Abstract
Individuals with schizophrenia consistently display deficits in a multitude of cognitive domains, but the neurobiological source of these cognitive impairments remains unclear. By analyzing the functional connectivity of resting-state functional magnetic resonance imaging (rs-fcMRI) data in clinical populations like schizophrenia, research groups have begun elucidating abnormalities in the intrinsic communication between specific brain regions, and assessing relationships between these abnormalities and cognitive performance in schizophrenia. Here we review studies that have reported analysis of these brain-behavior relationships. Through this systematic review we found that patients with schizophrenia display abnormalities within and between regions comprising 1) the cortico-cerebellar-striatal-thalamic loop and 2) task-positive and task-negative cortical networks. Importantly, we did not observe unique relationships between specific functional connectivity abnormalities and distinct cognitive domains, suggesting that the observed functional systems may underlie mechanisms that are shared across cognitive abilities, the disturbance of which could contribute to the “generalized” cognitive deficit found in schizophrenia. We also note several areas of methodological change that we believe will strengthen this literature.
Keywords: Schizophrenia, cognition, IQ, executive functioning, generalized cognitive deficit, resting-state fMRI, functional connectivity, functional brain networks, Go/NoGo, reinforcement learning, default mode network, task-positive networks, cognitive dysmetria
1. Introduction
Since the early 20th century, schizophrenia has been diagnosed based on the experience of “positive symptoms”, such as delusions and hallucinations, disorganized symptoms, and “negative symptoms”, including anhedonia and flattened affect. However, in the background of these overt symptoms is a milieu of deficits in cognitive function whose contribution to the disabling nature of the disorder appears substantial (Bowie et al., 2006). Over the past 50 years, a rich literature has developed characterizing these cognitive deficits in schizophrenia, with particular focus on their development, specificity, and neurobiological source. Although many tools have been employed in this effort, resting-state functional connectivity (rs-fcMRI) has emerged as a means of obtaining information about the intrinsic fluctuations in neural activity thought to support cognitive functioning. Through the use of neuroimaging, researchers have begun linking these neural signals with behavioral phenotypes like cognitive ability, allowing for richer characterizations of cognitive deficits in clinical populations like schizophrenia. The goal of this review is to synthesize current knowledge regarding the relationship between rs-fcMRI abnormalities in schizophrenia and deficits in cognitive functioning, in an effort to identify putative neural correlates of impaired cognitive performance in patients with schizophrenia.
Using largely behavioral data, researchers have identified several well-validated characteristics of cognitive deficits in schizophrenia. Cognitive deficits are present before the first onset of psychosis (Brewer et al., 2006) and persist across the entire course of illness (Chua & Murray, 1996). They can also be observed in healthy, first degree relatives of patients with schizophrenia, suggesting that the underlying neurobiological abnormality leading to cognitive impairment has a genetic component (Snitz et al., 2006). Cognitive deficits in schizophrenia patients are also associated with impairments in everyday functioning (Bowie et al., 2006), but are surprisingly unrelated to other symptoms of schizophrenia, in particular psychosis (O’Leary et al., 2000).
Additionally, deficits are present in a wide variety of cognitive domains, making it difficult to establish a clear pattern of specific deficits associated with the disorder. In fact, the global nature of cognitive impairment has been a thorn in the side of many researchers who hoped that specific patterns of deficit would reveal specific neurobiological abnormalities. Instead, patients with schizophrenia consistently display impairments ranging from 0.5 – 1.75 standard deviations below the mean of healthy individuals on neuropsychological tasks that measure a multitude of cognitive domains (Gold, 2004). Both in chronic states of illness and during the first episode of psychosis, cognitive deficits in schizophrenia can be observed in the domains of episodic memory, working memory, executive functioning, language, processing speed, attention, and perception (Mesholam-Gately, 2009; Reichenberg & Harvey, 2007). This consistent pattern of findings has led to the hypothesis of a generalized cognitive deficit in schizophrenia, which suggests that the wide range of observed cognitive impairments may be the result of a common neurobiological mechanism (Dickinson & Harvey, 2009). Measuring the relationship between impairments in specific cognitive domains and abnormalities in rs-fcMRI is one way of testing this generalized deficit hypothesis, as it may reveal patterns of brain-behavior relationships in patients with schizophrenia that either support the notion of specific mechanisms (i.e., differential relationship across cognitive domains) or general mechanisms (common relationships across cognitive domains).
In the current paper we review studies utilizing rs-fcMRI to better understand the neurobiological correlates of cognitive deficits in schizophrenia. Articles were systematically reviewed through an internet search of PubMed and Web of Science databases. Search terms included “schizophrenia, “schizoaffective”, “resting-state fMRI”, “executive function”, “cognition”, “neuropsychology”, and “neuropsychological”, which yielded 142 articles across the two databases. Articles were then individually assessed by reading the title, and when appropriate, the abstract and results section to determine inclusion in this review, yielding 16 studies that met inclusion criteria. Criteria for inclusion were 1) use of resting-state functional magnetic resonance imaging (fMRI) to measure functional connectivity (i.e. rs-fcMRI), 2) inclusion of at least one group of schizophrenia patients and a comparison group of healthy controls, 3) measurement of performance on at least one cognitive task, and 4) reported findings (either significant or null) of the relationship between rs-fcMRI and cognitive performance within the schizophrenia group. We begin with a brief overview of the methods of rs-fcMRI and previous findings of abnormalities in schizophrenia, followed by a review of the 16 studies identified as reporting rs-fcMRI abnormalities with cognitive deficits in schizophrenia. Following that review we provide an examination of how these findings fit within the framework of current theories of cognitive functioning, and argue that these theories address different aspects of the same neurobiological system that is affected in schizophrenia. We end with a discussion of the data and issues presented, with a particular emphasis on how these data may align with the notion of a global cognitive deficit in schizophrenia.
1.1 Resting-state Functional Connectivity
Recently scientists have begun utilizing rs-fcMRI data for understanding the on-going, intrinsic neural activity in the brain. Resting-state fMRI refers to the collection of neuroimaging data while an individual is laying quietly in the scanner (i.e. not performing a specific task), and measures spontaneous low-frequency fluctuations of the BOLD signal (0.01Hz – 0.10Hz). Interestingly, this resting-state activity is thought to consume the majority of energy used by the brain (about 60-80%), which itself expends 20% of the body’s energy to support ongoing neuronal activity. Given that task-induced changes in metabolism are typically <5%, ongoing resting-state activity provides a rich source of disease-related variability that complements changes observed due to task (Fox & Raichle, 2007).
The application of functional connectivity analysis to resting-state data has allowed for the assessment of relationships in spontaneous neural activity between different regions of the brain. The main inference of functional connectivity is that, if two regions have highly correlated BOLD activity (i.e. have high functional connectivity) then they are frequently co-activated, and are therefore more likely to be communicating with one another. Rs-fcMRI research has been critical in revealing intrinsic, stable networks of the human brain, which are comprised of brain regions that appear consistently functionally connected even during rest. Abnormalities in these functional connections in schizophrenia may lead to critical insights into the intrinsic neurobiological abnormalities that may underlie observed cognitive impairments.
Rs-fcMRI is a particularly useful tool for studying clinical populations like schizophrenia. One potential advantage of rs-fcMRI data is that it is not complicated by task performance, which has proved to be a difficult confound in studies comparing healthy individuals and schizophrenia patients (though it does leave open the question of what people are doing at “rest”). Rs-fcMRI data also allows for a broader, potentially more representative recruitment of individuals with schizophrenia, as inclusion in studies of rs-fcMRI does not depend on one’s ability to complete sometimes complex cognitive tasks in an fMRI scanner. Additionally, at least some of the variability in task-based BOLD signal fluctuations is due to the spontaneous, low-frequency fluctuations that make up resting-state data (Fox & Raichle, 2007), suggesting that previously identified associations between task-based BOLD activity and behavior could, in part, be influenced by resting-state activity. Therefore, rs-fcMRI represents a potentially powerful tool for understanding abnormalities in the intrinsic functional organization of the brain in schizophrenia, with variability that can be correlated with measures of cognitive ability.
1.2 Resting-state Functional Connectivity Methods
As with most imaging methods, rs-fcMRI introduces many choice-points for the researcher in terms of how functional connectivity should be calculated, how to identify different brain regions, and how noise should be handled. There is no clear consensus about a “best” method for all resting-state studies, though there are trends in the literature. For most studies, the main goal of rs-fcMRI is to assess relationships between different brain regions, either due to an interest in a specific, a priori region, or to better understand functional networks. The three most common analysis techniques used to address these questions are seed-based connectivity analysis, ROI-based connectivity analysis and independent component analysis (ICA). Each method has strengths and weaknesses, and researchers must decide which analysis will allow them to most accurately test the research question at hand. Previous reviews have outlined these methods in great detail (e.g. Cole et al., 2010; Fox & Raichle, 2007; Smith et al., 2013), therefore we only briefly overview them here.
In the studies reviewed in this paper, seed-based and ROI-based connectivity represent the most common methods employed to test hypotheses regarding functional connectivity abnormalities in schizophrenia and their relationship with cognition. Seed-based methods refer to the a priori selection of a small number of voxels, clusters, or atlas coordinates from which to extract the time series data, which is then used to create functional connectivity maps across the rest of the brain for those “seeds”. ROI-based connectivity refers to the selection of a set a priori ROIs from some type of parcellation scheme (e.g. Power et al., 2011; Tzourio-Mazoyer et al., 2002), with connectivity examined among those ROIs. Regarding the distinction between these methods, seed-based connectivity looks primarily at connections between a starting seed or ROI and all voxels in the brain. For example, one might take a seed in the dorsolateral prefrontal cortex, and examine its connectivity with every other voxel in the brain. ROI to ROI-based connectivity observes connections between averages across groups of voxels that comprise the regions of interest. For example, one might examine connectivity between a region in the dorsolateral prefrontal cortex with a region in the anterior cingulate, the dorsal parietal cortex and the anterior insula. We would argue that seed-based methods are somewhat more exploratory than ROI to ROI-based methods. However, many of the considerations for both seed-based and ROI-based functional connectivity are similar, and therefore are discussed here as “seed/ROI-based”. Likely the biggest advantage of seed/ROI-based connectivity is its flexibility in testing specific predictions of functional connectivity, but there are several limitations as well. Limitations of seed/ROI-based studies include the use of a priori seeds/ROIs that may or may not reliably represent the regions of interest, biases within the data if the seed/ROI is either unreliable or inappropriate for the hypothesis, and the use of a univariate analysis on a multivariate system.
