Abstract
Objective
The Lifestyle Interventions for Expectant Moms (LIFE-Moms) Consortium is designed to determine, in pregnant women with overweight or obesity, whether various behavioral and lifestyle interventions reduce excessive gestational weight gain (GWG) and subsequent adverse maternal and neonatal outcomes, and obesity in offspring. The design and planning process of the LIFE-Moms Consortium is described.
Design and Methods
The LIFE-Moms Consortium is a collaboration among seven clinical centers, a Research Coordinating Unit, and the NIH designed to support each clinical center’s conduct of a separate trial of a unique intervention. Specific common measures, procedures, and eligibility criteria are consistent across the 7 trials allowing data to be combined in exploratory analyses and/or compared readily.
Results
Numerous committees and working groups were created to define common measures and outcomes during pregnancy and through 1 year postpartum, develop Consortium policies and oversee progress of the trials. The primary outcome for the Consortium is excessive GWG. Secondary outcomes include maternal, neonatal and infant anthropometric measures, physical activity, sleep, and complications of pregnancy and delivery.
Conclusion
A multi-center consortium of independent, lifestyle interventions with common measures and outcomes may enhance the ability to identify promising interventions for improving outcomes in pregnant women and their offspring.
Keywords: pregnancy weight gain, lifestyle modifications
Introduction
Overweight and obesity in pregnancy are associated with numerous adverse maternal, fetal and neonatal outcomes.(1, 2, 3, 4, 5, 6, 7, 8,9, 10, 11) Maternal pre-pregnancy body mass index is an established predictor of exceeding gestational weight gain (GWG) recommendations, with women with overweight or obesity exceeding recommendations more often than normal weight women.(12) In 2009, the Institute of Medicine (IOM) and National Research Council issued revised guidelines for weight gain during pregnancy that, for the first time, included lower GWG goals for women with obesity at the time of conception.(13) The 2009 IOM guidelines were developed with the intent of defining GWG ranges that would result in optimal short- and long-term health outcomes for both mother and infant. Short-term outcomes associated with excessive GWG include gestational hypertension, preeclampsia, gestational diabetes, cesarean delivery, and large for gestational age (LGA) infants.(14, 15,16, 17, 18) Long-term outcomes include postpartum weight retention and obesity in the offspring.(19, 20, 21)
Pregnancy offers a unique window of opportunity to implement short-term lifestyle interventions to prevent excessive GWG. The request for applications for the Lifestyle Interventions for Expectant Moms (LIFE-Moms) Consortium was issued in January 2011, shortly after the IOM released revised GWG guidelines in recognition of the importance of overweight and obesity during pregnancy and appropriate GWG. At that time, several randomized clinical trials (RCTs) in the United States evaluating the effect of behavioral and lifestyle interventions on weight gain, healthy lifestyle patterns and glycemic status had shown mixed results.(22, 23, 24) In addition, few studies had enrolled sufficient women of low socioeconomic status and/or minority populations to rigorously evaluate the efficacy and feasibility of these interventions in such populations. A meta-analysis of antenatal dietary or lifestyle intervention RCTs reported no statistical differences in LGA and mean GWG and concluded that the uncertainty of both the effect of an antepartum intervention and its optimal intensity, along with inconsistency in maternal and infant outcome reporting, has yielded inadequate findings to conclude that limiting weight gain improves maternal and infant health measures.(25) Two trials have been published since the meta-analysis and after the initial design of the Consortium described in this manuscript. First, a trial conducted in a small number of women with obesity found weekly group-based weight management resulted in lower GWG and a lower prevalence of LGA infants.(26) Second, a large Australian RCT reported no differences in LGA and maternal pregnancy and birth outcomes with the exception of a reduction in macrosomia in the lifestyle advice group compared with standard care.(27) In addition, post-hoc analyses showed no difference in GWG between groups.(27) While recent studies have demonstrated that interventions in pregnancy may not be effective for modifying GWG, this emphasizes the importance of the LIFE-Moms Consortium which will evaluate the ability of different behavioral and lifestyle interventions to reduce excessive GWG in women with overweight or obesity and thereby reduce adverse maternal and neonatal outcomes.
Methods
LIFE-Moms Organization
LIFE-Moms is a research consortium that consists of seven clinical trials, a Research Coordinating Unit, and the NIH sponsoring Institutes and Centers. The majority of the Consortium is funded through cooperative agreement mechanisms which includes substantial NIH programmatic involvement.
The LIFE-Moms Consortium includes seven independent but collaborating RCTs that share the goal of identifying an effective intervention(s) that reduces GWG in women with overweight or obesity and a secondary goal of combining the data to perform a direct comparison of the interventions and additional exploratory analyses. This design is modeled after the experience of other NIH-funded multi-study collaborations, specifically the POWER (Practice-based Opportunities for Weight Reduction) and EARLY (Early Adult Reduction of Weight through Lifestyle Intervention) consortia.(28, 29) This rationale takes account of the lack of sufficient evidence to justify testing one single intervention in the traditional multi-center RCT. In addition, by identifying common measures to be collected across all studies and ensuring consistency of procedures, definitions and data collection, data from each trial may be combined resulting in a larger sample size to evaluate consortium primary and secondary outcomes.
Research Coordinating Unit (RCU)
LIFE-Moms is managed by a RCU located at The George Washington University Biostatistics Center. The primary goal of the RCU is to facilitate coordination of research activities and communications among the seven trials and the NIH. The RCU provides leadership in developing standardized variable definitions, common measures and methods; establishing the data collection and tracking system for storing and monitoring datasets that are common across studies; facilitating data sharing; and providing support for analysis of data for manuscripts that are common across studies or are collaborative.
Committees
The LIFE-Moms Consortium developed several Committees and Working Groups during the planning year. The following primary Committees are described in Table 1: Steering; Executive; Recruitment and Retention; Ancillary Studies; Publications and Presentations; Safety; and Design, Data Quality and Analysis. Monthly calls are scheduled (Executive is twice monthly) with discussions, decisions and action items recorded in written minutes. Each trial, NIH and the RCU elected at least one representative for each Committee.
