Highlights
-
•
Influenza-specific antibody levels were significantly increased after immunization with TIV + rOv-ASP-1 in aged mice.
-
•
rOv-ASP-1 was superior to the conventional adjuvant alum in inducing specific IgG after TIV immunization in aged mice.
-
•
Co-administration of rOv-ASP-1 induced cross-reactive antibody and enhanced cross-protection.
Keywords: Adjuvant, Influenza, Virus, Vaccine, Aged
Abbreviations: rOv-ASP-1, recombinant Onchocerca volvulus activation-associated secreted protein-1; TIV, trivalent inactivated influenza vaccine; HAU, hemagglutination unit
Abstract
Immunization is the best way to prevent seasonal epidemics and pandemics of influenza. There are two kinds of influenza vaccines available in the United States: an inactivated vaccine (TIV) and an attenuated vaccine; however, only TIV is approved for immunization of the elderly population. While the aged population has the highest rate of influenza vaccination, the protective efficacy is low as evidenced by elderly individuals having the highest mortality associated with influenza. Recently, we reported that an adjuvant derived from the helminth parasite Onchocerca volvulus, named O. volvulus activation-associated secreted protein-1 (Ov-ASP-1), can significantly enhance the protective efficacy of an inactivated vaccine (TIV) in young adult mice. In the current study, we examined whether this recombinant Ov-ASP-1 (rOv-ASP-1) can enhance the efficacy of TIV in aged mice as well. While primary immunization with TIV alone produced only a low level of influenza-specific antibodies (total IgG, IgG1, and IgG2c) in aged mice, the antibody levels were significantly increased after immunization with TIV + rOv-ASP-1. More importantly, the level of the total IgG in aged mice administered TIV + rOv-ASP-1 was comparable to that of young adult mice immunized with TIV alone. Co-administration of rOv-ASP-1 induced a low level of cross-reactive antibody and enhanced the protective efficacy of TIV in aged mice, reflected by significantly increased survival after challenge with a heterologous influenza virus. rOv-ASP-1 was also superior to the conventional adjuvant alum in inducing specific IgG after TIV immunization in aged mice, and in conferring protection after challenge. These results demonstrate that rOv-ASP-1 may serve as a potential adjuvant for influenza vaccine to improve the efficacy of protection in the elderly.
1. Introduction
Influenza virus is capable of causing serious disease resulting in hospitalization and death, especially in elderly individuals (>65 years of age). The influenza pandemic of 1918 killed nearly 50 million people worldwide, with the elderly having the highest mortality rate [1], [2]. A strong, primary immune response is important for protection against influenza viruses, since changes in the antigenic epitopes of the viruses occur rapidly. The immune response alters and diminishes with aging [3], [4], leaving the elderly more susceptible to infectious diseases. CDC estimates that from the 1976–1977 season to the 2006–2007 flu season, flu-associated deaths ranged from 3,000 to 49,000 people (CDC, 2010), and 90% of these deaths are among the elderly [5].
Immunization is the best way to prevent seasonal epidemics and pandemics of influenza [6]. Two different vaccines are presently used in the United States: a killed vaccine (trivalent inactivated influenza vaccine, TIV) that has been utilized since 1945 [7], and a live, attenuated vaccine that was approved by the FDA in 2003 for people between the ages of 2–49 [8], [9]. While both vaccines are effective in young adults, a diminished efficacy of TIV is consistently found in elderly recipients [10], [11]. The live, attenuated influenza vaccine is not approved for use in individuals with weakened immune systems or in the elderly [8], [9]. A high dose TIV vaccine has been reported to increase antibody response and to reduce the development of influenza by 22% in the elderly compared to the traditional dose of TIV [12], [13], [14]. However, neither dose totally prevents the disease.
In addition to increasing the antigen dose, the use of adjuvants is a proven approach to enhance the immunogenicity and protective efficacy of vaccines. Adjuvants have also been shown to enhance cross-reactive responses [15]. However, at present, there are very few adjuvants commercially available for use in humans [16]. Aluminum salt (Alum) is the first and only adjuvant approved in the United States for general use in vaccines [17], [18]. During the 1960s and 1970s, many influenza vaccines commercially available in both the United States and Europe were alum-adsorbed. However, alum was removed from influenza vaccine formulations in the early 1980s because large clinical data showed that it only marginally enhanced the antibody response, while inducing increased adverse effects [19], [20], [21].
A protein with adjuvant potential in mice has been discovered recently from the helminth parasite Onchocerca volvulus, O. volvulus activation-associated secreted protein-1 (Ov-ASP-1) [22], [23], [24], [25], [26], [27]. It is a member of a family of proteins found in both free-living and parasitic nematodes [28]. The recombinant Ov-ASP-1 (rOv-ASP-1) has a predicted molecular weight of 24.9 kD [28]. The adjuvanticity of rOv-ASP-1 has been studied using ovalbumin, HIV-1polypeptide, SARS-CoV peptide antigens, and several commercially available vaccines [23], [24], [26], [29]. rOv-ASP-1 functions as an adjuvant that enhances not only Th2, but also strong Th1 immune responses [23], the latter of which is critical for stimulating antiviral immune responses. We previously reported that rOv-ASP-1, when co-administrated with TIV using an aqueous formulation, significantly improves the influenza-specific antibody response and protection against lethal infection in young mice [25]. In the current study, we found rOv-ASP-1 similarly enhances influenza-specific response to TIV in aged mice.
