Abstract
Objective
Skeletal muscle fat infiltration (known as myosteatosis) is greater in African compared with European ancestry men and may play an important role in the development of type 2 diabetes (T2D). However, prospective studies examining the magnitude of changes in myosteatosis with aging and their metabolic consequences are sparse.
Methods
We examined longitudinal changes in peripheral quantitative computed tomography measured calf myosteatosis [inter-muscular fat (mm2) and skeletal muscle density as a measure of intra-muscular fat (mg/cm3)] in 1,515 Afro-Caribbean men aged 40+ years recruited without regard to their health status.
Results
During an average of 6.2 years of follow-up, we observed an age-related increase in inter-muscular fat and a decrease in skeletal muscle density (all P<0.0001), which remained significant in those who lost weight, gained weight, or remained weight-stable (all P<0.0001). In addition, muscle density loss accelerated with increasing age (P<0.0001). Increased inter-muscular fat during follow-up was associated with an increased incident risk of T2D independent of factors known to be associated with T2D (Odds ratios per 1-SD increase in inter-muscular fat=1.29; 95% CI=1.08-1.53).
Conclusions
Our findings suggest that both inter- and intra- muscular fat increase with advancing age and that inter-muscular fat contributes to development of T2D among African ancestry men.
Keywords: Myosteatosis, inter-muscular fat, muscle density, intramuscular triglyceride, aging, diabetes, African ancestry
INTRODUCTION
Increased accumulation of fat around and within organs that normally contain only small amounts of fat, such as liver and skeletal muscle (referred to as ectopic fat), can impair the normal physiological function of those organs[1]. Accumulation of ectopic fat may be explained by inadequate capacity of subcutaneous fat storage[2, 3], and/or increased fatty acid storage and reduced fatty acid oxidation[4-6]. Skeletal muscle fat infiltration (also known as myosteatosis) is a unique ectopic fat depot with broad sequelae including poor metabolic and musculoskeletal health[7, 8]. Despite the clinical significance, little is known about the effects of aging on myosteatosis. Although cross-sectional studies suggest that myosteatosis may increase with age[9-11], longitudinal studies are sparse. Previous studies have shown that African ancestry men have more myosteatosis, independent of differences in general adiposity[12-14], and greater risk for type 2 diabetes (T2D) compared to Caucasian men[15-17]. In addition, myosteatosis has been associated with a greater prevalence of T2D and insulin resistance[10, 18-20]. Therefore, a better documentation of aging effects on and the metabolic consequences of myosteatosis are particularly needed in large longitudinal studies among African ancestry individuals. In the present study in a large cohort of middle aged and elderly Afro-Caribbean men who were followed for more than 6 years on average, we tested whether increasing age is associated with increased myosteatosis independent of body weight changes, and whether myosteatosis increases the greatest among the eldest men. In addition, we sought to determine if change in myosteatosis predicts subsequent development of T2D in this cohort.
METHODS
Study population
From 1997-2003, 3,170 predominantly Afro-Caribbean men aged 40 and older were recruited for population-based prostate cancer screening for the first time on the island of Tobago, Trinidad & Tobago[21]. To be eligible, men had to be ambulatory, non-institutionalized and not terminally ill. Recruitment for the initial screening was accomplished by flyers, public service announcements, posters, informing health care workers at local hospital and health centers, and word of mouth. Approximately 60% of all age-eligible men on the island participated and participation was representative of the island parishes.
From 2004-2007, men in the original cohort were invited to complete a peripheral quantitative computed tomography (pQCT) scan. A total of 2,152 men underwent pQCT scans of the tibia at this exam[22]. From 2010-2013, we invited these men to return for repeat pQCT scans. Both the baseline and follow-up visits followed the same procedures for questionnaire interviews, biospecimen collection, and pQCT scans[22]. A total of 1,515 men completed the follow-up assessment, including the pQCT exam (82% of survivors). The Institutional Review Boards of the University of Pittsburgh and the Tobago Ministry of Health and Social Services approved this study and all participants provided written informed consent before data collection.
Peripheral Quantitative Computed Tomography (pQCT) Quantification of Myosteatosis
Myosteatosis studies that utilize CT directly measure either inter-muscular fat (visible fat beneath the fascia lata) and/or skeletal muscle density (lower skeletal muscle density is indicative of greater intra-muscular fat content)[23, 24]. In our study, measures of myosteatosis were obtained by pQCT scans of the calf, which were performed using the Stratec XCT-2000. At both visits, 2.2 mm cross-sectional images of the calf skeletal muscle composition were obtained at 66% of the tibia length, proximal to the terminal end of the tibia, because this is the region with the largest circumference of the calf and has less variability between individuals[25]. Standardized procedures for participant positioning and data analysis were used for all scans[26]. A scout view was obtained prior to the tomographic scan to define an anatomic reference line, to which the relative location of the subsequent tomographic slice is automatically adjusted. It is recommended that the reference line is placed through the flat portion of the distal tibia endplate[26]. Different tissues in the analyses were separated according to different density thresholds, appropriate for soft tissue segmentation, using the manufacturer's suggested analysis parameters[26]. Based on calibration of the XCT scanner, fat, muscle or lean tissue, and cortical bone were measured with mineral equivalent densities of 0, 80, and 1200 mg/cm3, respectively. Therefore, muscle, fat and bone with an image can be separated using appropriate fixed and gradient thresholds to separate the tissue compartments. Changes in muscle tissue to fat tissue were detected as a shift in mineral equivalent density of the muscle from 80 to 0 mg/cm3. Automatic threshold-based iterative edge detection-guided segmentation of muscle from bone was performed using a density threshold of 280 mg/cm3 with contour mode 1 and peel mode 2 (bone area and mass). Segmentation of muscle from subcutaneous fat followed a threshold of 40 mg/cm3 with contour mode 3 and peel mode 1 (total muscle + bone mass/content). The threshold of 40 mg/cm3 was selected as it is halfway between the densities for fat and water or muscle like tissues and represents a standard image processing threshold selection for defining the gradient (or edge) between tissues. The units of bone mass/content (or bone mineral content) are mg/cm. To determine muscle cross-sectional area, bone area was subtracted from total bone mass/content + muscle area. Similarly, bone mass/content was subtracted from total bone + muscle mass/content to derive muscle mass/content. After subtraction of intermuscular adipose tissue cross-sectional area, muscle density was computed by dividing total muscle mass (mg/cm) by muscle cross-sectional area (cm2).
