Abstract
Objective
The goal of this study was to determine whether endosomal Toll-like receptors (TLRs) contribute to the clinical manifestation of systemic autoimmunity exhibited by mice that lack the lysosomal nuclease DNaseII.
Methods
DNaseII/IFNaR double deficient mice were intercrossed with Unc93b13d/3d mice to generate DNaseII−/− mice with non-functional endosomal TLRs. The resulting triple deficient mice were evaluated for arthritis, autoantibody production, splenomegaly, and extramedullary hematopoiesis. B cells from both strains were evaluated for their capacity to respond to endogenous DNA by using small oligonucleotide based TLR9 ligands and a novel class of bifunctional anti-DNA antibodies.
Results
Mice that fail to express DNaseII, IFNaR, and Unc93b1 still develop arthritis but do not make autoantibodies, develop splenomegaly, or exhibit extramedullary hematopoiesis. DNaseII−/− IFNaR−/− B cells can respond to synthetic ODNs, but not to endogenous dsDNA.
Conclusion
RNA-reactive TLRs, presumably TLR7, are required for autoantibody production, splenomegaly, and extramedullary hematopoiesis in the DNaseII−/− model of systemic autoimmunity.
Keywords: TLR7, TLR9, DNaseII, arthritis, autoantibody, anti-nuclear antibody, splenomegaly, extramedullary hematopoiesis, STING, bifunctional antibody
Introduction
Toll-like receptors (TLRs) were initially identified in the context of host defense, where they were implicated in the detection of pathogen-associated molecular patterns uniquely expressed by infectious agents (1). However, it is now clear that TLRs also detect mammalian ligands released from stressed or dying cells and thereby play a key role in IFN-driven systemic autoimmune diseases such as SLE (2, 3). Endosomal TLRs are particularly important in the activation of autoreactive B cells (4, 5). Both in vivo and in vitro studies have demonstrated that the production of autoantibodies reactive with DNA or DNA-associated proteins is promoted by TLR9, while the production of autoantibodies reactive with RNA or RNA-associated proteins is promoted by TLR7 (6). Beyond autoantibody production, TLR-activated B cells also effectively activate autoreactive T cells and contribute to antibody-independent pathogenic processes (7).
TLRs are not the only nucleic acid sensing receptors that can detect endogenous ligands. In recent years, numerous cytosolic receptors have been found to also detect the aberrant cytosolic accumulation of both microbial and mammalian DNA and RNA (8). These include DNA sensors upstream of STING, such as cGAS or Ifi204 (9, 10), DNA sensors that can activate inflammasomes, such as AIM2 (11), and RNA sensors upstream of MAVS (12). Activation of these receptors by endogenous ligands has been shown to promote a variety of “autoinflammatory” conditions. Perhaps the best-described example is Aicardi-Goutieres syndrome, an early onset neuroinflammatory disease that occurs in individuals with loss of function mutations in cytosolic nucleases such as Trex1 or SAMHD1 (13, 14). Related mutations have also been associated with clinical manifestations of lupus and even autoantibody production. In addition, gain-of-function mutations of STING can also have pathological consequences, as evident in SAVI patients where the spontaneous dimerization of STING leads to a vasculopathy often associated with pulmonary fibrosis (15, 16).
DNaseII-deficiency and systemic autoimmunity
The enzyme DNaseII also constrains access of nucleic acids to cytosolic sensors. DNaseII is considered a lysosomal DNase and is responsible for the degradation of DNA engulfed as a result of processes such as apoptotic cell death or the extrusion of reticulocyte nuclei (17). DNaseII also plays a role in non-phagocytic cells, where it facilitates the autophagosome-dependent removal of damaged DNA extruded through the nuclear pores of stressed cells (18). Whether DNaseII is involved in the degradation of other potential sources of cytosolic DNA, (e.g. retroelement intermediates or mitochondrial DNA), remains to be determined. The importance of DNaseII to innate immune homeostasis is apparent from the initial observations of Nagata and colleagues. They found that DNaseII deficiency in mice causes embryonic lethality, due to severe anemia associated with excessively high levels of type I IFN (19). DNaseII−/− mice can be rescued by intercrossing them with mice that fail to express either the type I IFN receptor (IFNaR) or STING (19, 20). These data demonstrate that IFN production in this model is downstream of STING and thus depends on the cytosolic DNA sensors. As adults, DNaseII−/− IFNaR−/− double knockout (DKO) mice develop a severe inflammatory arthritis (21), not found in DNaseII/IFNaR/STING triple knockout (STING TKO) mice (22). Thus STING dependent pathways drive the production of proinflammatory cytokines through IFN-independent pathways. Intriguingly, a SNP in the 5′-regulatory region of DNaseII has been shown to confer a significant increase in RA disease susceptibility in a German Caucasian cohort (23), and the same SNP has been linked to an increased risk for renal complications in a group of Korean SLE patients (24). Thus DNaseII polymorphisms appear to contribute to human systemic autoimmune disease.