The most common alternative to seed/ROI-based connectivity analysis is ICA, which is a data-driven approach to identifying functional networks of the brain. ICA is an algorithm that decomposes BOLD signals into spatially segregated, maximally statistically independent components. In doing so, ICA addresses several of the problems associated with seed/ROI-based connectivity that were discussed above by not depending on a priori selection of ROIs and utilizing a multivariate analysis. Not surprisingly, ICA is also susceptible to biasing data and therefore introduces several limitations. Most notably, although ICA identifies components of the data, the researcher must determine what each component represents - a process that is complicated by the fact that the researcher must also determine a priori the number of components in the data, introducing a methodological bias. In addition, interpretation of ICA data is not nearly as straight-forward as seed-based results, since ICA provides information on the magnitude of a component, as opposed to the strength of specific functional connections. Therefore, questions regarding individual differences in ICA components encompass multiple factors, such as the magnitude of the fluctuations, temporal coherence, and spatial mapping, making it difficult to interpret the mechanism underlying any observed differences.
Overall, seed/ROI-based connectivity analysis and ICA are both common and relatively well-validated methods for analyzing and understanding resting-state functional connectivity data, both within and between subject groups. The majority of studies reviewed in this paper utilize seed/ROI-based analysis. This introduces challenges in comparing findings and converging upon common patterns in the data because many studies asking questions about the same cognitive domain used different seeds/ROIs to do so, leading some studies to ignore relationships that are the focus of others.
1.3 Previous Resting-state Findings in Schizophrenia
Although no papers to this point have reviewed the relationship between rs-fcMRI abnormalities in schizophrenia and cognitive deficits, many articles have synthesized the overall nature of rs-fcMRI in schizophrenia, describing the complex landscape on which the current review must be understood. Previous reviews have revealed several patterns of abnormality in schizophrenia that appear convergent across multiple studies. One pattern is alterations in the default mode network (DMN), though the nature of these abnormalities are inconsistent, with some studies reporting hyper-connectivity, some reporting hypo-connectivity, and others reporting stronger connectivity between DMN and non-DMN regions (Karbasforoushan & Woodward, 2012). In addition, most of the reviews note that connectivity of the prefrontal cortex is reduced in schizophrenia, particularly for intra-PFC connectivity, with three independent studies reporting reduced connectivity within the PFC in schizophrenia (Woodward et al., 2011; Rotarska-Jagiela et al., 2010; Cole et al., 2011). Lastly, altered connectivity between cortical and subcortical regions in schizophrenia, such as between the thalamus and frontal cortex and between the posterior cingulate cortex and cerebellum, has been frequently found in schizophrenia (Karbasforoushan & Woodward, 2012; Zhou et al., 2010; Zhang & Raichle, 2010). Although these reviews have identified broad categories of abnormalities in schizophrenia, the heterogeneity of specific findings within each is significant, with nearly all reviews noting the inconsistency of findings within the schizophrenia rs-fcMRI literature.
A common hypothesis for why these rs-fcMRI findings are so heterogeneous in schizophrenia is that the disorder itself is phenotypically complex – a fact that is frequently unaddressed in these types of studies. One approach for addressing this heterogeneity is by measuring associations between rs-fcMRI abnormalities and behavioral phenotypes in schizophrenia, such as cognitive ability. Accounting for some of the variance in rs-fcMRI using measures of cognitive performance may allow for a better understanding of the complex differences observed in this clinical population. Therefore, we review the current state of the literature on associations between rs-fcMRI and cognitive ability in schizophrenia, in an effort to understand how abnormalities in rs-fcMRI, using the methodologies outlined above, are related to different domains of cognitive performance.
2. Review of Resting-state Functional Connectivity and Cognitive Deficits in Schizophrenia
In this section we review 16 studies that have correlated rs-fcMRI abnormalities in schizophrenia with performance on cognitive tasks. As discussed above, rs-fcMRI measures the relatively stable, intrinsic co-activation of different brain areas, and these approximated neural signals are thought to underlie cognitive functioning across all domains of cognition. Therefore, any significant associations between irregular rs-fcMRI and cognitive performance in patients with schizophrenia would suggest that a) the functional connectivity between those regions is abnormal in patients with schizophrenia, and b) the magnitude of abnormal rs-fcMRI has functional consequences for the individual’s cognitive ability, and therefore may underlie the cognitive deficits observed in schizophrenia.
This review is organized into sections describing results for commonly used categories of cognitive function, including executive functioning, working memory, processing speed, attention, episodic memory, and verbal knowledge, followed by a summary and integration of findings across domains. A summary of the studies and their findings can be seen in Table 1. Unless otherwise specified, the patients included in these studies were medicated, chronic schizophrenia patients.
Table 1.
| Study | Sample Size (HC/SZ) |
Analysis Method |
Cognitive Tasks | Findings (associations with worse task performance for schizophrenia group) FC = Functional Connectivity |
|---|---|---|---|---|
| Argyelan et al., 2014 |
32/18/19 Bipolar | ROI-Based (266 ROIs) |
MATRICS Battery: Working Memory: WMS-III Processing Speed: Trail Making Test Attention: Attention Network Task Episodic Memory: HVLT |
Working Memory: ↑global disconnectivity*
(r=−0.40) Processing Speed: ↓left caudate FC (r=0.53), ↓left thalamus FC*(r=0.45), ↑paracingulate gyrus FC (r=−0.62), ↑lingual gyrus FC* (r=−0.56), ↑global disconnectivity*(r=−0.36) Attention: ↑global disconnectivity*(r=0.46) Episodic Memory: ↑global disconnectivity* (r=−0.45) |
| Bassett et al., 2012 |
29/29 | ROI-Based (90 ROIs); Network Analysis |
Attention & Concentration: WAIS digit symbol, digit span, symbol search, letter-number sequence, trails numbers-letters test, tower test Memory: CVLT-II, Wechsler Memory Scale |
Attention: ↑network size (interpreted as more variance in whole brain FC)* (r=.50) Episodic Memory: ↓network size (interpreted as more variance in whole brain FC)* (r=0.27) |
| Camchong et al., 2011 |
29/29 (same sample as Bassett) |
ICA-GLM ‘hybrid’ |
Same as Bassett (2012) |
Attention: ↓medial frontal gyrus FC (r=0.45), ↓right dorsal anterior cingulate gyrus FC**, ↓superior frontal gyrus FC** Episodic Memory: ↓medial frontal gyrus FC (r=0.40) |
| Cole et al., 2011 | 22/23 | Seed-Based (PFC/dlPFC) |
Executive Function: WAIS Matrix Reasoning Working Memory: Sternberg Working Memory Task Vocabulary: WAIS Vocabulary |
Executive Function: ↓dlPFC – withinPFC FC (r=0.59), ↑dlPFC – nonPFC FC (r=−0.52) Working Memory: ↑dlPFC – nonPFC FC (r=− 0.50) Vocabulary: ↓dlPFC – withinPFC FC (r=0.45), ↑dlPFC – nonPFC FC (r=−0.49) |
| Collin et al., 2011 |
41/62 | Seed-Based (Cerebellum) |
WAIS IQ | No significant relationships with cognition |
| He et al., 2013 | 72/80 (first- episode) |
Seed-Based (PCC) |
Memory and Attention: Logical Memory Test, Pattern Recognition Memory test, RVIP Processing Speed: Trail Making Test, Digit Symbol Test |
Attention: ↓PCC – left insula connectivity strength (regions were negatively correlated) (r=0.30) |
| Lynall et al., 2010 |
15/12 | ROI-Based (72 ROIs) |
Verbal Fluency Task |
Verbal Fluency: ↓whole brain FC strength (r=0.46), ↓small worldness (r=0.43), ↓clustering (r=0.48), ↓hub-dominated networks (r=−0.50) |
| Moran et al., 2013 |
23/40 | Seed-Based (insula) |
RVIP |
Attention: ↓anterior insula – DMN FC*
(r=0.46) |
| Mwansisya et al., 2013 |
33/41 (first episode) |
ROI-Based (90 ROIs) |
WAIS Information Processing Speed: WAIS Digit- Symbol Coding |
Processing Speed: ↓right – left medial frontal gyrus FC (rho=0.26), ↓right – left pallidum FC (rho=0.34) |
| Repovs et al., 2011 |
15/25 |
A priori
networks from meta-analysis (DMN, FPN, CON, and CER) |
Executive Function: Trails, Verbal Fluency, Matrix Reasoning, WCST Working Memory (letter-number sequencing, digit span, spatial span, 2-back, CPT) Episodic Memory: Wechsler Memory Tests, CVLT; WAIS Vocabulary |
Executive Function: ↓FPN – CER FC (r=0.49) Working Memory: ↓FPN – CER FC (r=0.52) Episodic Memory: ↓FPN – CER FC* (β=0.25) Vocabulary: ↓FPN – CER FC* (β =0.28) |
| Su et al., 2013 | 25/25 | Seed-Based (dlPFC) |
WCST |
Executive Function: ↓left dlPFC – right caudate FC (r=−0.46), ↓left dlPFC – left caudate FC (r=−0.61) |
| Tu et al., 2012 | 30/30 | Seed-Based (dACC) |
N-Back | No significant relationships with cognition in schizophrenia group |
| Tu et al., 2013 | 36/36 | Seed-Based (FPN) |
Color Trails Test, N-Back |
Executive Function: ↓right middle cingulate cortex – right thalamus FC (r=−.55), ↓right middle cingulate cortex – left thalamus FC (r=−0.56) |
| Unschuld et al., 2014 |
63/102/70 schizophrenia relatives |
Seed-Based (dlPFC seed → FPN; mPFC seed → DMN; dACC seed → CON) |
Attention & Working Memory Composite: BTA, HVLT, BVMT |
Composite Working Memory & Attention:
↑within-FPN connectivity* (r=−0.36), ↑within-DMN connectivity* (r=−0.24) |
| Wang et al., 2014 |
20/21 (“minimally treated”) |
Voxel-Based (all voxels) |
MATRICS Battery: Executive Function: Neuropsychological Assessment Battery – mazes subtest Processing Speed: BACS symbol coding Episodic Memory: HVLT |
Executive Function: ↓lateral PFC connectivity strength for “long distance” connections (r=−0.59) Processing Speed: ↑ right lateral PFC FC (r=− 0.72), ↑long range FC for precuneus (r=− 0.61), Episodic Memory: ↓left lateral temporal cortex FC (r=0.74), ↓long range connections with the visual cortex (r=0.57) |
| Yan et al., 2012 | 30/30 | Seed-Based (ACC) |
Stroop Task |
Executive Function: ↑ACC – PCC connectivity strength (regions are negatively correlated) (r=−0.43) |
only significant when groups combined;
no stats reported, relationship reported by the study authors as ‘varying together’
ACC = anterior cingulate cortex; BACS = Brief Assessment of Cognition in Schizophrenia; BTA = Brief Test of Attention; BVMT-R = Brief Visuospatial Memory Test-Revised; CER = cerebellar network; CON = cingulo-opercular network; CVLT = California Verbal Learning Task; dlPFC = dorsolateral prefrontal cortex; DMN = default mode network; FPN = fronto-parietal network; HVLT = Hamilton Verbal Learning Task; mPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; PFC = prefrontal cortex; RVIP = Rapid Visual Information Processing; WCST = Wisconsin Card Sorting Task; WAIS = Wechsler Adult Memory Scale; WMS-III = Wechsler Memory Scale – 3rd Edition.
