Abstract
Objective:
To investigate the association between neuromyelitis optica spectrum disorder (NMOSD) and pregnancy outcome.
Methods:
An international cohort of women with aquaporin-4 antibody–positive NMOSD and ≥1 pregnancy was studied retrospectively. Multivariate logistic regression was used to investigate whether pregnancy after NMOSD onset was associated with an increased risk of miscarriage (cohort of 40 women) or preeclampsia (cohort of 57 women).
Results:
Miscarriage rate was higher in pregnancies after NMOSD onset (42.9% [95% confidence interval 17.7%–71.1%] vs 7.04% [2.33%–15.7%]). Pregnancies conceived after, or up to 3 years before, NMOSD onset had an increased odds ratio of miscarriage (7.28 [1.03–51.6] and 11.6 [1.05–128], respectively), independent of maternal age or history of miscarriage. Pregnancies after, or up to 1 year before, NMOSD onset ending in miscarriage were associated with increased disease activity from 9 months before conception to the end of pregnancy, compared to viable pregnancies (mean annualized relapse rate 0.707 vs 0.100). The preeclampsia rate (11.5% [6.27%–18.9%]) was significantly higher than reported in population studies. The odds of preeclampsia were greater in women with multiple other autoimmune disorders or miscarriage in the most recent previous pregnancy, but NMOSD onset was not a risk factor.
Conclusions:
Pregnancy after NMOSD onset is an independent risk factor for miscarriage, and pregnancies conceived at times of high disease activity may be at increased risk of miscarriage. Women who develop NMOSD and have multiple other autoimmune disorders have greater odds of preeclampsia, independent of NMOSD onset timing.
Neuromyelitis optica spectrum disorder (NMOSD) is a severe recurrent antibody-mediated inflammatory disorder of the CNS, mainly characterized by optic neuritis (ON) and longitudinally extensive transverse myelitis,1 but also affecting other areas in the CNS (e.g., brainstem and hypothalamus). The presence of immunoglobulin G (IgG) that binds to aquaporin-4 (AQP4),2,3 known to be key in the pathogenic process of this disorder, distinguishes NMOSD from other CNS inflammatory disorders.1 NMOSD is up to 8 times more prevalent in women,4 many of whom have active disease during childbearing years.5–7 Recently, experimental and clinical reports have demonstrated the presence of AQP4 in human and animal placenta, and have linked AQP4-mediated placental inflammation to fetal death.8–10 It is clear that the annualized relapse rate (ARR) of NMOSD is significantly increased in the 0- to 3-month postpartum period,11–13 but there is a lack of information on the influence of NMOSD on the course of pregnancy.
We investigated the effect of NMOSD on miscarriage and preeclampsia rates using multivariate logistic regression analysis in a retrospective international cohort of 60 women with AQP4-positive NMOSD.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by the medical ethics committee of each center and conducted in accordance with internationally recognized ethical standards. Written consent to collect and use anonymized clinical data was obtained from each patient before the study.
Cohort and data collection.
Every woman with a history of ≥1 pregnancy and a diagnosis of AQP4-IgG–positive neuromyelitis optica (NMO) (fulfilling the 2006 revised diagnostic criteria14 or NMOSD as defined by Wingerchuk et al.1), registered at 1 of 3 hospitals in 3 countries (Oxford, UK; Porto, Portugal; Sendai, Japan), was enrolled in the study. Sixty women (126 pregnancies) met these inclusion criteria. All patients were tested for AQP4-IgG by cell-based assay in Oxford 15 or Sendai.16 Thirty percent of patients were first tested at clinical presentation; the remaining patients were tested at varying time points after the disease onset, at diagnosis. Disease onset was defined as the time of presenting attack.
Because this was not a prospective study, AQP4-IgG titers were not measured at regular intervals and were not included in the analyses. Titers in patients where blood samples were collected around the time of pregnancy are shown in figure e-1 on the Neurology® Web site at Neurology.org.
Pregnancy details were collected retrospectively (2011–2013) from 60 women (126 pregnancies from 1963 to 2013) using clinical notes and clinical interview. In the Japanese cohort (19 women, 39 pregnancies) pregnancy information was not available for women with an obstetric history consisting only of miscarriages. Consequently, the Japanese cohort potentially underestimated the true miscarriage rate and was not included in the miscarriage analysis. One woman with a history of only 2 ectopic pregnancies was excluded from the analysis. Miscarriage analysis was therefore performed on 40 women (85 pregnancies) from the United Kingdom and Portugal cohorts. The preeclampsia analysis excluded ectopic pregnancies and miscarriages, and used pregnancies from all 3 centers (57 women, 113 pregnancies). Figure e-2 summarizes the study design.
Miscarriage was defined as a spontaneous loss of intrauterine pregnancy during the first 24 weeks.17 There were no spontaneous losses of intrauterine pregnancy after this time. Preeclampsia was defined as new gestational hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg on at least 2 occasions 4 hours apart), developing after 20 weeks’ gestation, with proteinuria (≥300 mg in 24 hours, or 2 ++ readings on urine dipstick).18
Statistical analysis.
The association between maternal variables and miscarriage or preeclampsia was assessed using univariate logistic regression. Independent variables were race, maternal age at conception, temporal relationship to NMOSD onset, previous pregnancy, medical history of miscarriage (specifically: most recent pregnancy ending in miscarriage compared to most recent pregnancy ending in live birth; recurrent miscarriages, defined as ≥2 consecutive miscarriages; and obstetric history of only miscarriages, as per reference 19), medical history of preeclampsia, and clinical or immunologic evidence of one or ≥2 other autoimmune disorders (analyzed as a single group, as small sample sizes prevented analysis of individual disorders). The temporal relationship of pregnancy to NMOSD onset was assessed both as a continuous variable and discrete variable with 3 variations (pregnancy no earlier than 0, 1, and 3 years before NMOSD onset, compared to the remaining pregnancies). Similarly, maternal age was assessed both as a continuous variable and as a discrete variable with 3 variations (pregnancy ≥34, ≥33, and ≥32 years, compared to the remaining pregnancies). These values were selected to allow a numerically balanced comparison between “maternal age at pregnancy” and “timing of pregnancy relative to NMOSD onset” variables. Odds ratios (ORs) were calculated for all independent variables (i), using the formula eβi where βi is equal to the partial regression coefficient for variable “i.” Variables associated with a significant increase in OR of miscarriage or preeclampsia in univariate analysis were further analyzed using multivariate logistic regression. We compared variables in women of different racial backgrounds using the Fisher exact test of independence (dichotomous variables) and the Student 2-tailed unpaired t test (continuous variables).