Table 1.
LIFE-Moms Committees
| Committee | Purpose |
|---|---|
| Steering | Designs the research study activities and establishes priorities; develops common protocols and manuals, questionnaires and other data recording forms; establishes and maintains quality control among awardees; reviews progress; monitors participant recruitment and retention; coordinates and standardizes data management; and collaborates on the publication of results. Major scientific decisions are determined by the Steering Committee. |
| Executive | Manages the day-to-day operations of the Consortium and recommends potential solutions and policies for consideration by the Steering Committee for discussion and vote. |
| Recruitment & Retention | Develops plans to track recruitment and retention across all clinical trials; regularly monitors recruitment and retention at individual centers; provides a mechanism to share the best practices and lessons learned about recruitment and retention strategies; and addresses study-specific recruitment and retentions issues. |
| Ancillary Studies | Develops a policy for submission and evaluation of ancillary proposals intended for securing external funding to collect or conduct additional assessments, using existing study data, and/or utilizing commonly-collected bio-specimens stored in the repository; and reviews and approves ancillary study proposals. |
| Publications & Presentations | Develops a policy for publications and presentations; reviews proposals, abstracts and manuscripts; and identifies opportunities for other Consortium-wide publications. |
| Safety | Develops definitions of safety-related exclusion criteria, adverse and serious adverse events, and safety alert values for participant assessments; and reviews all adverse and serious adverse events in aggregate on a regular basis. |
| Design, Data Quality and Analysis | Develops the template for the DSMB reports including data quality measures; defines the Consortium’s primary and secondary outcomes and the analysis plan; develops strategies to facilitate data sharing across trials; recommends approaches to primary data analysis common across the trials; develops standardized methods to handle missing and censored data; develops strategies for pooled data analyses and potential meta-analyses among trials; and responds to analytic issues raised by the DSMB. |
In addition, specialized Committees were formed to determine common measures for key study questions and procedures and were later inactivated once their goals were accomplished. For example, as their titles suggest, the Physical and Clinical Measures, Surveys and Questionnaires, Diet and Physical Activity Assessment, and Biospecimens Committees were discontinued once they provided final recommendations to the Steering Committee, which is comprised of the PIs from each site, the RCU and the NIH.
NIH Programmatic Management
Each of the funding NIH Institutes in the Consortium is represented by a Project Scientist who helps guide the overall consortium of studies, serves on committees, and contributes as an author on Consortium publications and presentations. The NIDDK Project Scientist is responsible for leading the overall scientific project. The NIDDK Program Official oversees the operations of the Data and Safety Monitoring Board (DSMB). Other NIH Program Officials manage funding/administrative actions in accordance with Institute policies.
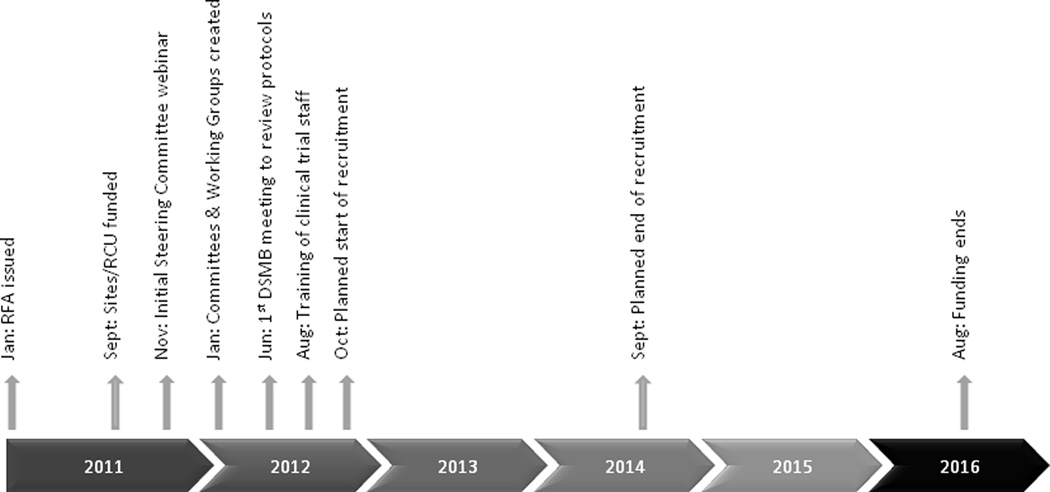
Timeline
Requests for Applications (RFAs), one for the clinical trials and another for the RCU, were issued in January 2011 with a planned start date of September 2011 and a 5-year funding period. The RFAs anticipated one-year for the collaborative planning phase, initial DSMB review, and IRB approval process. Additional timeline details are specified in Figure 1.
Figure 1.
LIFE-Moms Consortium timeline
Study Sites
The LIFE-Moms Consortium includes seven individual trials being conducted by investigators from California Polytechnic State University & Brown University (two-site trial, San Luis Obispo, CA & Providence, RI), St. Luke’s – Roosevelt Hospital and Columbia University (New York, NY), University of Puerto Rico (San Juan, PR), Northwestern University (Chicago, IL), Washington University in St. Louis (St. Louis, MO), Pennington Biomedical Research Center (Baton Rouge, LA), and NIDDK/Phoenix Indian Medical Center (PIMC, Phoenix, AZ). Details of each trial are described in Table 2 and below.
Table 2.