Image processing and quality control procedures were completed by a single investigator who was blinded to subjects’ outcome status (Stratec analysis software, Version 5.5). We obtained cross-sectional area of total fat (mm2), subcutaneous fat (mm2), inter-muscular fat (mm2), total muscle area (mm2), and skeletal muscle density (mg/cm3). The coefficients of variation (CV) were determined by repeat pQCT scanning in 15 individuals. The CVs for total, subcutaneous, and inter-muscular fat, muscle density, and muscle area were 0.98%, 1.5%, 7.6%, 1.1%, and 0.95% respectively.
Anthropometric measurements
Body weight was measured in kilograms with participants wearing light clothing and without shoes using a calibrated balance beam scale. Height was measured in centimeters without participants wearing shoes using a wall-mounted height board. Two height measurements were made and the average used in analysis. Waist circumference was measured at the umbilicus with an inelastic tape measure.
Other measurements
Trained interviewers and nurses administered questionnaires to participants. We collected information pertaining to demographic characteristics, medical history, medication use, personal and family medical history, physical activity, and lifestyle habits. Ethnicity/race was self-reported and participants provided detailed information on the ethnic/racial origin of their parents and grandparents. Men were asked to report their history of selected medications as well as current medication use and were instructed to bring in all prescription medications taken in the past 30 days to their clinic visit. Participants also rated their overall health status compared to men their own age. Smoking was defined as having smoked at least 100 cigarettes in their lifetime. Alcohol use was defined as having consumed 4 or more drinks per week in the past 12 months. Self-reported information on walking was recorded as walking is the predominant form of physical activity on the island of Tobago. We used hours of walking per week as a measure of physical activity, and hours of television watching per week as a measure of sedentary lifestyle.
Medical Conditions
Obesity was defined as BMI ≥30 kg/m2. T2D was defined as fasting serum glucose ≥126mg/dl or currently taking anti-diabetic medication. Hypertension was defined as a systolic blood pressure of ≥140 mmHg and/or diastolic blood pressure of ≥90 mmHg or currently taking antihypertensive medication.
Statistical Analysis
Percent changes in adiposity and muscle variables were calculated for each individual as [(follow-up–baseline)/baseline]*100%. Mean percent changes were then calculated as the average of individual percent changes for the whole sample. We present baseline and follow-up variables as median (IQR) instead of mean±SD for very skewed traits (total, subcutaneous and inter-muscular fats). Analysis of covariance (ANCOVA) was used to compare changes in these phenotypes across age groups 40-54, 55-64, and 65+ years. Covariates of interest included follow-up time, and baseline adiposity/muscle phenotype of interest, BMI (for myosteatosis), lifestyle factors (smoking status, physical activity level, and alcohol intake), muscle area, and T2D status. To examine the effect of body weight change on myosteatosis, participants were stratified into three categories: those who gained >3% of their baseline body weight were classified as having gained weight; those who lost >3% of their body weight were considered to have lost weight[27]; and the remaining participants were considered to be weight-stable. The association of weight change category with inter-muscular fat and muscle density changes were analyzed using ANCOVA with adjustment for the same covariates as in the analysis across age groups. Finally, logistic regression was used to determine the odds of incident diabetes among men free of diabetes at baseline by change in myosteatosis and other measured adiposity phenotypes while adjusting for follow-up time and covariates associated with diabetes in our study including baseline age, baseline adiposity phenotype of interest, lifestyle factors, and BMI (for myosteatosis). Odds ratios (OR) and 95% CI were calculated per 1-SD increase in body weight, BMI, waist circumference, and inter-muscular fat, and per 1-SD decrease in muscle density. All statistical analyses were performed using the Statistical Analysis System (SAS, version 9.1; SAS Institute, Cary, NC).
RESULTS
Baseline Characteristics
A total of 1,515 men had complete calf skeletal muscle composition data at the follow-up visit (Table 1). Men who participated in the follow-up exam were younger (56.9 vs. 62.7 years), more likely to report excellent/good health (93.7% vs. 85.7%), and had a lower prevalence of T2D (17.2% vs. 26.7%) compared to those who did not participate in the follow-up exam (data not shown, p<0001 for all).
