Abstract
Objective
To assess the stability of metabolic status and BMI status, and their relative contribution to risk of diabetes, cardiovascular events, and mortality.
Methods
14,685 participants from ARIC, and 4,990 from CARDIA. We defined people with healthy obesity (HO) as meeting all 3 indices of blood pressure, blood glucose, and blood lipids. People with unhealthy obesity crossed the risk threshold for all 3 criteria.
Results
In both healthy and unhealthy subgroups, risks for CHD, stroke, and mortality were comparable among BMI status during a mean 18.7-year follow-up. When compared with HO, hazard ratios were increased for diabetes (5.56, 95% CI 4.12–7.48), CHD (5.60, CI 3.14–9.98), stroke (4.84, CI 2.13–10.97), and mortality (2.6, CI 1.88–3.61) in people with unhealthy obesity. BMI only moderately increased the risks for diabetes among healthy subjects. In CARDIA over 20 years, 17.5 % of lean subjects and 67.3% of overweight subjects at baseline became obese during follow-up. Despite rising BMI, metabolic status remained relatively stable.
Conclusions
Metabolic status is relatively stable despite rising BMI. HO had lower risks for diabetes, CHD, stroke, and mortality than unhealthy subjects, but increased diabetes risks than healthy lean people. Cardiometabolic risk factors confer much higher risk than obesity per se.
Keywords: Risk Factors, Cardiovascular Risk, Cardiovascular Disease, Diabetes, Obesity
Introduction
Obesity is associated with elevated risk for morbidity and mortality (1), and has become an epidemic both in the United States and worldwide (2, 3). Body Mass Index (BMI) is widely used as a population-based tool to assess adiposity (2), and is also used in individual patients as a basis for therapeutic decisions according to guidelines for obesity management (4). However, BMI does not directly reflect the degree of excess adiposity or how it impacts health risks in individual patients. While obesity can exacerbate insulin resistance and impel the progression of cardiometabolic disease, lean individuals can be afflicted with insulin resistance and cardiometabolic disease, albeit with relative fat distribution to the intra-abdominal compartment, and individuals with obesity can be relatively insulin sensitive and without manifestations of cardiometabolic disease (5–9). Thus obesity and cardiometabolic disease can segregate independently, at least in part.
Consistent with the above formulation, we have developed the Cardiometabolic Disease Staging (CMDS) system, which predicts differential risk in people with obesity for future T2DM and cardiovascular disease mortality based on the presence or absence of metabolic syndrome traits (10). Individuals with obesity who are free of cardiometabolic disease risk factors, such as hypertension, dyslipidemia, and hyperglycemia, have been termed the ‘metabolically healthy obesity’ (11, 12). Physiological studies have demonstrated that these subjects with obesity without metabolic syndrome traits are relatively insulin sensitive (5–9, 12), and epidemiological data indicate low risk of progression to diabetes and cardiovascular disease (10, 13–16).
However, there has been an escalating debate (17–24) disputing whether people with metabolically healthy obesity are indeed at low risk, and the degree to which uncomplicated obesity places individuals at risk of cardiometabolic disease. “Metabolically healthy obesity” in these studies routinely included subjects with risk factors, and, therefore, would predictably be relatively insulin resistant (6) and at higher risk than those lacking all manifestations of cardiometabolic disease (10). To more definitively address this controversy, we have employed exacting definitions of metabolic health status and studied large cohorts, including both young and older adults, featuring rigorous follow-up for identification of health outcomes including incident diabetes, coronary heart disease, stroke, and all-cause mortality.
Methods
Cardiometabolic Heath
We categorized cardiometabolic health status as healthy, unhealthy, and suboptimal health using criteria from the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) (25) and the American Heart Association (26) for blood pressure, fasting blood glucose, and lipid profile. Cardiometabolically healthy subjects exhibit normal values for all 3 risk factors: (i) blood pressure (untreated systolic <130 mmHg and diastolic <85); (ii) blood glucose (untreated fasting <100 mg/dl or HbA1c <5.7%); (iii) blood lipids (untreated total cholesterol <240 mg/dl, and HDL ≥40 mg/dl in men and ≥50 in women). Cardiometabolically unhealthy individuals cross the risk threshold for all 3 factors, and meet Adult Treatment Panel III criteria for metabolic syndrome. All other subjects met criteria for one or two risk factors, and were defined as having suboptimal cardiometabolic health status. Obesity was defined according to BMI status: ≥30 kg/m2, overweight: 25–29.9 kg/m2, and lean: < 25 kg/m2.
Study Population
To test our hypotheses regarding interactions between obesity and cardiometabolic health, we employed data of participants from the Atherosclerosis Risk in Communities (ARIC) Study enrolled as young elders, and the Coronary Artery Risk Development in Young Adults (CARDIA) Study enrolled as young adults. Detailed information about the ARIC study and the CARDIA study can be found elsewhere (27, 28). After excluding participants with missing information on BMI status (n=35 in CARDIA, n=867 in ARIC) or cardiometabolic health status (n=90 in CARDIA, n=240 in ARIC), we got 4,990 participants from CARDIA and 14,685 participants from ARIC. For the analysis of cardiometabolic outcomes, we also excluded participants who had already developed those outcomes at baseline. We assessed stability of the cardiometabolic health status category over 10 years from Visit 1 to Visit 4 in ARIC, and during year 0 to year 10 and year 10 to year 20 in CARDIA, which is when the required data on body measures and metabolic health status were available. ARIC data were also used to examine the impact of BMI and health status on outcome events, including incident diabetes, coronary heart disease (CHD) event (myocardial infarction, coronary death), stroke, and mortality with follow up through December, 2009 (mean follow-up time 18.7 years).