Overview of the seven trials in the LIFE-Moms Consortium
| Study | Healthy Beginnings |
Lifestyle Intervention for Two (LIFT) |
Pregnancy and EARly Life improvement Study (PEARLS) |
Maternal- Offspring Metabolics: Family Intervention Trial (MOMFIT) |
PreGO | Expecting Success |
LIFE-Moms Phoenix |
|---|---|---|---|---|---|---|---|
| Institution | California Polytechnic State University & Brown University | St. Luke’s – Roosevelt Hospital and Columbia University | University of Puerto Rico | Northwestern University | Washington University in St Louis | Pennington Biomedical Research Center | NIDDK/Phoenix Indian Medical Center (PIMC) |
| Recruitment location | OB practices servicing major delivery hospitals in San Luis Obispo and Women & Infants Hospital in Providence | OB practices and clinics whose patients deliver at St. Luke’s – Roosevelt | University of Puerto Rico Hospital | OB practices whose patients deliver at Prentice Women’s Hospital | Women’s Health Clinic at Washington University | OB practices and clinics whose patients deliver at Women’s Hospital Baton Rouge | Women’s Clinic at the PIMC |
| Trial primary outcome | GWG per week | Newborn percent body fat by air displacement plethysmography | GWG per week below or above IOM guidelines | Total GWG | Total GWG exceeding IOM guidelines | GWG per week exceeding IOM guidelines | Total GWG |
| Target sample size | 350, 175 per arm | 210, 105 per arm | 200, 100 per arm | 300, 150 per arm | 266, 133 per arm | 306, 102 per arm | 200, 100 per arm |
| Common inclusion criteria | Singleton viable pregnancy, gestational age at randomization no earlier than 9 weeks 0 days and no later than 15 weeks 6 days, body mass index ≥ 25 based on first trimester measured weight (with adjustments) and height | ||||||
| Trial specific inclusion criteria | None | BMI ≤ 35 if having MRI | None | BMI ≤ 40 | African American, SES disadvantaged, BMI ≤ 45 | Gestational age < 13 weeks, 5 days | Receiving prenatal care at PIMC, 75g oral glucose tolerance test at < 16 weeks |
| Common exclusion criteria | Age < 18 years, diagnosis of diabetes prior to pregnancy, or an HbA1c ≥ 6.5% or other test result suggestive of pre-pregnancy diabetes, known fetal anomaly, planned termination of pregnancy, history of three or more consecutive first trimester miscarriages, past history of anorexia or bulimia, current eating disorder, active suicidal ideation, prior or planned bariatric surgery, current use of exclusionary medications, contraindications to aerobic exercise in pregnancy, participation in another interventional study that influences weight control, enrollment in this trial in a previous pregnancy, intention of the participant or of the care provider for the delivery to be outside the LIFE-Moms Consortium hospital, participant’s unwillingness or inability to commit to a 1 year follow-up of herself or her child | ||||||
| Trial specific exclusion criteria | Untreated medical or psychiatric complication | Asthma, smoking, drugs / alcohol, chronic health problems, binge-eating disorder, claustrophobia & implanted metal objects (when MRI is involved), medical/ psychiatric/ social/ behavioral factors | IV drug use, HIV infection, inability to participate in group sessions, non-Spanish speaking | Maternal age > 45, IVF conception / ovulation induction with gonadotropins, weight gain > 15 lbs from pre-pregnancy weight, smoking, in weight loss program within 3 months of conception, drugs/alcohol, no access to internet, condition that limits walking or following diet, not fluent in English | Maternal age > 45, prior unexplained spontaneous preterm birth < 34 weeks, drugs/alcohol | Maternal age > 40, not fluent in English, not medically cleared, not willing to avoid pregnancy for 12 months following delivery, smoking, drugs/alcohol, psychotic disorder, HIV, contraindications to physical activity | Unwilling to provide informed consent in English, conditions that interfere with consent, treatment or follow-up |
| Treatment groups | |||||||
| Intervention |
Antepartum Individual counseling session (2/mo to 20 wks GA; 1–2/mo from 20 wks to delivery); meal replacement product; weight graphing; weekly behavior change materials, physical activity (30 mins most days of week / 10,000 steps) |
Antepartum Individual counseling(biweekly in-person on diet modification & physical activity, with behavioral modification and social support strategies); weekly phone/e-mail contacts; group classes every 8 wks to aid in weight loss and support a healthy lifestyle Postpartum Group classes every 8 wks to aid in weight loss and support a healthy lifestyle |
Antepartum 2 individual, 7 group sessions & monthly calls for improving dietary carbohydrate & fat quality; providing food – brown rice, whole grain pasta; physical activity (NEAT), decrease sitting time; breastfeeding Postpartum 2 individual, 2 group sessions and monthly calls on infant feeding practices, sleep and physical activity |
Antepartum 6 group and 3 individual sessions with phone coaching on diet and physical activity (30 mins or 10,000 steps at least 5 days a week); weekly electronic feedback from participant self-monitoring using the “Loseit!” app Postpartum 1 group,2 individual sessions, monthly emails on weight loss, diet physical activity, infant nutrition |
Antepartum 10 Parents as Teachers (PAT) home visits incorporating diet and exercise (150 mins / wk or 10,000 steps/days) focusing on control of weight gain reflecting the PAT philosophy Postpartum 18 monthly PAT visits focused on returning to pre-pregnancy weight, positive child-feeding behaviors and interactive parent-child activities |
Antepartum Two groups (clinic vs phone) with similar dietary intake (55% carbs, 15% protein, 30% fat) and exercise (150 mins / wk) advice through 18 lessons; focus on appropriate weight gain, postpartum weight loss and infant health (breastfeeding); clinic group has individual and group sessions and phone group receives feedback from weight and physical activity data transmitted to the center |
Antepartum Weekly group or individual sessions focused on individualized managed weight gain goals through caloric and fat gram recommendations, physical activity, decreased sedentary time |
| Control |
Antepartum Usual obstetric care; newsletters |
Antepartum Usual obstetric care; educational materials; group meetings every 8 wks until delivery based on wellness curriculum for the Look AHEAD Diabetes Support and Education (DSE) group Postpartum 3 group meetings based on LA DSE |
Antepartum Usual obstetric care (brochures for improving diet quality and WIC); 2 group sessions on pregnancy health related issues Postpartum 1 group session |
Antepartum Usual obstetric care; websites with diet and physical activity recommendations, 2 group sessions (infant CPR, newborn care), educational materials on childcare Postpartum 2 group sessions on infant CPR, care for newborns |
Antepartum Usual obstetric care plus usual 10 PAT home visits focused on parenting and child development Postpartum 18 monthly PAT visits using standard PAT curriculum |
Antepartum Usual obstetric care |
Antepartum Usual obstetric care; educational materials to promote healthy pregnancy behaviors |
Clinical Trial Interventions
The intensive lifestyle interventions and comparison groups for the seven trials are specified in Table 2 and described below. By design, the interventions vary across the seven trials. All interventions focus on diet and physical activity modifications although each uses different methods to accomplish these goals. Comparison group participants in one trial (Expecting Success, Pennington) receive standard practice from their prenatal care provider while the remaining six trials use standard practice with some additional educational content unrelated to GWG, which is provided mainly for retention purposes.
