Abstract
Objective
The prevalence of obstructive sleep apnea (OSA) in women increases significantly after menopause. However, identifying at-risk women in this population is difficult because they tend to underreport symptoms and their complaints may differ from those traditionally associated with OSA. We investigated whether OSA risk factors are associated with the presence of a “non-traditional” complaint such as nocturnal enuresis in postmenopausal women.
Methods
A cross-sectional study of postmenopausal women ages 50–79, who participated in the Women Health Initiative Observational and Clinical Trial Studies (1993–2005) at 40 Clinical Centers in the United States, was performed. Multiple variable logistic regression analysis was employed to determine the association of OSA risk factors with nocturnal enuresis.
Results
A cohort of 2,789 women (1.7%) reported having nocturnal enuresis. Obesity (Odds ratio (OR)=2.29, 95% Confidence Interval (CI) 2.00–2.62), snoring (OR=2.01, 95% CI 1.74–2.32), poor sleep quality (OR=1.70, 95% CI 1.52–1.91), sleep fragmentation (OR=2.44, 95% CI 2.14–2.79), daytime sleepiness (OR=1.50, 95% CI 1.33–1.68), and hypertension (OR=1.13, 95% CI 1.01–1.26) were associated with nocturnal enuresis. Each additional OSA risk factor in a predefined OSA score significantly increased the odds of having nocturnal enuresis in a dose-response fashion (OR=1.38, 2.00, 2.80, 3.87, 5.10, and 7.02 for scores of 1–6, respectively) compared to no risk factors.
Conclusion
OSA risk factors are associated with nocturnal enuresis in postmenopausal women. Mechanisms relating nocturnal enuresis to OSA may include apnea-associated changes of intra-thoracic pressure leading to increased urine output. Questioning at-risk postmenopausal women presenting with nocturnal enuresis about other OSA risk factors should be considered.
Keywords: Obstructive sleep apnea, nocturnal enuresis, postmenopausal women
Introduction
Obstructive Sleep Apnea (OSA) is characterized by repetitive upper airway closure, resulting in disrupted sleep and nocturnal hypoxemia. Prolonged occurrence of frequent cyclic oxygen desaturations during sleep is associated with adverse medical outcomes, especially cardiovascular disease. Best prevalence estimates suggest that OSA affects 4% of adult men and 2% of adult women. (1) Although OSA appears to predominantly affect men, the risk for women to develop OSA increases significantly after menopause, (2) narrowing the difference in gender prevalence. Redline et al (3) observed the ratio of men to women reporting apnea in a community cohort to be 2:1, which is greater than the previously reported ratio of 10:1 from a sleep laboratory cohort. This increase in risk may be attributed to hormonal changes related to variation in estrogen and progesterone levels (4–5), differences in airway collapsibility (6), changes in adiposity, or differential referral rates between men and women for polysomnography. It has been hypothesized that men report classic symptoms of OSA, which leads to referral for OSA evaluation, whereas women tend to underreport their symptoms or report other atypical symptoms, directing the clinician to the evaluation of depression or insomnia instead of OSA. (7) Underreporting of symptoms in women may be due to a different perception of the disease process or due to the actual differences in disease presentation. This highlights the importance of investigating whether other “non-traditional” symptoms are associated with OSA in postmenopausal women.
Recognition of “non-traditional” symptoms in postmenopausal women may prompt earlier evaluation for OSA in this population to reduce the risk of acquiring comorbid conditions such as hypertension and cardiovascular disease, especially stroke. (8–9) Treatment of OSA with PAP improves blood pressure control and cardiovascular morbidity and mortality. (10–11) A recent study from the Women’s Health Initiative (WHI) demonstrated that short and long sleep durations increase the risk of coronary heart disease (CHD) and cardiovascular disease (CVD) in postmenopausal women. (12) This association may be linked to poor sleep quality, which may be attributed to frequent arousals either from respiratory compromise or urinary urgency. In this population, snoring, which is a symptom of OSA, has been associated with a modest increase in risk for CHD, CVD, and stroke. (13)
Commonly reported symptoms of OSA include snoring, snorting, choking, gasping, periods of interrupted breathing, morning headaches, diaphoresis during sleep, interrupted sleep, and excessive daytime sleepiness. (14) Young et al provided a thorough review on risk factors and symptoms of OSA. (15) Established risk factors that are associated with OSA include obesity, central body fat distribution, large neck diameter, and craniofacial and upper airway irregularities, such as tonsillar hypertrophy and retrognathia. They increase the one’s risk of airway collapsibility during sleep by reducing airway diameter and lung volume. Other associated risk factors include genetics, smoking, menopause, alcohol use before sleep, and nighttime nasal congestion. They have been shown in a few epidemiological and small experimental studies to be associated with OSA, but causal relationships have not been ascertained. (15)
Nocturia, waking up at night to urinate is a common urinary symptom of OSA. The overall prevalence of nocturia is 47.8%, and the prevalence is greater in females. (16) It is of interest to the clinician if one voids more than 2 times a night because sleep is disrupted. (17) Previous investigation suggests that women seek medical attention when they void more than 3 times a night. (18) The incidence of nocturia increases with age but appears to be not affected with being postmenopausal. (19) As being an expected norm of aging, pathologic nocturia is frequently overlooked. Nocturnal polyuria or pathological nocturia is also associated with disorders, including chronic kidney disease, congestive heart failure, neurological diseases, and venous insufficiency. (21) The increase in urination is mediated by atrial natriuretic peptide (ANP), which causes increased excretion of sodium and water. The release of ANP is triggered by the increase in negative intrathoracic pressure resulting from inspiratory effort against a closed glottis and thus, distending the heart. (21) Screening for nocturia is comparable to screening for snoring in recognizing high-risk patients for OSA. (22) Nocturia is reversible with the application of positive airway pressure (PAP). (23)
Nocturnal enuresis, a possible surrogate for nocturia, is uncontrollable loss of urine during sleep, and it may be the result of bladder dysfunction or altered anatomy. Its association with OSA has been studied extensively in children. (24) We postulate that nocturnal enuresis occurs in postmenopausal women through a mechanism of increased urine production and bladder and urethral dysfunction from hormonal changes causing overflow incontinence. Therefore, postmenopausal women may present with nocturnal enuresis rather than nocturia.