Statistical Analysis
Cox regression models and Kaplan-Meier survival curves were constructed to analyze long-term health outcomes as a function of BMI and cardiometabolic health status. For the ARIC study, follow-up time was calculated as the difference between the baseline and the year when events were first identified, or the year a participant was censored, whichever came first. Multivariable adjusted cox model was adjusted for age (log transformed), sex, race, income, education, tobacco smoking and alcohol drinking. For models assessing risk for incident diabetes, multivariable adjusted cox model was further adjusted for parental diabetes history. The proportional hazards assumption for cox models was assessed using Schoenfeld residuals. We analyzed the added discriminative power offered by including BMI category or cardiometabolic health category to a basic multivariable model including above-mentioned clinical characteristics using the Harrell C-index (29, 30). Statistical analyses were carried out with SAS for Windows version 9.3 (SAS Institute). A 2-sided P <0.05 was determined to be statistically significant.
Results
Baseline characteristics of individuals in the ARIC study are delineated in Table 1, stratified by BMI status and cardiometabolic health. The study population was comprised of 4007 participants with obesity, 5808 overweight participants, and 4870 lean participants, including 6694 men and 7991 women, and 3679 Blacks and 11006 Whites. Regarding cardiometabolic health status, 2641(18.0%) subjects were metabolically healthy, 2540 (17.3%) were unhealthy, and 9504 (64.7%) subjects had suboptimal cardiometabolic health.
Table 1.
Characteristics of ARIC participants.
| Healthy | Suboptimal | Unhealthy | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | Lean | Overweight | Obesity | Lean | Overweight | Obesity | Lean | Overweight | Obesity | |
| All | 14685 | 1499 | 882 | 260 | 3020 | 3962 | 2522 | 351 | 964 | 1225 |
| Men | 6694 | 411 | 399 | 75 | 1275 | 2324 | 995 | 161 | 559 | 495 |
| Women | 7991 | 1088 | 483 | 185 | 1745 | 1638 | 1527 | 190 | 405 | 730 |
| Black | 3679 | 169 | 157 | 75 | 532 | 930 | 939 | 104 | 291 | 482 |
| White | 11006 | 1330 | 725 | 185 | 2488 | 3032 | 1583 | 247 | 673 | 743 |
| age (Year) | 54.3(54.2–54.4) | 52.3(52.0–52.6) | 52.7(52.3–53.0) | 52.8(52.1–53.5) | 54.8(54.6–55.0) | 54.4(54.2–54.5) | 53.7(53.5–54.0) | 57.0(56.5–57.5) | 56.3(55.9–56.6) | 55.2(54.9–55.6) |
| BMI (kg/m2) | 27.7(27.6–27.8) | 22.2(22.1–22.3) | 27.0(27.0–27.1) | 33.3(32.8–33.7) | 22.6(22.6–22.7) | 27.3(27.2–27.3) | 34.2(34.1–34.4) | 23.1(23.0–23.3) | 27.6(27.5–27.7) | 35.2(34.9–35.4) |
| Waist Circumference (cm) | 97.1(96.9–97.3) | 82.1(81.7–82.5) | 94.9(94.4–95.4) | 107.5(106.2–108.8) | 85.2(85.0–85.5) | 97.2(96.9–97.4) | 111.7(111.3–112.2) | 88.3(87.5–89.0) | 98.8(98.4–99.2) | 115.0(114.3–115.6) |
| Systolic Blood Pressure (mmHg) | 121.3(121.0–121.6) | 107.1(106.6–107.7) | 110.9(110.2–111.5) | 113.8(112.6–115.0) | 118.8(118.1–119.5) | 120.6(120.1–121.2) | 124.4(123.7–125.1) | 136.0(134.2–137.9) | 134.3(133.1–135.5) | 135.0(134.0–136.1) |
| Diastolic Blood Pressure (mmHg) | 73.6(73.4–73.8) | 66.6(66.2–67.0) | 69.3(68.8–69.8) | 70.1(69.3–71.0) | 71.7(71.2–72.1) | 73.7(73.4–74.1) | 76.0(75.6–76.5) | 78.4(77.2–79.6) | 79.0(78.3–79.8) | 79.8(79.2–80.4) |
| Fasting Glucose (mg/dl) | 109.1(108.5–109.8) | 91.1(90.8–91.4) | 92.5(92.2–92.8) | 92.7(92.1–93.3) | 100.9(99.9–101.8) | 105.9(104.9–106.9) | 112.4(110.8–114.1) | 128.9(122.7–135.1) | 130.4(127.0–133.9) | 147.8(143.9–151.8) |
| Total Cholesterol (mg/dl) | 215.1(214.4–215.7) | 194.5(193.2–195.9) | 196.9(195.1–198.6) | 196.6(193.4–199.8) | 216.3(214.8–217.8) | 217.4(216.1–218.7) | 214.7(213.1–216.3) | 235.0(229.7–240.4) | 233.0(230.0–236.1) | 227.6(225.0–230.3) |
| HDL Cholesterol (mg/dl) | 51.5(51.2–51.8) | 65.7(64.9–66.5) | 58.8(57.9–59.7) | 58.7(57.3–60.0) | 55.9(55.2–56.5) | 48.4(47.9–48.9) | 48.4(47.8–48.9) | 47.7(45.8–49.5) | 42.7(41.8–43.6) | 40.6(40.0–41.3) |
| HOMA-IR | 3.92(3.66–4.18) | 1.05(1.01–1.08) | 1.49(1.43–1.55) | 1.96(1.83–2.09) | 2.13(1.70–2.55) | 3.21(2.87–3.54) | 5.11(4.57–5.65) | 6.00(3.31–8.69) | 8.27(5.57–10.96) | 9.84(8.93–10.75) |
Data are presented as n or mean (95% confidence interval).
Obesity was defined according to BMI status: ≥30 kg/m2 (obesity), 25–29.9 kg/m2 (overweight), and < 25 (lean).
Cardiometabolically healthy subjects exhibit normal values for all 3 risk factors: (i) blood pressure (untreated systolic <130 mmHg and diastolic <85); (ii) blood glucose (untreated fasting <100 mg/dl or HbA1c <5.7%); (iii) blood lipids (untreated total cholesterol <240 mg/dl, and HDL ≥40 mg/dl in men and ≥50 in women). Cardiometabolically unhealthy individuals cross the risk threshold for all 3 factors. All other subjects met criteria for one or two risk factors, and were defined as having suboptimal cardiometabolic health status.
ARIC: Atherosclerosis Risk in Communities.