Healthy Beginnings (California Polytechnic State University & Brown University) focuses on a comprehensive behavioral program which incorporates partial meal replacements (PMR) and encourages increased physical activity. The program is delivered by trained staff in individual sessions. The PMR plan provides a caloric prescription of ~20 kcal/kg of body weight to adhere to the 2009 IOM guidelines, and the PMR are study provided. The physical activity goal is 30 minutes of activity on most days of the week. Behavioral strategies (e.g., daily recording of food intake, activity, and weight; stimulus control techniques; problem-solving skills) and home environmental strategies (e.g., cabinet “cleanouts”, placement of visual cues) are encouraged. Weight graphs are provided to women at each visit. Additionally, women receive weekly educational tips via mail that are designed to reinforce healthy eating, physical activity, and behavioral recommendations.
The Lifestyle Intervention for Two (LIFT, St. Luke’s-Roosevelt Hospital and Columbia University) intervention focuses on diet modification (reduced caloric intake) and increased physical activity (30 min most days of the week) along with behavioral and social support strategies delivered in individual sessions by study counselors. The intervention program is derived from the lifestyle intervention curriculum developed by the Diabetes Prevention Program and Look AHEAD study, with the focus modified from an emphasis on weight loss to control of GWG as recommended by the 2009 IOM guidelines.(30, 31) During the postpartum period, group classes are offered every 8 weeks on health and weight management topics.
The Pregnancy and EARly Life improvement Study (PEARLS, University of Puerto Rico) intervention focuses on modifying total calorie consumption and improving diet quality (by reducing the intake of refined carbohydrates and sugar-sweetened beverages) and increasing physical activity levels (through promotion of non-structured physical activity). The intervention is delivered by study staff using an empowerment theoretical framework through individual and group-based counseling and communications.(32) The postpartum intervention focuses on breast-feeding, physical and cognitive activation of the infant, infant feeding patterns, sleep and healthy diet choices for the infant.
The Maternal-Offspring Metabolics: Family Intervention Trial (MOMFIT, Northwestern University) intervention follows the “MAMA DASH” diet (increased fruits, vegetables and low-fat dairy products) which is adapted from the Dietary Approaches to Stop Hypertension (DASH) study(33). Participants are also encouraged to gradually increase physical activity with a goal of achieving a goal of 10,000 steps per day. The intervention is delivered by study interventionists in both individual and group sessions. Weekly coaching phone calls/texts encourage adherence to the intervention. Participants are encouraged to self-monitor using the “Lose It!” smart phone application. The value and importance of breastfeeding is also reinforced. The postpartum intervention encourages adherence to the DASH diet, breastfeeding and discourages introduction of solid foods until four months but preferably six months of age.
The PreGO (Washington University in St. Louis) intervention is organized around social cognitive behavior change theory (e.g., self-assessment, reinforcement, behavioral capability, observational learning, environment) and targets: a) goals and strategies for achieving appropriate GWG, b) substituting water or low-fat milk for high-sugar beverages, c) substituting healthy for unhealthy foods, d) portion control, e) increasing access and availability of nutritious foods in the home, f) regular eating patterns, g) food cravings, and h) breastfeeding barriers and strategies and i) increasing physical activity (increase in walking with an ultimate goal of 150 minutes [2.5 hours] of moderate-intensity exercise [e.g., brisk walking] and/or lifestyle activity per week). The intervention is built on the scaffolding of the existing nationwide Parents as Teachers (PAT) program.(34) The PAT program provides free-of-charge parent-child education and services to women during the prenatal and post-partum period and is delivered by peer counselors in 10 weekly and bi-weekly visits. The lifestyle intervention (PAT+) is incorporated into the PAT curriculum and delivered by specially-trained PAT counselors.
The Expecting Success (Pennington Biomedical Research Center) intervention focuses on comprehensive behavior change in a 3-arm trial in which intervention participants are assigned to either a SmartMoms intervention delivered in-person (SmartMoms-Clinic) or through a smartphone app (SmartMoms-Phone). The SmartMoms-Clinic group receives the intervention, weight gain recommendations and feedback in group or individual sessions via an assigned counselor, whereas the SmartMoms-Phone group participants receive the intervention and feedback via the multi-media functions of the Smartphone. The intervention includes structured lessons (including diet, behavior change theory) delivered to participants weekly throughout the second trimester and semi-weekly throughout the third trimester. Participants in both intervention groups receive a scale and accelerometer to encourage self-monitoring along with regular dietary intake and exercise recommendations, and a weight graph, which presents weight gain trajectories recommended by the 2009 IOM guidelines.(13)
The Phoenix LIFE-Moms (NIDDK/PIMC) intervention focuses on individualized GWG goals. The intervention uses both structured group and individual sessions with study counselors. Participants are encouraged to meet those goals by monitoring their dietary intake (achieving individualized caloric and fat gram recommendations), increasing daily physical activity such as walking, and limiting sedentary behaviors (i.e., time spent sitting or lying). Key concepts in the lifestyle intervention are based on a modified version of the Diabetes Prevention Program Lifestyle Balance curriculum, which includes weight monitoring at each intervention visit, diet and physical activity counseling, and psychosocial support.
Each of the trials/interventions are considered to test highly innovative strategies to modify gestational weight gain (e.g., liquid meal replacements, modified DPP intervention, the DASH diet, smart-phone based intervention, parent educator intervention) and/or apply interventions to under-represented populations (e.g., minority, low socioeconomic status).
