Abstract
Purpose
Hematopoietic cell transplantation (HCT) is performed in select centers in the United States (U.S.), and patients are often required to temporarily relocate to receive care. The purpose of this study was to identify housing barriers impacting access to HCT and potential solutions.
Methods
A mixed-methods primary study of HCT social workers was conducted to learn about patient housing challenges and solutions in place that help address those barriers. Three telephone focus groups were conducted with adult and pediatric transplant social workers (n=15). Focus group results informed the design of a national survey. The online survey was e-mailed to a primary social worker contact at 133 adult and pediatric transplant centers in the U.S. Transplant centers were classified based on the patient population cared for by the social worker.
Results
The survey response rate was 49 %. Among adult programs (n=45), 93 % of centers had patients that had to relocate closer to the transplant center to proceed with HCT. The most common type of housing option offered was discounted hotel rates. Among pediatric programs (n=20), 90 % of centers had patients that had to relocate closer to the transplant center to proceed with HCT. Ronald McDonald House was the most common option available.
Conclusions
This study is the first to explore housing challenges faced by patients undergoing HCT in the U.S. from the perspective of social workers and to highlight solutions that centers use. Transplant centers will benefit from this knowledge by learning about options for addressing housing barriers for their patients.
Keywords: Hematopoietic cell transplantation, Housing, Barriers
Introduction
Hematopoietic cell transplantation (HCT) is a life-saving treatment for hematologic cancers (e.g., leukemia, lymphoma, myeloma) and non-malignant hematological diseases (e.g., aplastic anemia, sickle cell disease). Approximately 20,000 HCTs utilizing autologous (patient’s own hematopoietic progenitor cells) or allogeneic (using donor hematopoietic progenitor cells) grafts are performed annually in the United States (U.S.) [1]. The need for HCT is projected to increase in the coming years due to an increasing population of older patients with a high incidence of hematologic malignancies, emerging indications for transplantation, and virtually all patients now being able to find a donor source [2, 3].
To examine the health care system’s ability to handle this increase in the number of HCTs and to improve access to HCT by understanding system and infrastructural barriers, the National Marrow Donor Program (NMDP)/Be The Match® and the American Society for Blood and Marrow Transplantation sponsored the System Capacity Initiative (SCI) [2, 4]. The SCI was a multi-year initiative involving a number of stakeholders, with working groups focused on workforce, financial, and facility capacity and care delivery issues in HCT [2, 4]. The care delivery issues working group identified patient housing as a potential barrier to transplantation. While some research [5–9] exists that analyzes access issues such as availability of suitable donors [5], out-of-pocket costs [6, 7], and geographic region [8, 9], very limited literature exists on the housing barriers to HCT.
HCT is a specialized procedure that is available at select U.S. centers. Hence, patients and their caregivers frequently must temporarily relocate closer to the transplant center for 3–4 months during the transplant procedure and early recovery period. This can be a burden to patients, as it may separate the patient and caregiver from their family and community [10] at a time when they need them the most. Finding temporary lodging presents other challenges such as locating housing resources in an unfamiliar location, leaving or resigning from a job resulting in lost income, and displacing children or family members from school or work [11]. Temporary relocation can lead to increased costs for patients and their families [6, 12], as they typically have to pay for maintaining both temporary and permanent residences.
The purpose of this study was to explore housing barriers and caregiver availability for patients undergoing HCT in the U.S. from the perspective of HCT social workers, and to identify current practices that transplant centers utilize to address those barriers.
Methods
Study design and sample
This mixed-methods study used semi-structured focus groups and a survey of HCT social workers to obtain both qualitative and quantitative data to identify barriers and current practices relative to the housing and caregiver availability for patients undergoing HCT. This manuscript presents findings regarding housing barriers.
The NMDP facilitates unrelated donor transplantation in the U.S. through its Be The Match® donor registry. In 2014, 92.5 % of all U.S. transplant centers that perform unrelated donor HCT were members of the NMDP network. Each NMDP network center identifies a primary social worker contact; these social workers were the target study sample for this study.
The NMDP Institutional Review Board classified this research as exempt, determining that this research was not considered human subject research as defined by 45 CFR 46.102(d). Funding for the monetary incentive for study participants was provided by the SCI.
