuSC self-renewal in response to injury.
Binding of ECM molecules to specific integrin receptors affects intracellular signaling by, for example, by triggering activation of PI3K and Rac1–MAPK pathways that induce MuSC activation and differentiation96,97. Syndecan3, a transmembrane heparan sulfate proteoglycan (HSPG) facilitates Notch activation, which promotes MuSC self renewal and inhibits differentiation98. Collagen VI increases satellite cell numbers and promotes regeneration99. Fibronectin binding to the Syndecan4/Frizzled complex disrupts the asymmetric self-renewal of MuSCs, thereby promoting Wnt7a-mediated symmetrical muscle stem cell expansion100. The Par3 complex mediates asymmetric segregation of MuSCs with active (phosphorylated) and inactive p38α/β MAPK, which yields committed and uncommitted daughter cells55,58. Perturbations in the FGFR–p38 signaling axis that arise in aging disrupt this asymmetric division mechanism, leading to an increase in symmetrically committed cells55. Thus, alterations in ECM homeostasis can have a major impact on stem cell function in regeneration. Although the effects of ECM changes on MuSC function in the course of aging remain to be determined, they are likely to contribute to the disruption of muscle tissue homeostasis and regenerative failure.
Cells are sensitive to changes in matrix rigidity, which is generally characterized by the Young's modulus (Fig. 4). Exposure of multipotent mesenchymal stem cells to such changes in biophysical properties of substrates triggered altered gene expression typical of myogenic, chondrogenic, adipogenic, and osteogenic cell types101. The maintenance of MuSC self-renewal function relies heavily on rigidity, as when plated on traditional plastic dishes, stem cell function is lost. By contrast, if the matrix is synthesized to mimic the rigidity of muscle tissue (five orders of magnitude less stiff), stem cell function is retained, as shown by transplantation of young MuSCs after culture on soft hydrogels (12 kPa)31.
Cells sense their mechanical and topological environments through a number of membrane and cytoskeletal proteins that trigger signal transduction cascades. Although these mechanisms have not been fully elucidated with respect to MuSC mechanosensitivity, in other cell systems, matrix elasticity is sensed by integrin-focal adhesion complexes and their engagement with the actin-myosin network, causing alterations in RhoA GTPase activity102. Such alterations could result in translocation of the YAP/TAZ complex from the cytoplasm to the nucleus of MuSCs, leading to changes in differentiation-associated gene expression, as shown in various cell types including mammary epithelial and mesenchymal stem cells103,104. Notably, rigidity per se does not maintain aged stem cell function in culture. Instead, a combination of biophysical (12-kPa rigidity) and biochemical cues (p38α/β MAPK inhibitor) is necessary29. These findings highlight the interplay between extrinsic and intrinsic regulators of MuSC function and that homeostasis between the two is crucial. Remarkably, although as shown by parabiosis, extrinsic serum factors can rejuvenate regenerative function, intrinsic changes within MuSCs that accumulate with aging can also profoundly impact regeneration (Figs. 2 and 3).
Cell fate polarity
Stem cell dysfunction can be caused by changes in biophysical properties, which alter cell polarity. Asymmetrical stem-cell division, which entails production of two daughter cells with different cell fates, is typical of normal myogenesis. Apical-basal polarity cues orient the mitotic spindle perpendicular to the basement membrane (in skin and gut epithelium) or basal lamina (in muscle fibers) to induce asymmetric stem cell division, leading to the generation of a daughter cell that proliferates and one that remains quiescent7,105,106. The ECM and signaling proteins in the microenvironment, such as fibronectin or Wnt7a, direct symmetric MuSC self-renewal divisions, and a decline in their abundance in muscle stem cell niches in aging could result in exhaustion of the stem cell pool100, The underlying mechanisms for these cell fate changes may include epigenetic modifications. As many as 1,800 genes are associated with repressive H3K4me3 and H3K27me3 epigenetic marks in aged mouse muscles107. Specific examples include a complex of Pax7 to the histone methyltransferases ASH2L and MLL, which activates a number of myogenic genes108. Additionally, p38α/β MAPK, which is known to be in a complex with polycomb repressive complex 2, leads to the H3K27me repressive mark on the Pax7 promoter, could have a role in this gene repression57. Thus, we posit that aging-related aberrant p38 MAPK signaling may lead to specific heritable epigenetic changes in MuSCs that limit their self-renewal function.
