Abstract
Objective
Neural network models that guide neuropsychological assessment practices are increasingly used to explicate depression, though a paucity of work has focused on regulatory systems that are under development in adolescence. The purpose of this study was to evaluate subsystems of attention related to executive functioning including alerting, orienting, and executive attention networks, as well as sustained attention with varying working memory load, in a sample of depressed and well adolescents.
Method
Neuropsychological functioning in 99 adolescents diagnosed with major depressive disorder (MDD) and 63 adolescent healthy controls (M = 16.6 years old) was assessed on the Attention Network Task (ANT) and the Continuous Performance Test, Identical Pairs (CPT).
Results
Adolescents with MDD, particularly those who were not medicated, were slower to process conflict (slower reaction time on the executive attention scale of the ANT) compared to controls, particularly for those who were not undergoing psychopharmacological treatment. Tentative evidence also suggests that within the MDD group, orienting performance was more impaired in those with a history of comorbid substance use disorder, and alerting was more impaired in those with a history of a suicide attempt.
Conclusions
Adolescents with depression showed impaired executive attention, although cognitive performance varied across subgroups of patients. These findings highlight the importance of examining neurocognitive correlates associated with features of depression and suggest an avenue for future research to help guide the development of interventions.
Keywords: adolescent, major depression, executive function, attention networks, sustained attention, conflict detection
Depressive disorders impact about 16% of the population (Kessler et al., 2005). Depression is associated with impairment in interpersonal relationships, lower productivity in the work place, and may result in chronic suffering and early death (Zlotnick, Kohn, Keitner, & Grotta, 2000; Lerner et al., 2004). Depression is the third leading cause of global burden of disease worldwide, and is the leading cause of disability among Americans between 15 and 44 years old (World Health Organization, 2008). Not only is depression commonly first evident during adolescence, but early onset of depression is also associated with a poor prognosis (Berndt et al., 2000; Lewinsohn et al., 1994; Weissman et al., 1999). There remains much work to be done in advancing our understanding of the neurodevelopmental underpinnings of depression (Sanislow et al., 2010). Interrogation of existing models of psychopathology commonly involves considering performance in key neural networks and the associated behavioral indices developed through experimental neuropsychology. The focus here is on regulatory control functions (e.g., executive functioning).
Neural network models are increasingly used to explicate psychopathology (Nemeroff, Kilts, & Berns, 1999). Performance on neuropsychological indices will advance understanding of risk for and adaptation from psychopathology for as we now know, exaggerations or aberrations of typical maturational changes in brain functioning occur in serious psychiatric disorders (Paus, Keshavan, & Giedd, 2008). Influential models of adult depression centrally implicate cortical-limbic dysregulation (Furman, Hamilton, & Gotlib, 2011; Drevets, Savitz, & Trimble, 2008; Johansen-Berg et al., 2008; Mayberg, 1997). Broadly, these models posit that symptoms of depression stem from complex disruptions to interconnected brain circuitry in cortical brain regions (e.g., the prefrontal cortex [PFC]), the cingulate cortex (e.g., the anterior cingulate cortex [ACC]), and subcortical brain regions implicated in motivated behavior supporting self-preservation (e.g., the limbic system that is activated under threat conditions) and reward processing (e.g., striatal system).
These cortical-limbic neural systems are under development during adolescence. Thus, it is not clear how applicable existing neural network models of adult psychopathology are relevant for understanding adolescent depression. Frontal and limbic brain regions exhibit different time courses for maturation. Subcortical brain regions associated with motivated behavior show more pronounced activation during adolescence (Ernst, 2014). Cortical regulatory brain regions and performance on regulatory tasks undergo a protracted developmental course, not reaching maturity for many until the mid-twenties (Luciana, 2013; Kelly et al., 2009; Tamnes et al., 2010). Given these unique features associated with developmental course, continued evaluation of these brain networks and associated neurocognitive functioning in adolescents is needed.
Executive Functioning and Other Attention Processes in Depression
Executive functioning (EF) encompasses a broad range of cognitive processes including domains of attention, working memory, conflict resolution, the ability to flexibly shift perspectives, and decision-making. There is now considerable evidence that EF deficits are associated with depression in adults (for reviews see Degl'Innocenti & Backman, 1998; McClintock, Husain, Greer, & Cullum, 2010; Ottowitz, Dougherty, & Savage, 2002; Tavares, Drevets, & Sahakian, 2003). By contrast, there is a relative paucity of research on EF in adolescents with depression (reviewed by Baune, Fuhr & Hering, 2014), and little agreement across studies as to whether EF deficits are consistently implicated in adolescent depression. Some of the inconsistencies across studies may be due to the broad array of neural networks represented and task demands of different EF measures (e.g., explicit versus implicit task demands, set maintenance versus set shifting). Focusing the investigation on specific networks underlying EF and relevant to depression will be helpful for identifying functional impairments associated with depressive illness.
Posner's theory of attention and EF provides a useful neural network perspective for evaluating conflict processing in the context of depressive illness (Posner & Peterson, 1990). This theory of attention includes three separate networks: alerting, orienting, and conflict/executive attention. Alerting is related to sustained attention and vigilance to prepare for an impending stimulus. The alerting network has been linked to the right dorsolateral PFC, ACC, right inferior parietal cortex, and the norepinephrine system. Orienting is the ability to shift attention, and is associated with bilateral regions of the superior parietal cortex, bilateral fusiform gyrus, left superior frontal gyrus, frontal eye fields, right postcentral gyrus, left precentral gyrus, and the cholinergic system (Posner & Raichle, 1998). Executive attention refers to the network involved in resolving the correct response in the face of incongruent stimuli (i.e., conflict). The executive attention network involves the dorsolateral PFC, ACC, thalamus, and the mesocortical dopamine system. Applications of a neural network perspective can guide the selection of neuropsychological assessment tools that measure neural circuits related to specific types of attentional and regulatory features that are thought to be relevant to depression (Ottowitz, Dougherty, & Savange, 2002). The Attention Network Test (Fan, McCandliss, Sommer, Raz, & Posner, 2002) is a measure developed to assess these three networks of alerting, orienting and executive attention. Measurement of the executive attention network is based on the well-established flanker task (developed by Ericksen & Ericksen, 1974) and shares some similarities with Stroop tasks (Epp, Dobson, Dozois, & Frewen, 2012). Similarly, there are a host of well-established measures that assess some related aspects of attention, including the frequently used Continuous Performance Test (CPT; Cornblatt, Risch, Faris, Friedman, & Erlenmeyer-Kimling, 1988).
Some research has evaluated executive attention in depression. While the findings have been fairly consistent in adults, there are fewer studies of adolescents, and less consistent findings in these studies. Some of the existing research is based on the classic (color-word) Stroop task. For example, a recent meta-analysis of the Stroop task literature evaluated 14 studies which were primarily focused on comparing performance of depressed and well adults (Epp, Dobson, Dozios, & Frewen, 2012). Their findings showed that depressed participants exhibited longer reaction times (RTs) than healthy controls on incongruent trials. Beyond examining task performance, some studies have used event related potential to show atypical brain oscillation patterns in adults with depression during conflict processing (Holmes & Pizzagalli, 2008). Although Favre et al. (2009) and Ladouceur et al. (2012) failed to find group differences in behavioral performance on executive attention tasks in depressed adolescents, Ladouceur et al. (2012) showed that depressed adolescents exhibited smaller amplitudes of error-related negativity measured with event related potential on a flanker task compared to controls, similar to research with adults. Nevertheless, there is some evidence that executive attention may be impaired in at risk youth. For example, Han and colleagues (2012) showed tentative evidence that adolescents with MDD (n = 31) exhibited conflict processing deficits compared to typically developing counterparts (n = 30), although severity of depressive symptoms was not related to performance. In another study, adolescents who were at familial risk for depression, but did not have a current diagnosis of MDD, exhibited ANT conflict processing deficits (Belleau, Phillips, Birmaher, Axelson, & Ladouceur, 2013). By contrast, other research evaluating neurocognitive performance analogous to functions assessed by ANT alerting and orienting indices showed largely intact performance in adolescents with depression (e.g., as reviewed by Baune, Fuhr, Air, & Hering, 2014), although they failed to include one study in their review that had found impairment in sustained attention (assessed via the CPT) in depressed adolescents (Han et al., 2012). In part, these inconsistent findings across studies may reflect the wide range of EF measures used. Larger samples that focus on key features of EF that are thought to be more specifically implicated in depression should eliminate some of these spurious findings.