Developing core and super-shared measures
To identify common measures across the trials, each trial provided a list of criteria and measures specified in their individual study. Committees identified common measures based on the number of sites already incorporating that measure, the scientific rationale, and discussion and approval by the Steering Committee which took into consideration participant burden. Measures that are collected in all seven trials are defined as ‘core’ data and measures collected by 4 to 6 trials are defined as ‘super-shared’ data. All core and super-shared measures have a standardized definition and/or detailed procedures to facilitate uniform collection, a standardized training and certification process, and are entered into a common dataset at the RCU. Measures collected by three or fewer trials are defined as local (only one trial) or ‘shared’ (2 to 3 trials). The Consortium did not create standardized definitions and methods for local or shared measures as there is no pre-specified intent to pool these data.
Common measures include eligibility confirmation, assessments at study visits (e.g., blood pressure and weight) and outcome measures and evaluations (e.g., neonatal anthropometrics). Core inclusion and exclusion criteria are detailed in Table 2. Trials were allowed to use additional exclusion criteria if desired. Core and super-shared measures were developed for assessments performed at baseline, 24–27 weeks gestation, 35–36 weeks gestation, delivery and 1 year postpartum. Core measures include height, weight, blood pressure, ultrasound for dating gestational age, HbA1c (measured locally), physical activity and sleep assessed using the Actigraph GT3X+, medical history, medications, previous pregnancy, 2-hour 75 gram oral glucose tolerance test (OGTT) at 24–27 weeks gestation, development of contraindications to moderate- to high-intensity physical activity(35), maternal complications, delivery and neonatal outcomes, neonatal anthropometrics (weight, length, head circumference and skinfold thickness) and participant questionnaires (Beck Depression Inventory II, Eating Disorder Examination Questionnaire, demographics & social history, Modified-Pregnancy-Unique Quantification of Emesis and Nausea Index, Household Food Insecurity, breast feeding, maternal sedentary behavior, frequency of self-weighing, sleep, SF-12 Health Survey). A central trainer for neonatal and infant anthropometrics will visit each site initially and then annually to train and certify staff. Super-shared measures include maternal circumferences of the waist, arm and thigh, air displacement plethysmography for maternal (BODPOD) or infant (PEAPOD) body composition, OGTT at other time points and participant questionnaires (Physical Activity Neighborhood Environment Survey, NHS Physical Activity, Automated Self-Administered 24-hour Dietary Recall and Infant Feeding Styles).
Core biospecimen collections include maternal blood and urine at enrollment, 35–36 weeks gestation and 1 year postpartum, and cord blood. Super-shared biospecimen collections include placenta, breast milk at 4–8 weeks postpartum and infant blood at 12 months. Collection and processing procedures are standardized across all centers. All core and super-shared biospecimens are sent to the NIDDK Central Biosample Repository for storage and future analysis.
Primary & Secondary Outcomes
The primary outcome for the combined LIFE-Moms data is GWG per week above the 2009 Institute of Medicine’s upper limit of second and third trimester weight gain for pregnant women with overweight or obesity (greater than 0.32 kg/week and 0.27 kg/week respectively). Three factors were taken into account when defining GWG. First, the Consortium used the baseline study-measured weight rather than self-reported pre-pregnancy weight based on 1) the recognized unreliable nature of participant self-report, 2) the assumption that first-trimester weight should be a close representation of pre-pregnancy weight, and 3) the important goal of ensuring standardized assessment of baseline weight across all trials. Women with weights measured at 14 weeks will have 0.45 kilograms (1 pound) subtracted and women at 15 weeks 0.91 kilograms (2 pounds) subtracted for an estimate of their first-trimester baseline weight (i.e., pre-conception weight). These adjustments are based on data from women overweight and with obesity enrolled in the Eunice Kennedy Shriver NICHD Combined Antioxidant and Preeclampsia Prediction Studies.(36) Second, the weight measured by study staff at 35–36 weeks gestation rather than delivery will be used as the end weight based on 1) the recognized improvement in the accuracy of weight measured by trained study staff (using a calibrated scale), 2) weight measured at 35–36 weeks gestation should be less affected by the variation in fluid retention seen during the last month of pregnancy, and 3) the recognition that women are not routinely weighed when they are admitted for delivery. Third, to account for the differences in gestational age at delivery (and thus total pregnancy weeks), GWG per week is defined as the difference between study measured weight at 35–36 weeks and baseline weight divided by the number of days between the two dates divided by 7 to calculate weight gain per week. Secondary outcomes are detailed in Table 3 with definitions provided in Table S1.
Table 3.
Consortium primary and secondary outcomes
| Primary outcome | GWG above the 2009 Institute of Medicine’s guideline for pregnant women with overweight or obesity |
|---|---|
| Secondary outcomes | GWG per week |
| Early (second trimester) GWG per week and excessive early GWG | |
| Late (third trimester) GWG per week and excessive late GWG | |
| Excessive GWG using pre-pregnancy and weight closest to delivery | |
| Gestational diabetes | |
| Lipids (LDL, HDL, triglycerides, total cholesterol) | |
| Insulin resistance | |
| Blood pressure | |
| Gestational hypertension and preeclampsia | |
| Iatrogenic delivery | |
| Mode of delivery (vaginal, operative, cesarean) | |
| Preterm and early preterm delivery | |
| Shoulder dystocia | |
| Small and large for gestational age | |
| Macrosomia | |
| Birth trauma | |
| Neonatal intensive care unit or intermediate care nursery admission for 12 hours or more | |
| Respiratory morbidity | |
| Neonatal hypoglycemia requiring treatment | |
| Neonatal and infant anthropometrics at 1 year (ponderal index, body mass index, weight for age and length and adiposity) | |
| Physical activity | |
| Sedentary behavior | |
| Sleep duration | |
| Postpartum pre-diabetes and diabetes | |
| Postpartum hypertension | |
| Postpartum weight retention | |
| Frequency of self weighing | |
| Postpartum maternal quality of life | |
| Initiation of breast feeding | |
GWG = gestational weight gain, Definitions included in Table S1
Data collection / management
The RFA stated that sites were responsible for data collection and quality control and the RCU was responsible for establishing and maintaining a computer system to receive and store any datasets that are common. Given the large number of core and super-shared measures and to ensure consistency in collection, the RCU created the data collection forms to capture all of these data. To aid in the data transfer, the RCU specified variable names and formats on the data forms and provided a detailed manual of transfer procedures. Data checks for missing, out of range values, and data inconsistencies will be generated and sent to each of the centers on a weekly basis, regardless of whether data are entered directly into the RCU data management system or transferred to the RCU.