Conclusion and future outlook
Muscle tissue homeostasis actively maintains a balance between quiescence and repair to ensure future tissue regeneration. Extrinsic factors, which include inflammatory cytokines, growth factors and SASP components, promote embryonic muscle development and early adult myogenesis. These factors can derive from the circulation or neighboring cell types such as FAPs, fibroblasts and immune cells, as discussed herein, and other cell types which also play important roles, including adipocytes, endothelial cells, mesoangioblasts, PW1+ interstitial cells and myofibroblasts109–111. However, the cumulative exposure to these factors over an organism's lifetime alters the MuSC-intrinsic capacity to regenerate muscle damage. These intrinsic changes include constitutively phosphorylated p38α/β MAPK, Rb, Stat3, and p16Ink4a. Resolving whether intrinsic or extrinsic influences within the stem cell compartment or tissue are causal or dominant will require the deconstruction of the niche, which can be addressed with biomimetic tools in which the matrix and MuSC functions can be varied independently. By targeting genes that are transiently active in the course of muscle regeneration important insights can be gained into the regulatory networks that go awry in the aging process. In particular, resolving the dynamics and cell sources of inflammatory cytokine signals that are altered in aging should greatly enhance our understanding of the dysregulated repair process. A more comprehensive understanding of the mechanisms underlying the progressive disruption of tissue homeostasis will illuminate new therapeutic targets for regeneration.
MuSCs are not a homogeneous population even in young mice, and MuSC heterogeneity increases during aging14,16,25,29,56,62–64. To understand the significance of these changes with aging, single-cell studies (for example, transcriptome, proteome, and time-lapse lineage tracking) are needed in which multiple parameters can be simultaneously correlated. Further, prospective isolation of stem cell subsets harboring a constellation of markers identified by such single-cell analyses must be assayed for their ability to repair muscle damage in vivo. Such studies will enhance our understanding of the parameters that regulate muscle tissue homeostasis in young and aged muscles and how they might be perturbed to rejuvenate aged muscles.
The findings from serial transplantation experiments show that aged MuSCs have a persistent impairment—a “memory” that is inherited in their progeny (Fig. 3). Aberrantly regulated signaling and transcription factors are likely to lead to epigenetic changes in aged MuSCs that account for their heritable memory in serial transplants; aged or geriatric MuSCs serially transplanted into young recipients have potent regenerative capacity, whereas rejuvenated MuSCs transplanted into aged or geriatric mice are impaired25,27,29,55,56. We hypothesize that, in part, a short-term stem cell memory may be re-enforced and committed to long-term memory by feedback signaling between more ‘impaired senescent’ MuSCs and ‘youthful’ MuSCs, similar to that of the ‘bad neighbors, good citizens’ model described during cellular senescence112. What constitutes the molecular constituents of this memory and whether MuSCs can be taught to forget are the goals of future studies. Notably, the model of MuSC regenerative decline described herein for mice must be validated for human MuSCs and muscle tissues. Clearly, both intrinsic and extrinsic regulators will need to be taken into account in designing therapeutic strategies to enhance regeneration of damaged muscles.
Acknowledgments
We apologize to those investigators whose important work we were unable to cite or describe in depth owing to the limited scope and space constraints of this Perspective. We are grateful for support from Muscular Dystrophy Association grant 217821 (A.T.V.H.); support from the US National Institutes of Health (NIH) grant R00AG042491 (B.D.C.); and The Baxter Foundation, California Institute for Regenerative Medicine (CIRM) grants RB5-07469 and TR3-05501, and NIH grants AR063963, AG020961, AG044815, and NS089533 (H.M.B.).
