Abstract
Chemical modifications in cellular RNA are diverse and abundant. Commonly found in ribosomal RNA (rRNA), transfer RNA (tRNA), long-noncoding RNA (lncRNA), and small nuclear (snRNA), these components play various structural and functional roles. Until recently, the roles of chemical modifications within messenger RNA (mRNA) have been understudied. Recent maps of several mRNA modifications have suggested regulatory functions for these marks. This review summarizes recent advances in identifying and understanding biological roles of posttranscriptional mRNA modification, or ‘RNA epigenetics’, with an emphasis on the most common internal modification of eukaryotic mRNA, N6-methyladenosine (m6A). We also discuss YTH proteins as direct mediators of m6A function and the emerging role of this mark in a new layer of gene expression regulation.
Graphical abstract
Messenger RNA Modifications
Posttranscriptional modifications in RNA were discovered nearly 60 years ago with the identification of pseudouridine (ψ) as an abundant component of tRNA. Since this time over 100 additional modifications of RNA have been documented, including internal modifications within coding transcripts [1,2].
N6-methyladenosine (m6A)
The most abundant internal modification in eukaryotic mRNA is methylation at the N6 position of adenosine, which is present between ~3 times per mRNA on average in mammalian cells [3]. This modification is installed by a multicomponent methyltransferase complex [4–6] and can be reversed by functionally significant demethylases [7,8]. Two independent efforts in 2012 mapped the location of m6A in mRNA using antibody-based affinity capture coupled to high throughput sequencing [9,10]. These experiments revealed a previously unknown enrichment for m6A within coding regions and the 3’ untranslated region, peaking sharply near the stop codon. This distribution, together with noted conservation of the RRACH (R=A,G; H=A,C,U) sequence motif [11,12] between mouse and human methylomes is suggestive of a mark with fundamental importance in RNA biology. Additionally, studies have identified a unique methylation pattern in Arabidopsis thaliana [13] and rice [14], revealing additional enrichment near the start codon and the requirement for m6A in plant development [15]. m6A is also an abundant component of viral RNA [16] and occurs in bacterial mRNA [17].
Identifying the precise location of m6A within a transcript remains a challenge. Current methods rely on chemical fragmentation of mRNA to increase resolution, but fail to provide single base information. Site-specific cleavage and radioactive-labeling followed by ligation-assisted extraction and thin-layer chromatography (SCARLET) can deliver base-resolution information on location and modification fraction of m6A, but is not applicable to high-throughput analysis [18]. Recent antibody-based crosslinking strategies have increased the resolution of m6A methylomes, and utilized unique mutation signatures to map sites at the individual-nucleotide level [19,20]. Such high-resolution data will enable researchers to observe perturbations of individual m6A loci in a variety of biological contexts.
m6A Methyltransferases – ‘Writers’
m6A is installed posttranscriptionally by a methyltransferase complex consisting of METTL3 and METTL14, as well as the regulatory subunit WTAP (Wilms’ tumor 1-associating protein) and in mammalian cells [4–6]. Both METTL3 and METTL14 are capable of transferring a methyl group from cofactor S-adenosyl methionine (SAM) to GGACU and GGAUU sequences within single stranded and stem-loop RNA in vitro, with METTL14 showing the greatest individual activity well short of that observed for the complex. Perturbation of individual subunits each leads to significant decreases in the m6A abundance in mRNA. Knockdown of METTL3 or METTL14 in mouse embryonic stem cells (mESCs) results in decreased capacity for self-renewal [21]. Knockout models of mettl3 in mESCs have revealed the requirement for m6A methylation in early differentiation processes [22,23]. Each study highlights a failure of mESCs to downregulate pluripotency markers and upregulate transcripts required for differentiation, potentially due to the absence of the m6A-dependent mRNA decay (discussed below). Geula et al. also show this phenotype in mettl14 mESCs, indicating this process requires methylation activity of the multiprotein complex [23]. Despite an essential role of m6A in mammalian development and viability, we currently do not know how the m6A methyltransferase complex is regulated. Recent work has identified the protein interaction network of METTL3 and two distinct subsets of m6A modification as WTAP-dependent or WTAP-independent, adding an additional element of complexity to the m6A epitranscriptome [24]. However, consensus methylation motifs are common to nearly every mRNA transcript, yet only a very small fraction contains methylation. Selectivity of the methyltransferase complex, perhaps driven by guide RNAs or chromatin marks, as well as regulation of its catalytic activity, is an active area of research.