SLE-related autoantibody production in DNaseII−/− IFNaR−/− mice is TLR dependent
In line with the human genetic studies mentioned above, DKO mice also develop clinical manifestations of SLE, including the production of anti-nuclear antibodies (ANAs) and splenomegaly (22, 25). We have further explored the connection between DNaseII deficiency and SLE, with particular attention to the involvement of nucleic acid sensing TLRs. Our group has had a particular interest in the connection between pattern recognition receptors and autoantibody production in murine models of SLE, where autoantibody production is invariably TLR dependent. Previous studies suggested that DKO mice made rheumatoid factor (RF), anti-citrullinated protein antibodies (ACPA), and anti-DNA antibodies. We could only detect minimal RF and ACPA titers in DKO mice in our colony using standard ELISAs. However we were able to show that DKO mice appeared to make antibodies against an extensive panel of autoantigens, including DNA and histones, as detected by autoantigen arrays. Remarkably, autoantibody production was almost entirely TLR-dependent, as DKO mice expressing the loss-of-function 3D mutation of Unc93b1 (Unc93b1 TKO mice) completely failed to produce ANAs (25). Unc93b1 is a chaperone protein required for the functional activity of all endosomal TLRs. By contrast, STING TKO mice maintained autoantibody production. Therefore both cytosolic and endosomal nucleic acid sensors contribute to the clinical manifestations of systemic autoimmunity exhibited by DKO mice.
To more carefully evaluate the specificity of the DKO autoantibody repertoire, we also compared sera from DKO, Unc93B1 TKO, and STING TKO mice by immunofluorescent staining of HEp2 cells. As predicted by the autoantigen arrays, only the DKO and STING TKO, and not the Unc93b1 TKO sera, stained HEp2 cells. However, quite unexpectedly, we found that the vast majority of DKO sera showed staining patterns, either speckled nuclear or cytoplasmic, consistent with BCR/TLR7-driven responses, not BCR/TLR9-driven responses (25). TLR9-driven responses, directed against dsDNA or dsDNA-binding proteins, are normally associated with a homogeneous nuclear staining pattern and the detection of mitotic plates, and these were notably absent in the DKO sera. These results point to the importance of confirming actual autoantibody specificities as revealed by autoantigen arrays, and also raise the question as to why anti-dsDNA antibodies were lacking in a model of DNA accrual.
DNAseII is required for TLR9 detection of dsDNA
To confirm that TLR9 was still functional in the DKO mice, we isolated B cells from DKO, Unc93b1 TKO, and IFNaR−/− single KO mice and measured the responses of all three strains to a standard experimental TLR9 ligand, the CpGB ODN 1826. We found that both DKO and DNase+/− IFNaR−/− B cells responded normally to 1826 under conditions where the Unc93b1 TKO mice were completely unresponsive. Nevertheless, it was important to test the response of DKO mice to endogenous ligands, where the response is dependent on the uptake of autoantigen by the BCR and subsequent BCR-mediated delivery to endosomal TLR9. In previous studies we have used mice expressing a transgene-encoded BCR reactive for IgG2a. These rheumatoid factor (RF) B cells can bind IgG2a-associated immune complexes (ICs) that incorporate endogenous DNA-associated autoantigens (26). We have used defined ICs, consisting of experimentally modified dsDNA fragments, such as biotinylated CG-rich clone 11 bound by IgG2a anti-biotin antibody, to activate RF B cells (27). We have also used IgG2a autoantibodies where the autoantigen is undefined and presumably derived from cell debris (2). However the experimental use of such IgG2a ICs is limited to RF B cells since these ICs do not activate non-Tg B cells.