Footnotes
Competing Financial Interests: The authors declare no competing financial interests.
References
- 1.Buchman TG. The community of the self. Nature. 2002;420:246–251. doi: 10.1038/nature01260. [DOI] [PubMed] [Google Scholar]
- 2.Seale P, Asakura A, Rudnicki MA. The potential of muscle stem cells. Dev Cell. 2001;1:333–342. doi: 10.1016/s1534-5807(01)00049-1. [DOI] [PubMed] [Google Scholar]
- 3.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montarras D, L'Honore A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280:4036–4050. doi: 10.1111/febs.12372. [DOI] [PubMed] [Google Scholar]
- 5.Bosnakovski D, et al. Prospective isolation of skeletal muscle stem cells with a Pax7 reporter. Stem Cells. 2008;26:3194–3204. doi: 10.1634/stemcells.2007-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cerletti M, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lepper C, Fan CM. Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis. 2010;48:424–436. doi: 10.1002/dvg.20630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montarras D, et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 10.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Maltzahn J, Jones AE, Parks RJ, Rudnicki MA. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci USA. 2013;110:16474–16479. doi: 10.1073/pnas.1307680110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Günther S, et al. Myf5-positive satellite cells contribute to Pax7-dependent long-term maintenance of adult muscle stem cells. Cell Stem Cell. 2013;13:590–601. doi: 10.1016/j.stem.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012;490:355–360. doi: 10.1038/nature11438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sambasivan R, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- 15.Gopinath SD, Webb AE, Brunet A, Rando TA. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Rep. 2014;2:414–426. doi: 10.1016/j.stemcr.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012;148:112–125. doi: 10.1016/j.cell.2011.11.049. [DOI] [PubMed] [Google Scholar]
- 17.Fry CS, et al. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21:76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumont NA, Wang YX, Rudnicki MA. Intrinsic and extrinsic mechanisms regulating satellite cell function. Development. 2015;142:1572–1581. doi: 10.1242/dev.114223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilbert PM, Blau HM. Engineering a stem cell house in to a home. Stem Cell Res Ther. 2011;2:3. doi: 10.1186/scrt44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev. 2013;93:23–67. doi: 10.1152/physrev.00043.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornelison DD, Filla MS, Stanley HM, Rapraeger AC, Olwin BB. Syndecan-3 and syndecan-4 specifically mark skeletal muscle satellite cells and are implicated in satellite cell maintenance and muscle regeneration. Dev Biol. 2001;239:79–94. doi: 10.1006/dbio.2001.0416. [DOI] [PubMed] [Google Scholar]
- 22.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Fukada S, et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007;25:2448–2459. doi: 10.1634/stemcells.2007-0019. [DOI] [PubMed] [Google Scholar]
- 25.Price FD, et al. Inhibition of JAK-STAT signaling stimulates adult satellite cell function. Nat Med. 2014;20:1174–1181. doi: 10.1038/nm.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishijo K, et al. Biomarker system for studying muscle, stem cells, and cancer in vivo. FASEB J. 2009;23:2681–2690. doi: 10.1096/fj.08-128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tierney MT, et al. STAT3 signaling controls satellite cell expansion and skeletal muscle repair. Nat Med. 2014;20:1182–1186. doi: 10.1038/nm.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keefe AC, et al. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun. 2015;6:7087. doi: 10.1038/ncomms8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cosgrove BD, et al. Rejuvenation of the muscle stem cell population restores strength to injured aged muscles. Nat Med. 2014;20:255–264. doi: 10.1038/nm.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cosgrove BD, Sacco A, Gilbert PM, Blau HM. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78:185–194. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbert PM, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: age of host determines recovery. Am J Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- 33.Carlson BM, Dedkov EI, Borisov AB, Faulkner JA. Skeletal muscle regeneration in very old rats. J Gerontol A Biol Sci Med Sci. 2001;56:B224–B233. doi: 10.1093/gerona/56.5.b224. [DOI] [PubMed] [Google Scholar]