m6A Demethylases – ‘Erasers’
The discovery of m6A demethylating enzymes showed that mRNA methylation is a reversible process in vivo, further indicating a role in gene regulation [25]. Two members of the Fe(II)- and 2-oxoglutarate-dependent oxygenase superfamily, FTO and ALKBH5, exhibit catalytic activity in vitro and in vivo. FTO (fat mass and obesity-associated protein) oxidizes m6A to A through N6-hydroxymethyladenosine (hm6A) and N6-formyladenosine (f6A) intermediates and is sensitive to secondary structure [7,26]. A recent study showed that FTO regulates adipogenesis by affecting splicing patterns of preadipocyte differentiation markers, suggesting a mechanism by which m6A influences metabolism [27]. ALKBH5 (alkylation repair homologue protein 5) also shows highest activity towards ssRNA in vitro, and notable sequence preference for m6A consensus methylation motifs. Knockdown of ALKBH5 affects mRNA processing and export in HeLa cells, though the mechanism of this function remains unclear. Mice deficient in alkbh5 show increased levels of m6A in isolated organs and display defective spermatogenesis. Furthermore, transcriptome analysis of mRNA from knockout mice testis shows differential expression of over 1,500 genes, 127 of which are spermatogenesis-related [8]. As with m6A methyltransferases, mechanisms of regulation and selectivity for these demethylases are unknown.
m6A Effector Proteins –‘Readers’
RNA modifications influence protein binding behavior, generating specificity for ‘reader’ proteins and deterring potential ‘anti-reader’ proteins. m6A ‘reader’ proteins of the YTH family were identified by affinity chromatography using methylated RNA as bait [9]. Mammals encode five YTH proteins: YTH Domain Family (YTHDF) proteins 1, 2 and 3, and YTH Domain Containing (YTHDC) proteins 1 and 2. [28]. To date, four of these proteins have been shown to exhibit m6A selectivity in vitro and in vivo [29,30] A crystal structure of the YTH domain of YTHDF2 as well as that of YTHDC1 bound to methylated RNA have been solved, revealing a conserved hydrophobic binding pocket specific for m6A [30–32]. These m6A ‘reader’ proteins of the YTH family provide a direct mechanistic link between mRNA methylation and transcript fate.
Recently, two m6A binding proteins have been functionally characterized: YTHDF1 and YTHDF2 [29, 33]. While all methyltransferase and demethylase components are nuclear, these two proteins reside strictly within the cytoplasm. As expected of m6A ‘readers’, high-resolution mapping of transcript binding sites reveals a preference for GGACU sequence motifs in mRNA with high overlap with sites of m6A methylation. YTHDF1 enhances translation efficiency of methylated mRNAs by interacting with initiation factors, enhancing ribosome loading of its targets, and promoting translation initiation. YTHDF2 promotes mRNA decay of methylated transcripts by localizing targets to cytoplasmic processing bodies, reducing the half-life of the protein-coding message. Together, these effector proteins are capable of facilitating robust gene expression within a confined time frame and regulate a dynamic set of genes involved in many levels of genetic regulation.
YTHDF3 and YTHDC1 are also known to bind with specificity for the m6A modification in mRNA. YTHDF3 is similar in sequence to YTHDF1 and YTHDF2 and also localized to the cytoplasm but may play unique roles in tissue-specific processes that have yet to be thoroughly explored. Unlike the YTHDF proteins, YTHDC1 is strictly nuclear, and has previously been reported to influence splice-site selection of several mRNAs [28]. YTHDC2 contains a highly conserved YTH domain, but binding specificity and molecular function of this potential ‘reader’ have yet to be elucidated.