The relationship between NMOSD ARR and pregnancy outcome was investigated in the subset of all pregnancies in which NMOSD onset was before, or within 1 year after, the end of pregnancy (27 women, 15 pregnancies), as in references 12 and 13. The relationship between pregnancy outcome and peri-pregnancy disease activity (any relapse from 9 months before conception to 9 months after pregnancy including onset attack) was analyzed using the Student 2-tailed unpaired t test. The relationship between pregnancy outcome and concomitant immunosuppression was analyzed in the subset of pregnancies occurring after NMOSD onset. Statistical analysis was performed using MATLAB (MathWorks Inc., Natick, MA).
RESULTS
Study cohort.
Sixty AQP4-seropositive women with 126 pregnancies were included in this study to assess the influence of NMOSD on pregnancy outcome. Clinical and obstetric details are summarized in tables e-1 and e-2, respectively. Disease onset, at mean age of 46.4 years, was with isolated ON (42%), transverse myelitis (38%), or brain involvement (18%). The majority of patients with only ON (15/25) or brain/brainstem involvement (9/11) at onset progressed to fulfill the diagnostic criteria for NMO, while none of the patients with transverse myelitis developed ON. Immunotherapy was commenced an average of 47.2 months from disease onset, and 8 pregnancies occurred while the patient was on immunotherapy.
Maternal race.
There were no significant differences in parity, mean age of pregnancy, or mean age of NMOSD onset between women of Caucasian, Afro-Caribbean, and Japanese origin (mean parity = 2.11 [SD 0.913], 2.14 [1.07], and 2.05 [0.621], respectively; mean age of pregnancy = 28.4 [5.60], 27.3 [5.92], and 29.3 [4.66] years, respectively; and mean age of NMOSD onset = 46.6 [12.9], 37.7 [8.88], and 42.6 [14.6] years, respectively). There was a trend for women of Caucasian origin to have a later age of NMOSD onset compared to Afro-Caribbean women (p = 0.0896). There was sizeable overlap between the mean age of pregnancy and the mean age of NMOSD onset (figure e-3), and 22 pregnancies (17.5%) occurred after NMOSD (no significant difference with maternal race).
There was clinical or immunologic evidence of other autoimmune disorder (systemic lupus erythematosus, antiphospholipid syndrome, Sjögren syndrome, thyroid disease, and myasthenia gravis) in 63.3% of women in this study, and 16.7% of women had evidence of multiple other autoimmune disorders (no significant difference with maternal race).
Miscarriage.
Eighty-five informative pregnancies from 40 women were included in the analysis of the influence of NMOSD on the OR of miscarriage (figure e-2). Eleven pregnancies in 6 women ended in miscarriage (12.9%, 95% confidence interval [CI] 6.64%–22.0%): 7 in the first trimester, 1 in the second trimester, and 3 at an unknown time within the first 24 weeks. There was no significant difference in miscarriage rate between Caucasian and Afro-Caribbean women (p = 0.202).
Six of 14 pregnancies (42.9% [17.7%–71.1%]) after NMOSD onset ended in miscarriage, compared to 5 of 71 pregnancies (7.04% [2.33%–15.7%]) before NMOSD onset (figure 1A). Miscarriage rates after NMOSD onset were 60% in Caucasian women and 0% in Afro-Caribbean women (p = 0.0849).
Figure 1. Relationships between adverse pregnancy outcome, NMOSD onset, and maternal age.
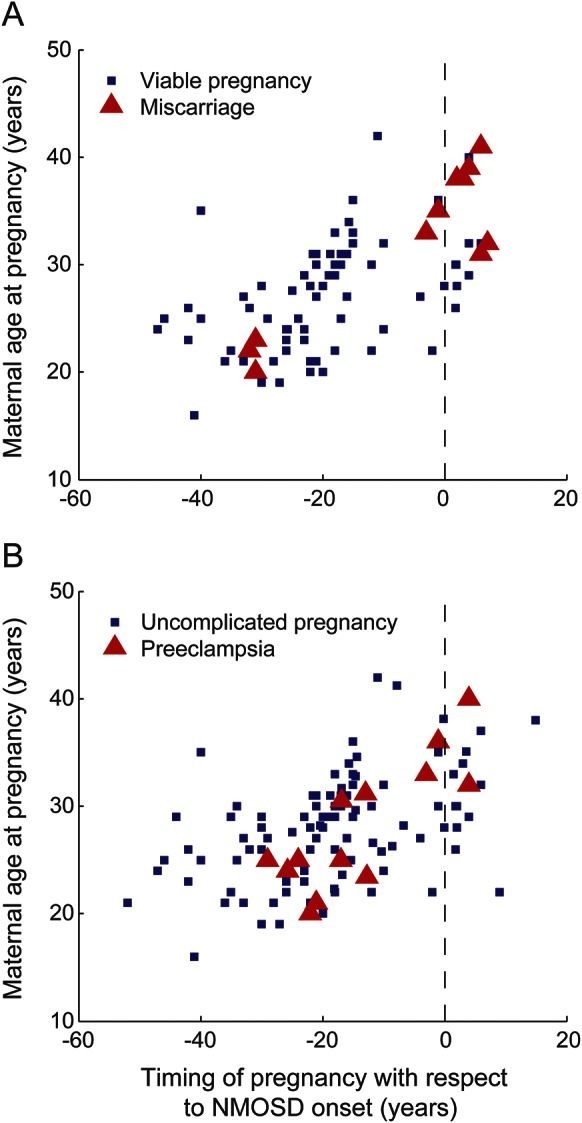
(A) Distribution of pregnancies ending in miscarriage, plotted against maternal age and pregnancy timing with respect to NMOSD onset. Blue squares denote viable pregnancies at time of delivery. Red triangles denote pregnancies ending in miscarriage, at time of miscarriage. Black vertical line marks onset of NMOSD. (B) Distribution of pregnancies complicated by preeclampsia, plotted against maternal age and pregnancy timing with respect to NMOSD onset. Blue squares denote viable pregnancies without preeclampsia, at time of delivery. Red triangles denote viable pregnancies complicated by preeclampsia, at time of delivery. Black vertical line marks onset of NMOSD. NMOSD = neuromyelitis optica spectrum disorder.