The inconsistency in findings on EF deficits in adolescent depression also likely stems in part from heterogeneity within depressed samples. While some limited efforts have attempted to account for or control for potential effects of clinical subgroups, small sample sizes of depressed adolescents have largely constrained existing efforts to sufficiently address these issues. First, previous results have been inconsistent with regard to the association of EF and psychopharmacological treatment status (Han et al., 2012; Gualtieri, Johnson, & Benedict, 2006). Given the frequent use of antidepressants in this age group (Zito et al., 2002), it is critical to evaluate whether and how medication status might be related to executive attention and other cognitive skills relevant to depression. Second, the severity of depression and the accompanying phenotypic presentations may be important to consider more fully as we attempt to account for inconsistent findings across studies. Some research with adults has shown that severity of MDD is related to the degree of neurocognitive impairment (e.g., Ottowitz et al., 2002) and yet studies have not consistently shown this pattern of performance in adolescents (e.g., Han et al., 2012). Third, closely associated with severity is the specific type of symptoms that are presented in depressed adolescents. Disinhibition may increase suicide threat and is associated with poorer overall cognitive functioning. There is growing evidence that EF deficits are particularly pronounced in depressed individuals who are suicidal (Bridge et al., 2012; Jollant et al., 2005). For example, deficient performance on Stroop interference (but not CPT performance) was reported for adult depressed patients with a history of suicidal behavior as compared to those with no history of suicidal behavior (Keilp, Gorlyn, Oquendo, Burke, & Mann, 2008). Additionally, EF impairment may be particularly pronounced in depressed individuals when they show comorbid externalizing pathology, including substance use disorders (e.g., Worley, Tate, Granholm, & Brown, 2014). By contrast, it is less clear what patterns of EF may be evidenced when internalizing comorbid conditions are considered. Anxiety is the most frequent comorbid condition associated with depression, and while research has rarely addressed this pattern of comorbidity per say in youth (for an exception see Ladouceur, Dahl, Williamson, Birmaher, Ryan, & Casey, 2005), there is evidence that anxiety may interfere with attention (Emerson, Mollet, & Harrison, 2005; Micco, Henin, Biederman et al., 2009).
Study Objectives
The purpose of this study was to replicate and extend the results of Han et al. (2012). The primary objective was to evaluate disturbances in the attention networks in adolescent participants with MDD and healthy controls (HC), particularly focusing on executive attention performance given the strong evidence that corresponding brain regions (e.g., ACC) are implicated in depression. We examined group differences in executive attention across the total sample as well as a subsample recruited after Han et al. (2012)'s initial report. We also explored a wider range of skills involved in attention via the ANT (e.g., orienting and alerting) as well as the CPT (a measure that largely overlaps with the latent construct represented by the alerting network on the ANT, but also provides an index for assessing varying loads of working memory capacity within the context of vigilance). We predicted that deficits in executive attention would be evident in adolescents with MDD, but made no specific hypotheses regarding group differences on the performance of alerting and orienting attention networks or performance on the CPT. This larger sample also allowed us to more fully explore a broader range of clinical characteristics represented in adolescent depression including treatment status (pharmacotherapy), critical features of depression (severity, suicidality), and common externalizing (i.e., substance use disorder) and internalizing (i.e., anxiety disorders) comorbid conditions.
Methods
Participants
One hundred and sixty-two adolescents between the ages of 12 and 20 years old (M age = 16.6; SD = 1.9) participated in this study; 99 participants were diagnosed with MDD and 63 were healthy controls (HC; Table 1). This age range was selected to cover the steepest slope of prefrontal cortex cortical thickness pruning across childhood to young adulthood development (Shaw et al., 2008). A majority of the participants were female (68.5%). Most participants identified as Caucasian (72.2%), followed by African American (7.4%), Asian American (4.3%), and Native American (1.9%); the remaining participants self-identified as other (14.2%), and were primarily bi-racial. HC participants were eligible if they had no current or past psychiatric diagnoses. Exclusion criteria for both groups included the presence of a neurological or chronic medical condition, intellectual disability (IQ < 80), pervasive developmental disorder, bipolar disorder, or schizophrenia.
Table 1.
Sample demographics and characteristics.
| Control | All MDD | Comparisons Control × MDD | |
|---|---|---|---|
| N | 63 | 99 | 162 |
| Sex | |||
| % Female | 65.10% | 70.70% | χ(1) = .565 |
| N | 41F, 22M | 70F, 29M | p = .45 |
| Age | |||
| Mean (SD) | 16.9 (1.9) | 16.4 (1.9) | t(160) = 1.61 |
| Range | 12.3 -19.9 | 12.3 - 20.0 | p = .11 |
| IQ | |||
| Mean (SD) | 112.5 (12.1) | 107.3 (14.7) | t(158) = 2.33 |
| Range | 83 - 134 | 80 - 140 | p = .02 |
| Race | |||
| White | 68.30% | 74.70% | χ(1) = .81 |
| Minority status | 31.70% | 25.30% | p = .37 |
| Black/African American | 1.60% | 11.10% | |
| Asian American | 7.90% | 2.00% | |
| Native American | 1.60% | 2.00% | |
| Other | 20.60% | 10.10% | |
| BDI | |||
| mean (SD) | 3.0 (3.4) | 22.8 (12.1) | t(122) = −15.19 |
| range | 0.0 - 16.0 | 0.0 - 52.3 | p < .001 |
Note. BDI = Beck Depression Inventory. M = males, F = females.
Procedures
Participants were recruited through community postings and inpatient and outpatient clinical services at the University of Minnesota, Twin Cities and the surrounding area. The University's Institutional Review Board approved this study. All participants completed signed informed consent and/or assent (if under 18), and received monetary compensation for their participation after completing each visit. Participants completed multiple visits as part of the study. In the first visit, diagnostic interviews were conducted. In the second visit, participants completed a battery of computerized neurocognitive tasks (including the CPT and ANT), which are the focus of this report, as well as the vocabulary and matrices subtests of the Wechsler Abbreviated Scale of Intelligence as an estimate of IQ (Wechsler, 1999). Although not addressed in the current report, a subset of the participants also underwent more extensive neurobiological assessments including paradigms to assess functioning of the hypothalamic pituitary adrenal axis, and brain structure and function via MRI brain scans (Cullen et al., 2014; Klimes-Dougan et al., 2014).
Measures
Diagnosis and symptom assessment
The presence or absence of a DSM-IV-TR Axis I disorder(s) was confirmed by a semi-structured diagnostic interview. Participants under 18 years of age and a legal guardian completed independent interviews using the Kiddie Schedule for Affective Disorders and Schizophrenia - Present and Lifetime Version (KSADS-PL; Kaufman et al. 1997). The KSADS-PL interviews were conducted by trained clinical psychologists, child psychiatrists, or advance trainees enrolled in graduate clinical psychology doctoral programs under the direct supervision of a senior clinician. Interviewers were extensively trained. As part of their training, interviewers provided ratings on 4 training tapes rated against the consensus of three lead investigator ratings considered here as the “gold standard.” The average percentage in agreement across the 8 evaluators was 96% for the specific symptoms in the screen, 84.63% for the specific symptoms of depression, and 100% for a diagnosis of depression. Diagnosis was based on consensus of parent and child reports, except for participants over 18 years of age for which a parent interview was not conducted. Participants completed the Beck Depression Inventory II (BDI; Beck, Steer, & Brown, 1996) (M = 23; SD = 12) at each study visit. BDI data was missing for 7 HC and 1 MDD. Since we had different quantities of BDI assessments for different participants depending on how many study visits they completed, we used the mean of all completed BDIs in order to make full use of the data available for each participant.
Within the context of the KSADS-PL, information about features of depression, and comorbid conditions were obtained for MDD participants (Table 2). The present usage of antidepressant medication was assessed (35.4% were taking medication for depression). Because this was not a medication trial, there was likely variation in the type, history, and dosage of medication. Although most medicated adolescents were taking fluoxetine. Suicide history was also assessed based on history of recent or past suicide attempts (reports of a suicide attempt(s) versus no reports of suicide attempt). Two comorbid conditions were also assessed: anxiety disorder (including generalized anxiety, obsessive compulsive, separation anxiety, social anxiety, panic, specific phobia, social phobia, or post traumatic stress disorders) and substance use disorders, which are largely reflective of internalizing and externalizing conditions. Comorbid anxiety disorders were dichotomized into evidence of any current or past diagnosis of an anxiety disorder and no diagnosis of an anxiety disorder. Finally, a history of substance use disorder versus no history of a substance use disorder was compared.
Table 2.
Subgroups within MDD group.
| Medication status | |
| Medicated | N = 35 (35.4%) |
| Un-medicated | N = 64 (64.6%) |
|
Suicide Attempt | |
| With | N = 28 (32.6%) |
| Without | N = 58 (67.4%) |
|
Comorbid Anxiety | |
| With | N = 62 (65.3%) |
| Without | N = 33 (34.7%) |
|
Substance Use Disorder | |
| With | N = 11 (12.8%) |
| Without | N =75 (87.2%) |
Neurocognitive assessments
The Attention Network Task (ANT) is a widely used task comprised of a combined cued reaction time task (Posner, 1980) and a flanker task (Eriksen & Eriksen, 1974; Fan, McCandliss, Sommer, Raz, & Posner, 2002). Participants are instructed to press either the left or right key to indicate the direction that a central arrow points. During various presentations, the arrow may appear above or below a center fixation point; in addition, the target stimuli may be presented with or without flankers. Four types of warning cues precede the presentation of the target stimuli: no cue, center cue, double cue, or spatial cue. Flankers may be neutral (the arrow appears with two dash lines in both sides), congruent (the central arrow appears with two arrows flanked on each side so that all arrows are pointing in the same direction), or incongruent (the central arrow points one way and the flanker arrows on each side point the opposite direction; for a more detailed description, see Fan et al., 2002). Dependent variables considered here are alerting, orienting, conflict, and mean accuracy. Alerting was calculated by subtracting the mean reaction time of double-cue conditions from the mean reaction time of no-cue conditions (averaging across all flanker conditions). Orienting was calculated by subtracting the mean reaction time of spatial cue conditions from the mean reaction time of center cue conditions (averaging across all flanker conditions). Conflict was calculated by subtracting the mean reaction time of incongruent flanker conditions from the mean reaction time of congruent flanker conditions (averaging across all cue conditions). Lower scores reflect better performance for these three variables. A final index of potential importance is accuracy of responding, measured by percentage of correct responses. Previous research has reported on reliability of this scale with adults (e.g., Ishigami & Klein, 2010). Two MDD participants were excluded due to accuracies below chance (<50%) and one control was excluded due to extremely high (3 SD above the sample mean) reaction times. Two HC and two MDD participants had missing data for this task.
