Abstract
The Wnt/β-catenin signaling cascade is an evolutionarily conserved, highly complex pathway that is known to be involved in kidney injury and repair after a wide variety of insults. While the kidney displays an impressive ability to repair and recover after injury, these repair mechanisms can be overwhelmed, leading to maladaptive responses and eventual development of chronic kidney disease (CKD). Emerging evidence demonstrates that Wnt/β-catenin signaling possesses dual roles in promoting repair/regeneration or facilitating progression to CKD after acute kidney injury (AKI), depending on the magnitude and duration of its activation. In this review, we summarize the expression, intracellular modification, and secretion of Wnt family proteins and their regulation in a variety of kidney diseases. We also explore our current understanding of the potential mechanisms by which transient Wnt/β-catenin activation positively regulates adaptive responses of the kidney after AKI, and discuss how sustained activation of this signaling triggers maladaptive responses and causes destructive outcomes. A better understanding of these mechanisms may offer important opportunities for designing targeted therapy to promote adaptive kidney repair/recovery and prevent progression to CKD in patients.
Keywords: Wnt signaling, β-catenin, kidney injury, renal fibrosis, AKI, CKD
Introduction
The mammalian kidney is a highly specialized organ that, while vulnerable to hypoxic and toxic injuries, has a significant capacity for repair and regeneration.1–3 The balance between tissue damage and repair/regeneration is critical for determining the ultimate outcome of the kidney following an insult. Extensive studies have demonstrated that the ability of the kidney to repair is inversely correlated with the severity, frequency and duration of initial injury.4, 5 Contradictory to the long-held belief that kidney repair/regeneration may only occur in acute kidney injury (AKI), increasing evidence illustrates that tissue repair and remodeling can take place even in the established and progressive chronic kidney disease (CKD).6–9
The biologic events in kidney repair include trophic growth factor/cytokine production and secretion, activation of interstitial fibroblasts, tubular epithelial cell proliferation and differentiation, subsequent resolution of renal fibroblasts and infiltrated inflammatory cells, and restoration of the damaged microvasculature.10–12 Evidence is emerging that several key developmental pathways, including Wnt, hedgehog and Notch signaling, as well as the hepatocyte growth factor (HGF) signal cascade, play an essential role in promoting kidney repair and regeneration after injury.13–19 In this context, tissue repair in response to injury in adult kidney can be viewed as a cascade of biological processes that recapitulates the developmental program.
Wnt/β-catenin signaling is an evolutionarily conserved, key developmental signaling pathway implicated in organogenesis, tissue homeostasis and disease development in multicellular organisms.20–23 Over the past several years, substantial studies have demonstrated that Wnt/β-catenin signaling could have a pivotal role in promoting tubular repair and regeneration after AKI induced by either ischemia-reperfusion injury (IRI) or nephrotoxins.24, 25 However, data are also mounting that aberrant activation of Wnt/β-catenin is associated with proteinuria, renal function decline and kidney fibrosis in many forms of CKD, regardless of whether the injury initially occurs in renal tubulointerstitium or glomeruli.26–31 We have recently reviewed the relevant studies on Wnt/β-catenin signaling in regulating podocyte dysfunction and kidney fibrosis in proteinuric and fibrotic kidney diseases, such as diabetic nephropathy and obstructive nephropathy.14, 15 Therefore, the scope of this article is limited to the discussion of Wnt/β-catenin signaling in regulating kidney repair and regeneration after injury, particularly AKI. We propose that Wnt/β-catenin is a double-edged sword in kidney repair, and the duration of its activation seems to be the major determinant for the long-term outcome of the injured kidney. While transient Wnt/β-catenin signaling is essential for accelerating tubular repair and kidney recovery, an exaggerated and sustained activation of this signaling triggers maladaptive responses, leading to AKI- CKD transition.32
Wnt ligands: expression, modification and secretion
Wnt proteins are a family of secreted lipid-modified glycoproteins with established roles in embryonic development and tissue homeostasis.33 The term Wnt combines the name of the Drosophila segment polarity gene wingless and the name of the vertebrate homolog, integrated or int-1.22, 23 The Wnt family contains 19 different members in mammalian species: Wnt1, 2, 2b, 3, 3a, 4, 5a, 5b, 6, 7a, 7b, 8a, 8b, 9a, 9b, 10a, 10b, 11, and 16.34 These proteins are vital to kidney development, and defects in Wnts are associated with various human diseases including developmental abnormalities and cancers.
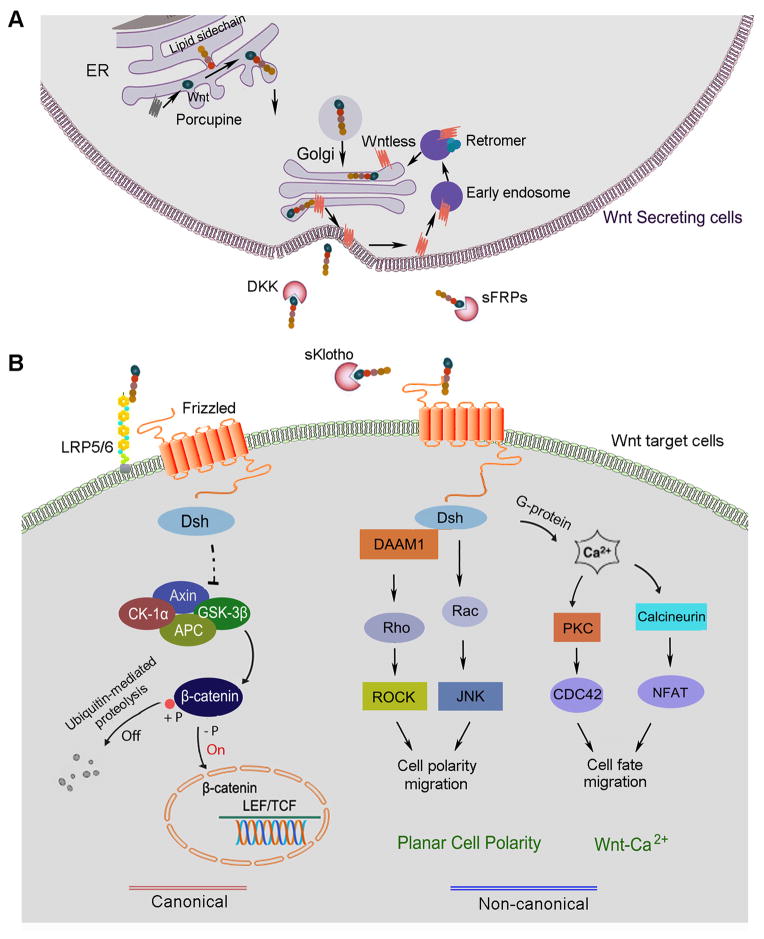
Wnt ligands are modified as glycolipoproteins and secreted into the extracellular environment as morphogens, which are signaling proteins acting in a concentration-dependent manner to determine tissue patterning during development.35 Mounting evidence has demonstrated that multiple layers of regulation are linked with the intracellular trafficking of Wnt proteins along their secretory pathway (Figure 1A). Wnt proteins are glycosylated in the endoplasmic reticulum (ER) and then are palmitolated by a membrane-bound acyltransferase, known as Porcupine, on at least two distinct sites: the N-terminal cysteine rich residues and a C-terminal serine 209 residue.36–38 Loss of Porcupine function causes Wnts to accumulate abnormally in the ER, leading to a defect in secretion.39
Figure 1. The principle of Wnt secretion and signaling. (A).
Wnt expression, modification and secretion. In Wnt producing cells, Wnt proteins are lipid modified in the lumen of endoplasmic reticulum (ER) by Porcupine and then transported to the Golgi, where they encounter Wntless (Wls). Supported by Wls, Wnt ligands are directly delivered to the plasma membrane through Trans-Golgi network (TGN). Wls is then taken up by the clathrin-mediated endocytosis and the retromer complex routes Wls back to the Golgi from the plasma membrane. (B) The principle of Wnt signaling. Once released from the producing cells, Wnt initiates signal by interacting with the Frizzled receptor and co-receptors LRP5/6 at the plasma membrane of target cells and activates Dsh. At Dsh level, Wnt signaling is branched into canonical and non-canonical pathways. Activation of the canonical Wnt signaling leads to β-catenin stabilization, accumulation and nuclear translocation, which enables β-catenin to influence the expression of downstream target genes. Non-canonical Wnt signaling invokes several β-catenin independent pathways via the activation of the Frizzled receptor. In Wnt/planar cell polarity (PCP) pathway, Dsh transfers signal to the small GTP-binding proteins Rho and Rac. In Wnt/Ca2+ pathway, the Frizzled receptor also interfaces with a trimeric G-protein and results in the release of calcium from ER. When concentration of calcium rises, protein kinase C (PKC) and calcineurin will be activated. Calcineurin induces the nuclear factor of activated T-cells (NFAT), which regulates cell fate and migration.
