Abstract
p53 mutations are mostly single amino acid changes resulting in expression of a stable mutant protein with “gain of function” (GOF) activity having a dominant oncogenic role rather than simple loss of function of wild-type p53. Knock-down of mutant p53 in human lung cancer cell lines with different endogenous p53 mutants results in loss of GOF activity as shown by lowering of cell growth rate. Two lung cancer cell lines, ABC1 and H1437 carrying endogenous mutants p53–P278S and –R267P, both show reduction in growth rate on knock-down on p53 levels. However, whereas reduction of the p53 level induces loss of tumorigenicity in nude mice for ABC1 cells, it escalates tumorigenicity for H1437 cells. We have tested their transactivation potential on p53 target gene promoters by performing transient transcriptional assays in the p53-null H1299 lung cancer cell line. Interestingly, while the mutant p53 target promoter Axl was activated by both the mutants, the p21 promoter was activated by p53-R267P and wild-type p53 but not by p53-P278S; showing a clear difference in transcriptional activity between the two mutants. Our result demonstrates allele specificity between GOF p53 mutants and attempt to show that the specificity is dependent on the transactivation property of GOF p53; it also suggests importance of p21 activation in tumor suppression by p53.
Keywords: mutant, p53, allele, specificity, in vivo
Introduction
p53 is the most frequently mutated “driver” gene in lung cancer, and frequencies range from 33% in adenocarcinomas to 70% in small cell lung cancers [1–4]. Studies suggest that lung cancers and cells with p53 mutations carry a worse prognosis and are more resistant to chemotherapeutic drugs and radiation [5–10] suggesting acquisition of gain-of-function (GOF) properties (i.e., acquired growth enhancing and oncogenic functions) by p53 mutations.
Unlike other tumor suppressors, in addition to loss of tumor suppressor function, in many cases mutations in p53 result in GOF activity [11]. A number of different phenotypes have been ascribed to the GOF activities of mutant p53 including increased tumorigenicity [12,13], increased metastasis and invasiveness [14], decreased sensitivity to chemotherapeutic drugs [9,15], increased growth rate [16], and increased motility [17], among others. We have demonstrated that knockdown of endogenous mutant p53 in lung cancer cells causes loss of tumorigenicity [18]. Whether different p53 mutations differ in their GOF ability (allele specificity) has not been deciphered at any length, particularly mechanistically.
We and others have shown that GOF p53 mutants modulate transcription of a series of genes, many of which are involved in cell proliferation and oncogenesis [15,19,20,21,22,23,24]. The mechanism of GOF has not been completely established, although mutant p53-mediated up-regulation seems to play a significant role along with GOF p53’s ability to interact with p53 family members p63 and p73 [11]. However, some examples of differential GOF activities for different p53 mutants including transactivation properties, growth in agar, and tumorigenicity have been demonstrated [9,25,26] (http://p53.free.fr/our_work/structure.html), although not studied in depth. Earlier we have shown differences in the transactivation potential of different p53 mutants [19].
We have used naturally occurring human lung cancer cell lines with endogenous mutant p53 to investigate allele specificity in GOF activity of different p53 mutants observed in lung cancer. Knock-down of mutant p53 in human lung cancer cell lines with different endogenous p53 mutants results in loss of GOF activity as shown by lowering of cell growth rate. Two of the lung adenocarcinoma cell lines, ABC1 and H1437, carrying two different p53 mutants, showed reduction in GOF activities upon lowering of p53 levels while their tumorigenicity and transcriptional properties showed dramatic differences. Our results clearly demonstrated allele specificity between GOF p53 mutants and attempted to show for the first time that the allele specificity is dependent on the transactivation property of GOF p53.
Materials and Methods
Cells
H1299, ABC1, VMRC-LCD, H1437, KNS-62, and H2405 cells were all purchased from commercial sources, and were maintained in media as suggested by the suppliers. Mutant p53 knock down cell lines were generated by using lentivirus expressing short hairpin RNA (shRNA) against p53 (or control green fluorescence protein, GFP) utilizing lentivirus systems (Open Biosystems) following the manufacturer’s protocol. Clones were isolated using puromycin selection at 1 μg/ml.
