Abstract
Objective
Women experience multiple co-occurring symptoms (symptom clusters) during the menopausal transition and early postmenopause. Although symptom clusters have been identified among community-dwelling midlife women, to date there have been no studies of midlife participants in clinical trials for hot flashes. Our objective was to identify symptom clusters using standardized measures completed by participants in the Menopausal Strategies: Finding Lasting Answers to Symptoms and Health (MsFLASH) clinical trial at baseline including: hot flash interference, and sleep, depressive, anxiety, and pain symptoms.
Methods
Data from all women randomized to interventions and controls from MsFLASH studies 1, 2, and 3 (N=899) were included; 797 with complete data were used in the analyses. Scores from standardized measures obtained at baseline included: Hot Flash Related Daily Interference Scale (HFRDIS), Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), Patient Health Questionnaire (PHQ 9) measure of depressed mood, Generalized Anxiety Disorder (GAD), and Brief Pain Inventory PEG scores. Latent class analysis was used to identify symptom clusters using standardized scale scores and their established cut points.
Results
We identified 5 classes using the BIC and AIC criteria. Women in classes 1 and 2 had high hot flash interference levels relative to the others, and class 1 (10.5% of total) included severe hot flash interference, severe sleep symptoms, and moderately severe pain symptoms (hot flash, sleep, pain). In class 2 (14.1%), severe hot flash interference was paired with the severe sleep symptoms, and moderate to severe depressed and anxious mood symptoms and pain (hot flash, sleep, mood, pain). In class 3 (39.6%) women reported moderately severe sleep symptoms with moderate hot flash interference, and low severity mood and pain symptoms (hot flash, sleep). Those in class 4 (7.0%) reported moderate hot flash interference with severe levels of anxiety and depressed mood symptoms, but low levels of other symptoms (hot flash, mood). Women in class 5 (28.7%) reported the lowest levels of all 5 symptoms (low severity symptoms).
Conclusions
Women meeting hot flash frequency criteria for inclusion in clinical trials exhibited multiple co-occurring symptoms that clustered into identifiable groups according to symptom interference and severity. Variability of symptom profiles between the classes was evident, indicating that the classes were composed of differing symptom types and not simply differing severity levels. These symptom clusters may be useful phenotypes for differentiating treatment effects or evaluating associations with biomarkers or genes.
Keywords: symptom clusters, menopause, hot flashes, sleep disturbances, mood, pain
Although a majority of women experience hot flashes during the menopausal transition and early postmenopause, many women experience multiple co-occurring symptoms (symptom clusters) during this period. Cray and colleagues found 3 predominant symptom clusters in the population-based Seattle Midlife Women’s Health Study cohort: cluster 1 included high severity hot flashes and moderate levels of sleep, mood, cognitive, and pain symptoms (12% of women studied); cluster 2 included moderate levels of sleep, mood, cognitive and pain symptoms with low severity hot flashes (13%), and cluster 3 included low severity or absent symptoms (75%).1 These clusters were differentially associated with menopausal transition stages: the late menopausal transition stage and early postmenopause were significantly more highly associated with symptom cluster 1 than with the other clusters.1 In addition, higher FSH, lower estrone, higher norepinephrine and lower epinephrine levels were associated with cluster 1.2
Despite the common co-occurrence of multiple symptoms, to date the majority of treatment trials have focused on hot flashes as a single or primary end-point, with few including endpoint measures that incorporate multiple co-occurring symptoms. The Menopausal Strategies: Finding Lasting Answers to Symptoms and Health (MsFLASH) network, established in response to a National Institutes of Health request for applications and charged with conducting rapid throughput randomized trials of novel interventions to alleviate vasomotor and other menopausal symptoms, has recruited women with bothersome hot flashes to participate in clinical trials and included measures of hot flashes as well as sleep, depressive, anxiety, and pain symptoms at baseline and following treatment. To date, MsFLASH investigators have reported outcomes of trials of escitalopram, yoga, exercise, omega-3, low dose estradiol and venlafaxine, examining changes in hot flashes, sleep, depressive, anxiety, pain symptoms and overall quality of life analyzed as individual outcomes.3–11
Some of these therapies appear promising for symptoms other than hot flashes, such as sleep, but it is not clear that the treatment effects pertain to women with multiple, co-occurring symptoms. Analyses are needed that examine the therapeutic effects on clusters or combinations of symptoms in ways that would inform clinicians as they select optimal therapies for women reporting co-occurring symptoms. A long-term goal of this research is to determine the effectiveness of therapies such as those tested in MsFLASH trials, including pharmaceuticals such as escitalopram, estradiol, venlafaxine, and behavioral therapies such as yoga and exercise on hot flashes and co-occurring symptoms (clusters) that women experience during the menopausal transition and early postmenopause as a basis for personalizing treatment and determining for whom certain therapies are most efficacious. Exploration of the patterns of symptom clusters in this population is a necessary early step in determining the likely efficacy of therapies on symptom clusters. In addition, each of the clusters could indicate a unique phenotype for use in future genotype or biomarker association studies.
The purposes of these analyses were to:
Identify symptom clusters from baseline measures of hot flashes, sleep, depressive, anxiety, and pain symptoms in MsFLASH participants from all 3 trials using standardized measures of: hot flashes (Hot Flash Daily Interference Scale), sleep (Insomnia Severity Index, Pittsburgh Sleep Quality Index), depressive symptoms (Patient Health Questionnaire-9), anxiety symptoms (Generalized Anxiety Disorder-7), and pain (PEG scale from the Brief Pain Inventory).
Explore associations of demographic characteristics with clusters of symptoms.