Wnts secretion is mainly regulated by the cargo receptor encoded by wntless gene, which was identified by three groups in 2006.40–44 As a putative G-protein coupled receptor, Wntless (Wls), also known as Evenness Interrupted (Evi) in Drosophila and G protein-coupled receptor 177 (GPR177) in mammals, is obligatory for the secretion of all Wnt proteins. Wls localizes to the entire Wnt secretory route including ER, Golgi, vesicles and plasma membrane and binds to the hydrophobic palmitate groups in mature Wnts by virtue of its lipocalin-like structure.38, 40, 41 The posttranslational modifications of Wnts contribute to their transport and secretion from ligand-producing cells. In the absence of Wls, a number of Wnt proteins are sequestered in the secretory pathway of Wnt-producing cells and fail to reach the plasma membrane, resulting in strong Wnt loss-of-function phenotypes. In addition, physical parameters such as environmental pH also have a strong impact on Wnts secretion.38
A multiprotein complex known as the retromer may also play a role in regulating Wnt protein secretion. As Wls accompanies Wnts to the cell surface for secretion, the Wls can be recovered and sent back to the Golgi. The retromer complex may govern this recycling of Wls from endosomes to the Golgi and allow for further Wnt binding (Figure 1A).45
The principle of Wnt signaling
Wnt signaling is extremely complex, and there are approximately more than 50 proteins that participate in Wnt signaling at various stages, which include 19 Wnt ligands, 10 Frizzled receptors and 2 co-receptors, a dozen of various kinds of inhibitors, multiple intracellular mediators, transcription factors and co-activators. In the extracellular milieu, Wnt diffusion and signaling abilities are limited due to stabilization by heparan sulfate proteoglycans including Dally and glypican.46, 47 In addition, secreted inhibitors such as a family of the secreted Frizzled-related proteins (sFRP1~5) bind to Wnts to prevent their interaction with cell surface receptors, effectively antagonizing Wnt signaling.48–51 The anti-aging protein Klotho, which is predominantly expressed in the tubular epithelium of normal kidneys, is also an endogenous Wnt antagonist, and both full-length, membranous Klotho and its truncated, soluble form effectively bind to and sequesters Wnt ligands, thereby negatively controlling Wnts action.48 Dickkopf (DKK) family of proteins (DKK1~4) are shown to disrupt Wnt binding to its co-receptors and inhibit β-catenin activation.
Wnts bind to the plasma membrane receptors known as the Frizzled receptor family of proteins, and co-receptors, the low density lipoprotein-related protein 5 and 6 (LRP-5/6), to mediate their signaling.52 After binding to the receptor complex, Wnt signal is transduced to the cytoplasmic phosphoprotein, Dishevelled (Dsh/Dvl) (Figure 1B). At the level of Dsh, the Wnt signal branches into the canonical, β-catenin-dependent pathway and non-canonical, β-catenin-independent pathway, the latter of which can be divided into the planar cell polarity pathway (PCP) and the Wnt/Ca2+ pathway. Dsh is an important downstream component and the first cytoplasmic protein that is indispensably involved in all branches of Wnt signaling.53
In canonical signaling, Wnts induces changes in the so-called ‘destruction complex’ comprised of Dsh, axin, adenomatosis polyposis coli (APC), casein kinase-1 and glycogen synthase kinase (GSK)-3β. In the normal, quiescent state, β-catenin is constitutively phosphorylated by GSK-3β and undergoes ubiquitin-mediated proteolytic degradation (Figure 1B). However, when Wnt engages with its receptor complex, it induces inhibition of GSK-3β and ultimately results in dephosphorylation of β-catenin. This causes the stabilization and activation of β-catenin and allows it to translocate into the nucleus, wherein it binds to T cell factor (TCF)/lymphoid enhancer-binding factor (LEF) to stimulate the transcription of downstream target genes (Figure 1B). The canonical Wnt pathway regulates gene transcription and thus often leads to cell survival, proliferation and differentiation.54 In addition, there appears to be some evidence that GSK-3β can also phosphorylate LRP 5/6 and be a new way that Wnt signaling is regulated.55
The non-canonical Wnt pathway has two major branches: the PCP pathway and the Wnt/Ca2+ pathway. In the PCP pathway, Frizzled receptors activate Dsh independently of LRP-5/6 and lead to Rho/Rho-associated protein kinase (ROCK) and c-Jun N-terminal kinase (JNK) activation (Figure 1B). This pathway mediates cytoskeletal organization and coordinated polarization of cells within the plane of epithelial sheets.56 The other non-canonical pathway leads to the release of intracellular Ca2+, possibly via G-proteins.57 This pathway involves activation of phospholipase C (PLC) and protein kinase C (PKC).58, 59 Elevated Ca2+ can activate the phosphatase calcineurin, which leads to dephosphorylation of the nuclear factor of activated T-cells (NFAT) and its accumulation in the nucleus. The calcium-mediated pathway has defined roles in dorsal/ventral patterning, gastrulation, and cardiac development.36 Overall, these non-canonical pathways are known to be involved in determination of cell polarity as well as mesodermal cell migration during development.60 While the involvement of canonical Wnt signaling in regulating kidney development and diseases is well studied, little is known about the potential role of the non-canonical pathway of Wnts in these processes.13, 15, 61
Regulation and function of Wnt/β-catenin after kidney injury
Wnt/β-catenin signaling in adult kidney is relatively silenced, although this signaling has an essential role in kidney development.61 Renal Wnt/β-catenin, however, is upregulated in virtually every experimental animal model tested up to date, arranging from AKI to various forms of CKD (Table 1). Re-activation of Wnt/β-catenin underscores that this developmental signaling may play a crucial role in the subsequent repair or disease development after a wide variety of injury in adult kidney. A comprehensive understanding of Wnt/β-catenin regulation and its roles in kidney injury repair and development of nephropathies would provide opportunities to target key components of Wnt signaling for designing rational therapeutic strategies.
Table 1.
Regulation and function of Wnt signaling in kidney injury repair and diseases
| AKI | ||||
|---|---|---|---|---|
| Models | Species | Major finding | Role | Ref. |
| IRI | Rat | Wnt4 is induced after AKI and promotes cell cycle progression of renal tubular cells in vitro | Protective | 62 |
| IRI | Mouse | Macrophage-derived Wnt7b promote tubular repair and regeneration | Protective | 25 |
| IRI | Mouse | Tubule-specific ablation of β-catenin aggravates kidney injury | Protective | 24 |
| Folic acid | Mouse | Tubule-specific ablation of β-catenin aggravates kidney injury | Protective | 24 |
| IRI | Rat | Wnt agonist reduces tissue damage and improves renal function | Protective | 64 |
| AKI-to-CKD | ||||
| IRI | Mouse | Transient activation of Wnt/β-catenin is associated with AKI recovery | Transient: protective | 32 |
| Sustained activation of Wnt/β-catenin accelerates AKI-to-CKD progression | Sustained: detrimental | |||
| CKD | ||||
| UUO | Mouse | sFRP4↓, β-catenin↑, sFRP4 inhibits fibrosis | Detrimental | 28 |
| Multiple Wnts↑, β-catenin↑, DKK1 inhibits fibrosis | Detrimental | 26 | ||
| Disruption of β-catenin/CBP by small molecule inhibitor ICG-001 ameliorates fibrosis | Detrimental | 30 | ||
| Klotho antagonizes Wnt and inhibits fibrosis↓ | Detrimental | 48, 126 | ||
| ADR-induced nephropathy | Mouse | Wnts↑, β-catenin↑, podocyte-specific deletion of β-catenin and DKK1 inhibits proteinuria | Detrimental | 27, 127 |
| Sequestration of β-catenin by VDR, reduces proteinuria and fibrosis | Detrimental | 29 | ||
| Antagonism of Wnt/β-catenin by Klotho reduces proteinuria and fibrosis | Detrimental | 48 | ||
| Small molecule inhibitor ICG-001 ameliorates established proteinuria and fibrosis | Detrimental | 77 | ||
| Ang II-induced nephropathy | Mouse | Wnt1↑, β-catenin↑, DKK1 reduces proteinuria | Detrimental | 71 |
| Remnant kidney after 5/6NX | Mouse | Klotho↓, β-catenin↑, antagonism of Wnt by Klotho ameliorates injury, hypertension and fibrosis | Detrimental | 68 |
| Diabetic nephropathy | Akita mice | Multiple Wnts↑,β-catenin↑, anti-LRP6 ameliorates proteinuria and fibrosis | Detrimental | 69 |
| db/db mouse | Multiple Wnts↑, β-catenin↑ | Detrimental | 69 | |
| STZ-diabetic rats | Multiple Wnts↑, β-catenin↑ | Detrimental | 69 | |
| Human | Wnt1↑, β-catenin↑ | Detrimental | 27 | |
| HIV- associated nephropathy | Mouse | Wnt↑, β-catenin↑, DKK1 ameliorates proteinuria and podocytopathy | Detrimental | 72 |
| Human | Wnt↑, β-catenin↑, | Detrimental | 72 | |
| TGF-β1- induced nephropathy | Mouse | TGF-β1 induces Wnt1 in vivo, DKK1 reduces proteinuria | Detrimental | 128 |
| Genetic model | Mouse | Podocyte-specific activation of β-catenin induces proteinuria | Detrimental | 75 |
| Fibroblast-specific activation of β-catenin induces fibrosis | Detrimental | 31 | ||
| Polycystic kidney disease | Mouse | Dysregulated β-catenin, sFRP4, Wnt9b, PCP | Detrimental | 74, 87, 88 |
| IgA nephropathy | Human | β-catenin↑, Inversin↓ | Detrimental | 78 |
| FSGS | Human | Wnt1↑, β-catenin↑ | Detrimental | 27 |
| Lupus nephritis | Human | β-catenin↑, plasma DKK1↑ | Detrimental | 82 |
Abbreviations: IRI, ischemia reperfusion injury; UUO, unilateral ureteral obstruction; ADR, adriamycin; Ang II, angiotensin II; DKK1,Dickkopf 1; LRP-6, low density lipoprotein-related protein 6; STZ, streptozotocin; sFRP4, secreted Frizzled-related protein-4; PCP, planar cell polarity pathway; FSGS, Focal segmental glomerulosclerosis.
