Abstract
Drug addiction is characterized by widespread abnormalities in brain function and neurochemistry, including drug-associated effects on concentrations of the excitatory and inhibitory neurotransmitters glutamate and gamma-aminobutyric acid (GABA), respectively. In healthy individuals, these neurotransmitters drive the resting state, a default condition of brain function also disrupted in addiction. Here, our primary goal was to review in vivo magnetic resonance spectroscopy and positron emission tomography studies that examined markers of glutamate and GABA abnormalities in human drug addiction. Addicted individuals tended to show decreases in these markers compared with healthy controls, but findings also varied by individual characteristics (e.g., abstinence length). Interestingly, select corticolimbic brain regions showing glutamatergic and/or GABAergic abnormalities have been similarly implicated in resting-state functional connectivity deficits in drug addiction. Thus, our secondary goals were to provide a brief review of this resting-state literature, and an initial rationale for the hypothesis that abnormalities in glutamatergic and/or GABAergic neurotransmission may underlie resting-state functional deficits in drug addiction. In doing so, we suggest future research directions and possible treatment implications.
Keywords: Drug addiction, glutamate, GABA, neurochemistry, magnetic resonance spectroscopy, positron emission tomography, resting-state, fMRI
1. Introduction
Drug addiction is characterized by dysfunction in corticolimbic networks subserving attentional, emotional, and inhibitory processes (Goldstein and Volkow, 2011). Insights into these systems-level deficits have been primarily advanced through in vivo, non-invasive brain imaging methodologies, such as functional magnetic resonance imaging (fMRI). Increasingly, these methods are being used to examine resting-state functional connectivity (RSFC), a measure of intrinsic activity that provides information on network-level function and its disruption in neuropsychiatric disorders (Rosazza and Minati, 2011), including substance use disorders (Fedota and Stein, 2015; Lu and Stein, 2014; Sutherland et al., 2012) (for an overview of the resting state, see Box 1).
Box 1. Overview of the Resting State.
Resting-state activity is a heterogeneous concept (Cabral et al., 2014; Mantini et al., 2013; Morcom and Fletcher, 2007; Northoff, 2014). The term resting state itself is an operational term that describes the absence of any particular external stimulus, such as a specific tactile or visual (or otherwise) stimulus or cognitive task. Instead, resting state focuses on internally generated mental activity with internal mental contents (e.g., thoughts or imagery) as distinguished from externally generated mental contents (e.g., perceptions). Psychologically, resting-state activity of the brain can manifest in what is described as mind-wandering (Smallwood and Schooler, 2015), random thoughts (Doucet et al., 2012), or self-generated thoughts (Smallwood and Schooler, 2015).
Different terms are used to describe the resting state, largely reflecting different investigative perspectives. In addition to “resting-state activity,” other terms include “spontaneous activity,” “baseline,” or “intrinsic activity” (Deco et al., 2014; Fox et al., 2015; Mantini et al., 2013; Northoff, 2014). The term “spontaneous activity” highlights the idea that resting-state activity is not induced by any particular external stimulus or task but instead is generated naturally (Cabral et al., 2014; Deco et al., 2014; Mantini et al., 2013). Imaging specialists often prefer the term “baseline,” indicating that the resting state (which occurs pre-stimulus, pre-task, or between stimuli/tasks) can serve as a reference condition that is subtracted from a task condition (Morcom and Fletcher, 2007). The term “intrinsic activity” highlights the idea that the resting-state has its origin within the brain itself [i.e., as distinguished from extrinsic activity that originates from stimulus-induced or task-evoked activity (Northoff, 2014)].
Both spatial and temporal measures can assess resting-state activity. Spatially precise modalities such as fMRI use RSFC approaches to target different neural networks that co-activate spontaneously within and between different networks (Cabral et al., 2014; Raichle et al., 2001). This method captures the synchronicity of low-frequency, spontaneous fluctuations in blood-oxygen-level-dependent (BOLD) signals that reflect fluctuations in neuronal activity (Shmuel and Leopold, 2008) between brain regions in the absence of external stimulation (Fox and Raichle, 2007), but that are linked to task-related functioning of brain regions comprising the same circuits (Hampson et al., 2006) and to corresponding behavior (Hampson et al., 2006; Kelly et al., 2008). These synchronous fluctuations are confined to gray matter and can be observed for monosynaptic or polysynaptic anatomical connections (Damoiseaux and Greicius, 2009; Shmuel and Leopold, 2008). Temporally precise modalities, such as EEG or MEG, can measure resting-state activity in electrophysiological or magnetic activity (Cabral et al., 2014; Mantini et al., 2013), which targets neural activity changes in different frequency ranges and their cross-frequency coupling (Engel et al., 2013). Research participants, while undergoing these assessments, are often instructed to close their eyes and not think about anything in particular (Logothetis et al., 2009); this eyes-closed condition is taken as the operational or methodological gold standard to measure resting-state activity.
Although the neurochemical bases of RSFC differences between addicted individuals and healthy controls are presently unclear, evidence from studies of healthy research participants suggests important contributions of the excitatory and inhibitory neurotransmitters glutamate and gamma-aminobutyric acid (GABA), respectively, to the resting state. In particular, glutamate and GABA appear to drive the metabolic and neuronal mechanisms underlying the resting state to sustain the excitation-inhibition balance (Duncan et al., 2014). Indeed, resting-state metabolic activity of the brain is linearly coupled to its neuronal activity (Hyder et al., 2013), largely reflecting the actions of glutamatergic and GABAergic neurons (Hyder et al., 2006; Rothman et al., 2011). Several combined fMRI-magnetic resonance spectroscopy (MRS) studies have provided evidence supporting such relationships. For example, the higher the glutamate concentrations and the lower the GABA concentrations in the posterior cingulate cortex (PCC), the higher was the RSFC between PCC and pregenual anterior cingulate cortex (pACC) (Duncan et al., 2014; Hu et al., 2013; Kapogiannis et al., 2013). GABA concentrations, measured with MRS in the resting-state, were also negatively correlated with task-evoked fMRI activity in the pACC, visual cortex, and somatomotor cortex (Duncan et al., 2014). The relationship between resting-state glutamate level and task-related activity is less clear (Duncan et al., 2014), though it appears that glutamate mainly exerts transregional effects by acting on the long-range axons of pyramidal cells to enable cortico-cortical connections (whereas GABA and GABAergic interneurons mainly exert local effects by acting on pyramidal-cell dendrites to affect the regional processing of inputs). In support of this view are observations that glutamate mediates the transition from resting-state activity in one region (e.g., pACC or PCC) to stimulus-induced and resting-state activity in the same or different regions (Duncan et al., 2011; Duncan et al., 2013; Hu et al., 2013).
The goals of the current article were: primarily to review evidence that human drug addiction is marked by abnormalities in brain glutamate and GABA; and secondarily to use findings of this literature in combination with select RSFC findings to build toward an initial plausible neurochemical framework underlying RSFC deficits in drug addiction, emphasizing important roles for glutamate and GABA. In the primary section, we reviewed in vivo neurochemical imaging studies that tested for glutamatergic and GABAergic abnormalities in drug-addicted individuals as compared with healthy controls. The addictions considered were alcohol, nicotine/tobacco, opiates, cocaine, methamphetamine, and cannabis, each reviewed in turn. Imaging methods included magnetic resonance spectroscopy (MRS), which provides information on neurotransmitter or metabolite concentrations; and positron emission tomography (PET) and single proton emission computed tomography (SPECT). PET and SPECT are nuclear medicine procedures that use tracer kinetic modeling to provide indices of neurotransmitter receptor binding, including: binding potential (BPND), the product of receptor density and affinity; volume of distribution (VT), the ratio at equilibrium of the sum of the concentrations of specifically bound, nonspecifically bound, and free radiotracer to that of parent radioligand in plasma, separated from radiometabolites; and distribution volume ratio (DVR), the volume of distribution normalized to nonspecific binding. Because abnormal activity of metabotropic glutamate receptors predisposes an individual to multiple disorders including addiction, the glutamate system has been assessed by PET with [11C]ABP688, a radioligand for the metabotropic glutamate receptor subtype 5 (mGluR5) (Terbeck et al., 2015). In examining GABA neurotransmission, PET/SPECT studies have concentrated on the GABAA receptor, a Cl−ion channel that produces fast electrical signals and directly controls the efficacy of GABAergic synaptic transmission (Luscher et al., 2011; Xu et al., 2014). These studies have primarily utilized three radiotracers: [11C]flumazenil and [123I]iomazenil, which bind to the benzodiazepine site on the GABAA receptor; and [11C]Ro15 4513, which binds to the GABAA receptor alpha-5 subunit (Ravan et al., 2014). Signaling through GABAA receptors, particularly those containing an alpha-5 subunit, contributes to the reinforcing effects of alcohol in non-human animal studies (Cook et al., 2005; McKay et al., 2004; Stephens et al., 2005).
We stress from the outset that many interpretative difficulties emerge in reviewing this MRS and PET/SPECT literature, including multiple sources of variation between studies that can produce inconsistent findings. One notable difficulty is variation in participant characteristics. Participants often report the use of multiple drugs of abuse in varying amounts and at different times relative to testing, and their self-reports may contain inaccuracies; this difficulty is often accentuated in individuals addicted to illicit drugs, who regularly have more expansive drug use histories. For example, many drug abusers are also cigarette smokers, and smoking independently affects glutamate and other metabolites (below). Although most studies employ safeguards against effects of recent use (e.g., exclusionary urine toxicology), fine-grained information about participants’ secondary drug use histories are not routinely provided; understandably, most studies concentrate on the primary substance of abuse. Other sources of participant variation could include psychiatric comorbidities and their treatments. Methodologically, sources of variation include the use of small sample sizes in some imaging studies, and differing and/or evolving sets of approaches and dependent variables. For example, MRS studies have measured glutamate signals in multiple ways [e.g., glutamate, glutamine, glutamate/glutamine, glutamine/glutamate, and/or glutamate+glutamine (Glx)]. In keeping with the glutamate-glutamine cycle [i.e., the conversion of glutamate to glutamine in astrocytes is catalyzed by glutamine synthetase, and, in turn, glutamine is reconverted into glutamate in neurons by glutaminase (e.g., Walls et al., 2015)], ratios reflecting increased glutamate and/or decreased glutamine both putatively indicate increased brain glutamate levels. Glutamate-related concentrations are sometimes further expressed as a ratio to creatine, often used as an internal reference metabolite (Licata and Renshaw, 2010). Given this heterogeneity of reporting, we attempted throughout to focus on effects from the perspective of glutamate or Glx. Such difficulties can also occur for GABA, although less so. Finally, it is possible that changes in the MRS glutamate/GABA resonance more immediately reflect changes in energy metabolism, and that changes in functional networks measurable by RSFC may stem more directly from such metabolic changes rather than from specific neurotransmission per se. This potential issue provides an important reason for including PET/SPECT studies that measured markers of glutamate and GABA neurotransmission. Taken together, even if these issues preclude full clarity regarding the directionality of effects at this time, our review of this literature serves to provide a macroscopic overview of the field and brings to light some inconsistencies that can be specifically addressed in future work.
In the second section of this article, we reviewed select RSFC studies of addiction that followed from, and were informed by, the primary section (and by neurochemical/RSFC studies in health, above). We did not intend for this section to exhaustive, nor did we describe each constituent study in complete depth and report every available analysis. Rather, we described representative RSFC findings in drug addiction, obtained using seed-based and data-driven methodologies, which examined connectivity differences between addicted individuals and healthy controls in select corticolimbic brain regions [e.g., ACC, medial prefrontal cortex (PFC), striatum, and insula]. We marshaled this RSFC evidence, as well as evidence from the primary MRS/PET section, to support a new neurochemical framework in which we hypothesize that glutamatergic and/or GABAergic deficits may underlie RSFC abnormalities in drug addiction. RSFC, then, may serve as an intermediate phenotype bridging neurochemical abnormalities and addiction-relevant behaviors (e.g., craving, drug-seeking, or engagement with treatment). We anticipate that this perspective can spearhead future hypothesis-driven research on this topic. In addition, an understanding of the neurochemical bases of the resting-state in drug addiction can also help advance the development of new therapeutics that target the relevant neurotransmitters and/or RSFC deficits.
2. Glutamatergic Alterations in Drug Addiction (Table 1)
Table 1.
MRS and PET glutamate studies in drug addiction.