Additional Functions of m6A
RNA methylation has been associated with a broad set of biological functions, few of which are currently understood in mechanistic detail. Methylation at the N6 position of adenosine destabilizes RNA duplexes by 0.5–1.7 kcal/mol, and conversely favors single-stranded conformation when located in unpaired positions due to increased base stacking interactions [34]. RNA secondary structure mapping shows increased icSHAPE reactivity at m6A sites consistent with reduced secondary structure, and may be used to predict sites of methylation [35]. To this end, m6A may regulate biological processes dependent on RNA structure, bypassing the need for a direct ‘reader’ mechanism. This is indeed apparent for hnRNPC, which binds to U-rich sites adjacent to ‘m6A structural switches’ and regulates pre-mRNA splicing [36]. In addition to roles in splicing, translation, and stability, m6A controls circadian rhythms via methylation-dependent RNA-processing [37], as well as the processing [38] and abundance [39] of miRNAs. m6A methylation is conversely regulated by miRNAs via seed regions enriched within consensus m6A sequences [40]. Understanding how RNA methylation controls these processes is still a major challenge for the field.
Pseudouridine (ψ)
Globally, the most abundant RNA modification is pseudouridine (ψ) generated from isomerization of uridine. Extensively explored in rRNA and tRNA, the function of ψ as a regulatory element in mRNA is unknown. Pseudouridine can be mapped by reacting with cyclohexyl-N’-(2-morpholinoethyl) carbodiimide metho-p-toluenesulphonate (CMC) to generate reverse-transcription stop sites one base from selectively labeled ψ [41] The presence of ψ in yeast mRNA was reported in roughly 300 unique sites [42,43], while Carlile et al. additionally revealed 96 ψ sites in 89 unique human mRNAs [36]. A chemical biology approach was developed to more effectively label and enrich ψ in mammalian mRNA with over 2,000 new sites discovered in 1,929 transcripts [44]. Members of the pseudouridine synthase family enzymes were shown to mediate U to ψ conversions in mRNA, enabling future studies of these proteins as potential ψ ‘writers’. ψ may play pivotal roles by offering additional hydrogen bonding thus altering secondary structure and by mediating nonsense-to-sense codon conversion (recoding) [45]. The role for ψ to increase protein production when synthetically introduced into mRNA also offers a potential mechanism by which this posttranscriptional modification could function within cells [46].
5-methylcytosine (m5C)
A widespread epigenetic marker in DNA, m5C is less studied in RNA. In addition to structural and functional roles in rRNA and tRNA, its presence has been previously reported in mammalian mRNA. Despite low abundance, m5C was identified in over ten thousand sites in HeLa RNA using RNA bisulfite conversion coupled to high throughput sequencing [47]. Though statistically underrepresented in mRNA, the distribution of m5C is biased towards untranslated regions and relatively depleted within the coding sequence. Interestingly, sites in the 3’ untranslated region correlate with sites of Argonaute I-IV, suggesting potential for m5C-guided miRNA targeting. The tRNA m5C methyltransferase NSun2 is responsible for a subset of these sites, regulating roughly 10% of the m5C methylome in mRNA, lncRNA and vault RNAs [48]. Direct analysis of m5C methyltransferase binding sites by 5-acacytosine-mediated RNA immunoprecipitation (Aza-IP) exploits a covalent bond between protein and RNA, revealing additional targets for NSUN2 in low copy RNAs at single nucleotide resolution [49]. Base resolution, quantitative mapping of m5C in RNA makes it amenable to study in several biological contexts.
Concluding Remarks
RNA modifications are emerging as critical components of the gene regulatory landscape. The most common modification of mRNA is analogous to modifications in DNA and protein in that it is reversible and dynamic. Though methyltransferase and demethylase components have been identified, a thorough understanding of their function is lacking. There likely exist modes of selectivity and activity regulation for each of these enzymes that have yet to be elucidated, and will shed light on when and how m6A is installed, removed, and utilized within the cell. There may be additional factors that have not been identified in this system. Technological advances in identifying methylation are needed as well. Particularly a quantitative, base resolution method will enable researchers to dissect mechanistic roles of m6A, ideally capable of detecting methylation with limited input. Functionally, direct or indirect ‘reader’ proteins offer an opportunity to study the effects of m6A methylation in biological systems, and full characterization of such proteins in development and disease will be continuing challenges. ψ and m5C may serve diverse regulatory roles in mRNA as well, and transcriptome-wide maps of these modifications suggest this is the case. Chemical methods for their identification will be invaluable in studying the biology of these modifications, as will further identification of potential ‘reading’ mechanisms. Together, modifications in mRNA deposit a unique chemical message on each transcript. Deciphering the meaning of these messages offers insight into new modes of posttranscriptional gene regulation.