Table 1.
Selected characteristics of 1,515 African Ancestry men at the baseline exam
| Baseline Visit 2004-2008 | |
|---|---|
| Age (years) | 56.9 ± 9.1 |
| Current smokers (%) | 10.6% |
| Walking per week (hours) | 3.2 ± 6.4 |
| Excellent/Good Health (%) | 93.8% |
| TV watching per week (hours) | 13.3 ± 8.2 |
| > 3 drinks per week (%) | 10.8% |
| Obesity (%) | 23.5% |
| Type 2 Diabetes (%) | 17.0% |
| Hypertension (%) | 48.5% |
| Hypoglycemic drugs or insulin (%) | 10.8% |
| Lipid-lowering (statins) (%) | 4.3% |
| Anti-hypertensive drugs (%) | 24.8% |
Values are means ± SD or percent
Percent change in anthropometric measures and skeletal muscle composition
Over an average of 6.2 years follow-up (range: 4.9-9.1 years), we observed a significant (Table 2) increase in waist circumference and total, subcutaneous, and inter-muscular fat, and a significant decrease in skeletal muscle density and muscle cross-sectional area (all p<0.001). There was no significant mean change in body weight or BMI during follow-up.
Table 2.
Baseline and follow-up anthropometric measures and skeletal muscle composition and their 6-year percent changes in 1515 African Ancestry men
| Values at Baseline Exam | Values at Follow-Up Exam | Percent Change | |
|---|---|---|---|
| Body weight (kg) | 84.2 ± 15.0 | 83.9 ± 14.3 | −0.14 ± 7.6 |
| BMI (kg/m2) | 27.4 ± 4.6 | 27.3 ± 4.3 | −0.12 ± 7.5 |
| Waist circumference (cm) | 92.6 ± 10.1 | 97.0 ± 12.8 | 5.1 ± 11.1† |
| Calf total fat (mm2)# | 1703.0 (1261.3, 2195.3) | 1745.38 (1288.6, 2310.0) | 6.9 ± 30.6† |
| Calf subcutaneous fat (mm2)# | 1326.38 (906.5, 1774.0) | 1332.0 (910.8, 1804.5) | 8.8 ± 49.2† |
| Calf inter-muscular fat (mm2)# | 175.5 (90.0, 324.5) | 209.75 (105.5, 396.3) | 78.7 ± 230.7† |
| Calf muscle density (mg/cm3)* | 73.8 ± 3.9 | 71.3 ± 5.1 | −3.4 ± 4.4† |
| Calf skeletal muscle area (mm2) | 7596.5 ± 1271.4 | 7530.2 ± 1482.9 | −0.94 ± 10.0† |
Values are presented mean ± SD #median (IQR) for skewed traits. Percent change values were calculated for each individual as [(follow-up value – baseline value)/baseline value]*100% then the whole sample of 1515 men was averaged together to get the values shown above.
*For skeletal muscle density: Total N= 975
P-value for mean percent change different than zero: †<0.001
Percent change in skeletal muscle composition across age groups
At baseline, older men from our study (aged 65+ years) had greater inter-muscular fat and lower muscle density compared to younger men (Table 3; p<0.0001 for both). Over the follow-up period, the percent increase in total and subcutaneous fat, and the percent decrease in muscle density and muscle area were greatest at older ages (all p-values for trend<0.0071). However, increases in inter-muscular fat showed no differences across age groups. Results remained similar after adjustment for follow-up time and baseline BMI, lifestyle factors, muscle cross-sectional area, myosteatosis measure of interest, and presence of diabetes (data not shown, all p-values for age-group trend<0.05, except for inter-muscular fat where p=0.49).
Table 3.
Baseline calf skeletal muscle composition measures and their 6-year percent change by age groups* among 1515 African ancestry men
| Values at Baseline Exam | Percent Change | ||||||
|---|---|---|---|---|---|---|---|
| Age 40-54 years | Age 55-64 years | Age 65+ years | Age 40-54 years |
Age 55-64 years |
Age 65+ years |
Trend P-value |
|
| Total fat (mm2)# | 1738.0 (1258.9, 2251.5) | 1655.3 (1247.3, 2096.3) | 1733.6 (1294.8, 2239.5) | 4.3 ± 29.8 | 6.5 ± 28.8 | 13.0 ± 34.0 | <0.0001 |
| Subcutaneous fat (mm2)# | 1390.5 (956.0, 1860.5) | 1257.5 (898.0, 1705.3) | 1259.6 (888.3, 1662.0) | 6.6 ± 49.5 | 6.7 ± 38.5 | 16.6 ± 60.6 | 0.0071 |
| Inter-muscular fat (mm2)# | 142.5 (78.5, 257.5) | 178.6 (89.0, 330.3) | 264.6 (142.8, 478.3) | 85.9 ± 269.7 | 75.7 ± 204.6 | 67.6 ± 166.8 | 0.2190 |
| Muscle density* (mg/cm3) | 75.0 ± 3.3 | 73.8 ± 3.4 | 71.5 ± 4.6 | −2.9 ± 3.7 | −3.1 ± 3.9 | −4.7 ± 5.9 | <0.0001 |
| Muscle area (mm2) | 7915.3 ± 1217.9 | 7492.2 ± 1271.8 | 7057.9 ± 1177.1 | 0.43 ± 9.1 | −1.1 ± 10.1 | −3.7 ± 11.2 | <0.0001 |
Values are means ± SD or #median (IQR) for skewed traits. Percent change values were calculated for each individual as [(follow-up value – baseline value)/baseline value]*100% then the whole sample of 1515 men was averaged together to get the values shown above.