Focus groups
Focus groups are useful to explore a topic when little information is known about it [13, 14]. For focus group inclusion, a convenience sample of transplant center social workers were invited based on center annual HCT volume, primary patient population served (adult, pediatric), and geographic location (Midwest, Northeast, South, West). The primary social worker contact at the selected centers was invited to participate via e-mail. Due to low enrollment from pediatric social workers, an invitation was placed on the Association of Pediatric Oncology Social Workers website to facilitate recruitment.
A discussion guide was developed by members of the protocol team. Questions focused on centers’ geographic location, housing and caregiver requirements, resources available to assist patients, and availability of innovative housing and caregiver solutions.
Three 60–90 min focus groups were conducted via teleconference (adult centers=2 groups, pediatric centers=1 group). Focus groups were audio-recorded with participants’ consent and facilitated by an experienced moderator with knowledge of HCT. A $50 gift card was provided to participants upon completion of the focus group.
Survey
Survey questions were developed based on focus group findings and inquired about: center requirements, perceived patient barriers and center solutions regarding housing (16 questions), and caregiver availability (20 questions). Seven questions were devoted to demographics. The 43-item survey was sent to primary social worker contacts at U.S. NMDP network transplant centers via e-mail using the Internet-based survey administration system, SurveyGizmo (Boulder, CO), and was in the field from August to December 2013. Non-responders were contacted by two follow-up e-mails, a follow-up phone call, and a final reminder e-mail. Respondents received a $25 gift card upon completion of the survey. Focus group participants were eligible to complete the survey.
Analysis
Focus group analysis
A codebook based on themes identified in a literature review, data collection, and study objectives was used to code transcriptions of the audio tapes. The codebook consisted of six basic components: the code, a brief definition, a full definition, guidelines for when to use the code, guidelines for when not to use the code, and examples [15]. Computer-assisted qualitative data analysis software, NVivo 10.0, was used. Two reviewers, familiar with the area of study, analyzed the data so that the reliability of the coded data could be assessed through inter-agreement measures [16]. Because initial coding instructions often yield poor agreement, the coders independently coded one of the focus groups twice. The codebook was amended after both times, with a third study team member resolving any discrepancies. Final coding was completed and the final kappa statistic was 0.86.
Survey data analysis
Survey response data were exported from SurveyGizmo to SPSS version 19 (Armonk, NY) for analysis. Descriptive statistics were computed for all demographic and survey items. Transplant centers were classified based on the patient population cared for such as: adult, pediatric, or both. Three social workers at children’s hospitals identified caring for both populations; they were classified as pediatric centers. Center volumes were obtained from the Center for International Blood and Marrow Transplantation Research, and were classified as low volume (<50 HCT/year), medium volume (51–150 HCT/year), or high volume (more than 150 HCT/year).
Results
Focus group results
Fifteen social workers participated in the focus groups. Two focus groups were devoted to adult centers (n=3 and n=6 participants) and one to pediatric centers (n=6 participants). Geographic representation included the Midwest (n=8), West (n=2), South (n=4), and Northeast (n=1). Participants represented centers with various HCT volumes, ranging from 16 to 812 total HCTs in 2011.
Many of the transplant centers served patients who had to travel to receive an HCT, including some that served international patients. Requirements for patients to stay near the transplant center varied by center. Common housing barriers and solutions that social workers identified seeing their patients face are summarized in Table 1; similarities existed across the adult and pediatric transplant centers. All of the pediatric centers had access to a Ronald McDonald House [17] for their patients, though requirements to stay at the Ronald McDonald House varied. Within the core HCT team, social workers were most often the primary provider responsible for sharing housing information and resources with patients, though in one center, a dedicated staff member helped patients with housing, and in some centers, a multi-disciplinary approach was taken.
Table 1.