2.1 Executive Functioning
Executive functioning is a broad cognitive construct that encompasses the ability to flexibly use context and rules in order to guide behavior towards a goal (Banich et al., 2009). Much of the literature in healthy individuals points to the frontal lobe as critical for executive functioning, as evidenced in part by the co-development of the frontal lobe and executive abilities throughout childhood and adolescence (Stuss et al., 1992). In line with this previous literature, rs-fcMRI data reveals converging evidence that the frontal lobe is a correlate of executive functioning deficits in schizophrenia; however, it also reveals important associations with sub-cortical regions of the brain, that are less frequently discussed when considering executive functioning ability.
Six studies looked for associations between functional connectivity abnormalities and executive functioning deficits in schizophrenia. Overall, these studies suggest that executive functioning impairments in schizophrenia are related to rs-fcMRI, a) within the PFC, b) between the PFC and other cortical regions, and c) between frontal cortex and sub-cortical regions. Studies from Cole et al. (2011) and Wang et al. (2014) revealed abnormal connectivity between the PFC and the rest of the brain, with Cole et al. showing that stronger connectivity between the dlPFC and non-PFC regions (r=−0.52), and weaker connectivity between the dlPFC and other-PFC voxels (r=0.59), were associated with poorer executive functioning in schizophrenia. Wang et al. found that, in minimally treated chronic patients (<3 months lifetime medication use), weaker long range connections of the lateral PFC in schizophrenia were related to impaired executive functioning (r=−0.59). Additionally, Su et al. (2013), Tu et al. (2013) and Repovs et al. (2011) provided evidence that weaker connections between the frontal regions (dlPFC, dACC, and the fronto-parietal network) and subcortical regions (thalamus (right: r=− 0.55; left: r=−0.56), cerebellum (r=0.49) and caudate (right: r=−0.46; left: r=−0.61)) were associated with executive functioning deficits in schizophrenia.
In interpreting these studies, the limitations of rs-fcMRI methods discussed earlier should be considered. Because executive functioning has often been associated with frontal cortex function, and in particular the dlPFC, both Su et al. and Tu et al. used the dlPFC as an a priori ROI to create correlation maps. However Su et al. also looked at whole-brain correlation maps between bilateral dlPFC and all other voxels in the brain, though Tu et al. looked only at connections within the fronto-parietal network. Therefore, these papers’ methods biased the potential findings towards connections with the dlPFC. Cole and colleagues, on the other hand, used a slightly broader method of looking for group differences in the correlation strength between all voxels within the PFC, in order to inform further analyses. Importantly, only the dlPFC and inferior frontal junction (IFJ) showed group differences, and only the dlPFC’s connectivity correlated with executive functioning, providing more data-driven support for the importance of the dlPFC. Finally, Repovs et al. used a priori defined network-assignments to look at connectivity both within and between networks, while still assessing relationships with the fronto-parietal network, which includes the dlPFC as a major hub. Thus, despite differing methods and findings, these papers all point to a role of the dlPFC in supporting executive functioning, with abnormalities in dlPFC rs-fcMRI being associated with executive functioning impairments in schizophrenia, particularly when considering its connections with subcortical or cerebellar regions.
2.2 Working Memory
Working memory deficits have also been consistently observed in schizophrenia and are often attributed, at least in part, to abnormal functioning of the prefrontal cortex (Perlstein et al., 2001; Manoach, 2003). Working memory processes encompass the ability to temporarily store and manipulate information “on-line” and given its relationship to the functioning of the dlPFC, is closely linked with executive functioning ability (for review, see Barch & Sheffield, 2014). Due to such reliable impairments in working memory in schizophrenia, five studies assessed its relationship with functional connectivity.
In studies mentioned above, Repovs et al. (2011) and Cole et al. (2011) found associations between working memory ability and prefrontal cortex rs-fcMRI in patients with schizophrenia. Similar to their findings with executive functioning, Repovs et al. reported that weaker between-network connectivity of the fronto-parietal network and cerebellar network was related to poorer working memory ability in the schizophrenia group (r=0.52), while Cole et al. found that working memory deficits were associated with stronger connectivity between the dlPFC and non-PFC regions of the brain (r=−0.50). Adding to these findings, Unschuld and colleagues (2014) found that schizophrenia patients had stronger within-network connectivity for fronto-parietal network and DMN, as compared to first-degree relatives and healthy controls. Across all subjects, working memory ability was negatively correlated with within-network connectivity of the fronto-parietal network (r=−0.36) and the DMN (r=−0.24), indicating that reduced working memory ability was associated with stronger within-network connectivity.
In addition, two studies assessed associations with working memory, but did not find strong relationships with functional connectivity. Tu et al. (2012) found that weaker connectivity between the right putamen and the dACC, as well as the right putamen and the inferior parietal lobe, was associated with poorer working memory ability in the healthy control group (r=−0.39 & r=−0.54, respectively), but not in schizophrenia (r=0.12 & r=0.34, respectively). Argyelan et al. (2014) quantified “global disconnectivity” as the first principle component from a principle components analysis that included connectivity strength from 266 a priori ROIs. Greater global disconnectivity was associated with reduced working memory ability (r=−0.40), but this was only true when the bipolar and schizophrenia groups were combined.
These data do not provide a consistent set of findings in regards to rs-fcMRI correlates of working memory in schizophrenia. The most consistent findings come from the Repovs et al. and Unschuld et al. studies, both of which found associations between working memory performance and connectivity of the fronto-parietal network. Repovs and colleagues showed that reduced fronto-parietal network-cerebellar connectivity was associated with poorer working memory performance, while Unschuld et al. showed that increased within-fronto-parietal network connectivity was associated with poorer working memory performance. Unfortunately, Repovs et al. looked for but did not find significantly reduced within-network fronto-parietal network connectivity in schizophrenia, and therefore did test whether within-fronto-parietal network connectivity was associated with working memory. Though the findings across these three studies were not identical, they all found significant associations between working memory ability and rs-fcMRI of the prefrontal cortex in schizophrenia. Therefore, this literature again suggests an important role of prefrontal cortex rs-fcMRI abnormalities in working memory deficits in schizophrenia, although much more work is needed to further understand and replicate this relationship.
2.3 Processing Speed
In meta-analyses, processing speed is often identified as one of the most impaired cognitive domains in schizophrenia, leading many to believe that it represents a core feature of cognitive deficits in the disorder (Dickinson et al., 2007; Schatz, 1998). Despite this, the functional connectivity findings associated with processing speed impairments in schizophrenia do not point to a clear pattern of abnormality.
Of the three studies that measured processing speed, two of them reported relationships to connectivity of regions within the default mode network. Mwansisya and colleagues (2013) found that reduced processing speed was associated with weaker inter-hemispheric functional connectivity between left/right medial frontal gyrus (rho=0.26) and left/right pallidum (rho=0.34) in a group of first episode patients (<18 months since diagnosis, <12 weeks medication treatment), while Wang et al. (2014) showed that overall functional connectivity of the right lateral prefrontal cortex (r=−0.72) and long-range connections of the bilateral precuneus (r=− 0.61) were negatively associated with processing speed in minimally treated patients with schizophrenia. The third study, from Argyelan et al. (2014) found that impaired processing speed in the schizophrenia group was associated with lower average functional connectivity of the left caudate nucleus (r=0.53) and stronger average connectivity of the paracingulate gyrus (r=−0.62); however only the average connectivity strength of the left caudate nucleus significantly differed between schizophrenia and controls. Perhaps due to increased power and/or variance, this study revealed more associations between processing speed and rs-fcMRI when their schizophrenia and bipolar groups were combined. They found that poorer processing speed was associated with greater global disconnectivity(r=−0.36), reduced average connectivity of the left caudate nucleus (r=0.48) and left thalamus (r=0.45), and increased average connectivity of the lingual gyrus (r=−0.48). Interestingly, their observed relationships between processing speed deficits and reduced connectivity of the caudate nucleus are consistent with findings in executive functioning (Su et al., 2013).
Although two studies revealed that processing speed deficits were related to the connectivity of regions within the DMN (i.e. precuneus and medial frontal gyrus), the nature of these associations were inconsistent, with one study reporting a negative correlation and the other a positive correlation between connectivity strength and processing speed. Additionally, the methods used across these papers were highly variable. For example, Wang et al. thresholded their rs-fcMRI correlations at r=.20 for both schizophrenia patients and controls, meaning that they ignored negative correlations but also may have eliminated a larger proportion of connections in the schizophrenia group, since schizophrenia patients often have lower rs-fcMRI correlations on average than controls (Fornito et al., 2011). Additionally, the characteristics of the schizophrenia cohorts differed between studies. One study included “minimally treated” chronic schizophrenia patients who had over six years of illness duration but less than three months of exposure to antipsychotics (Wang et al., 2014), while another assessed first episode patients who had been diagnosed with schizophrenia, schizoaffective disorder, or schizophreniform disorder within the past 18 months (Mwansisya et al., 2013). It is difficult to know how these methodological differences may have affected the associations between processing speed and functional connectivity, and together these findings do not point to a consistent picture of connectivity abnormalities that may underlie processing speed deficits in schizophrenia.