- 34.Brack AS, et al. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science. 2007;317:807–810. doi: 10.1126/science.1144090. [DOI] [PubMed] [Google Scholar]
- 35.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 36.Johansson CB, et al. Extensive fusion of haematopoietic cells with Purkinje neurons in response to chronic inflammation. Nat Cell Biol. 2008;10:575–583. doi: 10.1038/ncb1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;2:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 39.Bjornson CR, et al. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mourikis P, et al. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- 41.Elabd C, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha M, et al. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egerman MA, et al. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22:164–174. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loffredo FS, et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153:828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakashima M, Toyono T, Akamine A, Joyner A. Expression of growth/differentiation factor 11, a new member of the BMP/TGFβ superfamily during mouse embryogenesis. Mech Dev. 1999;80:185–189. doi: 10.1016/s0925-4773(98)00205-6. [DOI] [PubMed] [Google Scholar]
- 46.Lee SJ. Regulation of muscle mass by myostatin. Annu Rev Cell Dev Biol. 2004;20:61–86. doi: 10.1146/annurev.cellbio.20.012103.135836. [DOI] [PubMed] [Google Scholar]
- 47.Lee SJ, et al. Regulation of muscle mass by follistatin and activins. Mol Endocrinol. 2010;24:1998–2008. doi: 10.1210/me.2010-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee YS, Lee SJ. Regulation of GDF-11 and myostatin activity by GASP-1 and GASP-2. Proc Natl Acad Sci USA. 2013;110:E3713–E3722. doi: 10.1073/pnas.1309907110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci USA. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kambadur R, Sharma M, Smith TP, Bass JJ. Mutations in myostatin (GDF8) in double-muscled Belgian Blue and Piedmontese cattle. Genome Res. 1997;7:910–916. doi: 10.1101/gr.7.9.910. [DOI] [PubMed] [Google Scholar]
- 51.McPherron AC, Lawler AM, Lee SJ. Regulation of anterior/posterior patterning of the axial skeleton by growth/differentiation factor 11. Nat Genet. 1999;22:260–264. doi: 10.1038/10320. [DOI] [PubMed] [Google Scholar]
- 52.Brack AS, Rando TA. Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev. 2007;3:226–237. doi: 10.1007/s12015-007-9000-2. [DOI] [PubMed] [Google Scholar]
- 53.García-Prat L, Sousa-Victor P, Munoz-Canoves P. Functional dysregulation of stem cells during aging: a focus on skeletal muscle stem cells. FEBS J. 2013;280:4051–4062. doi: 10.1111/febs.12221. [DOI] [PubMed] [Google Scholar]
- 54.Notta F, et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 55.Bernet JD, et al. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med. 2014;20:265–271. doi: 10.1038/nm.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sousa-Victor P, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014;506:316–321. doi: 10.1038/nature13013. [DOI] [PubMed] [Google Scholar]
- 57.Palacios D, et al. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–469. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Troy A, et al. Coordination of satellite cell activation and self - renewal by Par-complex -dependent asymmetric activation of p38alpha/beta MAPK. Cell Stem Cell. 2012;11:541–553. doi: 10.1016/j.stem.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baker DJ, et al. Opposing roles for p16Ink4a and p19Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol. 2008;10:825–836. doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baker DJ, et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hall JK, Banks GB, Chamberlain JS, Olwin BB. Prevention of muscle aging by myofiber-associated satellite cell transplantation. Sci Transl Med. 2010;2:57ra83. doi: 10.1126/scitranslmed.3001081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chakkalakal JV, et al. Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state. Development. 2014;141:1649–1659. doi: 10.1242/dev.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- 65.Bouché M, Munoz-Canoves P, Rossi F, Coletti D. Inflammation in muscle repair, aging, and myopathies. Biomed Res Int. 2014;2014:821950. doi: 10.1155/2014/821950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tidball JG. Mechanisms of muscle injury, repair, and regeneration. Compr Physiol. 2011;1:2029–2062. doi: 10.1002/cphy.c100092. [DOI] [PubMed] [Google Scholar]