In the rat model of AKI induced by IRI, Wnt4 mRNA is reported to increase at 3 to 12 h while its protein level rises at 6 to 24 h after ischemia.62 This is associated with upregulation of cyclin D1 and cyclin A at 24 to 48 h, suggesting that this signaling may be instrumental for tubular cells entering the cell cycle, a key event in tubular repair and regeneration after damage. Indeed, overexpression of Wnt4 or β-catenin promotes cell cycle progression and increases the protein expression of cyclin D1 in tubular epithelial cells in vitro, indicating that stimulation of Wnt/β-catenin could be reparative in ischemic AKI.62 In a mouse model of ischemic AKI, renal expression of multiple Wnts including Wnt1, 2, 3, 3a, 4, 7a, 8a, 8b, 10a and 16, and β-catenin are upregulated, and the magnitude of their induction is closely associated with the severity of IRI.32 In AKI induced by nephrotoxin such as folic acid, β-catenin is also activated specifically in renal tubular epithelium.24 Therefore, upregulation of Wnt/β-catenin is a common and shared response of the kidney after acute injury induced by either ischemia or nephrotoxins (Table 1).
Appropriate activation of Wnt/β-catenin in the setting of AKI is reno-protective, leading to reduced kidney injury and accelerated recovery of renal structure and function. This notion is substantiated by numerous observations that Wnt/β-catenin is a survival signal that protects renal tubular epithelial cells against apoptosis in vitro and in vivo.24, 63 This property, together with its ability to promote tubular epithelial cell proliferation,62 renders Wnt/β-catenin not only able to minimize tubular damage but also accelerate tubular repair and regeneration. By using conditional knockout mice in which β-catenin is ablated in a tubule-specific fashion, it has been demonstrated that loss of β-catenin, the sole intracellular mediator of the canonical Wnt signaling, aggravates AKI induced by either ischemic or nephrotoxic insults, underscoring a protective potential of Wnt/β-caenin in the setting of AKI.24 Recent studies further show that administration of Wnt agonist at 1 h prior to ischemia reduces kidney damage and improves renal function in a rat model of IRI, thereby directly validating the role of Wnt/β-catenin in conferring renal protection after AKI.64
Repetitive and/or chronic injury to the kidney will lead to development of CKD, characterized by progressive renal fibrosis, which is widely viewed as a consequence of maladaptive, failed injury repair.65–67 In all animal models of CKD tested thus far, Wnt/β-catenin has been shown to be activated, with no exception (Table 1). This suggests that activation of Wnt/β-catenin is a generalized, and perhaps intuitive, response of the damaged kidney in an attempt to repair and heal. However, chronic injury such as ureteral obstruction or hyperglycemia often overwhelms the kidney, leading to an exaggerated and sustained activation of Wnt/β-catenin. For instance, in a mouse model of unilateral ureteral obstruction (UUO), 16 out of 19 members of the Wnt family proteins are upregulated in the obstructed kidney with distinct dynamics.26 Consequently, this leads to a dramatic and sustained activation of β-catenin in renal tubular epithelial cells.26 Upregulation of Wnt/β-catenin is also found in many other forms of CKD including remnant kidney after 5/6 nephrectomy (5/6NX),68 adriamycin (ADR)-induced nephropathy,27, 29 diabetic nephropathy,69, 70 angiotensin II infusion-induced nephropathy,71 HIV-associated nephropathy72 and polycystic kidney disease73, 74 (Table 1).
Contrary to the setting of AKI, Wnt/β-catenin in CKD appears to be detrimental in the evolution of nephropathies. Genetically modified mice with constitutive activation of β-catenin in cell type-specific fashion have been shown to spontaneously develop renal lesions and fibrosis,31, 75 suggesting that chronic activation of Wnt/β-catenin signaling is sufficient to cause kidney disorders in vivo. In a mouse model of UUO, inhibition of Wnt/β-catenin signaling using various antagonists including sFRP4,28 DKK1,26 Klotho48 and the small molecule inhibitor ICG-00130 represses myofibroblast activation and reduces renal fibrosis. In ADR-induced nephropathy, blockade of Wnt signaling with DKK1,27 vitamin D receptor (VDR) agonist,29 Klotho48, 76 and ICG-00177 also attenuates kidney injury and reverses established proteinuria. Wnt antagonists such as Klotho and DKK-1 also significantly reduce renal β-catenin accumulation and inhibit the expression of Wnt/β-catenin target genes in animal models of remnant kidney after 5/6NX, angiotensin II-induced nephropathy and HIV-associated nephropathy, respectively (Table 1).68, 71, 72 These data illuminate that Wnt/β-catenin signaling is a double-edged sword in kidney repair and regeneration after injury: it minimizes tissue damage and accelerates recovery in the setting of AKI, but its sustained activation clearly leads to a failed wound-healing response and drives the onset and progression of CKD.
Wnt/β-catenin signaling in human kidney disease
Similar to animal models, data are accumulating that link Wnt/β-catenin to the pathogenesis of human kidney disorders in patients (Table 1). IgA nephropathy (IgAN) is the most common primary glomerulonephritis worldwide. A microarray study, conducted by using peripheral blood leukocytes, demonstrates distinct gene expression profile in Wnt signaling that strongly discriminates between the IgAN patients and controls. Inversin (INV), a negative regulator of Wnt/β-catenin pathway, is decreased in IgAN patients, suggesting hyperactivation of Wnt signaling. Interestingly, a subset of 21 Wnt-related genes is able to specifically separate IgAN from healthy controls, and all of which is associated with enhanced peripheral blood mononuclear cell proliferation and activation.78
In human focal segmental glomerulosclerosis (FSGS), Wnt1 and β-catenin nuclear translocation is increased in podocytes and tubular cells from diseased kidneys, demonstrating the activation of canonical Wnt signaling.27 Several studies have implicated members of the Wnt signaling pathway in human diabetic kidney disease (DKD), although the data are conflicting.69, 79 Using a case-control design, analyses of single nucleotide polymorphisms (SNPs) and haplotypes in multiple key Wnt pathway genes (CTNNB1, AXIN2, LRP5, LRP6, GSK-3β, DAAM1 and NFAT5) suggest that genetic changes in the genes encoding Wnt signaling components are not strongly associated with diabetic nephropathy induced by type 1 diabetes among white individuals.80, 81 However, a microarray analysis has reported statistically significant increases in mRNA expression of Wnt1, Wnt2b, Wnt4, Wnt6, Wnt16, DKK3, and LEF1 in human DKD glomeruli. In human DKD kidneys, there is also increased expression of β-catenin in the glomeruli compared to healthy control kidneys.27
Canonical Wnt signaling is also induced in patients with lupus nephritis and with crescentic glomerulonephritis.82 In systemic lupus, hyperactivation of Wnt signaling might be through the p53/p21 pathway.83 In addition, a complex consisting of Pan-cadherin/p120 catenin/β-catenin was observed in abundance in the cellular crescents characterizing pauci-immune glomerulonephritis. The plethora of this complex diminishes as the cresents undergo cellular to fibrotic progression.84
Studies show that cystic kidney diseases have been associated with defective Wnt signaling as well.85–87 Perturbations of cystic disease genes cause impaired balance of non-canonical and canonical Wnt signaling, leading to cyst formation.73 Interestingly, inversin was identified as a causal gene of nephronophthisis type II, an autosomal recessive cystic kidney disease. Inversin is known to inhibit the canonical β-catenin pathway while promoting non-canonical PCP signaling.88 Therefore, disrupting the delicate balance between canonical and non-canonical Wnt/β-catenin signaling may be linked to cyst formation in patients.