Housing barriers and solutions: key themes from focus groups
| Housing barriers | Solutions |
|---|---|
|
|
Survey respondent and center characteristics
Among the 133 primary social worker contacts invited to participate in the survey, 65 (49 %) responded. Adult centers (n= 45) were fairly equally distributed in terms of geographic region, with seven centers in the Northeast (16 %), 15 in the Midwest (33 %), 14 in the South (31 %), and nine in the West (20 %). In terms of volume, most adult centers were medium (44 %, n=20) or high (42 %, n=19), with only 13 % (n=6) from low volume centers. Adult center respondents were asked the average number of hours they dedicated to addressing housing needs for patients and families per week; the median was 5 hours (range: 1–32). Twenty-nine centers (65 %) had less than two social worker full-time equivalents (FTEs); 14 centers (31 %) had 2–3 social worker FTEs, and two centers (4 %) had ≥4 social work FTEs (range 0.2–4.8).
Pediatric centers (n=20) were also fairly equally distributed in terms of geographic region, with six centers located in the Northeast (30 %), 5 in the Midwest (25 %), 6 in the South (30 %), and 3 in the West (15 %). In contrast to the adult centers, most of the pediatric centers (55 %, n=11) were low volume centers, with 25 % (n=5) from medium centers; and 20 % (n=4) from high volume centers. Respondents dedicated a median of 3.5 hours (range: 0–20) to addressing housing needs for patients and families per week. Eleven centers (58 %) had less than two social worker FTEs; five centers (26 %) had 2–3 social worker FTEs, and three centers (16 %) had ≥4 social work FTEs (range: 0.5–5.0).
Housing requirements
How close are patients required to stay near the center?
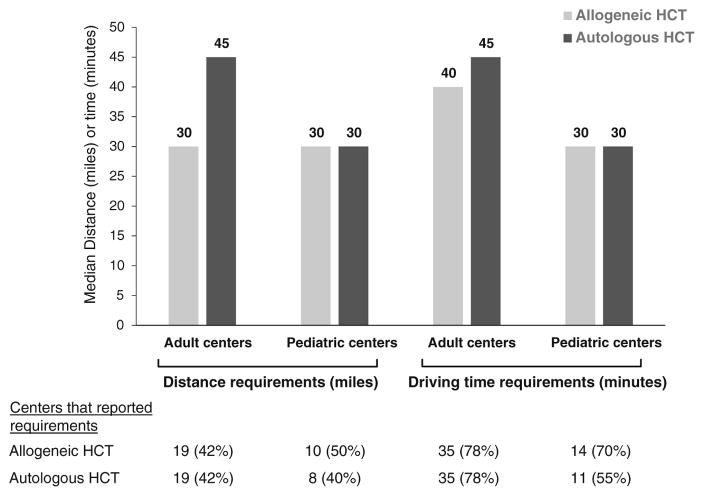
Most centers had requirements on how close to the transplant center patients were required to stay. The requirements were in time, distance, or both, depending on the center, and could vary based on whether a patient had an autologous or allogeneic HCT (Fig. 1). Three adult centers and two pediatric centers indicated that they had no specific requirements and provided recommendations on a case-by-case basis.
Fig. 1.
Transplant center distance and driving time requirements by type of transplant
Required time period of stay
The period of time that transplant centers required patients to stay nearby varied by the type of transplant a patient received. The majority of adult centers (57 %) required patients receiving allogeneic HCT to stay close to the center for 100 days after HCT, while only 4 % of autologous patients were required to stay that amount of time. The most frequent time period of stay required for autologous patients (53 %) was 14–45 days, while only 21 % of allogeneic patients had that requirement. One center had a requirement of 6 months for their patients undergoing allogeneic or autologous HCTs. Sixteen percent indicated that the allogeneic and autologous requirement was based on clinical condition. Two centers had no requirement for patients receiving allogeneic HCT to stay near the transplant center, while nine centers (20 %) indicated there was no requirement for patients receiving autologous HCT.
Similarly, many pediatric centers (45 %) required allogeneic patients to stay close to the center for 100 days, while 35 % had that requirement for autologous patients. For autologous patients, 40 % of the centers said the requirement was based on clinical condition, while 20 % said the same for allogeneic patients. Twenty-five percent indicated the allogeneic requirement was 14–45 days, while 10 % had that requirement for autologous. One center had no requirement for patients receiving allogeneic HCT, while two centers did not have a requirement for patients receiving autologous HCT.