2. Materials and methods
2.1. Mice
Two-month- and 22-month-old C57BL/6 (B6) mice were obtained from the NIA at Harlan Sprague Dawley (Indianapolis, IN). All mice were maintained in AAALAC-approved barrier facilities at Drexel University (Philadelphia, PA). Mice were allowed to acclimate for at least one week in the animal facilities prior to use. All experiments involving mice were conducted with the approval of the Institutional Animal Care and Use Committee (IACUC) at Drexel University. Mice exhibiting enlarged spleens or tumors were eliminated from this study.
2.2. Immunization
The influenza vaccine used in this study was a commercially available TIV which contains 15 μg of influenza virus hemagglutinin (HA) from each of the following 3 viruses: A/California/7/2009 NYMC X-181 (H1N1); A/Victoria/210/2009 NYMC X-187 (H3N2); and B/Brisbane/60/2008 (2010-2011 Formula, FLUARIX, GlaxoSmithKline) in a 0.5 ml volume. Mice were immunized intramuscularly (i.m.) with 0.6 μg of TIV or TIV mixed with purified rOv-ASP-1 derived from a recombinant Escherichia coli [24], [29], [30] (20 μg/mouse) in saline. As an adjuvant comparison control, mice were immunized with TIV mixed with Imject Alum (Thermo Scientific, 0.4 mg/mouse) at a 1:3 ratio (Alum:antigen volume). All mice were bled from their tails weekly for 3 weeks post-immunization. Plasma was utilized for all assays.
2.3. Influenza virus and infection
Influenza A/Puerto Rico/8/34 (PR8; H1N1) virus strain was propagated in specific pathogen-free fertile chicken eggs, as previously described [3], [31]. Groups of 7 mice were challenged intranasally (i.n.) on day 21 post primary immunization. Mice were anesthetized intraperitoneally with ketamine/xylazine (2 mg ketamine and 0.15 mg xylazine) before i.n. infection with 30 μl of sterile saline containing 2xLD50 of PR8 (20 HAU/mouse). The protective efficacy of immunization was measured by survival up to 13 days, and mice that lost ≥25% of their initial body weight were sacrificed according to institutional guidelines.
2.4. ELISA for total influenza-specific IgG, IgG1, and IgG2c
The influenza-specific antibody response was measured by ELISA as previously described [23], [27]. Briefly, 96 well ELISA plates were coated with TIV (0.6 μg/ml for each HA) or a UV-inactivated PR8 strain of influenza virus (140 HAU/ml). Bound antibody in plasma was detected with goat anti-mouse IgG, IgG1 or IgG2c antibody (Molecular Probes-Invitrogen). The titer of antibody was defined as the reciprocal of the highest dilution of plasma giving an optical density (OD) greater than 2 times that of a naïve mouse sample. The limit of detection (LOD) of IgG titer is indicated for each experiment in its respective figure.
2.5. Statistical analysis
The unpaired, two-tailed Student's t-test was used to determine if the difference in immune responses between groups of mice was significant. Survival rates were analyzed by the log-rank (Mantel-Cox) test. Antibody titers are expressed as mean ± SEM. Since antibody in plasma was quantitated using 1:2 serial dilutions in the ELISA, we compared the difference in the level of antibody between different groups with log2-converted antibody titers. Results were considered statistically significant when the p value was less than 0.05.
3. Results
3.1. rOv-ASP-1 enhances influenza-specific IgG response to TIV in aged mice
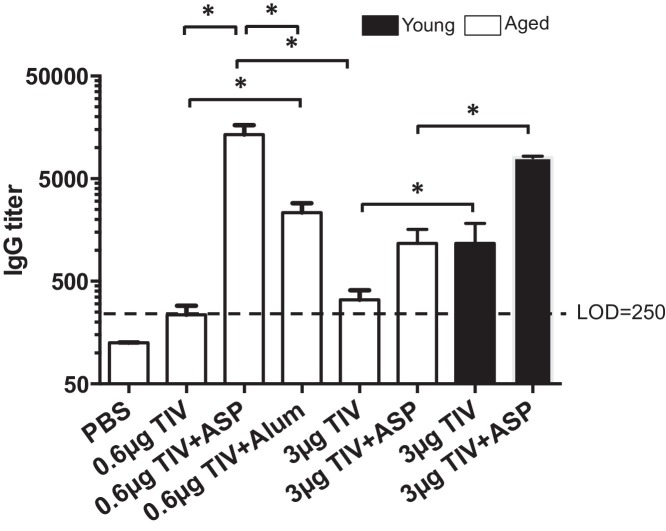
To examine if rOv-ASP-1 can enhance the specific IgG response to TIV in aged mice, aged B6 mice were immunized i.m. with TIV with or without rOv-ASP-1. Three weeks after immunization, the influenza-specific IgG antibody in plasma was determined by ELISA. As shown in Fig. 1 , when immunized with TIV alone, specific IgG was observed in young adult mice as previously reported [25], while the level of the antibody in aged mice was below the LOD. However, after immunization with TIV + rOv-ASP-1, the IgG levels were significantly increased in both young adult and aged mice (TIV vs TIV + rOv-ASP-1: Young: p = 0.0014; Aged: p = 0.0066). The IgG titer increased 9.3 times in aged mice and 8.7 times in young adult mice. Importantly, the level of IgG in aged mice after immunization with TIV + rOv-ASP-1 was comparable to that of young adult mice immunized with TIV alone (p = 0.74). These data demonstrate that rOv-ASP-1 can significantly enhance the specific antibody response to TIV in both young adult and aged mice.