Wnt/β-catenin and adaptive repair after AKI
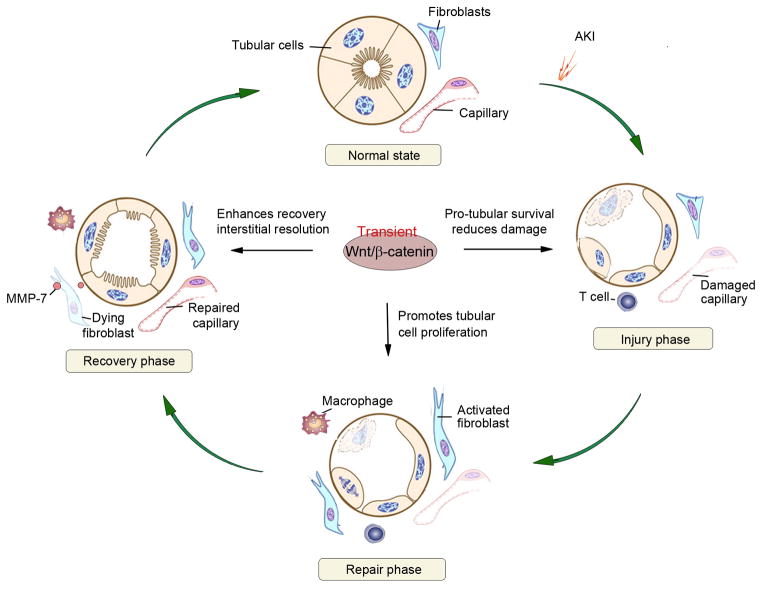
Following AKI, the kidney often recovers its structure and function via adaptive repair and regeneration. This process can be considered as an innate wound-healing response consisting of the injury, repair and recovery phases (Figure 2). Both gain- and loss-of-function studies indicate that Wnt/β-catenin plays an essential role in minimizing initial kidney damages and promoting adaptive repair and regeneration after AKI.24, 25, 64 Kidney injury repair following AKI involves several distinct biologic events including tubular damage/cell death, epithelial-mesenchymal communication (EMC), tubular cell proliferation, followed by resolution of renal fibroblasts and inflammation, and restoration of microvasculature (Figure 2). It appears that Wnt/β-catenin signaling participates in the most majority, if not all, of these reparative events, leading to accelerated regeneration and recovery.
Figure 2. Wnt/β-catenin signaling facilitates adaptive repair after AKI.
Following ischemic or toxic insults, kidneys possess an intrinsic ability to recover by undergoing adaptive repair and regeneration. (A) Injury phase. Shortly after AKI, tubular damage includes loss of brush border and apical-basal polarity, cell detachment, apoptosis and necrosis. Injury to capillary and infiltration of inflammatory cells are also evident. (B) Regeneration phase. Following the initial damage, peritubular fibroblasts are activated, and tubular cells undergo proliferation to repair and regeneration. (C) Recovery phase. Tubular cells re-differentiate, while interstitial fibroblasts and inflammatory cells are resolved and capillary integrity restored. Wnt/β-catenin signaling promotes adaptive repair after AKI by promoting tubular cells survival and mitigating damage in the injury phase, by stimulating tubular cell proliferation in the regeneration phase, and by facilitating the resolution of interstitial fibroblasts and restoring capillary integrity in the recovery phase.
The hallmarks of AKI following either ischemic or toxic insults are tubular damage, characterized by loss of proximal tubular cell brush border, demolition of epithelial cell apical-basal polarity, cell detachment from tubular basement membrane, cellular cast formation in the lumen of renal tubules, apoptosis and necrosis.89–92 As a potent survival factor, Wnt/β-catenin has been shown to protect tubular epithelial cells against apoptosis both in vitro and in vivo.24, 63 In either immortalized or primary proximal tubular epithelial cells, constitutively active β-catenin decreases apoptosis and improves cell survival after metabolic stress. Active β-catenin also inhibits Bax activation, oligomerization, and translocation to mitochondria, whereas dominant negative β-catenin exhibits the opposite effects, suggesting that Wnt/β-catenin signaling promotes survival of renal epithelial cells by inhibiting Bax.63 Recent studies also illustrate that in vitro activation of β-catenin by Wnt1 protects tubular epithelial cells from apoptosis, activates Akt, induces survivin, and represses p53 and Bax expression. In conditional knockout mice with tubule-specific ablation of β-catenin, ischemic or toxic AKI causes higher mortality, elevated serum creatinine, and more severe morphologic injury, compared to control mice.24 Consistently, apoptosis is more prevalent in kidneys of the knockout mice, which is accompanied by increased expression of p53 and Bax, and decreased phosphorylated Akt and survivin. Taken together, these data suggest that Wnt/β-catenin is pivotal survival factor that promotes renal tubular cell survival through multiple mechanisms.24 As such, early and appropriate activation of Wnt/β-catenin signaling is required for minimizing the initial renal damages after AKI.
Injured tubular cells after AKI often produce and secrete a variety of cytokines, chemokines and growth factors, which trigger and/or augment the infiltration of inflammatory cells and activation of fibroblasts in renal interstitium. While the contribution of renal inflammation to the pathophysiology of AKI is well established,93, 94 the role of renal interstitial fibroblast activation in AKI is largely neglected. Transient fibroblast activation is an integral part of wound-healing responses, and is important in promoting injury repair via providing trophic factors. Studies show that fibroblast activation is an early event after injury, and probably plays an unexpected, protective role in AKI. For example, in uranyl acetate-induced AKI in rats, peritubular α-smooth muscle actin (α-SMA)-positive fibroblasts are identified surrounding damaged, dilated proximal tubules before disappearing after tubular recovery.95 These cells attach to the tubular basement membrane via elongated cytoplasm-containing microfilament bundles, and cover large areas of denuded epithelium after AKI. Specific inhibition of fibroblast activation results in an aggravation of renal dysfunction and decrease in regenerative repair compared to control rats.95
Recent studies demonstrate that there are active communications between the injured tubular epithelial cells and interstitial fibroblasts via soluble factors. Both tubular cells and fibroblast cells produce and secrete Wnt ligands, which can target themselves and each other in autocrine and paracrine fashions.96, 97 Therefore, Wnts can mediate epithelial-mesenchymal communication (EMC) in both directions between the tubular and interstitial compartments of the kidney after AKI. Besides Wnts, other signaling proteins such as sonic hedgehog (Shh) and hepatocyte growth factor (HGF) also mediate EMC. However, these proteins often mediate EMC in one-way direction. For instance, injured tubular epithelial cells after AKI secrete Shh, while the Shh-responding cells are predominantly interstitial fibroblasts.16, 98 Tubular cells also express HGF receptor, c-met, and its activation is shown to be important for tubular cell survival and proliferation after AKI.99 However, tubular cells themselves do not produce and secrete HGF, and have to rely on other interstitial cells such as fibroblasts to produce this polytrophic growth factor. Similarly, a constructive crosstalk between infiltrated macrophages and tubular cells is mediated by Wnt7b, which is important for kidney repair and regeneration after ischemic AKI.25
Tubular repair following AKI requires the proliferation of surviving cells, and studies show that Wnt/β-catenin signaling participates in this process. The best characterized targets of Wnt/β-catenin are cyclin D1 and c-myc, two of the most crucial proteins in regulating cell proliferation and cell cycle progression.22, 23 As discussed above, Wnt4 and β-catenin could induce cell cycle progression in renal tubular cells and enable these cells to restore denuded epithelium after AKI. In the IRI model, Wnt4 is co-localized with proliferating cell nuclear antigen (PCNA), suggesting that Wnt4 is a mitotic signal in regenerating renal tubules in vivo.62 Wnt may also interact with other signaling molecules to coordinate the regeneration process in diseased kidneys. For example, HGF has been shown to stimulate β-catenin by inducing its tyrosine phosphorylation and translocation into the nucleus, or inducing LRP-5/6 phosphorylation, via a Wnt-independent pathway.100, 101 Since HGF, its c-met receptor and β-catenin are markedly activated after AKI,99 these suggest that HGF and Wnt signaling may work in concert to promote tubular cell proliferation.102, 103 In addition, other developmental signaling such as Shh and Notch may crosstalk with Wnt in promoting tissue repair after injury.104–107
AKI is often followed by complete recovery of kidney structure and function, if the injury is not appallingly severe.32, 92 Such complete recovery requires not only full tubular repair and regeneration but also the subsequent resolution of renal infiltrated cells and activated fibroblasts. Indeed, in rat model of uranyl acetate-induced acute renal failure, renal fibroblast activation is transient in the cortex and outer stripe of outer medulla, and then gradually disappears when renal function restores.108 The mechanism underlying such resolution of activated fibroblasts during the recovery phase of AKI is not entirely clear, but it could be related to the Wnt/β-catenin-mediated induction of matrix metalloproteinase 7 (MMP-7).97 It has been shown that activation of Wnt/β-catenin induces its target MMP-7,109 a zinc- and calcium-dependent endopeptidase secreted by tubular epithelium.110 We demonstrate that MMP-7 induces Fas ligand (FasL) expression in fibroblasts and potentiates fibroblast apoptosis and depletion in renal interstitium.97 Therefore, in the scenario of AKI, tubular Wnt/β-catenin signaling not only promotes epithelial cell survival, proliferation and repair but also triggers MMP-7 expression and secretion, which subsequently leads to fibroblast apoptosis and resolution in the interstitium and results in kidney hemostasis.