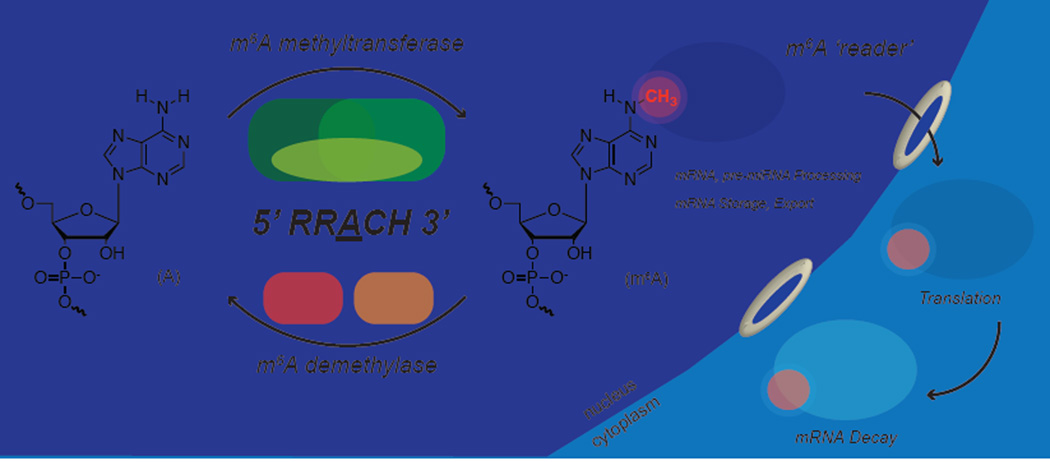
Figure 1. Reversible m6A Methylation of mRNA.
m6A is installed by a methyltransferase complex containing catalytic subunits METTL3/METTL14 and the regulatory subunit WTAP. FTO and ALKBH5 oxidatively demethylate mRNA within the nucleus. The m6A-modified mRNAs are recognized by YTH family proteins via the YTH domain both within the nucleus and the cytoplasm.
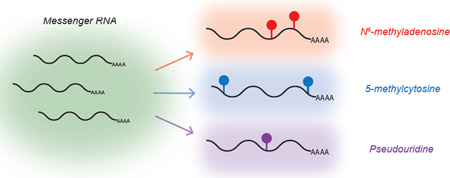
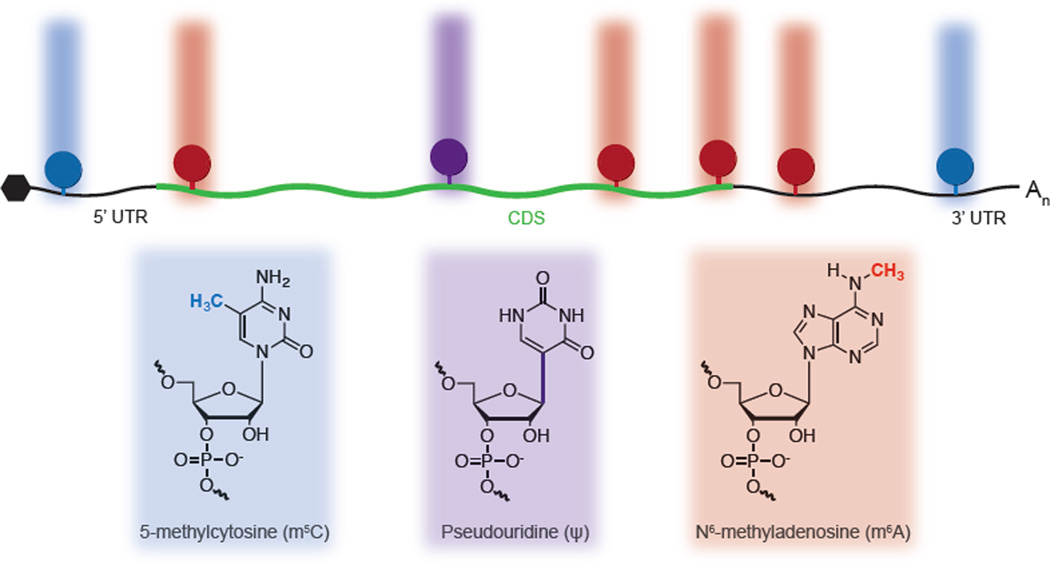
Figure 2. Diverse Chemical Modifications of mRNA.