Methods
Sample
All women who were randomized to interventions and control groups from the MsFLASH studies 1, 2 and 3 were included (N=899). Study 1, a trial of escitalopram,3 included 100 women in the intervention and 100 in the placebo control group. Study 2 used a 3×2 factorial design to test yoga and exercise vs. controls 6–9 crossed with omega-3 supplement vs. placebo, including 112 women in the yoga trial, 112 in the exercise trial, and 150 in the usual activity control group. Omega-3 and placebo were received by 187 women each. Study 3, a trial of estradiol vs. venlafaxine11,13 randomized 87 women to estradiol, 87 to venlafaxine, and 130 to placebo control. Of these women, a total of 797 participants provided complete data at baseline prior to randomization for the measures used in these analyses. The inclusion and exclusion criteria for the trials are discussed in detail elsewhere. 12 In brief, each of the trials required women to be: 40–62 years of age; in the late menopausal transition stage or within 5 years of their final menstrual period; not using hormone therapy in the past 2 months and no non-hormonal therapies for hot flashes; and experiencing at least 14–28 hot flashes per week, depending on the trial, and at least 4 reports of moderate to severe vasomotor symptoms and or bother for each of 3 weeks of screening. Medical history requirements were for generally good health, including not pregnant, breast-feeding or intending to become pregnant, no abnormal mammogram results in past two years, no current depression, no major severe depressive episode in past 3 months or suicide attempts in last 3 years, no diagnosis of psychosis/psychotic disorder. In addition, women were not eligible if they experienced drug/alcohol abuse in the past year or used psychotropic medications within the past 30 days. (See Newton12 for further details).
Measures
The MsFLASH trials incorporated standardized scales with known cutpoints which are described below. The data presented here are from the standardized measures for hot flashes, sleep, depressive, anxiety, and pain symptoms.
Hot flashes were assessed using the 10 item Hot Flash Related Daily Interference Scale (HFRDIS) which ascertains the degree to which hot flashes interfered with daily activities. 14 The scale requires women to rate the degree (0=does not interfere to 10 completely interferes) to which hot flashes interfere with nine daily life activities, such as work and social activities. 14 In this trial the HFRDIS score indicated the degree of interference associated with the symptom over the past week. Scores of 0–39 were rated mild, 40–69 moderate, and 70–100 severe (see Table 1).
Table 1.
Symptom Scores by Cluster Number
| Total N (%) 797 |
Symptom cluster number
|
|||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
|
| ||||||
| N (%) 84 (10.5) |
N (%) 112 (14.1) |
N (%) 316 (39.6) |
N (%) 56 (7.0) |
N (%) 229 (28.7) |
||
| HFRDIS | ||||||
| None (0) | 6 (0.8) | 0 (0.0) | 0 (0.0) | 1 (0.3) | 1 (1.8) | 4 (1.7) |
| Mild (1–39) | 398 (49.9) | 1 (1.2) | 7 (6.2) | 187 (59.2) | 28 (50.0) | 175 (76.4) |
| Moderate (40–69) | 328 (41.2) | 50 (59.5) | 82 (73.2) | 119 (37.7) | 27 (48.2) | 50 (21.8) |
| Severe (70–100) | 65 (8.2) | 33 (39.3) | 23 (20.5) | 9 (2.8) | 0 (0.0) | 0 (0.0) |
| ISI | ||||||
| No clinically significant insomnia (≤ 7) | 231 (29.0) | 11 (13.1) | 0 (0.0) | 7 (2.2) | 14 (25.0) | 199 (86.9) |
| Subthreshold insomnia (8–14) | 328 (41.2) | 30 (35.7) | 9 (8.0) | 218 (69.0) | 41 (73.2) | 30 (13.1) |
| Clinical insomnia (moderate, 15–21) | 203 (25.5) | 25 (29.8) | 88 (78.6) | 89 (28.2) | 1 (1.8) | 0 (0.0) |
| Clinical insomnia (severe, 22–28) | 35 (4.4) | 18 (21.4) | 15 (13.4) | 2 (0.6) | 0 (0.0) | 0 (0.0) |
| PSQI | ||||||
| < 5 | 147 (18.4) | 2 (2.4) | 0 (0.0) | 1 (0.3) | 5 (8.9) | 139 (60.7) |
| 5 – <8 | 259 (32.5) | 24 (28.6) | 4 (3.6) | 125 (39.6) | 31 (55.4) | 75 (32.8) |
| ≥ 8 | 391 (49.1) | 58 (69.0) | 108 (96.4) | 190 (60.1) | 20 (35.7) | 15 (6.6) |
| PHQ | ||||||
| No depression (0–4) | 559 (70.1) | 78 (92.9) | 4 (3.6) | 260 (82.3) | 0 (0.0) | 217 (94.8) |
| Mild depression (5–9) | 179 (22.5) | 6 (7.1) | 66 (58.9) | 54 (17.1) | 41 (73.2) | 12 (5.2) |
| Moderate+ depression (≥ 10) | 59 (7.4) | 0 (0.0) | 42 (37.5) | 2 (0.6) | 15 (26.8) | 0 (0.0) |
| GAD | ||||||
| No anxiety (0–4) | 612 (76.8) | 74 (88.1) | 34 (30.4) | 287 (90.8) | 0 (0.0) | 217 (94.8) |
| Mild anxiety (5–9) | 136 (17.1) | 10 (11.9) | 47 (42.0) | 26 (8.2) | 41 (73.2) | 12 (5.2) |
| Moderate+ anxiety (≥ 10) | 49 (6.1) | 0 (0.0) | 31 (27.7) | 3 (0.9) | 15 (26.8) | 0 (0.0) |
| PEG | ||||||
| None (0) | 340 (42.7) | 33 (39.3) | 36 (32.1) | 110 (34.8) | 19 (33.9) | 142 (62.0) |
| Mild (1–3.9) | 318 (39.9) | 4 (4.8) | 40 (35.7) | 178 (56.3) | 32 (57.1) | 64 (27.9) |
| Moderate (4–6.9) | 117 (14.7) | 30 (35.7) | 33 (29.5) | 28 (8.9) | 5 (8.9) | 21 (9.2) |
| Severe (7–10) | 22 (2.8) | 17 (20.2) | 3 (2.7) | 0 (0.0) | 0 (0.0) | 2 (0.9) |
All percentages within the column. HFRDIS = Hot Flash Related Daily Interference, ISI = Insomnia Severity Index, PSQI = Pittsburgh Sleep Quality Index, PHQ = Patient Health Questionnaire-Depression, GAD = Generalized Anxiety Disorder Scale, PEG = Pain Intensity (P), Interference with Enjoyment of Life (E), and Interference with Daily Activity (G)
Sleep symptoms were assessed using two standardized sleep measures: the Insomnia Severity Index (ISI) and the Pittsburgh Sleep Quality Inventory (PSQI). The ISI is used to assess insomnia symptoms over the past two weeks with seven items that assess difficulty falling asleep, difficulty staying asleep, problems with early awakening, satisfaction with current sleep pattern, interference of sleep problem with daily functioning, noticeability of impairment attributed to the sleep problem and degree of distress caused by the sleep problem. Women rated each item from 0 to 4 with higher scores suggesting more severe symptoms. Scores of 0–7 indicate absence of insomnia, 8–14 mild insomnia, 15–21 clinical insomnia of moderate severity and 22–28 severe clinical insomnia.15,16