Growth assay
Growth assays were carried out as described by us earlier [15,19]. Multiple cell clones were used for each assay. All experiments were done in triplicate, and repeated multiple times. The error bars represent standard deviation from the triplicates. The Student’s t-test was used for statistical analysis.
Xenograft assay
Nu/nu mice were used for the tumorigenicity studies. For all injections, 1×107 cells/250μl media were used. Mice were injected subcutaneously on the flanks and tumors allowed to grow to a maximum size of 1.2cm, measuring periodically as described before (17, 18). Two different clones of ABC1 and H1437 cells with mutant p53 levels reduced by shRNA were used in comparison to two GFP shRNA control cell lines to rule out clonal variations. The Student’s t-test was used for statistical analysis.
Transient promoter assays
Axl and p21 promoters were cloned in pGL3-basic vector using sequence information available in NCBI or as published. Transient transfection was performed in H1299 p53-null lung cancer cells with 200ng of promoter and 1ug of expression plasmid using Lipofectamine 2000 (Invitrogen) following the manufacturer’s instructions. Luciferase analysis was carried out using the luciferase assay system (E1500) and instructions from Promega. Both transfection and luciferase assays were performed as described previously in triplicate [27]. The Student’s t-test was used for statistical analysis.
Western blotting
Immunoblottings were carried out as described [15]. p53 was detected using the p53 antibody PAb 1801, and Erk2 was detected using ERK2 (sc-154) antibody from Santa Cruz Biotechnology. Westerns blots were developed by the ECL method (GE Healthcare; Piscataway, NJ).
Results
Lung cancer cells expressing GOF mutant p53 show lowering of growth potential on reduction of the mutant p53 levels
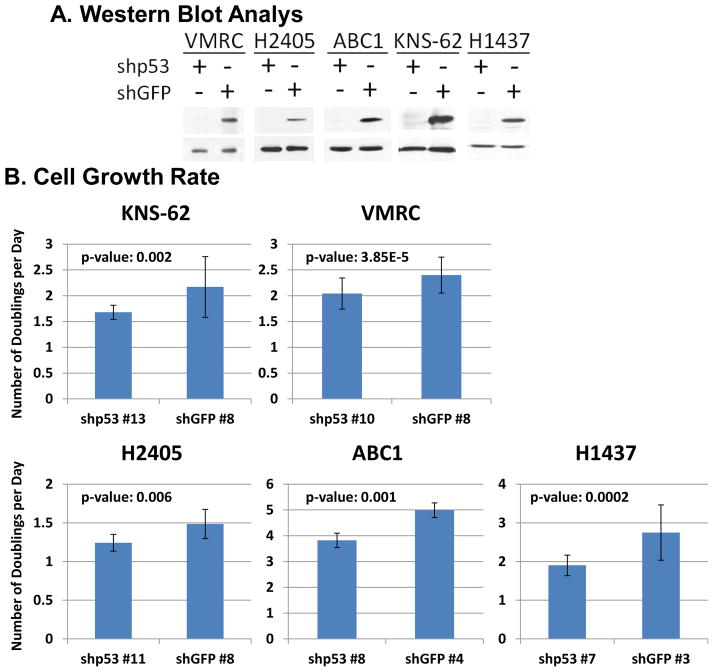
We have analyzed 5 lung cancer cell lines ABC1 (p53-P278S), VMRC-LCD (p53-R175H), H1437 (p53-R267P), KNS-62 (p53-R249S), and H2405 (p53-R273H) for its GOF activity. For this we have generated mutant p53 knock-down cells with corresponding controls as described in Materials and Methods. For each cell line, multiple clones with significantly reduced p53 levels have been generated. Data in Figure 1A shows an immunoblot demonstrating such clones with reduced p53 levels. For illustrative purposes only single clones have been shown. Rates of growth between the different cell lines are shown in Figure 1B, depicting decrease of rate of growth with reduction of p53 level for a number of cell lines under study. Other knock-down clones showed a similar trend and the data is representative of all cell clones. We have earlier demonstrated that knock-down of mutant p53 in H1437 cell also results in lowering GOF activity as shown by a reduction of rate of growth [28].
Figure 1. Human lung cancer cell lines with endogenous mutant p53 show loss of gain of function activities on mutant p53 knock-down.