Diabetes, CHD and Mortality
During a mean 18.7-year follow-up in the ARIC study, there were 3,667 cases of new-onset diabetes, 2,102 cases of CHD (myocardial infarction and coronary death), 1,044 cases of stroke, and 3,960 cases of deaths.
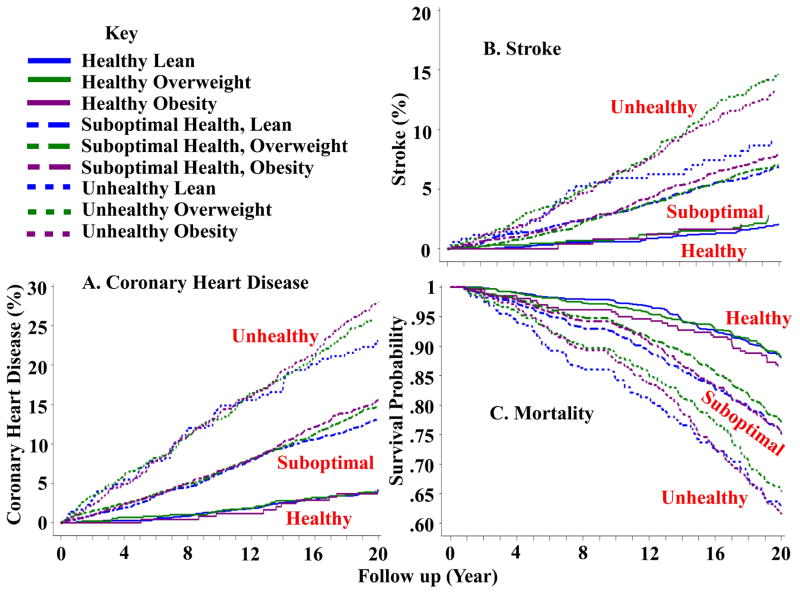
Kaplan-Meier plots for event probability as a function of cardiometabolic health and body weight status are shown in Figures 1 and 2, and multivariate adjusted hazard ratios with healthy obesity as reference are shown in Table 2 for incident diabetes, CHD, stroke, and all-cause mortality. Overall, the presence of metabolic syndrome risk factors was more predictive of outcomes than BMI status (i.e., lean, overweight, obesity). BMI status had no effect on the risks for CHD, all-cause mortality, or stroke, either in the healthy or unhealthy subgroups. We also assessed risk of cardiovascular disease outcomes in subjects with one or two metabolic syndrome traits (i.e., suboptimal health). It was clear that cumulative incidence rates for CHD, stroke, and survival probability over the 20 year period in subjects with suboptimal health were intermediate between those in the healthy and unhealthy subgroups with no effect of BMI status. Subjects with 2 risk factors had higher risks for those outcomes than subjects with only 1 risk factor (Table 2). In contrast to BMI, metabolic health status was a powerful predictor of cardiovascular events. When compared with healthy lean participants, overweight participants and participants with obesity, their BMI counterparts who were unhealthy or with suboptimal health exhibited increased cumulative incidence rates of CHD and stroke, and decreased survival probability.
Figure 1.
CHD, stroke, and mortality according to cardiometabolic health and body status in subjects from the ARIC study.
A. CHD; B. Stroke; C. All-cause Mortality.
CHD: coronary heart disease (myocardial infarction, coronary death).
ARIC: Atherosclerosis Risk in Communities.
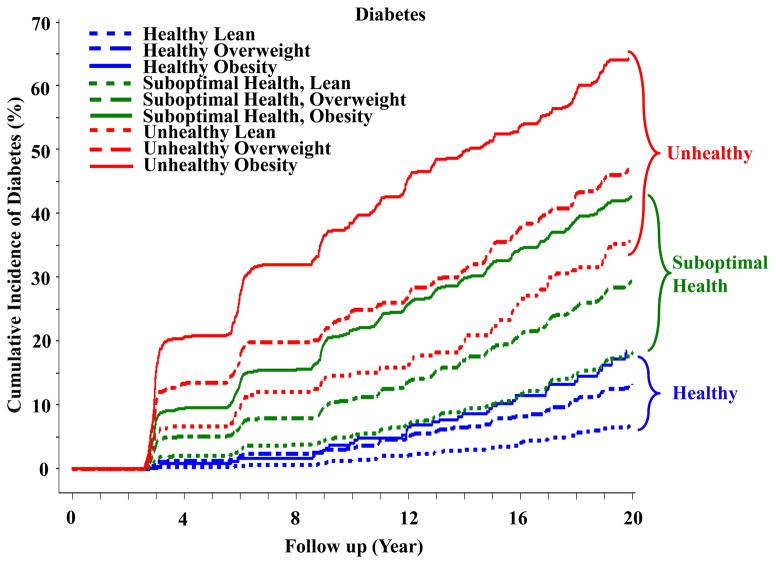
Figure 2.
Incident diabetes according to cardiometabolic health and body status in subjects from the ARIC study.
ARIC: Atherosclerosis Risk in Communities.
Table 2.
Multivariable-adjusted hazard ratio of long-term health outcomes according to cardiometabolic health and body weight status in the ARIC study.