The Continuous Performance Test, Identical Pairs version evaluates sustained attention and has shown evidence of reliability in adolescents (Cornblatt et al., 1988). Participants view a series of 2-, 3-, or 4-digit numbers as they appear one at a time on the screen. Participants respond to consecutive identical stimuli by pressing a key. Discrimination sensitivity (d’) takes into account both correct and false positive responses, and is considered a measure of an individual's ability to discriminate a signal from background noise. Higher d’ represents better performance. Discrimination sensitivity scores for the 2-, 3-, and 4-digit blocks (respectively CPT2, CPT3, CPT4) were analyzed. One HC and three MDD participants had missing data for this task.
Statistical Analyses
Analyses were conducted using the Statistical Package for the Social Sciences (SPSS), version 20.0. First, we conducted preliminary analyses to determine if there were differences between MDD and HC on critical demographic characteristics including age, sex, or IQ (Table 1). IQ estimates were significantly lower (t(158) = 2.331, p = .021) for MDD (M = 107.3, SD = 14.7) compared to HC (M = 112.5, SD = 12.1), so all between-group comparisons included IQ as a covariate.
We then carried out a series of univariate general linear models (GLM) for all performance measures on the ANT and CPT separately, considering main effects of group (MDD versus HC). Follow up analyses were then conducted in which we considered how medication status was related to neurocognitive functioning between HC and medicated and un-medicated MDD. Medicated and un-medicated participants with MDD did not differ on age, sex, IQ, severity (BDI scores), or presence of absence of a past suicide attempt, history of substance use disorder, or comorbid anxiety. Correlations for depressive symptom severity (BDI) and neurocognitive indices were conducted to determine the association across the whole sample and the MDD group.
Analyses were also conducted to address possible issues relevant to variation in neurocognitive functioning within the group of participants diagnosed with MDD. We conducted a series of GLM analyses to assess if specific features of depression (suicidality) or comorbid conditions (substance use disorder or anxiety disorder) differentiated neurocognitive performance within the MDD group. If these characteristics (e.g., comorbid anxiety disorder) differed for sample characteristics (age, sex, IQ) or severity of depression (BDI), we controlled for any variable that was represented differently across groups. Given that MDD participants with comorbid anxiety had significantly higher BDI scores (M = 25.16, SD = 11.26) compared to those without comorbid anxiety (M = 19.22, SD = 13.06; t(92) = 2.306, p = .02), we conducted analyses with BDI as a covariate for the analyses evaluating neurocognitive performance differences in MDD participants with and without a comorbid anxiety disorder.
Results
Preliminary Analyses
Differences from previous sample (Han et al., 2012)
Efforts were increasingly made to recruit a diverse sample of MDD and HC participants over time, resulting in significant differences in background characteristics between the preliminary sample (N = 61; many who had been hospitalized; Han et al., 2012) and the subsequently recruited participants (N = 101; who were recruited primarily from outpatient services). In comparison to the participants who were subsequently recruited, the Han et al. (2012) subsample had more participants who were male, older, ethnically less diverse, performed higher on average on the IQ test, and had a history of comorbid substance use disorder and suicide attempts. Follow-up analyses to evaluate comparability of findings across subsamples were conducted for key study questions.
Relation between variables
Presented in Table 3 are correlations between demographic/background variables, suicidality and comorbid disorders within the MDD group, and neurocognitive measures. The results indicate that increasing age was related to better performance on the ANT (faster alerting and conflict, and higher accuracy), and better performance on the CPT (for all 3 conditions). Increasing IQ was also related to better performance on the ANT (faster orienting and conflict, and higher accuracy), and better performance on the CPT (for all 3 conditions). MDD status was related to lower performance on the ANT conflict measure and the CPT (CPT2 and CPT4). There were few correlations within the MDD group for illness characteristics and neurocognitive performance (only history of substance use disorder was associated with slower ANT orienting, and history of a suicide attempt was associated with slower ANT alerting). Preliminary analyses also indicated that for the ANT, orienting and conflict were positively correlated and conflict and accuracy were negatively correlated. For the CPT, all three scales were positively correlated. The three CPT measures were negatively correlated with ANT alerting, and also negatively correlated with ANT conflict. CPT measures were positively correlated with ANT mean accuracy.
Table 3.
Correlations between variables in the study.
| Age | IQ | Sex | HC/MDD | BDI | Medicated* | Suicide attempt* | SUD* | Comorbid anxiety* | ANT Alerting | ANT Orienting | ANT Conflict | ANT Accuracy | CPT2 | CPT3 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IQ | 0.27 | ||||||||||||||
| 0.00 | |||||||||||||||
| Sex | −0.12 | −0.21 | |||||||||||||
| 0.13 | 0.01 | ||||||||||||||
| HC/MDD | −0.13 | −0.18 | |||||||||||||
| 0.11 | 0.02 | ||||||||||||||
| BDI | −0.11 | −0.29 | 0.14 | 0.70 | |||||||||||
| 0.16 | 0.00 | 0.09 | 0.00 | ||||||||||||
| Medicated* | −0.13 | −0.11 | 0.04 | N/A | 0.11 | ||||||||||
| 0.19 | 0.29 | 0.73 | N/A | 0.27 | |||||||||||
|
Suicide attempt* |
−0.01 | −0.03 | 0.08 | N/A | −0.12 | 0.08 | |||||||||
| 0.95 | 0.81 | 0.45 | N/A | 0.28 | 0.46 | ||||||||||
| SUD* | −0.06 | 0.10 | −0.10 | N/A | −0.07 | 0.07 | 0.25 | ||||||||
| 0.59 | 0.37 | 0.36 | NA | 0.52 | 0.55 | 0.02 | |||||||||
|
Comorbid anxiety* |
−0.05 | 0.03 | −0.01 | N/A | −0.23 | 0.18 | 0.05 | 0.11 | |||||||
| 0.66 | 0.81 | 0.90 | N/A | 0.02 | 0.09 | 0.66 | 0.31 | ||||||||
|
ANT Alerting |
−0.19 | −0.06 | 0.11 | −0.02 | 0.01 | −0.11 | −0.23 | 0.03 | 0.04 | ||||||
| 0.02 | 0.43 | 0.17 | 0.81 | 0.88 | 0.31 | 0.04 | 0.79 | 0.69 | |||||||
|
ANT Orienting |
0.08 | −0.18 | 0.09 | −0.00 | −0.06 | 0.04 | −0.03 | −0.26 | 0.01 | −0.01 | |||||
| 0.34 | 0.03 | 0.26 | 0.98 | 0.46 | 0.68 | 0.81 | 0.02 | 0.90 | 0.94 | ||||||
|
ANT Conflict |
−0.25 | −0.39 | 0.15 | 0.24 | 0.25 | 0.13 | 0.10 | 0.02 | 0.03 | 0.10 | 0.22 | ||||
| 0.00 | 0.00 | 0.07 | 0.00 | 0.00 | 0.20 | 0.39 | 0.87 | 0.80 | 0.23 | 0.01 | |||||
|
ANT Accuracy |
0.37 | 0.17 | −0.03 | −0.12 | −0.02 | −0.12 | −0.04 | −0.05 | −0.08 | −0.15 | 0.06 | −0.32 | |||
| 0.00 | 0.04 | 0.69 | 0.16 | 0.83 | 0.27 | 0.76 | 0.63 | 0.47 | 0.07 | 0.43 | 0.00 | ||||
| CPT2 | 0.33 | 0.45 | −0.03 | −0.16 | −0.17 | −0.13 | 0.14 | −0.03 | 0.10 | −0.19 | −0.05 | −0.23 | 0.32 | ||
| 0.00 | 0.00 | 0.70 | 0.04 | 0.03 | 0.22 | 0.22 | 0.77 | 0.33 | 0.02 | 0.54 | 0.01 | 0.00 | |||
| CPT3 | 0.33 | 0.38 | −0.08 | −0.13 | −0.16 | −0.07 | 0.13 | −0.13 | 0.05 | −0.20 | −0.13 | −0.31 | 0.31 | 0.71 | |
| 0.00 | 0.00 | 0.29 | 0.10 | 0.05 | 0.48 | 0.24 | 0.24 | 0.61 | 0.01 | 0.11 | 0.00 | 0.00 | 0.00 | ||
| CPT4 | 0.34 | 0.32 | −0.08 | −0.18 | −0.14 | −0.15 | −0.02 | −0.18 | −0.08 | −0.20 | −0.08 | −0.27 | 0.20 | 0.51 | 0.71 |
| 0.00 | 0.00 | 0.30 | 0.03 | 0.09 | 0.14 | 0.89 | 0.10 | 0.47 | 0.01 | 0.36 | 0.00 | 0.02 | 0.00 | 0.00 | |
Note. The table shows for each correlation r values on top and p values below with p < .05 bolded. For sex 1 = females, 2 = males; For HC/MDD HC (healthy control) = 0; MDD (depressed) = 1; For medicated 1 = medicated, 2 = not medicated; For history of suicide attempt, SUD (history of substance use disorder), and comorbid anxiety, 1 = present, 2 = absent; BDI = Beck Depression Inventory (an index of severity); ANT = Attention Network Test; CPT = Continuous Performance Test;
data only available for MDD subjects.