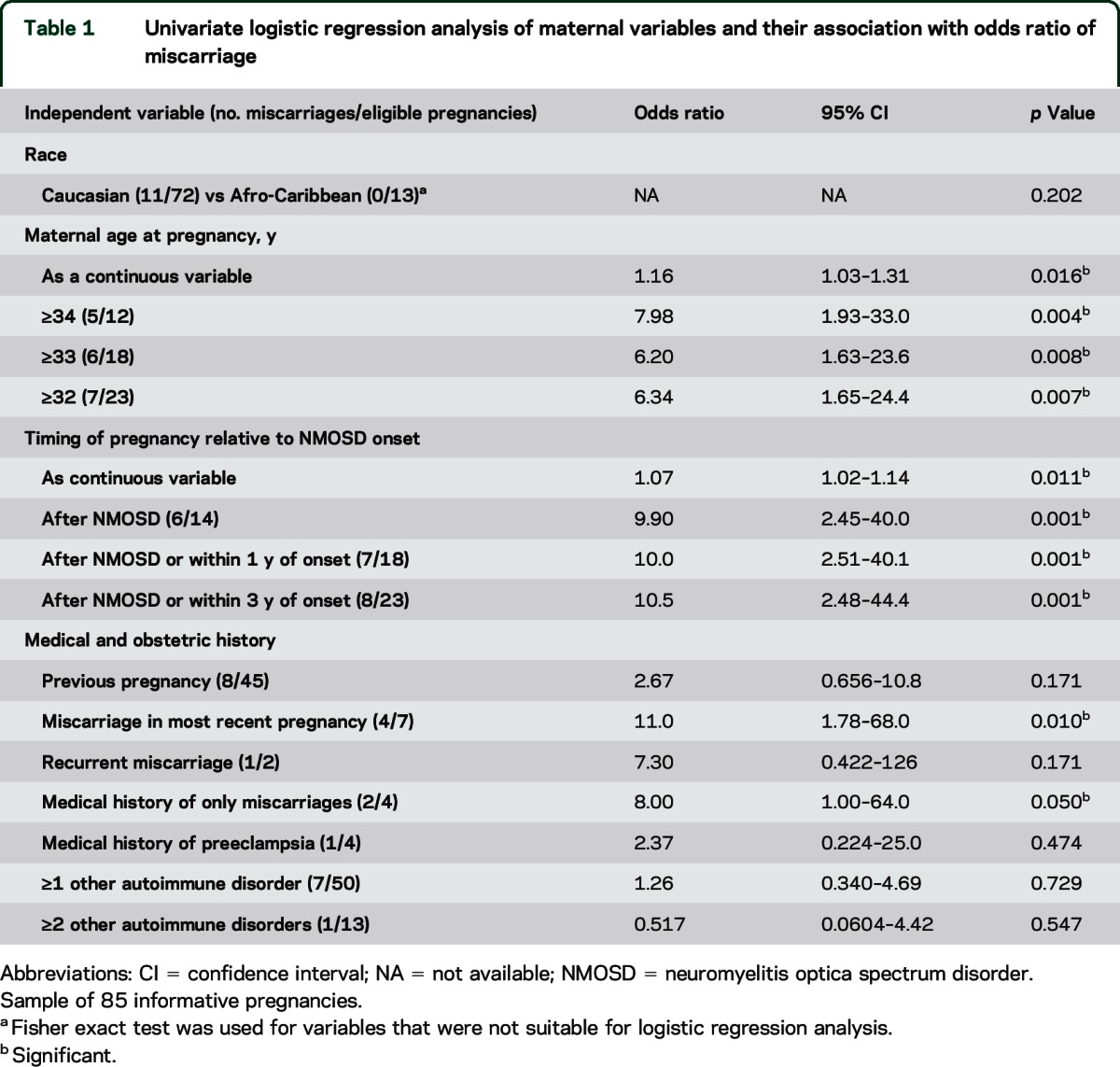
Univariate logistic regression identified maternal age at pregnancy, timing of pregnancy relative to NMOSD onset, most recent pregnancy ending in miscarriage, and obstetric history consisting only of miscarriages as being associated with increased OR of miscarriage (table 1). There was no association between the presence of other autoimmune disorders (analyzed as a heterogeneous single group) and increased odds of miscarriage. Furthermore, there was no association between antiphospholipid antibodies and miscarriage rate.
Table 1.
Univariate logistic regression analysis of maternal variables and their association with odds ratio of miscarriage
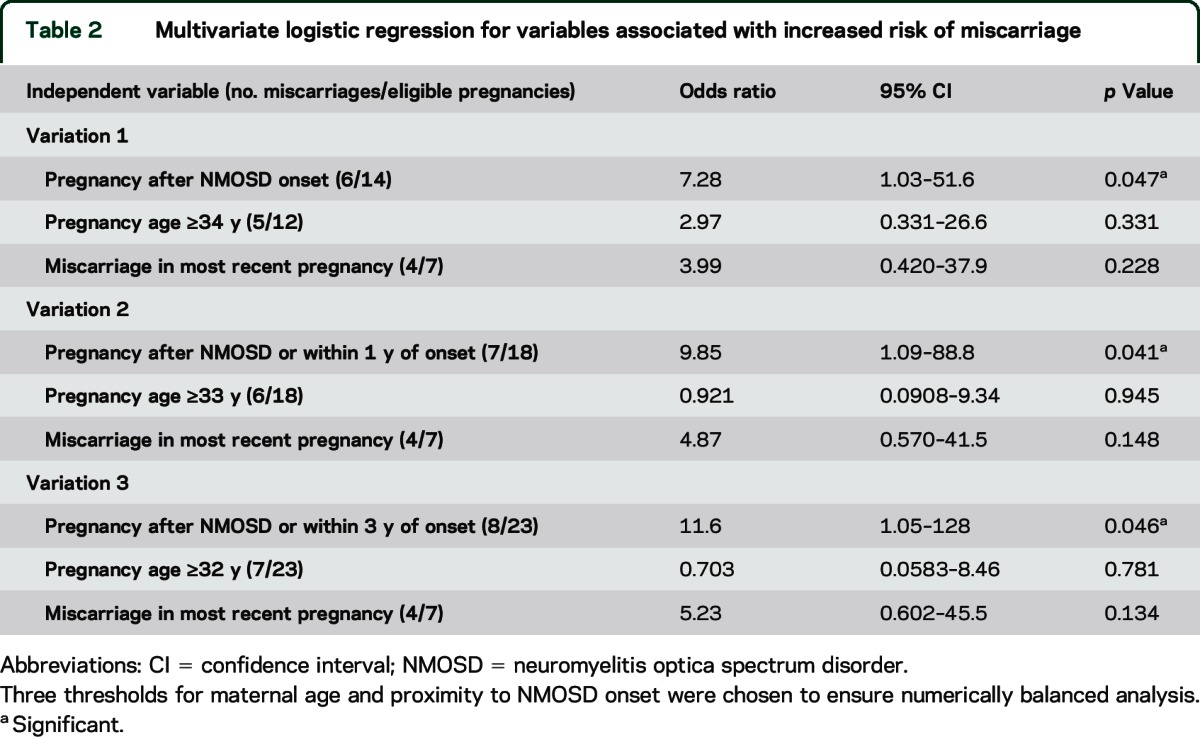
Multivariate logistic regression analyzed the independent influences of timing relative to NMOSD onset, maternal age at pregnancy, and history of miscarriage in the most recent pregnancy, on miscarriage rate. Timing of pregnancy with respect to NMOSD emerged as the strongest predictor of miscarriage (table 2).