Fig. 1.
rOv-ASP-1 enhances antibody response to TIV in aged mice. Aged B6 mice were immunized i.m. with TIV alone (0.6 μg or 3 μg/mouse), or 0.6 μg or 3 μg TIV admixed with rOv-ASP-1 (TIV + ASP), or 0.6 μg TIV with alum (0.4 mg/mouse). As controls, young adult B6 mice were immunized i.m. with 3 μg TIV alone (3 μg TIV), or 3 μg TIV admixed with rOv-ASP-1 (3 μg TIV + ASP). Three weeks after immunization, influenza-specific IgG levels were determined in plasma using ELISA. Pooled data represent 9–13 mice in each group from 2 to 3 experiments. * p < 0.05.
To compare the adjuvant effect of rOv-ASP-1 to the conventional adjuvant alum, we immunized aged B6 mice i.m. with 0.6 μg of TIV alone, or with 20 μg rOv-ASP-1, or 0.4 mg Alum (Fig. 1). Mice immunized with a 5-fold higher dose of TIV alone (3 μg/mouse) was included to investigate if rOv-ASP-1 can provide antigen sparing. Three weeks after vaccination, the influenza-specific IgG in plasma was determined by ELISA. As shown in Fig. 1, addition of either rOv-ASP-1 or alum significantly enhanced influenza-specific IgG response compared to TIV alone (TIV vs TIV + rOv-ASP-1: p = 0.0007; TIV vs TIV + alum: p = 0.0015). However, the titer of IgG was significantly higher using rOv-ASP-1 compared to alum (p = 0.003). Interestingly, no significant difference in IgG levels was observed between the low and high dose of TIV given alone (p = 0.12) indicating that the antibody response to TIV cannot be improved in aged mice by simply increasing the dose of the vaccine.
3.2. rOv-ASP-1 enhances both Th1- and Th2-associated influenza-specific antibody response in aged mice
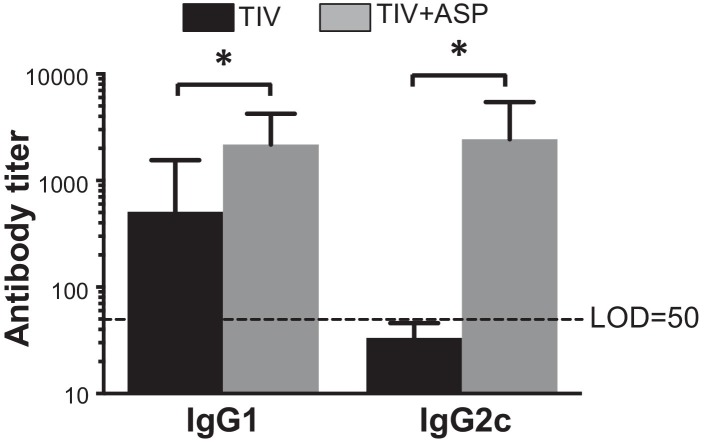
The Th1 antibody response induced by influenza vaccine plays an important role in protection against influenza infection [32]. Therefore, we investigated the ability of rOv-ASP-1 to enhance Th1 (IgG2a) and Th2 (IgG1) antibody responses in aged mice. It has been reported that unlike BALB/c mice, B6 mice do not have an IgG2a gene, but express a different Th1 antibody, IgG2c, which cross-reacts with IgG2a [33], [34], [35]. We, therefore, determined influenza-specific IgG2c titers. Three weeks after i.m. immunization with TIV or TIV + rOv-ASP-1, titers of IgG1 and IgG2c in plasma were determined by ELISA. As shown in Fig. 2 , TIV alone induced an IgG1 response, and rOv-ASP-1 significantly enhanced IgG1 levels compared to TIV alone (p = 0.045). TIV alone, however, did not induce detectable IgG2c, while co-administration of rOv-ASP-1 induced high levels of IgG2c compared to TIV alone (p = 0.028), which was also comparable to levels of IgG1. These results demonstrate that primary immunization of aged mice with rOv-ASP-1 + TIV induces an increase in both IgG1 and IgG2c responses, and, importantly, elicits a significant influenza-specific IgG2c response that does not occur with TIV alone.
Fig. 2.
rOv-ASP-1 enhances both Th1- and Th2-associated influenza-specific antibody response in aged mice. Twenty-two month old B6 mice were immunized i.m. with TIV alone (0.6 μg/mouse) or admixed with rOv-ASP-1 (TIV + ASP). Three weeks after immunization, influenza-specific IgG1 and IgG2c levels were examined in plasma using ELISA. Pooled experimental data represent 12 mice in each group in two experiments. * p < 0.05.