| Metabolic Health Status | BMI Status | Hazard Ratio (95% Confidence Interval) | |||
|---|---|---|---|---|---|
| Diabetes | CHD | Stroke | Mortality | ||
| Healthy | Lean | 0.47 (0.34–0.66) | 0.92 (0.50–1.71) | 1.17 (0.49–2.78) | 0.97 (0.69–1.37) |
| Overweight | 0.77 (0.56–1.08) | 0.95 (0.50–1.81) | 1.53 (0.64–3.69) | 0.90 (0.63–1.29) | |
| Obesity | Reference | Reference | Reference | Reference | |
| 1 Risk Factor | Lean | 0.84(0.62–1.13) | 2.00(1.12–3.59) | 2.42(1.06–5.49) | 1.24(0.89–1.72) |
| Overweight | 1.33(0.99–1.79) | 2.02(1.13–3.61) | 2.17(0.95–4.93) | 1.18(0.85–1.63) | |
| Obesity | 2.28(1.69–3.07) | 2.09(1.15–3.81) | 2.45(1.06–5.68) | 1.38(0.98–1.94) | |
| 2 Risk Factors | Lean | 1.57(1.16–2.14) | 2.71(1.51–4.87) | 2.86(1.25–6.55) | 1.63(1.17–2.28) |
| Overweight | 2.50(1.87–3.34) | 2.95(1.66–5.25) | 3.10(1.37–7.02) | 1.48(1.07–2.05) | |
| Obesity | 3.35(2.50–4.48) | 3.57(2.00–6.36) | 3.37(1.49–7.63) | 1.86(1.34–2.57) | |
| Suboptimal Health (1 or 2 Risk Factors) | Lean | 1.07 (0.80–1.43) | 2.26 (1.27–4.03) | 2.58 (1.14–5.82) | 1.39 (1.01–1.92) |
| Overweight | 1.81 (1.36–2.41) | 2.48 (1.40–4.40) | 2.65 (1.18–5.95) | 1.33 (0.97–1.83) | |
| Obesity | 2.84 (2.13–3.78) | 3.01 (1.69–5.35) | 3.03 (1.35–6.84) | 1.68 (1.22–2.32) | |
| Unhealthy | Lean | 2.33 (1.64–3.30) | 3.61 (1.96–6.66) | 2.87 (1.19–6.92) | 1.95 (1.37–2.78) |
| Overweight | 3.33 (2.46–4.51) | 4.43 (2.48–7.92) | 4.59 (2.02–10.4) | 1.81 (1.30–2.53) | |
| Obesity | 5.42 (4.03–7.29) | 5.47 (3.08–9.74) | 4.53 (2.00–10.3) | 2.52 (1.82–3.50) | |
Multivariable adjusted cox model was adjusted for age (log transformed), sex, race, income, education, tobacco smoking and alcohol drinking. For models assessing risk for incident diabetes, multivariable adjusted cox model was further adjusted for parental diabetes history.
CHD: coronary heart disease (myocardial infarction, coronary death).
ARIC: Atherosclerosis Risk in Communities.
For diabetes, cumulative diabetes rates over 20 years of follow-up remained low in healthy subjects regardless of their BMI status (Figure 2). However, incident diabetes was increased in all unhealthy subgroups. Importantly, lean subjects who were unhealthy displayed a >2-fold increase in cumulative diabetes when compared with healthy individuals with obesity. However, among unhealthy subjects, diabetes risk rose progressively as BMI status increased from lean to overweight to obesity. Cumulative diabetes rates in subjects with suboptimal health status (with 1 or 2 metabolic syndrome traits) were observed to be intermediate between unhealthy and healthy subgroups, and higher BMI status also tended to increase diabetes risk in subjects with suboptimal health. We interpret the data in Figure 1A and Table 2 to mean that weight gain against an insulin sensitive background (i.e., metabolically healthy) exerts a relatively small effect to increase risk of diabetes, but can significantly augment diabetes risk in insulin resistant patients with suboptimal health and more so in unhealthy individuals. Along these lines, it is important to consider that the unhealthy subjects (mean HOMA-IR 8.71, 95% CI 7.54–9.89) and the suboptimal health subgroup were more insulin resistant (mean HOMA-IR 3.37, 95% CI 3.13–3.61) than the healthy subjects (HOMA-IR 1.29, 95% CI 1.25–1.32, Table 1).
The Harrell C-statistic was significantly increased when adding the cardiometabolic health category into the basic model for both diabetes (increased from 0.6097 to 0.6771) and CHD (0.6934 to 0.7239). When only the BMI category was included in the basic model, the Harrell C-statistic was significantly increased for diabetes (0.6097 to 0.6721) albeit to a lesser degree than for cardiometabolic health status, whereas BMI category exerted no significant effect regarding CHD (0.6934 to 0.7024).
Stability of BMI and Cardiometabolic Health Status
BMI Status
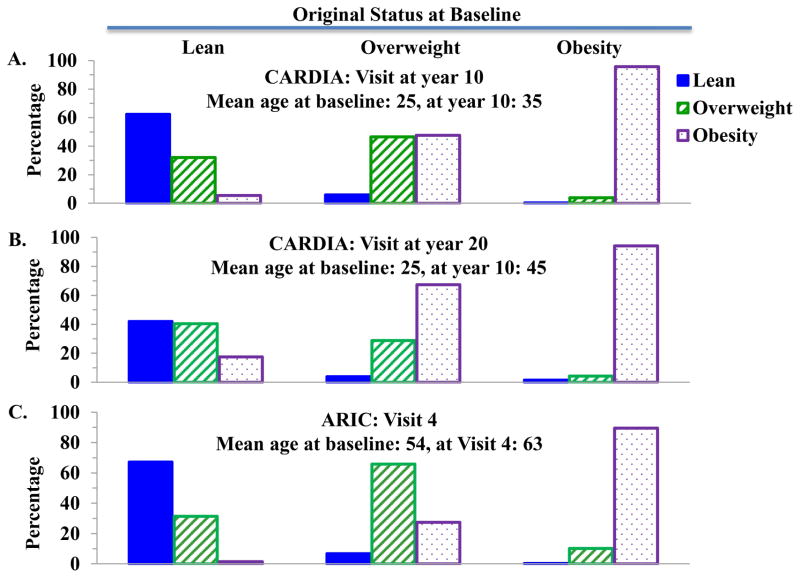
The stability of BMI status and metabolic health (i.e., healthy and unhealthy categories) were assessed in both ARIC and CARDIA. These two cohorts allowed us to study changes over a 10-year period in ARIC which featured older subjects (mean age at baseline was 54 years) and over 20 years in CARDIA comprised of younger subjects (mean age 25 years at baseline). During follow-up, the prevalence of obesity progressively increased, concomitant with a diminishing prevalence of lean in both men and women, Blacks and Whites. In both studies, however, subjects with obesity at baseline rarely became lean or overweight during the follow-up period (Figure 3). Specifically, over 20 years in CARDIA, only 1.5% (4 women) individuals with obesity at baseline became lean, and as few as 0.4% of subjects with obesity became lean over 10 years in ARIC. On the other hand, individuals who were lean or overweight at baseline did demonstrate a proclivity for transition to obesity in young adults but not older adults. In CARDIA over 20 years, 17.5 % of lean subjects at baseline and 67.3% of overweight subjects at baseline transitioned to obesity at the year 20 examination. Fewer of the older patients in ARIC transitioned from lean to obesity, or overweight to obesity, relative to the younger patients in CARDIA, indicating that BMI status was more stable over a 10 year period in subjects in their 6th and 7th decade compared with middle-aged adults.