AKI is also associated with microvascular injury in the early phase after ischemic or toxic injury,5 and appropriate restoration of damaged microvasculature is critical for a complete recovery. Vascular repair mechanisms include proliferation and migration of endothelial cells after renal injury. Modulation of endothelial progenitor cells (EPC) and hematopoietic stem cells (HSC) may also contribute to microvascular repair.111 In rat IRI model, erythropoietin (EPO) could promote endothelial cell viability and prevent rarefication of microvasculature via Wnt/β-catenin activation.112 The macrophage is also involved in microvasculature repair phase by its interaction with endothelium, which is co-mediated by Wnt and Notch signaling.106, 113 Wnt5a induces several pro-inflammatory and pro-angiogenic cytokines expression such as IL-6 and IL-8 in macrophages in an autocrine manner,114, 115 and subsequently directly affects endothelial cells proliferation and migration.116, 117 Wnt7b is also required for the interaction between macrophage and endothelial cells in microvasculature repair, by stimulating endothelial cells entry into the S phase in the cell cycle.118, 119 Therefore, an appropriate activation of Wnt signaling is beneficial for microvasculature regeneration after AKI.
Wnt/β-catenin and maladaptive response in CKD
While activation of Wnt/β-catenin is clearly reno-protective in AKI, this same signaling appears detrimental in the setting of chronic or repetitive kidney injury and drives the onset and progression of CKD.34, 120 What cause this pathway to behave so differently in AKI and CKD remains largely elusive. However, recent studies on the model of AKI-to-CKD progression have begun to shed new light on this mystery.
It is increasingly recognized that patients who survive an episode of AKI will have a significant risk of developing progressive CKD.121–123 The long-term outcome of AKI patients is divergent. While some patients may fully recover kidney function, others progress to CKD with declining renal function. Although host factors such as pre-existing conditions and genetic backgrounds are important, the severity of AKI seems to be the most important and robust predictor of poorer outcome.4, 124 Consistent with this notion, mice with 20 min IRI display transient AKI followed by recovery of renal function, whereas 30 min IRI causes severe AKI followed by progressing to CKD.32 Therefore, by merely altering the duration of ischemia, one can establish both models of moderate/reversible AKI with full recovery and severe/irreversible AKI destined to CKD in the same setting. Such IRI models with different outcomes provide an unparalleled system to interrogate the different mechanisms by which Wnt/β-catenin plays during AKI-to-CKD transition.
Studies show that although Wnt/β-catenin is upregulated in both moderate and severe AKI, the magnitude and duration of its activation are distinct.32 As discussed above, transient activation of Wnt/β-catenin facilitates tubular repair and regeneration, as well as resolution of interstitial fibroblasts and infiltrated cells after recovery. Sustained activation of the same signaling, however, triggers maladaptive responses, ultimately leading to development of progressive CKD (Figure 3). This view is supported by gain- or loss-of-function manipulations of Wnt/β-catenin in vivo. Prolonged activation of Wnt/β-catenin via in vivo expression of Wnt1 after AKI accelerates CKD progression, whereas blockade of Wnt/β-catenin prevents AKI-CKD progression.32 In vitro, Wnt ligands induce renal interstitial fibroblasts activation. However, activated fibroblasts readily revert to a quiescent phenotype after Wnts are removed, suggesting that fibroblast activation requires persistent Wnt signaling. Collectively, these findings support the notion that an early and transient activation of Wnt/β-catenin after AKI is reno-protective by facilitating tubular repair and regeneration, while sustained activation of the same signaling promotes AKI-to-CKD progression.
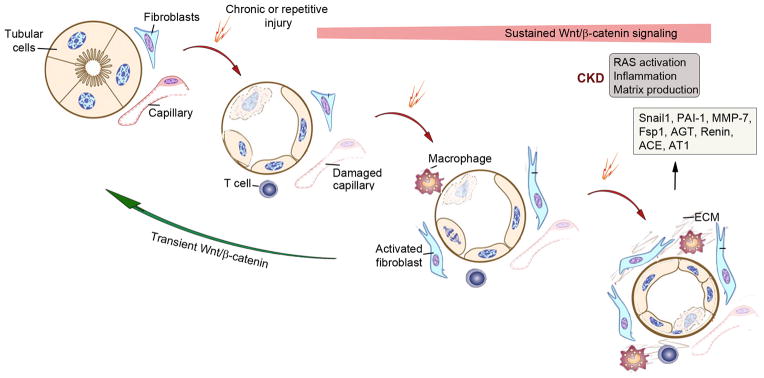
Figure 3. Wnt/β-catenin signaling promotes maladaptive responses after chronic injury.
Following chronic, repetitive or severe injury, kidneys endure maladaptive responses that lead to CKD after the initial attempts of repair have failed. Sustained, but not transient, activation of Wnt/β-catenin signaling is crucial in triggering such maladaptive responses and causing destructive outcomes. Sustained Wnt//b-catenin signaling causes uncontrolled fibroblast activation, renin-angiotensin system (RAS) activation, inflammation and excessive deposition of extracellular matrix (ECM). Hyperactive Wnt/β-catenin also induces Snial1, plasminogen activator inhibitor 1 (PAI-1), MMP-7, fibroblast-specific protein 1 (Fsp1) and multiple components of RAS, all of which are relevant to CKD progression.
As reviewed recently,15 there are several reasons why a sustained activation of Wnt/β-catenin promotes kidney injury and renal fibrosis after injury (Figure 3). In the setting of CKD or irreversible AKI, activation of Wnt/β-catenin is never-ending, because the injury is chronic, repetitive or severe. As such, persistent activation of Wnt/β-catenin would result in sustained and irreversible fibroblast activation, which contributes to the relentless production and deposition of extracellular matrix, leading to development of fibrotic lesions. Furthermore, sustained activation of Wnt/β-catenin in CKD causes an exaggerated induction of several key target genes such as Snail1, plasminogen activator inhibitor-1 (PAI-1), MMP-7, fibroblast-specific protein 1 (Fsp1) and multiple components of the renin-angiotensin system (RAS) (Figure 3), all of which are relevant to the progression of CKD.27, 77, 109, 125 These findings suggest that a sustained activation of Wnt/β-catenin in the settings of either severe AKI or CKD would result in maladaptive responses characterized by persistent fibroblast activation, excessive matrix accumulation and renal fibrosis.
Given the importance of sustained Wnt signaling in driving CKD progression, it is not surprising that inhibition of this signaling is renoprotective and ameliorates kidney injury and fibrosis.14, 15, 34 Over the last several years, tremendous efforts have been made on developing therapeutic strategies to block Wnt/β-catenin signaling for the treatment of a wide variety of CKD in preclinical setting (Table 1). Detailed discussion of the therapeutic approaches and CKD models used is beyond the scope of this article, and interested readers are referred to several recent reviews.13–15, 34 Carefully designed clinical trials are needed to validate the safety and efficacy of these remedies in CKD patients.
Summary
Kidney repair is the ‘Holy Grail’ of medicine in the nephrology field. Harnessing the kidney’s innate and robust regenerative capacity could enable the recovery of renal function and health after injury. During the past several years, substantial evidence has pointed to a crucial role of Wnt/β-catenin signaling, a key developmental pathway that is essential for nephron formation in embryogenesis, in dictating kidney repair or disease after a great diversity of insults. While transient activation of Wnt/β-catenin is reno-protective by promoting adaptive repair and recovery (Figure 2), sustained activation of the same signaling is detrimental and triggers maladaptive responses, leading to onset and progression of CKD (Figure 3). These findings make it possible to understand the mechanisms determining whether kidney structure and function are "reversible" or "irreversible" after injury.
There are many challenges in understanding exactly how different Wnt/β-catenin activation in AKI versus CKD transforms a reparative signaling into pathologic mediator. It also remains to be determined whether various kidney cells such as tubular epithelial cells, fibroblasts, endothelial cells behave differently when they are transiently or persistently exposed to Wnt ligands. Furthermore, we know little about the multifaceted cell-cell communication in the kidney after injury, as well as the complex crosstalk between Wnt and other signaling pathways involved in injury repair and regeneration. Nevertheless, recent studies have inspired multiple novel treatment remedies for nephropathies in preclinical setting (Table 1). Therefore, further elucidation of the mechanisms by which Wnt/β-catenin plays in AKI and CKD could hold promise to offer new therapeutic options for patients suffering from various kidney diseases.