Neurocognitive Performance for MDD and HC
We assessed for differences in neurocognitive performance between MDD and HC participants using univariate general linear models (GLM), controlling for IQ and considering all neurocognitive performance measures separately (Table 4). A main effect for group was found for the ANT conflict (F(1, 152) = 5.70, p = .02). Participants with MDD (M = 119.63, SE = 5.18) had significantly slower reaction times on the conflict index of the ANT than HC (M = 99.50, SE = 6.57). There were no group differences for the alerting or orienting indices, or accuracy on the ANT. No main effects for group were found for the four indices of the CPT (when IQ was included as a covariate).
Table 4.
Performance on CPT and ANT.
| HC M (SE) | MDD M (SE) | F | p | |
|---|---|---|---|---|
|
CPT
| ||||
| Omnibus | 1.31 | 0.27 | ||
| 2-digit d’ | 3.51 (.09) | 3.35 (.07) | 1.71 | 0.19 |
| 3-digit d’ | 2.48 (.119) | 2.35 (.10) | 0.71 | 0.40 |
| 4-digit d’ | 1.29 (.10) | 1.07 (.08) | 2.71 | 0.10 |
|
ANT | ||||
| Alerting | 45.66 (3.71) | 44.02 (2.92) | 0.12 | 0.73 |
| Orienting | 49.05 (3.19) | 47.59 (2.51) | 0.13 | 0.72 |
| Conflict | 99.50 (6.57) | 119.63 (5.18) | 5.70 | 0.02* |
| Accuracy | .96 (.01) | .95 (.01) | 1.18 | 0.28 |
Note. IQ was included as a covariate in these between-group univariate GLM comparisons;
p < .05
To test replication, we compared neurocognitive performance between HC and MDD excluding the entire previous sample from Han et al. (2012). Here again ANT conflict was significantly different between groups (F(1, 93) = 5.10, p = .03), with MDD (M = 124.06, SD = 60.82) having significantly slower reaction times compared to HC (M = 97.52, SD = 34.32).
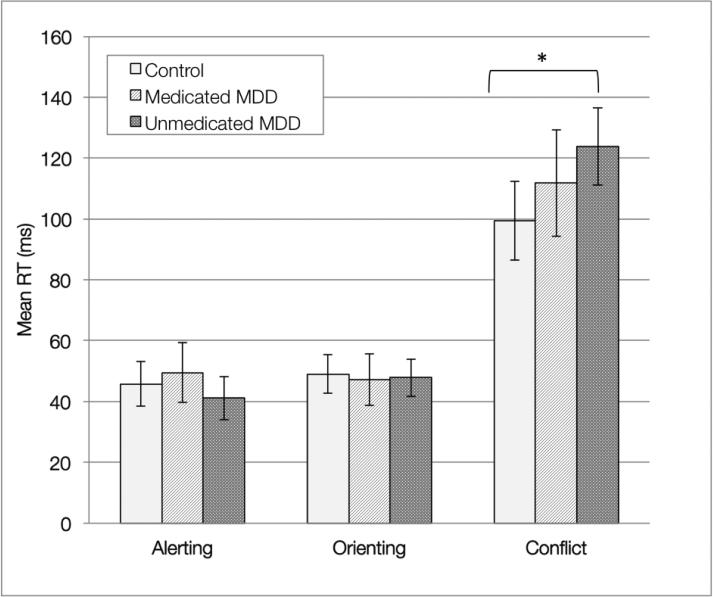
We conducted a series of additional analyses to see how medication status was related to neurocognitive performance measures. First, we evaluated performance between HC, participants with MDD who were taking medication for depression, and participants with MDD who were not taking medication. There was a significant difference in reaction time between groups for conflict scores on the ANT (F(2, 149) = 3.47, p = .03). Analyses using a pairwise comparison yielded significant differences between un-medicated MDD (M = 128.43, SD = 70.51) and HC's (M = 95.04, SD = 29.43) performance on the conflict index of the ANT (Figure 1). There were no significant differences in performance between medicated participants compared to un-medicated MDD participants (M = 110.81, SD = 47.23) or compared to HC (M = 95.04, SD = 29.43).
Figure 1.
Between-group comparisons of ANT performance. Error bars represent 95% confidence intervals. Pair wise comparison yielded a significant different between the HC and un-medicated MDD on the ANT conflict z-score index (p < .049).
Severity of depressive symptoms as measured by the BDI assessed across the whole study group was significantly associated with ANT conflict (r = .25, p = .002) and with CPT2 d’ (r = −.17, p = .03). However, BDI was not significantly correlated with any of the dependent measures when analyses were restricted to the subgroup of MDD participants (e.g., ANT conflict r = .13, p = .22).
Individual Differences within Participants with MDD
Additional analyses of MDD adolescents explored neurocognitive performance in subgroups of participants with MDD, as shown in Table 5. A few differences were noted on various indices of the ANT, though none of these subgroup analyses were significant for the CPT. MDD participants who had attempted suicide demonstrated significantly slower alerting reaction times on the ANT compared to MDD participants who had not attempted suicide (F(1, 80) = 4.38, p = .04). MDD participants with a past substance use disorder had significantly slower orienting reaction times compared to participants without a past substance use disorder (F(1, 80) = 5.91, p = .02). There were no significant differences between depressed adolescents with and without anxiety on any of the ANT or CPT measures. As there was a correlation between the presence of a past suicide attempt and a substance use disorder, multiple regressions were conducted to predict alerting and orienting from medication status, age, IQ, gender, co-morbid anxiety, suicide attempt, past comorbid substance use disorder, and mean BDI, with results supporting findings of the GLM analyses whereby alerting performance is uniquely related to history of a suicide attempt, and orienting performance is related to history of substance use disorder. ANT alerting was significantly predicted by age (β=−4.115, t(78)=−2.161, p=.034) and past suicide attempt (β=−17.151, t(78)=−2.202, p=.031). ANT orienting was significantly predicted by IQ (β=−.501, t(78)=−2.470, p=.016), and history of substance use disorder (β=−19.623, t(78)=−2.193, p=.032).
Table 5.
Comparisons within the MDD group.
| Suicide Attempt | Comorbid Anxiety Disorder | History of Substance Use Disorder | ||||
|---|---|---|---|---|---|---|
| With | Without | With | Without | With | Without | |
| N (%) | 28 (32.6%) | 58 (67.4%) | 62 (65.3%) | 33 (34.7%) | 11 (12.8%) | 75 (87.2%) |
| Alerting | F(1, 80) = 4.38, p = .04* | F(1, 87) = .29, p = .59 | F(1, 80) = .07, p = .79 | |||
| M = 54.33 (SD = 34.13) | M = 39.31 (SD = 28.66) | M = 42.62 (SE = 4.15) | M = 46.39 (SE = 5.50) | M = 41.93 (SD = 41.21) | M = 44.62 (SD = 29.68) | |
| Orienting | F(1, 80) = .06, p = .81 | F(1, 87) = .00, p = .96 | F(1, 80) = 5.91, p = .02* | |||
| M = 48.83 (SD = 26.4) | M = 47.35 (SD = 25.50) | M = 46.97 (SE = 3.52) | M = 46.70 (SE = 4.65) | M = 64.82 (SD =21.52) | M = 45.21 (SD = 25.34) | |
| Conflict | F(1, 80) = .77, p = .39 | F(1, 87) = .29, p = .59 | F(1, 80) = .03, p = .87 | |||
| M = 112.09 (SD = 52.58) | M = 125.58 (SD = 71.13) | M = 118.92 (SE = 8.55) | M = 126.60 (SE = 11.30) | M = 118.15 (SD = 60.32) | M = 121.60 (SD = 66.75) | |
Note: Reported values represent estimated marginal means for co-morbid anxiety disorder.
Discussion
This study examined EF in adolescent depression. Adolescents with MDD showed evidence of impaired executive attention and this pattern was most pronounced in adolescents with MDD who were un-medicated. An additional study goal was to explore whether functioning of the different attention systems evaluated were associated with features of depression. Results suggested that functioning of attention systems were differentially associated with clinical characteristics of depression and comorbid conditions within the MDD group.