Safety monitoring / DSMB
The DSMB, an independent group of experts not affiliated with any of the participating institutions in the Consortium, was convened by NIDDK. Initially the DSMB reviewed and approved the protocols and informed consent documents before any of the individual studies could launch. Any subsequent major changes to a trial’s protocol are also reviewed by the DSMB. The LIFE-Moms DSMB has overall responsibility for monitoring the progress of each trial as well as the overall research program. The DSMB focuses on safety, recruitment and retention, protocol adherence, and data quality.
In addition to the DSMB meetings, the NIDDK Program Official and DSMB Chair review each serious adverse event (SAE) in real time. The LIFE-Moms Safety Committee reviews aggregated data on adverse events, SAEs, and safety alerts on a monthly basis. Safety alerts developed by the Safety Committee include weight loss during pregnancy, high blood pressure, contraindications to moderate- to high-intensity physical activity during pregnancy(35), active suicidal ideation or moderate/severe depression, abnormal or out-of-range findings on imaging and/or laboratory studies, child abuse/neglect, and poor infant growth.
Analyses
Consortium analyses will encompass the core and super-shared data and are considered exploratory. Analyses will be based on intent-to-treat principles and will include only baseline variables as covariates. The main analyses will compare all participants in the intervention groups with all participants in the standard of care/enhanced standard of care groups. Data will be analyzed in aggregate using forest plots showing the individual results of the seven trials and as an individual participant data meta-analysis which will include a random effect for each trial. Subgroup analyses are planned for race/ethnicity, socioeconomic status, maternal age, gestational age at randomization, baseline accelerometry, parity and BMI category. An analysis that incorporates a taxonomy of the lifestyle intervention is also being investigated with the intent of determining which aspects of the interventions were successful.(37)
Discussion
LIFE-Moms is modeled on previously-funded NIH consortia, including primarily the EARLY studies and the POWER trials.(28, 29) With this consortium-based model in mind, the NIH-issued RFAs that led to LIFE-Moms invited clinical applications proposing randomized clinical trials in pregnant women with overweight or obesity, with an emphasis on enrollment of women from diverse racial/ethnic groups and lower socioeconomic status. Seven clinical trials and a RCU were selected. LIFE-Moms applicants were required to convene a multi-disciplinary investigative team with wide clinical and research expertise relevant to the focus of the consortium, including obstetricians, behaviorists, pediatricians, statisticians, and clinical trialists. LIFE-Moms differs from the previous models in having a very short period for recruitment (prior to 16 weeks gestation), a single population (pregnant women with overweight or obesity), and a larger number of trials.
Described are the establishment and development phases of the LIFE-Moms Consortium that occurred during the first year prior to the launch of recruitment. The Consortium first developed consensus regarding common measures to be assessed across all the trials, thus ensuring standardized definitions and collection procedures. Other administrative and scientific characteristics of this Consortium are further described.
Supplementary Material
What is already known about this subject
Preconception, maternal overweight and obesity are risk factors for adverse birth outcomes
Excessive gestational weight gain is associated with numerous adverse maternal, fetal and neonatal outcomes
Several randomized clinical trials aimed at reducing excessive weight gain during pregnancy have produced mixed results
What this study adds
The Consortium is designed to identify effective interventions to control gestational weight gain in pregnant women with overweight or obesity
Identifying common measures and ensuring standardization across the seven trials will allow data to be compared across the trials as well as combined in a pre-planned meta-analysis
The design of the Consortium may be useful in planning future consortia or other lifestyle intervention studies
Acknowledgments
Funding:
LIFE-Moms is supported by the National Institutes of Health through The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK, U01 DK094418, U01 DK094463, U01 DK094416, 5U01 DK094466 (RCU)), The National Heart, Lung, and Blood Institute (NHLBI, U01 HL114344, U01 HL114377), and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD, U01 HD072834). In addition, the National Center for Complementary and Integrative Health (NCCIH), the NIH Office of Research in Women’s Health (ORWH), the Office of Behavioral and Social Science Research (OBSSR), the Indian Health Service and the Intramural Research Program of the NIDDK contributed support.
Additional support was received from the NIDDK Obesity Nutrition Research Centers (P30 DK026687, P30 DK072476, P30 DK56341), National Center for Advancing Translational Sciences Clinical and Translational Science Awards (U54 GM104940, U54 MD007587, UL1 RR024992), National Institute on Minority Health and Health Disparities (S21MD001830) and EXODIAB-Excellence of diabetes research in Sweden.