3.3. rOv-ASP-1 induction of a cross-reactive antibody response
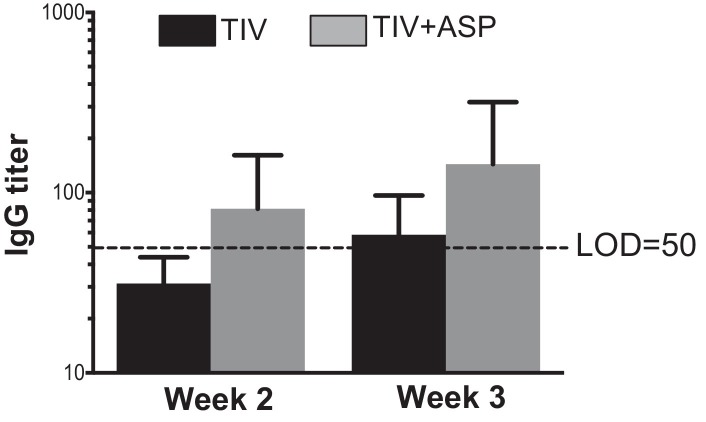
Induction of cross-reactive antibodies is particularly important for influenza vaccines since the frequent change of HA requires yearly immunization and, in some years, leads to loss of vaccine efficacy due to the mismatch of vaccine components and the circulating viruses. PR8 virus is a mouse-adapted H1N1 influenza virus that was isolated in 1934. This virus differs substantially from the recently isolated A/California/7/2009 NYMC X-181 (H1N1) strain that is a component of TIV. We reported that rOv-ASP-1 enhanced a cross-reactive IgG response in young adult mice and provided protection against an infection induced by the antigenically different PR8 strain[25]. To test if rOv-ASP-1 could enhance a cross-reactive antibody response in aged mice as well, mice were immunized i.m. with TIV or TIV + rOv-ASP-1. The cross-reactive responses at weeks 1 through 3 post primary immunization were determined by ELISA in which the plates were coated with inactivated PR8 virus. No cross-reactive antibodies were detectable at week 1 with either immunization (data not shown). At weeks 2 and 3, cross-reactive antibody levels were detected in mice immunized with TIV + rOv-ASP-1, while no cross-reactive antibody was detected in TIV alone group at week 2, and only a marginal level was detected at week 3 (Fig. 3 ). These data demonstrate that TIV + rOv-ASP-1 can induce a cross-reactive antibody response, however, at a lower level than we previously observed in young adult mice [25].
Fig. 3.
rOv-ASP-1 induce a low level of cross-reactive antibody response after immunization with TIV. Aged B6 mice were immunized i.m. with 0.6 μg of TIV alone or combined with rOv-ASP-1 (TIV + ASP). Plasma was collected at weeks 2 and 3 after each immunization and influenza-specific IgG was determined by ELISA using inactivated PR8 virus. Similar experiments were performed 3 times with similar results (3–4 mice/group).
3.4. rOv-ASP-1 induces protection by TIV in aged mice after primary immunization
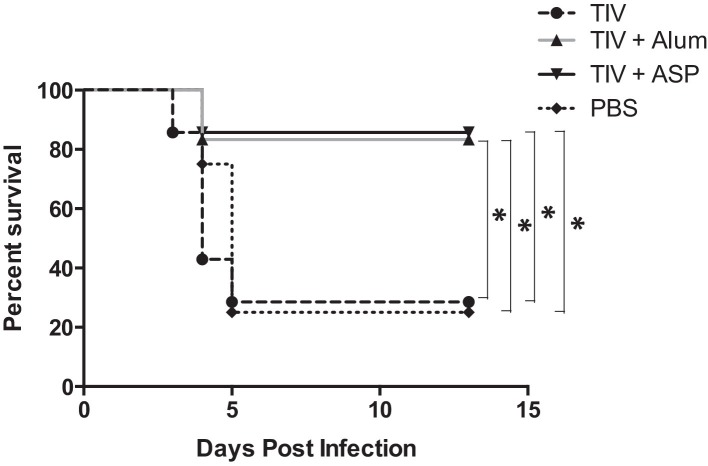
The ability of rOv-ASP-1 to enhance the efficacy of TIV against an influenza virus challenge was examined in aged mice. Aged B6 mice were immunized i.m. with TIV alone, or with rOv-ASP-1 or alum, and were challenged i.n. three weeks later with influenza virus PR8. As shown in Fig. 4 , while TIV alone afforded no protection against PR8 virus infection (28.5% survival vs 25% survival in controls), 86% of the TIV + rOv-ASP-1 group and 83% of the TIV + Alum group survived. Importantly, 100% of the TIV immunized group and 72% of TIV + Alum immunized mice demonstrated influenza disease based on >5% loss of body weight, while only 43% of TIV + rOv-ASP-1 showed weight loss. Further, the average weight loss on Day 7 of the TIV + Alum group was 10.0%, while of the TIV + rOv-ASP-1 was only 1.7% (p < 0.05). These results indicate that rOv-ASP-1 has the potential to enhance with survival and disease protection in aged mice, even against an antigenically distinct virus.
Fig. 4.