Disruptions in Executive Attention in Adolescents with MDD
Adolescents with depression demonstrated impaired ability to focus attention in the presence of incongruent stimuli. Specifically, they were significantly slower on the ANT conflict index, which is a critical feature of executive attention exhibited by the difference in reaction times between incongruent and congruent flanker trials. The confidence in this finding is strengthened by the fact that executive attention deficits were found not only for the entire sample contained in the current study, but also the sample reported in Han et al's (2012) preliminary report, as well as separately in the subsample of 101 participants that were subsequently added to make up the current study sample. Interestingly, adolescents with MDD were not significantly slower at responding to congruent flankers, which would have demonstrated a more general slowing in MDD.
Executive attention involved in conflict detection and resolution has reliably been associated with activity in the ACC (Carter & van Veen, 2007; Kerns et al., 2004; Bush, Luu, & Posner, 2000; Fan, McCandliss, Fosella, Flombaum, & Posner, 2005), a brain region consistently implicated in depression. Identified abnormalities include reduced gray matter volume (Drevets et al., 1997; Botteron, Raichle, Drevets, Heath, & Todd, 2002; Hajek, Kozeny, Kopecek, Alda, & Hoschl, 2008) and dysregulation of resting-state connectivity and functional task activation in both depressed adolescents and adults (Cullen et al., 2009; Drevets, Savitz, & Trimble, 2008; Beauregard et al., 1998). The ACC is thought to detect conflict (when multiple incompatible response channels are activated by the presence of conflicting information in a stimulus) and trigger additional processing by frontal regions in order to determine an appropriate response.
Minor slowing on executive attention measures could indicate considerable impairment in the context of real-world daily interactions where competing response options might be both more ubiquitous and more socially-relevant. Indeed, difficulties in error monitoring and more broadly, cognitive control, have been posited to lead to challenges with emotion regulation, as exemplified by difficulties disengaging from negative thoughts and feelings (Joormann & Quinn, 2014). Conflict detection and error monitoring may also be related to negative cognitive biases in depression. Executive attention deficits may make it difficult to recognize when thoughts do not match reality (e.g., when the interpretation of a social interaction is more negative than what the facts of the situation would suggest) and accordingly make the needed adjustments to these thought patterns.
Another way in which deficits in cognitive control and monitoring affect adolescents is through undermining cognitive resources necessary to navigate social affiliations including friendships and romantic relationships (Davey, Yücel, & Allen, 2008). Difficulties with cognitive monitoring and control may further maintain interpretation biases surrounding negative affect and prolong their experience by making it difficult to disengage from current representations of negative emotions, which prevents access to more positive memories or options in decision-making. Finally, we posit that conflict monitoring deficits found in adolescents with MDD would impact their valuation of the rewards and costs associated with decision making and this may further incur interpersonal difficulties, which could exacerbate cognitive deficits via altering stress neuroendocrine responses (e.g., Rahdar & Galván, 2014).
Neurocognitive Deficits for Different Presentations of Adolescent Depression
A secondary study goal was to address the heterogeneity of depression by applying appropriate controls and examining subgroups defined by different clinical characteristics. The findings point to the need to continue to examine critical features of depression and related problems that pertain to neurocognitive anomalies. The severity and symptom configuration for those meeting criteria for depression varies widely across individuals. Our results with regard to severity of symptoms were generally consistent with the existing literature on adolescents (e.g, Kavanaugh & Holler, 2014; Favre et al., 2009).
The impairments noted also vary according to the patterns of identified comorbid conditions associated with depression. Deficient orienting attention network functioning was observed in the small subgroup of MDD participants with a history of a substance use disorder (n = 11). In contrast, Abdullaev, Posner, Nunnally, and Dishion (2010) found that adolescents with chronic cannabis use had impaired ANT conflict performance, but intact ANT alerting and orienting. Additional studies comparing adolescents with both isolated and comorbid substance use disorder and depression can help to better dissociate the attentional deficiencies in these disorders and their interaction. There may also be differences between individuals who abuse different types of substances (e.g., stimulants versus marijuana), and thus further sub-groupings of individuals with substance use disorders may need to be considered.
It is well established that impairments in attention and comorbid attention deficit disorder are associated with lethal suicide attempts (e.g., Nasser & Overholzer, 1999), yet there remain inconsistencies in the existing literature that deserve further examination. Our findings show that participants with MDD who had a history of suicide attempts demonstrated slower ANT alerting than MDD participants with no history of suicide attempts. However, despite evidence that ANT alerting and CPT were correlated in our sample, our results failed to yield group differences between suicide attempt histories for the CPT indices. The existing literature using the CPT to evaluate attention has yielded mixed findings, with one study with depressed adolescents showing group differences (Horesh, 2001) and another study failing to show evidence of group differences (e.g., Keilp et al, 2008). By contrast, the results have been more consistent with indices measuring executive attention (e.g., as measured by the Stroop test), particularly in those with a history of highly lethal suicide attempts (e.g., Keilp et al., 2008). Contrary to predictions, our results for suicide attempt histories failed to identify group differences in executive attention, perhaps reflective of the relatively low levels of lethality. If intervention is not successful in addressing the problems identified in adolescence, it is possible that over time, some of these adolescents suffering from attentional impairments go on to engage in more highly lethal suicide attempts.
Growing evidence suggests that depression accompanied by anxious arousal may impede top-down processing (Engels et al., 2010). Anxiety has been associated with hypervigilant attention for threat-related stimuli. The attention tasks used in the current study contain only neutral (not emotionally salient) stimuli that would not be expected to activate the same threat system. It is possible that our results failed to identify differences between depressed adolescents with and without anxiety disorders because of the absence of emotionally charged stimuli in the tasks administered. Similarly, Gunther et al. (2004) found no differences in attentional performance between youth with anxiety, depression, or no psychiatric disorder on attentional tasks without emotionally-salient stimuli. Reinholdt-Dunne, Mogg, and Bradley (2009) found that a combination of high anxiety and poor attentional control (as measured by the ANT) predicted poor processing of emotionally salient information. There is also some evidence that adults with anxiety had significantly slower ANT orienting and conflict response times on a slightly different version of the ANT (e.g., with auditory tones for alerting; Pacheco-Unguetti, Acosta, Marqués, & Lupiáñez, 2011), which may suggest that cognitive load alterations may come later in the course of the disorder. Or perhaps, specific types of anxiety disorders differentially impact functioning, as shown by a study that generalized anxiety disorder was associated with impaired verbal learning but social phobia was associated with impaired performance on the CPT. It may also be that findings from studies of anxiety alone may not be generalizable to individuals with comorbid anxiety and depressive disorders (e.g., Taghavi, Neshat-Doost, Moradi, Yule, & Dalgleish, 1999). One recent study conducted a chart review on adolescents hospitalized for depression and found no associations between EF and anxiety symptoms (Holler, Kavanaugh, & Cook, 2014). Given that depression is more broadly conceived as an internalizing disorder (along with anxiety), and anxiety and depressive disorders commonly co-occur, it would be important to further evaluate contributions of anxiety to EF deficits associated with diverse samples of adolescents with MDD.
In summary, considering some of the heterogeneity in the presentation of depression will likely be critically important in advancing our understanding of the underlying neurocognitive processes implicated in adolescent depression, as these processes appear to vary depending on the presence of other conditions. While largely preliminary, the findings of this study suggest important avenues to pursue in the future.
Implications for Treatment
A continued inquiry is needed to more definitively evaluate the impact of medication on brain functioning in adolescent MDD before we can conclude that pharmacotherapy may show some benefits. While a randomized controlled longitudinal design would be best suited to evaluate the effects of medication on executive functioning in adolescent MDD, the results of this study bring up the possibility that psychopharmacological interventions may ameliorate neurocognitive functioning. We found that the MDD participants receiving pharmacotherapy did not show significantly different ANT conflict scores compared to HC; performance differed most between the group of un-medicated depressed adolescents and HC. Most of the participants in this study were taking SSRIs (typically fluoxetine) although in this open-label study there was considerable variation of dose and duration of treatment. Additionally, most of the participants (at least 57.6%) had or were currently in some form of psychotherapy. Study designs using randomized control trials are now needed to evaluate this important question and further explore the possible neuroprotective effects of pharmacotherapy and establish a causal relationship between medication and neurocognitive functioning.
Specific interventions targeting attention networks may prove useful, and treatments that enhance the efficiency of conflict monitoring and associated ACC functioning may be particularly effective during adolescence, when the ACC is still developing (e.g., van Leijenhorst et al., 2010). Previous studies have associated depression with both dysregulated top-down and bottom-up processes (Fales et al. 2008), whereby the amygdala may be hyperactive and the prefrontal cortex hypoactive. The ACC is a critical circuit in that it recruits prefrontal resources to regulate limbic responses, and targeting this circuit first may be the most effective route to treating depression. If executive functioning is improved, frontal regions will be better able to down-regulate overactive limbic regions. On the other hand, simply attempting to minimize hyperactivity of limbic regions would not solve problems related to deficits in executive function, which affect functioning outside of emotion regulation. Thus, targeting conflict processing and ACC function are important avenues to explore in adolescent MDD.