The PSQI is used to assess subjective sleep quality and sleep disturbances over the past month. Women assess their sleep quality, latency, duration, and efficiency, sleep disturbances, use of sleeping medication, and daytime dysfunction. Higher scores indicate poorer sleep quality and cutoff values of 5 and 8 have been used to indicate poor sleep quality.17–19 We used established scores of <5 to indicate low level severity, 5–<8 to indicate moderate severity, and >8 to indicate the most severe sleep disturbances.
Depressive symptoms were assessed using the Patient Health Questionnaire (PHQ) 8 item depression scale 20 and anxiety symptoms with the Generalized Anxiety Disorder −7. 21 We used published criteria of scores from 0–4 to indicate no depression, 5–9 to indicate mild depression, and >10 to indicate moderate to severe depressive symptoms. In addition we used published criteria of 0–4 to indicate no anxiety, 5–9 to indicate mild anxiety, and >10 to indicate moderate or more severe anxiety symptoms for the GAD-7.
Finally, pain was assessed using the PEG (Pain Intensity (P), Interference with Enjoyment of Life (E), and Interference with Daily Activity (G)) scale from the Brief Pain Inventory.22,23 The pain (PEG) scores were classified such that 0 indicated no pain, 1–3.9 indicated mild, 4–6.9 moderate, and 7–10 higher severity pain.
Analysis
All women randomized to interventions and controls from MsFLASH studies 1, 2 and 3 (N=899) were eligible to be included in the study; the analytic cohort was comprised of 797 women with complete baseline data. Demographic characteristics were summarized using descriptive statistics. To identify symptom clusters we used latent class analysis to group participants into unobserved (latent) classes based on observed (manifest) symptom scores, with cut points based on established standards. Members of the same class are likely to have similar symptom characteristics. We included age, education, ethnicity, MsFLASH trial number, menopause status, and whether the participant had an oophorectomy or hysterectomy as covariates in a latent class regression model.
Two methods are commonly used to estimate latent class regression models that include covariates to predict an individual’s class membership. We used a “one step” method, in which the covariate associations and class membership are estimated simultaneously in the model. In the alternative “three-step” method, a simple latent class model is estimated without covariates. The results of this model are used to categorize participants according to estimated class membership. Finally, this estimated class membership is treated as a dependent variable in a multinomial logistic model that includes the covariates of interest. The three-step method has been shown to result in biased estimation of the association of covariates with class membership. In contrast to the three-step method, an individual’s initial probability of class membership varies based on the covariates in the one step method.25
We assessed 5 potential models with 1 through 5 classes. We used both the Akaike Information Criterion (AIC) and the Bayesian Information Criterion (BIC) to compare and select a range of models for consideration. The BIC imposes a higher penalty for model complexity than the AIC. Thus, when the criteria disagree, a model selected on the basis of the BIC will tend to be overly conservative, whereas one selected on the basis of the AIC tend to be overly complex. In the context of latent class analysis, where differing complexity means a different number of latent classes, the AIC and BIC can be thought of as setting upper and lower bounds on a range of acceptable latent class models. We also evaluated model fit using a measure of relative entropy. The significance of each covariate in the selected model was assessed using a likelihood ratio test comparing the model with the covariate to a model without. Analysis was conducted using R 3.0 and the poLCA package. 24, 25
Results
Participants were midlife women (mean age 54.5, SD=3.8) who were well educated, with 55% having completed college and only 11% having a high school diploma or less formal education. Women reported their race/ethnicity as Black (n=249), white (510) and another racial/ethnic group (38), including American Indian (13), Asian American (20), Hispanic (21). The majority (82%) indicated they were in the early postmenopause and 28% were in the early or late menopausal transition, based on the STRAW+10 definitions (26). Eighteen percent reported having had a hysterectomy and 10% an oophorectomy.
Overall, nearly 50% of women reported moderate or severe HFRDIS scores, but approximately 50% scored below 38 on the HFRDIS (maximal score of 100), suggesting that their hot flashes interfered mildly with their daily lives As measured by ISI scores, nearly 29% had no clinically significant insomnia, but 30% had moderate to severe clinical insomnia. PSQI scores were 8 or higher for 50% of women, indicating they reported the poorest sleep quality. Nearly 23% of women had mild depressive mood symptoms and 7% moderate to high levels as measured by the PHQ. The GAD scores indicated mild anxiety symptoms for 17% of women and moderate or more severe anxiety for 6%. Most women had PEG scores indicating no to mild pain severity (40%), but 15% reported moderate and 3% reported severe pain.