We sought to determine if OSA risk factors, such as obesity, snoring, poor sleep quality, sleep fragmentation, daytime sleepiness, and hypertension, are associated with nocturnal enuresis in a large cohort of postmenopausal women.
Methods
Study Population
The Women’s Health Initiative consists of three clinical trials and an observational study (1993–2005) with an enrolled population of 161,808 postmenopausal women. The WHI cohort consists of multiethnic women with ages between 50 and 79 years at baseline. All of these women completed the same baseline questionnaires. The methods of recruitment and study design have been previously published. (25–27) The WHI cohort was utilized for the study because it provided a large target population of postmenopausal women to investigate the proposed question. Due to the low prevalence of nocturnal enuresis and paucity of literature of its association with OSA, baseline questionnaires from the complete cohort were utilized to ensure maximal power to test our hypothesis. Therefore, a pre-hoc sample size calculation was not performed.
Outcome of Nocturnal Enuresis
Since the WHI clinical trials and observational study focused on comprehensive postmenopausal women’s health issues, subjects were inquired about urinary incontinence in a set of questions relating to experiences of daily life but were not thoroughly assessed with an existing instrument. Nocturnal enuresis was defined based on the answers to the following questions. First, women were asked: “Have you ever leaked even a small amount of urine involuntarily and you couldn’t control it?” If yes, then they were asked: “How often does this urine leaking occur?” and “When do you usually leak urine?” The response options provided for the former were as follows: “Not once during the past year”, “Less than once a month”, “More than once a month but less than once a week”, “One or more times a week but less than every day”, and “daily”. The response options provided for the latter were as follows: “When I cough, laugh, sneeze, lift, stand up, or exercise”, “When I feel the need to urinate and can’t get to a toilet fast enough”, and “When I am sleeping”. If they answered, “when I am sleeping” to the latter question regardless of frequency, this was defined as nocturnal enuresis.
Obstructive Sleep Apnea Risk Factors
Obesity, snoring, poor sleep quality, and sleep fragmentation are predictors of OSA. (28) BMI was categorized into 4 groups: <25, 25–30, 30–35, 35+ kg/m2, similar to the National Institute of Health cutoffs. (29) Obesity was defined as BMI > 30 kg/m2. Snoring, sleep quality, and sleep fragmentation were evaluated using a previously validated Women’s Health Initiative Insomnia Rating Scale (12–13, 30–32) with the following questions: “Did you snore?”, “Overall, how was your typical night’s sleep during the past 4 weeks:”, and “Did you wake up several times at night?”, respectively. For snoring, the response options were categorized into 4 groups: “No”, “Don’t know”, “Yes, less than or equal to 2 times per week”, and “Yes, greater or equal to 3 times per week”. For sleep quality, the response options were categorized into 3 groups: “Average”, “Sound or restful”, and “Restless or worse”. For sleep fragmentation, the response options were categorized into 3 groups: “No”, “Yes, less than or equal to 2 times per week”, and “Yes, greater than or equal to 3 times per week”.
Diagnosis of hypertension and questions regarding presence of daytime sleepiness are included as risk factors in several validated OSA screening questionnaires, including the Epworth sleepiness scale (33), Pittsburgh Sleep Quality Index (34), Berlin (35), and STOP-Bang questionnaires (36). Hypertension was defined as subjects reporting having been told by a physician that they had high blood pressure or hypertension. Daytime sleepiness was defined by the response to the question, “Did you fall asleep during quiet activities like reading, watching TV, or riding a car?”
Covariates
The following baseline demographic, anthropometric, behavioral, and disease variables have been either included in previous models on sleep-related outcomes (12–13) or are associated with OSA risk factors and nocturnal enuresis. Since nocturnal enuresis may be a surrogate of nocturia, confounders of the OSA and nocturia relationship also were included. (15, 22, 37) The baseline demographic characteristics included race, education, annual household income, and marital status. Race was categorized into black/African American, white/Caucasian, Hispanic/Latina, Asian, and other. Education was categorized into 4 groups: less than high school, high school graduate, some college, and college graduate. The annual household income was categorized into 3 ranges: less than $20,000, between $20,000 and $49,999, and greater than or equal to $50,000. Marital status was categorized into 2 categories: married or partnered and single, divorced, or widowed.
Physical measurements included body-mass index (BMI) and waist-to-hip ratio. BMI was categorized into 3 groups: less than 25 kg/m2, 25 to 30 kg/m2, and > 30 kg/m2. Diabetes mellitus was defined as the use of diabetic medications. Coronary heart disease, congestive heart failure, atrial fibrillation, and stroke were defined as being diagnosed previously with the disease. Diuretic and sedative/hypnotic use was defined as having one of the corresponding medications listed in the medication record. Hormone therapy (HT) was defined as the use of exogenous hormone for replacement. HT may be protective against having sleep-disordered breathing. (4)
The modified Charlson comorbidity index predicts the 10-year mortality risk for a subject with given comorbidities. (38) The list of comorbidities include previous myocardial infarction or cardiac arrest, heart failure, peripheral arterial disease, cardiovascular disease, asthma, emphysema, stomach or duodenal ulcer, lupus, rheumatoid arthritis, liver disease, diabetes, leukemia, lymphoma, and several cancer types. The index was defined as a modified score ranging from 1 to 3. A score of 1 indicated no comorbidities. A score of 2 indicated 1 comorbid condition, and a score of 3 indicated 2 or more comorbidities.