There is evidence that individuals dynamically change the level of control given the task demands. For example, basic research has shown that when a high proportion of the stimuli are incongruent, individuals exhibit smaller interference effects than when a low proportion of the stimuli are incongruent (Lindsay & Jacoby, 1994). When disruptions in these error-monitoring processes occur it may be possible to “train” more normative responding by altering stimuli exposure schedules. Computerized cognitive control training is starting to be used with depression (Calkins, McMorran, Siegle, & Otto, 2014) and attention bias modification procedures may be important to shape more affective responses (e.g., Linden, Habes, Johnston et al., 2012; Wells & Beevers, 2010).
Similarly, other broad-based psychological interventions may also serve to enhance EF. Most of the participants in this study had engaged in psychotherapy or counseling although there was little evidence of engagement in a specific validated treatment for adolescent depression such as Cognitive Behavioral Therapy or Interpersonal Psychotherapy. Contemplative practices may be an additional avenue to pursue, for not only is meditation associated with improved attention (Kozasa et al., 2012; Jha, Krompinger, & Baime, 2007; Zylowska et al., 2008) and altered ACC structure and function (Tang, Lu, Geng, Stein, Yang, & Posner, 2010; Kozasa et al., 2012), but mindfulness meditation has also been shown to alleviate depressive and anxious symptoms (Ramel, Goldin, Carmona, & McQuaid, 2004; Hofmann, Sawyer, Witt, & Oh, 2010; Evans et al., 2008).
Finally, considering applications of this work as it might apply to developing or validating personalized treatments for depression may be worthwhile in the future. Past research has identified functional activity of the ACC as an important predictor of treatment response in adults with MDD (Davidson, Irwin, Anderle, & Kalin, 2003; Jappe, Klimes-Dougan, & Cullen, 2013; Mayberg et al., 1997; Pizzagalli et al., 2001). Though relevant to personalizing treatments for depression, this approach has yet to be extensively applied to the use of more feasible neurocognitive assessment batteries with depressed adolescents, which could identify specific neurocognitive deficits in individuals that would guide the selection of treatments targeted at ameliorating the specific deficient networks.
Limitations and Future Directions
This study provided evidence for significantly different neuropsychological profiles within individuals with MDD. The approach used here was to examine a sample of depressed adolescents that may be representative of the heterogeneity of depression in the community rather than providing strict restrictions on inclusion criteria. More restricted approaches to characterizing depression provide alternative advantages (e.g., determining what is specific to MDD), however, considering empirically driven systems of classification of symptoms (Hudziak, Achenbach, Althoff, & Pine, 2007; Krueger & Bezdjian, 2009; Krueger & Piasecki, 2002) or multi-method assessments of domain impairments (e.g., RDoC; Cuthbert & Insel, 2013) are likely to advance our understanding of pathology. While consistently demonstrated similarities across individuals diagnosed with the syndrome of MDD support the relevance of studying the disorder as a syndrome, more discrete constructs rather than complex sets of symptoms may be better suited to investigations of the neural and neuropsychological substrates of MDD (RDoC, NIMH, 2011). This study was limited by small sub-groups within the sample of adolescents with MDD (e.g., only 11 MDD adolescents with a past substance use disorder) limited the power to detect differences. Lack of differences within the MDD group as a function of clinical characteristics (e.g., anxiety, suicidality, substance use disorder) should therefore be interpreted cautiously.
Additional study limitations pertain to the measurements of attention and EF. For example, although Posner's (1978) development of the concept of “isolable” sub-systems of attention is initiatively appealing, some have criticized the evidence supporting this theory stating, “Housed in one brain and implemented by distributed and somewhat overlapping neural networks, strong independence between the networks of attention is unlikely,” (Macleod et al., 2010). Finally, because the main study questions regarding executive attention functioning are hypothesis driven but the secondary questions pertaining to within group variability were largely exploratory, we have not controlled for multiple comparisons. This study's ethnically diverse, primarily outpatient population of depressed adolescents with many common comorbid conditions makes it generalizable to a broader population in many ways, nevertheless, some features of the depression are likely to be underrepresented (e.g., current substance use disorder comorbidities) due to sample exclusion criteria.
In conclusion, the results of this study document a pattern of EF impairment associated with adolescent depression and provide some possible avenues to pursue with regard to how variation in phenotypic presentation of adolescent depression may map onto different patterns of neurocognitive functioning. It may be fruitful to direct attention to incorporating EF enhancement training into existing interventions to treat depression in adolescence more effectively.
References
- Abdullaev Y, Posner MI, Nunnally R, Dishion TJ. Functional MRI evidence for inefficient attentional control in adolescent chronic cannabis abuse. Behavioural Brain Research. 2010;215(1):45–57. doi: 10.1016/j.bbr.2010.06.023. doi:10.1016/j.bbr.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Baune BT, Fuhr M, Air T, Hering C. Neuropsychological functioning in adolescents and young adults with major depressive disorder–A review. Psychiatry research. 2014;218(3):261–271. doi: 10.1016/j.psychres.2014.04.052. doi:10.1016/j.psychres.2014.04.052. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Leroux JM, Bergman S, Arzoumanian Y, Beaudoin G, Bourgouin P, Stip E. The functional neuroanatomy of major depression: an fMRI study using an emotional activation paradigm. NeuroReport. 1998;9:3253–3258. doi: 10.1097/00001756-199810050-00022. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Belleau EL, Phillips ML, Birmaher B, Axelson DA, Ladouceur CD. Aberrant executive attention in unaffected youth at familial risk for mood disorders. Journal of affective disorders. 2013;147(1):397–400. doi: 10.1016/j.jad.2012.08.020. doi:10.1016/j.jad.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berndt ER, Koran LM, Finkelstein SN, Gelenberg MD, Kornstein SG, Miller IM, Thase ME, Keller MB. Lost human capital from early-onset chronic depression. The American Journal of Psychiatry. 2000;157(6):940–947. doi: 10.1176/appi.ajp.157.6.940. [DOI] [PubMed] [Google Scholar]
- Bridge JA, McBee-Strayer SM, Cannon EA, Sheftall AH, Reynolds B, Campo JV, Pajer KA, Brent DA. Impaired decision-making in adolescent suicide attempters. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(4):394–403. doi: 10.1016/j.jaac.2012.01.002. doi:10.1016/j.jaac.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4(6):215–222. doi: 10.1016/s1364-6613(00)01483-2. doi:10.1016/S1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Botteron KN, Raichle ME, Drevets WC, Heath AC, Todd RD. Volumetric reduction in left subgenual prefrontal cortex in early onset depression. Biological Psychiatry. 2002;51(4):342–344. doi: 10.1016/s0006-3223(01)01280-x. doi:10.1016/S0006-3223(01)01280-X. [DOI] [PubMed] [Google Scholar]
- Calkins AW, McMorran KE, Siegle GJ, Otto MW. The effects of computerized cognitive control training on community adults with depressed mood. Behavioural and cognitive psychotherapy. 2014:1–12. doi: 10.1017/S1352465814000046. doi:10.1017/S1352465814000046. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. doi:10.3758/CABN.7.4.367. [DOI] [PubMed] [Google Scholar]
- Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry. 1996;39:896–899. doi: 10.1016/0006-3223(95)00295-2. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, Identical Pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Research. 1988;26(2):223–238. doi: 10.1016/0165-1781(88)90076-5. doi:10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Gee DG, Klimes-Dougan B, Gabbay V, Hulvershorn L, Mueller BA, Milham MP. A preliminary study of functional connectivity in comorbid adolescent depression. Neuroscience Letters. 2009;460(3):227–231. doi: 10.1016/j.neulet.2009.05.022. doi:10.1016/j.neulet.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, Lim KO. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry. 2014;71:1138–1147. doi: 10.1001/jamapsychiatry.2014.1087. doi:10.1001/jamapsychiatry.2014.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Medicine. 2013;11(126):1–8. doi: 10.1186/1741-7015-11-126. doi:10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davey CG, Yücel M, Allen NB. The emergence of depression in adolescence: development of the prefrontal cortex and the representation of reward. Neuroscience & Biobehavioral Reviews. 2008;32(1):1–19. doi: 10.1016/j.neubiorev.2007.04.016. doi:10.1016/j.neubiorev.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W, Anderle MJ, Kalin NH. The neural substrates of affective processing in depressed patients treated with venlafaxine. American Journal of Psychiatry. 2003;160(1):64–75. doi: 10.1176/appi.ajp.160.1.64. doi:10.1176/appi.ajp.160.1.64. [DOI] [PubMed] [Google Scholar]