Figure 3.
Body Weight status changes in participants from the CARDIA study and the ARIC study.
A. Changes from baseline to year 10 in participants from the CARDIA study; B. Changes from baseline to year 20 in participants from the CARDIA study; C. Changes from baseline to Visit 4 in participants from the ARIC study.
CARDIA: Coronary Artery Risk Development in Young Adults.
ARIC: Atherosclerosis Risk in Communities.
Cardiometabolic Health Status
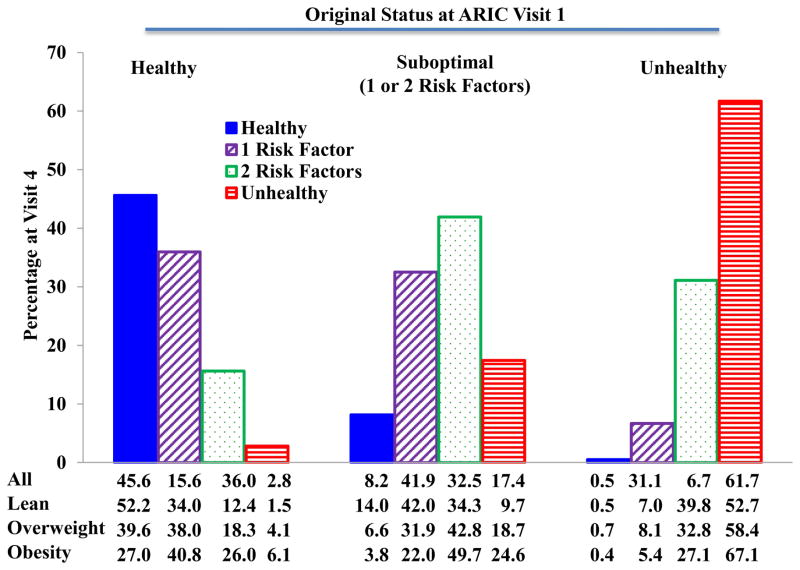
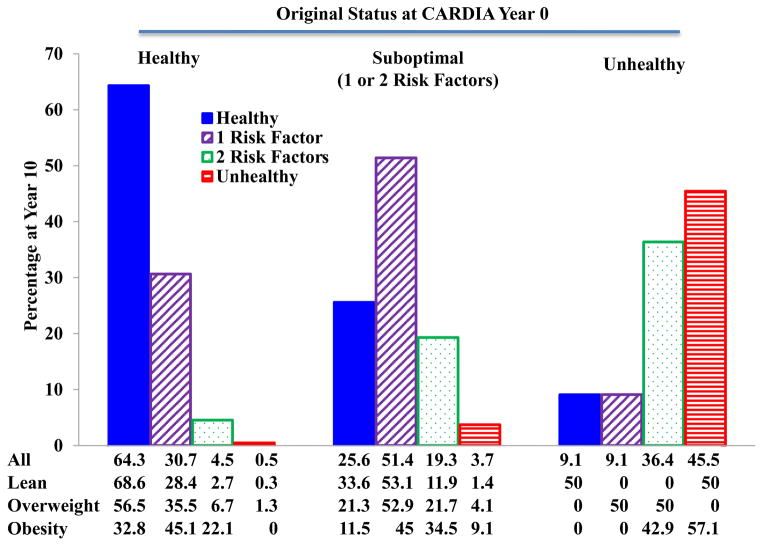
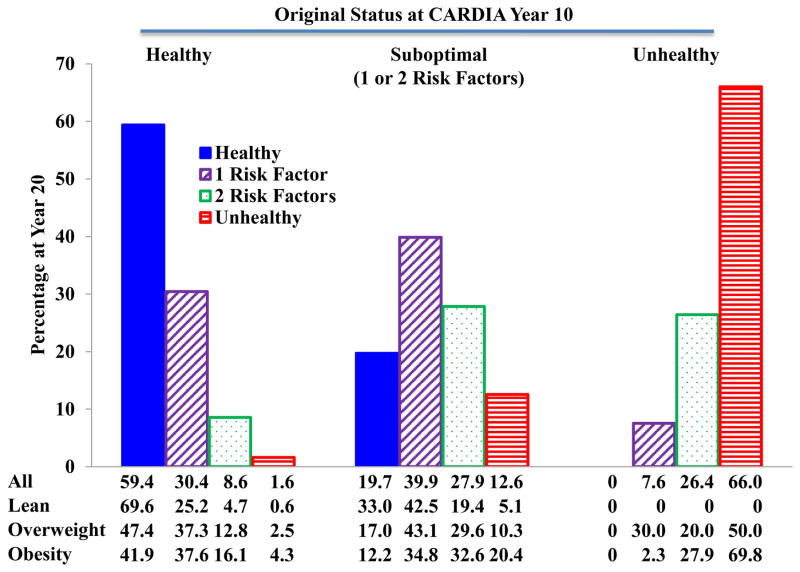
We also assessed stability of cardiometabolic health categories. Figures 4 and 5 show the cardiometabolic health status after 10 years follow-up in both ARIC and CARDIA. Very few subjects who were metabolically healthy at baseline transitioned to unhealthy status (2.8% in ARIC and 0.5% in CARDIA) after 10 years. Similarly, few if any subjects who were unhealthy at baseline became metabolically healthy (0.5% in ARIC and 9.1% in CARDIA. However, significant percentages of healthy subjects did develop 1 or 2 risk factors (51.6% in ARIC and 35.2% in CARDIA), as did smaller percentages of subjects who were unhealthy at baseline (37.8% in ARIC and 45.5% in CARDIA). Most of the individuals with 1 or 2 risk factors at baseline remained within this category of suboptimal health after 10 years. These same patterns were maintained in CARDIA during the follow-up from year 10 to year 20 as shown in Figure 6.