A. Western blot analysis showing knock down of mutant p53 level in human lung cancer cell lines. VMRC, H2405, ABC1, H1437 and KNS62 cells were infected with GFP or p53-specific shRNA expressing lentivirus, and p53 knock-down cell clones were generated as described in Materials and Methods. The Western blot analysis shows representative examples of different clones. B. Growth rate of VMRC, H2405, ABC1, H1437 and KNS62 lung cancer cells depends on the mutant p53 level. Different cell clones are plated in equal numbers for growth assays as described in Materials and Methods, and harvested each day for five days to determine the rate of doubling. The cell clones showed are a representative sample of all cell clones which showed similar trends.
Lung adenocarcinoma ABC1 and H-1437 cells expressing GOF mutant p53 show differing effect on tumorigenicity on reduction of the mutant p53 levels
We wanted to see if lowering mutant p53 level would affect one of the most important GOF properties: tumorigenicity in nude mice. Figure 2 shows xenograft assay data of ABC1 and H1437 cells (control and p53 knock downs) obtained after injections in athymic nude mice. The ABC1 and H1437 cell lines showed the fastest growth compared to the other three cell lines and thus were selected for the tumorigenicity assay. The data show an interesting situation. As expected, both ABC1 and H1437 control (GFP) cells formed growing tumors. On reduction of mutant p53, tumorigenicity of ABC1 was reduced; however, tumorigenicity of H1437 increased on reduction of mutant p53 level, although both cell lines showed reduced growth potential on reduction of the p53 level (see above). This suggested a functional difference between either the cellular milieu or the mutant p53s.
Figure 2. p53 knock down in cancer cell lines with naturally occurring p53 mutations causes a change in rate of tumor growth.
Mutant p53 levels were knocked down in the lung cancer cell lines ABC1 (p53-P278S) and H1437 (p53-R267P) by lentivirus expressing p53 shRNA or GFP shRNA (used as our control cell line) as indicated and its tumorigenic ability measured after subcutaneous injection into nude mice. For all injections, 1×107 cells/250μl media were used. Mice were injected subcutaneously and tumors allowed to grow to a maximum size of 1cm, measuring periodically as described before (17, 18). Two different clones of ABC1 and H1437 cells with mutant p53 levels knocked down by shRNA were used in comparison to two GFP shRNA control cell lines to eliminate clonal variations. Average tumor size was calculated by taking the average of the width and length of each tumor, then taking the average of all tumors from the particular cell line.
Transcriptional properties of GOF p53 mutants expressed in p53-null H1299 lung cancer cells
To characterize the p53 mutants: p53-P278S and p53-R267, endogenous to ABC1 and H1437 for their transcriptional activities, we tested the transactivation potential on Axl and p21 gene promoters by performing transient transcriptional assays in the H1299 lung cancer cell line with no endogenous p53. Axl is a mutant p53 target gene whose expression gets up-regulated by GOF p53 mutants [15] and p21 is a well established target for WT p53. The data in Figure 3 interestingly show a clear difference in transcriptional activity between the two mutants, p21 promoter being activated by p53-R267P and WT p53 but not p53-P278S. The Axl promoter, on the other hand, has been transactivated by WT and the two other mutants as expected. WT p53 is expected to transactivate the Axl promoter as Axl has a p53/63 binding site [29].
Figure 3. Wild-type and mutant p53 target promoters are differentially modulated by p53 endogenous to ABC1 and H1437 cells, and WT p53 in H1299 cells.
H1299 cells were transfected with 200ng of pGL3-basic vector containing the human Axl or p21 promoter upstream of the luciferase reporter gene, and 1ug of the indicated p53 expression plasmid. Cells lysates were prepared 48hr after transfection, and luciferase activity was determined. Data is shown as fold activation over control. Experiments were done in triplicate. Error bars showing standard deviations are indicated.
Discussion
Here we demonstrate that lung cancer p53 mutants have differential GOF properties (Figures 1–3). The exact reason for the quantitative and qualitative differences between them is not easily discernible and definable. This manifests perhaps in part the heterogeneity of lung cancer itself in terms of aggressiveness and prognosis, a mechanistic reason for which is not clear yet. This is our attempt to delve into that.