Sample size for age groups: N=708 for 40-54 years N=481 for 55-64 years, and N=326 for 65+ years N=326 (except for muscle density where: N=439 for 40-54 years N=318 for 55-64 years, and N=218, 65+years)
Percent change in myosteatosis by weight change categories
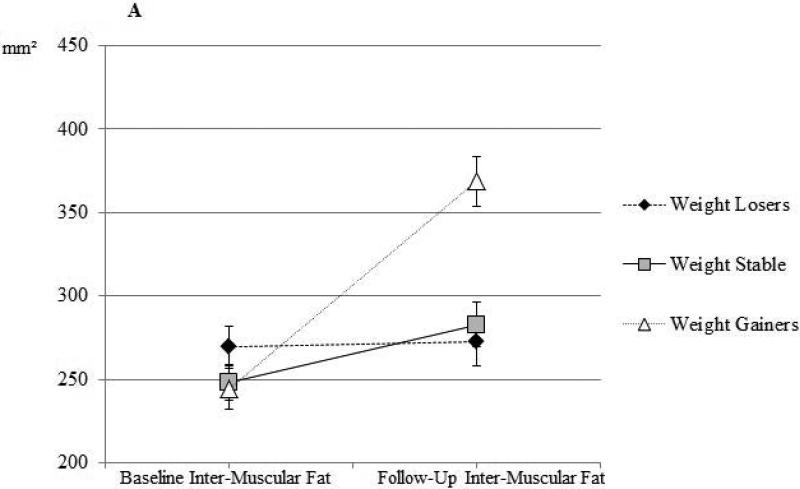
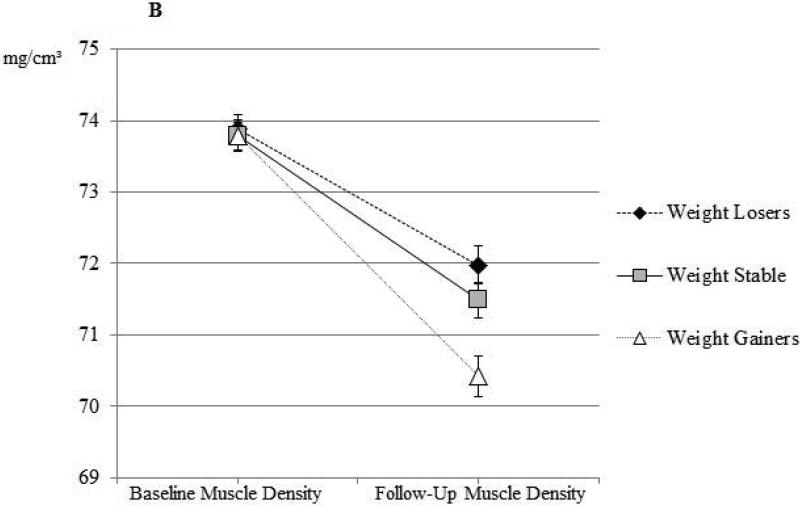
Percent change in inter-muscular fat and muscle density by weight change group are shown in Figure 1. The percent increase in inter-muscular fat was significant among men who experienced >3% weight loss, remained weight stable, or experienced >3% weight gain (Figure 1A, all percent change p-values<0.0001). Despite some amount of increase in inter-muscular fat among all weight-change categories, changes in men who lost weight or were weight stable were statistically similar (p>0.05), while men who gained weight had statistically greater increase in inter-muscular fat (pairwise p-values<0.0001). Similarly, the percent decrease in muscle density was significant among men who experienced weight loss, remained weight stable, or experienced weight gain (Figure 1B, all percent change p-values<0.0001). Despite some amount of decrease in muscle density among all weight-change categories, changes in men who lost weight or were weight stable were statistically similar (p>0.05), while men who gained weight had statistically greater decrease in muscle density (pairwise p-values<0.0001). The results remained similar after adjustment for follow-up time and baseline BMI, lifestyle factors, muscle cross-sectional area, myosteatosis measure of interest, and presence of diabetes (all p-values<0.001, data not shown). There was no significant difference in baseline inter-muscular fat or muscle density between weight change groups (data not shown).
Figure 1a.
Calf inter-muscular fat at baseline and at follow-up among African ancestry men by weight-change categories among African ancestry men
Figure 1b.