Figure 4.
Caridometabolic health status changes from baseline to Visit 4 in participants from the ARIC study.
Data are presented as percentages.
ARIC: Atherosclerosis Risk in Communities.
Figure 5.
Caridometabolic health status changes from year 0 to year 10 in participants from the CARDIA study.
Data are presented as percentages.
CARDIA: Coronary Artery Risk Development in Young Adults.
Figure 6.
Caridometabolic health status changes from year 10 to year 20 in participants from the CARDIA study.
Data are presented as percentages.
CARDIA: Coronary Artery Risk Development in Young Adults.
Discussion
Using data from two large cohorts, the CARDIA study and the ARIC study, we assessed body weight status and cardiometabolic health over an extended period of follow up in both young adults and young-elder adults. Importantly, we have defined metabolic health status as healthy only if no metabolic syndrome risk factors were present, unhealthy if subjects met criteria for metabolic syndrome, and also included a category for suboptimal health for those individuals meeting criteria for one or two metabolic syndrome risk factors. It allowed us to determine the relative impact of metabolic health status and BMI on long-term health outcomes, such as incident diabetes, coronary heart disease, stroke, and mortality. We found that metabolic health status predominated in determining cardiovascular disease risk for outcomes including coronary artery disease, stroke, and mortality, while BMI status exerted no influence on the risks. With respect to diabetes risk, metabolic health status again proved to be a more powerful indicator of risk compared with healthy subjects in all BMI strata.
The relatively small contribution of generalized adiposity measured by BMI to individual differences in insulin sensitivity has been well documented (5–9) and insulin resistance is central in the pathophysiology responsible for cardiometabolic disease. In the current study, the metabolically unhealthy were markedly insulin resistant compared with healthy subjects based on HOMA values, independent of BMI in agreement with the previous reports. We have shown that increasing obesity leads to much greater absolute increases in diabetes incidence rates in metabolically unhealthy compare to healthy subjects. We interpret this to mean that excess adiposity will substantially augment incident diabetes in subjects if weight gain occurs against a background of insulin resistance, while a comparable amount of weight gain will not lead to high incidence rates if acquired in insulin sensitive individuals.
We were also able to assess the stability of BMI and metabolic health status over time in adults. We found that subjects with obesity at baseline remained obesity and rarely became lean whether in the young adults with obesity or in the young-elder adults with obesity during the extended follow-up periods. On the other hand, ~18% of young lean adults became obese during a 20-year follow-up period in CARDIA, whereas lean young-elder adults seldom became obese during the ensuing 10-year follow-up period in ARIC. However, a significant portion of lean subjects at baseline became overweight and overweight subjects became obese. Despite this overall progressive increase in mean BMI, metabolic health status stood in contrast as being rather stable. In both ARIC and CARDIA, most of the healthy participants with obesity remained healthy; and, while a minority did develop one or two metabolic syndrome risk factors (i.e., suboptimal health), very few became unhealthy over 10 years of follow-up. Similarly, the clear majority of metabolically unhealthy subjects with obesity at baseline remained unhealthy during follow-up in both cohorts. Interestingly, the majority of subjects with suboptimal metabolic health at baseline also remained in this category over the course of follow-up. Thus, despite rising BMI in these adult cohorts the metabolic status remained relatively stable.
Cardiometabolic Health in People with Healthy Obesity
Subjects with obesity who are free of cardiometabolic disease risk factors are termed as ‘metabolically healthy obesity’ (11, 12). However, the existence of the metabolically healthy obesity remains controversial and it has been debated whether the healthy obesity are in fact at lower risk for developing future cardiometabolic disease compared with subjects with equal BMI who are metabolically unhealthy (17–22). For example, Thomsen et al studied 71,527 individuals from the Copenhagen General Population Study with a median of 3.6 years’ follow-up and concluded that subjects with obesity, whether or not they had metabolic syndrome, had significantly higher risk for myocardial infarction compared with normal weight individuals (18). These authors defined metabolically healthy and unhealthy obesity as the absence or presence of the metabolic syndrome. They used a modified definition of metabolic syndrome by defining glycemic criteria as registry-documented diagnosis of diabetes mellitus and/or self-reported diabetes mellitus and/or antidiabetic treatment and/or nonfasting plasma glucose level more than 200 mg/dl. Thus, in this study, over 10% of participants with obesity had overt diabetes, which is accepted as equivalent to pre-existing coronary artery disease as a vascular disease risk factor (26). The key consideration in this study is that only 0.1% of subjects with obesity had no metabolic syndrome traits and would satisfy the current criteria for metabolically healthy (18). We (5, 6, 10) and others (14–16) have shown that one or two metabolic syndrome traits confer increased risk of diabetes and cardiovascular disease, and that these individuals are relatively insulin resistant. Even Thomsen et al (18) demonstrated that their subjects with 1 or 2 traits had increased risk of myocardial infarction compared with the small group with no risk factors, and that the risk of infarction was statistically similar in comparing these subjects with those meeting criteria for the metabolic syndrome. Thus, the population studied by Thomsen et al was highly co-morbid, and their ‘healthy’ population with obesity was almost entirely comprised of insulin resistant subjects with risk factors.
This discussion highlights the importance of a rigorous definition for metabolically healthy obesity, and the risk conferred by even 1 or 2 traits when addressing the relative importance of BMI versus metabolic status as risk factors for cardiometabolic disease outcomes. Several other authors have examined this issue with respect to both diabetes and vascular disease events, and tended to belittle the concept of the metabolically healthy obesity (17–22). However, in all these studies, the metabolically healthy group similarly included a large proportion of subjects who had at least one risk factor. Moreover, the data in these papers demonstrated that regardless of BMI status, subjects with the metabolic syndrome consistently had 2- to 4-fold higher incident rates of diabetes and cardiovascular disease than their weight-matched counterparts assigned to the metabolically healthy subgroup. Our data support previous epidemiological (10–16) and physiological (5–9) studies demonstrating that significant numbers of individuals with obesity are insulin sensitive, lack cardiometabolic disease risk factors, and are at markedly reduced risk of diabetes and cardiovascular disease.