*The LIFE-Moms Research Group
The following individuals and institutions are members of the LIFE-Moms Research Group (asterisks indicate principal investigators):
Clinical Centers
California Polytechnic State University & Brown University: S. Phelan*, R.R. Wing, T.A. Hagobian, A. Schaffner, C. Hart, E.K. Yin, M.G. Phipps, B. Abrams, T.O. Scholl, D.A. Savitz
St. Luke’s-Roosevelt Hospital and Columbia University: D. Gallagher*, X. Pi-Sunyer*, J. Thornton, B. Rosenn, C. Paley, S. Gidwani, M. Horowitz
University of Puerto Rico: K. Joshipura*, P.W. Franks*, C. Palacios, M. Campos, J. Rivera, W.C. Willett, C. Zorrilla, S. Soltero, F. Hu, J. Cordero, M.A. Trak, M. Meléndez
Washington University in St. Louis: A.G. Cahill*, S. Klein*, D. Haire-Joshu*, R. Stein, A. Mathur, W.T. Cade, K. Moley
Northwestern University: A.M. Peaceman*, L. Van Horn*, M. Kwasny, J.L. Josefson, L. Neff, B. Spring
Pennington Biomedical Research Center: L.M. Redman*, C.K. Martin, K. Elkind-Hirsh, J. Breaux, W. Johnson, E.A. Frost
NIDDK/Phoenix Indian Medical Center: W.C. Knowler*, K.A. Couch*, J.M. Curtis, D.L. Dunnigan, R.L. Hanson, M. Hoskin, K. Kavena, G.Y. Kishi, C. Moffett, R.G. Nelson, J. Pomeroy, L. Shovestull, Rachel Williams
Research Coordinating Unit, George Washington University Biostatistics Center: R.G. Clifton*, E.A. Thom*, K. Drews, T. Boekhoudt
NIH: M. Evans (NIDDK), S.Z. Yanovski (NIDDK), S. Arteaga (NHLBI), D.L. Alekel (NCCIH, now at NIAMS)
Writing group with institutions
Rebecca G. Clifton, Ph.D.
The George Washington University Biostatistics Center, Washington, DC, USA,
Mary Evans, Ph.D.
The National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, USA
Alison G. Cahill, M.D., M.S.C.I.
Washington University in St. Louis School of Medicine, St. Louis, MO, USA
Paul W. Franks, Ph.D.
Department of Clinical Sciences, Genetic and Molecular Epidemiology Unit, Lund University, Skåne University Hospital Malmö, Malmö, Sweden; Department of Nutrition, Harvard School of Public Health, Boston, MA, USA
Dympna Gallagher, Ed.D.
Department of Medicine, St. Luke’s-Roosevelt Hospital and Columbia University, New York, NY, USA
Suzanne Phelan, Ph.D.
Department of Kinesiology, California Polytechnic State University, San Luis Obispo, CA, USA
Jeremy Pomeroy, Ph.D.
Phoenix Epidemiology and Clinical Research Branch, National Institute of Diabetes and Digestive and Kidney Diseases, Phoenix, AZ, USA
Leanne M. Redman, Ph.D.
Pennington Biomedical Research Center, Baton Rouge, LA
Linda Van Horn, Ph.D., R.D.
Department of Preventive Medicine, Northwestern University, Feinberg School of Medicine, Chicago, IL
Footnotes
Disclosure: LMR reported one of the interventions being evaluated in the Expecting Success trial at Pennington Biomedical Research Center (the SmartMoms™ smartphone application) has a pending trademark and is available for licensure. The other writing group members declared no conflict of interest.
References
- 1.Metwally M, Ong KJ, Ledger WL, Li TC. Does high body mass index increase the risk of miscarriage after spontaneous and assisted conception? A meta-analysis of the evidence. Fertil Steril. 2008;90:714–726. doi: 10.1016/j.fertnstert.2007.07.1290. [DOI] [PubMed] [Google Scholar]
- 2.Alanis MC, Goodnight WH, Hill EG, Robinson CJ, Villers MS, Johnson DD. Maternal super-obesity (body mass index >or = 50) and adverse pregnancy outcomes. Acta obstetricia et gynecologica Scandinavica. 2010;89:924–930. doi: 10.3109/00016341003657884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brost BC, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, et al. The Preterm Prediction Study: association of cesarean delivery with increases in maternal weight and body mass index. American journal of obstetrics and gynecology. 1997;177:333–337. doi: 10.1016/s0002-9378(97)70195-9. discussion 337–341. [DOI] [PubMed] [Google Scholar]
- 4.Chen A, Feresu SA, Fernandez C, Rogan WJ. Maternal obesity and the risk of infant death in the United States. Epidemiology. 2009;20:74–81. doi: 10.1097/EDE.0b013e3181878645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards LE, Hellerstedt WL, Alton IR, Story M, Himes JH. Pregnancy complications and birth outcomes in obese and normal-weight women: effects of gestational weight change. Obstetrics and gynecology. 1996;87:389–394. doi: 10.1016/0029-7844(95)00446-7. [DOI] [PubMed] [Google Scholar]
- 6.Ehrenberg HM, Iams JD, Goldenberg RL, Newman RB, Weiner SJ, Sibai BM, et al. Maternal obesity, uterine activity, and the risk of spontaneous preterm birth. Obstetrics and gynecology. 2009;113:48–52. doi: 10.1097/AOG.0b013e318191c818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendler I, Goldenberg RL, Mercer BM, Iams JD, Meis PJ, Moawad AH, et al. The Preterm Prediction Study: association between maternal body mass index and spontaneous and indicated preterm birth. American journal of obstetrics and gynecology. 2005;192:882–886. doi: 10.1016/j.ajog.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Landon MB, Leindecker S, Spong CY, Hauth JC, Bloom S, Varner MW, et al. The MFMU Cesarean Registry: factors affecting the success of trial of labor after previous cesarean delivery. American journal of obstetrics and gynecology. 2005;193:1016–1023. doi: 10.1016/j.ajog.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 9.McDonald SD, Han Z, Mulla S, Beyene J Knowledge Synthesis G. Overweight and obesity in mothers and risk of preterm birth and low birth weight infants: systematic review and meta-analyses. Bmj. 2010;341:c3428. doi: 10.1136/bmj.c3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson HE, O'Connell CM, Joseph KS, McLeod NL. Maternal outcomes in pregnancies complicated by obesity. Obstetrics and gynecology. 2005;106:1357–1364. doi: 10.1097/01.AOG.0000188387.88032.41. [DOI] [PubMed] [Google Scholar]
- 11.Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: a systematic review and meta-analysis. JAMA : the journal of the American Medical Association. 2009;301:636–650. doi: 10.1001/jama.2009.113. [DOI] [PubMed] [Google Scholar]