Based on the BIC, the 3 class solution was optimal (BIC=8776 for the three cluster solution), and based on the AIC, the 5 class solution was optimal (AIC=8341 for the five cluster solution). All models had a relative entropy greater than .99, meaning that all had a low level of uncertainty when classifying individuals into particular classes. Since every model had a high relative entropy, this measure was not useful in differentiating between models. The 3 class solution yielded 3 clusters that differed only in severity of the symptoms, e.g. low, medium, and high symptom severity clusters, whereas the 4 and 5 class solutions differed based on type and seerity of symptoms. The 5 class solution offered greater differentiation of the classes based on symptom type than the 4 class solution, yielding clinically relevant profiles.
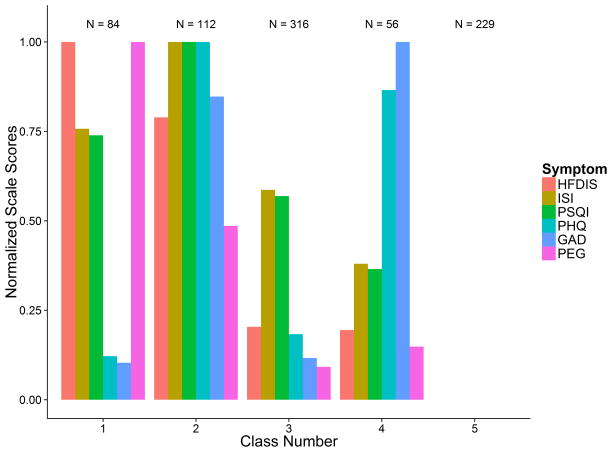
The posterior class membership probabilities were applied to assign each woman to the appropriate symptom cluster: class 1 included 84 women (10.5%), class 2 included 112 women (14.1%), class 3 included 316 women (39.6%), class 4 included 56 women (7.0%), and class 5 included 229 women (28.7%). Figure 1 shows the mean symptom interference or severity per class on a normalized scale from 0 to 1. Because women in class 5 reported the lowest severity levels for all symptoms, the symptom means appear as 0 in this figure.
Figure 1.
Plot of Normalized Mean Symptom Level by Cluster: 5 Cluster Solution.
HFDIS = Hot Flash Daily Interference Scale; ISI=Insomnia Severity Index; PSQI = Pittsburgh Sleep Quality Inventory; PHQ = Patient Health Questionnaire depression scale; GAD=Generalized Anxiety Disorder Scale.
Based on the sample class assignments, we investigated the symptom distributions within each class (Table 1). Women in classes 1 and 2 had high hot flash interference levels relative to the others. Class 1, termed hot flashes/sleep/pain, (10.5% of the total sample) included severe hot flash interference (39%), severe sleep symptoms ((69% with scores >8 on the PSQI; 51% with moderate or severe clinical insomnia on the ISI,), and 46% with moderate to severe pain scores on PEG). Class 2, termed hot flashes/sleep/mood (14.1% of the total sample), included severe hot flash interference (21%), severe sleep symptoms (96% with scores >8 on PSQI, 92% with moderate to severe clinical insomnia on ISI), 38% with moderate depressed mood on the PHQ, 28% with moderate levels of anxiety on the GAD, and 32% with moderate to severe pain on the PEG scale. Class 3, termed poor sleep (39.6% of the sample), had only 3% with severe hot flash interference, but 60% reported sleep scores >8 on PSQI and 29% indicated moderate to severe clinical insomnia on the ISI; mood and pain symptoms were none to mild. Class 4, termed poor sleep/mood (7.0% of the sample), had no participants with severe hot flash interference, but 36% had scores >8 on PSQI, 27% had moderate to severe depression scores, and 27% had moderate to severe anxiety scores. Class 5, termed low severity (28.7% of the sample), had 22% with moderate hot flash interference. The majority of women in this cluster rated all other symptoms as none or mild.
Age, education, race/ethnicity, and trial were statistically significant in determining cluster assignment. College graduates accounted for the majority of clusters 3 and 5 (low severity symptoms). The majority of those with no more than a high school education or equivalent were over-represented in cluster 1 (32.6%) with the most severe hot flash interference, insomnia symptoms, and pain symptoms. Black women were over-represented in cluster 1, those with the most severe hot flash interference, clinical insomnia, and pain symptoms. Although age was significantly associated with cluster assignment, there were no apparent clinically important differences across the clusters. There were no significant differences among the clusters with respect to menopausal status, oophorectomy or hysterectomy. There were differences across the trials in the proportion of women in each cluster, with women in cluster 1 being most likely to be in trial 1 or 3, those in clusters 2, 3 and 4 being most likely to be in trial 2, and those in cluster 5 being most likely to be in trial 3.
Discussion
Latent class analysis of data from 797 women with frequent hot flashes participating in the MsFLASH trials at baseline revealed five clusters of symptoms. Class 1 (10.5%) included women with highest hot flash interference, moderately high sleep, and severe pain symptoms; class 2 (14.1%) high hot flash interference paired with high severity sleep, depressed mood, and anxiety symptoms, but mild to moderate pain symptoms; class 3 (39.6%) low to moderate hot flash interference, moderate severity sleep symptoms and low severity levels of depressed mood, anxiety and pain symptoms; class 4 (7.0%) high severity depressed mood and anxiety symptoms, but lower levels of hot flash interference, sleep, and pain symptoms; and class 5 (28.7%) lowest severity of each type of symptom.
These data indicate that individual symptoms did cluster empirically among participants who were screened for bothersome hot flashes as a clinical trial inclusion criterion. Although each MSFlash trial required a minimum number of hot flashes for inclusion, there was considerable range in the number and the reported severity (HFRDIS scores) of hot flashes at baseline and the clusters differentiated between higher and lower severity of the hot flashes.