In an effort to harmonize the discrepant conclusions offered by all authors, it is important to consider several salient points. First, with respect to the vascular component of cardiometabolic disease, the presence of metabolic syndrome and its risk factors clearly predominate over BMI as a predictor of cardiovascular disease events. Second, with respect to the metabolic component of cardiometabolic disease, increasing BMI does increase the hazard ratio for diabetes in metabolically healthy subjects, but, importantly, the cumulative incidence rates remain quite low. In contrast, the presence of the metabolic syndrome results in much higher rates of incident diabetes regardless of BMI (even when comparing unhealthy lean with healthy obesity). However, BMI does interact with metabolic status to significantly augment cumulative incident rates of diabetes. Whether one accepts the term metabolically healthy obesity may revolve around the relative importance attributed to the fold increase in diabetes risk due to BMI in healthy subjects even though cumulative incidence rates remain quite low (i.e., a nay vote), or the much higher incident rates observed in subjects with the metabolic syndrome irrespective of BMI status (i.e., an aye vote). Suffice it to say that cardiometabolic risk factors confer much higher risk of diabetes, CVD, stroke and mortality, than obesity per se, and that there is a population of individuals with obesity devoid of metabolic syndrome risk factors who are at markedly reduced risk of cardiometabolic disease.
Clinical Implications
Without intensive interventions, people with obesity will stay obesity and will not lose enough body weight to become lean, whether in young adults or young-elder adults. On the other hand, young-elder lean people seldom become obesity, but ~18% of young lean adults will become obesity in their middle age. These data imply that among adults primary efforts to prevent obesity should be directed at young adults.
Our data also have potential implications regarding the treatment of obesity. The American Association of Endocrinologists have advanced a complications centric approach to weight loss therapy, which advocates for more aggressive treatment in those patients with obesity with complications or at higher risk of complications in order to optimize outcomes, the benefit/risk ratio of the intervention, and cost effectiveness of care (31, 32). In conformity with this approach regarding cardiometabolic disease, weight loss therapy should be more aggressively targeted to those individuals at highest risk of diabetes and cardiovascular disease. We have demonstrated that metabolically healthy obesity have significantly lower risks for diabetes, CHD, stroke, and mortality compared with unhealthy obesity. Thus, in terms of preventing diabetes and improving cardiovascular risk factors, a far greater percentage of individuals with metabolically unhealthy obesity will realize the benefits of weight loss pharmacotherapy compared with individuals with healthy obesity. It is for this reason we established CMDS as a guide to clinical decision making in obesity (10). However, even after the implementation of weight loss strategies, metabolically unhealthy obesity still have a higher risk profile compared to metabolic healthy subjects with obesity (33–35).
Strength and Limitations
The main strengths of this study involve using data from participants of two large longitudinal cohorts, young adults from the CARDIA study and young-elders from the ARIC study. CARDIA and ARIC included multiple follow up visits and ARIC also had data on long period of annual follow up. Those cohorts included both men and women, and Blacks and Whites.
Limitations in this study include that the sample size in the CARDIA study is not large enough to permit extensive subgroup analyses. Further, the ARIC study only recruited young-elder adults (age 45–65 years), and our findings regarding the long-term health outcomes in metabolically healthy obesity may not be readily applied to other age groups.
Conclusions
In conclusion, (i) in free-living populations people with obesity rarely become lean, but lean young adults may become obesity; (ii) people with healthy obesity have lower risks for diabetes, CHD, stroke, and mortality compared with unhealthy subjects regardless of their BMI status. Obesity did not affect risks of CHD, stroke, and mortality, but did increase diabetes risk although cumulative incidence remained low in healthy people. The data support risk stratification in an effort to identify those patients with obesity who will realize greater benefits of weight loss interventions with respect to treatment and prevention of cardiometabolic disease.
What is already known about this subject?
Obesity is associated with elevated risk for morbidity and mortality.
Adults with obesity and no metabolic syndrome traits are at lower risks of future diabetes and mortality compared to those with risk factors.
What does this study add?
People with obesity will not become lean in the free-living population, and lean people may become people with obesity if they are young.
Metabolic status remains relatively stable. Very few metabolically healthy people at baseline transition to unhealthy status.
Metabolically healthy people with obesity do not have increased risks for cardiovascular outcomes or mortality, but have a small increase in risks for future diabetes compared with healthy lean people.
Acknowledgments
Primary Funding Source: Dr. Guo is currently a postdoctoral fellow supported by an institutional training grant (National Research Service Award T32HD055163) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) at the National Institutes of Health (NIH). This study is supported by the Merit Review program of the U.S. Department of Veterans Affairs, National Institutes of Health (DK-038765 and DK-083562), and the UAB Diabetes Research Center (P60-DK079626).
Role of the Sponsors: Data from the CARDIA study and the ARIC study are obtained through The National Heart, Lung, and Blood Institute (NHLBI) Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC).
Footnotes
Part of the results has been presented as a poster at the 74th Scientific Session of American Diabetes Association 2014 at San Francisco, California.
Disclaimer: The findings and conclusions in this paper are those of the authors and do not necessarily represent the views of the U.S. Department of Veterans Affairs, National Institutes of Health, or the National Heart, Lung, and Blood Institute.
Conflicts of Interest: Dr. Garvey is an advisor for Astra Zeneca, Boehringer-Ingelheim, Daiichi-Sankyo, Inc., Eisai, Janssen Pharmaceuticals, LipoScience, Novo Nordisk, Takeda, and VIVUS, Inc.; is a stockholder for Bristol-Myers Squibb Company, Eli Lilly and Company, Isis/Genzyme, Merck, Novartis, and Pfizer, Inc.; and has received research support from Astra Zeneca, Eisai, Lexicon, Merck & Co., Pfizer, Inc., Sanofi, and Weight Watchers International, Inc. Dr. Guo has no conflict of interest or financial disclosure to declare.