Messenger RNA is modified with several distinct chemical marks, each with a unique distribution pattern. Recent maps of m5C, ψ, and m6A suggest that the combination of these modifications can impart an additional chemical message on top of the underlying genetic sequences.
Highlights.
N6-methyladenosine is an abundant and reversible chemical modification in mRNA.
YTH proteins mediate functions of N6-methyladenosine in regulating gene expression.
5-methylcytosine and pseudouridine are abundant components of mRNA.
A diverse set of chemical modifications in mRNA combine to uniquely mark transcripts.
Acknowledgement
The authors apologize to colleagues whose work was not cited owing to space limitation. I.A.R is supported by the University of Chicago Medical Scientist Training Program training grant. C.H. is an investigator of the Howard Hughes Medical Institute (HHMI) and is supported by National Institutes of Health GM071440.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Machnicka MA, Milanowska K, Osman Oglou O, Purta E, Kurkowska M, Olchowik A, Januszewski W, Kalinowski S, Dunin-Horkawicz S, Rother KM, et al. MODOMICS: a database of RNA modification pathways--2013 update. Nucleic Acids Res. 2013;41:D262–D267. doi: 10.1093/nar/gks1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grosjean H. Fine-tuning of RNA functions by modification and editing. Berlin: Springer-Verlag; 2005. [Google Scholar]
- 3.Desrosiers RC, Friderici KH, Rottman F. Identification of methylated nucleosides in messenger RNA from Novikoff hepatoma cells. Proc Natl Acad Sci USA. 1974;71:3971–3975. doi: 10.1073/pnas.71.10.3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bokar JA, Rath-Shambaugh ME, Ludawiczak RL, Narayan P, Rottman FM. Characterization and partial purification of mRNA N6-adenosine methyltransferase from HeLa cell nuclei. J Biol Chem. 1994;269:17679–17704. [PubMed] [Google Scholar]
- 5. Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, Jia G, Yu M, Lu Z, Deng X, et al. A METTL3–METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. Identification of catalytic and regulatory components of the m6A methyltransferase complex
- 6.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS, et al. Mammalian WTAP is a regulatory subunit of the N6-methyladenosine methyyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindhal T, Pan T, Yang YG, He C. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. Demonstrate that m6A is a reversible chemical modification in messenger RNA with significant biological function
- 9.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-DIvon M, Ungar L, Osenberg S, Cesarkas K, Jacob-Hirsch J, Amariglio N, Kupiec M, Sorek R, Rechavi G. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 10. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive Analysis of mRNA Methylaiton Reveals Enrichment in 3’ UTRs and Near Stop Codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. Provide transcriptome-wide maps of m6A in messenger RNA
- 11.Wei CM, Moss B. Nucleotide sequences at the N6-methyladenosine sited of HeLa cell messenger ribonucleic acid. Biochem. 1977;15:397–401. doi: 10.1021/bi00627a023. [DOI] [PubMed] [Google Scholar]
- 12.Csepany T, Lin A, Baldick CJ, Jr, Beeman K. Sequence specificity of the mRNA N6-adenosine methyltransferase. 1990;265:20117–20122. [PubMed] [Google Scholar]