- 67.Bosurgi L, Manfredi AA, Rovere-Querini P. Macrophages in injured skeletal muscle: a perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Front Immunol. 2011;2:62. doi: 10.3389/fimmu.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chazaud B. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology. 2014;219:172–178. doi: 10.1016/j.imbio.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Arnold L, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cheng M, Nguyen MH, Fantuzzi G, Koh TJ. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am J Physiol Cell Physiol. 2008;294:C1183–C1191. doi: 10.1152/ajpcell.00568.2007. [DOI] [PubMed] [Google Scholar]
- 71.Perdiguero E, et al. p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol. 2011;195:307–322. doi: 10.1083/jcb.201104053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 73.Coppé JP, et al. Senescence-associated secretory phenotypes reveal cell-nonauto-nomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Demaria M, et al. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014;31:722–733. doi: 10.1016/j.devcel.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Joe AW, et al. Muscle injury activats resident fibro/adipogenic progenitors that facili tate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 77.Pretheeban T, Lemos DR, Paylor B, Zhang RH, Rossi FM. Role of stem/progenitor cells in reparative disorders. Fibrogenesis Tissue Repair. 2012;5:20. doi: 10.1186/1755-1536-5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heredia JE, et al. Type 2 innate signals stimulate fibro/adipogenic progenitors to facilitate muscle regeneration. Cell. 2013;153:376–388. doi: 10.1016/j.cell.2013.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lemos DR, et al. Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors. Nat Med. 2015;21:786–794. doi: 10.1038/nm.3869. [DOI] [PubMed] [Google Scholar]
- 80.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rodeheffer MS. Tipping the scale: muscle versus fat. Nat Cell Biol. 2010;12:102–104. doi: 10.1038/ncb0210-102. [DOI] [PubMed] [Google Scholar]
- 82.Serrano AL, et al. Cellular and molecular mechanisms regulating fibrosis in skeletal muscle repair and disease. Curr Top Dev Biol. 2011;96:167–201. doi: 10.1016/B978-0-12-385940-2.00007-3. [DOI] [PubMed] [Google Scholar]
- 83.Thorsteinsdóttir S, Deries M, Cachaco AS, Bajanca F. The extracellular matrix dimension of skeletal muscle development. Dev Biol. 2011;354:191–207. doi: 10.1016/j.ydbio.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 84.Kothari P, et al. IL-6-mediated induction of matrix metalloproteinase-9 is modulated by JAK-dependent IL-10 expression in macrophages. J Immunol. 2014;192:349–357. doi: 10.4049/jimmunol.1301906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu Q, Stamenkovic I. Cell surface-localized matrix metalloproteinase-9 proteolyti-cally activates TGF-β and promotes tumor invasion and angiogenesis. Genes Dev. 2000;14:163–176. [PMC free article] [PubMed] [Google Scholar]
- 86.Philippou A, Maridaki M, Koutsilieris M. The role of urokinase-type plasminogen activator (uPA) and transforming growth factor beta 1 (TGFβ1) in muscle regeneration. In Vivo. 2008;22:735–750. [PubMed] [Google Scholar]
- 87.Snow MH. The effects of aging on satellite cells in skeletal muscles of mice and rats. Cell Tissue Res. 1977;185:399–408. doi: 10.1007/BF00220299. [DOI] [PubMed] [Google Scholar]
- 88.Kovanen V, Suominen H, Risteli J, Risteli L. Type IV collagen and laminin in slow and fast skeletal muscle in rats–effects of age and life-time endurance training. Coll Relat Res. 1988;8:145–153. doi: 10.1016/s0174-173x(88)80026-8. [DOI] [PubMed] [Google Scholar]