Table 2.
Multivariate logistic regression for variables associated with increased risk of miscarriage
Preeclampsia.
One hundred thirteen informative pregnancies in 57 women were included in the analysis of the influence of NMOSD on OR of preeclampsia (figure e-2). Thirteen cases of preeclampsia were distributed among 11 women, corresponding to a preeclampsia rate of 11.5% (95% CI 6.27%–18.9%). The incidence of preeclampsia in pregnancies occurring before and after NMOSD onset was 11.2% (5.74%–9.2%) and 13.3% (1.66%–40.5%), respectively (figure 1B).
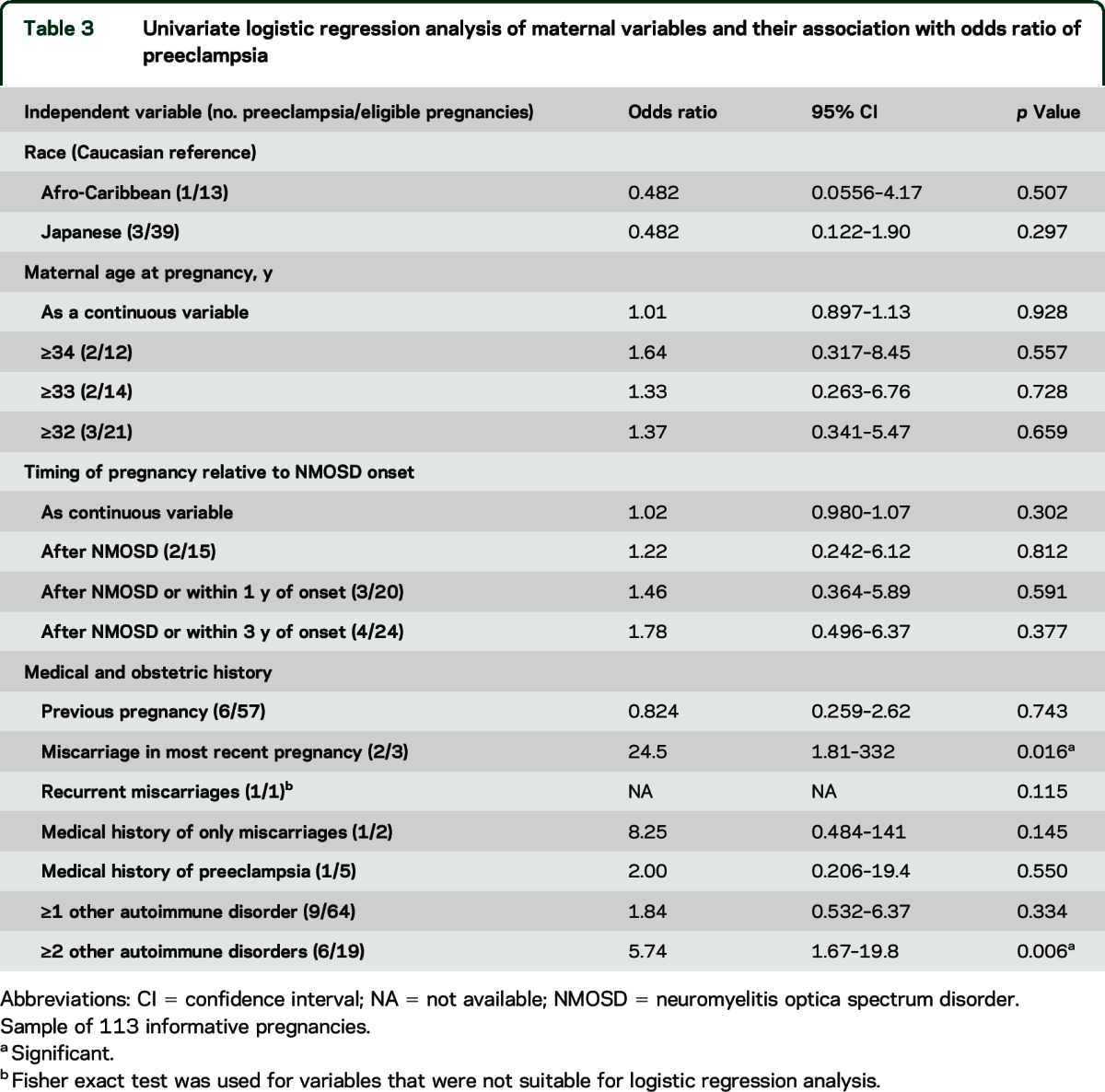
Univariate logistic regression was used in an identical manner to the miscarriage analysis (table 3). Miscarriage in the most recent pregnancy and presence of multiple other autoimmune diseases were associated with an increased OR of preeclampsia. No significant association was found between preeclampsia risk and maternal age or timing of pregnancy relative to NMOSD onset (table 3). Multivariate logistic regression found that both miscarriage in the most recent pregnancy (OR 27.2 [1.57–475], p = 0.023) and maternal history of at least 2 other autoimmune diseases (OR 8.01 [1.03–62.6], p = 0.047) were independently significantly associated with an increased OR of preeclampsia (table e-3).