Behavior risk factors included smoking, alcohol consumption, and physical activity level. Smoking was categorized into 3 groups: never, past, and current smoker. Alcohol consumption was expressed as servings per week.
Physical activity was estimated from questions asking about recreational activity and expressed in estimated Metabolic Equivalent of Task (MET)-hours per week. Parity was defined as number of term pregnancies and categorized into six groups: 0, 1, 2, 3, 4, and 5+.
OSA Score
Due to the unavailability of the diagnosis of OSA in the WHI, an “OSA” score, incorporating the following OSA risk factors: BMI ≥ 30 kg/m2, snoring ≥ 3 times per week, restless or worse sleep quality, wake up at night ≥ 3 times per week, diagnosis of hypertension, and presence of daytime sleepiness ≥ 3 times per week, was created. For those who reported, “don’t know” for snoring, was coded as “no” snoring. A summary score (0=no risk factors, 1=one risk factor, and so forth) was determined based on the presence or absence of these risk factors in any order. The association of each additional risk factor as a categorical variable with nocturnal enuresis was determined using multiple variable regression analysis.
Data Synthesis and Statistical Analysis
To account for any residual confounding between the clinical trials and observational study cohorts, we utilized an indicator variable for the sub-cohorts. We created two models, one with and one without the sub-cohort indicator variable, and compared the change in effect estimates between the two models.
We performed chi-square analysis for categorical variables and analysis of variance (ANOVA) for continuous variables to determine the significance of the association of the outcome, nocturnal enuresis, with the OSA risk factors and clinical covariates as previously described. Missing baseline data were infrequent (< 1%). We employed multiple variable logistic regression analyses to adjust for known confounders of OSA risk factors and nocturnal enuresis relationship.
Other types of incontinence and categorization of BMI may confound or decrease the precision of the proposed comparison. Due to nocturnal enuresis having a similar mechanism to stress and urge incontinence, they may be misclassified as nocturnal enuresis or vice versa. As for BMI, since it was categorized into 4 groups, loss of precision may be present with using categories instead of a continuous measure. We performed sensitivity analyses of stress and urge incontinence and of continuous and categorical BMI. We created two different models, one with and one without stress and urge incontinence, to examine the effect of daytime incontinence on nocturnal enuresis. As for BMI, we created a model utilizing both continuous and categorical BMI and a model utilizing only categorical BMI. If the changes in effect estimate were less than 15%, residual confounding from daytime incontinence and continuous BMI is negligible, and they are not included in the model.
BMI may modify the effect between OSA risk factors and nocturnal enuresis. We tested for interactions between the OSA risk factors and BMI and stratified the cohort according to BMI categories (<25, 25–30, 30–35, 35+ kg/m2) when appropriate.
All statistical analyses were performed using SAS, version 9.2 (SAS Institute, Cary, North Carolina).
Results
Of the 161,808 postmenopausal women, 2,789 women (1.7%) reported that they leaked urine when sleeping, and > 80% of these women reported the occurrence to be at least “more than once a month” or more frequent. Baseline characteristics are presented in Table 1. Women who reported nocturnal enuresis were mostly obese (50.7%). The number of comorbid conditions, including stroke, atrial fibrillation, heart failure, diabetes, and coronary heart disease, was higher in women who reported nocturnal enuresis. The increasing amount of comorbid conditions also correlated with higher Charlson comorbidity indices. Use of diuretics and sedatives/hypnotics was higher in women who reported nocturnal enuresis. Fewer women who reported nocturnal enuresis were on HT. Women who had nocturnal enuresis were more often current and past smokers and had lower physical activity level. Parity was similar across the groups with the exception of the group that reported 5 or more term pregnancies and nocturnal enuresis. Those who reported nocturnal enuresis had more snoring, worse sleep quality, more sleep fragmentation, and more daytime sleepiness compared to those who did not report nocturnal enuresis. Hypertension was also more prevalent in those who reported nocturnal enuresis.
Table 1.