Calf skeletal muscle density at baseline and at follow-up among African ancestry men by weight-change categories among African ancestry men
The relationship between percent change in myosteatosis and incident type 2 diabetes
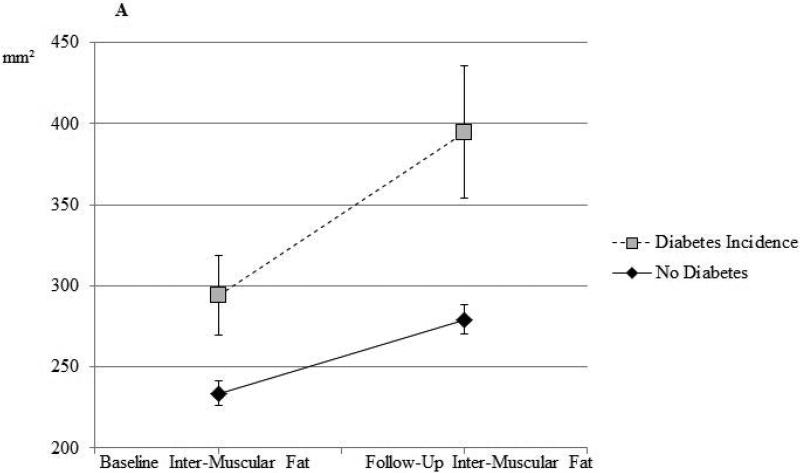
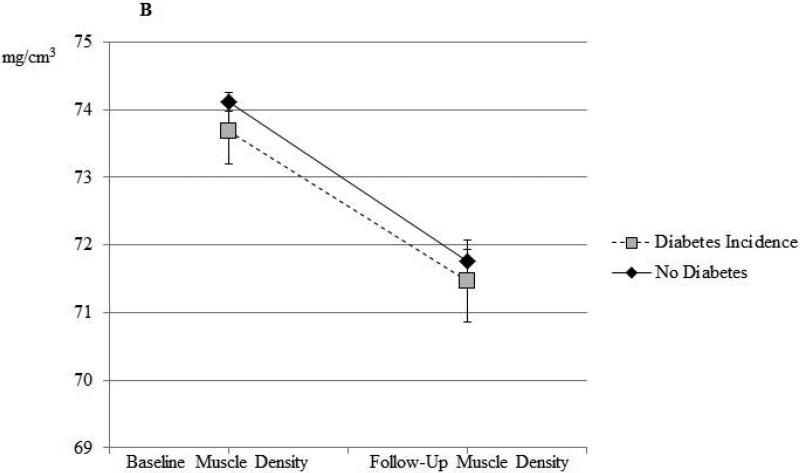
Among the 1212 men who did not have T2D at baseline, we identified 96 incident cases of T2D at follow-up. We observed a significant positive association between inter-muscular fat and odds of T2D after adjustment for baseline age (OR=1.27 per SD increase in inter-muscular fat; 95% CI=1.04-1.56), which remained statistically significant (OR=1.29; 95% CI=1.08-1.53) after additional adjustment for follow-up time and other factors known to be associated with diabetes risk, including baseline inter-muscular fat and baseline lifestyle factors. After additional adjustment for baseline BMI, this association was slightly weakened, but remained borderline statistically significant (OR=1.20; 95% CI=0.995-1.43). Adjusted means by incident diabetes status are shown in the Supplemental Table. No significant relationships between incident T2D and 6-year change in skeletal muscle density, body weight, BMI, or waist circumference were observed, regardless of the degree of adjustment (data not shown). The values of inter-muscular fat and muscle density at baseline and follow-up visit for non-diabetic men who did and did not develop T2D are shown illustratively in Figure 2. Men who developed T2D at follow-up had greater inter-muscular fat than those who did not develop T2D at both baseline (p=0.051) and follow-up (p=0.0021) exams (Figure 2a). There was no difference in baseline or follow-up muscle density in men who did or did not develop T2D (Figure 2b).
Figure 2a.
Calf inter-muscular fat at baseline and at follow-up among African ancestry men without diabetes at baseline
Figure 2b.
Calf skeletal muscle density at baseline and at follow-up among African ancestry men without diabetes at baseline
DISCUSSION
Our study examined longitudinal changes in calf measures of myosteatosis among 1,515 middle-aged and elderly men of African ancestry. To our knowledge, this is the largest study in a population recruited without regard to health status and across a wide age range. Over an average of 6 years, we observed a significant increase in inter-muscular fat and decrease in skeletal muscle density. These changes in myosteatosis were independent of changes in body weight. We also found that older men have even greater decreases in skeletal muscle density, but at the same time they have a lower increase in inter-muscular fat. Finally, we found that the increase in inter-muscular fat with aging is associated with development of T2D, suggesting for the first time that inter-muscular fat may be an independent predictor of the development of type 2 diabetes in African ancestry men.
The Health Aging and Body Composition Study previously examined longitudinal changes in myosteatosis in older adults, with a focus on inter-muscular fat[28]. This study reported that inter-muscular fat increases with aging among African-American (N=246) and white (N=567) men as well as African-American (N=340) and white (N=525) women. Participants in this study were all recruited to be 70-79 years old, healthy and very high-functioning[28]. Nonetheless, the annualized rate of increase in inter-muscular fat in this study was (~9.7%/year) similar to the rate among men 65 and older in our study (11.3%/year). Other longitudinal studies have shown that inter-muscular fat increases with aging, but these studies were conducted in highly selected or very small samples, such as a osteoarthritis case-control study of 86 Caucasian women[29], and a longitudinal study of 26 healthy, elderly African-American women[11]. The exact mechanisms underlying increases in myosteatosis with aging in humans are largely unknown. There may be age-related changes in the activation, proliferation and differentiation of skeletal muscle precursor cells into adipocytes[30], and/or there may be increased fatty acid transport and reduced fatty acid oxidation[4-6] with aging. Further studies are needed to better understand the clinical and biological factors that cause myosteatosis increases with aging.