Acknowledgments
This work was supported by the National Institutes of Health Grants DK064005, DK091239 and DK106049, National Science Foundation of China Grants 81130011 and 81521003, and American Heart Association grant FTF 16990086.
Footnotes
Disclosure/Conflict of interest
The authors declare no conflict of interest.
References
- 1.Berger K, Moeller MJ. Mechanisms of epithelial repair and regeneration after acute kidney injury. Semin Nephrol. 2014;34:394–403. doi: 10.1016/j.semnephrol.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida M, Honma S. Regeneration of injured renal tubules. J Pharmacol Sci. 2014;124:117–122. doi: 10.1254/jphs.13r12cp. [DOI] [PubMed] [Google Scholar]
- 3.Pulkkinen K, Murugan S, Vainio S. Wnt signaling in kidney development and disease. Organogenesis. 2008;4:55–59. doi: 10.4161/org.4.2.5849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla LS, Amdur RL, Amodeo S, et al. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79:1361–1369. doi: 10.1038/ki.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 6.Xu W, Ge Y, Liu Z, et al. Glycogen synthase kinase 3beta orchestrates microtubule remodeling in compensatory glomerular adaptation to podocyte depletion. J Biol Chem. 2015;290:1348–1363. doi: 10.1074/jbc.M114.593830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi K, Sasamura H, Nakamura M, et al. KLF4-dependent epigenetic remodeling modulates podocyte phenotypes and attenuates proteinuria. J Clin Invest. 2014;124:2523–2537. doi: 10.1172/JCI69557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden MR, Chowdhury NA, Cooper SA, et al. Proximal tubule microvilli remodeling and albuminuria in the Ren2 transgenic rat. Am J Physiol Renal Physiol. 2007;292:F861–867. doi: 10.1152/ajprenal.00252.2006. [DOI] [PubMed] [Google Scholar]
- 9.Mene P, Polci R, Festuccia F. Mechanisms of repair after kidney injury. J Nephrol. 2003;16:186–195. [PubMed] [Google Scholar]
- 10.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Rev Nephrol. 2015;11:264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Besschetnova TY, Brooks CR, et al. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. doi: 10.1038/nm.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonventre JV. Pathophysiology of AKI: injury and normal and abnormal repair. Contrib Nephrol. 2010;165:9–17. doi: 10.1159/000313738. [DOI] [PubMed] [Google Scholar]
- 13.Maarouf OH, Ikeda Y, Humphreys BD. Wnt signaling in kidney tubulointerstitium during disease. Histol Histopathol. 2015;30:163–171. doi: 10.14670/HH-30.163. [DOI] [PubMed] [Google Scholar]
- 14.Zhou L, Liu Y. Wnt/β-catenin signalling and podocyte dysfunction in proteinuric kidney disease. Nat Rev Nephrol. 2015 doi: 10.1038/nrneph.2015.1088. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan RJ, Zhou D, Zhou L, et al. Wnt/β-catenin signaling and kidney fibrosis. Kidney Int Suppl. 2014;4:84–90. doi: 10.1038/kisup.2014.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding H, Zhou D, Hao S, et al. Sonic hedgehog signaling mediates epithelial-mesenchymal communication and promotes renal fibrosis. J Am Soc Nephrol. 2012;23:801–813. doi: 10.1681/ASN.2011060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasagni L, Ballerini L, Angelotti ML, et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells. 2010;28:1674–1685. doi: 10.1002/stem.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai C, Yang J, Liu Y. Single injection of naked plasmid encoding hepatocyte growth factor prevents cell death and ameliorates acute renal failure in mice. J Am Soc Nephrol. 2002;13:411–422. doi: 10.1681/ASN.V132411. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Sirin Y, Susztak K. The story of Notch and chronic kidney disease. Curr Opin Nephrol Hypertens. 2011;20:56–61. doi: 10.1097/MNH.0b013e3283414c88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peifer M, Polakis P. Wnt signaling in oncogenesis and embryogenesis--a look outside the nucleus. Science. 2000;287:1606–1609. doi: 10.1126/science.287.5458.1606. [DOI] [PubMed] [Google Scholar]
- 21.Lancaster MA, Louie CM, Silhavy JL, et al. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med. 2009;15:1046–1054. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angers S, Moon RT. Proximal events in Wnt signal transduction. Nat Rev Mol Cell Biol. 2009;10:468–477. doi: 10.1038/nrm2717. [DOI] [PubMed] [Google Scholar]
- 23.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 24.Zhou D, Li Y, Lin L, et al. Tubule-specific ablation of endogenous beta-catenin aggravates acute kidney injury in mice. Kidney Int. 2012;82:537–547. doi: 10.1038/ki.2012.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin SL, Li B, Rao S, et al. Macrophage Wnt7b is critical for kidney repair and regeneration. Proc Natl Acad Sci USA. 2010;107:4194–4199. doi: 10.1073/pnas.0912228107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He W, Dai C, Li Y, et al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20:765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai C, Stolz DB, Kiss LP, et al. Wnt/beta-catenin signaling promotes podocyte dysfunction and albuminuria. J Am Soc Nephrol. 2009;20:1997–2008. doi: 10.1681/ASN.2009010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol. 2005;16:2373–2384. doi: 10.1681/ASN.2004110949. [DOI] [PubMed] [Google Scholar]
- 29.He W, Kang YS, Dai C, et al. Blockade of Wnt/β-catenin signaling by paricalcitol ameliorates proteinuria and kidney injury. J Am Soc Nephrol. 2011;22:90–103. doi: 10.1681/ASN.2009121236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hao S, He W, Li Y, et al. Targeted inhibition of β-catenin/CBP signaling ameliorates renal interstitial fibrosis. J Am Soc Nephrol. 2011;22:1642–1653. doi: 10.1681/ASN.2010101079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DiRocco DP, Kobayashi A, Taketo MM, et al. Wnt4/beta-catenin signaling in medullary kidney myofibroblasts. J Am Soc Nephrol. 2013;24:1399–1412. doi: 10.1681/ASN.2012050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xiao L, Zhou D, Tan RJ, et al. Sustained activation of Wnt/β-catenin signaling drives AKI to CKD progression. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2015040449. In revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 34.Kawakami T, Ren S, Duffield JS. Wnt signalling in kidney diseases: dual roles in renal injury and repair. J Pathol. 2013;229:221–231. doi: 10.1002/path.4121. [DOI] [PubMed] [Google Scholar]
- 35.Das S, Yu S, Sakamori R, et al. Wntless in Wnt secretion: molecular, cellular and genetic aspects. Front Biol. 2012;7:587–593. doi: 10.1007/s11515-012-1200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanaka K, Okabayashi K, Asashima M, et al. The evolutionarily conserved porcupine gene family is involved in the processing of the Wnt family. Eur J Biochem. 2000;267:4300–4311. doi: 10.1046/j.1432-1033.2000.01478.x. [DOI] [PubMed] [Google Scholar]
- 38.Coombs GS, Yu J, Canning CA, et al. WLS-dependent secretion of WNT3A requires Ser209 acylation and vacuolar acidification. J Cell Sci. 2010;123:3357–3367. doi: 10.1242/jcs.072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franch-Marro X, Wendler F, Griffith J, et al. In vivo role of lipid adducts on Wingless. J Cell Sci. 2008;121:1587–1592. doi: 10.1242/jcs.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banziger C, Soldini D, Schutt C, et al. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125:509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 41.Bartscherer K, Pelte N, Ingelfinger D, et al. Secretion of Wnt ligands requires Evi, a conserved transmembrane protein. Cell. 2006;125:523–533. doi: 10.1016/j.cell.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 42.Goodman RM, Thombre S, Firtina Z, et al. Sprinter: a novel transmembrane protein required for Wg secretion and signaling. Development. 2006;133:4901–4911. doi: 10.1242/dev.02674. [DOI] [PubMed] [Google Scholar]
- 43.Hausmann G, Banziger C, Basler K. Helping Wingless take flight: how WNT proteins are secreted. Nat Rev Mol Cell Biol. 2007;8:331–336. doi: 10.1038/nrm2141. [DOI] [PubMed] [Google Scholar]
- 44.Ching W, Nusse R. A dedicated Wnt secretion factor. Cell. 2006;125:432–433. doi: 10.1016/j.cell.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 45.Belenkaya TY, Wu Y, Tang X, et al. The retromer complex influences Wnt secretion by recycling wntless from endosomes to the trans-Golgi network. Dev Cell. 2008;14:120–131. doi: 10.1016/j.devcel.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, Belenkaya TY, Lin X. Dual roles of Drosophila glypican Dally-like in Wingless/Wnt signaling and distribution. Methods Enzymol. 2010;480:33–50. doi: 10.1016/S0076-6879(10)80002-3. [DOI] [PubMed] [Google Scholar]