- 89.Alexakis C, Partridge T, Bou-Gharios G. Implication of the satellite cell in dystro-phic muscle fibrosis: a self-perpetuating mechanism of collagen overproduction. Am J Physiol Cell Physiol. 2007;293:C661–C669. doi: 10.1152/ajpcell.00061.2007. [DOI] [PubMed] [Google Scholar]
- 90.Scimè A, et al. Transcriptional profiling of skeletal muscle reveals factors that are necessary to maintain satellite cell integrity during ageing. Mech Ageing Dev. 2010;131:9–20. doi: 10.1016/j.mad.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 91.Paliwal P, Pishesha N, Wijaya D, Conboy IM. Age dependent increase in the levels of osteopontin inhibits skeletal muscle regeneration. Aging (Albany, NY) 2012;4:553–566. doi: 10.18632/aging.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosant C, Nagel MD, Perot C. Aging affects passive stiffness and spindle function of the rat soleus muscle. Exp Gerontol. 2007;42:301–308. doi: 10.1016/j.exger.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 93.Gao Y, Kostrominova TY, Faulkner JA, Wineman AS. Age-related changes in the mechanical properties of the epimysium in skeletal muscles of rats. J Biomech. 2008;41:465–469. doi: 10.1016/j.jbiomech.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Engler AJ, et al. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boonen KJ, Rosaria-Chak KY, Baaijens FP, van der Schaft DW, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol. 2009;296:C1338–C1345. doi: 10.1152/ajpcell.00015.2009. [DOI] [PubMed] [Google Scholar]
- 96.Liu H, Niu A, Chen SE, Li YP. Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J. 2011;25:1914–1921. doi: 10.1096/fj.10-170449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang HV, et al. Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. J Cell Biol. 2008;180:1037–1049. doi: 10.1083/jcb.200707175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pisconti A, Cornelison DD, Olguin HC, Antwine TL, Olwin BB. Syndecan-3 and Notch cooperate in regulating adult myogenesis. J Cell Biol. 2010;190:427–441. doi: 10.1083/jcb.201003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Urciuolo A, et al. Collagen VI regulates satellite cell self - renewal and muscle regeneration. Nat Commun. 2013;4:1964. doi: 10.1038/ncomms2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bentzinger CF, et al. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell. 2013;12:75–87. doi: 10.1016/j.stem.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 102.Halder G, Dupont S, Piccolo S. Transduction of mechanical and cytoskeletal cues by YAP and TAZ. Nat Rev Mol Cell Biol. 2012;13:591–600. doi: 10.1038/nrm3416. [DOI] [PubMed] [Google Scholar]
- 103.Dupont S, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 104.Pelissier FA, et al. Age-related dysfunct ion in mechanotransduction impairs differentiation of human mammary epithelial progenitors. Cell Rep. 2014;7:1926–1939. doi: 10.1016/j.celrep.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quyn AJ, et al. Spindle orientation bias in gut epithelial stem cell compartments is lost in precancerous tissue. Cell Stem Cell. 2010;6:175–181. doi: 10.1016/j.stem.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 107.Liu L, et al. Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep. 2013;4:189–204. doi: 10.1016/j.celrep.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.McKinnell IW, et al. Pax7 activates myogenic genes by recruitment of a histone methyltransferase complex. Nat Cell Biol. 2008;10:77–84. doi: 10.1038/ncb1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Paylor B, Natarajan A, Zhang RH, Rossi F. Nonmyogenic cells in skeletal muscle regeneration. Curr Top Dev Biol. 2011;96:139–165. doi: 10.1016/B978-0-12-385940-2.00006-1. [DOI] [PubMed] [Google Scholar]
- 110.Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013;14:1062–1072. doi: 10.1038/embor.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonfanti C, et al. P W1/Peg 3 expression regluates key properties that determine mesoangioblast stem cell competence. Nat Commun. 2015;6:6364. doi: 10.1038/ncomms7364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 113.Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 115.Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol. 2014;15:802–812. doi: 10.1038/nrm3896. [DOI] [PMC free article] [PubMed] [Google Scholar]