Data from the MsFLASH trial participants, in contrast to the community-based sample1, supported identification of 5 different profiles of symptoms. As anticipated, the lowest severity of hot flashes (and other symptoms) was seen among only 29% of the sample (class 5), a substantially smaller proportion than identified in a community based sample studied by Cray and colleagues.1 Indeed, the community based sample included a large proportion of women with low severity symptoms (>70%). Moreover, variability of symptom severity levels among the five classes was evident, indicating that the classes were composed of differing types of symptoms and not simply differing severity levels. These clusters may represent groups of women who may respond to treatments differently, thus further study is needed of whether they represent potentially useful profiles of symptoms for clinicians to consider when prescribing therapies.
Of interest is that cluster 3 had the largest membership, accounting for 39.5% of the total sample, suggesting that moderately severe levels of sleep symptoms accounted for an important proportion of women experiencing symptoms. Given that there were moderately severe sleep symptoms reported in clusters 1 and 3 and high severity sleep symptoms reported in cluster 2, sleep symptoms warrant further attention in this population of women. Nearly 65% of women in the MsFLASH trials reported sleep symptoms severe enough to be included in these three clusters. Moreover, there is evidence of association of poor quality sleep, including night-time awakening, with hot flashes, pain, and negative mood.28–30
Although the primary symptom used as an inclusion criterion in the MsFLASH trials was bothersome hot flashes, only two clusters included women reporting the highest levels of hot flash interference: clusters 1 and 2. Despite inclusion of women meeting the criteria for bothersome symptoms in the MsFLASH trials, some women had considerably more severe symptoms that interfered with their lives than did others. The co-occurrence of hot flash, sleep, and pain symptoms in cluster 1 and hot flashes, sleep, and mood symptoms in cluster 2 resembles a cluster in which high hot flash severity was associated with moderate levels of mood and sleep symptoms seen in the community-based Seattle Midlife Women’s Health Study population.1 Association of sleep and hot flash symptoms has been noted by Joffe and colleagues,28 in the Seattle Midlife Women’s Health Study,29 and in the Study of Women Across the Nation (SWAN). 30
Mood symptoms (depressed mood, anxiety) were noteworthy in clusters 2 and 4, and in cluster 2 were paired with high severity sleep symptoms and hot flash interference. In cluster 4, high severity depressed mood and anxiety were not accompanied by high severity of other symptoms, including hot flashes, sleep, or pain. The association of hot flashes and anxiety, as seen in cluster 2, also has been reported by Freeman and colleagues’ from the Penn Ovarian Aging Study.31 In addition, the association of depressed mood with sleep symptoms has been reported in the SWAN cohort.32 These findings suggest that mood symptoms may be bothersome for women during the menopausal transition or early postmenopause, regardless of their experience of hot flashes and sleep disruption, and are worthy of clinical attention.
Of note, the MsFLASH trial participants differ importantly from community samples because recruitment was restricted to women who had hot flashes that were sufficiently bothersome to prompt them to seek enrollment in a treatment trial. Also participants were selected because they did not have current or recent clinical depression or other specific health problems and they were not using hormones or other selected medications that potentially would confound the results of the trials.
The co-occurrence of pain symptoms with high levels of hot flash interference and moderately severe sleep symptoms reported by MsFLASH participants also has been reported in the Seattle Midlife Women’s Health Study population. Women with the most severe pain symptoms also reported more severe hot flashes, night-time awakening, depressed mood, anxiety, and cognitive symptoms.33
Further analyses are needed to determine if the identified clusters could serve as phenotypes for differentiating the type of and/or effects of interventions needed for these subgroups of midlife women. Given that the goal of the MsFLASH clinical trials was reducing symptom severity, in particular hot flashes, the use of the clusters as potential outcomes may have utility in tracking multiple co-occurring symptoms before and after treatment. Analyses might focus on whether there is stability in the clusters before, during and after treatment, and whether an intervention directed at hot flashes as a primary outcome produces a shift in cluster membership in which women exposed to the experimental treatment shift from a cluster with more severe symptoms to one in which severity levels of several symptoms are lower post-treatment. Comparing the impact of an intervention on cluster membership should be accompanied by examination of effects on individual symptoms to determine the extent to which outcomes may be dependent on only one or a few symptoms.
In addition, evidence that education was associated with class membership iprompts consideration of the potential effectiveness of an educational intervention directed to self-management of symptoms. This finding is consistent with other studies that reveal the benefit of formal education on women’s health in general and the experience of symptoms (1, 29–31, 35–39). An educational intervention might be tested in conjunction with other behavioral interventions in future trials.
Additional research is needed to determine whether the clusters could serve as phenotypes in clinical studies. For example, it would be useful to investigate the association of biomarkers, including endocrine levels such as estradiol and FSH, or genes polymorphisms with the cluster membership. Woods and colleagues found that levels of urinary estrone, FSH, epinephrine, and norepinephrine differentiated the three symptoms clusters identified in their earlier analyses.1,2 Women in a cluster including severe hot flashes had higher overnight norepinephrine and FSH levels and lower estrone and epinephrine levels than those with low severity symptoms.2 In addition women’s progression through the menopausal transition stages to the late menopausal transition and early postmenopause resulted in a greater likelihood of a symptom cluster with high severity hot flashes.1 These analyses could be replicated with the MsFLASH trial participants. Finally, gene polymorphisms related to estrogen synthesis, metabolism, and receptor function could be explored in relation to these clusters. Such analyses could help identify potential genetic markers for symptom experiences, including not only the type of symptom but the level of distress associated with their experience.