- 13.Luo GZ, MacQueen A, Zheng G, Duan H, Dore LC, Lu Z, Liu J, Chen K, Jia G, Bergelson J, He C. Unique features of the m6A methylome in Arabidopsis thaliana. Nat Commun. 2014;5:5630. doi: 10.1038/ncomms6630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Wang X, Li C, Hu S, Yu J, Song S. Transcriptome-wide N6-methyladenosine profiling of rice callus and leaf reveals the presence of tissue-specific competitors involved in mRNA modification. RNA Biol. 2014;11:1180–1188. doi: 10.4161/rna.36281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bodi Z, Zhong S, Mehra S, Song J, Graham N, Li H, May S, Fray RG. Adenosine Methylation in Arabidopsis mRNA is Associated with the 3’ End and Reduced Levels Cause Developmental Defects. Front Plant Sci. 2012;3:48. doi: 10.3389/fpls.2012.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lavi S, Shatkin AJ. Methylated simian virus 40-specific RNA from nuclei and cytoplasm of infected BSC-1 cells. Proc Natl Acad Sci USA. 1975;72:2012–2016. doi: 10.1073/pnas.72.6.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng X, Chen K, Luo GZ, Weng X, Ji Q, Zhou T, He C. Widespread occurrence of N6-methyladenosine in bacterial mRNA. Nucleic Acids Res. 2015;43:7557–6567. doi: 10.1093/nar/gkv596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu N, Parisien M, Dai Q, Zheng G, He C, Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19:1848–1856. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen K, Luo GZ, He C. High-Resolution N6-methyladenosine (m6A) map using photo-crosslinking-assisted m6A sequencing. Angew Chem Int Ed Engl. 2015;54:1587–1590. doi: 10.1002/anie.201410647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linder B, Grozhik AV, Olarerin-George AO, Maydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12:767–772. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z, Zhao JC. N6-methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol. 2014;16:191–198. doi: 10.1038/ncb2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batista PJ, Molinie B, Wang J, Qu K, Zhang J, Li L, Bouley DM, Lujan E, Haddad B, Daneshvar K, Carter AC, et al. m6A RNA modification controls cell fate transition in mammalian embryonic stem cells. Cell Stem Cell. 2014;15:707–719. doi: 10.1016/j.stem.2014.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geula S, Moshitch-Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon-Divon M, Hershkovitz V, Peer E, Mor N, Manor YS, et al. m6A mRNA methylation facilitates resolution of naïve pluripotency toward differentiation. Science. 2015;347:1002–1006. doi: 10.1126/science.1261417. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter-Ovanesyan D, Habib N, Cacchiarelli D, et al. Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal 5’ sites. Cell Rep. 2014;8:284–296. doi: 10.1016/j.celrep.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He C. Grand challenge commentary: RNA epigenetics? Nat Chem Biol. 2010;6:863–865. doi: 10.1038/nchembio.482. [DOI] [PubMed] [Google Scholar]
- 26.Fu Y, Jia G, Pang X, Wang RN, Wang X, Li CJ, Smemo S, Dai Q, Bailey KA, Nobrega MA, Han KL, Cui Q, He C. FTO-mediated formation of N6-hydroxymethyladenosine and N6-formyladenosine in mammalian RNA. Nat Commun. 2013;4:1798. doi: 10.1038/ncomms2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Yang Y, Sun BF, Shi Y, Yang X, Xiao W, Hao YJ, Ping XL, Chen YS, Wang WJ, et al. FTO-dependent demethylation of N6-methyladenosine regulates mRNA splicing and is required for adipogenesis. Cell Res. 2014;24:1403–1419. doi: 10.1038/cr.2014.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang G, Theler D, Kaminska KH, Hiller M, de la Grange P, Pudimat R, Rafalska I, Heinrich B, Bujnicki JM, Allain FH, Stamm S. The YTH domain is a novel RNA binding domain. J Biol Chem. 2010;285:14701–14710. doi: 10.1074/jbc.M110.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G, Ren B, Pan T, He C. N6-methyladenosine-dependent regulation of messenger RNA stability. Nature. 2014;505:117–120. doi: 10.1038/nature12730. Identifies YTHDF2 as a direct mediator of m6A-dependent mRNA stability
- 30.Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y, Lu Z, He C, Min J. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10:927–929. doi: 10.1038/nchembio.1654. [DOI] [PubMed] [Google Scholar]