Baseline characteristics in the Women’s Health Initiative study population distributed by nocturnal enuresis status
| Variable | Overall (n = 161,808) | Presence of Nocturnal Enuresis
|
P-value | |
|---|---|---|---|---|
| Yes (n = 2,789) | No (n = 152,342) | |||
| Age (yrs) | 63.2 (7.2) | 64.7 (7.4) | 63.3 (7.2) | < 0.001 |
| Race | ||||
| Black/African American | 9.2% | 8.6% | 9.2% | < 0.001 |
| White/Caucasian | 82.7% | 84.8% | 82.6% | |
| Hispanic/Latina | 3.9% | 3.5% | 3.9% | |
| Asian | 2.7% | 1.3% | 2.7% | |
| Other | 1.6% | 1.8% | 1.6% | |
| Education | ||||
| Less than high school | 5.3% | 8.1% | 5.3% | < 0.001 |
| High school graduate | 17.2% | 20.1% | 17.2% | |
| Some college | 37.9% | 40.9% | 37.8% | |
| College graduate | 39.6% | 30.9% | 39.8% | |
| Income (% annual household) | ||||
| < $20,000 | 16.7% | 25.0% | 16.6% | < 0.001 |
| $20,000 – $49,999 | 44.7% | 49.2% | 44.6% | |
| ≥ $50,000 | 38.6% | 25.9% | 38.8% | |
| Marital status | ||||
| Married or partnered | 62.2% | 55.4% | 62.3% | < 0.001 |
| Single/divorced/widowed | 37.8% | 44.6% | 37.7% | |
| Waist-to-hip ratio | 0.81 (0.08) | 0.83 (0.08) | 0.81 (0.08) | < 0.001 |
| Diabetes mellitus | 4.5% | 8.0% | 4.4% | < 0.001 |
| Hyperlipidemia | 14.3% | 17.4% | 14.2% | < 0.001 |
| Coronary heart disease | 7.0% | 12.6% | 6.9% | < 0.001 |
| Heart failure | 1.3% | 2.5% | 1.3% | < 0.001 |
| Atrial fibrillation | 4.4% | 6.9% | 4.3% | < 0.001 |
| Stroke | 1.3% | 2.8% | 1.3% | < 0.001 |
| Urge incontinence | 37.5% | 64.2% | 37.0% | < 0.001 |
| Stress incontinence | 42.6% | 56.4% | 42.3% | < 0.001 |
| Hormone therapy | 43.4% | 40.2% | 43.5% | < 0.001 |
| Diuretics | 14.0% | 18.1% | 13.9% | < 0.001 |
| Sedatives/hypnotics | 13.5% | 22.4% | 13.4% | < 0.001 |
| Modified Charlson Comorbidity Indexa | ||||
| 1 | 57.4% | 42.2% | 57.7% | < 0.001 |
| 2 | 23.1% | 26.0% | 23.1% | |
| 3 | 19.5% | 31.8% | 19.3% | |
| Smoker | ||||
| Never | 51.0% | 48.8% | 51.0% | < 0.001 |
| Past | 42.0% | 43.0% | 42.0% | |
| Current | 7.0% | 8.3% | 7.0% | |
| Alcohol (servings/wk) | 2.4 (4.9) | 1.8 (5.1) | 2.4 (4.9) | < 0.001 |
| Physical activity (MET-hrs/wk)b | 12.4 (13.7) | 9.3 (12.1) | 12.5 (13.8) | < 0.001 |
| Parityc | ||||
| 0 | 2.9% | 2.7% | 2.9% | < 0.001 |
| 1 | 9.8% | 9.9% | 9.8% | |
| 2 | 27.7% | 25.6% | 27.7% | |
| 3 | 26.6% | 24.1% | 26.7% | |
| 4 | 16.8% | 16.8% | 16.8% | |
| 5+ | 16.2% | 21.0% | 16.1% | |
| Clinical Trials Sub-cohort | 40.6% | 40.4% | 40.6% | 0.85 |
| Observational Study Sub-cohort | 59.4% | 59.6% | 59.4% | |
| Body mass index | ||||
| < 25 kg/m2 | 35.4% | 19.7% | 35.7% | < 0.001 |
| 25–30 kg/m2 | 34.6% | 29.6% | 34.7% | |
| > 30 kg/m2 | 30.0% | 50.7% | 29.6% | |
| Snoring | ||||
| No | 20.8% | 13.5% | 20.9% | < 0.001 |
| Don’t know | 51.5% | 51.1% | 51.5% | |
| Yes, ≤ 2 times per week | 11.0% | 9.7% | 11.0% | |
| Yes, ≥ 3 times per week | 16.8% | 25.7% | 16.6% | |
| Sleep quality | ||||
| Average | 42.0% | 43.5% | 42.0% | < 0.001 |
| Sound or restful | 41.7% | 31.0% | 41.9% | |
| Restless or worse | 16.3% | 25.5% | 16.2% | |
| Sleep fragmentation | ||||
| No | 22.0% | 12.7% | 22.2% | < 0.001 |
| Yes, ≤ 2 times per week | 38.4% | 26.6% | 38.6% | |
| Yes, ≥ 3 times per week | 39.6% | 60.7% | 39.3% | |
| Daytime sleepiness | < 0.001 | |||
| No | 24.9% | 20.9% | 24.9% | |
| Yes, ≤ 2 times per week | 48.6% | 44.6% | 48.6% | |
| Yes, ≥ 3 times per week | 26.6% | 34.5% | 26.4% | |
| Hypertension | 33.9% | 42.8% | 33.7% | < 0.001 |
Data are presented as mean (SD) or %.
Modified Charlson Comorbidity Index: 1 is defined as no comorbidities, 2 is defined as the presence of 1 comorbid condition, 3 is defined as the presence of 2 or more comorbidities.
MET is the abbreviation for Metabolic Equivalent of Task.
Parity is defined as the number of term pregnancies.
Unadjusted and adjusted odds ratios of nocturnal enuresis
The prevalence of nocturnal enuresis was not significantly different between the clinical trials or observational cohorts. We did not find a significant difference in effect estimates between the models with or without the sub-cohort indicator variable. Therefore, the sub-cohort indicator variable was not included in the final model.
The unadjusted and adjusted odds ratios (OR) of nocturnal enuresis given BMI, snoring, sleep quality, sleep fragmentation, daytime sleepiness, and hypertension are presented in Table 2. The odds of having nocturnal enuresis increased with BMI. They were greater with more frequent snoring (≥3 times per week) compared to no snoring. Those who did not know if they snored had higher odds of having nocturnal enuresis relative to those who did not snore. After adjustment, an association remained for those who reported more frequent snoring (≥3 times per week).
Table 2.