It will be interesting in future research to evaluate whether other characteristics such as somatosensory awareness are associated with these symptom clusters. Somatosensory or symptom awareness has been recognized as an important factor in midlife women’s ratings of the severity or bother associated with their symptoms.33,34 Given that a major distinction among the clusters identified in these analyses is the perceived awareness of the severity of different symptoms, further analyses with behavioral correlates of symptom experience, including somatosensory awareness, may help explain individual differences associated with symptom cluster membership.35,36 I
Limitations to the generalizability of this analysis included the exclusion from trials 1 and 3 of women with major depressive or anxiety disorders as well as women using antidepressants for hot flashes, mood, or pain symptoms. Therefore, the clusters identified here likely under-represent women with midlife mood disorders and/or pain. In addition, while women were required to experience hot flashes, minimum frequencies to be included in the studies varied between the trials. Thus, all women reported a moderate level of hot flashes at baseline, precluding identification of a cluster in which women had no or only infrequent hot flashes and more severe symptoms of other types as seen in the community-based Seattle Midlife Women’s Health Study sample.1 It is possible that the symptom clusters we identified might vary if more frequent and severe hot flashes had been required for inclusion in the trials. Finally, cognitive symptoms, such as changes in memory and concentration, were part of the symptom clusters documented in Cray and colleagues’ community-based samples1 but these symptoms were not assessed in the MsFLASH studies.
Future studies should also attend to the potential role of sleep disruption, including sleep apnea, in symptom clusters that include sleep, mood, and pain symptoms as well as hot flashes. Evidence linking disrupted sleep to mood, pain, and cognitive outcomes38,39, 40 warrants further investigation of these relationships in women experiencing clusters such as these during themenopausal transition and early postmenopause.
Conclusions
In conclusion, clinical trial participants experience symptoms that cluster together into different groups such that those with greater hot flash interference tend to experience more severe sleep, depressed mood, anxiety, and/or pain symptoms. In addition, clusters were characterized by more severe sleep or mood symptoms in the absence of severe hot flashes. Clusters were distinguished not only by the severity but also the type of symptoms. Studying symptom clusters may provide an opportunity to evaluate the impact of clinical trial interventions on multiple co-occurring symptoms in future studies and may also provide useful phenotypes for investigating influence of biomarkers, gene polymorphisms, or other individual characteristics on symptom experiences.
Table 2.
Association of Baseline Participant Characteristics with Symptom Clusters
| Total N (%) 797 (100) |
Symptom cluster number
|
||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | P-value | ||
|
| |||||||
| N (%) 84 (10.5) |
N (%) 112 (14.1) |
N (%) 316 (39.6) |
N (%) 56 (7.0) |
N (%) 229 (28.7) |
|||
| Age | 0.001 | ||||||
| Mean (SD) | 54.5 (3.8) | 53.2 (3.7) | 54.6 (3.9) | 54.5 (3.7) | 54.7 (3.6) | 54.8 (3.9) | |
| Education | <.001 | ||||||
| <= High school diploma/GED | 86 (10.8) | 28 (33.3) | 11 (9.8) | 16 (5.1) | 5 (8.9) | 26 (11.4) | |
| Some school after high school | 272 (34.1) | 41 (48.8) | 46 (41.1) | 97 (30.7) | 26 (46.4) | 62 (27.1) | |
| College graduate | 439 (55.1) | 15 (17.9) | 55 (49.1) | 203 (64.2) | 25 (44.6) | 141 (61.6) | |
| Ethnicity | <.001 | ||||||
| White | 510 (64.0) | 21 (25.0) | 61 (54.5) | 245 (77.5) | 41 (73.2) | 142 (62.0) | |
| Black | 249 (31.2) | 55 (65.5) | 47 (42.0) | 67 (21.2) | 10 (17.9) | 70 (30.6) | |
| Other | 38 (4.8) | 8 (9.5) | 4 (3.6) | 4 (1.3) | 5 (8.9) | 17 (7.4) | |
| MsFLASH trial | 0.02 | ||||||
| 1 | 186 (23.3) | 37 (44.0) | 28 (25.0) | 57 (18.0) | 11 (19.6) | 53 (23.1) | |
| 2 | 320 (40.2) | 9 (10.7) | 50 (44.6) | 151 (47.8) | 32 (57.1) | 78 (34.1) | |
| 3 | 291 (36.5) | 38 (45.2) | 34 (30.4) | 108 (34.2) | 13 (23.2) | 98 (42.8) | |
| Menopause status | 0.8 | ||||||
| Menopausal Transition | 143 (17.9) | 7 (8.3) | 20 (17.9) | 65 (20.6) | 12 (21.4) | 39 (17.0) | |
| Postmenopausal | 654 (82.1) | 77 (91.7) | 92 (82.1) | 251 (79.4) | 44 (78.6) | 190 (83.0) | |
| Oophorectomy | 0.4 | ||||||
| No | 718 (90.1) | 69 (82.1) | 95 (84.8) | 299 (94.6) | 51 (91.1) | 204 (89.1) | |
| Yes | 79 (9.9) | 15 (17.9) | 17 (15.2) | 17 (5.4) | 5 (8.9) | 25 (10.9) | |
| Hysterectomy | 0.5 | ||||||
| No | 653 (81.9) | 56 (66.7) | 88 (78.6) | 277 (87.7) | 50 (89.3) | 182 (79.5) | |
| Yes | 144 (18.1) | 28 (33.3) | 24 (21.4) | 39 (12.3) | 6 (10.7) | 47 (20.5) | |
All percentages within the column. SD = standard deviation. P-values calculated from a likelihood ratio test
Acknowledgments
Funding/Support: This study was funded by the National Institutes of Health as a cooperative agreement issued by the National Institute on Aging (NIA), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Research on Women’s Health (ORWH), and grants U01 AG032656, U01AG032659, U01AG032669, U01AG032682, U01AG032699, U01AG032700 from the NIA. At Indiana University, the project was funded in part with support from the Indiana Clinical and Translational Sciences Institute, grant UL1RR02571 from the NIH, National Center for Research Resources, Clinical and Translational Sciences Award.