Table 3.
Univariate logistic regression analysis of maternal variables and their association with odds ratio of preeclampsia
NMOSD disease activity, immunosuppression, and AQP4-IgG titers.
Twenty-seven pregnancies occurred after (n = 21), or up to 1 year before (n = 6), NMOSD onset (figure e-4). Mean ARR during the 3 trimesters before conception, the pregnancy period, and the 3 trimesters after pregnancy, was calculated for pregnancies ending in miscarriage (n = 7) and live birth (n = 20) separately (figure 2).
Figure 2. NMOSD ARR in pregnancies after NMOSD or within 1 year of NMOSD onset.
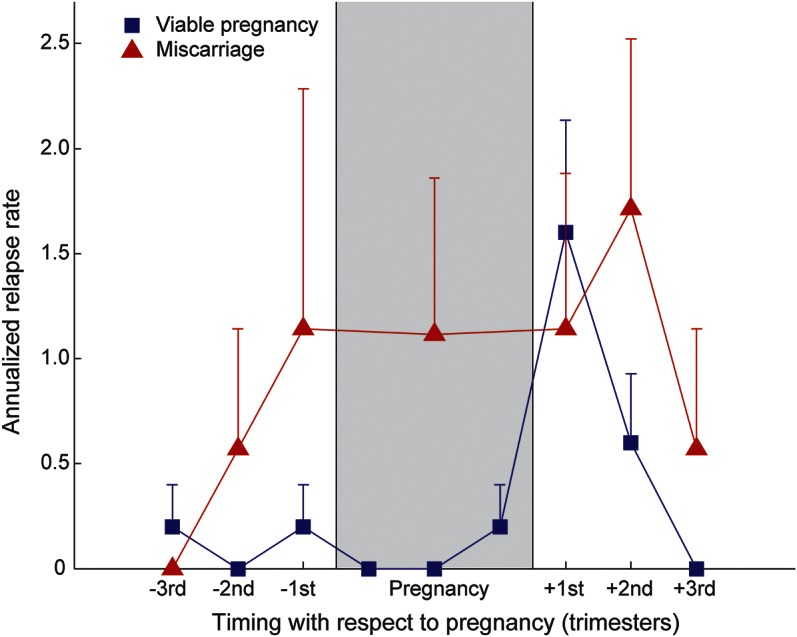
ARR is shown for the 9 months before conception, intrapregnancy period, and the 9 months postpregnancy. Shaded gray region represents the intrapregnancy period (9 months in viable pregnancies, 1–4 months for miscarriages). Blue squares represent viable pregnancies (n = 20). Red triangles represent pregnancies ending in miscarriage (n = 7). All data points show the ARR derived from the relapse rate per 3-month period (with the exception of the intrapregnancy period for pregnancies ending in miscarriage, where the single data point represents the ARR calculated from the number of relapses during the truncated pregnancy-specific duration). Error bars are +1 SEM. ARR = annualized relapse rate; NMOSD = neuromyelitis optica spectrum disorder.
Viable pregnancies were associated with increased mean ARR in the 0- to 3-month postpartum period compared to 0 to 9 months preconception (1.60 vs 0.133, p = 0.01). This difference was not significant in pregnancies ending in miscarriage (1.14 and 0.571, p = 0.508). Mean ARR from the 9 months preconception to the end of pregnancy was higher in the miscarriage subgroup compared to the viable pregnancy subgroup (0.707 vs 0.100, p = 0.0152). Moreover, there was a trend toward significance for the ability of the ARR during this period to predict miscarriage using univariate logistic regression analysis (p = 0.0726). The difference in mean ARR between miscarriage and viable pregnancies during the 9-month postpregnancy period, however, was not significant (1.43 vs 0.733, p = 0.415).
Standard immunotherapy included high-dose steroids ± plasma exchange to treat attacks and maintenance therapy (started before conception) of prednisolone alone, azathioprine alone, or both agents combined (see figure e-4 for details). Of the 21 pregnancies that occurred after NMOSD onset, 8 were associated with concurrent maintenance immunosuppressive therapy. Two of these pregnancies in 2 women were uncomplicated by miscarriage or preeclampsia. They received prednisolone 5 mg/d or a combination of azathioprine 100 mg/d and prednisolone 5 mg/d. Five pregnancies in 2 women ended in miscarriage. These patients were treated with prednisolone 5 to 10 mg/d in the first woman, and azathioprine 150 mg/d in the second. One patient whose pregnancy was complicated with preeclampsia was on a combination of azathioprine 250 mg/d and prednisolone 20 mg/d. No patients interrupted treatment just before or during pregnancy. Two patients with viable pregnancies received acute treatment during pregnancy. The attacks occurred in the second to third trimester in one patient, and third trimester and at 3 months postpartum in the second patient. These patients were not receiving any chronic treatment. Attacks were treated with high-dose steroids (both patients; 1 g/d for 3 days) and plasma exchange (one patient; 5 consecutive days).
Finally, 7 patients with pregnancy up to 3 years before, or after the disease onset, had been tested for AQP4-IgG more than once around the time of pregnancy. There was no clear correlation between the AQP4-IgG titers and pregnancy outcome. In the majority of cases, levels reduced shortly after starting immunosuppressive treatment (figure e-1).
Neonatal complications.
One pregnancy resulted in an infant with hydrocephalus and permanent neurologic disability. This pregnancy occurred 4 years after the onset of AQP4-IgG–positive relapsing ON, although this had not been diagnosed or treated. The pregnancy was also complicated by preeclampsia. There were no stillbirths.