Both of the two p53 mutants under scrutiny here: p53-P278S (from ABC1) and p53-R267P (from H1437), have demonstrated GOF activity as shown by decrease of cell growth upon their knock-down in their native cell surroundings (Figure 1). However, after p53 knock-down the two cell lines behaved differently towards tumorigenicity in nude mice, while decrease of p53-P278S caused reduction of tumor formation, knockdown of p53-R267P resulted in an increase. This apparent paradox was partially resolved by examining transcriptional properties of the two p53 mutants in H1299 cells. As expected, both of the mutants activated the promoter for the Axl gene as did WT p53. WT p53 is expected to up-regulate the Axl promoter since it has a p53/p63 binding site [29]. As expected, the p21 promoter was transactivated by WT p53; surprisingly, however, mutant p53-R267P also up-regulated this promoter although this position of p53 mutation is conserved between p53, p73 and p63 [30]. This suggests that p53-R267P retains partial WT p53 functions as it may induce expression of the p21 gene. The implication of this observation is highly significant in that it suggests p21 activation to be crucial in tumor suppression as inability to activate p21 is related to formation of tumor formation in nude mice.
The mechanism of GOF activities by tumor-derived mutant p53 is still under development. Two mutually non-exclusive possibilities are: (1) GOF functions are attained by mutant p53 interacting with p53 family members, p73 and p63, neutralizing their growth suppressive functions leading to the GOF phenotype. In a modification of that hypothesis, mutant p53 may interact with other cellular proteins to gain oncogenic activities. (2) GOF mutant p53 modulates a series of genes involved in cell growth and oncogenesis. Therefore, the differential nature of GOF activities that are reported here should be explained in the light of these two theories.
Since p53 mutations under consideration in this report occupy structurally different positions in the DNA binding domain of p53, the structural effects caused by them could be varied. This could possibly result in an alteration of the three dimensional structure of the p53 protein in different ways which might impact its interaction with other proteins differentially. This differential protein-protein interaction may be responsible for the observed functional difference, and could be explained by both the hypotheses, as both of these rely ultimately on protein-protein and or protein/nucleic acid interactions, either with p67/p73 or with other factor(s) involved in transcription.
Highlights.
We show that different p53 mutants endogenously expressed in human lung cancer cells demonstrate gain of function activity
Not all the p53 mutants in these cells show identical gain of function (GOF) activities
We demonstrate that knock-down of mutant p53 of a majority of lung cancer cells with endogenous mutant p53 causes reduction of growth rate universally, although effect on xenograft assays differ remarkably.
We relate the difference in physiological (GOF) activities with differences in transcriptional functions.
Acknowledgments
This work was supported by grants from the NIH to Sumitra Deb (CA70712 and CA121144) and Swati Palit Deb (CA74172), Pilot Project Awards from Massey Cancer to Sumitra Deb, Swati Palit Deb, Andrew Yeudall and Brad Windle, and a grant from Jeffress Trust Foundation to Brad Windle. We thank Arnold Levine, and Bert Vogelstein, for providing us with reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chang YL, Wu CT, Shih JY, Lee YC. Comparison of p53 and epidermal growth factor receptor gene status between primary tumors and lymph node metastases in non-small cell lung cancers. Ann Surg Oncol. 18:543–550. doi: 10.1245/s10434-010-1295-6. [DOI] [PubMed] [Google Scholar]