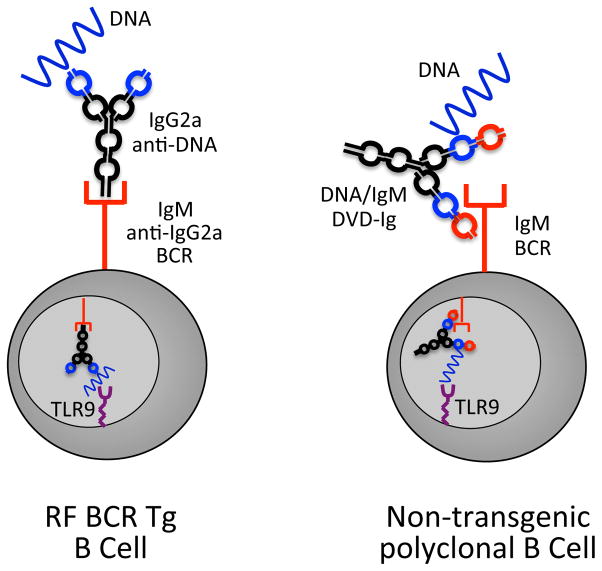
To directly test the capacity of DKO B cells to respond to endogenous ligands, we used a novel bifunctional antibody constructed to express one variable domain reactive with DNA and second variable domain reactive with IgM. These DNA/IgM dual variable domain antibodies, DVD-Ig™ can direct endogenous dsDNA ligands to all BCRs in the same way as an IgG2a IC can direct endogenous nucleic acid ligands to RF BCRs (Figure 1). Activation of both RF B cells by the original IgG2a anti-DNA autoantibody, as well as non-Tg B cells by DNA/IgM DVD-Ig™s, is entirely TLR9 dependent. Importantly we found that DKO B cells could not respond to DNA/IgM DVDs (25). These data suggested that DNAseII is required for the degradation of dsDNA in order to facilitate either the uptake of dsDNA by the BCR or the detection of dsDNA by TLR9.
Figure 1.
Comparison of IgG2a anti-DNA activation of RF B cells and DNA/IgM DVD-Ig activation of polyclonal B cells. Activation of both IgG2a-reactive RF B cells by an IgG2a anti-DNA autoantibody, and activation of polyclonal B cells by a DNA/IgM DVD-Ig, depends on co-engagement of the BCR and TLR9. The BCR delivers both kinds of immune complexes to TLR9 (purple receptor) inside an endosome (light grey circle).
The latter possibility is supported by a recent report from Miyake and colleagues who found that DNAseII is required for dendritic cell detection of microbial DNA as well as a CpGA ODNs (28). The inability of TLR9 to recognize endogenous DNA ligands in DNaseII-deficient mice accounts for the failure of the DKO mice to produce anti-dsDNA antibodies. Therefore the requirement for functional Unc93b1 in DKO ANA production most likely reflects a role for TLR7 in the detection of RNA-associated autoantigens. Presumably STING-dependent inflammation results in the release of immunogenic ligands from RNA-associated cell debris. Our previous studies had reported a close association between type 1 IFN, B cell expression of TLR7, and B cell responses to TLR7 ligands (29). However, in the context of DNaseII deficiency, DKO B cells can make autoantibodies reactive with RNA-associated autoantigens even though they cannot respond to type I IFN.
Splenomegaly and extramedullary hematopoiesis are also TLR-dependent
Not only do DKO mice develop arthritis and make autoantibodies, but they also exhibit markedly enlarged spleens from an early age. This splenomegaly has been attributed to extramedullary hematopoiesis due to the inability of erythroid island macrophages (EIM) to degrade reticulocyte nuclei and the ensuing disruption of erythropoiesis in the bone marrow. In fact DKO mice do have a significantly increased percentage of Ter119+ (erythroid lineage) cells in the spleen, again from an early age. However, both splenomegaly and extramedullary hematopoiesis are greatly reduced in Unc93b1 TKO mice, despite the persistent inability of EIM to degrade reticulocyte nuclei. STING TKO mice still develop massively enlarged spleens and extramedullary hematopoiesis. Therefore splenomegaly and extramedullary hematopoiesis in DKO mice are most likely due to factors produced by TLR-dependent mechanisms.
Summary and Conclusions
In the absence of DNaseII, undegraded DNA gains access to the cytosol where it can activate cytosolic nucleic acid sensors upstream of STING, leading to excessive type I IFN production and embryonic lethality. In the absence of a type I IFN response because of a failure to express either the type I IFN receptor or STING, DNaseII deficient mice develop clinical manifestations of systemic autoimmunity that include arthritis, ANA production, splenomegaly, and extramedullary hematopoiesis. Despite the excessive accrual of DNA in phagolysosomes and other endocytic compartments, DNaseII-deficient mice cannot respond to endogenous dsDNA because the DNA is not sufficiently degraded to effectively bind TLR9. TLR9 is functional as DKO B cells respond normally to DNA ODNs. However DKO mice can respond to endogenous RNA or RNA-associated ligands through a mechanism that depends on Unc93b1 and therefore most likely RNA-sensing TLRs. Thus both cytosolic and endosomal nucleic acid sensors contribute to the disease profile of DKO mice. Future studies will be required to better understand the interplay between these two classes of nucleic acid sensors in additional aspects of immune dysregulation in this model and other examples of systemic autoimmune disease.