2.4 Attention
Attention is a cognitive construct that refers to the ability to filter information both externally and internally, and describes the allocation of resources to either goal-relevant or stimulus-driven objects. Through a dynamic system, the process of attention is thought to help select stimuli for use by other cognitive domains, such as what will be stored in working memory or what will be selected in a cognitive control task (Bundesen, 1990; Desimone & Duncan, 1995). Similar to all other cognitive domains in this review, attention is impaired in patients with schizophrenia. Some researchers have hypothesized that misallocation of attentional resources leads patients to assign salience to otherwise unimportant objects in the environment, and this saliency leads the individual to build explanations about why that object is important, resulting in delusional thought (Braff, 1993). Therefore, rs-fcMRI abnormalities that are associated with attentional deficits may represent important factors in the positive symptoms of schizophrenia, as well as providing insight into neuropsychological impairments. Six reports looked for links between attention and functional connectivity, and results from these papers largely converged on the notion of attention deficits as related to abnormalities in the DMN, in conjunction with global reductions in connectivity.
The study from Bassett and colleagues (2012) and to some degree the findings from Argeylan et al. (2014) suggest that reduced attention performance is related to global reductions in connectivity strength, such that more variability in functional connections (r=0.50) and weakened functional integration (r=0.46) are associated with impaired attention in schizophrenia. In addition, Camchong et al. (2011) observed that schizophrenia patients with lower attention scores had lower functional connectivity in the MFG (r=0.45), right dorsal ACG, and the superior frontal gyrus (SFG); however it should be noted that no effect sizes were given for the relationships with the MFG and ACG, and they were reported only as “varying together”. Unschuld and colleagues calculated composite scores for attention and working memory and, as reported earlier, this composite was associated with DMN connectivity (r=−0.24), as well as fronto-parietal network connectivity (r=−0.36), such that impaired attention and working memory ability was related to stronger within-network connectivity. This was only true when all subjects were included, and again the composite score included a sustained attention task as well as working memory tasks, making these findings more difficult to interpret.
Based on the other studies reviewed, it is possible that these seemingly global abnormalities related to attention in schizophrenia are, at least in part driven by abnormal connections within and between the DMN. In particular, based on consistent findings from Moran et al. (2013) and He et al. (2013), it appears that reduced connectivity between the insula and DMN may be critical to attention deficits, possibly due to the insula’s hypothesized role as a hub modulating activity between the DMN and task-positive networks, which are otherwise functionally segregated (Fox et al., 2005b). Although these findings are promisingly convergent, it should be noted that Camchong et al., Bassett et al. and Unschuld et al. used composite scores to measure the construct of attention that included a wide variety of tasks that are often thought to reflect ability in the domains of working memory, executive function, and processing speed. Although the authors discuss these tasks as measuring an ‘attention’ construct, this composite score may more likely reflect overall cognitive functioning, as opposed to something attention-specific.
2.5 Episodic Memory
Episodic memory is a memory system that supports the ability to retrieve or remember information from the past (Tulving, 2002). Patients with schizophrenia display deficits in episodic memory, which can result in a multitude of functional consequences, such as forgetting to take medication or go to appointments, and having trouble remembering therapeutic skills that have been learned. Episodic memory encompasses many aspects of memory, ranging from autobiographical memories to free recall of a recently read list of words. Perhaps because of this heterogeneity, a diffuse constellation of neurobiological abnormalities have been associated with episodic memory deficits, including in the hippocampus, the DMN and the different regions of the prefrontal cortex (McIntosh et al., 1997; Sesteiri et al., 2011). The results from the current review are equally complex, pointing to associations between episodic memory ability and default mode regions, as well as more global, diffuse connectivity abnormalities in schizophrenia.
Using the Wechsler Memory Scale, Camchong et al. (2011) found that poorer episodic memory in schizophrenia was associated with reduced rs-fcMRI between the medial frontal gyrus (a part of the DMN) and the whole brain(r=0.40), providing some evidence that episodic memory is associated with DMN abnormalities. In the same group of subjects, Bassett et al. (2012) showed that episodic memory impairments were related to smaller network size (r=0.27), a metric thought to reflect reduced global integration. However, Bassett’s findings were only significant (p=.04) when both patients and controls were included in the analysis. Repovs et al. (2011) also used the Wechsler Memory Scale and observed a negative relationship between episodic memory ability and connectivity between the fronto-parietal network and the cerebellum (β=0.25). Adding further to this picture, Wang et al. (2014) found that impaired memory in the schizophrenia patients was related to reduced whole brain rs-fcMRI of both the left lateral temporal cortex (r=0.74) and the visual cortex (r=0.57) in minimally treated chronic patients. The lateral temporal cortex has been shown to be activated in healthy individuals during encoding on an episodic memory task (Kirschhoff et al., 2010), providing further evidence that connectivity of this area may be important for episodic memory ability. In a study also looking at verbal learning, Argyelan et al. (2014) found that impaired verbal learning was associated with reduced global rs-fcMRI (r=−0.45), but only when schizophrenia and bipolar groups were combined.
Together, these findings reveal an unclear picture of associations between rs-fcMRI abnormalities and episodic memory deficits in schizophrenia. As noted previously, episodic memory is a cognitive construct that includes many different facets of memory. Therefore, the haziness of these findings may reflect variability in the tasks chosen by researchers as defining “episodic memory ability”. For example, several of the studies used the Wechsler Memory Scale, which measures a wide range of memory abilities, including auditory memory, visual memory, visual working memory, and immediate and delayed recall (Wechsler, 1997), while several other studies used a simple measure of verbal learning to assess episodic memory ability. These methodological differences ultimately led to fairly distinct measurements of episodic memory ability, given their differing levels of complexity and the nature of the memory that was being measured. That said, all researchers included tasks that have been frequently used and are well validated for measuring episodic memory, suggesting that either a clearer delineation of different facets of episodic memory are important for detecting neurobiological correlates, or that this group of findings has not provided the best representation of how functional connectivity abnormalities may influence episodic memory ability.
2.6 Verbal Knowledge
Two cognitive tasks were included in the reviewed studies that did not fit closely into any of the above cognitive domains, and are included under the heading of “verbal knowledge”. These tasks are the Vocabulary module from the WAIS, which requires the subject to define a series of words, and a verbal fluency task, which requires the subject to produce as many words starting with F, A, and S as they can think of within one minute. Given that only two studies reported significant findings using these tasks, it is difficult to make any conclusive statements about relationships with functional connectivity. Looking at Vocabulary, Cole et al. (2011) reported that better scores were significantly positively associated with strength of the functional connectivity between the dlPFC and other PFC voxels (r=0.45), whereas scores were negatively associated with the connectivity between the dlPFC and non-PFC voxels within the schizophrenia group (r=−0.49). The Vocabulary module is thought to reflect verbal IQ, which is often considered akin to crystallized intelligence or pre-morbid IQ (Kaufman et al., 1989). Therefore, associations with the dlPFC may be more reflective of a generalized cognitive deficit that is thought to develop early in the course of schizophrenia.
Regarding verbal fluency, Lynall et al. (2010) used graph analysis to measure network metrics of small-worldness, clustering, and hubs for the whole brain. They found that impaired verbal fluency ability was significantly associated with reduced whole brain rs-fcMRI strength (r=0.46), as well as reduced small worldness (r=0.43), reduced clustering (r=0.48), and less hub-dominated networks in schizophrenia (r=−0.50), all of which were significantly reduced in schizophrenia compared to controls. Findings from this paper suggest that verbal fluency impairments may be associated with less strongly connected and less globally integrated whole brain networks.
2.7 Summary
Taken together, these findings present a complex picture of the relationships between cognitive deficits in schizophrenia and rs-fcMRI, but they do converge on several important neurobiological correlates of cognition. Across several studies, abnormalities in the relationship between cortical and sub-cortical regions, in particular the PFC, thalamus, basal ganglia, and cerebellum, were observed in patients with schizophrenia and correlated primarily with deficits in executive functioning, as well as deficits in processing speed and working memory. These relationships were largely in the direction of reduced rs-fcMRI between cortical and sub-cortical regions associated with impaired cognition in schizophrenia. In addition, many studies reported abnormal rs-fcMRI between task positive regions (e.g. anterior cingulate cortex and insula) and default mode network regions (e.g. precuneus, posterior cingulate cortex, and medial frontal gyrus). Regions from these networks are typically anti-correlated in healthy adults, however both increased and decreased negative correlations were found in schizophrenia. Overall, it appears that reduced strength of negative rs-fcMRI between task positive and default mode regions is associated with cognitive deficits in schizophrenia within the domains of attention and working memory, with increased rs-fcMRI potentially relating to deficits in executive function and processing speed. Finally, several studies that assessed global rs-fcMRI found it to be reduced in schizophrenia, and these global reductions were associated with processing speed, working memory, episodic memory, attention, and verbal learning.
As previously mentioned, heterogeneity in clinical characteristics of patient samples may be impacting the observed results. Ideally, results from studies that assessed similar relationships between rs-fcMRI and cognitive domain, but varied on a single clinical domain (e.g. medicated vs. unmedicated; first episode vs. chronic), could be directly compared. However, at this point in the literature, only 16 studies were identified as correlating rs-fcMRI and cognitive ability in schizophrenia, all of which used varying methods, making such comparisons difficult. Of the 16 studies reviewed, all but three used chronic, medicated patients, and all patient samples included a mix of those taking second and first-generation antipsychotics, yielding shallow ground for inferences about the impact of medication and illness status on the observed results. We hope that, as this field grows, more nuanced questions regarding the impact of clinical characteristics on the data can be addressed.
Despite the limitations of this review, the evidence for common rs-fcMRI correlates across cognitive domains is notable, and may provide evidence that common mechanisms are influencing functioning across multiple domains of cognition. In addition, findings from these studies appear consistent with two major literatures on the neurobiological underpinnings of cognitive ability, namely cortical-subcortical connectivity surrounding the frontal cortex, striatum, thalamus, and cerebellum, as well as the competition between task positive and default mode networks. Below, we discuss the integration of these literatures and speculate about their role in the generalized cognitive deficit in schizophrenia.