Footnotes
Conflict of interest/financial disclosures: Dr. Cohen has been a consultant for Noven pharmaceutical, and has received research support from: Astra-Zeneca Pharmaceuticals; Bristol-Myers Squibb; Cephalon, Inc.; GlaxoSmithKline; Ortho-McNeil Janssen; Pfizer Inc.; and Sunovion Pharmaceuticals, Inc. Dr. Freeman received research support from Forest Laboratories, Inc., Bionovo, Inc., and Xanodyne Pharmaceuticals, Inc. Dr. Joffe has received grant support from Cephalon/Teva, serves as a consultant to Merck, and on an advisory board for Noven and Merck. Dr. Ensrud is a consultant on a Data Monitoring Committee for Merck, Sharp & Dohme. Dr. All other authors have no direct conflicts of interest or financial disclosures relevant to this manuscript.
References
- 1.Cray L, Woods NF, Mitchell ES. Symptom Clusters during the Late Reproductive Stage through the eary postmenopause: Observations from the Seattle Midlife Women’s Health Study. Menopause. 2012;19:964–869. doi: 10.1097/gme.0b013e31824790a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woods NF, Cray L, Herting J, Mitchell ES. Endocrine Biomarkers and Symptom Clusters during the Menopausal Transiton and Early Postmenopause. Menopause: Observations from the Seattle Midlife Women’s Health Study. Menopause. 2014;21:646–652. doi: 10.1097/GME.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman EW, Guthrie KA, Caan B, Sternfeld B, Cohen LS, Joffe H, Carpenter JS, Anderson GL, Larson JC, Ensrud KE, Reed SD, Newton KM, Sherman S, Sammel MD, LaCroix AZ. Efficacy of escitalopram for hot flashes in healthy menopausal women: a randomized controlled trial. JAMA. 2011;305(3):267–274. doi: 10.1001/jama.2010.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ensrud KE, Joffe H, Guthrie KA, Larson JC, Reed SD, Newton KM, Sternfeld B, LaCroix AZ, Landis CA, Woods NF, Freeman EW. Effect of escitalopram on insomnia symptoms and subjective sleep quality in healthy menopausal women with hot flashes: a randomized controlled trial. Menopause. 2012;19(8):848–855. doi: 10.1097/gme.0b013e3182476099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carpenter JS, Guthrie KA, Larson JC, Freeman EW, Joffe H, Reed SD, Ensrud KE, LaCroix AZ. Effect of escitalopram on hot flash interference and day/night hot flashes: a randomized, controlled trial. Fertil Steril. 2012;97(6):1399–1404. doi: 10.1016/j.fertnstert.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sternfeld BS, Guthrie KA, Ensrud KE, LaCroix AZ, Larson JC, Dunn AL, Anderson GL, Sequin RA, Carpenter JS, Newton KM, Reed SD, Freeman EW, Cohen LS, Joffe H, Roberts M, Caan BJ. Efficacy of exercise for menopausal symptoms: a randomized controlled trial. Menopause. 2014;21(4):330–338. doi: 10.1097/GME.0b013e31829e4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newton KM, Reed SD, Guthrie KA, Sherman KJ, Booth-LaForce C, Caan B, Sternfeld B, Carpenter JS, Learman LA, Freeman EW, Cohen LS, Joffe H, Anderson GL, Larson JC, Hunt JR, Ensrud KE, LaCroix AZ. Efficacy of yoga for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21(4):339–346. doi: 10.1097/GME.0b013e31829e4baa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen LS, Joffe H, Guthrie KA, Ensrud KE, Freeman MP, Carpenter JS, Learman LA, Newton KM, Reed SD, Manson JE, Sternfeld B, Caan BJ, Freeman EW, LaCroix AZ, Tinker LF, Booth-LaForce C, Larson JC, Anderson GL. Efficacy of Omega-3 treatment for vasomotor symptoms: a randomized controlled trial. Menopause. 2014;21(4):347–354. doi: 10.1097/GME.0b013e31829e40b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reed SD, Guthrie KA, Newton KM, Anderson GL, Booth-LaForce C, Caan B, Carpenter JS, Cohen LS, Dunn AL, Ensrud KE, Freeman EW, Hunt JR, Joffe H, Larson JC, Learman LA, Rothenberg R, Seguin RA, Sherman KJ, Sternfeld BS, LaCroix AZ. Menopausal quality of life: a RCT of yoga, exercise, and omega-3 supplements. Am J Obstet Gynecol. 2014;210(3):244.e1–244.e11. doi: 10.1016/j.ajog.2013.11.016. Available on 2015/3/1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joffe H, Guthrie KA, LaCroix AZ, Reed SD, Ensrud KE, Manson JE, Newton KM, Freeman EW, Anderson GL, Larson JC, Hunt JR, Shifren J, Rexrode KM, Caan B, Sternfeld B, Carpenter JS, Cohen LS. Randomized controlled trial of low-dose estradiol and the SNRI venlafaxine for vasomotor symptoms. JAMA Int Med. 2014 doi: 10.1001/jamainternmed.2014.1891. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensrud KE, Guthrie KA, Hohensee C, Caan B, Carpenter JS, Freeman EW, LaCroix AZ, Landis CA, Manson J, Newton KM, Otte J, Reed SD, Shifren JL, Sternfeld B, Woods NF, Joffe H. Effects of estradiol and venlafaxine on insomnia symptoms and sleep quality in women with hot flashes. Under revision, Sleep. 2014 doi: 10.5665/sleep.4332. PMC Journal – In Process. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newton KM, Carpenter JS, Guthrie KA, Anderson GL, Caan B, Cohen LS, Ensrud KE, Freeman EW, Joffe H, Sternfeld B, Reed SD, Sherman S, Sammel MD, Kroenke K, Larson JC, LaCroix AZ. Methods for the design of vasomotor symptom trials: the Menopausal Strategies: Finding Lasting Answers to Symptoms and Health network. Menopause. 2014;21(1):45–58. doi: 10.1097/GME.0b013e31829337a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LaCroix AZ, Freeman EW, Larson J, Carpenter JS, Joffe H, Reed SD, Newton KM, Seguin RA, Sternfeld B, Cohen L, Ensrud KE. Effects of escitalopram on menopause-specific quality of life and pain in healthy menopausal women with hot flashes: a randomized controlled trial. Maturitas. 2012;73(4):361–368. doi: 10.1016/j.maturitas.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpenter JS. The Hot Flash Related Daily Interference Scale: a tool for assessing the impact of hot flashes on quality of life following breast cancer. J Pain Symptom Manage. 2001;22:979–989. doi: 10.1016/s0885-3924(01)00353-0. [DOI] [PubMed] [Google Scholar]