DISCUSSION
A number of studies have investigated the effect of pregnancy on the clinical activity of AQP4-IgG–positive NMOSD.11–13 This study explicitly focused on adverse pregnancy outcomes in NMOSD mediated by AQP4-IgG. Pregnancies after NMOSD onset were associated with a significantly increased OR of miscarriage compared to pregnancies before NMOSD onset, independent of the risk associated with advanced maternal age. By contrast, the OR of preeclampsia was independent of NMOSD onset timing, but was increased in women with multiple other autoimmune diseases or a history of miscarriage in the most recent pregnancy. Moreover, pregnancies after NMOSD onset ending in miscarriage are associated with higher rates of disease activity in the preconception and intrapregnancy period compared with viable pregnancies, and were consequently more often receiving treatment.
The clinical observation of increased miscarriage in AQP4-IgG–seropositive patients suggests AQP4-IgG as a possible causative agent. AQP4-IgG is abundant in the periphery,20 and during pregnancy there is expression of AQP4 in syncytiotrophoblasts and stroma of placental villi, suggesting that it may have an important role in maternal–fetal fluid exchange.9 A recent mouse passive transfer study showed that intraperitoneal injection of both AQP4-IgG and human complement resulted in placental inflammation with increased fetal death,10 reproducing key histologic features seen in NMO lesions of the CNS, as well as in placental lesions in a case report of a miscarriage at 20 weeks in a patient with NMO.8 This adds to the growing clinical8,21,22 and experimental10,23 evidence of AQP4-IgG–mediated organ involvement beyond the CNS.
Our cohort miscarriage rate (12.9%) is consistent with levels in the general population (12%–24%).17,19 In pregnancies conceived after NMOSD onset, the miscarriage rate was 42.9%, and there was an increased OR of miscarriage in pregnancies conceived after, or up to 3 years before, NMOSD onset, independent of maternal age. This finding may be explained by subclinical levels of circulating AQP4-IgG, which may be present years before clinical presentation.24,25
The miscarriage rate of 42.9% is outside the 95% CI that would be expected if the true miscarriage rate post-NMOSD was equal to reported rates in multiple sclerosis (expected 95% CI 1.29%–40.9%)26,27 and just within the expected range calculated from published rates in systemic lupus erythematosus (expected 95% CI 2.56%–45.5%).28 This suggests that the miscarriage rate in patients with NMOSD is higher than in patients with multiple sclerosis, although larger prospective studies with adequate control groups are needed to test this hypothesis.
There were no reported miscarriages among the 13 pregnancies in Afro-Caribbean women, 4 of which occurred after NMOSD onset, with 2 patients receiving acute treatment for attacks in the second to third and third trimesters. Previous studies have reported differences in NMOSD characteristics between different racial groups,1,29 and the relationship between NMOSD and pregnancy outcomes in women of different racial backgrounds should be investigated in larger studies.
We found that pregnancies after NMOSD onset ending in miscarriages were associated with a higher ARR in the 9 months before conception and during the pregnancy compared to viable pregnancies. This is consistent with the hypothesis that circulating AQP4-IgG, in the context of active disease, can cause placental autoimmune-mediated damage and miscarriage.
Our study revealed that the majority of miscarriages occurred in patients receiving immunosuppression, but this finding must be interpreted in the context of elevated disease activity in pregnancies ending in miscarriage. Similarly, in patients with systemic lupus erythematosus, disease activity before conception is a predictor of pregnancy loss.30 The available literature suggests that prednisolone and azathioprine may be used safely in pregnant woman who have chronic autoimmune conditions.30,31 Based on this evidence, it has been widely recommended that patients with antibody-mediated diseases who plan pregnancy continue on treatment that is recognized as safe (e.g., steroids, azathioprine, cyclosporine),31 before, during, and after pregnancy, to reduce disease activity and improve pregnancy outcome.
The overall rate of preeclampsia was higher in our cohort than in control populations (11.5% vs 3.1%, p < 0.0001).18,32,33 We found an increased risk of preeclampsia in women with multiple other autoimmune disorders, consistent with previous findings.34–36 No association was found between preeclampsia and NMOSD onset age or maternal age.
AQP4 in fetal CNS is expressed as early as 14 weeks,37 and disturbances in fetal CNS fluid balance caused by AQP4 abnormalities could theoretically contribute to neonatal hydrocephalus.38 Hydrocephalus has also been recognized in patients with NMOSD with brain involvement.39 In our cohort, there was one case of severe neonatal hydrocephalus with permanent neurologic disability. However, without pathologic confirmation of AQP4-IgG–mediated disease in this neonate, the link between maternal NMOSD and neonatal NMOSD with brain involvement remains speculative.
The main limitations of our study are the small sample size (attributed to the rarity of the disease), the retrospective design, and the lack of pathologic evidence from placenta samples. Nevertheless, we show that (1) patients with NMOSD have high risk of miscarriage particularly in pregnancies occurring within the 3 years before, or after, the disease onset; (2) higher disease activity appears to precede pregnancies that end in miscarriage; (3) patients with NMOSD who have other autoimmune manifestations have high risk of preeclampsia in pregnancies occurring at any time in their life; and (4) a single case of hydrocephalus in this cohort suggests the possibility of fetal brain developmental abnormalities due to AQP4-IgG. Furthermore, preventing disease activity before and during pregnancy appears to be essential to improve pregnancy outcome in patients with NMOSD. Larger prospective studies with age-matched controls are required to extend our findings and to allow better evidence-based treatment approaches before, during, and after pregnancy to reduce disease activity, prevent attacks, and avoid complications of both pregnancy and NMOSD.