3. Review of Cognitive Hypotheses and Their Relationship to Functional Connectivity
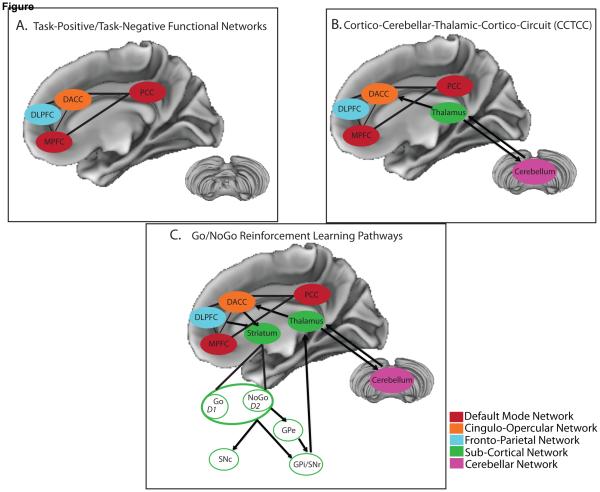
In 1986, Alexander and colleagues described the existence of at least five parallel circuits in the human brain connecting nuclei in the basal ganglia, thalamus, and cortex. These circuits were thought to consist of both ‘open’ and ‘closed’ loops, indicating that portions of each pathway receive inputs from and terminate within a single cortical region (closed), but also project to and receive projections from ancillary regions (open). Among those circuits were three that appeared potentially relevant in supporting higher order cognition, given their connection with the dlPFC, lateral orbitofrontal cortex, and anterior cingulate cortex. Importantly, the literature reviewed above points to converging evidence of abnormal connectivity within these circuits as associated with a range of cognitive deficits in schizophrenia. Several theories that focus on these circuits have been put forth in an effort to better understand cognitive functioning in schizophrenia, including cognitive dysmetria, models of reinforcement learning, and the growing literature on task-positive and task-negative functional networks. Although these three sets of hypotheses have developed largely in parallel, their integration (Figure 1) may provide a framework for testing specific hypotheses regarding global cognitive deficits in schizophrenia.
Figure 1.
Here we present a heuristic model of how several neural systems - whose functional connectivity has been associated with cognitive ability in schizophrenia - may interact with one another to contribute to cognitive deficits. This model is based on results from the review of 16 articles that correlated resting-state functional connectivity (rs-fcMRI) measures with cognitive performance in schizophrenia. Although the reviewed studies yielded fairly heterogenous findings, they converged on associations between cognitive ability and the rs-fcMRI of regions comprising: A) task-positive and task-negative functional brain networks, B) Cortico-Cerebellar-Thalamic-Cortico Circuit (CCTCC), and C) the Go/NoGo pathway of reinforcement learning. Here, we present how these models build on one another, to create a final integrated model (presented in (C)), such that the cortical networks presented in (A) interact with the thalamus and cerebellum, as presented in (B), which in turn are connected with the striatum, integrating the Go/NoGo reinforcement learning pathways, as presented in (C). Based on findings from the current review, we suggest that interactions between task-positive/task-negative functional networks provide the basis for a more complex interaction between the cortex, thalamus, and cerebellum than was originally proposed by cognitive dysmetria (i.e. CCTCC). Additionally, the cortex, thalamus, and cerebellum interact with the striatum, integrating the Go/NoGo pathways involved in the process of reinforcement learning. These Go/NoGo pathways are believed to influence activity in the thalamus and cerebellum, which feedback to cortical networks, in order to control cognitive functioning. In sum, the regions presented in this figure not only represent common hubs for multiple models of cognitive ability, but also all exhibited abnormal functional connectivity in schizophrenia, which in turn was related to cognitive impairment. These findings suggest that abnormal functional connectivity between regions that comprise the task-positive/task-negative networks, the CCTCC, and reinforcement learning pathways together may contribute to the generalized cognitive deficit observed in schizophrenia. Go/NoGo Model modified from Frank et al. (2004). CCTCC model modified from Andreasen (1999).
DLPFC =Dorsolateral Prefrontal Cortex; DACC = Dorsal Anterior Cingulate Cortex; GPe = external segment of the Globus Pallidus; GPi = internal segment of the Globus Pallidus; MPFC = Medial Prefrontal Cortex; PCC = Posterior Cingulate Cortex; SNc = Substantia Nigra pars compacta; SNr = Substantia Nigra pars reticula
3.1 Cognitive Dysmetria and the Cortico-Cerebellar-Thalamic-Cortico-Circuit (CCTCC)
In the 1990s, Nancy Andreasen published a series of positron emission tomography (PET) studies looking at differential activation during performance of cognitive tasks in patients with schizophrenia and healthy controls. These studies revealed that healthy individuals activated prefrontal, thalamic, and cerebellar areas during memory retrieval, but that patients had significantly reduced cerebral blood flow in this circuit (Andreasen et al., 1996). Based on these findings, Andreasen theorized that schizophrenia is a disorder characterized by “cognitive dysmetria” as a result of inappropriate connections within this key circuit in the brain. The cognitive dysmetria theory states that cognitive abilities, similar to motor functions, are supported by a fluid coordination of activity between the prefrontal cortex, cerebellum, and thalamus (cortico-cerebellar-thalamic-cortico circuit; CCTCC). This CCTCC feedback loop is thought to monitor and control the mental activity that supports cognitive abilities, and in schizophrenia this feedback loop is hypothesized to be disrupted, leading to a multitude of cognitive problems in individuals with this disorder.
Over the past several decades, evidence has accumulated to support the presence of abnormalities in the nodes of this circuit in patients with schizophrenia. Likely the most comprehensive literature on the CCTCC has come from studies documenting abnormalities in task activation (Callicot et al., 2003; Heckers et al., 2000; Weinberger et al., 1986) and connectivity (Minzenberg et al., 2009; Zhou et al., 2007) of the prefrontal cortex in schizophrenia, as well as structural abnormalities (Schlaepfer et al., 1994; Andreasen et al.,1994) in this region. More recently, studies on thalamic connectivity have provided some specificity to the relationship between thalamic nuclei and the PFC. For instance, in healthy individuals, Zhang and colleagues (2008) identified robust patterns of functional connectivity between non-overlapping voxels of the thalamus and different cortical regions, including between the mediodorsal nucleus of the thalamus and the PFC, which have a previously established anatomic and structural connectivity (Zhang et al., 2010). This pattern of functional connectivity has been shown to be abnormal in schizophrenia in a number of studies (Woodward et al., 2012; Anticevic et al., 2013; Zhou et al., 2007; Welsch et al., 2010; Klingner et al., 2014), and reduced thalamic functional connectivity was found to be associated with multiple cognitive deficits in this review (Tu et al., 2013; Argyelan et al., 2014), providing support for dysregulation of this portion of the CCTCC in schizophrenia.
In addition, abnormalities in the cerebellum have also been observed in schizophrenia, with evidence of white matter abnormalities within certain cerebellar lobes (Kim et al, 2014), as well as abnormal size and decreased blood flow in the cerebellum during a broad range of cognitive tasks (Andreasen et al., 2008; Barch, 2014). Importantly, reduced functional connectivity between the cerebellum and medial dorsal nucleus of the thalamus has been observed in schizophrenia, providing evidence of abnormalities in this portion of the CCTCC as well (Anticevic et al., 2014; Collin et al., 2011). Some conflicting results regarding the cerebellum’s relationship with cognition were observed in this review, with one paper reporting no significant associations between cerebellar rs-fcMRI and cognition in schizophrenia (Collin et al., 2011), while another found that reduced rs-fcMRI between the cerebellum and the fronto-parietal network was associated with impairments in executive functioning, working memory, and episodic memory (Repovs et al., 2011), pointing to a need for further work to understand the role of the cerebellum in schizophrenia.
Andreasen’s theory of cognitive dysmetria in schizophrenia presents a unitary model to explain a highly complex and heterogeneous disorder, using known anatomic circuits of the brain as a hypothesized source of dysfunction. Importantly, rs-fcMRI abnormalities in the regions outlined by cognitive dysmetria were found to be associated with a multitude of cognitive abnormalities in patients with schizophrenia. Although these associations are not sufficient for proving Andreasen’s theory, they lend support to the notion that abnormal functional connections between regions in the CCTCC underlie a variety of cognitive deficits in schizophrenia.
3.2 Reinforcement Learning
Importantly, there exists another theory that specifically hypothesizes a role of the CCTCC in cognitive functioning, by describing this circuit through the framework of reinforcement learning. One particular model put forth by Frank et al. (2004) describes relationships between the cortex, striatum, and thalamus that are thought to modulate the processing of reward and punishment in order to guide behavior. Briefly, Frank’s model describes how Go/NoGo pathways in the brain – thought to depend on communication between the cortex, striatum, and thalamus, as modulated by dopamine – adjust their signaling based on both task goals and the negative or positive reinforcement of different stimuli. Recently, researchers have adopted this reinforcement learning model to explain symptom expression in schizophrenia. Studies have found that patients with schizophrenia have impaired reinforcement learning to positive feedback (a PFC-dependent process), but intact learning following negative feedback (a basal ganglia-dependent process), suggesting that schizophrenia is characterized by impaired PFC-dependent learning to reward but intact basal ganglia-dependent learning to negative outcomes (Waltz et al., 2007; Strauss et al., 2011; Waltz et al., 2011). These learning abnormalities are thought to be the result of dopamine dysfunction in schizophrenia, particularly of phasic dopamine believed to facilitate rapid updating of information in the PFC.