| Reference | Sample | Mean Age (SD) |
Sex | Mean Abst Duration (SD) |
Medication and/or Tx |
Other Drug Use |
Psychiatric or Medical |
Regions of Interest |
Imaging Method |
Primary Glutamate Imaging Outcome (vs Controls) |
Clinical or Neuropsych Correlates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol | |||||||||||
| (Bagga et al., 2014) | 35 AUD 35 HC |
36.5 (5) 35.2 (3.7) |
35M/0F 35M/0F |
17.5 (4.3) d | NR | NR | NR | R Occip | MRS | ↓ Glu-Gln/Cr | AUD: ↓ Glu-Gln/Cr corr ↓ visual-motor functioning |
| (Bauer et al., 2013) | 29 AUD 31 HC |
29M/0F 31M/0F |
40.2 (7.5) 36.6 (10.1) |
≤ 10 d | Inpatient Detox; Benzos |
Nic | NR | pACC, VS | MRS | ↑ VS Glu | AUD: ↑ VS & ACC Glx corr ↑ craving |
| (Ende et al., 2013) | 21 AUD 9 HC |
13M/8F 8M/1F |
48.5 (10.7) 40.9 (4.5) |
Unspec | NR | Nic | NR | dACC into adjacent WM) |
MRS | NS Glu (modulation by loss of control) |
AUD: ↑ Glu corr ↓Alc severity |
| (Hermann et al., 2012b) | 47 AUD 57 HC |
46.3 (1.5) 45.1 (1.5) |
38M/9F 44M/13F |
1 d, 14 d (within) |
Inpatient Detox; clonidine, Benzos |
Nic | NR | ACC | MRS | 1 d: ↑Glu | AUD, 1 d: ↑ Glu corr ↑ breath Alc & 3-mo severity |
| (Jalan et al., 2000) | 6 AUD 11 HC |
61 55 |
4M/2F 8M/3F |
≥ 6 mo | NR | NR | Cirrhosis | Cereb, Cerebel, BG |
MRS | ↑ Glx/Cr | NA |
| Lee et al., 2007) | 13 AUD 18 HC |
33.8 (5.8) 32.9 (0.9) |
13M/0F 18M/0F |
15.5 d (min 14 d) |
Thiamine | Nic | NR | ACC, Ins | MRS | ↑ ACC Glu/Cr | ↑ ACC Glu/Cr corr ↑ memory & recent Alc use |
| (Miese et al., 2006) | 26 AUD 18 HC |
61.1 (12.4) 54.3 (12.7) |
18M/8M 14M/5M |
During study (Unspec) |
NR | NR | Cirrhosis | BG, Occip |
MRS | ↑ Glx/Cr | NA |
| (Mon et al., 2012) | 44 AUD 16 HC |
53.9 (8.8) 49.0 (10.1) |
39M/5F 14M/2F |
TP1: 9 (4) TP2: 34 (7) |
VA Medical Center, Other Tx; Benzos |
Nic | Hep C, type 2 diabetes, ↑ BP, depress |
pACC, DLPFC, Occip |
MRS | ↓ ACC Glu | ↑ ACC Glu corr ↑ Abst |
| (Nery et al., 2010) | 22 AUD 54 HC |
39.1 (11.4) 38.7 (12.4) |
6M/16F 14M/40F |
6.8 (6.5) y | Lithium, AntiDepress, antiPsy, Benzos |
Mari, Coc, Stim, opioids |
Bipolar, anxiety | DLPFC | MRS | NS Glu, Glx | NS |
| (Pennington et al., 2014) | 10 AUD 28 PTSD 20 HC |
51.9 (13.9) 35.4 (10.5) 36.3 (12.4) |
10M/0F 28M/0F 20M/0F |
NS | VA Medical Center |
NR | AUD also had PTSD |
ACC Occip Temp |
MRS | ↓Glu | AUD: ↑ Glu corr ↑ divided attention |
| (Seitz et al., 1999) | 11 AUD 10 HC |
44 (10) 38 (12) |
8M/3F 9M/3F |
3–6 d | Inpatient Detox |
NR | Frontal & cerebellar atrophy |
Vermis | MRS | NS Glx/Cr | NA |
| (Thoma et al., 2011) | 7 AUD 6 AUD Abst 17 HC |
35.5 (8.2) 35.4 (5.7) 32.3 (7.9) |
5M/2F 5M/1F 9M/8F |
≥48 h | University Tx | Nic, sedatives, Marij, opioids, Coc, Halluc |
Abnormal liver function, bipolar, Depress, Psy, anxiety, OCD, PTSD, phobias |
dACC | MRS | ↓Glu (AD Abst only), ↑ Gln (AD only) |
AUD: ↓Glu corr ↑drinking consequences |
| (Yeo et al., 2013) | 213 AUD 66 HC |
31.0 (9.0) 29.5 (8.3) |
153M/60F 37M/29F |
≥24 h | Unspec Tx (n=97/213) |
Nic, Marij | Depres | dACC | MRS | NS Glu-Gln | ↑ Glu-Gln corr ↑ years drinking & ↑ smoking severity |
| Nicotine/Smoking | |||||||||||
| (Akkus et al., 2013) | 14 SM 14 SM Abst 14 HC |
36.1 (10.2) 37.7 (10.1) 36.8 (9.6) |
6M/8F 8M/6F 6M/8F |
None 25.0 (19.4) |
NR | NR | Depress, anorexia |
6 Cereb, 3 Limbic, STR, Thal, 1 brainste m |
PET [11C]ABP6 88 |
↓ DVR Amyg, Striat (caudate), PFC, Temp, PCC |
Sh Abst: ↓ BPND brainstem corr ↑ cigs per day |
| (Akkus et al., 2015) | 14 SM 14 Sh Abst 14 Lg Abst 14 HC |
36.1 (10.2) 37.7 (10.1) 37.8 (10.1) 36.8 (9.6) |
6M/8F 8M/6F 8M/6F 6M/8F |
None 25.0 (19.4) 473.6 (336.1) |
NR | NR | Depress, anorexia |
6 Cereb, 3 Limbic, STR, Thal |
PET [11C]ABP6 88 |
↓ BPND ACC, Temp, MedOFC (SM, Sh Abst only) |
NS |
| (Durazzo et al., 2015) | 35 SM 30 HC |
48.6 (10.1) 49.1 (12.0) |
26M/4F 31M/4F |
Unspec | NR | Alc | NR | pACC, DLPFC |
MRS | ↓ Glu (acceleration with ↑ age) |
All: ↑ DLPFC Glu corr ↑ NP function |
| (Gallinat and Schubert, 2007) | 13 SM 9 SM Abst 16 HC |
35.4 (10.0) 41.5 (9.7) 32.8 (9.6) |
5M/8F 5M/4F 8M/8F |
None 16.5 (2.9) y |
NR | Alc | NR | ACC, Hipp |
MRS | NS Glu | NS |
| (Gutzeit et al., 2013) | 14 SM 10 HC |
50.4 (12.6) 36.6 (9.2) |
14M/0F 10M/0F |
None, 24 h (within) |
NR | NR | NR | Ins | MRS | ↑ Gln (withdrawal) |
NA |
| (Mennecke et al., 2014) | 12 SM 12 HC |
25.7 (3.1) 26.2 (2.7) |
6M/6W 6M/6W |
None, 3 d (within) |
NR | NR | NR | dACC, Hipp |
MRS | ↑ left dACC Glx/Cr |
NA |
| (O’Neill et al., 2014) | 18 SM 16 HC |
35.4 (2.1) 33.2 (2.6) |
11M/7F 8M/8F |
Unspec | NR | Marij, Alc, anorexia |
NR | Thal | MRS | NS Glu | SM: ↓ Glu & Glx corr ↑ Cigarettes/day and pack-years |
| Opiates | |||||||||||
| (Greenwald et al., 2015) | 7 HD 5 HC |
42.4 (7.5) 45.2 (7.2) |
6M/1F 3M/2F |
Unspec | Methadone (high vs. low, within) |
Nic | NR | pACC, Thal |
MRS | NS Glu (versus either methadone dose) |
NS |
| (Hermann et al., 2012a) | 17 HD 20 HC |
36.9 (9.7) 36.4 (10.3) |
11M/6F 13M/9F |
Unspec | Opiate maintenance |
Marij, Alc, Benzos, Amph, MDMA |
NR | dACC | MRS | NS Glu or Glx (moderation by age) |
HD: ↑ Glu & Glx corr ↑ #withdrawals |
| (Verdejo-Garcia et al., 2013) | 24 HD 24 HC |
29.8 (6.9) 29.6 (6.5) |
13M/11F 13M/11F |
≥24 h | Methadone (n=10), Bup (n=14) |
Marij, benzos, heroin |
NR | dACC | MRS | ↓ Glx (hemisphere x medication effects) |
HD: ↑ Glx corr ↑ methadone dose |
| (Yücel et al., 2007) | 24 HD 24 HC |
29.8 (6.9) 29.6 (6.5) |
13M/11F 13M/11F |
≥24 h | Methadone (n=10), Bup (n=14) |
Marij, Benzos, heroin |
NR | dACC | MRS | ↓ Glx (across medication) |
NS |
| Cocaine | |||||||||||
| (Chang et al., 1997) | 11 CD 16 HC |
32.0 (7.9) 32.6 (9.1) |
26M/0F 26M/0F |
66.4 (26.2) mo | NR | Alc, Marij, Amph, PCP |
NR | Mid Occip |
MRS | NS Glx/Cr | CD: ↓ Glx corr ↓ Coc use yrs |
| (Hulka et al., 2014a) | 18 CD 18 HC |
36.2 (7.6) 35.8 (8.3) |
18M/0F 18M/0F |
7.6 (6.8) d | NR | Nic, Alc, Marij, MDMA |
Depress | pACC, R DLPFC |
MRS | NS Glu/Cr or Gln/Cr |
CD: ↓ pACC Gln/Cr corr ↑ Coc frequency |
| (Martinez et al., 2014) | 15 CD 14 HC |
43 (3) 41 (4) |
14M/1F 13M/1F |
10–14 d | University Inpatient |
Nic | NR | STR | MRS; PET [11C]ABP6 88 |
↓ BPND VS, putamen; NS Glx/H2O |
NS |
| (Milella et al., 2014) | 9 CD 9 HC |
37.7 (8.7) 37.2 (8.8) |
7M/2F 6M/3F |
5.7 (4.1) d | NR | Nic, Alc, Marij |
NR | 3 STR, 4 PFC, 4 Limbic |
PET [11C]ABP6 88 |
↓ BPND striatum, Amyg, Ins |
↓ BPND striatum, Amyg, Ins |
| (Yang et al., 2009) | 14 CD 14 HC |
37 (4.9) 34 (9.0) |
10M/4F 7M/7F |
44 (21) h | NR | Nic | NR | pACC | MRS | ↓Glu/Cr | ↓ Glu/Cr corr ↓ Coc use yrs |
| Methamphetamine | |||||||||||
| (Crocker et al., 2014) | 29 MA 29 Psy 45 HC |
25.6 (2.6) 21.9 (3.4) 22.4 (3.3) |
18M/11F 23M/6F 36M/9F |
370 d (median) |
Unspec Tx; Psy: Antipsychotic |
NR | NR | ACC / MedPFC |
MRS | ↓ Glu | NS |
| (Ernst and Chang, 2008) | 25 MA 28 HC |
31.8 (7.4) 32.6 (8.8) |
11M/14W 14M/14W |
2.1 (3.0) mo | Unspec Tx | Nic | NR | PFC, BG | MRS | ↓ PFC Glx (≤ 1 mo Abst) |
MA: ↓ PFC Glx corr ↓ Abst & ↑ craving |
| (Howells et al., 2014) | 16 MA 10 MA- Psy 19 HC |
24.0 (3.7) 24.0 (1.3) 25.0 (5.7) |
13M/3F 10M/1F 16M/3F |
56 (60) d 60 (53) d |
Unspec Rehab, Hospital; MA- Psy: Haloperidol |
Nic, Alc, Marij, Quaalude |
NR | Bilat ACC, DLPFC |
MRS | NS: Glu or Glx | NA |
| (O’Neill et al., 2015) | 44 MA 24 HC |
33.0 (9.4) 33.0 (7.4) |
25M/19F 13M/11F |
7–10 d | University Inpatient |
Nic, Marij, Alc |
NR | PCC, Precun, Bilat PFC |
MRS | ↓ Glx PCC, Precun, R PFC |
MA: ↓ Glx PCC corr ↑ use yrs |
| (Sailasuta et al., 2010) | 18 MA 22 HC |
35.0 (9.3) 31.9 (9/7) |
11M/7F 12M/10F |
3–8 wk (7), >20 wk (11) |
Rehab or “halfway house” |
Coc, Marij, Alc |
NR | Med Occip |
MRS | NS Glu | NA |
| Cannabis | |||||||||||
| (Muetzel et al., 2013) | 27 MJ 26 HC |
19.5 (0.6) 19.3 (3.1) |
16M/11F 10M/16F |
≥12 h | NR | Alc | NR | Dorsal STR |
MRS | ↓ Glx (F only) | NA |
| (Chang et al., 2006) | 24 MJ 30 HC |
36.3 (2.3) 42.2 (2.2) |
20M/4F 24M/6F |
51.8 (16.7) mo | NR | Nic, Alc, Coc | NR | BG, Thal | MRS | ↓ Glu BG | NS |
| (Prescot et al., 2011) | 17 MJ 17 HC |
17.8 (1.1) 16.2 (2.1) |
15M/2F 8M/9F |
Unspec | AntiD | Alc | Depress | ACC | MRS | ↓ Glu | NS |
| Multiple Substances | |||||||||||
| (Hulka et al., 2014b) | 18 CD 18 HC |
36.2 (7.6) 36.2 (8.4) |
18M/0W 18M/0W |
Unspec | Unspec Tx centers & hospitals |
Nic, Alc, Marij, Amph, MDMA |
Depress | 5 PFC, 2 STR, 5 limbic |
PET [11C]ABP6 88 |
↓Vnorm (all regions) in smokers (no CD effect) |
↓ Vnorm corr more recent smoking |
| (Mason et al., 2006) | 12 AD 8 HC |
39 (8.2) 39 (9.0) |
12M/0F 8M/0F |
5.0 (4.0) d | Inpatient Tx | Marij, Nic, Coc |
NR | Occip | MRS | NS Glx; AD: ↑ Glx SM versus non-SM |
NA |
Abbreviations: (p or d)ACC = (pregenual or dorsal) anterior cingulate cortex, Abst = abstinence, Alc = alcohol, AntiD = antidepressant medication, Approx = approximated values, AUD = alcohol use disorder, Amyg = amygdala, Amph = amphetamine, BG = basal ganglia, Benzos = benzodiazepines, BP = blood pressure, Bup = buprenorphine, Cereb = cerebral cortex, Coc = cocaine, corr = was correlated with, Cr = creatine, Depress = depression, Detox = detoxification program, F= females, GM = gray matter, Glu = glutamate, Hall = hallucinogen use disorder, Hipp = hippocampus, Ins = insula, Lg = long, M = males, MA = methamphetamine use disorder, MJ = marijuana, MDMA = ecstasy, Med = medial, Mid = middle, MJ = marijuana use disorder, mo = month, MRS = magnetic resonance spectroscopy, NA = not applicable, Nic = nicotine, NR = none reported, NS = not significant, Occip = occipital lobe, OCD = obsessive compulsive disorder, OFC = orbitofrontal cortex, ParHipp = parahippocampal gyrus, Pariet = parietal cortex, PET = positron emission tomography, PFC = prefrontal cortex, Pos = posterior, PS = polysubstance users, Psy = psychosis, PTSD, posttraumatic stress disorder, R =right, Sh = short, SM = smoker, Stim = stimulants, STR = striatum, Subco = subcortical regions, Temp = temporal cortex, Thal = thalamus, TP = time point, Tx = treatment, Unspec = unspecified, VS = ventral striatum, wk = week
2.1. Alcohol
2.1.1. MRS
Compared with controls, alcohol-addicted individuals had lower glutamate levels in the occipital cortex (Bagga et al., 2014) and ACC (Pennington et al., 2014) [at least among individuals who had achieved remission (Thoma et al., 2011)]. These lower occipital or ACC (into adjacent white matter) glutamate levels were correlated with greater drinking severity [e.g., more alcohol-related consequences (Thoma et al., 2011), loss of control over drinking (Ende et al., 2013)] or poorer neuropsychological functioning [e.g., more impaired visual-motor or attentional functioning (Bagga et al., 2014; Pennington et al., 2014)]. In contrast, other studies reported no differences between alcohol-addicted individuals and controls in glutamate or Glx in the cerebellar vermis (Seitz et al., 1999), dorsolateral PFC (DLPFC) (Nery et al., 2010), or dACC (Yeo et al., 2013). For the latter, somewhat surprisingly, higher Glx was correlated with more years of drinking (Yeo et al., 2013). Finally, earlier studies reported higher Glx in alcohol-addicted individuals compared with controls (e.g., in basal ganglia) (Jalan et al., 2000; Miese et al., 2006). However, it is important to note that in these latter studies, the samples of alcohol-addicted individuals were older than in most studies and were not medically healthy (presence of cirrhosis). Moreover, these latter studies examined Glx, which includes both glutamate and glutamine, and this difference also might have contributed to inconsistencies between studies.
Other MRS studies have examined the effects of short-term alcohol abstinence on glutamate concentrations. Multiple studies reported that, compared with healthy controls, short-term abstinent alcohol-addicted individuals exhibited higher glutamate levels in the ACC (Hermann et al., 2012b; Lee et al., 2007) and ventral striatum (Bauer et al., 2013). In these studies, higher ACC and/or striatal glutamate levels were also correlated with more alcohol craving (Bauer et al., 2013) and more recent (e.g., 1 month, 3 month) alcohol consumption (Hermann et al., 2012b; Lee et al., 2007), and with better memory retention (Lee et al., 2007). However, this view is complicated by other studies reporting that pACC concentrations of glutamate, which were initially lower in alcohol-addicted individuals (who were abstinent for approximately 1-day or 1-week) than healthy controls, returned to control levels over 2–5 weeks of abstinence (Hermann et al., 2012b; Mon et al., 2012). Higher glutamate levels were also correlated with more days of current abstinence (Mon et al., 2012).
2.1.2. PET/SPECT
No studies were found.