Unadjusted and adjusted odds ratios of nocturnal enuresis in postmenopausal women
| Characteristics | Model Unadjusted
|
Model Fully Adjusteda
|
||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P-value | Odds Ratio | 95% CI | P-value | |
| Body mass index | ||||||
| < 25 kg/m2 (referent) | 1.00 | Referent | 1.00 | Referent | ||
| ≥25 and < 30 kg/m2 | 1.55 | 1.39 – 1.73 | < 0.001 | 1.47 | 1.30 – 1.67 | < 0.001 |
| ≥30 and < 35 kg/m2 | 2.57 | 2.29 – 2.87 | < 0.001 | 2.29 | 2.00 – 2.62 | < 0.001 |
| ≥35 kg/m2 | 3.97 | 3.54 – 4.45 | < 0.001 | 3.54 | 3.06 – 4.09 | < 0.001 |
| Snoring | ||||||
| No (referent) | 1.00 | Referent | 1.00 | Referent | ||
| Yes, ≤ 2 times per week | 1.37 | 1.17 – 1.60 | < 0.001 | 1.33 | 1.12 – 1.59 | 0.002 |
| Yes, ≥ 3 times per week | 2.39 | 2.11 – 2.71 | < 0.001 | 2.01 | 1.74 – 2.32 | < 0.001 |
| Don’t know | 1.53 | 1.37 – 1.72 | < 0.001 | 1.29 | 1.14 – 1.48 | < 0.001 |
| Sleep quality | ||||||
| Sound or restful (referent) | 1.00 | Referent | 1.00 | Referent | ||
| Average | 1.40 | 1.28 – 1.53 | < 0.001 | 1.22 | 1.11 – 1.35 | < 0.001 |
| Restless or worse | 2.13 | 1.93 – 2.35 | < 0.001 | 1.70 | 1.52 – 1.91 | < 0.001 |
| Sleep fragmentation | ||||||
| No (referent) | 1.00 | Referent | 1.00 | Referent | ||
| Yes, ≤ 2 times per week | 1.20 | 1.06 – 1.36 | 0.005 | 1.25 | 1.08 – 1.44 | 0.003 |
| Yes, ≥ 3 times per week | 2.69 | 2.40 – 3.02 | < 0.001 | 2.44 | 2.14 – 2.79 | < 0.001 |
| Daytime sleepiness | ||||||
| No | 1.00 | Referent | 1.00 | Referent | ||
| Yes, ≤ 2 times per week | 1.20 | 1.06 – 1.36 | 0.005 | 1.13 | 1.01 – 1.26 | 0.039 |
| Yes, ≥ 3 times per week | 2.69 | 2.40 – 3.02 | < 0.001 | 1.50 | 1.33 – 1.68 | < 0.001 |
| Hypertension | ||||||
| No | 1.00 | Referent | 1.00 | Referent | ||
| Yes | 1.47 | 1.36 – 1.59 | < 0.001 | 1.13 | 1.01 – 1.26 | 0.039 |
Model adjusted for age, race, education, income, marital status, waist-to-hip ratio, diabetes, hyperlipidemia, coronary heart disease, heart failure, atrial fibrillation, stroke, hormone therapy, diuretic use, sedatives/hypnotics use, modified Charlson comorbidity index, smoking, alcohol consumption, physical activity, and parity.
Those with restless or worse sleep quality had higher odds of having nocturnal enuresis compared to sound or restful sleep quality. After adjustment, an association remained for those who had restless or worse sleep quality. Those who woke up at night ≥ 3 times per week had greater odds of having nocturnal enuresis compared to those who did not woke up. Even after adjustment, an association remained for those who woke up at night ≥ 3 times per week.
Those who had daytime sleepiness ≥ 3 times per week had higher odds of having nocturnal enuresis compared to those who had no daytime sleepiness. After adjustment, an association remained. Those with hypertension had higher odds of having nocturnal enuresis compared to those who did not have hypertension. However, this association was attenuated after adjustment but remained significant.
Misclassification and effect measure modification
Urge and stress incontinence are more commonly reported in women who have nocturnal enuresis (64.2% and 56.4%, respectively) compared to those who do not have nocturnal enuresis (37.0% and 42.3%, respectively). Our sensitivity analysis revealed that the change in effect estimates of nocturnal enuresis with and without urge and stress incontinence is less than 15%. Therefore, urge and stress incontinence were not included in the final model. As for BMI, due to possible loss of precision, we compared the change in effect estimates of using either continuous or categorical BMI in 4 groups. The change was < 1%. Therefore, categorical BMI was used in all the analyses.
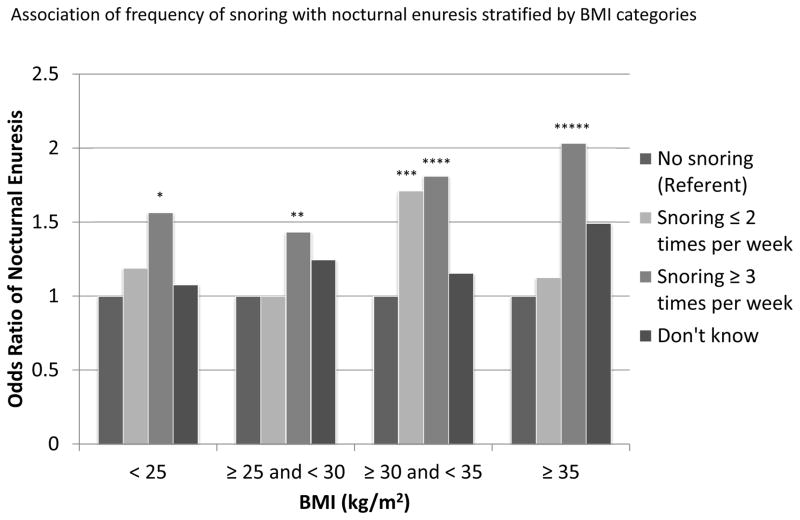
BMI is known to be associated with OSA risk factors and nocturnal enuresis. These effects vary across different strata of BMI. The interactions between BMI and each of these risk factors, sleep quality, sleep fragmentation, daytime sleepiness, and hypertension were not significant (p=0.45, p=0.67, p=1.00, and p=0.53, respectively). However, the interaction between snoring and BMI was significant (p=0.02). Nocturnal enuresis was plotted against snoring frequency stratified by BMI categories. (Figure 1) Compared to the no snoring group, those who had the highest frequency of snoring had higher odds of having nocturnal enuresis. The odds of having nocturnal enuresis is also higher in those who are obese (BMI ≥ 30 kg/m2) compared to those who are not obese.