Interestingly, computational modeling work has shown how gating of information in the PFC is critical for intact cognitive abilities (O’Reilly et al., 2006). Researchers have argued that the PFC is capable of both robustly maintaining information in the face of distractors (i.e. working memory) but also of rapidly updating what is being maintained in order to facilitate behavioral flexibility. The Go/NoGo pathway represents a potential gating mechanism for information in the PFC, implying that dysregulation of this system could result in impaired cognitive functioning across many cognitive domains. Importantly, abnormal functional connectivity of brain region implicated in this reinforcement learning pathway are associated with cognitive deficits in schizophrenia, particularly the caudate (Su et al., 2013), pallidum (Mwansisya et al., 2013), thalamus (Tu et al., 2013; Argyelan et al., 2014), and PFC (Cole et al., 2011). These regions largely showed reduced functional connectivity in patients with schizophrenia, and were associated with a multitude of cognitive domains, including processing speed, executive functioning, and working memory. Given that reinforcement learning is a domain-general process that is critical for multiple areas of cognition, these findings implicate abnormal rs-fcMRI between regions of this pathway in the general cognitive deficit of schizophrenia. In addition, some researchers have posited a role of the cerebellum in reinforcement learning (Swain et al., 2011; Thompson, 1986), allowing for more direct convergence between the theories of cognitive dysmetria and impaired reinforcement learning in schizophrenia
3.3 The Push and Pull Between Task-Positive and Task-Negative Functional Networks
Both cognitive dysmetria and reinforcement learning hypothesize that cognitive deficits in schizophrenia are the result of abnormalities in circuits comprised of the frontal cortex, basal ganglia, thalamus, and cerebellum. These theories bridge cortical and sub-cortical regions in an effort to understand a wide range of cognitive functions and symptoms in schizophrenia. Recently, parallel work has been done to better understand functional networks of the brain that are defined almost exclusively through functional connectivity and that are largely comprised of cortical regions. Similar to how Go/NoGo pathways may fit within the CCTCC framework, these functional networks are likely critical components of the cortical activity outlined in both reinforcement learning and cognitive dysmetria. Therefore, they should not be considered competing theories of cognitive ability, but can hopefully be understood as closer examinations into pieces of a much larger puzzle.
As described earlier in the review, one of these cortical networks is the Default Mode Network (DMN), which includes regions whose BOLD activation is decreased during the performance of goal-directed tasks as compared to during rest, leading to its conceptualization as a “task-negative network”. The role of the DMN has been conceptualized as “stimulus-independent thought”, encompassing mental explorations that are detached from the external environment (for review, see Whitfield-Gabrieli & Ford, 2012). Importantly, the more that DMN activity is suppressed, the better an individual performs on tasks that require attention to external stimuli (Daselaar et al., 2004, 2009), suggesting a competitive relationship for cognitive and attentional resources between internal and external stimuli.
This competition for neural resources may occur between the DMN and other functional networks that consistently increase their activity during cognitive tasks - networks that are often referred to as “task-positive networks”. In particular, two task-positive networks, the fronto-parietal network and cingulo-opercular network, have been identified as supporting task control across a wide variety of cognitive tasks (Dosenbach et al., 2006). These networks are comprised of largely neocortical regions, such as the dlPFC, intraparietal sulcus, intraparietal lobule, precuneus, and dorsal frontal cortex for the fronto-parietal network, and anterior insula, anterior PFC, and the dorsal anterior cingulate cortex for the cingulo-opercular network. Importantly, regions within these networks are functionally connected to the cerebellum and thalamus, making them critical for bridging connections with sub-cortical regions, as discussed in the previous two sections.
Furthermore, the fronto-parietal and cingulo-opercular networks have a reciprocal relationship with the DMN, such that they are anti-correlated. Similar to studies showing that suppression of the DMN is related to better task performance, greater anti-correlation between task-positive and task-negative networks is also associated with better task performance in healthy individuals (Hampson et al., 2010; Kelly et al., 2008). An interesting model of these three networks was put forth by Bressler & Menon (2010), who suggest that the cingulo-opercular network mediates the antagonistic relationship between the fronto-parietal network and DMN, primarily through the insula and dorsal anterior cingulate cortex. Task-positive and task-negative functional networks are therefore thought to support an array of cognitive abilities that are largely domain-free, making them intriguing sources of abnormality in schizophrenia that may underlie the generalized cognitive deficit.
In fact, several studies from this review reported that aberrant functional connectivity within and between these networks is associated with impaired cognition in schizophrenia, across multiple domains. For instance, reduced rs-fcMRI between the fronto-parietal network and the cerebellum was associated with poorer executive functioning, working memory, and episodic memory in patients with schizophrenia (Repovs et al., 2011), and increased within-network connectivity of both the DMN and fronto-parietal network were related to impaired working memory and attention (Unschuld et al., 2014). Additionally, several hub regions within these networks, particularly the insula and anterior cingulate, showed abnormal rs-fcMRI in schizophrenia, and this abnormality was associated with cognitive deficits, primarily in the domains of attention and executive functioning (Yan et al., 2012; He et al., 2013; Moran et al., 2013). Abnormal functional connectivity of the fronto-parietal and cingulo-opercular networks has also been observed while individuals with schizophrenia perform cognitive control tasks (Fornito et al., 2011; Fox et al., 2005a), providing further evidence that coordinated neural activity in these functional networks is compromised in schizophrenia, leading to impaired cognitive performance in patients with the disorder.
3.4 Summary
These literatures on cognitive functioning provide a framework for understanding the findings from this review, given that abnormal functional connectivity within and between regions from the CCTCC, Go/NoGo pathways, and task-positive/task-negative networks were consistently found to correlate with cognitive deficits in schizophrenia. Studies looking at task-positive and task-negative functional networks observed abnormal connectivity between these typically anti-correlated networks, and these abnormalities were associated with deficits in attention, working memory, and executive functioning (Camchong et al., 2011; Unschuld et al., 2014; Repovs et al., 2011; Moran et al., 2013). In addition, many studies reported abnormal connectivity between the PFC, thalamus, striatum and cerebellum, most of which were related to deficits in executive functioning, processing speed, and working memory (Repovs et al., 2011; Argyelan et al., 2014; Mwansisya et al, 2013; Su et al., 2013; Tu et al., 2013). Therefore, this circuitry, which is abnormal in schizophrenia and correlates with cognitive ability, represents a plausible common mechanism for the generalized cognitive deficit in schizophrenia.
Based on the findings from our review, unifying these models of CCTCC, reinforcement learning, and task-positive/task-negative networks may provide a more nuanced model of cognitive impairment in schizophrenia that could lead to the testing of more specific predictions regarding the hypothesized generalized deficit. In such a model (visualized in Figure 1), the Go/NoGo system and task positive/task negative functional networks perform dynamic processes within the circuitry of the CCTCC. More specifically, dopamine modulated Go/NoGo circuitry can be conceptualized as a critical component of the CCTCC that explains major functions supported by communication between the frontal cortex, thalamus, striatum, and cerebellum. The computations performed within the frontal cortex can be understood through the dynamic competition between task-positive and task-negative networks, which are also thought to involve connectivity with the thalamus and cerebellum. Therefore, the reviewed studies of cognitive deficits in schizophrenia point to the notion that components of this larger framework are abnormal in schizophrenia and contribute to cognitive impairments.
4. Limitations and Future Directions
It is important to note the challenges in reviewing and synthesizing data from this literature, given the use of differing methodologies. As outlined in the introduction, rs-fcMRI is a method full of choice-points, any of which can influence the type of question being answered and the results themselves. One solution to this may be an increase in replications and extensions. Although all groups using the exact same methods is not a reasonable (or likely even useful) solution, there are certain aspects of these methods for which uniformity would be extremely helpful for comparing findings. Motion correction is one example, given that motion artifacts are known to influence functional connectivity data, and are particularly prevalent in clinical populations like schizophrenia; therefore more consistency in protocols for how to handle motion correction would help future integration of studies. In addition, agreeing upon seemingly simple choices like whether the subject should leave their eyes open or closed or the length of resting-state scans would help when comparing studies, since it would reduce concerns that such methodological choices are driving differences in results.
Another layer of methodological complexity within this literature review are the various tasks used to measure each cognitive domain. In the last decade, significant efforts have been made regarding the validation and availability of standardized cognitive batteries that are sensitive to differences in ability between patients with schizophrenia and healthy controls (e.g. Nuechterlein et al., 2008; Carter & Barch, 2007). However, across the reviewed studies, there was a wide range in tasks chosen to represent each cognitive domain. One would hope that tasks designed to measure the same cognitive domain would have high construct validity, and using a single task would limit the generalizability of findings and bias the literature. However, measuring multiple cognitive domains within the same study would benefit the field and allow for easier comparison across studies. If a goal is to understand the specificity of cognitive deficits in regards to specific functional abnormalities, then analyzing relationships between functional connectivity and performance on multiple cognitive domains is necessary for parsing out those differential associations.
That said, another important limitation to the current use of neuropsychological tasks is the dependency of many task on multiple cognitive processes. For example, as outlined by Gold and colleagues (2009), a Digit Symbol task, which is often used as a measure of “processing speed”, requires the maintenance of stimuli pairs in working memory, rapidly shifting visual attention, and the transformation of cognitive representations into written responses. Impairment in any of these processes could result in, what is interpreted as, a “processing speed deficit”, making it difficult to determine what cognitive functions are truly impaired in schizophrenia. Relevant to this review, the inter-dependency of cognitive processes also makes it difficult to know which aspects of task performance are truly correlated with rs-fcMRI abnormalities; a fact that likely contributed to the non-specific patterns of rs-fcMRI correlations with “distinct” cognitive domains. Although the current review did not clearly identify differential patterns for specific cognitive domains, future efforts with additional studies that assess more cognitive domains within each study, as well as increased sensitivity of neuropsychological tasks for specific cognitive processes, could help better identify such patterns if they exist.
One major objective for parsing out the variance associated with methodological choices is the future assessment of possible neurodevelopmental mechanisms that lead to the observed associations between cognition and rs-fcMRI in chronic patients, as well as greater understanding of how abnormalities in rs-fcMRI may impact everyday functioning. Neurodevelopmental theories of schizophrenia suggest several mechanisms contributing to the presentation of cognitive deficits early in the course of illness. In fact, significant deficits in IQ can be observed in individuals who later go on to develop schizophrenia as early as 13 years old (Dickson et al., 2012), suggesting that cognitive deficits are an early (but non-specific) marker of the disorder. Therefore, theories of abnormal synaptic pruning (Keshevan et al., 1994), dysregulated dopamine, and the impact of early life stress on brain function (for comprehensive review, see Howes & Murray, 2014) provide testable hypotheses regarding the developmental mechanisms underlying abnormal rs-fcMRI and cognitive impairment in schizophrenia. Overall, these theories suggest that early neural insult and genetic risk lead to abnormal development of functional and structural brain networks, that are influenced by dopamine function, and all of which are mediated by environmental stressors. Furthermore, longitudinal studies that assess both rs-fcMRI and cognitive ability across development (e.g. the Philadelphia Neurodevelopmental Cohort; Satterthwaite et al., 2014) will be powerful tools for determining the point at which abnormal rs-fcMRI can be observed in individuals who later develop schizophrenia. This data will also aid in our understanding of the link between rs-fcMRI abnormalities, cognitive deficits, and real-world functional outcome, by helping to reveal the state of rs-fcMRI that corresponds with an individual’s declines in daily living activities, and the association of those two measures with cognitive functioning.