2.2 Smoking
2.2.1. MRS
An interesting recent study indicated that smokers had lower glutamate levels in the pACC and DLPFC than nonsmokers, and that such differences were accentuated with increasing age (Durazzo et al., 2015). Moreover, in the DLPFC, higher glutamate levels were correlated with better neuropsychological functioning, measured by a battery of tasks (Durazzo et al., 2015). Other studies did not report differences in glutamate levels between smokers and ex-smokers in either the hippocampus or ACC (Gallinat and Schubert, 2007), or in the thalamus (O’Neill et al., 2014). In the latter study, however, glutamate levels measured in smokers were negatively correlated with the frequency and duration of smoking (O’Neill et al., 2014), further supporting the idea that lower glutamate levels are associated with poorer outcomes (i.e., decreased functioning, increased use).
In examining effects of withdrawal, Glx levels were higher in the left dACC in smokers than in nonsmokers, but this effect did not emerge when the smokers were in withdrawal (Mennecke et al., 2014). Unlike Glx, which was lowered during withdrawal, glutamine in the insula was higher during withdrawal (Gutzeit et al., 2013).
2.2.2. PET/SPECT
In studies that used [11C]ABP688 as a radioligand for mGluR5, both DVR and BPND in multiple limbic and PFC brain regions were lowest in current smokers, followed respectively by short-term ex-smokers, long-term ex-smokers, and controls (highest) (Akkus et al., 2013; Akkus et al., 2015).
2.3. Opiates
2.3.1. MRS
Compared with controls, opiate-addicted individuals receiving methadone or buprenorphine maintenance therapy had lower dACC glutamate concentrations than controls (Verdejo-Garcia et al., 2013; Yücel et al., 2007) [but see (Greenwald et al., 2015)]. It is unclear to what extent this finding reflected a pre-existing condition or effects of the ongoing treatment. Concentrations of dACC glutamate were also positively correlated with the number of previous withdrawals (Hermann et al., 2012a).
2.3.2. PET/SPECT
No studies were found.
2.4. Cocaine
2.4.1. MRS
Compared with controls, chronic cocaine users had lower pACC glutamate levels (Yang et al., 2009). Other studies did not report MRS-measured group differences in glutamate in occipital cortex, pACC, DLPFC, or striatum (Chang et al., 1997; Hulka et al., 2014a; Martinez et al., 2014), although lower pACC glutamate measures were correlated with higher frequency of cocaine use (Hulka et al., 2014a) but also (again somewhat surprisingly) fewer years of cocaine use (Chang et al., 1997; Yang et al., 2009).
2.4.2. PET/SPECT
Compared with controls, cocaine-addicted individuals showed lower striatal BPND for [11C]ABP688, consistent with lower mGluR5 glutamate receptor availability (Martinez et al., 2014; Milella et al., 2014). Other regions showing lower BPND for [11C]ABP688 in cocaine-addicted individuals compared with controls included the amygdala and insula, and lower BPND in these regions was correlated with more days of abstinence (though still within a withdrawal period) (Milella et al., 2014).
2.5. Methamphetamine
2.5.1. MRS
Methamphetamine-addicted individuals had lower glutamate concentrations in the pACC/dorsomedial PFC compared with healthy controls and even with non-stimulant-using, psychotic patients (Crocker et al., 2014). Similar results were observed for Glx levels in the PCC, precuneus, and right inferior frontal cortex (O’Neill et al., 2015), but not glutamate levels in the occipital cortex (Sailasuta et al., 2010). Moreover, lower PCC Glx levels were correlated with more years of methamphetamine abuse (O’Neill et al., 2015). Glx concentrations in the frontal cortex were also reduced in addicted individuals with ≤ 1 month of abstinence (but not after longer abstinence), and reduced Glx levels were correlated with fewer days of abstinence and higher craving (Ernst and Chang, 2008). In another study, however, neither glutamate nor Glx levels in the dACC or DLPFC differed between methamphetamine-using participants and controls (Howells et al., 2014).
2.5.2. PET/SPECT
No studies were found.
2.6. Cannabis
2.6.1. MRS
Compared with controls, chronic marijuana users had lower glutamate levels in the ACC (Prescot et al., 2011) and in basal ganglia regions (Chang et al., 2006; Muetzel et al., 2013), although one study reported that the basal ganglia effect was specific to women (Muetzel et al., 2013).
2.6.2. PET/ SPECT
No studies were found
2.7. Multiple Substances
2.7.1. MRS
Although Glx levels in the occipital cortex did not differ between alcohol-addicted individuals and healthy controls, Glx levels were higher in alcohol-addicted smokers than in alcohol-addicted nonsmokers (Mason et al., 2006).
2.7.2. PET/SPECT
A study compared cocaine-addicted individuals and healthy controls using PET with [11C]ABP688. Results revealed no effects of cocaine use disorder, but robust effects of smoking status were observed. In particular, compared with nonsmokers, smokers (especially those who had smoked recently) had lower Vnorm (defined as BPND + 1) in multiple cortical (e.g., ACC, medial PFC, DLPFC) and subcortical brain regions (e.g., striatum, amygdala, hippocampus) (Hulka et al., 2014b).
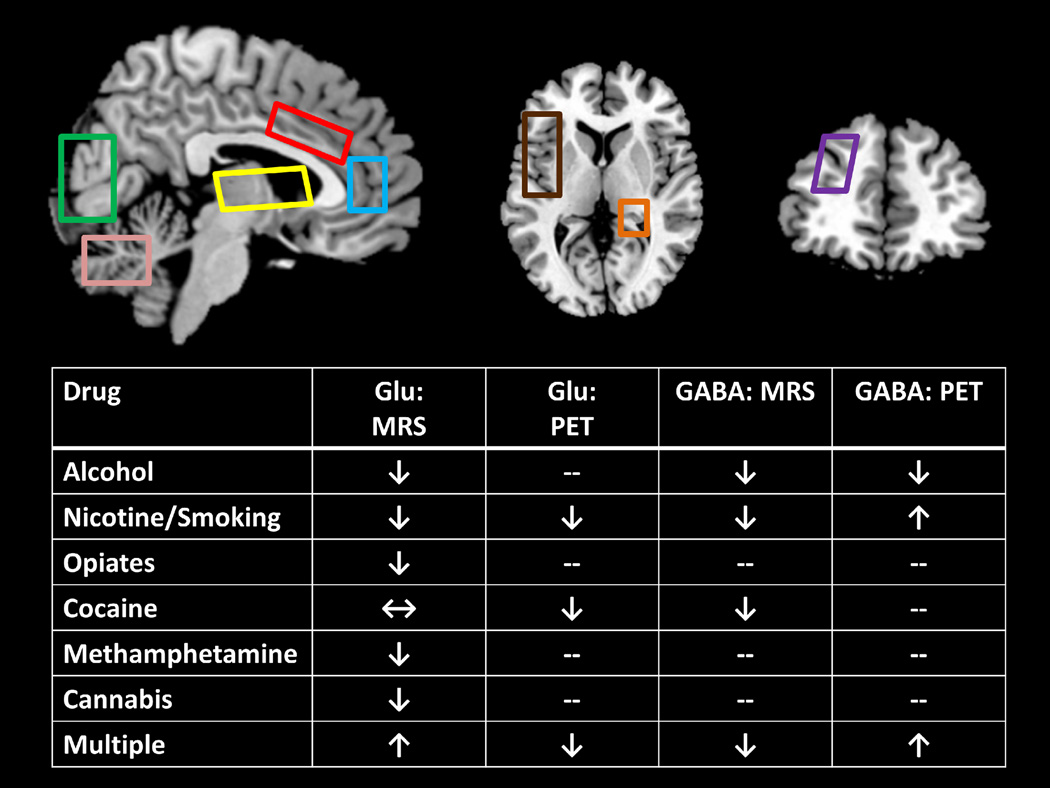
2.8. Summary (see also Figure 1)
Figure 1.
Overview of the MRS and PET/SPECT studies. Figure displays regions of interest common to many studies (blue: rostral/pregenual anterior cingulate cortex, sometimes extending into medial prefrontal cortex; red: dorsal anterior cingulate cortex; yellow: basal ganglia/thalamus; green: occipital cortex; pink: cerebellum; brown: insula; orange: hippocampus; purple: dorsolateral prefrontal cortex) and a table showing the general direction of effects. Arrows in the table reflect the preponderance of evidence while also prioritizing studies with larger and/or more homogeneous samples and not considering acute clinical features such as short-term withdrawal (which can be associated with opposite effects). ↓ = lower in addiction; ↑ higher in addiction; ↔ = nonsignificant differences between groups; -- no studies found.
Individuals who abused addictive substances spanning alcohol, nicotine/tobacco, opiates, methamphetamine, and cannabis showed lower brain glutamate concentrations and/or mGluR5 receptor availability than corresponding measurements in controls. Differences most consistently emerged in multiple subregions of the ACC and in basal ganglia regions (e.g., striatum). A few exceptions to the general pattern of lower glutamate in addiction deserve mention: (A) studies of cocaine users yielded some equivocal findings, although the findings were generally more consistent with the hypothesis of lower glutamate levels than higher glutamate levels; (B) more research is needed on cannabis, especially while incorporating PET, before firm conclusions can be drawn; and (C) when examining the use of multiple substances, MRS and PET studies yielded results that differed in direction, although each modality only had one relevant study and included different substances (alcohol versus cocaine, though both were examined in conjunction with cigarette smoking).
Although with multiple exceptions, markers of reduced glutamatergic neurotransmission were often correlated with greater drug-related impairment (e.g., higher craving and substance-related consequences, reduced neuropsychological function). A notable exception to this pattern was seen in some studies that showed positive correlation of glutamate levels with years of use. These findings suggest that the extent of dysregulation may vary with length of abuse (O’Neill et al., 2015). Interestingly, acute withdrawal (and possibly the number of withdrawals) instead tended to correlate with higher brain glutamate associated with the use of some substances (especially alcohol and opiates, which perhaps not coincidentally produce the most severe withdrawal syndromes). More work is needed to corroborate this withdrawal effect, however, as it was not consistently observed across all studies of early abstinent individuals even within the same substance (e.g., alcohol). Nevertheless, this pattern of effects squares with findings showing that glutamate neurotransmission may be accentuated during acute withdrawal (Burnett et al., 2015).
3. GABAergic Changes in Drug Addiction (Table 2)
Table 2.
MRS and PET GABA studies in drug addiction.
| Reference | Sample | Mean Age (SD) |
Sex | Mean Abst Duration (SD) |
Medication and/or Tx |
Other Drug Use |
Psychiatric or Medical |
Regions of Interest |
Imaging Method |
Primary Glutamate Imaging Outcome (vs. Controls) |
Clinical or Neuropsych Correlates |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alcohol | |||||||||||
| (Abi-Dargham et al., 1998) | 11 AUD 11 HC |
44 (8) 43 (10) |
11M/0F 11M/0F |
98 (46) d | VA Medical Center (Unspec Tx) |
NR | NR | 5 Cereb, 4 Subco, Cerebel |
SPECT [123I]iomazenil |
↓VT PFC ACC, Cerebel |
NS |
| (Behar et al., 1999) | 5 AUD 10 HC |
46 (11) 35 (7) |
Unspec | 34 (20) d | VA Medical Center |
Nic | NR | Occip | MRS | ↓ GABA (+homocarnosine ) |
↓ GABA corr ↑ delayed memory |
| (Gilman et al., 1996) | 17 AUD 14 HC |
52 (Approx) 46 (13) |
17M/0F 14M/0F |
≥17 d | VA Medical Center (Unspec Tx) |
NR | Cerebel degeneration (n=8) |
pACC, Cerebel, Pariet, Cereb |
PET [11C]flumazeni I |
↓ VT pACC | NA |
| (Jalan et al., 2000) | 6 AUD 11 HC |
61 55 |
4M/2F 8M/3F |
≥ 6 mo | NR | NR | Cirrhosis | Cereb, Cerebel, BG |
PET [11C]flumazeni I |
↑ VT | NA |
| (Lingford-Hughes et al., 1998) | 12 AUD 14 HC |
43.2 (10.9) 39.9 (8.5) |
12M/0F 14M/0F |
22.5 (50.2) | Unspec SSRI | MJ, MDMA | Depress | Whole brain |
PET [123I]iomazenil |
↑ VT MPFC Pariet |
↓ VT corr ↑ Alc severity |
| (Lingford-Hughes et al., 2000) | 9 AUD 13 HC |
42.9 (7.6) 38.1 (9.0) |
0M/9F 0M/13F |
30.4 (53.2) mo | fluoxetine, venlafaxine, amitriptyline |
Coc, Amph | Depress, anxiety, bulimia |
Whole brain |
PET [123I]iomazenil |
NS VT | NA |
| (Lingford-Hughes et al., 2005) | 11 AUD 10 HC |
44.5 (1.8) 46.2 (2.6) |
11M/0F 10M/0F |
7.3 (5) mo | midazolam challenge; paroxetine or clomipramine |
MJ | Depress | ACC, OFC, Occip, Thai, Cerebel |
PET [11C]flumazeni I |
NS VT | NA |
| (Lingford-Hughes et al., 2012) | 8 AUD 11 HC |
44 (7) 43 (7) |
8M/0F 11M/0F |
33.4 (44.7) mo |
Fluoxetine | Nic, MJ | Depress | STR, ACC, Hipp, Ins, OFC |
PET [11C]Ro15 4513 |
↓ VT Striat, Hipp |
AD: ↑ VT Hipp & ACC, ↑ delayed recall |
| (Litton et al., 1993) | 5 AUD 5 HC |
37 25 |
5M/0F 5M/0F |
17 d | Detox, oxazepam |
Nic | NR | DLPFC/ MedPFC, Temp, Occip, Cerebel |
PET [11C]flumazeni I |
NS Bmax | NA |
| (Pennington et al., 2014) | 10 AUD 28 PTSD 20 HC |
51.9 (13.9) 35.4 (10.5) 36.3 (12.4) |
10M/0F 28M/0F 20M/0F |
NS | VA Medical Center (Unspec Tx) |
NR | AUD also had PTSD |
ACC Occip Temp |
MRS | NS GABA | AD: ↑ GABA corr ↑ verbal memory |
| Nicotine/Smoking | |||||||||||
| (Stokes et al., 2013) | 8 SM 12 HC |
48.4 (6.8) 46.4 (7.9) |
8M/0F 12M/0F |
NS | NR | MJ, Alc, Stim, MDMA |
NR | STR, Ins, ACC, pACC, Amyg, Hipp, ParHipp |
PET [11C]Ro15 4513 |
↑ VT pACC, ParHipp |
NS |
| (Epperson et al., 2005) | 16 SM 20 HC |
36.0 (16.0) 32.3 (5.9) (Approx) |
10M/6W 7M/13W |
0h, 48 h (within) |
NR | NR | NR | Occip | MRS | ↓GABA (women only) |
NS |
| Opiates | |||||||||||
| None | |||||||||||
| Cocaine | |||||||||||
| (Ke et al., 2004) | 35 CD 20 HC |
43.2 (7.4) 39.2 (8.0) |
26M/9F 7M/13F |
NS | NR | Alc | NR | pACC/ MedPFC |
MRS | ↓UGABA | GABA corr years Coc use (Unspec direction) |
| Methamphetamince | |||||||||||
| None | |||||||||||
| Cannabis | |||||||||||
| None | |||||||||||
| Multiple Substances | |||||||||||
| (Abe et al., 2013) | 28 AUD/PS 40 AD 16 HC |
45.3 (9.6) 52.1 (9.1) 49.0 (10.1) |
26M/2F 37M/3F 15M/1F |
~1 mo | Local VA Tx | Opiates, MJ, MDMA |
Hep C, type-2 diabetes, ↑BP, depress |
pACC, DLPFC, Occip |
MRS | AD/PS: ↓ pACC GABA (uncorrected) |
AD/PS: ↓ pACC GABA, ↑ verbal memory |
| (Cosgrove et al., 2014) | 17 AUD/SM 10 AUD 15 HC/SM 10 HC |
42 (11) 49 (9) 40 (9) 48 (10) |
15M/2F 7M/3F 13M/2F 8M/2F |
Alc: Abst Sm: Unspec |
Inpatient Tx | NR | Abnormal liver function (3x higher allowed) |
Whole brain |
PET [123I]iomazenil |
↑ GABAA VT (buffered by smoking) |
AD/SM: ↑ GABAAVT corr ↑ crave alc and cig |
| (Mason et al., 2006) | 12 AUD 8 HC |
39 (8.2) 39 (9.0) |
12M/0F 8M/0F |
5.0 (4.0) d | Inpatient Tx | MJ, Nic, Coc | NR | Occip | MRS | ↑ GABA (non-SM) |
NA |
| (Staley et al., 2005) | 15 AUD/SM 8 AUD 5 HC/SM 10 HC |
40.9 (8.1) 39.9 (8.6) 41.2 (9.2) 35.9 (7.5) |
15M/0F 8M/0F 5M/0F 10M/0F |
5.0 (2.9) d 4.6 (1.8) d -- -- |
1 mo Tx | Nic, Benzos | Abnormal liver function (3x higher allowed) |
MedPFC, ACC, Amyg, Hipp, Cerebel |
PET [123I]iomazenil (baseline &1 mo later) |
AD (short Abst): ↑ GABAA VT |
AD: ↑ MPF GABAA VT corr Alc Abst d |
Abbreviations, (p or d)ACC = (pregenual or dorsal) anterior cingulate cortex, Abst = abstinence, Alc = alcohol, AntiD = antidepressant medication, Approx = approximated values, AUD = alcohol use disorder, Amyg = amygdala, Amph = amphetamine, BG = basal ganglia, Benzos = benzodiazepines, BP = blood pressure, Bup = buprenorphine, Cereb = cerebral cortex, Coc = cocaine, corr = was correlated with, Cr = creatine, Depress = depression, Detox = detoxification program, F = females GM = gray matter, Glu = glutamate, Hipp = hippocampus, Ins = insula, MA = methamphetamine use disorder, M = males, Marij = marijuana, MDMA = ecstasy, Med = medial, Mid = middle, MJ = marijuana, mo = month, MRS = magnetic resonance spectroscopy, NA = not applicable, Nic = nicotine, NP = neuropsychological, NR = none reported, NS = not significant, Occip = occipital lobe, ParHipp = parahippocampal gyrus, Pariet = parietal cortex, PET = positron emission tomography, PFC = prefrontal cortex, Pos = posterior, PS = polysubstance users, R =right, SM = smoker, Stim = stimulants, STR = striatum, Subco = subcortical regions, Temp = temporal cortex, Thal = thalamus, Tx = treatment, Unspec = unspecified, wk = week
3.1. Alcohol
3.1.1. MRS
Compared with controls, GABA levels in the occipital cortex were lower in alcohol-addicted individuals (Behar et al., 1999). In examining the dACC, groups comprising alcohol-addicted individuals with PTSD, PTSD only, and controls did not significantly differ on GABA levels (Pennington et al., 2014). However, within this comorbid group, higher GABA levels were correlated with better verbal learning/memory (Pennington et al., 2014).