- 15.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 16.Morin CM, Vallieres A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: a randomized controlled trial. JAMA. 2009;301:2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–895. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 19.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45:5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Zhong X, Theobald D, Wu J, Tu W, Carpenter JS. Somatic symptoms in patients with cancer experiencing pain or depression: prevalence, disability, and health care use. Arch Intern Med. 2010;170:1686–1694. doi: 10.1001/archinternmed.2010.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spitzer RL, Korenke K, Williams JB, Lowe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166:1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 22.Krebs EE, Bair MJ, Damush TM, Tu W, Wu J, Kroenke K. Comparative responsiveness of pain outcome measures among primary care patients with musculoskeletal pain. Med Care. 2010;48:1007–1014. doi: 10.1097/MLR.0b013e3181eaf835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24:733–738. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolck A, Croon M, Hagenaars J. Estimating Latent structure models with categorical variables: One-step versus three-step estimators. Political Analysis. 2004;12:3–27. [Google Scholar]
- 25.Linzer, Drew A, Lewis, Jeffrey B. poLCA: An R Package for Polytomous Variable Latent Class Analysis. Journal of Statistical Software. 2011;42(10):1–29. http://www.jstatsoft.org/v42/i10/ [Google Scholar]
- 26.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ, et al. STRAW+10 Collaborative Group. (2012) Executive Summary of the Stages of Reproductive Aging Workshop+10: addressing the unfinished agenda of Staging Reproductive Aging. Journal of Clinical Endocrinology and Metabolism. 97:1159–1166. doi: 10.1210/jc.2011-3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2005. http://www.R-project.org. [Google Scholar]
- 28.Joffe H, Crawford S, Economou N, Kim S, Regan S, Hall JE*, White DP* A Gonadotropin-Releasing Hormone Agonist Model Demonstrates that Nocturnal Hot Flashes Interrupt Objective Sleep. Sleep. 2013 Dec 1;36(12):1977–85. doi: 10.5665/sleep.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woods NF, Mitchell ES. Sleep symptoms during the menopausal transition and early menopause: observations from the Seattle Midlife Women’s Health Study. Sleep. 2010;33(4):539–549. doi: 10.1093/sleep/33.4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kravitz H, Zhao X, Bromberger J, et al. Sleep disturbance during the Menopausal Transition in a Multi-ethnic community sample of women. Sleep. 2009;31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 31.Freeman E, SAmmel M, Lin H, Gracia CR, Kapoor S, Ferdousi T. The role of anxiety and hormonal changes in menopausal hot flashes. Menopause. 2005;12:258–266. doi: 10.1097/01.gme.0000142440.49698.b7. [DOI] [PubMed] [Google Scholar]
- 32.Bromberger JT, Schott LL, Kravitz HM, Sowers M, Avis NE, Gold EB, Randolph JF, Jr, Matthews KA. Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN) Arch Gen Psychiatry. 2010;67:598–607. doi: 10.1001/archgenpsychiatry.2010.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell ES, Woods NF. Pain symptoms during the menopausal and early postmenopause: Observations from the Seattle Midlife Women’s Health Study. Climacteric. 2010;13:467–478. doi: 10.3109/13697137.2010.483025. [DOI] [PubMed] [Google Scholar]
- 34.Avis N, Stellato R, Crawford S, et al. Is there a menopausal syndrome? Menopausal status and symptoms across racial/ethcnic groups. Social Sci Med. 2001;52:345–356. doi: 10.1016/s0277-9536(00)00147-7. [DOI] [PubMed] [Google Scholar]
- 35.Gold EB, Colvin A, Avis N, et al. Longitudinal analysis of the association between vasomotor symptoms ad race/ethnicity across the menopausal transition. Study of women’s Health across the Nation. American J Public Health. 2006;96:1226–1235. doi: 10.2105/AJPH.2005.066936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carpenter JC, Igega CM, Otte JL, Burns DS, YM, Wu J. Somatosensory amplification and menopausal symptoms in breast cancer survivors and midlife women. Maturitas. 2013;78:51–55. doi: 10.1016/j.maturitas.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter MS, Chilcot J. Testing a cognitive model of menopausal hot flushes and night sweats. Journal of Psychosomatic Research. 2013;74:307–312. doi: 10.1016/j.jpsychores.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Ensrud K, Stone KL, Blackwell TL, Sawaya GF, Tagliaferri M, Diem SJ, Grady D. Frequency and severity of hot flashes and sleep disturbance in postmenopausal women with hot flashes. Menopause. 2009;16:286–292. doi: 10.1097/gme.0b013e31818c0485. [DOI] [PubMed] [Google Scholar]
- 39.Burleson MH, Todd M, Trevathan WR. Daily vasomotor symptoms, sleep problems, and mood: using daily data to evaluate the domino hypothesis in middle-aged women. Menopause. 2010;17:87–95. doi: 10.1097/gme.0b013e3181b20b2d. [DOI] [PubMed] [Google Scholar]
- 40.Lentz MJ, Landis CA, Rothermel J, Shaver JL. Effects of slow wave sleep disruption on musculoskeleal pain and fatigue in middle aged women. J Rheumatology. 1999;26:1586–1592. [PubMed] [Google Scholar]