Protection of TIV-immunized mice following influenza virus challenge. Aged B6 mice were immunized i.m. with TIV alone or with rOv-ASP-1 (TIV + ASP) or alum (TIV + Alum). Three weeks after immunization, mice were infected i.n. with a lethal dose of PR8 (20 HAU/mouse). Mice were monitored daily for 13 days. Death was defined as ≥25% weight loss. Data represent 7–8 mice in each group. * p < 0.05.
4. Discussion
Annual vaccination against influenza is a key strategy employed to combat this illness, and it is very effective in healthy, young adults [6]. However, it is much less successful in the elderly [36], [37], [38], [39]. The dysregulated immune response seen with aging contributes to the diminished ability of the vaccine to provide protection. While it has been reported that increasing the dose of antigen fourfold can effectively increase this diminished response of the elderly [12], [13], [14], we found that the level of influenza-specific IgG in the plasma of aged mice was not significantly enhanced by administration of a 5 fold higher dose of TIV (Fig. 1). We, therefore, suggest that additional alternatives need to be explored.
One potential strategy to improve vaccine efficacy is the inclusion of adjuvants in vaccine formulations. Adjuvants have been shown to improve antibody production [40], enable antigen dose-sparing [25], [41], and potentially increase cross-reactivity [15], [42]. These benefits are important in combating both seasonal influenza and pandemic influenza, as the effectiveness of current seasonal vaccine depends on closely matching the circulating virus, and pandemic vaccines need to be quickly produced.
There are very few adjuvants approved for commercial use in humans in the United States [16]. The adjuvanticity of rOv-ASP-1 discovered from the helminth parasite O. volvulus has been studied using ovalbumin, HIV-1 polypeptide, SARS-CoV peptide and subunit antigens, and several commercially available vaccines [23], [24], [26], [29]. We demonstrated in young adult mice that rOv-ASP-1 is an effective adjuvant that: accelerates and enhances the influenza-specific antibody response induced by TIV; allows antigen sparing; and augments a Th1-biased and cross-reactive antibody response that confers heterologous protection [25]. In the current study, we confirmed previous studies that immunization with an inactivated influenza vaccine induces significantly lower levels of IgG in aged vs young mice (Fig. 1) [43], [44]. However, when co-administered with rOv-ASP-1, influenza-specific IgG was significantly increased in aged mice, was higher than immunization with a 5× higher dose of TIV alone, and importantly, was comparable to that of young adult mice receiving TIV alone (Fig. 1). Further, TIV + rOv-ASP-1 increased both Th1 and Th2 antibody responses (Fig. 2). Interestingly, Bungener et al. [32] demonstrated that mice immunized with influenza vaccine plus alum only showed an enhanced Th2 response but suffered more severe weight loss and had significantly higher virus loads in their lungs than those receiving vaccine alone.
Since alum is the first and only adjuvant approved in the United States for general use in vaccines [17], [18], although it is not present in any of the currently available commercial influenza vaccines, we compared the efficacy of rOv-ASP-1 to that of alum for inducing influenza-specific IgG response to TIV. We observed that rOv-ASP-1 is superior to alum in enhancing an influenza-specific IgG response (Fig. 1). And although the survival rate is similar to that of alum (Fig. 4), rOv-ASP-1 was more effective in decreasing the signs of disease based on weight loss.
An ideal influenza vaccine would be able to offer broad cross-reactive immunity against different strains of influenza virus. It has been reported that both attenuated and inactivated influenza vaccines may induce cross-protective immunity against antigenically distinct virus strains in mice [42]. The cross-reactive antibody usually targets the HA stem region, since it is the conserved part of HA [45]. However, such antibodies are rarely seen in humans after infection or vaccination with seasonal influenza virus strains [46]. Our previous study in young adult mice demonstrated that rOv-ASP-1 could enhance the production of cross-reactive antibodies and confer protection against infection caused by a heterologous strain of influenza [25]. In the present study, we found that TIV + rOv-ASP-1 induced a low level of cross-reactive antibody to whole PR8 virus in aged mice 2 weeks after primary immunization while TIV alone did not (Fig. 3). These findings in aged mice are different from that observed in young adult mice where cross-reactive IgG antibody to HA of PR8 was detected in plasma after secondary, although not primary, immunization with TIV + rOv-ASP-1 [25]. Interestingly, however, after primary immunization of both young adult [25] and aged mice (Fig. 4), protection was observed after challenge with the antigenically different strain of influenza virus, PR8. The protection provided by TIV + rOv-ASP-1 may be associated with the low level of a cross-reactive IgG antibody in aged mice. This is similar to what has been observed in the aged population immunized with TIV: they have significantly lower levels of antibody responses to the circulating influenza virus and show influenza symptoms when infected with influenza virus, but demonstrate decreased hospitalization for pneumonia or influenza and also reduce the risk of death from all causes [47], [48], [49], [50].
The TIV used in this study was an inactivated flu vaccine that cannot induce CD8 T cell response. It is still unclear if rOv-ASP-1 can induce or enhance the T cell response which is important since specific CD8 T cell response is critical for elimination of virus infection. Further evaluation of rOv-ASP-1 as an effective adjuvant to prevent other infectious diseases should focus on the primary defense mechanism associated with the agent, including CD8 T cell responses and CD4 T cell induced cytokines.