3.1.2. PET/SPECT
In studies using [11C]flumazenil and [123I]iomazenil, alcohol-addicted individuals generally exhibited lower ratiotracer uptake VT than controls in the cerebellum and medial PFC including the pACC and/or dACC, consistent with reduced GABAA receptor availability (Abi-Dargham et al., 1998; Gilman et al., 1996; Lingford-Hughes et al., 1998), and such decreases have been correlated with greater severity of alcohol dependence in some studies (Lingford-Hughes et al., 1998) but not in others (Abi-Dargham et al., 1998). More recently, alcohol-addicted participants exhibited lower (than controls) [11C]Ro15 4513 VT in the nucleus accumbens, parahippocampal gyri, right hippocampus, and amygdala, suggesting reduced GABAA receptor availability (Lingford-Hughes et al., 2012). Within these alcohol-addicted individuals, higher VT in hippocampus and parahippocampal gyri was correlated with better performance on a delayed verbal memory task (Lingford-Hughes et al., 2012).
Other studies disagreed on whether there were differences in GABAA receptor measures between cases and controls. When a saturation method or VT was used with [11C]flumazenil or [123I]iomazenil, group differences were either absent (Lingford-Hughes et al., 2000; Lingford-Hughes et al., 2005; Litton et al., 1993) or reversed (Jalan et al., 2000). However, recall that this latter study included alcohol-addicted individuals of older age and with liver disease, which may have affected findings.
3.2. Smoking
3.2.1. MRS
Smokers had lower GABA in the occipital cortex than non-smokers, though this effect was only observed in women (Epperson et al., 2005).
3.2.2. PET/SPECT
GABAA receptor availability, indexed by [11C]Ro15 4513 VT, in the pACC and parahippocampal gyrus was higher in current/past smokers than non-smokers (Stokes et al., 2013).
3.3. Opiates
No studies were found.
3.4. Cocaine
3.4.1. MRS
In cocaine addiction, GABA levels were reduced in the pACC/dorsomedial PFC compared with controls (Ke et al., 2004).
3.4.2. PET/SPECT
No studies were found.
3.5. Methamphetamine
No studies were found.
3.6. Cannabis
No studies were found.
3.7. Multiple Substances
3.7.1. MRS
Compared with controls and alcohol-only addicted individuals, polysubstance-addicted individuals had lower pACC GABA levels (a difference that met nominal but not Bonferroni-corrected significance), and such lowered pACC GABA was correlated with worse verbal memory within the polysubstance users (Abe et al., 2013). In the occipital cortex, however, GABA levels were increased in non-smoking (but not smoking) alcohol-addicted individuals; these increased GABA levels declined after 1 month of alcohol abstinence (Mason et al., 2006).
3.7.2. PET/SPECT
Several studies reported increased radiotracer uptake (VT of [123I]iomazenil) in individuals with alcohol use disorder, but that these effects were blunted by active smoking. In particular, alcohol-addicted individuals in withdrawal (approximately 5-day abstinence) had higher GABAA receptor availability, indexed by [123I]iomazenil VT, in the medial PFC, ACC, hippocampus-amygdala, and cerebellum, but these effects were less pronounced either if they were active smokers or after they achieved 4 weeks of alcohol abstinence (Staley et al., 2005). In the alcohol-addicted smokers, higher GABAA receptor availability was correlated with longer initial abstinence from alcohol (1–7 days) (Staley et al., 2005). In a subsequent corroborative study, alcohol-addicted participants, further stratified by smoking status, were evaluated at 3, 10, and 30 days into withdrawal. Both alcohol-addicted smokers and non-smokers had higher GABAA receptor availability, indexed by [123I]iomazenil VT, compared with smoking-matched controls (Cosgrove et al., 2014). However, smoking status modulated the neuroadaptations seen during withdrawal. Alcohol-addicted non-smokers showed the highest and most widespread differences from controls at the 10-day assessment versus the 3-day and 4-week assessments, whereas the alcohol-addicted smokers had a more consistent pattern of differences from controls across all assessment time points. In the alcohol-addicted smokers, higher GABAA receptor availability was correlated with more craving for alcohol (at 10-day withdrawal) and cigarettes (at 3-day withdrawal) (Cosgrove et al., 2014).
3.8. Summary (see also Figure 1)
Overall, GABA was less studied than glutamate. MRS studies suggested lower GABA concentrations in abusers of alcohol, nicotine, and cocaine, which was also the typical direction of MRS effects for glutamate. Perhaps due to availability of more radiotracers, and/or because of their availability for a longer period of time, there were more PET/SPECT studies related to GABA than for glutamate, particularly for alcohol (which is unsurprising given alcohol’s known effects on the GABAA receptor). These studies generally showed decreased GABAA receptor availability/distribution volume in the addicted individuals compared with controls. Nicotine, however, showed an opposite pattern of effects. History of smoking was not only associated with higher GABAA receptor availability on its own, but smoking also modulated the early abstinence course of individuals with alcohol dependence. Interestingly, the effects of smoking on alcohol dependence showed an opposite pattern of effects to that of glutamate. Examining the joint effects of smoking and alcohol abuse, while incorporating markers of both glutamate and GABA neurotransmission, will be an interesting and important direction for future research.
It is also important to note that, similarly to glutamate, GABA effects appeared to be sensitive to study participant characteristics, such as the length of abstinence and/or drug-related medical diseases (less evidence for the latter). We did not locate any PET/SPECT studies labeling the GABAB receptor, which unlike the fast ligand-gated action of the GABAA receptor, is instead associated with long-term modulation through G protein-regulated gene transcription and protein synthesis (Xu et al., 2014). More research, both MRS and PET, is also needed in opiates, methamphetamine, and cannabis.
4. Evidence of Resting-State Functional Connectivity Deficits in Drug Addiction
A large literature has examined RSFC deficits in drug addiction (Fedota and Stein, 2015; Lu and Stein, 2014; Sutherland et al., 2012), and we did not reprise all of this important work here. Rather, our current goal was to provide evidence that some of the same regions implicated in glutamate and GABA MRS and PET studies in addiction are also functionally disrupted as revealed by RSFC. We focused on studies that examined RSFC differences between addicted individuals and healthy controls [using approaches that were seed-based and/or whole-brain (e.g., independent components analysis (ICA) or the graph theory-based metric degree (i.e., number of connections exceeding a specified correlation threshold)] in the (A) ACC extending into the dorsomedial and/or ventromedial PFC, (B) insula, and (C) striatum (for more discussion of the advantages and disadvantages of using RSFC in psychopathology, see Box 2).
Box 2. Methodological Advantages and Disadvantages of Resting-State Functional Connectivity (RSFC).
Assessment of RSFC is a sensitive and highly generalizable fMRI methodology. Using RSFC, group differences between cases and controls have emerged across numerous psychiatric and neurological conditions even in the absence of gross morphological abnormalities (Barkhof et al., 2014). Furthermore, because this approach does not depend on a particular experimental context (task), it becomes possible to identify commonalities and differences between individuals in clinical groups, who otherwise probably would not undergo similar experimental paradigms [e.g., addicted individuals are routinely exposed to drug cues (Jasinska et al., 2014), whereas depressed individuals are routinely exposed to emotional faces (Stuhrmann et al., 2011)]. It even becomes possible to scan individuals unable to perform task-based fMRI at all, including individuals with altered or diminished states of consciousness, or severe cognitive decline (e.g., coma, psychosis, Alzheimer’s disease, etc.) (Brier et al., 2014; Demertzi et al., 2014; Satterthwaite and Baker, 2015). Because many discrete psychopathologies share deficits in network-level functional connectivity, it is possible that this transdiagnostic tool can suggest previously unrecognized overlap among disorders that may be targeted for previously unrecognized therapeutic interventions [e.g., consistent with the Research Domain Criteria (RDoC) approach]. RSFC, then, may indeed be regarded an intermediate phenotype that may be compared across different diagnostic groups.
Nevertheless, it is also important to note some of the interpretative issues of RSFC, which have been well-articulated elsewhere (Weinberger and Radulescu, 2015). In brief, such issues include systematic differences in the use of (potentially multiple) substances; inability to discern what participants are thinking and feeling during the resting-state scan, with possible group differences in these psychological states; and the possibility of prominent artifacts resulting from head movement and other motion, which may also differ at the group level. However, even with these potential sources of variability, effect sizes of RSFC studies generally have been large in magnitude. In particular, the select studies/findings included in the current review were estimated to have following M ± SD effect sizes [Cohen’s d, calculated based on sample sizes and means ± standard deviations (or t-values), where available]: alcohol (d=0.84 ± 0.0), nicotine (d=1.32 ± 0.22), opiates (d=2.39 ± 1.66), cocaine (d=1.01 ± 0.22), methamphetamine (d=1.04 ± 0.0), and cannabis (d=0.98 ± 0.0). (Note that because this manuscript is not a meta-analysis and includes only a portion of possible resting-state studies and a portion of possible effects within those studies, extensive discussion about these effect size estimates or their interpretations is outside the scope of this review; rather, the goal here was to provide macroscopic view of the magnitude of effects in these kinds of studies.)
The rationales for focusing on these regions are as follows. The ACC (especially, pACC) and adjacent medial PFC (encompassing dorsomedial and ventromedial subsections) form part of the default mode network (DMN), which is activated during the resting state (Gusnard et al., 2001; Molnar-Szakacs and Uddin, 2013). Moreover, the resting state is a condition replete with mind-wandering and self-generated thinking (Smallwood and Schooler, 2015), and these self-referential functions have been linked with activation of cortical midline regions, including the pACC and medial PFC, in healthy individuals (Abraham, 2013; D’Argembeau, 2013; de Greck et al., 2008; van der Meer et al., 2010) and addicted individuals (de Greck et al., 2009; Moeller and Goldstein, 2014). Thus, although larger regions, such as the ACC and medial PFC, have sometimes been selected as regions of interest in MRS studies for practical reasons (i.e., large area to position the sequence), effects in these regions are nonetheless highly anticipated for both MRS studies and RSFC studies; recent combined fMRI-MRS studies in healthy participants further speak to this point (see Introduction). The insula has a critical role in mediating interoception (Craig, 2009) and the detection of behaviorally relevant stimuli (Uddin, 2015). In drug addiction, these functions subserved by the insula appear vital for the experience of drug craving (Naqvi et al., 2007; Verdejo-Garcia et al., 2012). The striatum forms a key part of the mesocorticolimbic dopamine projections that mediate the reinforcing effects of addictive drugs; chronic perturbation of this system ultimately leads to enduring changes in striatal-PFC glutamatergic projections (Kalivas, 2007, 2009). Although MRS measurement of glutamate and GABA is more difficult in the striatum than in the insula (Wiebking et al., 2014), some studies included in this review indeed have reported striatal effects. Importantly, prior resting-state studies of healthy individuals have revealed functional connections between these three regions (Margulies et al., 2007; Uddin et al., 2009).
4.1. Alcohol
Alcohol-addicted individuals had weaker connectivity between subregions of the ACC with the insula (Sullivan et al., 2013) and subthalamic nucleus (Morris et al., 2015). Using ICA, it was shown that alcohol-addicted individuals had stronger connectivity than controls within and between various networks, including an orbitofrontal cortex (OFC) network, an amygdala-striatum network, and a DMN network (Zhu et al., 2015).
4.2. Smoking
Smokers in withdrawal showed stronger RSFC between the ACC and dorsal striatum compared with controls; these same smokers showed stronger RSFC between the ACC and bilateral insula in a withdrawal study condition compared with a satiated study condition (Huang et al., 2014). Similarly, 12-hour abstinent smokers showed stronger global connections to the insula compared with controls, and this difference was not observed when the smokers were satiated (Wang et al., 2014). Interestingly, strengthened connections with the insula (e.g., to regions of the DMN, such as the ventromedial and dorsomedial PFC) were abolished by a nicotine challenge (Sutherland et al., 2013). A different pattern of effects was observed with ICA, however. Relative to non-smokers, satiated smokers exhibited stronger connectivity between the medial PFC and a left fronto-parietal network (including ACC, DLPFC, and insula extending into putamen) (Janes et al., 2012).