- 47.Gallet A, Staccini-Lavenant L, Therond PP. Cellular trafficking of the glypican Dally-like is required for full-strength Hedgehog signaling and wingless transcytosis. Dev Cell. 2008;14:712–725. doi: 10.1016/j.devcel.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Zhou L, Li Y, Zhou D, et al. Loss of Klotho contributes to kidney injury by derepression of Wnt/beta-catenin signaling. J Am Soc Nephrol. 2013;24:771–785. doi: 10.1681/ASN.2012080865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Langhe SP, Sala FG, Del Moral PM, et al. Dickkopf-1 (DKK1) reveals that fibronectin is a major target of Wnt signaling in branching morphogenesis of the mouse embryonic lung. Dev Biol. 2005;277:316–331. doi: 10.1016/j.ydbio.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 50.Das DS, Wadhwa N, Kunj N, et al. Dickkopf homolog 3 (DKK3) plays a crucial role upstream of WNT/beta-CATENIN signaling for Sertoli cell mediated regulation of spermatogenesis. PLoS One. 2013;8:e63603. doi: 10.1371/journal.pone.0063603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li L, Mao J, Sun L, et al. Second cysteine-rich domain of Dickkopf-2 activates canonical Wnt signaling pathway via LRP-6 independently of dishevelled. J Biol Chem. 2002;277:5977–5981. doi: 10.1074/jbc.M111131200. [DOI] [PubMed] [Google Scholar]
- 52.Schweizer L, Varmus H. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 2003;4:4. doi: 10.1186/1471-2121-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kikuchi K, Niikura Y, Kitagawa K, et al. Dishevelled, a Wnt signalling component, is involved in mitotic progression in cooperation with Plk1. EMBO J. 2010;29:3470–3483. doi: 10.1038/emboj.2010.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stenman JM, Rajagopal J, Carroll TJ, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 55.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao B. Wnt regulation of planar cell polarity (PCP) Curr Top Dev Biol. 2012;101:263–295. doi: 10.1016/B978-0-12-394592-1.00008-9. [DOI] [PubMed] [Google Scholar]
- 57.Seitz K, Dursch V, Harnos J, et al. beta-Arrestin interacts with the beta/gamma subunits of trimeric G-proteins and dishevelled in the Wnt/Ca(2+) pathway in xenopus gastrulation. PLoS One. 2014;9:e87132. doi: 10.1371/journal.pone.0087132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dissanayake SK, Weeraratna AT. Detecting PKC phosphorylation as part of the Wnt/calcium pathway in cutaneous melanoma. Methods Mol Biol. 2008;468:157–172. doi: 10.1007/978-1-59745-249-6_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomez-Cambronero J, Kantonen S. A river runs through it: how autophagy, senescence, and phagocytosis could be linked to phospholipase D by Wnt signaling. J Leukoc Biol. 2014;96:779–784. doi: 10.1189/jlb.2VMR0214-120RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 61.Halt K, Vainio S. Coordination of kidney organogenesis by Wnt signaling. Pediatr Nephrol. 2014;29:737–744. doi: 10.1007/s00467-013-2733-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terada Y, Tanaka H, Okado T, et al. Expression and function of the developmental gene Wnt-4 during experimental acute renal failure in rats. J Am Soc Nephrol. 2003;14:1223–1233. doi: 10.1097/01.asn.0000060577.94532.06. [DOI] [PubMed] [Google Scholar]
- 63.Wang Z, Havasi A, Gall JM, et al. Beta-catenin promotes survival of renal epithelial cells by inhibiting Bax. J Am Soc Nephrol. 2009;20:1919–1928. doi: 10.1681/ASN.2009030253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuncewitch M, Yang WL, Corbo L, et al. WNT Agonist Decreases Tissue Damage and Improves Renal Function After Ischemia-Reperfusion. Shock. 2015;43:268–275. doi: 10.1097/SHK.0000000000000293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu Y. Renal fibrosis: New insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 66.Zeisberg M, Neilson EG. Mechanisms of tubulointerstitial fibrosis. J Am Soc Nephrol. 2010;21:1819–1834. doi: 10.1681/ASN.2010080793. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y. Cellular and molecular mechanisms of renal fibrosis. Nat Rev Nephrol. 2011;7:684–696. doi: 10.1038/nrneph.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou L, Mo H, Miao J, et al. Klotho ameliorates kidney injury and fibrosis by targeting the renin-angiotensin system. Am J Pathol. 2015 doi: 10.1016/j.ajpath.2015.08.004. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou T, He X, Cheng R, et al. Implication of dysregulation of the canonical wingless-type MMTV integration site (WNT) pathway in diabetic nephropathy. Diabetologia. 2012;55:255–266. doi: 10.1007/s00125-011-2314-2. [DOI] [PubMed] [Google Scholar]
- 70.Xiao L, Wang M, Yang S, et al. A glimpse of the pathogenetic mechanisms of Wnt/beta-catenin signaling in diabetic nephropathy. Biomed Res Int. 2013;2013:987064. doi: 10.1155/2013/987064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang L, Xu L, Song Y, et al. Calmodulin-dependent protein kinase II/cAMP response element-binding protein/Wnt/beta-catenin signaling cascade regulates angiotensin II-induced podocyte injury and albuminuria. J Biol Chem. 2013;288:23368–23379. doi: 10.1074/jbc.M113.460394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shkreli M, Sarin KY, Pech MF, et al. Reversible cell-cycle entry in adult kidney podocytes through regulated control of telomerase and Wnt signaling. Nat Med. 2012;18:111–119. doi: 10.1038/nm.2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lancaster MA, Gleeson JG. Cystic kidney disease: the role of Wnt signaling. Trends Mol Med. 2010;16:349–360. doi: 10.1016/j.molmed.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Romaker D, Puetz M, Teschner S, et al. Increased expression of secreted frizzled-related protein 4 in polycystic kidneys. J Am Soc Nephrol. 2009;20:48–56. doi: 10.1681/ASN.2008040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kato H, Gruenwald A, Suh JH, et al. Wnt/-catenin pathway in podocytes integrates cell adhesion, differentiation, and survival. J Biol Chem. 2011;286:26003–26015. doi: 10.1074/jbc.M111.223164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou L, Li Y, He W, et al. Mutual antagonism of Wilms' tumor 1 and β-catenin dictates podocyte health and disease. J Am Soc Nephrol. 2015;26:677–691. doi: 10.1681/ASN.2013101067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou L, Li Y, Hao S, et al. Multiple genes of the renin-angiotensin system are novel targets of Wnt/β-catenin signaling. J Am Soc Nephrol. 2015;26:107–120. doi: 10.1681/ASN.2014010085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cox SN, Sallustio F, Serino G, et al. Altered modulation of WNT-beta-catenin and PI3K/Akt pathways in IgA nephropathy. Kidney Int. 2010;78:396–407. doi: 10.1038/ki.2010.138. [DOI] [PubMed] [Google Scholar]
- 79.Lin CL, Wang JY, Ko JY, et al. Dickkopf-1 promotes hyperglycemia-induced accumulation of mesangial matrix and renal dysfunction. J Am Soc Nephrol. 2010;21:124–135. doi: 10.1681/ASN.2008101059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kavanagh DH, Savage DA, Patterson CC, et al. Association analysis of canonical Wnt signalling genes in diabetic nephropathy. PLoS One. 2011;6:e23904. doi: 10.1371/journal.pone.0023904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kavanagh DH, Savage DA, Patterson CC, et al. Haplotype association analysis of genes within the WNT signalling pathways in diabetic nephropathy. BMC Nephrol. 2013;14:126. doi: 10.1186/1471-2369-14-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang XD, Huang XF, Yan QR, et al. Aberrant activation of the WNT/beta-catenin signaling pathway in lupus nephritis. PLoS One. 2014;9:e84852. doi: 10.1371/journal.pone.0084852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gu Z, Tan W, Feng G, et al. Wnt/beta-catenin signaling mediates the senescence of bone marrow-mesenchymal stem cells from systemic lupus erythematosus patients through the p53/p21 pathway. Mol Cell Biochem. 2014;387:27–37. doi: 10.1007/s11010-013-1866-5. [DOI] [PubMed] [Google Scholar]
- 84.Usui J, Kanemoto K, Tomari S, et al. Glomerular crescents predominantly express cadherin-catenin complex in pauci-immune-type crescentic glomerulonephritis. Histopathology. 2003;43:173–179. doi: 10.1046/j.1365-2559.2003.01660.x. [DOI] [PubMed] [Google Scholar]
- 85.Wuebken A, Schmidt-Ott KM. WNT/beta-catenin signaling in polycystic kidney disease. Kidney Int. 2011;80:135–138. doi: 10.1038/ki.2011.87. [DOI] [PubMed] [Google Scholar]
- 86.Benzing T, Simons M, Walz G. Wnt signaling in polycystic kidney disease. J Am Soc Nephrol. 2007;18:1389–1398. doi: 10.1681/ASN.2006121355. [DOI] [PubMed] [Google Scholar]