Figure 1.
Bar graph of the interaction between snoring and BMI on nocturnal enuresis. Confidence intervals compared to referent as indicated: * 1.12–2.18; ** 1.10–1.87; *** 1.20–2.44; **** 1.33–2.47; ***** 1.41–2.94.
OSA Score
An OSA score derived from combining one to six OSA risk factors and its association with nocturnal enuresis is presented in Table 3. Of the 2,789 women who reported nocturnal enuresis, 10% had no OSA risk factors. With those who had a summary score of 1–6, 19% had one risk factor, 26% had two risk factors, 22% had three risk factors, 14% had four risk factors, 6% had five risk factors, and 2% had six risk factors. Each additional risk factor increased the odds of having nocturnal enuresis compared to having no risk factors, and the association is linear. Approximately half of the women had 2–3 OSA risk factors, and the odds of having nocturnal enuresis in this group is 3–4 times higher relative to having no risk factors. If one were to have all six risk factors, the odds of having nocturnal enuresis is seven times higher compared to having no risk factors.
Table 3.
Multiple variable adjusted odds ratio of nocturnal enuresis by Obstructive Sleep Apnea (OSA) score
| OSA score | Odds ratio | 95% CI | P-trend |
|---|---|---|---|
| 0 | 1.00 | Referent | < 0.0001 |
| 1a | 1.38 | 1.17 – 1.63 | |
| 2b | 2.00 | 1.70 – 2.34 | |
| 3c | 2.80 | 2.38 – 3.30 | |
| 4d | 3.87 | 3.23 – 4.62 | |
| 5e | 5.10 | 4.09 – 6.36 | |
| 6f | 7.02 | 4.85 – 10.15 |
Presence of one of the risk factors compared to no risk factors: BMI > 30 kg/m2, snoring ≥ 3 times per week, restless or worse sleep quality, wake up at night ≥ 3 times per week, diagnosis of hypertension, or presence of daytime sleepiness.
Presence of any 2 of the risk factors compared to no risk factors.
Presence of any 3 of the risk factors compared to no risk factors.
Presence of any 4 of the risk factors compared to no risk factors.
Presence of any 5 of the risk factors compared to no risk factors.
Presence of all 6 risk factors compared to no risk factors.
Discussion
The association between nocturnal enuresis and OSA risk factors, including obesity, frequent snoring, restless sleep quality, sleep fragmentation, daytime sleepiness, and hypertension, in postmenopausal women have not been previously studied. We found that OSA risk factors are associated with nocturnal enuresis in this population. Even though the associations were attenuated after adjusting for known confounders, the odds of having nocturnal enuresis with any of the OSA risk factors remained significantly higher compared to those who did not have nocturnal enuresis. Obesity is a prominent risk factor for nocturnal enuresis. Also, those with frequent snoring, a common symptom of OSA, along with greater BMI had higher odds of having nocturnal enuresis compared to those who did not snore or had lower BMI. According to the OSA score, each additional OSA risk factor increased the odds of having nocturnal enuresis. With the given findings, nocturnal enuresis may be an initial presenting symptom of OSA in postmenopausal women. Our study may help heighten awareness of this atypical presentation. Also, like snoring and nocturia it may be useful in screening for OSA in this population.
Half the overall cohort reported, “don’t know” in response to the snoring question. The increased odds of nocturnal enuresis with “not knowing” classification of snoring suggests the possibility that some of these women may snore. This finding implies that at-risk postmenopausal women who present with nocturnal enuresis but do not know if they snore should not be disregarded in the evaluation of OSA.
Our study confirms the findings of previous adult case reports and pediatric literature that there is a relationship between nocturnal enuresis and OSA, and diagnosis and treatment of OSA in these patients abolished enuresis. (39–41) However, the population we studied is comprised of postmenopausal women. Nonetheless, postmenopausal women may be more at risk due to anatomy and effects of hormonal variation. Although only 1.7% of the cohort reported nocturnal enuresis, the frequency of nocturnal enuresis may be underreported, likely due to one seeking medical attention only when it is problematic.
Limitations
Our study is the first to investigate the association between OSA risk factors and nocturnal enuresis in a large cohort of postmenopausal women. Although we found an association between these risk factors and nocturnal enuresis, there are a few limitations to our study. First, our study employed a cross-sectional design. With this design, the temporal relationship between acquiring OSA risk factors and nocturnal enuresis is unknown. However, this study provides stimulating evidence that nocturnal enuresis in the postmenopausal population may be a potential OSA risk factor.
Second, since subjects self-report incontinence, misclassification bias may be present and may bias the effect estimates toward the null. Due to the stigmata associated with nocturnal enuresis, those who have it may be less likely to report the symptom. Those who have it infrequently may underreport. Nocturia may also be misclassified. Many of the women with nocturnal enuresis may also have nocturia. The WHI questionnaires do not inquire about nocturia. We are unable to confirm the association between OSA risk factors and nocturia and to compare nocturnal enuresis with nocturia. However, although nocturnal enuresis and nocturia are pathophysiologically associated with ANP release during apnea-associated changes in intra-thoracic pressure, nocturnal enuresis is unique given that it occurs involuntary during sleep rather than while awake like nocturia, and it is likely a more salient event compared to nocturia. Therefore, misclassification of nocturia is less likely.