Patients required to relocate for HCT
The majority of centers (adult, 93 %; pediatric, 90 %) indicated that they had patients that needed to relocate closer to the center to proceed with HCT in the previous calendar year. Eighteen (40 %) adult and ten (50 %) pediatric centers indicated that 50 % or more of their patients had to relocate, and 24 (53 %) adult centers and eight (40 %) pediatric centers indicated that less than half of their patients had to relocate in order to proceed with transplant.
Barriers
Respondents were asked how frequently their patients faced certain housing barriers to HCT in the past year. The most frequent housing-related barrier that social workers indicated that their patients (regardless of age and type of transplant) faced was lack of insurance benefits for lodging, with the majority of respondents indicating their patients always or often faced this barrier (adult; allogenic, 78 %; autologous, 65 %; pediatric; allogeneic, 65 %; autologous, 63 %). The second most frequent housing barrier identified by the social workers was cost/affordability of housing. Again, the majority of respondents, regardless of age of the transplant recipient served and type of transplant, indicated that their patients always or often faced this barrier (adult; allogeneic, 64 %; autologous, 47 %; Pediatric; allogeneic, 60 %; autologous, 58 %). For the adult population of the three centers who cared for both adult and pediatric patients, the most common identified barrier by the social workers was lack of insurance benefits for lodging (allogeneic, 100 %; autologous, 100 %).
Other barriers always or often faced by adult patients included the following: housing options full or had long waiting lists (allogeneic, 33 %; autologous, 33 %), lack of housing options available (allogeneic, 33 %; autologous, 28 %), and eligibility for housing (e.g., distance, income, or diagnosis) (allogeneic, 31 %; autologous, 26 %).
For pediatric patients, social workers indicated that other barriers always or often faced included the following: lack of housing options available (allogeneic, 50 %; autologous, 47 %), restrictions on persons who can stay in housing (e.g., siblings) (allogeneic, 45 %; autologous, 42 %), eligibility for housing (e.g., distance, income, or diagnosis) (allogeneic, 35 %; autologous, 32 %), restrictions placed by housing provider (e.g., background checks) (allogeneic, 30 %; autologous, 32 %), and housing options full or had long waiting lists (allogeneic, 30 %; autologous, 28 %).
Center solutions to address housing barriers
Many centers had temporary housing available for patients. Adult centers most commonly had discounted hotel rates (82 %) and local hotel/motels (58 %) available for patients. Ronald McDonald House (85 %) and discounted hotel rates (75 %) were the most common types of temporary housing available for pediatric patients. Additional options included housing owned in collaboration with another entity, e.g., a foundation (adult, 36 %; pediatric, 20 %), Hope Lodge [18] (adult, 31 %; pediatric, 30 %), discounted apartments (adult, 24 %; pediatric, 10 %), housing fully owned and operated by the hospital (adult, 16 %; pediatric, 10 %), and local hotel/motel (pediatric, 45 %). For adult centers, respondents also reported hospitality homes (a patient and their family stay with a family in a room of a home) and hospitality apartments (free temporary housing for three months). Pediatric center social workers also reported hospitality houses and other non-profit housing complexes.
Centers also offered programs and resources to assist patients in finding housing. The most common resources offered by both adult and pediatric centers were print materials (84 and 80 %, respectively). Other programs and resources included a housing/accommodations department within the center (adult, 33 %; pediatric, 40 %), waiting lists (adult, 20 %; pediatric, 25 %), and a website as a housing resource (adult, 20 %; pediatric, 10 %). Only one adult center indicated they had no programs or resources available to assist patients. Both adult and pediatric centers indicated that social workers were a resource for providing patients with information and for helping address housing issues.
Transplant centers also provided housing assistance to patients (40 % of adult centers and 10 % of pediatric centers). For adult centers, assistance came in the form of grants, donated funds, contracted rates for hotels, and rent for local apartments being determined on a sliding fee scale based on patient income. Requirements to receive this assistance included income or financial need, insurance contracting, distance from the hospital, and diagnosis. Amounts available varied by center, and included specific dollar amounts and amounts based on patient need (e.g., “pending need and available funds”).
Pediatric centers provided assistance with Ronald McDonald House nightly costs (paying them directly), as well as connected patients to funds that could assist with expenses at home. For requirements, one center had a “financial approval process,” and another indicated that the requirement was “income eligibility.”
Additional solutions that centers developed to address housing issues were identified; common themes and illustrative quotes are identified in Table 2.