- 87.Karner CM, Chirumamilla R, Aoki S, et al. Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet. 2009;41:793–799. doi: 10.1038/ng.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim I, Ding T, Fu Y, et al. Conditional mutation of Pkd2 causes cystogenesis and upregulates beta-catenin. J Am Soc Nephrol. 2009;20:2556–2569. doi: 10.1681/ASN.2009030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alge JL, Arthur JM. Biomarkers of AKI: a review of mechanistic relevance and potential therapeutic implications. Clin J Am Soc Nephrol. 2015;10:147–155. doi: 10.2215/CJN.12191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romanovsky A, Morgan C, Bagshaw SM. Pathophysiology and management of septic acute kidney injury. Pediatr Nephrol. 2014;29:1–12. doi: 10.1007/s00467-013-2427-6. [DOI] [PubMed] [Google Scholar]
- 91.Linkermann A, Chen G, Dong G, et al. Regulated cell death in AKI. J Am Soc Nephrol. 2014;25:2689–2701. doi: 10.1681/ASN.2014030262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Okusa MD, Jaber BL, Doran P, et al. Physiological biomarkers of acute kidney injury: a conceptual approach to improving outcomes. Contrib Nephrol. 2013;182:65–81. doi: 10.1159/000349967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Comprehen Physiol. 2012;2:1303–1353. doi: 10.1002/cphy.c110041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fujigaki Y, Muranaka Y, Sun D, et al. Transient myofibroblast differentiation of interstitial fibroblastic cells relevant to tubular dilatation in uranyl acetate-induced acute renal failure in rats. Virchows Arch. 2005;446:164–176. doi: 10.1007/s00428-004-1155-5. [DOI] [PubMed] [Google Scholar]
- 96.Maarouf OH, Aravamudhan A, Rangarajan D, et al. Paracrine Wnt1 Drives Interstitial Fibrosis without Inflammation by Tubulointerstitial Cross-Talk. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014121188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou D, Tan RJ, Zhou L, et al. Kidney tubular beta-catenin signaling controls interstitial fibroblast fate via epithelial-mesenchymal communication. Sci Rep. 2013;3:1878. doi: 10.1038/srep01878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou D, Li Y, Zhou L, et al. Sonic hedgehog is a novel tubule-derived growth factor for interstitial fibroblasts after kidney injury. J Am Soc Nephrol. 2014;25:2187–2200. doi: 10.1681/ASN.2013080893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou D, Tan RJ, Lin L, et al. Activation of hepatocyte growth factor receptor, c-met, in renal tubules is required for renoprotection after acute kidney injury. Kidney Int. 2013;84:509–520. doi: 10.1038/ki.2013.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Monga SP, Mars WM, Pediaditakis P, et al. Hepatocyte growth factor induces Wnt-independent nuclear translocation of beta-catenin after Met-beta-catenin dissociation in hepatocytes. Cancer Res. 2002;62:2064–2071. [PubMed] [Google Scholar]
- 101.Koraishy FM, Silva C, Mason S, et al. Hepatocyte growth factor (Hgf) stimulates low density lipoprotein receptor-related protein (Lrp) 5/6 phosphorylation and promotes canonical Wnt signaling. J Biol Chem. 2014;289:14341–14350. doi: 10.1074/jbc.M114.563213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Surendran K, Simon TC, Liapis H, et al. Matrilysin (MMP-7) expression in renal tubular damage: association with Wnt4. Kidney Int. 2004;65:2212–2222. doi: 10.1111/j.1523-1755.2004.00641.x. [DOI] [PubMed] [Google Scholar]
- 104.Cordeiro BM, Oliveira ID, Alves MT, et al. SHH, WNT, and NOTCH pathways in medulloblastoma: when cancer stem cells maintain self-renewal and differentiation properties. Childs Nerv Syst. 2014;30:1165–1172. doi: 10.1007/s00381-014-2403-x. [DOI] [PubMed] [Google Scholar]
- 105.Winkler T, Mahoney EJ, Sinner D, et al. Wnt signaling activates Shh signaling in early postnatal intervertebral discs, and re-activates Shh signaling in old discs in the mouse. PLoS One. 2014;9:e98444. doi: 10.1371/journal.pone.0098444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shi Y, Shu B, Yang R, et al. Wnt and Notch signaling pathway involved in wound healing by targeting separately c-Myc and Hes1. Stem Cell Res Ther. 2015;6:120. doi: 10.1186/s13287-015-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stasiulewicz M, Gray S, Mastromina I, et al. A conserved role for Notch in priming the cellular response to Shh through ciliary localisation of the key Shh transducer, Smoothened. Development. 2015 doi: 10.1242/dev.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun DF, Fujigaki Y, Fujimoto T, et al. Possible involvement of myofibroblasts in cellular recovery of uranyl acetate-induced acute renal failure in rats. Am J Pathol. 2000;157:1321–1335. doi: 10.1016/S0002-9440(10)64647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He W, Tan RJ, Li Y, et al. Matrix metalloproteinase-7 as a surrogate marker predicts renal Wnt/β-catenin activity in CKD. J Am Soc Nephrol. 2012;23:294–304. doi: 10.1681/ASN.2011050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and disease. Am J Physiol Renal Physiol. 2012;302:F1351–F1361. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hohenstein B, Kuo MC, Addabbo F, et al. Enhanced progenitor cell recruitment and endothelial repair after selective endothelial injury of the mouse kidney. Am J Physiol Renal Physiol. 2010;298:F1504–1514. doi: 10.1152/ajprenal.00025.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chen X, Wang CC, Song SM, et al. The administration of erythropoietin attenuates kidney injury induced by ischemia/reperfusion with increased activation of Wnt/beta-catenin signaling. J Formosan Med Asso. 2015;114:430–437. doi: 10.1016/j.jfma.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 113.Outtz HH, Tattersall IW, Kofler NM, et al. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118:3436–3439. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li A, Dubey S, Varney ML, et al. IL-8 directly enhanced endothelial cell survival, proliferation, and matrix metalloproteinases production and regulated angiogenesis. J Immunol. 2003;170:3369–3376. doi: 10.4049/jimmunol.170.6.3369. [DOI] [PubMed] [Google Scholar]
- 115.Fan Y, Ye J, Shen F, et al. Interleukin-6 stimulates circulating blood-derived endothelial progenitor cell angiogenesis in vitro. J Cereb Blood Flow Metab. 2008;28:90–98. doi: 10.1038/sj.jcbfm.9600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Masckauchan TN, Shawber CJ, Funahashi Y, et al. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- 117.Linnskog R, Jonsson G, Axelsson L, et al. Interleukin-6 drives melanoma cell motility through p38alpha-MAPK-dependent up-regulation of WNT5A expression. Mol Oncol. 2014;8:1365–1378. doi: 10.1016/j.molonc.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Diez-Roux G, Argilla M, Makarenkova H, et al. Macrophages kill capillary cells in G1 phase of the cell cycle during programmed vascular regression. Development. 1999;126:2141–2147. doi: 10.1242/dev.126.10.2141. [DOI] [PubMed] [Google Scholar]
- 119.Lobov IB, Rao S, Carroll TJ, et al. WNT7b mediates macrophage-induced programmed cell death in patterning of the vasculature. Nature. 2005;437:417–421. doi: 10.1038/nature03928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Peng J, Dong Z. Role changes of beta-catenin in kidney injury and repair. Kidney Int. 2012;82:509–511. doi: 10.1038/ki.2012.155. [DOI] [PubMed] [Google Scholar]
- 121.Belayer LY, Palevsky PM. The link between acute kidney injury and chronic kidney disease. Curr Opin Nephrol Hypertens. 2014;23:149–154. doi: 10.1097/01.mnh.0000441051.36783.f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol. 2013;9:77–85. doi: 10.1038/nrneph.2012.280. [DOI] [PubMed] [Google Scholar]
- 123.Bonventre JV, Basile D, Liu KD, et al. AKI: a path forward. Clin J Am Soc Nephrol. 2013;8:1606–1608. doi: 10.2215/CJN.06040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 125.He W, Tan R, Dai C, et al. Plasminogen activator inhibitor-1 is a transcriptional target of the canonical pathway of Wnt/β-catenin signaling. J Biol Chem. 2010;285:24665–24675. doi: 10.1074/jbc.M109.091256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Satoh M, Nagasu H, Morita Y, et al. Klotho protects against mouse renal fibrosis by inhibiting Wnt signaling. Am J Physiol Renal Physiol. 2012;303:F1641–F1651. doi: 10.1152/ajprenal.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Heikkila E, Juhila J, Lassila M, et al. β-Catenin mediates adriamycin-induced albuminuria and podocyte injury in the adult mouse kidneys. Nephrol Dial Transplant. 2010;25:2437–2446. doi: 10.1093/ndt/gfq076. [DOI] [PubMed] [Google Scholar]
- 128.Wang D, Dai C, Li Y, et al. Canonical Wnt/beta-catenin signaling mediates transforming growth factor-beta1-driven podocyte injury and proteinuria. Kidney Int. 2011;80:1159–1169. doi: 10.1038/ki.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]