In summary, we found that rOv-ASP-1 can significantly enhance the influenza-specific IgG response after immunization with TIV + rOv-ASP-1 in aged mice. Importantly, the level of IgG in aged mice administered TIV + rOv-ASP-1 was comparable to that of young adult mice immunized with TIV alone. rOv-ASP-1 was superior to alum in inducing a high level of the specific IgG after TIV immunization in aged mice. Both TIV + rOv-ASP-1 and TIV + Alum, but not TIV alone, induced protective immunity in aged mice to an antigenically different strain of influenza virus, reflected by increased survival. While both decreased weight loss after challenge, rOv-ASP-1 was superior to alum in preventing disease as indicated by weight loss. These results suggest that rOv-ASP-1 may serve as a potential adjuvant to enhance the protective efficacy of influenza vaccine in the elderly which has important implications for decreasing the morbidity and mortality of influenza in this population.
Conflict of interest statement
J.J. was a part-time employee of DMX Inc.
Acknowledgments
This project was supported by research grants from the NIAID R43AI085783 and R43AG042993 to J.J. We thank BEI Resources for kindly providing FLUARIX (2010–2011 Formula) and purified HA of PR8 strain.
References
- 1.Johnson N.P., Mueller J. Updating the accounts: global mortality of the 1918–1920 Spanish influenza pandemic. Bull Hist Med. 2002;76(1):105–115. doi: 10.1353/bhm.2002.0022. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed R., Oldstone M.B., Palese P. Protective immunity and susceptibility to infectious diseases: lessons from the 1918 influenza pandemic. Nat Immunol. 2007;8(11):1188–1193. doi: 10.1038/ni1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Po J.L., Gardner E.M., Anaraki F., Katsikis P.D., Murasko D.M. Age-associated decrease in virus-specific CD8+ T lymphocytes during primary influenza infection. Mech Ageing Dev. 2002;123(8):1167–1181. doi: 10.1016/s0047-6374(02)00010-6. [DOI] [PubMed] [Google Scholar]
- 4.Linterman M.A. How T follicular helper cells and the germinal centre response change with age. Immunol Cell Biol. 2014;92(1):72–79. doi: 10.1038/icb.2013.77. [DOI] [PubMed] [Google Scholar]
- 5.Thompson W.W., Shay D.K., Weintraub E., Brammer L., Cox N., Anderson L.J. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 6.Velez I.D., Gilchrist K., Martinez S., Ramirez-Pineda J.R., Ashman J.A., Alves F.P. Safety and immunogenicity of a defined vaccine for the prevention of cutaneous leishmaniasis. Vaccine. 2009;28(2):329–337. doi: 10.1016/j.vaccine.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 7.Salk J.E., Pearson H.E. Immunization against influenza with observations during an epidemic of influenza A one year after vaccination. Am J Hyg. 1945;42:307–322. doi: 10.1093/oxfordjournals.aje.a119045. [DOI] [PubMed] [Google Scholar]
- 8.Harper S.A., Fukuda K., Cox N.J., Bridges C.B. Using live, attenuated influenza vaccine for prevention and control of influenza: supplemental recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2003;52(RR-13):1–8. [PubMed] [Google Scholar]
- 9.Belshe R.B., Gruber W.C., Mendelman P.M., Mehta H.B., Mahmood K., Reisinger K. Correlates of immune protection induced by live, attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine. J Infect Dis. 2000;181(3):1133–1137. doi: 10.1086/315323. [DOI] [PubMed] [Google Scholar]
- 10.Strassburg M.A., Greenland S., Sorvillo F.J., Lieb L.E., Habel L.A. Influenza in the elderly: report of an outbreak and a review of vaccine effectiveness reports. Vaccine. 1986;4(1):38–44. doi: 10.1016/s0264-410x(86)80002-0. [DOI] [PubMed] [Google Scholar]
- 11.Govaert T.M., Thijs C.T., Masurel N., Sprenger M.J., Dinant G.J., Knottnerus J.A. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994;272(21):1661–1665. [PubMed] [Google Scholar]
- 12.Fiore A.E., Uyeki T.M., Broder K., Finelli L., Euler G.L., Singleton J.A. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 13.Tsang P., Gorse G.J., Strout C.B., Sperling M., Greenberg D.P., Ozol-Godfrey A. Immunogenicity and safety of Fluzone(R) intradermal and high-dose influenza vaccines in older adults≥65 years of age: a randomized, controlled, phase II trial. Vaccine. 2014;32(21):2507–2517. doi: 10.1016/j.vaccine.2013.09.074. [DOI] [PubMed] [Google Scholar]