4.3. Opiates
The most RSFC studies have been conducted with individuals addicted to opiates. Opiate-addicted individuals had weaker RSFC between the pACC with the dACC, DLPFC, medial PFC, and PCC/precuneus (Ma et al., 2010; Ma et al., 2015; Wang et al., 2013; Yuan et al., 2010); and between the caudate and DLPFC (middle frontal gyrus) (Wang et al., 2013). In ICA or other whole-brain approaches, compared with controls, opiate-addicted individuals had weaker resting-state functional connectivity of the ACC and basal ganglia regions (including the striatum) (Liu et al., 2011a; Ma et al., 2011; Schmidt et al., 2015).
Other studies, however, have shown stronger connectivity in opiate-addicted individuals: higher RSFC between the pACC, dACC, or precuneus with the striatum (Ma et al., 2010; Zhang et al., 2015) and insula (Zhang et al., 2015) [but see (Upadhyay et al., 2010)]. Stronger connectivity between the IFG with the dACC and ventromedial PFC also has been reported (Ma et al., 2010; Wang et al., 2013). Connectivity between the insula and the amygdala was also stronger in opiate-addicted individuals compared with controls (Xie et al., 2011). In whole-brain RSFC approaches, heroin-addicted individuals had stronger overall connectivity of the ACC, midcingulate, insula, OFC, and putamen (Liu et al., 2009; Liu et al., 2011a).
4.4. Cocaine
Compared with controls, cocaine-addicted individuals generally had weaker RSFC of the pACC and dACC with subcortical regions, including the striatum, amygdala, thalamus, hippocampus, and parahippocampal gyrus (Gu et al., 2010; Hu et al., 2015; Verdejo-Garcia et al., 2014) [but see (Wilcox et al., 2011)]. In one study, pACC-amygdala connectivity was also associated with clinical outcome (30-day relapse after treatment) (McHugh et al., 2014).
Other studies have reported stronger connectivity between the ACC subregions and other cortical regions (e.g., middle frontal gyrus, inferior parietal lobe, or supramarginal gyrus) in cocaine-addicted individuals than controls (Camchong et al., 2011; Konova et al., 2013), with the dACC and ventromedial PFC in particular receiving an abnormally high number of short- and long-range functional connections (Konova et al., 2015).
The directionality of limbic-limbic connectivity was less clear. Cocaine-addicted individuals had weaker connectivity between the bilateral putamen and the left posterior insula compared with controls, and this effect was driven by data from individuals who relapsed 30 days after treatment discharge (McHugh et al., 2013). However, cocaine-addicted individuals had stronger connectivity between the ventral striatum and dorsal striatum than controls (Konova et al., 2013).
4.5. Methamphetamine
Compared with healthy controls, methamphetamine-addicted individuals exhibited greater RSFC between a midbrain seed and a number of subcortical (e.g., putamen and insula) and cortical regions (e.g., OFC) (Kohno et al., 2014).
4.6. Cannabis
Chronic marijuana users showed weaker RSFC between an insular seed and the ACC (Pujol et al., 2014).
4.7. Summary
As also articulated elsewhere (Lu and Stein, 2014), RSFC in addiction remains an emerging field, and conflicting findings have been quite common. One potentially interesting pattern of results for opiates and cocaine, perhaps the most widely studied addictions in this field, appears to be that cortical-cortical connections generally appear to be weakened, whereas corticolimbic connections generally appear to be strengthened; more research is clearly required, however, before firm conclusions can be drawn, especially for certain substances (e.g., methamphetamine, marijuana). Despite these inconsistencies of directionality, these studies have revealed reliable RSFC differences between cases and controls in regions that have also been investigated using MRS or PET/SPECT (e.g., ACC, medial PFC, insula, and striatum), and these group differences have been relatively large in magnitude (see Box 2).
5.0. Working Hypothesis: Abnormal Glutamatergic and/or GABAergic Neurotransmission Underlies Corticolimbic RSFC Deficits in Addiction
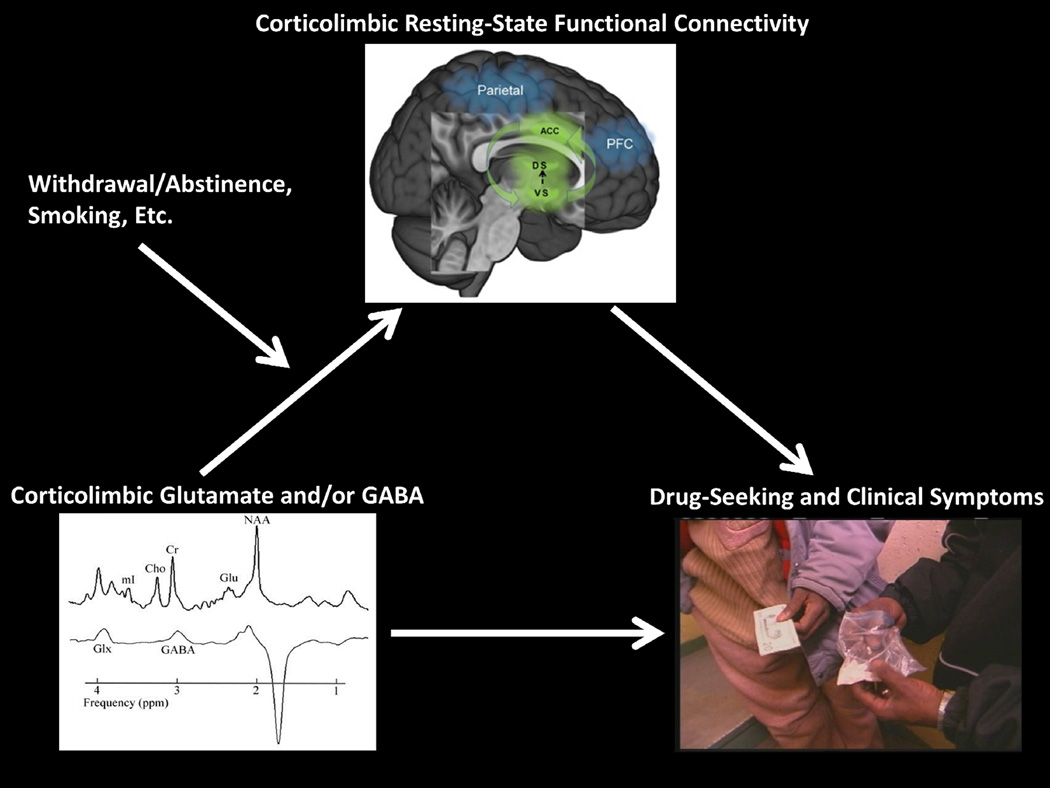
Taken together, the literature indicates that drug-addicted individuals exhibit abnormal neurotransmission involving glutamate and GABA in corticolimbic brain regions of core relevance to their disease (e.g., ACC, medial PFC insula, and striatum), and that these same regions also show disruptions in RSFC. Because glutamatergic and GABAergic neurotransmission in such regions also drive the resting state in health, we raise the hypothesis that corticolimbic RSFC can provide an intermediate phenotype to explain associations between addiction-relevant glutamatergic and/or GABA dysregulation and addiction symptomatology (e.g., craving, drug-seeking, engagement with treatment) (Figure 2). Future work can center on the following areas.
Figure 2.
Schematic of the hypothesized model. Deficits in glutamate and/or GABA, which are further modulated by clinical characteristics including withdrawal/abstinence, are associated with deficits in brain resting-state functional connectivity (e.g., anterior cingulate cortex with the dorsal and ventral striatum), which in turn are associated with drug-related symptoms. [The metabolite image (left) is adapted from (Abe et al., 2013), with permission from Elsevier; the brain image (top) is adapted from (Garland et al., 2014), under the Creative Commons Attribution License; and the drug image (right) is adapted from (Moeller et al., 2009), with permission from Elsevier].
5.1. Finer Specification of the Model
It is crucial to incorporate the modulating influences of clinical characteristics, especially withdrawal/abstinence and smoking (Figure 2). Withdrawal carries a high vulnerability to relapse, which may partially stem from associated perturbations in brain glutamate or GABA (Mashhoon et al., 2011). Smoking history, as shown above, exerts important independent effects on brain glutamate and GABA metabolites. Current smoking also modulates the effects of other substances, such as alcohol (especially during withdrawal), and the resulting effects on brain glutamate and GABA may differ depending on which neurotransmitter is examined. Future studies might also investigate whether neurochemical deficits in one corticolimbic brain region have reverberations across the brain. This may be especially true for deficits in glutamate, which has more global (transregional) effects (Duncan et al., 2013). RSFC methods, especially using whole-brain graph theory approaches, are ideally suited to test such hypotheses. Finally, future studies can incorporate direct measures of brain metabolism, such as PET with [18F]fluorodeoxyglucose. Indeed, energy metabolism may represent an intermediary process between fast neurotransmission and the slow RSFC blood-oxygen-level dependent (BOLD) response, and this kind of precision would increase mechanistic understanding.
5.2. Integration and Translation between Human Data and Animal Models
Another important future direction for enhancing mechanistic understanding is to conduct studies with tighter experimental control, as can be achieved in animal models. Animal models offer the advantages of more controlled drug histories and more invasive assessments, which could clarify how addiction may causally change glutamate/GABA neurotransmission and metabolite levels in select brain regions, as well as their consequent associations with RSFC.
In such animal studies, lower Glx levels in the dorsal striatum of rhesus monkeys due to chronic methamphetamine exposure showed a linear pattern of recovery with abstinence over one year (i.e., returning to control levels) (Yang et al., 2015) [but see (Liu et al., 2011b), where cocaine administration over the course of 9 months increased levels of glutamate and glutamine in squirrel monkeys]. In another study, rats received subcutaneous twice-daily injections of 2.5mg/kg methamphetamine for one week. This drug exposure resulted in decreased MRS-measured glutamate, glutamine, and GABA in hippocampus, nucleus accumbens, and PFC (Bu et al., 2013). Interestingly, a different study revealed decreased RSFC in cocaine-exposed rats between the nucleus accumbens and the dorsomedial PFC as a function of the degree of cocaine self-administration escalation (Lu et al., 2014). These combined studies generally support our hypothesized model.
Alternatively, drug-administration schedules not intended to produce addiction have largely produced opposite results. For example, following short-term administrations of cocaine (Li et al., 2012) or alcohol (Zahr et al., 2015), rats showed transient striatal (Li et al., 2012) or whole-brain (Zahr et al., 2015) increases in glutamate and/or GABA [but see (Lee et al., 2014)]. Such results are consistent with the idea that addiction-related decreases in glutamate or GABA could reflect neuroadaptations to chronic drug exposure. Such conclusions are difficult, if not impossible, to achieve in studies of already-addicted humans.
5.3. More Comprehensive Methods
Because human studies cannot achieve the level of precision attained in animal studies, mechanistic clarity needs to rely on more comprehensive and innovative experimental methods. A drug challenge model, if employed in combination with fMRI and with MRS or PET, can address causality by modulating underlying glutamate/GABA neurotransmission that can then be correlated with resting-state fMRI and then other clinical variables.
We are aware of no previous studies in this field that have attempted this kind of ambitious design, though some have incorporated various components. For example, one study showed that acute alcohol administration reduced occipital GABA levels (Gomez et al., 2012). However, because this experiment was conducted in social drinkers (not in alcohol-addicted individuals), the potential relevance to addiction is unclear. Another study found that a heroin challenge (versus placebo) in opiate-addicted individuals strengthened connectivity within an ICA-defined basal ganglia network (including striatum) (Schmidt et al., 2015). Similarly, opiate-addicted individuals receiving high methadone doses showed higher ACC glutamate levels (Greenwald et al., 2015; Verdejo-Garcia et al., 2013). However, these studies did not incorporate both neurochemical measurements and RSFC. Finally, perhaps the most methodologically rich study to date evaluated the effects of 12-week varenicline administration on dACC Glx levels and fMRI BOLD response (during a color-word Stroop task) (Wheelock et al., 2014). The varenicline regimen decreased dACC Glx levels, modulated DMN regions (including pACC and PCC) during task performance, and changed dACC-DMN connectivity as revealed by psychophysiological interaction (PPI) analysis (Wheelock et al., 2014). Future iterations of this study type would need to include a control group and could benefit from using a pharmacological probe that modulates the neurotransmitter system of interest more directly (i.e., because varenicline is a nicotinic receptor partial agonist, the Glx results could represent secondary effects). A future study that integrates these various components within a single design promises to be highly informative.
5.4. Testing for Substance-Specific Effects
It would be interesting to test whether addiction-related effects on brain glutamate and GABA are specific to addiction related to substances rather than behaviors. One could compare and contrast effects in individuals with substance use disorders with those in individuals who have behavioral addictions, such as gambling (Clark and Limbrick-Oldfield, 2013). We are aware of no MRS or PET studies that contrasted substance addiction and gambling addiction, but several studies on this front have been conducted using RSFC. For example, whereas increased intrinsic local connectivity of the PCC was observed for both behavioral (gambling) and substance (alcohol) addictions, decreased connectivity of the ACC was specific to alcohol addiction (Kim et al., 2015). Moreover, cocaine addiction was uniquely associated with enhanced connectivity between the subgenual ACC with OFC or striatum (in further correlation with measures of impulsivity) (Contreras-Rodriguez et al., 2015a; Contreras-Rodriguez et al., 2015b). In contrast, connectivity in cocaine addiction overlapped with that in gambling addiction in the OFC and dorsomedial PFC, and in the amygdala and insula (Contreras-Rodriguez et al., 2015b) [note that this latter connection was also reported in opiate dependence (Xie et al., 2011)].
5.5. Potential Applications to Treatment
A relatively small but growing literature suggests that glutamatergic and/or GABAergic medications modulate neural activity in brain regions spotlighted in this review. In smokers, the GABAB receptor agonist baclofen, given both acutely and after 3 weeks of treatment, decreased cerebral blood flow during perfusion fMRI in several regions including the dACC (Franklin et al., 2012; Franklin et al., 2011). In an animal model (rhesus monkeys), baclofen reversed neuropsychological deficits owing to acute cocaine injections in association with normalized metabolic activation in the PFC (Porrino et al., 2013). Acamprosate, despite continuing debates regarding its clinical mechanism of action, appears to exert effects on brain glutamate (Bolo et al., 1998). Consistent with this idea, 4-week treatment of acamprosate reduced MRS-measured pACC glutamate levels in recently abstinent alcohol-addicted individuals; such reductions appeared to be clinically warranted, as glutamate levels in cerebrospinal fluid were positively correlated with alcohol dependence severity (Umhau et al., 2010). Moreover, in an animal model (rats), acamprosate reduced Glx levels in the ventral striatum during alcohol withdrawal (Hinton et al., 2012). In healthy controls, the GABA reuptake inhibitor (i.e., transporter blocker) tiagabine, which notably has been shown to decrease cocaine-positive urines in pilot clinical trials (Gonzalez et al., 2003), increased (either significantly or at trend level) the VT and/or BPND of [11C]flumazenil and [11C]Ro15 4513 in multiple PFC regions, including the ACC (Frankle et al., 2012; Frankle et al., 2009; Stokes et al., 2014).
We hypothesize that these medications – as well as potentially novel medications yet to be developed that act on these respective systems – could also modulate corticolimbic RSFC, providing a potential therapeutic target for intervention in drug addiction. In this regard, modulation of brain glutamate and GABA signaling may be particularly important during acute withdrawal, a time period when neurotransmission seems especially perturbed.