Men who were older than 65 years had greater decrease in muscle density than younger men, a trend that was not observed for inter-muscular fat, despite its significant overall increase in the total study. This finding could be due to selective survival bias as we have found that inter-muscular fat, but not intra-muscular fat, is associated with increased risk of T2D in our cohort. It is possible that older men with a greater degree of inter-muscular fat may have not been able to participate in our follow-up visit due to poorer health related to T2D. For example, we found that men who did not return for the follow-up exam had a greater prevalence of T2D at study entry compared to men who participated in the follow-up exam. The less dramatic change in inter-muscular fat in the oldest group over time could also be due to their greater inter-muscular fat at the baseline compared to the younger men, although the same was true for intra-muscular fat. We can also speculate that in the elderly men skeletal muscle fat infiltration may be preferentially accumulated intra- instead of inter-muscularly. Future studies are warranted to test this hypothesis.
We also found that aging-related increases in inter-muscular fat and decreases in muscle density occur even in those who lose or maintain body weight, although the changes were greatest among those who gained body weight. Our findings confirm a previous report in older individuals from the Health Aging and Body Composition Study[28] and suggest that increases in myosteatosis may be a characteristic of skeletal muscle aging even in those who lose body weight.
Finally, our prospective analysis is the first, to our knowledge, to demonstrate an association of inter-muscular fat in the development of incident T2D. Several cross-sectional studies have reported a positive association between myosteatosis and insulin resistance[31-33]. We have found that inter-muscular fat is positively associated with T2D in African ancestry men even among those who are lean[10]. Additionally, Goodpaster et al. reported a positive cross-sectional association between thigh inter-muscular fat and T2D among 2964 elderly men and women[18]. A positive association of inter-muscular fat with insulin resistance[20, 34] and T2D[35] has also been reported in a few other small cross-sectional studies. Previous studies have also shown that while differences in total and central obesity account for almost 50% of the excess diabetes risk in African ancestry women, they fail to explain black-white differences in diabetes risk among men[16, 36]. Our findings support a role for inter-muscular fat in T2D risk in African ancestry men. Large studies in other populations are needed to more definitively test this hypothesis.
The mechanisms linking inter- and intra- muscular fat with T2D are still unclear and may be different. Increased accumulation of inter-muscular fat may induce changes in muscle metabolism and insulin sensitivity via local secretion of inflammatory adipokines from adipocytes surrounding muscle fibers[37]. Inter-muscular fat may also impair nutritive blood flow to muscle and thus contribute to insulin resistance by impairing insulin action and insulin diffusion capacity[38]. Additionally, a recent study reported that increased number of inter-muscular adipocytes has a direct local impact on skeletal myocyte metabolism, causing increased myotube mRNA expression of genes involved in oxidative metabolism[39]. This study suggests that even a relatively small amount of inter-muscular fat may be sufficient to drive myotubes into lipid oxidation and affect skeletal muscle metabolism. These effects depended on the metabolic state of the system. As for intra-muscular fat, it has been hypothesized that mitochondrial dysfunction present in T2D and aging leads to increased intra-muscular fat[40], and thus, it is possible that increased intra-muscular fat is actually a consequence of T2D. Further studies are needed to confirm if inter-muscular fat is causally liked with T2D and to clarify if intra-muscular fat is a marker or mediator of insulin resistance.
The present study has several potential limitations. Our cohort included middle-aged and elderly African ancestry men and our findings may not apply to younger men, women or other ethnic/racial groups. Also, there are likely cultural differences in the degree of westernization between the sub-Saharan Africans, Afro-Caribbeans, and African-Americans; thus, future studies should also include populations of African ancestry living outside of the Caribbean region. We were unable to assess fat depots in other important non-adipose tissues and organs, in particular in the visceral cavity and liver, so we were not able to test if the observed associations were independent of other relevant ectopic fat depots. Additionally, although a high proportion of eligible participants completed the follow-up exam (80% of survivors), it is possible that incomplete recruitment may have biased results, as some attrition may have been associated with diabetes risk. However, such bias would yield underestimates of the true magnitude of associations. Lastly, in the current study, we did not have objective measures of physical activity at baseline and follow-up exams. However, at an ongoing second follow-up exam, we are collecting objective measures of physical activity using arm-band accelerometers. So far in 48 men, we found a high correlation between self-reported hours of walking per week and arm-band recorded total hours of any physical activity per week (r=0.69, P=0.045). This confirms that the walking variable used in the current analysis is indeed a good measure of physical activity in our study.
In conclusion, among middle-aged and elderly African ancestry men, both inter- and intra- muscular fat accumulate with age, independent of body weight changes. Increases in intra-muscular fat are greater among older men, while increases in inter-muscular fat appear to be greater among younger men. Our study also adds new evidence to support a role of inter-muscular fat in the development of T2D, although additional research is needed to confirm these findings and better understand the mechanisms involved.
Supplementary Material
What is already known about this subject?