- 12.Restall A, Taylor RS, Thompson JM, Flower D, Dekker GA, Kenny LC, et al. Risk factors for excessive gestational weight gain in a healthy, nulliparous cohort. Journal of obesity. 2014;2014:148391. doi: 10.1155/2014/148391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine and National Research Council. Weight gain during pregnancy: reexamining the guidelines. Washington, DC: The National Academies Press; 2009. [PubMed] [Google Scholar]
- 14.Carreno CA, Clifton RG, Hauth JC, Myatt L, Roberts JM, Spong CY, et al. Excessive early gestational weight gain and risk of gestational diabetes mellitus in nulliparous women. Obstetrics and gynecology. 2012;119:1227–1233. doi: 10.1097/AOG.0b013e318256cf1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortner RT, Pekow P, Solomon CG, Markenson G, Chasan-Taber L. Prepregnancy body mass index, gestational weight gain, and risk of hypertensive pregnancy among Latina women. American journal of obstetrics and gynecology. 2009;200:167 e161, e167. doi: 10.1016/j.ajog.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 16.Johnson J, Clifton RG, Roberts JM, Myatt L, Hauth JC, Spong CY, et al. Pregnancy outcomes with weight gain above or below the 2009 Institute of Medicine guidelines. Obstetrics and gynecology. 2013;121:969–975. doi: 10.1097/AOG.0b013e31828aea03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kiel DW, Dodson EA, Artal R, Boehmer TK, Leet TL. Gestational weight gain and pregnancy outcomes in obese women: how much is enough? Obstetrics and gynecology. 2007;110:752–758. doi: 10.1097/01.AOG.0000278819.17190.87. [DOI] [PubMed] [Google Scholar]
- 18.Nohr EA, Vaeth M, Baker JL, Sorensen T, Olsen J, Rasmussen KM. Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. The American journal of clinical nutrition. 2008;87:1750–1759. doi: 10.1093/ajcn/87.6.1750. [DOI] [PubMed] [Google Scholar]
- 19.Amorim AR, Rossner S, Neovius M, Lourenco PM, Linne Y. Does excess pregnancy weight gain constitute a major risk for increasing long-term BMI? Obesity. 2007;15:1278–1286. doi: 10.1038/oby.2007.149. [DOI] [PubMed] [Google Scholar]
- 20.Oken E, Rifas-Shiman SL, Field AE, Frazier AL, Gillman MW. Maternal gestational weight gain and offspring weight in adolescence. Obstetrics and gynecology. 2008;112:999–1006. doi: 10.1097/AOG.0b013e31818a5d50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson CM, Strawderman MS, Dennison BA. Maternal weight gain during pregnancy and child weight at age 3 years. Maternal and child health journal. 2009;13:839–846. doi: 10.1007/s10995-008-0413-6. [DOI] [PubMed] [Google Scholar]
- 22.Asbee SM, Jenkins TR, Butler JR, White J, Elliot M, Rutledge A. Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstetrics and gynecology. 2009;113:305–312. doi: 10.1097/AOG.0b013e318195baef. [DOI] [PubMed] [Google Scholar]
- 23.Polley BA, Wing RR, Sims CJ. Randomized controlled trial to prevent excessive weight gain in pregnant women. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2002;26:1494–1502. doi: 10.1038/sj.ijo.0802130. [DOI] [PubMed] [Google Scholar]
- 24.Thornton YS, Smarkola C, Kopacz SM, Ishoof SB. Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. Journal of the National Medical Association. 2009;101:569–577. doi: 10.1016/s0027-9684(15)30942-1. [DOI] [PubMed] [Google Scholar]
- 25.Dodd JM, Grivell RM, Crowther CA, Robinson JS. Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG : an international journal of obstetrics and gynaecology. 2010;117:1316–1326. doi: 10.1111/j.1471-0528.2010.02540.x. [DOI] [PubMed] [Google Scholar]
- 26.Vesco KK, Karanja N, King JC, Gillman MW, Leo MC, Perrin N, et al. Efficacy of a group-based dietary intervention for limiting gestational weight gain among obese women: A randomized trial. Obesity. 2014;22:1989–1996. doi: 10.1002/oby.20831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dodd JM, Turnbull D, McPhee AJ, Deussen AR, Grivell RM, Yelland LN, et al. Antenatal lifestyle advice for women who are overweight or obese: LIMIT randomised trial. Bmj. 2014;348:g1285. doi: 10.1136/bmj.g1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lytle LSS, Patrick K, et al. The EARLY trials: a consortium of studies targeting weight control in young adults. Translational Behavioral Medicine. 2014 doi: 10.1007/s13142-014-0252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yeh HC, Clark JM, Emmons KE, Moore RH, Bennett GG, Warner ET, et al. Independent but coordinated trials: insights from the practice-based Opportunities for Weight Reduction Trials Collaborative Research Group. Clinical trials. 2010;7:322–332. doi: 10.1177/1740774510374213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diabetes Prevention Program Research Group. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Look AHEAD Research Group. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity. 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tucker CM, Lopez MT, Campbell K, Marsiske M, Daly K, Nghiem K, et al. The effects of a culturally sensitive, empowerment-focused, community-based health promotion program on health outcomes of adults with type 2 diabetes. Journal of health care for the poor and underserved. 2014;25:292–307. doi: 10.1353/hpu.2014.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. The New England journal of medicine. 2001;344:3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 34.Parents as Teachers 2012–2013 Annual Report. Available from: http://www.parentsasteachers.org/images/stories/documents/pat_ar2013-FA_sm.pdf. [Google Scholar]
- 35.American Congress of Obstetricians and Gynecologists. Exercise During Pregnancy and the Postpartum Period. Committee Opinion #267. January 2002 (reaffirmed 2009) Obstet Gynecol. 2002;99:171–173. doi: 10.1016/s0029-7844(01)01749-5. [DOI] [PubMed] [Google Scholar]
- 36.Roberts JM, Myatt L, Spong CY, Thom EA, Hauth JC, Leveno KJ, et al. Vitamins C and E to prevent complications of pregnancy-associated hypertension. The New England journal of medicine. 2010;362:1282–1291. doi: 10.1056/NEJMoa0908056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2013;46:81–95. doi: 10.1007/s12160-013-9486-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.