5. Conclusions
Over the last century, significant advances have been made in our understanding of the neurobiological correlates of cognition, due in part to the use of functional neuroimaging to non-invasively approximate neuronal activity in the human brain. Despite these efforts, much work needs to be done to understand how cognition goes awry in disorders like schizophrenia, and then how we can translate that information into effective treatments. The current review revealed two major patterns of functional connectivity abnormalities related to cognitive ability: 1) reduced connectivity between cortical regions, particularly the prefrontal cortex, and sub-cortical regions, including the basal ganglia, thalamus, and cerebellum, and 2) abnormal connectivity between cortical regions involved in task-positive and task-negative functional networks. Both of these patterns can be understood within current cognitive neuroscience theories of cognitive function, including Nancy Andreasen’s theory of cognitive dysmetria of the CCTCC in schizophrenia, work on reinforcement learning pathways, and the dynamic competition between task positive and task negative functional networks.
We have argued that these cognitive theories are not mutually exclusive, and instead can each be understood as pieces of a larger puzzle, with task-positive and task-negative networks influencing the dynamics of the cortex, and the cortex influencing the Go/NoGo pathways within the CCTCC. Additionally, we argued that the lack of specific associations between impairments in putatively different cognitive domains and patterns of functional connectivity abnormalities in schizophrenia may reflect the global cognitive deficit in schizophrenia, which we argue could be a psychological manifestation of abnormalities within this larger cortical-subcortical system. While more work must be done to directly test this hypothesis, we believe the findings from this review provide early evidence that cognitive impairments in schizophrenia are due, at least in part, to abnormal functional connectivity within this dynamic system.
Highlights.
Review 16 studies measuring rs-fcMRI abnormalities and cognition in schizophrenia
Lack of specificity between individual cognitive domains and specific brain regions
Nodes from 3 cognitive models were abnormal and associated with cognitive deficits
Propose a unified model of these 3 prior cognitive models, to guide future research
Acknowledgements
Work for this paper was completed while the author (JMS) was receiving funding from a T32 training grant: Training at the Interface of Psychology, Neuroscience and Genetics, 5T32GM081739, as well as from an F31 pre-doctoral fellowship: F31 MH108309-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annual review of neuroscience. 1986;9(1):357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen NC, Carpenter WT., Jr Diagnosis and classification of schizophrenia. Schizophrenia Bulletin. 1993;19(2):199. doi: 10.1093/schbul/19.2.199. [DOI] [PubMed] [Google Scholar]
- 3.Andreasen NC. A unitary model of schizophrenia: Bleuler's fragmented phrene as schizencephaly. Archives of general psychiatry. 1999;56(9):781–787. doi: 10.1001/archpsyc.56.9.781. [DOI] [PubMed] [Google Scholar]
- 4.Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Hichwa RD. Schizophrenia and cognitive dysmetria: a positron-emission tomography study of dysfunctional prefrontal-thalamic-cerebellar circuitry. Proceedings of the National Academy of Sciences. 1996;93(18):9985–9990. doi: 10.1073/pnas.93.18.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreasen NC, Arndt S, Swayze V, Cizadlo T, Flaum M, O'Leary D, Yuh WT. Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science. 1994;266(5183):294–298. doi: 10.1126/science.7939669. [DOI] [PubMed] [Google Scholar]
- 6.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biological psychiatry. 2008;64(2):81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anticevic A, Cole MW, Repovs G, Murray JD, Brumbaugh MS, Winkler AM, Glahn DC. Characterizing thalamo-cortical disturbances in schizophrenia and bipolar illness. Cerebral Cortex. 2013 doi: 10.1093/cercor/bht165. bht165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anticevic A, Yang G, Savic A, Murray JD, Cole MW, Repovs G, Glahn DC. Mediodorsal and Visual Thalamic Connectivity Differ in Schizophrenia and Bipolar Disorder With and Without Psychosis History. Schizophrenia bulletin. 2014;40(6):1227–1243. doi: 10.1093/schbul/sbu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Argyelan M, Ikuta T, DeRosse P, Braga RJ, Burdick KE, John M, Szeszko PR. Resting-state FMRI connectivity impairment in schizophrenia and bipolar disorder. Schizophrenia bulletin. 2013 doi: 10.1093/schbul/sbt092. sbt092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banich MT. Executive function the search for an integrated account. Current Directions in Psychological Science. 2009;18(2):89–94. [Google Scholar]
- 11.Barch DM. Cerebellar-Thalamic Connectivity in Schizophrenia. Schizophrenia Bulletin. 2014 doi: 10.1093/schbul/sbu076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bassett DS, Nelson BG, Mueller BA, Camchong J, Lim KO. Altered resting-state complexity in schizophrenia. Neuroimage. 2012;59(3):2196–2207. doi: 10.1016/j.neuroimage.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowie CR, Reichenberg A, Patterson TL, Heaton RK, Harvey PD. Determinants of real-world functional performance in schizophrenia subjects: correlations with cognition, functional capacity, and symptoms. American Journal of Psychiatry. 2006;163(3):418–425. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- 14.Bowie CR, Harvey PD. Treatment of cognitive deficits in schizophrenia. Current opinion in investigational drugs (London, England: 2000) 2006;7(7):608–613. [PubMed] [Google Scholar]
- 15.Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophrenia bulletin. 1993;19(2):233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- 16.Bressler SL, Menon V. Large-scale brain networks in cognition: emerging methods and principles. Trends in cognitive sciences. 2010;14(6):277–290. doi: 10.1016/j.tics.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Brewer WJ, Wood SJ, Phillips LJ, Francey SM, Pantelis C, Yung AR, McGorry PD. Generalized and specific cognitive performance in clinical high-risk cohorts: a review highlighting potential vulnerability markers for psychosis. Schizophrenia Bulletin. 2006;32(3):538–555. doi: 10.1093/schbul/sbj077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bundesen C. A theory of visual attention. Psychological review. 1990;97(4):523. doi: 10.1037/0033-295x.97.4.523. [DOI] [PubMed] [Google Scholar]
- 19.Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. American Journal of Psychiatry. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- 20.Camchong J, MacDonald AW, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophrenia bulletin. 2011;37(3):640–650. doi: 10.1093/schbul/sbp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Carter CS, Barch DM. Cognitive neuroscience-based approaches to measuring and improving treatment effects on cognition in schizophrenia: the CNTRICS initiative. Schizophrenia bulletin. 2007;33(5):1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chua SE, Murray RM. The neurodevelopmental theory of schizophrenia: evidence concerning structure and neuropsychology. Annals of medicine. 1996;28(6):547–555. doi: 10.3109/07853899608999119. [DOI] [PubMed] [Google Scholar]
- 23.Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Frontiers in systems neuroscience. 2010:4. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole MW, Bassett DS, Power JD, Braver TS, Petersen SE, Cole MW. Intrinsic and task-evoked network architectures of the human brain. Neuron. 2014 doi: 10.1016/j.neuron.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biological psychiatry. 2011;70(1):43–50. doi: 10.1016/j.biopsych.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collin G, Pol HEH, Haijma SV, Cahn W, Kahn RS, van den Heuvel MP. Impaired cerebellar functional connectivity in schizophrenia patients and their healthy siblings. Frontiers in psychiatry. 2011:2. doi: 10.3389/fpsyt.2011.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23(3):921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 28.Daselaar SM, Prince SE, Dennis NA, Hayes SM, Kim H, Cabeza R. Posterior midline and ventral parietal activity is associated with retrieval success and encoding failure. Frontiers in human neuroscience. 2009:3. doi: 10.3389/neuro.09.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual review of neuroscience. 1995;18(1):193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- 30.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of general psychiatry. 2007;64(5):532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 31.Dickinson D, Harvey PD. Systemic hypotheses for generalized cognitive deficits in schizophrenia: a new take on an old problem. Schizophrenia bulletin. 2009;35(2):403–414. doi: 10.1093/schbul/sbn097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickson H, Laurens KR, Cullen AE, Hodgins S. Meta-analyses of cognitive and motor function in youth aged 16 years and younger who subsequently develop schizophrenia. Psychological medicine. 2012;42(04):743–755. doi: 10.1017/S0033291711001693. [DOI] [PubMed] [Google Scholar]
- 33.Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50(5):799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fornito A, Yoon J, Zalesky A, Bullmore ET, Carter CS. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biological psychiatry. 2011;70(1):64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox MD, Snyder AZ, McAvoy MP, Barch DM, Raichle ME. The BOLD onset transient: identification of novel functional differences in schizophrenia. Neuroimage. 2005a;25(3):771–782. doi: 10.1016/j.neuroimage.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 36.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005b;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fox MD, Greicius M. Clinical applications of resting-state functional connectivity. Frontiers in systems neuroscience. 2010:4. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 39.Frank MJ, Seeberger LC, O'reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306(5703):1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- 40.Gold JM. Cognitive deficits as treatment targets in schizophrenia. Schizophrenia research. 2004;72(1):21–28. doi: 10.1016/j.schres.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 41.Gold JM, Gold JM, Hahn B, Strauss GP, Waltz JA. Turning it upside down: areas of preserved cognitive function in schizophrenia. Neuropsychology review. 2009;19(3):294–311. doi: 10.1007/s11065-009-9098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hampson M, Driesen N, Roth JK, Gore JC, Constable RT. Functional connectivity between task-positive and task-negative brain areas and its relation to working memory performance. Magnetic resonance imaging. 2010;28(8):1051–1057. doi: 10.1016/j.mri.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Z, Deng W, Li M, Chen Z, Jiang L, Wang Q, Li T. Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychological medicine. 2013;43(04):769–780. doi: 10.1017/S0033291712001638. [DOI] [PubMed] [Google Scholar]
- 45.Heckers S, Curran T, Goff D, Rauch SL, Fischman AJ, Alpert NM, Schacter DL. Abnormalities in the thalamus and prefrontal cortex during episodic object recognition in schizophrenia. Biological Psychiatry. 2000;48(7):651–657. doi: 10.1016/s0006-3223(00)00919-7. [DOI] [PubMed] [Google Scholar]