Cross-sectional studies strongly suggest that skeletal muscle fat infiltration (i.e. myosteatosis) may increase with advancing age, is associated with poor metabolic health, and is greater among African ancestry men compared to Caucasians. However, longitudinal studies examining changes in myosteatosis with aging, and its clinical consequences are very sparse. Such studies are particularly needed among high-risk race/ethnic population groups.
Existing studies have either been conducted among very small number of participants or among the participants recruited to be old, healthy and very high-functioning. No previous studies have examined myosteatosis and aging and metabolic consequences of myosteatosis in a large, prospective study among African ancestry individuals recruited across a wide age range and without regard to their health status.
What does this study add?
Our study shows that both inter- and intra- muscular fat increase with aging, independent of body weight changes in middle-aged and older African ancestry men, a population segment generally understudied. Our novelty also lies in the fact that, unlike previous studies, our participants were recruited across a wide age range and without regard to their health status.
We also made the novel observation that while intra-muscular fat accelerates with increasing age; in contrast, inter-muscular fat decelerates with increasing age among African ancestry men.
Finally, our study is of great public health importance as it the first prospective, large population-based study to examine and demonstrate a role of inter-muscular fat in the development of type 2 diabetes.
Acknowledgments
Funding: This research was supported by grant R01-AR049747 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and grants K01-DK083029 and R01-DK097084 from the National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Conflict of Interest: The authors have nothing to disclose.
References
- 1.Carobbio S, Rodriguez-Cuenca S, Vidal-Puig A. Origins of metabolic complications in obesity: ectopic fat accumulation. The importance of the qualitative aspect of lipotoxicity. Curr Opin Clin Nutr Metab Care. 2011;14(6):520–6. doi: 10.1097/MCO.0b013e32834ad966. [DOI] [PubMed] [Google Scholar]
- 2.Danforth E., Jr. Failure of adipocyte differentiation causes type II diabetes mellitus? Nat Genet. 2000;26(1):13. doi: 10.1038/79111. [DOI] [PubMed] [Google Scholar]
- 3.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley DE, Goodpaster BH. Skeletal muscle triglyceride. An aspect of regional adiposity and insulin resistance. Diabetes Care. 2001;24(5):933–41. doi: 10.2337/diacare.24.5.933. [DOI] [PubMed] [Google Scholar]
- 5.Kim JY, Hickner RC, Cortright RL, Dohm GL, Houmard JA. Lipid oxidation is reduced in obese human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279(5):E1039–44. doi: 10.1152/ajpendo.2000.279.5.E1039. [DOI] [PubMed] [Google Scholar]
- 6.Hegarty BD, Cooney GJ, Kraegen EW, Furler SM. Increased efficiency of fatty acid uptake contributes to lipid accumulation in skeletal muscle of high fat-fed insulin-resistant rats. Diabetes. 2002;51(5):1477–84. doi: 10.2337/diabetes.51.5.1477. [DOI] [PubMed] [Google Scholar]
- 7.Miljkovic I, Zmuda JM. Epidemiology of myosteatosis. Curr Opin Clin Nutr Metab Care. 2010;13(3):260–4. doi: 10.1097/MCO.0b013e328337d826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, et al. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci. 2005;60(3):324–33. doi: 10.1093/gerona/60.3.324. [DOI] [PubMed] [Google Scholar]
- 9.Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, et al. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab. 2004;89(8):3864–71. doi: 10.1210/jc.2003-031986. [DOI] [PubMed] [Google Scholar]
- 10.Miljkovic-Gacic I, Gordon CL, Goodpaster BH, Bunker CH, Patrick AL, Kuller LH, et al. Adipose tissue infiltration in skeletal muscle: age patterns and association with diabetes among men of African ancestry. Am J Clin Nutr. 2008;87(6):1590–1595. doi: 10.1093/ajcn/87.6.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song MY, Ruts E, Kim J, Janumala I, Heymsfield S, Gallagher D. Sarcopenia and increased adipose tissue infiltration of muscle in elderly African American women. Am J Clin Nutr. 2004;79(5):874–80. doi: 10.1093/ajcn/79.5.874. [DOI] [PubMed] [Google Scholar]
- 12.Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81(4):903–10. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Albu JB, Kovera AJ, Allen L, Wainwright M, Berk E, Raja-Khan N, et al. Independent association of insulin resistance with larger amounts of intermuscular adipose tissue and a greater acute insulin response to glucose in African American than in white nondiabetic women. Am J Clin Nutr. 2005;82(6):1210–7. doi: 10.1093/ajcn/82.6.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miljkovic I, Cauley JA, Petit MA, Ensrud KE, Strotmeyer E, Sheu Y, et al. Greater adipose tissue infiltration in skeletal muscle among older men of African ancestry. J Clin Endocrinol Metab. 2009;94(8):2735–42. doi: 10.1210/jc.2008-2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haffner SM, D'Agostino R, Saad MF, Rewers M, Mykkanen L, Selby J, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites. The Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45(6):742–8. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 16.Brancati FL, Kao WH, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults: the Atherosclerosis Risk in Communities Study. Jama. 2000;283(17):2253–9. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 17.Cowie CC, Rust KF, Byrd-Holt DD, Eberhardt MS, Flegal KM, Engelgau MM, et al. Prevalence of Diabetes and Impaired Fasting Glucose in Adults in the U.S. Population. Diabetes Care. 2006;29(6):1263–1268. doi: 10.2337/dc06-0062. [DOI] [PubMed] [Google Scholar]