- 14.Izurieta H.S., Thadani N., Shay D.K., Lu Y., Maurer A., Foppa I.M. Comparative effectiveness of high-dose versus standard-dose influenza vaccines in US residents aged 65 years and older from 2012 to 2013 using Medicare data: a retrospective cohort analysis. Lancet Infect Dis. 2015;15(3):293–300. doi: 10.1016/S1473-3099(14)71087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamouda T., Sutcliffe J.A., Ciotti S., Baker J.R., Jr. Intranasal immunization of ferrets with commercial trivalent influenza vaccines formulated in a nanoemulsion-based adjuvant. Clin Vaccine Immunol. 2011;18(7):1167–1175. doi: 10.1128/CVI.00035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McKee A.S., Munks M.W., Marrack P. How do adjuvants work? Important considerations for new generation adjuvants. Immunity. 2007;27(5):687–690. doi: 10.1016/j.immuni.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Cox J.C., Coulter A.R. Adjuvants—a classification and review of their modes of action. Vaccine. 1997;15(3):248–256. doi: 10.1016/s0264-410x(96)00183-1. [DOI] [PubMed] [Google Scholar]
- 18.Flach T.L., Ng G., Hari A., Desrosiers M.D., Zhang P., Ward S.M. Alum interaction with dendritic cell membrane lipids is essential for its adjuvanticity. Nat Med. 2011;17(4):479–487. doi: 10.1038/nm.2306. [DOI] [PubMed] [Google Scholar]
- 19.Davenport F.M., Hennessy A.V., Askin F.B. Lack of adjuvant effect of A1PO4 on purified influenza virus hemagglutinins in man. J Immunol. 1968;100(5):1139–1140. [PubMed] [Google Scholar]
- 20.D’Errico M.M., Grasso G.M., Romano F., Montanaro D. Comparison of anti-influenza vaccines: whole adsorbed trivalent, trivalent subunit and tetravalent subunit. Boll Ist Sieroter Milan. 1988;67(4):283–289. [PubMed] [Google Scholar]
- 21.Ionita E., Lupulescu E., Alexandrescu V., Matepiuc M., Constantinescu C., Cretescu L. Comparative study of the immunogenicity of aqueous versus aluminium phosphate adsorbed split influenza vaccine C.I. Arch Roum Pathol Exp Microbiol. 1989;48(3):265–273. [PubMed] [Google Scholar]
- 22.McElhaney J.E., Hooton J.W., Hooton N., Bleackley R.C. Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adults. Vaccine. 2005;23(25):3294–3300. doi: 10.1016/j.vaccine.2005.01.080. [DOI] [PubMed] [Google Scholar]
- 23.Xiao W., Du L., Liang C., Guan J., Jiang S., Lustigman S. Evaluation of recombinant Onchocerca volvulus activation associated protein-1 (ASP-1) as a potent Th1-biased adjuvant with a panel of protein or peptide-based antigens and commercial inactivated vaccines. Vaccine. 2008;26(39):5022–5029. doi: 10.1016/j.vaccine.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He Y., Barker S.J., MacDonald A.J., Yu Y., Cao L., Li J. Recombinant Ov-ASP-1, a Th1-biased protein adjuvant derived from the helminth Onchocerca volvulus, can directly bind and activate antigen-presenting cells. J Immunol. 2009;182(7):4005–4016. doi: 10.4049/jimmunol.0800531. [DOI] [PubMed] [Google Scholar]
- 25.Jiang J., Fisher E.M., Hensley S.E., Lustigman S., Murasko D.M., Shen H. Antigen sparing and enhanced protection using a novel rOv-ASP-1 adjuvant in aqueous formulation with influenza vaccines. Vaccine. 2014;32(23):2696–2702. doi: 10.1016/j.vaccine.2014.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao G., Du L., Xiao W., Sun S., Lin Y., Chen M. Induction of protection against divergent H5N1 influenza viruses using a recombinant fusion protein linking influenza M2e to Onchocerca volvulus activation associated protein-1 (ASP-1) adjuvant. Vaccine. 2010;28(44):7233–7240. doi: 10.1016/j.vaccine.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 27.Wang J., Tricoche N., Du L., Hunter M., Zhan B., Goud G. The adjuvanticity of an O. volvulus-derived rOv-ASP-1 protein in mice using sequential vaccinations and in non-human primates. PLoS ONE. 2012;7(5):e37019. doi: 10.1371/journal.pone.0037019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tawe W., Pearlman E., Unnasch T.R., Lustigman S. Angiogenic activity of Onchocerca volvulus recombinant proteins similar to vespid venom antigen 5. Mol Biochem Parasitol. 2000;109(2):91–99. doi: 10.1016/s0166-6851(00)00231-0. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald A.J., Cao L., He Y., Zhao Q., Jiang S., Lustigman S. rOv-ASP-1, a recombinant secreted protein of the helminth Onchocercavolvulus, is a potent adjuvant for inducing antibodies to ovalbumin, HIV-1 polypeptide and SARS-CoV peptide antigens. Vaccine. 2005;23(26):3446–3452. doi: 10.1016/j.vaccine.2005.01.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald A.J., Tawe W., Leon O., Cao L., Liu J., Oksov Y. Ov-ASP-1, the Onchocerca volvulus homologue of the activation associated secreted protein family is immunostimulatory and can induce protective anti-larval immunity. Parasite Immunol. 2004;26(1):53–62. doi: 10.1111/j.0141-9838.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- 31.Ilyushina N.A., Khalenkov A.M., Seiler J.P., Forrest H.L., Bovin N.V., Marjuki H. Adaptation of pandemic H1N1 influenza viruses in mice. J Virol. 2010;84(17):8607–8616. doi: 10.1128/JVI.00159-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bungener L., Geeraedts F., Ter Veer W., Medema J., Wilschut J., Huckriede A. Alum boosts TH2-type antibody responses to whole-inactivated virus influenza vaccine in mice but does not confer superior protection. Vaccine. 2008;26(19):2350–2359. doi: 10.1016/j.vaccine.2008.02.063. [DOI] [PubMed] [Google Scholar]