- 46.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. The Lancet. 2014;383(9929):1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaufman AS, Reynolds CR, McLean JE. Age and WAIS-R intelligence in a national sample of adults in the 20-to 74-year age range: A cross-sectional analysis with educational level controlled. Intelligence. 1989;13(3):235–253. [Google Scholar]
- 48.Karbasforoushan H, Woodward ND. Resting-state networks in schizophrenia. Current topics in medicinal chemistry. 2012;12(21):2404–2414. doi: 10.2174/156802612805289863. [DOI] [PubMed] [Google Scholar]
- 49.Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 50.Keshavan MS, Anderson S, Pettergrew JW. Is schizophrenia due to excessive synaptic pruning in the prefrontal cortex? The Feinberg hypothesis revisited. Journal of psychiatric research. 1994;28(3):239–265. doi: 10.1016/0022-3956(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 51.Kim DJ, Kent JS, Bolbecker AR, Sporns O, Cheng H, Newman SD, Hetrick WP. Disrupted Modular Architecture of Cerebellum in Schizophrenia: A Graph Theoretic Analysis. Schizophrenia bulletin. 2014 doi: 10.1093/schbul/sbu059. sbu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Klingner CM, Langbein K, Dietzek M, Smesny S, Witte OW, Sauer H, Nenadic I. Thalamocortical connectivity during resting-state in schizophrenia. European archives of psychiatry and clinical neuroscience. 2014;264(2):111–119. doi: 10.1007/s00406-013-0417-0. [DOI] [PubMed] [Google Scholar]
- 53.Liu H, Kaneko Y, Ouyang X, Li L, Hao Y, Chen EY, Liu Z. Schizophrenic patients and their unaffected siblings share increased resting-state connectivity in the task-negative network but not its anticorrelated task-positive network. Schizophrenia bulletin. 2010 doi: 10.1093/schbul/sbq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lynall ME, Bassett DS, Kerwin R, McKenna PJ, Kitzbichler M, Muller U, Bullmore E. Functional connectivity and brain networks in schizophrenia. The Journal of Neuroscience. 2010;30(28):9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia research. 2003;60(2):285–298. doi: 10.1016/s0920-9964(02)00294-3. [DOI] [PubMed] [Google Scholar]; McIntosh AR, Nyberg L, Bookstein FL, Tulving E. Differential functional connectivity of prefrontal and medial temporal cortices during episodic memory retrieval. Human brain mapping. 1997;5(4):323–327. doi: 10.1002/(SICI)1097-0193(1997)5:4<323::AID-HBM20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 56.McIntosh AR, Nyberg L, Bookstein FL, Tulving E. Differential functional connectivity of prefrontal and medial temporal cortices during episodic memory retrieval. Human brain mapping. 1997;5(4):323–327. doi: 10.1002/(SICI)1097-0193(1997)5:4<323::AID-HBM20>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 57.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23(3):315. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 58.Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of general psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moran LV, Tagamets MA, Sampath H, O’Donnell A, Stein EA, Kochunov P, Hong LE. Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biological psychiatry. 2013;74(6):467–474. doi: 10.1016/j.biopsych.2013.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mwansisya TE, Wang Z, Tao H, Zhang H, Hu A, Guo S, Liu Z. The diminished interhemispheric connectivity correlates with negative symptoms and cognitive impairment in first-episode schizophrenia. Schizophrenia research. 2013;150(1):144–150. doi: 10.1016/j.schres.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 61.Nuechterlein K, Green M, Kern R, Baade L, Barch D, Cohen J, Marder S. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. American Journal of Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 62.O'Leary DS, Flaum M, Kesler ML, Flashman LA, Arndt S, Andreasen NC. Cognitive correlates of the negative, disorganized, and psychotic symptom dimensions of schizophrenia. The Journal of neuropsychiatry and clinical neurosciences. 2000;12(1):4–15. doi: 10.1176/jnp.12.1.4. [DOI] [PubMed] [Google Scholar]
- 63.O'Reilly RC. Biologically based computational models of high-level cognition. Science. 2006;314(5796):91–94. doi: 10.1126/science.1127242. [DOI] [PubMed] [Google Scholar]
- 64.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Relation. 2001;158(7) doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 65.Power JS, Cohen AM, Nelson SM, Wig GS, Barnes KA, Church JA, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72(3):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: Integration of performance-based and brain imaging findings. Psychological bulletin. 2007;133(5):833. doi: 10.1037/0033-2909.133.5.833. [DOI] [PubMed] [Google Scholar]
- 68.Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biological psychiatry. 2011;69(10):967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophrenia research. 2010;117(1):21–30. doi: 10.1016/j.schres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Satterthwaite TD, Elliott MA, Ruparel K, Loughead J, Prabhakaran K, Calkins ME, Gur RE. Neuroimaging of the Philadelphia neurodevelopmental cohort. Neuroimage. 2014;86:544–553. doi: 10.1016/j.neuroimage.2013.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schatz J. Cognitive processing efficiency in schizophrenia: generalized vs domain specific deficits. Schizophrenia Research. 1998;30(1):41–49. doi: 10.1016/s0920-9964(97)00125-4. [DOI] [PubMed] [Google Scholar]
- 72.Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Pearlson GD. Decreased regional cortical gray matter volume in schizophrenia. The American Journal of Psychiatry. 1994 doi: 10.1176/ajp.151.6.842. [DOI] [PubMed] [Google Scholar]
- 73.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. The Journal of neuroscience. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Snitz BE, MacDonald AW, Carter CS. Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophrenia Bulletin. 2006;32(1):179–194. doi: 10.1093/schbul/sbi048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strauss GP, Frank MJ, Waltz JA, Kasanova Z, Herbener ES, Gold JM. Deficits in positive reinforcement learning and uncertainty-driven exploration are associated with distinct aspects of negative symptoms in schizophrenia. Biological psychiatry. 2011;69(5):424–431. doi: 10.1016/j.biopsych.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stuss DT. Biological and psychological development of executive functions. Brain and cognition. 1992;20(1):8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- 77.Su TW, Lan TH, Hsu TW, Biswal BB, Tsai PJ, Lin WC, Lin CP. Reduced neuro-integration from the dorsolateral prefrontal cortex to the whole brain and executive dysfunction in schizophrenia patients and their relatives. Schizophrenia research. 2013;148(1):50–58. doi: 10.1016/j.schres.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 78.Swain RA, Kerr AL, Thompson RF. The cerebellum: a neural system for the study of reinforcement learning. Frontiers in behavioral neuroscience. 2011;5:8. doi: 10.3389/fnbeh.2011.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thompson RF. The neurobiology of learning and memory. Science. 1986;233(4767):941–947. doi: 10.1126/science.3738519. [DOI] [PubMed] [Google Scholar]
- 80.Tu PC, Hsieh JC, Li CT, Bai YM, Su TP. Cortico-striatal disconnection within the cingulo-opercular network in schizophrenia revealed by intrinsic functional connectivity analysis: a resting fMRI study. Neuroimage. 2012;59(1):238–247. doi: 10.1016/j.neuroimage.2011.07.086. [DOI] [PubMed] [Google Scholar]
- 81.Tu PC, Lee YC, Chen YS, Li CT, Su TP. Schizophrenia and the brain's control network: Aberrant within-and between-network connectivity of the frontoparietal network in schizophrenia. Schizophrenia research. 2013;147(2):339–347. doi: 10.1016/j.schres.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 82.Tulving E. Episodic memory: from mind to brain. Annual review of psychology. 2002;53(1):1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 83.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 84.Unschuld PG, Buchholz AS, Varvaris M, van Zijl PC, Ross CA, Pekar JJ, Schretlen DJ. Prefrontal brain network connectivity indicates degree of both schizophrenia risk and cognitive dysfunction. Schizophrenia bulletin. 2014;40(3):653–664. doi: 10.1093/schbul/sbt077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Waltz JA, Frank MJ, Robinson BM, Gold JM. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biological psychiatry. 2007;62(7):756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Waltz JA, Frank MJ, Wiecki TV, Gold JM. Altered probabilistic learning and response biases in schizophrenia: behavioral evidence and neurocomputational modeling. Neuropsychology. 2011;25(1):86. doi: 10.1037/a0020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang X, Xia M, Lai Y, Dai Z, Cao Q, Cheng Z, He Y. Disrupted resting-state functional connectivity in minimally treated chronic schizophrenia. Schizophrenia research. 2014;156(2):150–156. doi: 10.1016/j.schres.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 88.Wechsler D. WAIS-III, Wechsler Adult Intelligence Scale: Administration and Scoring Manual. Psychological Corporation; Wechsler, D: 1997. 1997. [Google Scholar]
- 89.Wechsler memory scale (WMS-III) Psychological Corporation; [Google Scholar]
- 90.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia: I. Regional cerebral blood flow evidence. Archives of General Psychiatry. 1986;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 91.Whitfield-Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Faraone SV, McCarley RW, Seidman LJ. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proceedings of the National Academy of Sciences. 2009;106(4):1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Whitfield-Gabrieli S, Ford JM. Default mode network activity and connectivity in psychopathology. Annual review of clinical psychology. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 93.Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. American Journal of Psychiatry. 2012;169(10):1092–1099. doi: 10.1176/appi.ajp.2012.12010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yan H, Tian L, Yan J, Sun W, Liu Q, Zhang YB, Zhang D. Functional and anatomical connectivity abnormalities in cognitive division of anterior cingulate cortex in schizophrenia. PloS one. 2012;7(9):e45659. doi: 10.1371/journal.pone.0045659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang D, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. Journal of neurophysiology. 2008;100(4):1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cerebral cortex. 2010;20(5):1187–1194. doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang D, Raichle ME. Disease and the brain's dark energy. Nature Reviews Neurology. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Y, Liang M, Jiang T, Tian L, Liu Y, Liu Z, Kuang F. Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neuroscience letters. 2007;417(3):297–302. doi: 10.1016/j.neulet.2007.02.081. [DOI] [PubMed] [Google Scholar]
- 99.Zhou Y, Wang K, Liu Y, Song M, Song SW, Jiang T. Spontaneous brain activity observed with functional magnetic resonance imaging as a potential biomarker in neuropsychiatric disorders. Cognitive neurodynamics. 2010;4(4):275–294. doi: 10.1007/s11571-010-9126-9. [DOI] [PMC free article] [PubMed] [Google Scholar]