Supplementary Material
GLOSSARY
- AQP4
aquaporin-4
- ARR
annualized relapse rate
- CI
confidence interval
- IgG
immunoglobulin G
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disorder
- ON
optic neuritis
- OR
odds ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
M.M.N.: analyzed the data and wrote the paper, substantial contribution to the study conception, acquisition, analysis, and interpretation of data for the work, writing the manuscript, drafting and correction of all versions of the manuscript including figures, tables, and references, completion of the work to be submitted, final approval of the version to be published, agreement to be accountable for all aspects. I.N.: substantial contribution to the conception and design of the work, as well as supervision of the acquisition, analysis, and interpretation of data for the work, revising several versions of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. E.C.: acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. M.W.: substantial contribution to the acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. F.S.: acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. J.R.: substantial contribution to the planning of the work, as well as acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. Y.T.: substantial contribution to the acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. J.G.: acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. J.K.: acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. M.E.S.: acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. J.M.N.: substantial contribution to the conception and design of the work, and critical contribution to the statistical analysis and interpretation of data for the work, revising several versions of manuscript critically for important intellectual content, final responsibility and approval of the version to be published, agreement to be accountable for all aspects of the work. F.C.: acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. H.K.: acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. T.M.: acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. A.M.-d.-S.: acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. G.C.D.: contribution to the plan of the work, acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. A.V.: substantial contribution to the design of the work, as well as supervision of the acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. J.P.: substantial contribution to the acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. P.W.: substantial contribution to the conception and design of the work, as well as supervision of the acquisition, analysis, and interpretation of data for the work, revising several versions of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. K.F.: substantial contribution to the conception and design of the work, as well as supervision of the acquisition, analysis, and interpretation of data for the work, revising of manuscript critically for important intellectual content, final approval of the version to be published, agreement to be accountable for all aspects of the work. M.I.L.: substantial contribution to the conception and design of the work, as well as supervision of the acquisition, analysis, and interpretation of data for the work, supervision of the manuscript preparation, revising several versions of manuscript critically for important intellectual content, final responsibility and approval of the version to be published, agreement to be accountable for all aspects of the work.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
M. Nour reports no disclosures relevant to the manuscript. I. Nakashima has received funding for travel and received speaker honoraria from Bayer Schering Pharma and Biogen Idec and has received research funding from Mitsubishi Chemical Medicine Corporation and the Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Technology of Japan. E. Coutinho, M. Woodhall, F. Sousa, J. Revis, Y. Takai, and J. George report no disclosures relevant to the manuscript. J. Kitley was supported by the NHS National Specialised Commissioning Group for Neuromyelitis Optica and has received travel grants from Biogen Idec, Novartis, and Teva and speaker honoraria from Novartis. M. Santos, J. Nour, F. Cheng, and H. Kuroda report no disclosures relevant to the manuscript. T. Misu has received speaker honoraria from Bayer Schering Pharma, Biogen Idec Japan, Mitsubishi Tanabe Pharma Corporation, Asahi Kasei Medical Co., and Astellas Pharma Inc. and has received research support from Bayer Schering Pharma, Biogen Idec Japan, Asahi Kasei Kuraray Medical Co., The Chemo-Sero-Therapeutic Research Institute, Teva Pharmaceutical K.K., Mitsubishi Tanabe Pharma Corporation, Teijin Pharma, and Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Technology, and the Ministry of Health, Labor and Welfare of Japan. A. Martins-da-Silva reports no disclosures relevant to the manuscript. G. DeLuca is supported a Goodger Scholarship (University of Oxford) and the NIHR Biomedical Research Centre, Oxford. G.C.D. has received honoraria and travel expenses as an invited speaker for Bayer Schering and travel expenses from Novartis and Biogen Idec. A. Vincent and the Department of Clinical Neurology in Oxford hold patents and receive royalties and payments for performing antibody assays. J. Palace is supported by NHS National Specialised Commissioning Group for Neuromyelitis Optica, UK; she serves on the scientific advisory board for Charcot Foundation, and has performed advisory work for Biogen Idec, Merck Serono Ltd., Bayer Schering Pharma, Novartis Pharmaceuticals UK Ltd., Teva Pharmaceutical Industries Ltd., Gilenya, Ono Pharmaceutical Co. Ltd., Primary i-research, Chugai Pharma Europe, and CI Consulting. She receives research support from the MS Society, QIDIS, Merck Serono Ltd., and Bayer Schering Pharma, plus conference expenses from Novartis and Merck Serono Ltd. P. Waters is supported by the NHS National Specialised Commissioning Group for Neuromyelitis Optica, UK, and the NIHR Oxford Biomedical Research Centre. He has received speaker honoraria from Biogen Idec and Euroimmun AG, and travel grants from the Guthy-Jackson Charitable Foundation. He holds patents and has received royalties for antibody assays. K. Fujihara serves on scientific advisory boards for Bayer Schering Pharma, Biogen Idec, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, Merck Serono, Alexion Pharmaceuticals, MedImmune, and Medical Review; has received funding for travel and speaker honoraria from Bayer Schering Pharma, Biogen Idec, Eisai Inc., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Astellas Pharma Inc., Takeda Pharmaceutical Company Limited, Asahi Kasei Medical Co., Daiichi Sankyo, and Nihon Pharmaceutical; serves as an editorial board member of Clinical and Experimental Neuroimmunology (2009–present) and an advisory board member of Sri Lanka Journal of Neurology; has received research support from Bayer Schering Pharma, Biogen Idec Japan, Asahi Kasei Medical, the Chemo-Sero-Therapeutic Research Institute, Teva Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, and Genzyme Japan; is funded as the secondary investigator (22229008, 2010–2015) by the Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Technology of Japan and as the secondary investigator by the Grants-in-Aid for Scientific Research from the Ministry of Health, Welfare and Labor of Japan (2010–present). M. Leite is supported by NHS National Specialised Commissioning Group for Neuromyelitis Optica, UK, and by NIHR Oxford Biomedical Research Centre, and has received speaking honoraria from Biogen Idec and travel grants from Novartis. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815. [DOI] [PubMed] [Google Scholar]