- 33.Jouvin-Marche E., Morgado M.G., Leguern C., Voegtle D., Bonhomme F., Cazenave P.A. The mouse Igh-1a and Igh-1b H chain constant regions are derived from two distinct isotypic genes. Immunogenetics. 1989;29(2):92–97. doi: 10.1007/BF00395856. [DOI] [PubMed] [Google Scholar]
- 34.Martin R.M., Brady J.L., Lew A.M. The need for IgG2c specific antiserum when isotyping antibodies from C57BL/6 and NOD mice. J Immunol Methods. 1998;212(2):187–192. doi: 10.1016/s0022-1759(98)00015-5. [DOI] [PubMed] [Google Scholar]
- 35.Petrushina I., Tran M., Sadzikava N., Ghochikyan A., Vasilevko V., Agadjanyan M.G. Importance of IgG2c isotype in the immune response to beta-amyloid in amyloid precursor protein/transgenic mice. Neurosci lett. 2003;338(1):5–8. doi: 10.1016/s0304-3940(02)01357-5. [DOI] [PubMed] [Google Scholar]
- 36.Deans G.D., Stiver H.G., McElhaney J.E. Influenza vaccines provide diminished protection but are cost-saving in older adults. J Intern Med. 2010;267(2):220–227. doi: 10.1111/j.1365-2796.2009.02201.x. [DOI] [PubMed] [Google Scholar]
- 37.Nichol K.L., Nordin J.D., Nelson D.B., Mullooly J.P., Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 38.Vu T., Farish S., Jenkins M., Kelly H. A meta-analysis of effectiveness of influenza vaccine in persons aged 65 years and over living in the community. Vaccine. 2002;20(13–14):1831–1836. doi: 10.1016/s0264-410x(02)00041-5. [DOI] [PubMed] [Google Scholar]
- 39.Nordin J., Mullooly J., Poblete S., Strikas R., Petrucci R., Wei F. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J Infect Dis. 2001;184(6):665–670. doi: 10.1086/323085. [DOI] [PubMed] [Google Scholar]
- 40.Carter D., Reed S.G. Role of adjuvants in modeling the immune response. Curr Opin HIV AIDS. 2010;5(5):409–413. doi: 10.1097/COH.0b013e32833d2cdb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Diwan M., Elamanchili P., Cao M., Samuel J. Dose sparing of CpG oligodeoxynucleotide vaccine adjuvants by nanoparticle delivery. Curr Drug Delivery. 2004;1(4):405–412. doi: 10.2174/1567201043334597. [DOI] [PubMed] [Google Scholar]
- 42.Lu X., Edwards L.E., Desheva J.A., Nguyen D.C., Rekstin A., Stephenson I. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine. 2006;24(44-46):6588–6593. doi: 10.1016/j.vaccine.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 43.Katz J.M., Lu X., Todd C.W., Newman M.J. A nonionic block co-polymer adjuvant (CRL1005) enhances the immunogenicity and protective efficacy of inactivated influenza vaccine in young and aged mice. Vaccine. 2000;18(21):2177–2187. doi: 10.1016/s0264-410x(00)00022-0. [DOI] [PubMed] [Google Scholar]
- 44.Mbawuike I.N., Wyde P.R., Anderson P.M. Enhancement of the protective efficacy of inactivated influenza A virus vaccine in aged mice by IL-2 liposomes. Vaccine. 1990;8(4):347–352. doi: 10.1016/0264-410x(90)90093-2. [DOI] [PubMed] [Google Scholar]
- 45.Doms R.W. Immunology prime, boost, and broaden. Science. 2010;329(5995):1021–1022. doi: 10.1126/science.1195116. [DOI] [PubMed] [Google Scholar]
- 46.Li G.M., Chiu C., Wrammert J., McCausland M., Andrews S.F., Zheng N.Y. Pandemic H1N1 influenza vaccine induces a recall response in humans that favors broadly cross-reactive memory B cells. Proc Natl Acad Sci USA. 2012;109(23):9047–9052. doi: 10.1073/pnas.1118979109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bernstein E., Kaye D., Abrutyn E., Gross P., Dorfman M., Murasko D.M. Immune response to influenza vaccination in a large healthy elderly population. Vaccine. 1999;17(1):82–94. doi: 10.1016/s0264-410x(98)00117-0. [DOI] [PubMed] [Google Scholar]
- 48.Lang P.O., Mendes A., Socquet J., Assir N., Govind S., Aspinall R. Effectiveness of influenza vaccine in aging and older adults: comprehensive analysis of the evidence. Clin Interv Aging. 2012;7:55–64. doi: 10.2147/CIA.S25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murasko D.M., Bernstein E.D., Gardner E.M., Gross P., Munk G., Dran S. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002;37(2–3):427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 50.Nichol K.L., Nordin J., Mullooly J., Lask R., Fillbrandt K., Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348(14):1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]