- 2.Harris CC. p53 tumor suppressor gene: from the basic research laboratory to the clinic--an abridged historical perspective. Carcinogenesis. 1996;17:1187–1198. doi: 10.1093/carcin/17.6.1187. [DOI] [PubMed] [Google Scholar]
- 3.Bronte G, Rizzo S, La Paglia L, Adamo V, Siragusa S, Ficorella C, Santini D, Bazan V, Colucci G, Gebbia N, Russo A. Driver mutations and differential sensitivity to targeted therapies: a new approach to the treatment of lung adenocarcinoma. Cancer Treat Rev. 36(Suppl 3):S21–29. doi: 10.1016/S0305-7372(10)70016-5. [DOI] [PubMed] [Google Scholar]
- 4.Vandin F, Upfal E, Raphael BJ. De novo discovery of mutated driver pathways in cancer. Genome Res. doi: 10.1101/gr.120477.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campling BG, El-Deiry WS. Clinical implication of p53 mutation in lung cancer. Mol Biotechnol. 2003;24:141–156. doi: 10.1385/MB:24:2:141. [DOI] [PubMed] [Google Scholar]
- 6.Mogi A, Kuwano H. TP53 mutations in nonsmall cell lung cancer. J Biomed Biotechnol. 2011:583929. doi: 10.1155/2011/583929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakajima T, Yasufuku K, Nakagawara A, Kimura H, Yoshino I. Multi-gene mutation analysis of metastatic lymph nodes in non-small cell lung cancer diagnosed by EBUS-TBNA. Chest. doi: 10.1378/chest.10-3186. [DOI] [PubMed] [Google Scholar]
- 8.Lai SL, Perng RP, Hwang J. p53 gene status modulates the chemosensitivity of non-small cell lung cancer cells. J Biomed Sci. 2000;7:64–70. doi: 10.1007/BF02255920. [DOI] [PubMed] [Google Scholar]
- 9.Blandino G, Levine AJ, Oren M. Mutant p53 gain of function: differential effects of different p53 mutants on resistance of cultured cells to chemotherapy. Oncogene. 1999;18:477–485. doi: 10.1038/sj.onc.1202314. [DOI] [PubMed] [Google Scholar]
- 10.Li R, Sutphin PD, Schwartz D, Matas D, Almog N, Wolkowicz R, Goldfinger N, Pei H, Prokocimer M, Rotter V. Mutant p53 protein expression interferes with p53-independent apoptotic pathways. Oncogene. 1998;16:3269–3277. doi: 10.1038/sj.onc.1201867. [DOI] [PubMed] [Google Scholar]
- 11.Oren M, Rotter V. Mutant p53 gain-of-function in cancer. Cold Spring Harb Perspect Biol. 2:a001107. doi: 10.1101/cshperspect.a001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin J, Teresky AK, Levine AJ. Two critical hydrophobic amino acids in the N-terminal domain of the p53 protein are required for the gain of function phenotypes of human p53 mutants. Oncogene. 1995;10:2387–2390. [PubMed] [Google Scholar]
- 13.Lanyi A, Deb D, Seymour RC, Ludes-Meyers JH, Subler MA, Deb S. ‘Gain of function’ phenotype of tumor-derived mutant p53 requires the oligomerization/nonsequence-specific nucleic acid-binding domain. Oncogene. 1998;16:3169–3176. doi: 10.1038/sj.onc.1201857. [DOI] [PubMed] [Google Scholar]
- 14.Taylor WR, Egan SE, Mowat M, Greenberg AH, Wright JA. Evidence for synergistic interactions between ras, myc and a mutant form of p53 in cellular transformation and tumor dissemination. Oncogene. 1992;7:1383–1390. [PubMed] [Google Scholar]
- 15.Scian MJ, Stagliano KE, Anderson MA, Hassan S, Bowman M, Miles MF, Deb SP, Deb S. Tumor-derived p53 mutants induce NF-kappaB2 gene expression. Mol Cell Biol. 2005;25:10097–10110. doi: 10.1128/MCB.25.22.10097-10110.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy KL, Dennis AP, Rosen JM. A gain of function p53 mutant promotes both genomic instability and cell survival in a novel p53-null mammary epithelial cell model. Faseb J. 2000;14:2291–2302. doi: 10.1096/fj.00-0128com. [DOI] [PubMed] [Google Scholar]