- 2.Yanagawa K, Kawachi I, Toyoshima Y, et al. Pathologic and immunologic profiles of a limited form of neuromyelitis optica with myelitis. Neurology 2009;73:1628–1637. [DOI] [PubMed] [Google Scholar]
- 3.Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112. [DOI] [PubMed] [Google Scholar]
- 4.Quek AML, McKeon A, Lennon VA, et al. Effects of age and sex on aquaporin-4 autoimmunity. Arch Neurol 2012;69:1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornelio DB, Braga RP, Rosa MW, Ayub AC. Devic's neuromyelitis optica and pregnancy: distinction from multiple sclerosis is essential. Arch Gynecol Obstet 2009;280:475–477. [DOI] [PubMed] [Google Scholar]
- 6.Bencherifa F, Bourissa A, Mellal Z, Berraho A. Devic's neuro-optic myelitis and pregnancy. J Fr Ophtalmol 2007;30:737–743. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet F, Mercié P, Morlat P, et al. Devic's neuromyelitis optica during pregnancy in a patient with systemic lupus erythematosus. Lupus 1999;8:244–247. [DOI] [PubMed] [Google Scholar]
- 8.Reuss R, Rommer P, Bruck W, Paul F, Bolz M. A woman with acute myelopathy in pregnancy: case outcome. BMJ 2009;339:1372–1373. [DOI] [PubMed] [Google Scholar]
- 9.De Falco M, Cobellis L, Torella M, et al. Down-regulation of aquaporin 4 in human placenta throughout pregnancy. In Vivo 2007;21:813–817. [PubMed] [Google Scholar]
- 10.Saadoun S, Waters P, Leite MI, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica IgG causes placental inflammation and fetal death. J Immunol 2013;191:2999–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourre B, Marignier R, Zéphir H, et al. Neuromyelitis optica and pregnancy. Neurology 2012;78:875–879. [DOI] [PubMed] [Google Scholar]
- 12.Kim W, Kim SH, Nakashima I, et al. Influence of pregnancy on neuromyelitis optica spectrum disorder. Neurology 2012;78:1264–1267. [DOI] [PubMed] [Google Scholar]
- 13.Fragoso YD, Adoni T, Bichuetti DB, et al. Neuromyelitis optica and pregnancy. J Neurol 2013;260:2614–2619. [DOI] [PubMed] [Google Scholar]
- 14.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489. [DOI] [PubMed] [Google Scholar]
- 15.Waters PJ, McKeon A, Leite MI, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology 2012;78:665–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 2007;130:1235–1243. [DOI] [PubMed] [Google Scholar]
- 17.Jurkovic D, Overton C, Bender-Atik R. Diagnosis and management of first trimester miscarriage. BMJ 2013;346:f3676. [DOI] [PubMed] [Google Scholar]
- 18.Khalil A, Syngelaki A, Maiz N, Zinevich Y, Nicolaides KH. Maternal age and adverse pregnancy outcome: a cohort study. Ultrasound Obstet Gynecol 2013;42:634–643. [DOI] [PubMed] [Google Scholar]
- 19.Regan L, Braude PR, Trembath PL. Influence of past reproductive performance on risk of spontaneous abortion. BMJ 1989;299:541–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papadopoulos MC, Verkman AS. Aquaporin 4 and neuromyelitis optica. Lancet Neurol 2012;11:535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki N, Takahashi T, Aoki M, et al. Neuromyelitis optica preceded by hyperCKemia episode. Neurology 2010;74:1543–1545. [DOI] [PubMed] [Google Scholar]
- 22.Guo Y, Lennon VA, Popescu BFG, et al. Autoimmune aquaporin-4 myopathy in neuromyelitis optica spectrum. JAMA Neurol 2014;71:1025–1029. [DOI] [PubMed] [Google Scholar]
- 23.Ratelade J, Bennett JL, Verkman AS. Intravenous neuromyelitis optica autoantibody in mice targets aquaporin-4 in peripheral organs and area postrema. PLoS One 2011;6:e27412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leite M, Coutinho E, Lana-Peixoto M. Myasthenia gravis and neuromyelitis optica spectrum disorder: a multicenter study of 16 patients. Neurology 2012;78:1601–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishiyama S, Ito T, Misu T, et al. A case of NMO seropositive for aquaporin-4 antibody more than 10 years before onset. Neurology 2009;72:1960–1961. [DOI] [PubMed] [Google Scholar]
- 26.Amato MP, Portaccio E, Ghezzi A, et al. Pregnancy and fetal outcomes after interferon-β exposure in multiple sclerosis. Neurology 2010;75:1794–1802. [DOI] [PubMed] [Google Scholar]
- 27.Sandberg-Wollheim M, Alteri E, Moraga MS, Kornmann G. Pregnancy outcomes in multiple sclerosis following subcutaneous interferon beta-1a therapy. Mult Scler 2011;17:423–430. [DOI] [PubMed] [Google Scholar]
- 28.Smyth A, Oliveira GHM, Lahr BD, Bailey KR, Norby SM, Garovic VD. A systematic review and meta-analysis of pregnancy outcomes in patients with systemic lupus erythematosus and lupus nephritis. Clin J Am Soc Nephrol 2010;5:2060–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kitley J, Leite MI, Nakashima I, et al. Prognostic factors and disease course in aquaporin-4 antibody-positive patients with neuromyelitis optica spectrum disorder from the United Kingdom and Japan. Brain 2012;135:1834–1849. [DOI] [PubMed] [Google Scholar]
- 30.Ostensen M, Brucato A, Carp H, et al. Pregnancy and reproduction in autoimmune rheumatic diseases. Rheumatology 2011;50:657–664. [DOI] [PubMed] [Google Scholar]
- 31.Norwood F, Dhanjal M, Hill M, et al. Myasthenia in pregnancy: best practice guidelines from a UK multispecialty working group. J Neurol Neurosurg Psychiatry 2014;85:538–543. [DOI] [PubMed] [Google Scholar]
- 32.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens 2008;21:521–526. [DOI] [PubMed] [Google Scholar]
- 33.Klungsøyr K, Morken NH, Irgens L, Vollset SE, Skjaerven R. Secular trends in the epidemiology of pre-eclampsia throughout 40 years in Norway: prevalence, risk factors and perinatal survival. Paediatr Perinat Epidemiol 2012;26:190–198. [DOI] [PubMed] [Google Scholar]
- 34.Gordon C. Pregnancy and autoimmune diseases. Best Pract Res Clin Rheumatol 2004;18:359–379. [DOI] [PubMed] [Google Scholar]
- 35.Chakravarty EF, Nelson L, Krishnan E. Obstetric hospitalizations in the United States for women with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum 2006;54:899–907. [DOI] [PubMed] [Google Scholar]
- 36.Barnado A, Wheless L, Meyer AK, Gilkeson GS, Kamen DL. Pregnancy outcomes among African-American patients with systemic lupus erythematosus compared with controls. Lupus Sci Med 2014;1:e000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gömöri E, Pál J, Abrahám H, et al. Fetal development of membrane water channel proteins aquaporin-1 and aquaporin-4 in the human brain. Int J Dev Neurosci 2006;24:295–305. [DOI] [PubMed] [Google Scholar]
- 38.Owler B, Pitham T, Dongwei W. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res 2010;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clardy SL, Lucchinetti CF, Krecke KN, et al. Hydrocephalus in neuromyelitis optica. Neurology 2014;82:1841–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