Lastly, the diagnosis of OSA is not available in the WHI. We modeled OSA by incorporating several established OSA risk factors, like those found in validated screening questionnaires. All of the included OSA risk factors were significantly associated with nocturnal enuresis. The gold standard to diagnose OSA is through polysomnography, but this study provides the initial framework for future studies to investigate the causal relationship between OSA and nocturnal enuresis in the target population and outpatient clinical setting with polysomnographic findings.
Conclusion
OSA is under-diagnosed in postmenopausal women due to underreporting of symptoms. Since nocturnal enuresis may be a presenting complaint in postmenopausal women, it, as explained by an overflow mechanism, may be a surrogate for nocturia, a well-described symptom of OSA. We demonstrated that OSA risk factors are associated with nocturnal enuresis in postmenopausal women. Nocturnal enuresis may be useful as a symptom to inquire about when screening for OSA. Postmenopausal women who present with nocturnal enuresis along with suspicious history or exam findings should be questioned about other OSA risk factors.
Acknowledgments
We thank the WHI study investigators and participants for their contributions.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
For a list of all the investigators who have contributed to WHI science, please visit: https://www.whi.org/researchers/Documents%20%20Write%20a%20Paper/WHI%20Investigator%20Long%20List.pdf
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Sources of financial support: The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Additional contributions: We also thank Mary B. Roberts, M.S. and Guohui Pan, Ph.D. for their contributions in statistical programming.
The National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services fund the WHI program. The funding source had no role in the study.
Footnotes
All authors have no conflicts of interest.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993 Apr 29;328(17):1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Wilhout SC, Suratt PM. Obstructive sleep apnea in pre-menopausal women: a comparison with men and postmenopausal women. Chest. 1987;91:654–7. doi: 10.1378/chest.91.5.654. [DOI] [PubMed] [Google Scholar]
- 3.Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994 Mar;149(3 Pt 1):722–6. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 4.Bixler EO, Vgontzas AN, Lin HM, Have TT, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med. 2001;163:608–613. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 5.Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med. 2003;167:1181–1185. doi: 10.1164/rccm.200209-1055OC. [DOI] [PubMed] [Google Scholar]
- 6.Jordan AS, Wellman A, Edwards JK, et al. Respiratory control stability and upper airway collapsibility in men and women with obstructive sleep apnea. J Appl Physiol. 2005;99:2020–2027. doi: 10.1152/japplphysiol.00410.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shepertycky MR, Banno K, Kryger MH. Differences between men and women in the clinical presentation of patients diagnosed with obstructive sleep apnea syndrome. Sleep. 2005;28:309–314. [PubMed] [Google Scholar]
- 8.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 9.Campos-Rodriguez F, Martinez-Garcia MA, Reyes-Nuñez N, Caballero-Martinez I, Catalan-Serra P, Almeida-Gonzalez CV. Role of sleep apnea and continuous positive airway pressure therapy in the incidence of stroke and coronary artery disease in women. Am J Respir Crit Care Med. 2014;189(12):1544–50. doi: 10.1164/rccm.201311-2012OC. [DOI] [PubMed] [Google Scholar]
- 10.Martinez-Garcia MA, Capote F, Campos-Rodriguez F, et al. Effect of CPAP on blood pressure in patients with obstructive sleep apnea and resistant hypertension: the HIPARCO randomized clinical trial. JAMA. 2013;310(22):2407–15. doi: 10.1001/jama.2013.281250. [DOI] [PubMed] [Google Scholar]
- 11.Doherty LS, Kiely JL, Swan V, McNicholas WT. Long-term effects of nasal continuous positive airway pressure therapy on cardiovascular outcomes in sleep apnea syndrome. Chest. 2005;127(6):2076–84. doi: 10.1378/chest.127.6.2076. [DOI] [PubMed] [Google Scholar]
- 12.Sands-Lincoln M, Loucks EB, Lu B, Carskadon MA, Sharkey K, Stefanick ML, Ockene J, Shah N, Hairston KG, Robinson JG, Limacher M, Hale L, Eaton CB. Sleep duration, insomnia, and coronary heart disease among postmenopausal women in the Women’s Health Initiative. J Womens Health (Larchmt) 2013 Jun;22(6):477–86. doi: 10.1089/jwh.2012.3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sands M, Loucks EB, Lu B, Carskadon MA, Sharkey K, Stefanick M, Ockene J, Shah N, Hairston KG, Robinson J, Limacher M, Hale L, Eaton CB. Self-reported snoring and risk of cardiovascular disease among postmenopausal women (from the Women’s Health Initiative) Am J Cardiol. 2013 Feb 15;111(4):540–6. doi: 10.1016/j.amjcard.2012.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McNicholas WT. Diagnosis of Obstructive Sleep Apnea. Proc Am Thorac Soc. 2008 Feb 15;5(2):154–60. doi: 10.1513/pats.200708-118MG. [DOI] [PubMed] [Google Scholar]