- 17.Yeudall WA, Vaughan CA, Miyazaki H, Ramamoorthy M, Choi MY, Chapman CG, Wang H, Black E, Bulysheva AA, Deb SP, Windle B, Deb S. Gain-of-function mutant p53 upregulates CXC chemokines and enhances cell migration. Carcinogenesis. 2012;33:442–451. doi: 10.1093/carcin/bgr270. [DOI] [PubMed] [Google Scholar]
- 18.Vaughan CA, Singh S, Windle B, Sankala HM, Graves PR, Andrew Yeudall W, Deb SP, Deb S. p53 mutants induce transcription of NF-kappaB2 in H1299 cells through CBP and STAT binding on the NF-kappaB2 promoter and gain of function activity. Arch Biochem Biophys. 2012;518:79–88. doi: 10.1016/j.abb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scian MJ, Stagliano KE, Ellis MA, Hassan S, Bowman M, Miles MF, Deb SP, Deb S. Modulation of gene expression by tumor-derived p53 mutants. Cancer Res. 2004;64:7447–7454. doi: 10.1158/0008-5472.CAN-04-1568. [DOI] [PubMed] [Google Scholar]
- 20.Cadwell C, Zambetti GP. The effects of wild-type p53 tumor suppressor activity and mutant p53 gain-of-function on cell growth. Gene. 2001;277:15–30. doi: 10.1016/s0378-1119(01)00696-5. [DOI] [PubMed] [Google Scholar]
- 21.Sigal A, Rotter V. Oncogenic mutations of the p53 tumor suppressor: the demons of the guardian of the genome. Cancer Res. 2000;60:6788–6793. [PubMed] [Google Scholar]
- 22.Singer S, Ehemann V, Brauckhoff A, Keith M, Vreden S, Schirmacher P, Breuhahn K. Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis. Hepatology. 2007;46:759–768. doi: 10.1002/hep.21736. [DOI] [PubMed] [Google Scholar]
- 23.Strano S, Dell’Orso S, Di Agostino S, Fontemaggi G, Sacchi A, Blandino G. Mutant p53: an oncogenic transcription factor. Oncogene. 2007;26:2212–2219. doi: 10.1038/sj.onc.1210296. [DOI] [PubMed] [Google Scholar]
- 24.Weisz L, Damalas A, Liontos M, Karakaidos P, Fontemaggi G, Maor-Aloni R, Kalis M, Levrero M, Strano S, Gorgoulis VG, Rotter V, Blandino G, Oren M. Mutant p53 enhances nuclear factor kappaB activation by tumor necrosis factor alpha in cancer cells. Cancer Res. 2007;67:2396–2401. doi: 10.1158/0008-5472.CAN-06-2425. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa K, Hamada J, Tada M, Kameyama T, Nakagawa K, Suzuki Y, Ikawa M, Hassan NM, Kitagawa Y, Moriuchi T. Mutant p53 R248Q but not R248W enhances in vitro invasiveness of human lung cancer NCI-H1299 cells. Biomed Res. 31:401–411. doi: 10.2220/biomedres.31.401. [DOI] [PubMed] [Google Scholar]
- 26.Xu J, Reumers J, Couceiro JR, De Smet F, Gallardo R, Rudyak S, Cornelis A, Rozenski J, Zwolinska A, Marine JC, Lambrechts D, Suh YA, Rousseau F, Schymkowitz J. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat Chem Biol. 2011;7:285–295. doi: 10.1038/nchembio.546. [DOI] [PubMed] [Google Scholar]
- 27.Vaughan CA, Singh S, Windle B, Sankala HM, Graves PR, Andrew Yeudall W, Deb SP, Deb S. p53 mutants induce transcription of NF-kappaB2 in H1299 cells through CBP and STAT binding on the NF-kappaB2 promoter and gain of function activity. Arch Biochem Biophys. 518:79–88. doi: 10.1016/j.abb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan CA, Singh S, Windle B, Sankala HM, Graves PR, Andrew Yeudall W, Deb SP, Deb S. p53 mutants induce transcription of NF-kappaB2 in H1299 cells through CBP and STAT binding on the NF-kappaB2 promoter and gain of function activity. Arch Biochem Biophys. 2011;518:79–88. doi: 10.1016/j.abb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barton CE, Johnson KN, Mays DM, Boehnke K, Shyr Y, Boukamp P, Pietenpol JA. Novel p63 target genes involved in paracrine signaling and keratinocyte differentiation. Cell Death Dis. 2010;1:e74. doi: 10.1038/cddis.2010.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levrero M, De Laurenzi V, Costanzo A, Gong J, Wang JY, Melino G. The p53/p63/p73 family of transcription factors: overlapping and distinct functions. J Cell Sci. 2000;113(Pt 10):1661–1670. doi: 10.1242/jcs.113.10.1661. [DOI] [PubMed] [Google Scholar]