References
- 1.Hu FB. Obesity and mortality: watch your waist, not just your weight. Archives of internal medicine. 2007;167:875–876. doi: 10.1001/archinte.167.9.875. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 3.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9. 1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. National Institutes of Health. Obes Res. 1998;6 (Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 5.Lara-Castro C, Garvey WT. Diet, insulin resistance, and obesity: zoning in on data for Atkins dieters living in South Beach. J Clin Endocrinol Metab. 2004;89:4197–4205. doi: 10.1210/jc.2004-0683. [DOI] [PubMed] [Google Scholar]
- 6.Liao Y, Kwon S, Shaughnessy S, Wallace P, Hutto A, Jenkins AJ, et al. Critical evaluation of adult treatment panel III criteria in identifying insulin resistance with dyslipidemia. Diabetes Care. 2004;27:978–983. doi: 10.2337/diacare.27.4.978. [DOI] [PubMed] [Google Scholar]
- 7.Bogardus C, Lillioja S. Pima Indians as a model to study the genetics of NIDDM. J Cell Biochem. 1992;48:337–343. doi: 10.1002/jcb.240480402. [DOI] [PubMed] [Google Scholar]
- 8.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45:633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 10.Guo F, Moellering DR, Garvey WT. The progression of cardiometabolic disease: Validation of a new cardiometabolic disease staging system applicable to obesity. Obesity (Silver Spring) 2014;22:110–118. doi: 10.1002/oby.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 12.Wildman RP, Muntner P, Reynolds K, McGinn AP, Rajpathak S, Wylie-Rosett J, et al. The obese without cardiometabolic risk factor clustering and the normal weight with cardiometabolic risk factor clustering: prevalence and correlates of 2 phenotypes among the US population (NHANES 1999–2004) Arch Intern Med. 2008;168:1617–1624. doi: 10.1001/archinte.168.15.1617. [DOI] [PubMed] [Google Scholar]
- 13.Kip KE, Marroquin OC, Kelley DE, Johnson BD, Kelsey SF, Shaw LJ, et al. Clinical importance of obesity versus the metabolic syndrome in cardiovascular risk in women: a report from the Women’s Ischemia Syndrome Evaluation (WISE) study. Circulation. 2004;109:706–713. doi: 10.1161/01.CIR.0000115514.44135.A8. [DOI] [PubMed] [Google Scholar]
- 14.Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab. 2006;91:2906–2912. doi: 10.1210/jc.2006-0594. [DOI] [PubMed] [Google Scholar]
- 15.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 16.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 17.Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 18.Thomsen M, Nordestgaard BG. Myocardial infarction and ischemic heart disease in overweight and obesity with and without metabolic syndrome. JAMA Intern Med. 2014;174:15–22. doi: 10.1001/jamainternmed.2013.10522. [DOI] [PubMed] [Google Scholar]
- 19.Aung K, Lorenzo C, Hinojosa MA, Haffner SM. Risk of developing diabetes and cardiovascular disease in metabolically unhealthy normal-weight and metabolically healthy obese individuals. J Clin Endocrinol Metab. 2014;99:462–468. doi: 10.1210/jc.2013-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Appleton SL, Seaborn CJ, Visvanathan R, Hill CL, Gill TK, Taylor AW, et al. Diabetes and cardiovascular disease outcomes in the metabolically healthy obese phenotype: a cohort study. Diabetes Care. 2013;36:2388–2394. doi: 10.2337/dc12-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung HS, Chang Y, Eun Yun K, Kim CW, Choi ES, Kwon MJ, et al. Impact of body mass index, metabolic health and weight change on incident diabetes in a Korean population. Obesity (Silver Spring) 2014;22:1880–1887. doi: 10.1002/oby.20751. [DOI] [PubMed] [Google Scholar]
- 22.Chang Y, Ryu S, Suh BS, Yun KE, Kim CW, Cho SI. Impact of BMI on the incidence of metabolic abnormalities in metabolically healthy men. Int J Obes (Lond) 2012;36:1187–1194. doi: 10.1038/ijo.2011.247. [DOI] [PubMed] [Google Scholar]
- 23.Twig G, Afek A, Derazne E, Tzur D, Cukierman-Yaffe T, Gerstein HC, et al. Diabetes risk among overweight and obese metabolically healthy young adults. Diabetes Care. 2014;37:2989–2995. doi: 10.2337/dc14-0869. [DOI] [PubMed] [Google Scholar]
- 24.Jung CH, Lee MJ, Kang YM, Jang JE, Leem J, Hwang JY, et al. The risk of incident type 2 diabetes in a Korean metabolically healthy obese population: the role of systemic inflammation. J Clin Endocrinol Metab. 2015;100:934–941. doi: 10.1210/jc.2014-3885. [DOI] [PubMed] [Google Scholar]
- 25.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 26.Expert Panel on Detection Evaluation, Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 27.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 28.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 29.Harrell FE, Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 30.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 31.Garvey WT, Garber AJ, Mechanick JI, Bray GA, Dagogo-Jack S, Einhorn D, et al. American association of clinical endocrinologists and american college of endocrinology position statement on the 2014 advanced framework for a new diagnosis of obesity as a chronic disease. Endocr Pract. 2014;20:977–989. doi: 10.4158/EP14280.PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garvey WT. New tools for weight-loss therapy enable a more robust medical model for obesity treatment: rationale for a complications-centric approach. Endocr Pract. 2013;19:864–874. doi: 10.4158/EP13263.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karelis AD, Messier V, Brochu M, Rabasa-Lhoret R. Metabolically healthy but obese women: effect of an energy-restricted diet. Diabetologia. 2008;51:1752–1754. doi: 10.1007/s00125-008-1038-4. [DOI] [PubMed] [Google Scholar]
- 34.Janiszewski PM, Ross R. Effects of weight loss among metabolically healthy obese men and women. Diabetes Care. 2010;33:1957–1959. doi: 10.2337/dc10-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kantartzis K, Machann J, Schick F, Rittig K, Machicao F, Fritsche A, et al. Effects of a lifestyle intervention in metabolically benign and malign obesity. Diabetologia. 2011;54:864–868. doi: 10.1007/s00125-010-2006-3. [DOI] [PubMed] [Google Scholar]