6. Conclusion
Glutamatergic and GABAergic neurotransmission drives the resting state in healthy individuals. As drug-addicted individuals exhibit abnormalities in concentrations of these neurotransmitters and in the resting state, we posited that abnormal glutamate and/or GABA concentrations – especially in corticolimbic brain areas – might underlie the abnormal RSFC in addiction. This hypothesis remains to be empirically verified. If supported, our perspective can provide mechanistic insight into the disordered resting state in drug addiction, potentially also elucidating the mechanisms of existing therapeutics and ultimately even informing the development of novel therapeutics that target this disordered resting state. Future research can also expand concepts in our review to other psychopathologies marked by deficits in the resting state; the resting state, because it does not rely on disease-specific tasks, is one of the most robust, consistent, and far-reaching deficits in psychiatry and neurology. Accordingly, our framework could have important transdiagnostic mechanistic and therapeutic implications.
Highlights.
We review glutamate and GABA alterations in addiction, observed via MRS and PET/SPECT.
These neurotransmitters drive the resting state in healthy individuals.
Brain regions showing glutamate and GABA abnormalities are also functionally disrupted in addiction.
We link these literatures to suggest a neurochemical basis of resting-state abnormalities in addiction.
We identify possible research and treatment directions that can follow from this framework.
Acknowledgements
This work was supported by grants from the National Institute on Drug Abuse (K01DA037452; R21DA040046) (to SJM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support came from: Brain Imaging Center (BIC) pilot funds from the Icahn School of Medicine at Mount Sinai (to SJM); endowments from the Thomas P. and Katherine K. Pike Chair of Addiction Studies and the Marjorie Greene Trust (to EDL); grants from the Canadian Institutes of Health (CIHR 465 and CIHR-EJLB) (to GN); and the Michael Smith Chair for Neuroscience and Mental Health (to GN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest
None declared.
References
- Abe C, Mon A, Durazzo TC, Pennington DL, Schmidt TP, Meyerhoff DJ. Polysubstance and alcohol dependence: unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug Alcohol Depend. 2013;130:30–37. doi: 10.1016/j.drugalcdep.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Krystal JH, Anjilvel S, Scanley BE, Zoghbi S, Baldwin RM, Rajeevan N, Ellis S, Petrakis IL, Seibyl JP, Charney DS, Laruelle M, Innis RB. Alterations of benzodiazepine receptors in type II alcoholic subjects measured with SPECT and [123I]iomazenil. Am J Psychiatry. 1998;155:1550–1555. doi: 10.1176/ajp.155.11.1550. [DOI] [PubMed] [Google Scholar]
- Abraham A. The world according to me: personal relevance and the medial prefrontal cortex. Frontiers in human neuroscience. 2013;7:341. doi: 10.3389/fnhum.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus F, Ametamey SM, Treyer V, Burger C, Johayem A, Umbricht D, Gomez Mancilla B, Sovago J, Buck A, Hasler G. Marked global reduction in mGluR5 receptor binding in smokers and ex-smokers determined by [11C]ABP688 positron emission tomography. Proc Natl Acad Sci U S A. 2013;110:737–742. doi: 10.1073/pnas.1210984110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkus F, Treyer V, Johayem A, Ametamey SM, Mancilla BG, Sovago J, Buck A, Hasler G. Association of Long-Term Nicotine Abstinence with Normal Metabotropic Glutamate Receptor-5 Binding. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.02.027. [DOI] [PubMed] [Google Scholar]
- Bagga D, Khushu S, Modi S, Kaur P, Bhattacharya D, Garg ML, Singh N. Impaired visual information processing in alcohol-dependent subjects: a proton magnetic resonance spectroscopy study of the primary visual cortex. Journal of studies on alcohol and drugs. 2014;75:817–826. doi: 10.15288/jsad.2014.75.817. [DOI] [PubMed] [Google Scholar]
- Barkhof F, Haller S, Rombouts SA. Resting-state functional MR imaging: a new window to the brain. Radiology. 2014;272:29–49. doi: 10.1148/radiol.14132388. [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology. 2013;38:1401–1408. doi: 10.1038/npp.2013.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar KL, Rothman DL, Petersen KF, Hooten M, Delaney R, Petroff OA, Shulman GI, Navarro V, Petrakis IL, Charney DS, Krystal JH. Preliminary evidence of low cortical GABA levels in localized 1H–MR spectra of alcohol-dependent and hepatic encephalopathy patients. Am J Psychiatry. 1999;156:952–954. doi: 10.1176/ajp.156.6.952. [DOI] [PubMed] [Google Scholar]
- Bolo N, Nedelec JF, Muzet M, De Witte P, Dahchour A, Durbin P, Macher JP. Central effects of acamprosate: part 2. Acamprosate modifies the brain in-vivo proton magnetic resonance spectrum in healthy young male volunteers. Psychiatry Res. 1998;82:115–127. doi: 10.1016/s0925-4927(98)00017-1. [DOI] [PubMed] [Google Scholar]
- Brier MR, Thomas JB, Ances BM. Network dysfunction in Alzheimer’s disease: refining the disconnection hypothesis. Brain connectivity. 2014;4:299–311. doi: 10.1089/brain.2014.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu Q, Lv L, Yan G, Deng P, Wang Y, Zhou J, Yang Y, Li Y, Cen X. NMR-based metabonomic in hippocampus, nucleus accumbens and prefrontal cortex of methamphetamine-sensitized rats. Neurotoxicology. 2013;36:17–23. doi: 10.1016/j.neuro.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Burnett EJ, Chandler LJ, Trantham-Davidson H. Glutamatergic plasticity and alcohol dependence-induced alterations in reward, affect and cognition. Progress in neuro-psychopharmacology & biological psychiatry. 2015 doi: 10.1016/j.pnpbp.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral J, Kringelbach ML, Deco G. Exploring the network dynamics underlying brain activity during rest. Prog Neurobiol. 2014;114:102–131. doi: 10.1016/j.pneurobio.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW, 3rd, Nelson B, Bell C, Mueller BA, Specker S, Lim KO. Frontal hyperconnectivity related to discounting and reversal learning in cocaine subjects. Biol Psychiatry. 2011;69:1117–1123. doi: 10.1016/j.biopsych.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. J Neuroimmune Pharmacol. 2006;1:65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Mehringer CM, Ernst T, Melchor R, Myers H, Forney D, Satz P. Neurochemical alterations in asymptomatic abstinent cocaine users: a proton magnetic resonance spectroscopy study. Biol Psychiatry. 1997;42:1105–1114. doi: 10.1016/s0006-3223(97)00135-2. [DOI] [PubMed] [Google Scholar]
- Clark L, Limbrick-Oldfield EH. Disordered gambling: a behavioral addiction. Curr Opin Neurobiol. 2013;23:655–659. doi: 10.1016/j.conb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Contreras-Rodriguez O, Albein-Urios N, Perales JC, Martinez-Gonzalez JM, Vilar-Lopez R, Fernandez-Serrano MJ, Lozano-Rojas O, Verdejo-Garcia A. Cocaine-specific neuroplasticity in the ventral striatum network is linked to delay discounting and drug relapse. Addiction. 2015a doi: 10.1111/add.13076. [DOI] [PubMed] [Google Scholar]
- Contreras-Rodriguez O, Albein-Urios N, Vilar-Lopez R, Perales JC, Martinez-Gonzalez JM, Fernandez-Serrano MJ, Lozano-Rojas O, Clark L, Verdejo-Garcia A. Increased corticolimbic connectivity in cocaine dependence versus pathological gambling is associated with drug severity and emotion-related impulsivity. Addict Biol. 2015b doi: 10.1111/adb.12242. [DOI] [PubMed] [Google Scholar]
- Cook JB, Foster KL, Eiler WJ, 2nd, McKay PF, Woods J, 2nd, Harvey SC, Garcia M, Grey C, McCane S, Mason D, Cummings R, Li X, Cook JM, June HL. Selective GABAA alpha5 benzodiazepine inverse agonist antagonizes the neurobehavioral actions of alcohol. Alcohol Clin Exp Res. 2005;29:1390–1401. doi: 10.1097/01.alc.0000175073.94575.86. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, McKay R, Esterlis I, Kloczynski T, Perkins E, Bois F, Pittman B, Lancaster J, Glahn DC, O’Malley S, Carson RE, Krystal JH. Tobacco smoking interferes with GABAA receptor neuroadaptations during prolonged alcohol withdrawal. Proc Natl Acad Sci U S A. 2014;111:18031–18036. doi: 10.1073/pnas.1413947111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Crocker CE, Bernier DC, Hanstock CC, Lakusta B, Purdon SE, Seres P, Tibbo PG. Prefrontal glutamate levels differentiate early phase schizophrenia and methamphetamine addiction: a (1)H MRS study at 3Tesla. Schizophr Res. 2014;157:231–237. doi: 10.1016/j.schres.2014.05.004. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A. On the role of the ventromedial prefrontal cortex in self-processing: the valuation hypothesis. Frontiers in human neuroscience. 2013;7:372. doi: 10.3389/fnhum.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Greicius MD. Greater than the sum of its parts: a review of studies combining structural connectivity and resting-state functional connectivity. Brain structure & function. 2009;213:525–533. doi: 10.1007/s00429-009-0208-6. [DOI] [PubMed] [Google Scholar]
- de Greck M, Rotte M, Paus R, Moritz D, Thiemann R, Proesch U, Bruer U, Moerth S, Tempelmann C, Bogerts B, Northoff G. Is our self based on reward? Self-relatedness recruits neural activity in the reward system. Neuroimage. 2008;39:2066–2075. doi: 10.1016/j.neuroimage.2007.11.006. [DOI] [PubMed] [Google Scholar]
- de Greck M, Supady A, Thiemann R, Tempelmann C, Bogerts B, Forschner L, Ploetz KV, Northoff G. Decreased neural activity in reward circuitry during personal reference in abstinent alcoholics--a fMRI study. Hum Brain Mapp. 2009;30:1691–1704. doi: 10.1002/hbm.20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deco G, Ponce-Alvarez A, Hagmann P, Romani GL, Mantini D, Corbetta M. How local excitation-inhibition ratio impacts the whole brain dynamics. J Neurosci. 2014;34:7886–7898. doi: 10.1523/JNEUROSCI.5068-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demertzi A, Gomez F, Crone JS, Vanhaudenhuyse A, Tshibanda L, Noirhomme Q, Thonnard M, Charland-Verville V, Kirsch M, Laureys S, Soddu A. Multiple fMRI system-level baseline connectivity is disrupted in patients with consciousness alterations. Cortex. 2014;52:35–46. doi: 10.1016/j.cortex.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Doucet G, Naveau M, Petit L, Zago L, Crivello F, Jobard G, Delcroix N, Mellet E, Tzourio-Mazoyer N, Mazoyer B, Joliot M. Patterns of hemodynamic low-frequency oscillations in the brain are modulated by the nature of free thought during rest. Neuroimage. 2012;59:3194–3200. doi: 10.1016/j.neuroimage.2011.11.059. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Enzi B, Wiebking C, Northoff G. Involvement of glutamate in rest-stimulus interaction between perigenual and supragenual anterior cingulate cortex: a combined fMRI-MRS study. Hum Brain Mapp. 2011;32:2172–2182. doi: 10.1002/hbm.21179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans-a review of multimodal imaging studies. Neurosci Biobehav Rev. 2014;47:36–52. doi: 10.1016/j.neubiorev.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Tiret B, Marjanska M, Hayes DJ, Lyttleton O, Doyon J, Northoff G. Glutamate concentration in the medial prefrontal cortex predicts resting-state cortical-subcortical functional connectivity in humans. PLoS One. 2013;8:e60312. doi: 10.1371/journal.pone.0060312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Meyerhoff DJ, Mon A, Abe C, Gazdzinski S, Murray DE. Chronic Cigarette Smoking in Healthy Middle-Aged Individuals Is Associated with Decreased Regional Brain N-acetylaspartate and Glutamate Levels. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ende G, Hermann D, Demirakca T, Hoerst M, Tunc-Skarka N, Weber-Fahr W, Wichert S, Rabinstein J, Frischknecht U, Mann K, Vollstadt-Klein S. Loss of control of alcohol use and severity of alcohol dependence in non-treatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res. 2013;37:1643–1649. doi: 10.1111/acer.12149. [DOI] [PubMed] [Google Scholar]
- Engel AK, Gerloff C, Hilgetag CC, Nolte G. Intrinsic coupling modes: multiscale interactions in ongoing brain activity. Neuron. 2013;80:867–886. doi: 10.1016/j.neuron.2013.09.038. [DOI] [PubMed] [Google Scholar]
- Epperson CN, O’Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, Rothman DL, Krystal JH, Mason GF. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. 2005;57:44–48. doi: 10.1016/j.biopsych.2004.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol. 2008;3:165–172. doi: 10.1007/s11481-008-9108-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Stein EA. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann N Y Acad Sci. 2015;1349:64–82. doi: 10.1111/nyas.12882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KC, Spreng RN, Ellamil M, Andrews-Hanna JR, Christoff K. The wandering brain: Meta-analysis of functional neuroimaging studies of mind-wandering and related spontaneous thought processes. Neuroimage. 2015;111:611–621. doi: 10.1016/j.neuroimage.2015.02.039. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Mason NS, Chen CM, Himes M, Walker C, Lewis DA, Mathis CA, Narendran R. [11C]flumazenil binding is increased in a dose-dependent manner with tiagabine-induced elevations in GABA levels. PLoS One. 2012;7:e32443. doi: 10.1371/journal.pone.0032443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankle WG, Cho RY, Narendran R, Mason NS, Vora S, Litschge M, Price JC, Lewis DA, Mathis CA. Tiagabine increases [11C]flumazenil binding in cortical brain regions in healthy control subjects. Neuropsychopharmacology. 2009;34:624–633. doi: 10.1038/npp.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Shin J, Jagannathan K, Suh JJ, Detre JA, O’Brien CP, Childress AR. Acute baclofen diminishes resting baseline blood flow to limbic structures: a perfusion fMRI study. Drug Alcohol Depend. 2012;125:60–66. doi: 10.1016/j.drugalcdep.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Wang Z, Sciortino N, Harper D, Li Y, Hakun J, Kildea S, Kampman K, Ehrman R, Detre JA, O’Brien CP, Childress AR. Modulation of resting brain cerebral blood flow by the GABA B agonist, baclofen: a longitudinal perfusion fMRI study. Drug Alcohol Depend. 2011;117:176–183. doi: 10.1016/j.drugalcdep.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry. 2007;40:64–67. doi: 10.1055/s-2007-970144. [DOI] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Howard MO. Mindfulness training targets neurocognitive mechanisms of addiction at the attention-appraisal-emotion interface. Frontiers in psychiatry / Frontiers Research Foundation. 2014;4:173. doi: 10.3389/fpsyt.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Adams K, Johnson-Greene D, Junck L, Kluin KJ, Brunberg J, Martorello S, Lohman M. Positron emission tomographic studies of cerebral benzodiazepine-receptor binding in chronic alcoholics. Ann Neurol. 1996;40:163–171. doi: 10.1002/ana.410400207. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez R, Behar KL, Watzl J, Weinzimer SA, Gulanski B, Sanacora G, Koretski J, Guidone E, Jiang L, Petrakis IL, Pittman B, Krystal JH, Mason GF. Intravenous ethanol infusion decreases human cortical gamma-aminobutyric acid and N-acetylaspartate as measured with proton magnetic resonance spectroscopy at 4 tesla. Biol Psychiatry. 2012;71:239–246. doi: 10.1016/j.biopsych.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G, Sevarino K, Sofuoglu M, Poling J, Oliveto A, Gonsai K, George TP, Kosten TR. Tiagabine increases cocaine-free urines in cocaine-dependent methadone-treated patients: results of a randomized pilot study. Addiction. 2003;98:1625–1632. doi: 10.1046/j.1360-0443.2003.00544.x. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Woodcock EA, Khatib D, Stanley JA. Methadone maintenance dose modulates anterior cingulate glutamate levels in heroin-dependent individuals: A preliminary in vivo(1)H MRS study. Psychiatry Res. 2015;233:218–224. doi: 10.1016/j.pscychresns.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage. 2010;53:593–601. doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutzeit A, Froehlich JM, Hergan K, Graf N, Binkert CA, Meier D, Brugger M, Reischauer C, Sutter R, Herdener M, Schubert T, Kos S, Grosshans M, Straka M, Mutschler J. Insula-specific H magnetic resonance spectroscopy reactions in heavy smokers under acute nicotine withdrawal and after oral nicotine substitution. Eur Addict Res. 2013;19:184–193. doi: 10.1159/000345915. [DOI] [PubMed] [Google Scholar]
- Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT. Brain connectivity related to working memory performance. J Neurosci. 2006;26:13338–13343. doi: 10.1523/JNEUROSCI.3408-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann D, Frischknecht U, Heinrich M, Hoerst M, Vollmert C, Vollstadt-Klein S, Tunc-Skarka N, Kiefer F, Mann K, Ende G. MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addict Biol. 2012a;17:659–667. doi: 10.1111/j.1369-1600.2010.00290.x. [DOI] [PubMed] [Google Scholar]
- Hermann D, Weber-Fahr W, Sartorius A, Hoerst M, Frischknecht U, Tunc-Skarka N, Perreau-Lenz S, Hansson AC, Krumm B, Kiefer F, Spanagel R, Mann K, Ende G, Sommer WH. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry. 2012b;71:1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- Hinton DJ, Lee MR, Jacobson TL, Mishra PK, Frye MA, Mrazek DA, Macura SI, Choi DS. Ethanol withdrawal-induced brain metabolites and the pharmacological effects of acamprosate in mice lacking ENT1. Neuropharmacology. 2012;62:2480–2488. doi: 10.1016/j.neuropharm.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells FM, Uhlmann A, Temmingh H, Sinclair H, Meintjes E, Wilson D, Stein DJ. (1)H-magnetic resonance spectroscopy ((1)H-MRS) in methamphetamine dependence and methamphetamine induced psychosis. Schizophr Res. 2014;153:122–128. doi: 10.1016/j.schres.2014.01.029. [DOI] [PubMed] [Google Scholar]
- Hu Y, Chen X, Gu H, Yang Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J Neurosci. 2013;33:18566–18573. doi: 10.1523/JNEUROSCI.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72:584–592. doi: 10.1001/jamapsychiatry.2015.1. [DOI] [PubMed] [Google Scholar]
- Huang W, King JA, Ursprung WW, Zheng S, Zhang N, Kennedy DN, Ziedonis D, DiFranza JR. The development and expression of physical nicotine dependence corresponds to structural and functional alterations in the anterior cingulate-precuneus pathway. Brain and behavior. 2014;4:408–417. doi: 10.1002/brb3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulka LM, Scheidegger M, Vonmoos M, Preller KH, Baumgartner MR, Herdener M, Seifritz E, Henning A, Quednow BB. Glutamatergic and neurometabolic alterations in chronic cocaine users measured with H-magnetic resonance spectroscopy. Addict Biol. 2014a doi: 10.1111/adb.12217. [DOI] [PubMed] [Google Scholar]
- Hulka LM, Treyer V, Scheidegger M, Preller KH, Vonmoos M, Baumgartner MR, Johayem A, Ametamey SM, Buck A, Seifritz E, Quednow BB. Smoking but not cocaine use is associated with lower cerebral metabotropic glutamate receptor 5 density in humans. Mol Psychiatry. 2014b;19:625–632. doi: 10.1038/mp.2013.51. [DOI] [PubMed] [Google Scholar]
- Hyder F, Fulbright RK, Shulman RG, Rothman DL. Glutamatergic function in the resting awake human brain is supported by uniformly high oxidative energy. J Cereb Blood Flow Metab. 2013;33:339–347. doi: 10.1038/jcbfm.2012.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26:865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- Jalan R, Turjanski N, Taylor-Robinson SD, Koepp MJ, Richardson MP, Wilson JA, Bell JD, Brooks DJ. Increased availability of central benzodiazepine receptors in patients with chronic hepatic encephalopathy and alcohol related cirrhosis. Gut. 2000;46:546–552. doi: 10.1136/gut.46.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes AC, Nickerson LD, Frederick Bde B, Kaufman MJ. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine-dependent smokers and non-smoking controls. Drug Alcohol Depend. 2012;125:252–259. doi: 10.1016/j.drugalcdep.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. doi: 10.1016/j.neubiorev.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. Cocaine and amphetamine-like psychostimulants: neurocircuitry and glutamate neuroplasticity. Dialogues Clin Neurosci. 2007;9:389–397. doi: 10.31887/DCNS.2007.9.4/pkalivas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–119. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Streeter CC, Nassar LE, Sarid-Segal O, Hennen J, Yurgelun-Todd DA, Awad LA, Rendall MJ, Gruber SA, Nason A, Mudrick MJ, Blank SR, Meyer AA, Knapp C, Ciraulo DA, Renshaw PF. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res. 2004;130:283–293. doi: 10.1016/j.pscychresns.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim YK, Gwak AR, Lim JA, Lee JY, Jung HY, Sohn BK, Choi SW, Kim DJ, Choi JS. Resting-state regional homogeneity as a biological marker for patients with Internet gaming disorder: A comparison with patients with alcohol use disorder and healthy controls. Progress in neuro-psychopharmacology & biological psychiatry. 2015;60:104–111. doi: 10.1016/j.pnpbp.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Kohno M, Morales AM, Ghahremani DG, Hellemann G, London ED. Risky decision making, prefrontal cortex, and mesocorticolimbic functional connectivity in methamphetamine dependence. JAMA Psychiatry. 2014;71:812–820. doi: 10.1001/jamapsychiatry.2014.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Goldstein RZ. Effects of chronic and acute stimulants on brain functional connectivity hubs. Brain Res. 2015 doi: 10.1016/j.brainres.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konova AB, Moeller SJ, Tomasi D, Volkow ND, Goldstein RZ. Effects of methylphenidate on resting-state functional connectivity of the mesocorticolimbic dopamine pathways in cocaine addiction. JAMA Psychiatry. 2013;70:857–868. doi: 10.1001/jamapsychiatry.2013.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DW, Nam YK, Kim TK, Kim JH, Kim SY, Min JW, Lee JH, Kim HY, Kim DJ, Choe BY. Dose-dependent influence of short-term intermittent ethanol intoxication on cerebral neurochemical changes in rats detected by ex vivo proton nuclear magnetic resonance spectroscopy. Neuroscience. 2014;262:107–117. doi: 10.1016/j.neuroscience.2013.12.061. [DOI] [PubMed] [Google Scholar]
- Lee E, Jang DP, Kim JJ, An SK, Park S, Kim IY, Kim SI, Yoon KJ, Namkoong K. Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport. 2007;18:1511–1514. doi: 10.1097/WNR.0b013e3282ef7625. [DOI] [PubMed] [Google Scholar]
- Li Y, Yan GY, Zhou JQ, Bu Q, Deng PC, Yang YZ, Lv L, Deng Y, Zhao JX, Shao X, Zhu RM, Huang YN, Zhao YL, Cen XB. (1)H NMR-based metabonomics in brain nucleus accumbens and striatum following repeated cocaine treatment in rats. Neuroscience. 2012;218:196–205. doi: 10.1016/j.neuroscience.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Licata SC, Renshaw PF. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann N Y Acad Sci. 2010;1187:148–171. doi: 10.1111/j.1749-6632.2009.05143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingford-Hughes A, Reid AG, Myers J, Feeney A, Hammers A, Taylor LG, Rosso L, Turkheimer F, Brooks DJ, Grasby P, Nutt DJ. A [11C]Ro15 4513 PET study suggests that alcohol dependence in man is associated with reduced alpha5 benzodiazepine receptors in limbic regions. J Psychopharmacol. 2012;26:273–281. doi: 10.1177/0269881110379509. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Boddington SJ, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Levels of gamma-aminobutyric acid-benzodiazepine receptors in abstinent, alcohol-dependent women: preliminary findings from an 123I–iomazenil single photon emission tomography study. Alcohol Clin Exp Res. 2000;24:1449–1455. [PubMed] [Google Scholar]
- Lingford-Hughes AR, Acton PD, Gacinovic S, Suckling J, Busatto GF, Boddington SJ, Bullmore E, Woodruff PW, Costa DC, Pilowsky LS, Ell PJ, Marshall EJ, Kerwin RW. Reduced levels of GABA-benzodiazepine receptor in alcohol dependency in the absence of grey matter atrophy. Br J Psychiatry. 1998;173:116–122. doi: 10.1192/bjp.173.2.116. [DOI] [PubMed] [Google Scholar]
- Lingford-Hughes AR, Wilson SJ, Cunningham VJ, Feeney A, Stevenson B, Brooks DJ, Nutt DJ. GABA-benzodiazepine receptor function in alcohol dependence: a combined 11C–flumazenil PET and pharmacodynamic study. Psychopharmacology (Berl) 2005;180:595–606. doi: 10.1007/s00213-005-2271-x. [DOI] [PubMed] [Google Scholar]
- Litton JE, Neiman J, Pauli S, Farde L, Hindmarsh T, Halldin C, Sedvall G. PET analysis of [11C]flumazenil binding to benzodiazepine receptors in chronic alcohol-dependent men and healthy controls. Psychiatry Res. 1993;50:1–13. doi: 10.1016/0925-4927(93)90019-e. [DOI] [PubMed] [Google Scholar]
- Liu J, Liang J, Qin W, Tian J, Yuan K, Bai L, Zhang Y, Wang W, Wang Y, Li Q, Zhao L, Lu L, von Deneen KM, Liu Y, Gold MS. Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neurosci Lett. 2009;460:72–77. doi: 10.1016/j.neulet.2009.05.038. [DOI] [PubMed] [Google Scholar]
- Liu J, Qin W, Yuan K, Li J, Wang W, Li Q, Wang Y, Sun J, von Deneen KM, Liu Y, Tian J. Interaction between dysfunctional connectivity at rest and heroin cues-induced brain responses in male abstinent heroin-dependent individuals. PLoS One. 2011a;6:e23098. doi: 10.1371/journal.pone.0023098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Jensen JE, Gillis TE, Zuo CS, Prescot AP, Brimson M, Cayetano K, Renshaw PF, Kaufman MJ. Chronic cocaine exposure induces putamen glutamate and glutamine metabolite abnormalities in squirrel monkeys. Psychopharmacology (Berl) 2011b;217:367–375. doi: 10.1007/s00213-011-2292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logothetis NK, Murayama Y, Augath M, Steffen T, Werner J, Oeltermann A. How not to study spontaneous activity. Neuroimage. 2009;45:1080–1089. doi: 10.1016/j.neuroimage.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Lu H, Stein EA. Resting state functional connectivity: its physiological basis and application in neuropharmacology. Neuropharmacology. 2014;84:79–89. doi: 10.1016/j.neuropharm.2013.08.023. [DOI] [PubMed] [Google Scholar]
- Lu H, Zou Q, Chefer S, Ross TJ, Vaupel DB, Guillem K, Rea WP, Yang Y, Peoples LL, Stein EA. Abstinence from cocaine and sucrose self-administration reveals altered mesocorticolimbic circuit connectivity by resting state MRI. Brain connectivity. 2014;4:499–510. doi: 10.1089/brain.2014.0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, Fuchs T, Kilpatrick CL. GABAA receptor trafficking-mediated plasticity of inhibitory synapses. Neuron. 2011;70:385–409. doi: 10.1016/j.neuron.2011.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Fu XM, Li N, Wang CX, Zhang H, Qian RB, Xu HS, Hu X, Zhang DR. Abnormal brain default-mode network functional connectivity in drug addicts. PLoS One. 2011;6:e16560. doi: 10.1371/journal.pone.0016560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, Xu HS, Fu XM, Hu X, Zhang DR. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Qiu Y, Tian J, Wang J, Li S, Zhan W, Wang T, Zeng S, Jiang G, Xu Y. Aberrant default-mode functional and structural connectivity in heroin-dependent individuals. PLoS One. 2015;10:e0120861. doi: 10.1371/journal.pone.0120861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Corbetta M, Romani GL, Orban GA, Vanduffel W. Evolutionarily novel functional networks in the human brain? J Neurosci. 2013;33:3259–3275. doi: 10.1523/JNEUROSCI.4392-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies DS, Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Mapping the functional connectivity of anterior cingulate cortex. Neuroimage. 2007;37:579–588. doi: 10.1016/j.neuroimage.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Martinez D, Slifstein M, Nabulsi N, Grassetti A, Urban NB, Perez A, Liu F, Lin SF, Ropchan J, Mao X, Kegeles LS, Shungu DC, Carson RE, Huang Y. Imaging glutamate homeostasis in cocaine addiction with the metabotropic glutamate receptor 5 positron emission tomography radiotracer [(11)C]ABP688 and magnetic resonance spectroscopy. Biol Psychiatry. 2014;75:165–171. doi: 10.1016/j.biopsych.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashhoon Y, Janes AC, Jensen JE, Prescot AP, Pachas G, Renshaw PF, Fava M, Evins AE, Kaufman MJ. Anterior cingulate proton spectroscopy glutamate levels differ as a function of smoking cessation outcome. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:1709–1713. doi: 10.1016/j.pnpbp.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason GF, Petrakis IL, de Graaf RA, Gueorguieva R, Guidone E, Coric V, Epperson CN, Rothman DL, Krystal JH. Cortical gamma-aminobutyric acid levels and the recovery from ethanol dependence: preliminary evidence of modification by cigarette smoking. Biol Psychiatry. 2006;59:85–93. doi: 10.1016/j.biopsych.2005.06.009. [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse. 2013;39:424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Salmeron BJ, Devous MD, Stein EA, Adinoff B. Cortico-amygdala coupling as a marker of early relapse risk in cocaine-addicted individuals. Frontiers in psychiatry / Frontiers Research Foundation. 2014;5:16. doi: 10.3389/fpsyt.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay PF, Foster KL, Mason D, Cummings R, Garcia M, Williams LS, Grey C, McCane S, He X, Cook JM, June HL. A high affinity ligand for GABAA-receptor containing alpha5 subunit antagonizes ethanol’s neurobehavioral effects in Long-Evans rats. Psychopharmacology (Berl) 2004;172:455–462. doi: 10.1007/s00213-003-1671-z. [DOI] [PubMed] [Google Scholar]
- Mennecke A, Gossler A, Hammen T, Dorfler A, Stadlbauer A, Rosch J, Kornhuber J, Bleich S, Dolken M, Thurauf N. Physiological effects of cigarette smoking in the limbic system revealed by 3 tesla magnetic resonance spectroscopy. Journal of neural transmission (Vienna, Austria : 1996) 2014;121:1211–1219. doi: 10.1007/s00702-014-1190-6. [DOI] [PubMed] [Google Scholar]
- Miese F, Kircheis G, Wittsack HJ, Wenserski F, Hemker J, Modder U, Haussinger D, Cohnen M. 1H–MR spectroscopy, magnetization transfer, and diffusion-weighted imaging in alcoholic and nonalcoholic patients with cirrhosis with hepatic encephalopathy. AJNR. American journal of neuroradiology. 2006;27:1019–1026. [PMC free article] [PubMed] [Google Scholar]