- 31.Luo S, Tong L. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc Natl Acad Sci USA. 2014;111:13834–13839. doi: 10.1073/pnas.1412742111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu T, Roundtree IA, Wang P, Wang X, Wang L, Sun C, Tian Y, Li J, He C, Xu Y. Crustal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res. 2014;24:1493–1496. doi: 10.1038/cr.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H, He C. N6-methyladenosine Modulates Messenger RNA Translation Efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. Identifies YTHDF1 as a direct mediator of m6A-dependent translation initiation
- 34.Roost C, Lynch SR, Batista PJ, Qu K, Chang HY, Kool ET. Structure and thermodynamics of N6-methyladenosine in RNA: a spring-loaded base modification. J Am Chem Soc. 2015;137:2107–2115. doi: 10.1021/ja513080v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spitale RC, Flynn RA, Zhang QC, Crisalli P, Lee B, Jung JW, Kuchelmeister HY, Batista PJ, Torre EA, Kool ET, Chang HY. Structural imprints in vivo decode RNA regulatory mechanisms. Nature. 2105;519:486–490. doi: 10.1038/nature14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu N, Dai Q, Zheng G, He C, Parisien M, Pan T. N6-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fustin JM, Doi M, Yamaguchi Y, Hida H, Nishimura S, Yoshida M, Isagawa T, Morioka MS, Kakeya H, Manabe I, Okamura H. RNA-methylation-dependent RNA processing controls the speed of the circadian clock. Cell. 2013;155:793–806. doi: 10.1016/j.cell.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 38.Alarcón CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berulava T, Rahmann S, Rademacher K, Klein-Hitpass L, Horsthemke B. N6-adenosine methylation in miRNAs. PLoS One. 2015;10:e0118438. doi: 10.1371/journal.pone.0118438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen T, Hao YJ, Zhang Y, Li MM, Wang M, Han W, Wu Y, Lv Y, Hao J, Wang L, et al. m6A RNA methylation is regulated by microRNAs and promotes reprogramming to pluripotency. Cell Stem Cell. 2015;16:289–301. doi: 10.1016/j.stem.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 41.Bakin AV, Ofengand J. Mapping of pseudouridine residues in RNA to nucleotide resolution. Methods Mol Biol. 1998;77:297–309. doi: 10.1385/0-89603-397-X:297. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz S, Bernstein DA, Mumbach MR, Jovanovic M, Herbst RH, León-Richardo BX, Engreitz JM, Guttman M, Satija R, Lander ES, et al. Transcriptome-wide mapping reveals widespread dynamic-regulated pseudouridylation of ncRNA and mRNA. Cell. 2014;159:148–162. doi: 10.1016/j.cell.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carlile TM, Rojas-Duran MF, Zinshteyn B, Shin H, Bartoli KM, Gilbert WV. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143–146. doi: 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li X, Zhu P, Ma S, Song J, Bai J, Sun F, Yi C. Chemical pulldown reveals dynamic pseudouridylation of the mammalian transcriptome. 2015;11:592–599. doi: 10.1038/nchembio.1836. Provide transcriptome-wide maps of psdueouridine in diverse types of cellular RNA
- 45.Karijolich J, Yu YT. Converting nonsense codons into sense codons by targeted pseuroudidylation. Nature. 2011;474:395–398. doi: 10.1038/nature10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karikó K, Muramatsu H, Keller JM, Weissman D. Increased erythropoiesis in mice injected with submicrogram quantities of pseudouridine-containing mRNA encoding erythropoietin. Mol Ther. 2012;20:948–953. doi: 10.1038/mt.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Squires JE, Patel HR, Nousch M, Sibbritt T, Humphreys DT, Parker BJ, Suter CM, Preiss T. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hussain S, Aleksic J, Blanco S, Dietmann S, Frye M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biol. 2013;14:215. doi: 10.1186/gb4143. Provide transcriptome-wide maps of 5-methylcytosine in mRNA and lncRNA
- 49. Khoddami V, Cairns BR. Identification of direct targets and modified bases of cytosine methyltransferases. Nat Biotechnol. 2013;31:458–464. doi: 10.1038/nbt.2566. Describe methods for assigning 5-methylcytosine modification to methyltransferases transcriptome-wide