- 18.Goodpaster BH, Krishnaswami S, Resnick H, Kelley DE, Haggerty C, Harris TB, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26(2):372–9. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 19.Miljkovic I, Cauley JA, Wang PY, Holton KF, Lee CG, Sheu Y, et al. Abdominal myosteatosis is independently associated with hyperinsulinemia and insulin resistance among older men without diabetes. Obesity (Silver Spring) 2013;21(10):2118–25. doi: 10.1002/oby.20346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boettcher M, Machann J, Stefan N, Thamer C, Haring HU, Claussen CD, et al. Intermuscular adipose tissue (IMAT): association with other adipose tissue compartments and insulin sensitivity. J Magn Reson Imaging. 2009;29(6):1340–5. doi: 10.1002/jmri.21754. [DOI] [PubMed] [Google Scholar]
- 21.Bunker CH, Patrick AL, Konety BR, Dhir R, Brufsky AM, Vivas CA, et al. High prevalence of screening-detected prostate cancer among Afro-Caribbeans: the Tobago Prostate Cancer Survey. Cancer Epidemiol Biomarkers Prev. 2002;11(8):726–9. [PubMed] [Google Scholar]
- 22.Sheu Y, Cauley JA, Bunker CH, Wheeler VW, Patrick AL, Gordon CL, et al. Correlates of trabecular and cortical volumetric BMD in men of African ancestry. J Bone Miner Res. 2009;24(12):1960–8. doi: 10.1359/JBMR.090522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 24.Larson-Meyer DE, Smith SR, Heilbronn LK, Kelley DE, Ravussin E, Newcomer BR. Muscle-associated triglyceride measured by computed tomography and magnetic resonance spectroscopy. Obesity (Silver Spring) 2006;14(1):73–87. doi: 10.1038/oby.2006.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simonsick EM, Maffeo CE, Rogers SK, Skinner EA, Davis D, Guralnik JM, et al. Methodology and feasibility of a home-based examination in disabled older women: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci. 1997;52(5):M264–74. doi: 10.1093/gerona/52a.5.m264. [DOI] [PubMed] [Google Scholar]
- 26.Stratec XCT 2000 Manual Version 6.66 Medizintechnik GmbH Pforzheim Germany. 2005.
- 27.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky FA, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82(4):872–8. doi: 10.1093/ajcn/82.4.872. quiz 915-6. [DOI] [PubMed] [Google Scholar]
- 28.Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–85. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beattie KA, MacIntyre NJ, Ramadan K, Inglis D, Maly MR. Longitudinal changes in intermuscular fat volume and quadriceps muscle volume in the thighs of women with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012;64(1):22–9. doi: 10.1002/acr.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinanan ACM, Buxton PG, Lewis MP. Muscling in on stem cells. Biol. Cell. 2006;98(4):203–214. doi: 10.1042/BC20050050. [DOI] [PubMed] [Google Scholar]
- 31.Brehm A, Krssak M, Schmid AI, Nowotny P, Waldhausl W, Roden M. Increased lipid availability impairs insulin-stimulated ATP synthesis in human skeletal muscle. Diabetes. 2006;55(1):136–40. [PubMed] [Google Scholar]
- 32.Perseghin G, Scifo P, De Cobelli F, Pagliato E, Battezzati A, Arcelloni C, et al. Intramyocellular triglyceride content is a determinant of in vivo insulin resistance in humans: a 1H-13C nuclear magnetic resonance spectroscopy assessment in offspring of type 2 diabetic parents. Diabetes. 1999;48(8):1600–6. doi: 10.2337/diabetes.48.8.1600. [DOI] [PubMed] [Google Scholar]
- 33.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–2. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zoico E, Rossi A, Di Francesco V, Sepe A, Olioso D, Pizzini F, et al. Adipose Tissue Infiltration in Skeletal Muscle of Healthy Elderly Men: Relationships With Body Composition, Insulin Resistance, and Inflammation at the Systemic and Tissue Level. J Gerontol A Biol Sci Med Sci. 2009 doi: 10.1093/gerona/glp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallagher D, Kelley DE, Yim JE, Spence N, Albu J, Boxt L, et al. Adipose tissue distribution is different in type 2 diabetes. Am J Clin Nutr. 2009;89(3):807–14. doi: 10.3945/ajcn.2008.26955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, et al. Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health. 2007;97(12):2260–7. doi: 10.2105/AJPH.2006.094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, et al. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297(5):E987–998. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- 38.Lee DE, Kehlenbrink S, Lee H, Hawkins M, Yudkin JS. Getting the message across: mechanisms of physiological cross talk by adipose tissue. Am J Physiol Endocrinol Metab. 2009;296(6):E1210–29. doi: 10.1152/ajpendo.00015.2009. [DOI] [PubMed] [Google Scholar]
- 39.Kovalik J-P, Slentz D, Stevens RD, Kraus WE, Houmard JA, Nicoll JB, et al. Metabolic Remodeling of Human Skeletal Myocytes by Cocultured Adipocytes Depends on the Lipolytic State of the System. Diabetes. 2011;60(7):1882–1893. doi: 10.2337/db10-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–7. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.