- 15.Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA. 2004;291(16):2013–6. doi: 10.1001/jama.291.16.2013. [DOI] [PubMed] [Google Scholar]
- 16.Hadjuk IA, Strollo PJ, Jr, Jasani RR, Atwood CW, Jr, Houck PR, Sanders MH. Prevalence and predictors of nocturia in obstructive sleep apnea-hypopnea syndrome – a retrospective study. Sleep. 2003 Feb 1;26(1):61–4. [PubMed] [Google Scholar]
- 17.Ancoli-Israel S, Bliwise DL, Nørgaard JP. The effect of nocturia on sleep. Sleep Med Rev. 2011 Apr;15(2):91–7. doi: 10.1016/j.smrv.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen FY, Dai YT, Liu CK, Yu HJ, Liu CY, Chen TH. Perception of nocturia and medical consulting behavior among community-dwelling women. Int Urogynecol J Pelvic Floor Dysfunct. 2007 Apr;18(4):431–6. doi: 10.1007/s00192-006-0167-x. [DOI] [PubMed] [Google Scholar]
- 19.Lin TL, Ng SC, Chen YC, Hu SW, Chen GD. What affects the occurrence of nocturia more: menopause or age? Maturitas. 2005 Feb 14;50(2):71–7. doi: 10.1016/j.maturitas.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Asplund R. The nocturnal polyuria syndrome (NPS) Gen Pharmacol. 1995 Oct;26(6):1203–9. doi: 10.1016/0306-3623(94)00310-j. [DOI] [PubMed] [Google Scholar]
- 21.Krieger J, Follenius M, Sforza E, et al. Effects of treatment with nasal continuous positive airway pressure on atrial natriuretic peptide and arginine vasopressin release during sleep in patients with obstructive sleep apnoea. Clin Sci. 1991;80:443–49. doi: 10.1042/cs0800443. [DOI] [PubMed] [Google Scholar]
- 22.Romero E, Krakow B, Haynes P, Ulibarri V. Nocturia and snoring: predictive symptoms for obstructive sleep apnea. Sleep Breath. 2010 Dec;14(4):337–43. doi: 10.1007/s11325-009-0310-2. [DOI] [PubMed] [Google Scholar]
- 23.Krieger J, Imbs JL, Schmidt M, Kurtz D. Renal function in patients with obstructive sleep apnoea-effects of nasal continuous positive airways pressure. Arch Intern Med. 1988;148:1337–1340. [PubMed] [Google Scholar]
- 24.Su MS, Li AM, So HK, Au CT, Ho C, Wing YK. Nocturnal enuresis in children: prevalence, correlates, and relationship with obstructive sleep apnea. J Pediatr. 2011;159(2):238–42. doi: 10.1016/j.jpeds.2011.01.036. [DOI] [PubMed] [Google Scholar]
- 25.Hays J, Hung JR, Hubbell FA, Anderson GL, Limacher M, Allen C, Rossouw JE. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13(9 suppl):S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 26.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13(9 suppl):S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 27.Rossouw JE, Hurd S. The Women’s Health Initiative: recruitment complete – looking back and looking forward. J Womens Health. 1999;8:3–5. doi: 10.1089/jwh.1999.8.3. [DOI] [PubMed] [Google Scholar]
- 28.Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med. 2002 Apr 22;162(8):893–900. doi: 10.1001/archinte.162.8.893. [DOI] [PubMed] [Google Scholar]
- 29.National Institutes of Health, National Heart, Lung, and Blood Institute. . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adult: the evidence report. Obes Res. 1998;6(suppl 2):S51–S120. [PubMed] [Google Scholar]
- 30.Levine DW, Kripke DF, Kaplan RM, Lewis MA, Naughton MJ, Bowen DJ, Shumaker SA. Reliability and validity of the Women’s Health Initiative Insomnia Rating Scale. Psychol Assess. 2003;15(2):137–148. doi: 10.1037/1040-3590.15.2.137. [DOI] [PubMed] [Google Scholar]
- 31.Levine DW, Dailey ME, Rockhill B, Tipping D, Naughton MJ, Shumaker SA. Validation of Women’s Health Initiative Insomnia Rating Scale in a multi-center controlled trial. Psychosom Med. 2005;67(1):98–104. doi: 10.1097/01.psy.0000151743.58067.f0. [DOI] [PubMed] [Google Scholar]
- 32.Chen JC, Brunner RL, Ren H, Wassertheil-Smoller S, Larson JC, Levine DW, Allison M, Naughton MJ, Stefanick ML. Sleep duration and risk of ischemic stroke in postmenopausal women. Stroke. 2008;39:3185–3192. doi: 10.1161/STROKEAHA.108.521773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131(7):485–91. doi: 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]
- 36.Chung F, Subramanyam R, Liao P, Sasaki E, Shapiro C, Sun Y. High STOP-Bang score indicates a high probability of obstructive sleep apnea. Br J Anaesth. 2012;108(5):768–75. doi: 10.1093/bja/aes022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgio KL, Locher JL, Ives DG, Hardin JM, Newman AB, Kuller LH. Nocturnal enuresis in community-dwelling older adults. J Am Geriatr Soc. 1996;44:139–43. doi: 10.1111/j.1532-5415.1996.tb02429.x. [DOI] [PubMed] [Google Scholar]
- 38.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 39.Everaert K, Pevernagie D, Oosterlinck W. Nocturnal enuresis provoked by an obstructing sleep apnea syndrome. J Urol. 1995;153:1236. [PubMed] [Google Scholar]
- 40.Steers WD, Suratt PM. Sleep apnoea as a cause of daytime and nocturnal enuresis. Lancet. 1997 May 31;349(9065):1604. doi: 10.1016/S0140-6736(05)61633-9. [DOI] [PubMed] [Google Scholar]
- 41.Kramer NR, Bonitati AE, Millman RP. Enuresis and obstructive sleep apnea in adults. Chest. 1998 Aug;114(2):634–7. doi: 10.1378/chest.114.2.634. [DOI] [PubMed] [Google Scholar]