- Milella MS, Marengo L, Larcher K, Fotros A, Dagher A, Rosa-Neto P, Benkelfat C, Leyton M. Limbic system mGluR5 availability in cocaine dependent subjects: a high-resolution PET [(11)C]ABP688 study. Neuroimage. 2014;98:195–202. doi: 10.1016/j.neuroimage.2014.04.061. [DOI] [PubMed] [Google Scholar]
- Moeller SJ, Goldstein RZ. Impaired self-awareness in human addiction: deficient attribution of personal relevance. Trends Cogn Sci. 2014;18:635–641. doi: 10.1016/j.tics.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller SJ, Maloney T, Parvaz MA, Dunning JP, Alia-Klein N, Woicik PA, Hajcak G, Telang F, Wang GJ, Volkow ND, Goldstein RZ. Enhanced choice for viewing cocaine pictures in cocaine addiction. Biol Psychiatry. 2009;66:169–176. doi: 10.1016/j.biopsych.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar-Szakacs I, Uddin LQ. Self-processing and the default mode network: interactions with the mirror neuron system. Frontiers in human neuroscience. 2013;7:571. doi: 10.3389/fnhum.2013.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Meyerhoff DJ. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Depend. 2012;125:27–36. doi: 10.1016/j.drugalcdep.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morcom AM, Fletcher PC. Does the brain have a baseline? Why we should be resisting a rest. Neuroimage. 2007;37:1073–1082. doi: 10.1016/j.neuroimage.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Morris LS, Kundu P, Baek K, Irvine MA, Mechelmans DJ, Wood J, Harrison NA, Robbins TW, Bullmore ET, Voon V. Jumping the Gun: Mapping Neural Correlates of Waiting Impulsivity and Relevance Across Alcohol Misuse. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel RL, Marjanska M, Collins PF, Becker MP, Valabregue R, Auerbach EJ, Lim KO, Luciana M. In vivo H magnetic resonance spectroscopy in young-adult daily marijuana users. Neuroimage Clin. 2013;2:581–589. doi: 10.1016/j.nicl.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315:531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery FG, Stanley JA, Chen HH, Hatch JP, Nicoletti MA, Monkul ES, Lafer B, Soares JC. Bipolar disorder comorbid with alcoholism: a 1H magnetic resonance spectroscopy study. J Psychiatr Res. 2010;44:278–285. doi: 10.1016/j.jpsychires.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G. Vol I Coding. Oxford, NY: Oxford University Press; 2014. Unlocking the brain. [Google Scholar]
- O’Neill J, Tobias MC, Hudkins M, London ED. Glutamatergic neurometabolites during early abstinence from chronic methamphetamine abuse. Int J Neuropsychopharmacol. 2015:18. doi: 10.1093/ijnp/pyu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Tobias MC, Hudkins M, Oh EY, Hellemann GS, Nurmi EL, London ED. Thalamic glutamate decreases with cigarette smoking. Psychopharmacology (Berl) 2014;231:2717–2724. doi: 10.1007/s00213-014-3441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Abe C, Batki SL, Meyerhoff DJ. A preliminary examination of cortical neurotransmitter levels associated with heavy drinking in posttraumatic stress disorder. Psychiatry Res. 2014;224:281–287. doi: 10.1016/j.pscychresns.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Hampson RE, Opris I, Deadwyler SA. Acute cocaine induced deficits in cognitive performance in rhesus macaque monkeys treated with baclofen. Psychopharmacology (Berl) 2013;225:105–114. doi: 10.1007/s00213-012-2798-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescot AP, Locatelli AE, Renshaw PF, Yurgelun-Todd DA. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage. 2011;57:69–75. doi: 10.1016/j.neuroimage.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Blanco-Hinojo L, Batalla A, Lopez-Sola M, Harrison BJ, Soriano-Mas C, Crippa JA, Fagundo AB, Deus J, de la Torre R, Nogue S, Farre M, Torrens M, Martin-Santos R. Functional connectivity alterations in brain networks relevant to self-awareness in chronic cannabis users. J Psychiatr Res. 2014;51:68–78. doi: 10.1016/j.jpsychires.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravan S, Martinez D, Slifstein M, Abi-Dargham A. Molecular imaging in alcohol dependence. Handbook of clinical neurology. 2014;125:293–311. doi: 10.1016/B978-0-444-62619-6.00018-5. [DOI] [PubMed] [Google Scholar]
- Rosazza C, Minati L. Resting-state brain networks: literature review and clinical applications. Neurol Sci. 2011;32:773–785. doi: 10.1007/s10072-011-0636-y. [DOI] [PubMed] [Google Scholar]
- Rothman DL, De Feyter HM, de Graaf RA, Mason GF, Behar KL. 13C MRS studies of neuroenergetics and neurotransmitter cycling in humans. NMR in biomedicine. 2011;24:943–957. doi: 10.1002/nbm.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailasuta N, Abulseoud O, Hernandez M, Haghani P, Ross BD. Metabolic Abnormalities in Abstinent Methamphetamine Dependent Subjects. Subst Abuse. 2010;2010:9–20. doi: 10.4137/sart.s4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Baker JT. How can studies of resting-state functional connectivity help us understand psychosis as a disorder of brain development? Curr Opin Neurobiol. 2015;30:85–91. doi: 10.1016/j.conb.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Denier N, Magon S, Radue EW, Huber CG, Riecher-Rossler A, Wiesbeck GA, Lang UE, Borgwardt S, Walter M. Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution. Transl Psychiatry. 2015;5:e533. doi: 10.1038/tp.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz D, Widmann U, Seeger U, Nagele T, Klose U, Mann K, Grodd W. Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcohol Clin Exp Res. 1999;23:158–163. [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp. 2008;29:751–761. doi: 10.1002/hbm.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The science of mind wandering: empirically navigating the stream of consciousness. Annu Rev Psychol. 2015;66:487–518. doi: 10.1146/annurev-psych-010814-015331. [DOI] [PubMed] [Google Scholar]
- Staley JK, Gottschalk C, Petrakis IL, Gueorguieva R, O’Malley S, Baldwin R, Jatlow P, Verhoeff NP, Perry E, Weinzimmer D, Frohlich E, Ruff E, van Dyck CH, Seibyl JP, Innis RB, Krystal JH. Cortical gamma-aminobutyric acid type A-benzodiazepine receptors in recovery from alcohol dependence: relationship to features of alcohol dependence and cigarette smoking. Arch Gen Psychiatry. 2005;62:877–888. doi: 10.1001/archpsyc.62.8.877. [DOI] [PubMed] [Google Scholar]
- Stephens DN, Pistovcakova J, Worthing L, Atack JR, Dawson GR. Role of GABAA alpha5-containing receptors in ethanol reward: the effects of targeted gene deletion, and a selective inverse agonist. European journal of pharmacology. 2005;526:240–250. doi: 10.1016/j.ejphar.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Stokes PR, Benecke A, Myers J, Erritzoe D, Watson BJ, Kalk N, Barros DR, Hammers A, Nutt DJ, Lingford-Hughes AR. History of cigarette smoking is associated with higher limbic GABAA receptor availability. Neuroimage. 2013;69:70–77. doi: 10.1016/j.neuroimage.2012.12.010. [DOI] [PubMed] [Google Scholar]
- Stokes PR, Myers JF, Kalk NJ, Watson BJ, Erritzoe D, Wilson SJ, Cunningham VJ, Riano Barros D, Hammers A, Turkheimer FE, Nutt DJ, Lingford-Hughes AR. Acute increases in synaptic GABA detectable in the living human brain: a [(1)(1)C]Ro15–4513 PET study. Neuroimage. 2014;99:158–165. doi: 10.1016/j.neuroimage.2014.05.035. [DOI] [PubMed] [Google Scholar]
- Stuhrmann A, Suslow T, Dannlowski U. Facial emotion processing in major depression: a systematic review of neuroimaging findings. Biology of mood & anxiety disorders. 2011;1:10. doi: 10.1186/2045-5380-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Muller-Oehring E, Pitel AL, Chanraud S, Shankaranarayanan A, Alsop DC, Rohlfing T, Pfefferbaum A. A selective insular perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biol Psychiatry. 2013;74:547–555. doi: 10.1016/j.biopsych.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, Ross TJ, Hong LE, Stein EA. Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry. 2013;74:538–546. doi: 10.1016/j.biopsych.2013.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62:2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terbeck S, Akkus F, Chesterman LP, Hasler G. The role of metabotropic glutamate receptor 5 in the pathogenesis of mood disorders and addiction: combining preclinical evidence with human Positron Emission Tomography (PET) studies. Frontiers in neuroscience. 2015;9:86. doi: 10.3389/fnins.2015.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology. 2011;36:1359–1365. doi: 10.1038/npp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ. Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci. 2015;16:55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30:625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umhau JC, Momenan R, Schwandt ML, Singley E, Lifshitz M, Doty L, Adams LJ, Vengeliene V, Spanagel R, Zhang Y, Shen J, George DT, Hommer D, Heilig M. Effect of acamprosate on magnetic resonance spectroscopy measures of central glutamate in detoxified alcohol-dependent individuals: a randomized controlled experimental medicine study. Arch Gen Psychiatry. 2010;67:1069–1077. doi: 10.1001/archgenpsychiatry.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay J, Maleki N, Potter J, Elman I, Rudrauf D, Knudsen J, Wallin D, Pendse G, McDonald L, Griffin M, Anderson J, Nutile L, Renshaw P, Weiss R, Becerra L, Borsook D. Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain. 2010;133:2098–2114. doi: 10.1093/brain/awq138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer L, Costafreda S, Aleman A, David AS. Self-reflection and the brain: a theoretical review and meta-analysis of neuroimaging studies with implications for schizophrenia. Neurosci Biobehav Rev. 2010;34:935–946. doi: 10.1016/j.neubiorev.2009.12.004. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Contreras-Rodriguez O, Fonseca F, Cuenca A, Soriano-Mas C, Rodriguez J, Pardo-Lozano R, Blanco-Hinojo L, de Sola Llopis S, Farre M, Torrens M, Pujol J, de la Torre R. Functional alteration in frontolimbic systems relevant to moral judgment in cocaine-dependent subjects. Addict Biol. 2014;19:272–281. doi: 10.1111/j.1369-1600.2012.00472.x. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Lubman DI, Roffel K, Vilar-Lopez R, Bora E, MacKenzie T, Yucel M. Cingulate biochemistry in heroin users on substitution pharmacotherapy. Aust N Z J Psychiatry. 2013;47:244–249. doi: 10.1177/0004867412463088. [DOI] [PubMed] [Google Scholar]
- Walls AB, Waagepetersen HS, Bak LK, Schousboe A, Sonnewald U. The glutamine-glutamate/GABA cycle: function, regional differences in glutamate and GABA production and effects of interference with GABA metabolism. Neurochem Res. 2015;40:402–409. doi: 10.1007/s11064-014-1473-1. [DOI] [PubMed] [Google Scholar]
- Wang K, Yang J, Zhang S, Wei D, Hao X, Tu S, Qiu J. The neural mechanisms underlying the acute effect of cigarette smoking on chronic smokers. PLoS One. 2014;9:e102828. doi: 10.1371/journal.pone.0102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhu J, Li Q, Li W, Wu N, Zheng Y, Chang H, Chen J, Wang W. Altered fronto-striatal and fronto-cerebellar circuits in heroin-dependent individuals: a resting-state FMRI study. PLoS One. 2013;8:e58098. doi: 10.1371/journal.pone.0058098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger DR, Radulescu E. Finding the Elusive Psychiatric “Lesion” With 21st-Century Neuroanatomy: A Note of Caution. Am J Psychiatry. 2015 doi: 10.1176/appi.ajp.2015.15060753. appiajp201515060753. [DOI] [PubMed] [Google Scholar]
- Wheelock MD, Reid MA, To H, White DM, Cropsey KL, Lahti AC. Open label smoking cessation with varenicline is associated with decreased glutamate levels and functional changes in anterior cingulate cortex: preliminary findings. Frontiers in pharmacology. 2014;5:158. doi: 10.3389/fphar.2014.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebking C, Duncan NW, Tiret B, Hayes DJ, Marjanska M, Doyon J, Bajbouj M, Northoff G. GABA in the insula - a predictor of the neural response to interoceptive awareness. Neuroimage. 2014;86:10–18. doi: 10.1016/j.neuroimage.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox CE, Teshiba TM, Merideth F, Ling J, Mayer AR. Enhanced cue reactivity and fronto-striatal functional connectivity in cocaine use disorders. Drug Alcohol Depend. 2011;115:137–144. doi: 10.1016/j.drugalcdep.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie C, Li SJ, Shao Y, Fu L, Goveas J, Ye E, Li W, Cohen AD, Chen G, Zhang Z, Yang Z. Identification of hyperactive intrinsic amygdala network connectivity associated with impulsivity in abstinent heroin addicts. Behav Brain Res. 2011;216:639–646. doi: 10.1016/j.bbr.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Zhang W, Rondard P, Pin JP, Liu J. Complex GABAB receptor complexes: how to generate multiple functionally distinct units from a single receptor. Front Pharmacol. 2014;5:12. doi: 10.3389/fphar.2014.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Belcher AM, Chefer S, Vaupel DB, Schindler CW, Stein EA, Yang Y. Withdrawal from long-term methamphetamine self-administration ‘normalizes’ neurometabolites in rhesus monkeys: a (1) H MR spectroscopy study. Addict Biol. 2015;20:69–79. doi: 10.1111/adb.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Salmeron BJ, Ross TJ, Xi ZX, Stein EA, Yang Y. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users - A (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res. 2009;174:171–176. doi: 10.1016/j.pscychresns.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo RA, Thoma RJ, Gasparovic C, Monnig M, Harlaar N, Calhoun VD, Kalyanam R, Mayer AR, Durazzo TC, Hutchison KE. Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry Res. 2013;211:141–147. doi: 10.1016/j.pscychresns.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan K, Qin W, Dong M, Liu J, Liu P, Zhang Y, Sun J, Wang W, Wang Y, Li Q, Yang W, Tian J. Combining spatial and temporal information to explore resting-state networks changes in abstinent heroin-dependent individuals. Neurosci Lett. 2010;475:20–24. doi: 10.1016/j.neulet.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Yücel M, Lubman DI, Harrison BJ, Fornito A, Allen NB, Wellard RM, Roffel K, Clarke K, Wood SJ, Forman SD, Pantelis C. A combined spectroscopic and functional MRI investigation of the dorsal anterior cingulate region in opiate addiction. Mol Psychiatry. 2007;12(611):691–702. doi: 10.1038/sj.mp.4001955. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Rohlfing T, Mayer D, Luong R, Sullivan EV, Pfefferbaum A. Transient CNS responses to repeated binge ethanol treatment. Addict Biol. 2015 doi: 10.1111/adb.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gong J, Xie C, Ye EM, Jin X, Song H, Yang Z, Shao Y. Alterations in brain connectivity in three sub-regions of the anterior cingulate cortex in heroin-dependent individuals: Evidence from resting state fMRI. Neuroscience. 2015;284:998–1010. doi: 10.1016/j.neuroscience.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Zhu X, Cortes CR, Mathur K, Tomasi D, Momenan R. Model-free functional connectivity and impulsivity correlates of alcohol dependence: a resting-state study. Addict Biol. 2015 doi: 10.1111/adb.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]