Acknowledgments
We thank Dr. S. Nagata for providing DNaseII-deficient mice and Dr. T. Ghayur and C-H. Choi for the DVD-Ig™. This work was supported by National Institutes of Health grants AR050256 (A.M.R.), AI093752, AI067497, and AI083713 (K.A.F.), AR067394 (E.M.G.), F31 AR063597 (K.L.M.), AI095213, and by grants from the Lupus Research Institute (A.M.R.) and Alliance for Lupus Research Grant (S.S.).
Abbreviations
- ANA
anti-nuclear antibody
- DVD-Ig™
Dual variable domain immunoglobulin
- DKO
DNaseII−/− IFNAR−/− double knockout
- Het
DNaseII−/+ IFNAR−/−
- RF
rheumatoid factor
- STING
stimulator of type I IFN genes
- STING TKO
DNaseII−/− IFNAR−/−STING−/− triple knockout
- TLRs
Toll-like receptors
- Unc93 TKO
DNaseII−/− IFNAR−/−Unc93B3d/3d triple knockout
References
- 1.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–7. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 2.Leadbetter EA, Rifkin IR, Hohlbaum AH, Beaudette B, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate autoreactive B cells by dual engagement of sIgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 3.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined BCR/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–77. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teichmann LL, Schenten D, Medzhitov R, Kashgarian M, Shlomchik MJ. Signals via the adaptor MyD88 in B cells and DCs make distinct and synergistic contributions to immune activation and tissue damage in lupus. Immunity. 2013;38:528–40. doi: 10.1016/j.immuni.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson SW, Scharping NE, Kolhatkar NS, Khim S, Schwartz MA, Li QZ, Hudkins KL, Alpers CE, Liggitt D, Rawlings DJ. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J Immunol. 2014;192:4525–32. doi: 10.4049/jimmunol.1400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–28. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Chan OT, Hannum LG, Haberman AM, Madaio MP, Shlomchik MJ. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189:1639–48. doi: 10.1084/jem.189.10.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma S, Fitzgerald KA. Innate immune sensing of DNA. PLoS Pathog. 2011;7:e1001310. doi: 10.1371/journal.ppat.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun L, Wu J, Du F, Chen X, Chen ZJ. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science. 2013;339:786–91. doi: 10.1126/science.1232458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, Fitzgerald KA, Paludan SR, Bowie AG. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–8. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seth RB, Sun L, Ea CK, Chen ZJ. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell. 2005;122:669–82. doi: 10.1016/j.cell.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Crow YJ, Hayward BE, Parmar R, Robins P, Leitch A, Ali M, Black DN, van Bokhoven H, Brunner HG, Hamel BC, Corry PC, Cowan FM, Frints SG, Klepper J, Livingston JH, Lynch SA, Massey RF, Meritet JF, Michaud JL, Ponsot G, Voit T, Lebon P, Bonthron DT, Jackson AP, Barnes DE, Lindahl T. Mutations in the gene encoding the 3′-5′ DNA exonuclease TREX1 cause Aicardi-Goutieres syndrome at the AGS1 locus. Nat Genet. 2006;38:917–20. doi: 10.1038/ng1845. [DOI] [PubMed] [Google Scholar]
- 14.Crow YJ, Rehwinkel J. Aicardi-Goutieres syndrome and related phenotypes: linking nucleic acid metabolism with autoimmunity. Hum Mol Genet. 