- Degl'Innocenti A, Ågren H, Bäckman L. Executive deficits in major depression. Acta Psychiatrica Scandinavica. 1998;97(3):182–188. doi: 10.1111/j.1600-0447.1998.tb09985.x. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13(8):663–681. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson CS, Mollet GA, Harrison DW. Anxious-depression in boys: an evaluation of executive functioning. Archives of Clinical Neuropsychology. 2005;20(4):539–546. doi: 10.1016/j.acn.2004.10.003. doi:10.1016/j.acn.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Engels AS, Heller W, Spielberg JM, Warren SL, Sutton BP, Banich MT, Miller GA. Co-occurring anxiety influences patterns of brain activity in depression. Cognitive, Affective, & Behavioral Neuroscience. 2010;10:141–156. doi: 10.3758/CABN.10.1.141. doi:10.3758/CABN.10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp AM, Dobson KS, Dozois DJA, Frewen PA. A systematic meta-analysis of the Stroop task in depression. Clinical Psychology Review. 2012;32:316–328. doi: 10.1016/j.cpr.2012.02.005. doi:10.1016/j.cpr.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16(1):143–149. doi:10.3758/BF03203267. [Google Scholar]
- Ernst M. The triadic model perspective for the study of adolescent motivated behavior. Brain and Cognition. 2014;89:104–111. doi: 10.1016/j.bandc.2014.01.006. doi:10.1016/j.bandc.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S, Ferrando S, Findler M, Stowell C, Smart C, Haglin D. Mindfulness-based cognitive therapy for generalized anxiety disorder. Journal of anxiety disorders. 2008;22(4):716–721. doi: 10.1016/j.janxdis.2007.07.005. doi:10.1016/j.janxdis.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, Mintun MA, Snyder AZ, Cohen JD, Sheline YI. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological psychiatry. 2008;63(4):377–384. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of cognitive neuroscience. 2002;14(3):340–347. doi: 10.1162/089892902317361886. doi:10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. NeuroImage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. doi:10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Favre T, Hughes C, Emslie G, Stavinoha P, Kennard B, Carmody T. Executive functioning in children and adolescents with major depressive disorder. Child Neuropsychology. 2009;15:85–98. doi: 10.1080/09297040802577311. doi:10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman DJ, Hamilton JP, Gotlib IH. Frontostriatal functional connectivity in major depressive disorder. Biology of mood & anxiety disorders. 2011;1(1):1–11. doi: 10.1186/2045-5380-1-11. doi:10.1186/2045-5380-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri C, Johnson L, Benedict K. Neurocognition in depression: patients on and off medication versus healthy comparison subjects. The Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18(2):217–225. doi: 10.1176/jnp.2006.18.2.217. doi: 10.1016/j.jad.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Gunther T, Holtkamp K, Jolles J, Herpertz-Dahlmann B, Konrad K. Verbal memory and aspects of attentional control in children and adolescents with anxiety disorders or depressive disorders. Journal of Affective Disorders. 2004;82:265–269. doi: 10.1016/j.jad.2003.11.004. Doi: [DOI] [PubMed] [Google Scholar]
- Hajek T, Kozeny J, Kopecek M, Alda M, Höschl C. Reduced subgenual cingulate volumes in mood disorders: a meta-analysis. Journal of Psychiatry & Neuroscience. 2008;33(2):91–99. [PMC free article] [PubMed] [Google Scholar]
- Han G, Klimes-Dougan B, Jepsen S, Ballard K, Nelson M, Houri A, Cullen K. Selective neurocognitive impairments in adolescents with major depressive disorder. Journal of Adolescence. 2012;35(1):11–20. doi: 10.1016/j.adolescence.2011.06.009. doi:10.1016/j.adolescence.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Witt AA, Oh D. The effect of mindfulness-based therapy on anxiety and depression: a meta-analytic review. Journal of Consulting and Clinical Psychology. 2010;78(2):169–183. doi: 10.1037/a0018555. doi:10.1037/a0018555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holler K, Kavanaugh B, Cook NE. Executive functioning in adolescent depressive disorders. Journal of Child and Family Studies. 2013;23:1315–1324. doi:10.1007/s10826-013-9789-z. [Google Scholar]
- Holmes AJ, Pizzagalli DA. Response conflict and frontocingulate dysfunction in unmedicated participants with major depression. Neuropsychologia. 2008;46(12):2904–2913. doi: 10.1016/j.neuropsychologia.2008.05.028. doi:10.1016/j.neuropsychologia.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horesh N. Self-report vs. computerized measures of impulsivity as a correlate of suicidal behavior. Crisis. 2001;22:7–31. doi: 10.1027//0227-5910.22.1.27. [DOI] [PubMed] [Google Scholar]
- Hudziak JJ, Achenbach TM, Althoff RR, Pine DS. A dimensional approach to developmental psychopathology. International Journal of Methods in Psychiatric Research. 2007;16(S1):S16–S23. doi: 10.1002/mpr.217. doi:10.1002/mpr.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami Y, Klein RM. Repeated measurement of the components of attention using two versions of the Attention Network Test (ANT): Stability, isolability, robustness, and reliability. Journal of neuroscience methods. 2010;190(1):117–128. doi: 10.1016/j.jneumeth.2010.04.019. doi:10.1016/j.jneumeth.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Jappe LM, Klimes-Dougan B, Cullen KR. Brain Imaging and the Prediction of Treatment Outcomes in Mood and Anxiety Disorders in. Functional brain mapping and the endeavor to understand the working brain. 2013:279–300. Doi: 10.5772/55446. [Google Scholar]
- Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(2):109–119. doi: 10.3758/cabn.7.2.109. doi:10.3758/CABN.7.2.109. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Gutman DA, Behrens TEJ, Matthews PM, Rushworth MFS, Katz E, Mayberg HS. Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cerebral Cortex. 2008;18(6):1374–1383. doi: 10.1093/cercor/bhm167. Doi:10.1093/cercor/bhm167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jollant F, Bellivier F, Leboyer M, Astruc B, Torres S, Verdier R, Courtet P. Impaired decision making in suicide attempters. American Journal of Psychiatry. 2005;162(2):304–310. doi: 10.1176/appi.ajp.162.2.304. doi:10.1176/appi.ajp.162.2.304. [DOI] [PubMed] [Google Scholar]
- Joormann J, Quinn ME. Cognitive processes and emotion regulation in depression. Depression and anxiety. 2014;31(4):308–315. doi: 10.1002/da.22264. doi: 10.1002/da.22264. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao UMA, Flynn C, Moreci P, Ryan N. Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. doi:10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kavanaugh B, Holler K. Executive functioning and self-reported depressive symptoms within an adolescent inpatient population. Applied Neuropsychology: Child. 2014;3(2):126–134. doi: 10.1080/21622965.2012.731662. doi: 10.1080/21622965.2012.731662. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Gorlyn M, Oquendo MA, Burke AK, Mann JJ. Attention deficit in depressed suicide attempters. Psychiatry Research. 2008;159(1):7–17. doi: 10.1016/j.psychres.2007.08.020. doi:10.1016/j.psychres.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AC, Di Martino A, Uddin LQ, Shehzad Z, Gee DG, Reiss PT, Milham MP. Development of anterior cingulate functional connectivity from late childhood to early adulthood. Cerebral Cortex. 2009;19(3):640–657. doi: 10.1093/cercor/bhn117. doi:10.1093/cercor/bhn117. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. doi:10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. doi:10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimes-Dougan B, Eberly LE, Westlund-Schreiner MK, Kurkiewicz P, Houri A, Schlesinger A, Cullen KR. Multilevel assessment of the neurobiological threat system in depressed adolescents: Interplay between the limbic system and hypothalamic–pituitary–adrenal axis. Development and Psychopathology. 2014;26:1321–1335. doi: 10.1017/S0954579414001059. doi:10.1017/S0954579414001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa EH, Sato JR, Lacerda SS, Barreiros MA, Radvany J, Russell TA, Amaro E., Jr Meditation training increases brain efficiency in an attention task. NeuroImage. 2012;59(1):745–749. doi: 10.1016/j.neuroimage.2011.06.088. doi:10.1016/j.neuroimage.2011.06.088. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Piasecki TM. Toward a dimensional and psychometrically-informed approach to conceptualizing psychopathology. Behaviour Research and Therapy. 2002;40(5):485–499. doi: 10.1016/s0005-7967(02)00016-5. doi:10.1016/S0005-7967(02)00016-5. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Bezdjian S. Enhancing research and treatment of mental disorders with dimensional concepts: toward DSM-V and ICD-11. World Psychiatry. 2009;8(1):3–6. doi: 10.1002/j.2051-5545.2009.tb00197.x. doi:10.1002/j.2051-5545.2009.tb00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Ryan ND, Casey BJ. Altered emotional processing in pediatric anxiety, depression, and comorbid anxiety-depression. Journal of Abnormal Child Psychology. 2005;33(2):165–177. doi: 10.1007/s10802-005-1825-z. doi: 10.1007/s10802-005-1825-z. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Slifka JS, Dahl RE, Birmaher B, Axelson DA, Ryan ND. Altered error-related brain activity in youth with major depression. Developmental Cognitive Neuroscience. 2012;2:351–362. doi: 10.1016/j.dcn.2012.01.005. doi:10.1016/j.dcn.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner D, Adler DA, Chang H, Lapitsky L, Hood MY, Perissinotto C, Rogers WH. Unemployment, job retention, and productivity loss among employees with depression. Psychiatric Services. 2004;55(12):1371–1378. doi: 10.1176/appi.ps.55.12.1371. doi:10.1176/appi.ps.55.12.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn PM, Clarke GN, Seeley JR, Rohde P. Major depression in community adolescents: age at onset, episode duration, and time to recurrence. Journal of the American Academy of Child & Adolescent Psychiatry. 1994;33(6):809–818. doi: 10.1097/00004583-199407000-00006. [DOI] [PubMed] [Google Scholar]
- Linden DE, Habes I, Johnston SJ, Linden S, Tatineni R, Subramanian L, Goebel R. Real-time self-regulation of emotion networks in patients with depression. PloS one. 2012;7(6):e38115. doi: 10.1371/journal.pone.0038115. doi:10.1371/journal.pone.003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay DS, Jacoby LL. Stroop process dissociations: the relationship between facilitation and interference. Journal of Experimental Psychology: Human Perception and Performance. 1994;20(4):219–234. doi: 10.1037//0096-1523.20.2.219. [DOI] [PubMed] [Google Scholar]
- Luciana M. Adolescent brain development in normality and psychopathology. Development and Psychopathology. 2013;25:1325–1345. doi: 10.1017/S0954579413000643. doi:10.1017/S0954579413000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod JM, McConnel M,M, Lawrence MA, Eskes GA, Klein RM, Shore DI. Appraising the ANT: psychometric and theoretical considerations of the Attention Network Test. Neuropsychology. 2010;24:637–651. doi: 10.1037/a0019803. doi: 10.1037/a0019803. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Mahurin RK, Jerabek PA, Brickman JS, Tekell JL, Fox PT. Cingulate function in depression: a potential predictor of treatment response. NeuroReport. 1997:1057–1061. doi: 10.1097/00001756-199703030-00048. [DOI] [PubMed] [Google Scholar]
- McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology. 2010;24(1):9. doi: 10.1037/a0017336. doi:10.1037/a0017336. [DOI] [PubMed] [Google Scholar]
- Micco JA, Henin A, Biederman J, Rosenbaum JF, Petty C, Rindlaub LA, Hirschfield-Becker DR. Executive functioning in offspring at risk for depression and anxiety. Depression and Anxiety. 2009;26:780–790. doi: 10.1002/da.20573. doi:10.1002/da.20573. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Kilts CD, Berns GS. Functional brain imaging: twenty-first century phrenology or psychobiological advance for the millennium?. The American Journal of Psychiatry. 1999;156(5):671. doi: 10.1176/ajp.156.5.671. [DOI] [PubMed] [Google Scholar]
- NIMH . Research Domain Criteria (RDoC) Project. Rockville, MD.: 2011. [Google Scholar]
- Ottowitz WE, Dougherty DD, Savage CR. The neural network basis for abnormalities of attention and executive function in major depressive disorders: Implications for application of the medical disease model to psychiatric disorders. Harvard Review of Psychiatry. 2002;10:86–99. doi: 10.1080/10673220216210. doi:10.1080/10673220216210. [DOI] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, Lupiáñez J. Attention and anxiety different attentional functioning under state and trait anxiety. Psychological Science. 2010;21(2):298–304. doi: 10.1177/0956797609359624. doi:10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Marqués E, Lupiáñez J. Alterations of the attentional networks in patients with anxiety disorders. Journal of Anxiety Disorders. 2011;25(7):888–895. doi: 10.1016/j.janxdis.2011.04.010. doi:10.1016/j.janxdis.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence?. Nature Reviews Neuroscience. 2008;9(12):947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Pascual-Marqui RD, Nitschke JB, Oakes TR, Larson CL, Abercrombie HC, Davidson RJ. Anterior cingulate activity as a predictor of treatment response in major depression: Evidence from brain electrical tomography analysis. American Journal of Psychiatry. 2001;158:405–415. doi: 10.1176/appi.ajp.158.3.405. [DOI] [PubMed] [Google Scholar]
- Posner MI. Chronometric explorations of mind. Lawrence Erlbaum; 1978. [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32(1):3–25. doi: 10.1080/00335558008248231. doi:10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Posner MI, Raichle ME. The neuroimaging of human brain function. Proceedings of the National Academy of Sciences. 1998;95(3):763–764. doi: 10.1073/pnas.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahdar A, Galván A. The cognitive and neurobiological effects of daily stress in adolescents. NeuroImage. 2014;92:267–273. doi: 10.1016/j.neuroimage.2014.02.007. [DOI] [PubMed] [Google Scholar]
- Ramel W, Goldin PR, Carmona PE, McQuaid JR. The effects of mindfulness meditation on cognitive processes and affect in patients with past depression. Cognitive Therapy and Research. 2004;28(4):433–455. doi:10.1023/B:COTR.0000045557.15923.96. [Google Scholar]
- Reinholdt-Dunne ML, Mogg K, Bradley BP. Effects of anxiety and attention control on processing pictorial and linguistic emotional information. Behaviour Research and Therapy. 2009;47:410–417. doi: 10.1016/j.brat.2009.01.012. doi: 10.1016/j.brat.2009.01.012. [DOI] [PubMed] [Google Scholar]
- Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. Journal of abnormal psychology. 2010;119(4):631. doi: 10.1037/a0020909. doi:doi.org/10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. The Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. doi:10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghavi MR, Neshat-Doost HT, Moradi AR, Yule W, Dalgleish T. Biases in visual attention in children and adolescents with clinical anxiety and mixed anxiety-depression. Journal of Abnormal Child Psychology. 1999;27(3):215–223. doi: 10.1023/a:1021952407074. doi:10.1023/A:1021952407074. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Østby Y, Fjell AM, Westlye LT, Due-Tønnessen P, Walhovd KB. Brain maturation in adolescence and young adulthood: Regional age-related changes in cortical thickness and white matter volume and microstructure. Cerebral Cortex. 2010;20:534–548. doi: 10.1093/cercor/bhp118. doi:10.1093/cercor/bhp118. [DOI] [PubMed] [Google Scholar]
- Tang YY, Lu Q, Geng X, Stein EA, Yang Y, Posner MI. Short-term meditation induces white matter changes in the anterior cingulate. Proceedings of the National Academy of Sciences. 2010;107(35):15649–15652. doi: 10.1073/pnas.1011043107. doi:10.1073/pnas.1011043107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares JT, Drevets WC, Sahakian BJ. Cognition in mania and depression. Psychological Medicine. 2003;33:959–967. doi: 10.1017/s0033291703008432. doi:10.1017/S0033291703008432. [DOI] [PubMed] [Google Scholar]
- Ursache A, Raver CC. Trait and state anxiety: Relations to executive functioning in an at-risk sample. Cognition & Emotion. 2013. Advance online publication doi:10.1080/02699931.2013.855173. [DOI] [PMC free article] [PubMed]
- van Leijenhorst L, Moor BG, Op de Macks ZA, Rombouts SA, Westenberg PM, Crone EA. Adolescent risky decision-making: neurocognitive development of reward and control regions. NeuroImage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. doi:10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) American Psychological Association; San Antonio, Texas: 1999. [Google Scholar]
- Weissman MM, Wolk S, Goldstein RB, Moreau D, Adams P, Greenwald S, Wickramaratne P. Depressed adolescents grown up. The Journal of the American Medical Association. 1999;281(18):1707–1713. doi: 10.1001/jama.281.18.1707. [DOI] [PubMed] [Google Scholar]
- Wells TT, Beevers CG. Biased attention and dysphoria: manipulating selective attention reduces subsequent depressive symptoms. Cognition and Emotion. 2010;24(4):719–728. doi:10.1080/02699930802652388. [Google Scholar]
- World Health Organization . The global burden of disease. Geneva, Switzerland: 2008. p. 2004. update. [Google Scholar]
- Worley MJ, Tate SR, Granholm E, Brown SA. Mediated and moderated effects of neurocognitive impairment on outcomes of treatment for substance dependence and major depression. Journal of Consulting and Clinical Psychology. 2014. Advance online publication doi:10.1037/a0036033. [DOI] [PMC free article] [PubMed]
- Zlotnick C, Kohn R, Keitner G, Grotta SA. The relationship between quality of interpersonal relationships and major depressive disorder: findings from the National Comorbidity Survey. Journal of Affective Disorders. 2000;59:205–215. doi: 10.1016/s0165-0327(99)00153-6. doi:10.1016/S0165-0327(99)00153-6. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, Gardner JF, Soeken K, Boles M, Lynch F. Rising prevalence of antidepressants among US youths. Pediatrics. 2002;109(5):721–727. doi: 10.1542/peds.109.5.721. doi:10.1542/peds.109.5.721. [DOI] [PubMed] [Google Scholar]
- Zylowska L, Ackerman DL, Yang MH, Futrell JL, Horton NL, Hale TS, Smalley SL. Mindfulness meditation training in adults and adolescents with ADHD a feasibility study. Journal of Attention Disorders. 2008;11(6):737–746. doi: 10.1177/1087054707308502. doi:10.1177/1087054707308502. [DOI] [PubMed] [Google Scholar]