2009;18:R130–6. doi: 10.1093/hmg/ddp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Y, Jesus AA, Marrero B, Yang D, Ramsey SE, Montealegre Sanchez GA, Tenbrock K, Wittkowski H, Jones OY, Kuehn HS, Lee CC, DiMattia MA, Cowen EW, Gonzalez B, Palmer I, DiGiovanna JJ, Biancotto A, Kim H, Tsai WL, Trier AM, Huang Y, Stone DL, Hill S, Kim HJ, St Hilaire C, Gurprasad S, Plass N, Chapelle D, Horkayne-Szakaly I, Foell D, Barysenka A, Candotti F, Holland SM, Hughes JD, Mehmet H, Issekutz AC, Raffeld M, McElwee J, Fontana JR, Minniti CP, Moir S, Kastner DL, Gadina M, Steven AC, Wingfield PT, Brooks SR, Rosenzweig SD, Fleisher TA, Deng Z, Boehm M, Paller AS, Goldbach-Mansky R. Activated STING in a vascular and pulmonary syndrome. N Engl J Med. 2014;371:507–18. doi: 10.1056/NEJMoa1312625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jeremiah N, Neven B, Gentili M, Callebaut I, Maschalidi S, Stolzenberg MC, Goudin N, Fremond ML, Nitschke P, Molina TJ, Blanche S, Picard C, Rice GI, Crow YJ, Manel N, Fischer A, Bader-Meunier B, Rieux-Laucat F. Inherited STING-activating mutation underlies a familial inflammatory syndrome with lupus-like manifestations. J Clin Invest. 2014;124:5516–20. doi: 10.1172/JCI79100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagata S. Autoimmune diseases caused by defects in clearing dead cells and nuclei expelled from erythroid precursors. Immunol Rev. 2007;220:237–50. doi: 10.1111/j.1600-065X.2007.00571.x. [DOI] [PubMed] [Google Scholar]
- 18.Lan YY, Londono D, Bouley R, Rooney MS, Hacohen N. Dnase2a deficiency uncovers lysosomal clearance of damaged nuclear DNA via autophagy. Cell Rep. 2014;9:180–92. doi: 10.1016/j.celrep.2014.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida H, Okabe Y, Kawane K, Fukuyama H, Nagata S. Lethal anemia caused by interferon-beta produced in mouse embryos carrying undigested DNA. Nat Immunol. 2005;6:49–56. doi: 10.1038/ni1146. [DOI] [PubMed] [Google Scholar]
- 20.Ahn J, Gutman D, Saijo S, Barber GN. STING manifests self DNA-dependent inflammatory disease. Proc Natl Acad Sci U S A. 2012;109:19386–91. doi: 10.1073/pnas.1215006109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawane K, Ohtani M, Miwa K, Kizawa T, Kanbara Y, Yoshioka Y, Yoshikawa H, Nagata S. Chronic polyarthritis caused by mammalian DNA that escapes from degradation in macrophages. Nature. 2006;443:998–1002. doi: 10.1038/nature05245. [DOI] [PubMed] [Google Scholar]
- 22.Baum R, Sharma S, Carpenter S, Li Q, Busto P, Fitzgerald KA, Marshak-Rothstein A, Gravallese EM. Cutting Edge: AIM2 and Endosomal TLRs Differentially Regulate Arthritis and Autoantibody Production in DNase II-Deficient Mice. J Immunol. 2014 doi: 10.4049/jimmunol.1402573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rossol M, Pierer M, Arnold S, Keysser G, Burkhardt H, Baerwald C, Wagner U. Homozygosity for DNASE2 single nucleotide polymorphisms in the 5′-regulatory region is associated with rheumatoid arthritis. Ann Rheum Dis. 2009;68:1498–503. doi: 10.1136/ard.2008.092239. [DOI] [PubMed] [Google Scholar]
- 24.Shin HD, Park BL, Cheong HS, Lee HS, Jun JB, Bae SC. DNase II polymorphisms associated with risk of renal disorder among systemic lupus erythematosus patients. J Hum Genet. 2005;50:107–11. doi: 10.1007/s10038-004-0227-3. [DOI] [PubMed] [Google Scholar]
- 25.Pawaria S, Moody K, Busto P, Nundel K, Choi CH, Ghayur T, Marshak-Rothstein A. Cutting Edge: DNase II Deficiency Prevents Activation of Autoreactive B Cells by Double-Stranded DNA Endogenous Ligands. J Immunol. 2015;194:1403–7. doi: 10.4049/jimmunol.1402893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–35. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–47. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 28.Chan MP, Onji M, Fukui R, Kawane K, Shibata T, Saitoh S, Ohto U, Shimizu T, Barber GN, Miyake K. DNase II-dependent DNA digestion is required for DNA sensing by TLR9. Nat Commun. 2015;6:5853. doi: 10.1038/ncomms6853. [DOI] [PubMed] [Google Scholar]
- 29.Green NM, Laws A, Kiefer K, Busconi L, Kim YM, Brinkmann MM, Trail EH, Yasuda K, Christensen SR, Shlomchik MJ, Vogel S, Connor JH, Ploegh H, Eilat D, Rifkin IR, van Seventer JM, Marshak-Rothstein A. Murine B cell response to TLR7 ligands depends on an IFN-beta feedback loop. J Immunol. 2009;183:1569–76. doi: 10.4049/jimmunol.0803899. [DOI] [PMC free article] [PubMed] [Google Scholar]