Table 2.
Themes and selected quotes: solutions centers have implemented to address housing issues
| Theme | Adult transplant center responses (n=43) | Pediatric transplant center responses (n=19) |
|---|---|---|
| Patient education and navigation |
|
|
| Collaboration with community and national organizations |
|
|
| Collaboration with local facilities |
|
|
| Staff and infrastructure |
|
|
| Financial assistance |
|
|
The survey also asked about what respondents thought solutions were to address housing barriers. Respondents identified additional housing for patients, provided either on or off-hospital grounds, as a solution. Other proposed solutions focused on removing housing requirements, placing patients on housing waiting lists sooner in the transplant process, and having a dedicated housing coordinator to assist patients.
Discussion
Our study systematically explores the prevalence of housing challenges faced by patients undergoing HCT from the perspective of social workers and highlights solutions that centers use to address them. Our study showed that nearly all transplant centers had patients who needed temporary relocation and local housing to proceed with transplantation. Actual numbers of patients who were denied transplantation due to housing barriers were not reflected in the survey. Focus group results suggested that denial was rare, but two participants said delays due to housing barriers combined with insurance coverage changes or lack of informal resources had occurred.
The need for temporary relocation is largely dictated by transplant center requirements for their patients. Most centers had some distance/time requirements for which patients could stay at their primary residence versus those who needed to relocate and stay close to the transplant center. However, there was variation among centers for distance/time guidelines and duration post-transplant for which these guidelines applied, which may partly reflect local commuting and geographic considerations. Requirements also differed by transplant type (allogeneic versus autologous), reflecting the quicker recovery that occurs in autologous HCT recipients. It is unclear what might be an optimal distance threshold and time period for staying near a center. Different geographic areas may present different challenges and opportunities. For example, in a densely populated area, it may take an hour to cross a city, or, as suggested in the focus groups, events (such as sporting events, concerts, etc.) in cities can affect the amount of time/distance a patient may have to travel. A previous study by Abou-Nassar et al. found that long driving time to the center was associated with decreased overall survival [8], though the center’s requirements were not examined. Further research is needed to determine if differences in distance and time requirements affect patient outcomes.
Two of the major barriers that social workers identified their patients as having were lack of insurance coverage for housing needs and the cost/affordability of temporary housing. A systems-level approach is needed to advocate for housing coverage, including policies which mandate appropriate housing benefits, and for insurers and re-insurers to evaluate benefit packages to accommodate the widespread need for housing coverage, which would allow patients and their families to focus on treatment rather than the additional expense and other barriers associated with temporary lodging. Transplant centers need to continue to work with local and regional or national organizations to develop cost-effective solutions. It is also important to identify ways to help lower costs for patients, including working with hotels/motels on contracting for lower rates. Transplant centers could also work within their hospitals or with partners to increase availability or housing capacity, or possibly analyze their current residency requirements for HCT patients. In one of the focus groups for example, a participant mentioned that their center changed their distance requirement from 60 to 90 miles after receiving numerous requests from patients and an analysis by their physicians that showed neutral effects of distance on patient outcomes.
Both in the focus groups and survey, we learned that centers have multiple resources and services available to help their patients and that the social workers work to identify solutions based on those services. Social workers also work closely with the patient to identify resources they may not readily identify in their own community or among their family and friends. This is an area for future research.
While the two most frequent barriers faced by pediatric patients identified by the respondents were insurance coverage and cost/affordability, it is of note that 20 % of pediatric centers indicated that all of their allogeneic HCT recipients and 16 % of autologous recipients faced restrictions on who can use local housing options (e.g., number of people allowed to stay, financial requirements, or background checks). Additionally, a few of the adult center respondents indicated that it was the availability of a caregiver that limited access to transplant, rather than housing; one respondent pointed out the availability of a caregiver to relocate with the patient can be a barrier.
Another barrier was that housing options were full or had long waiting lists. The timing of when social workers discuss housing with patients (e.g., at time of initial transplant center consultation versus at work-up), could be studied to see if it impacts the timeline of placing patients on housing lists.
Some limitations of our study have to be considered. Responses may not be generalizable to all centers, though respondents and non-respondents were fairly similar in geographic region and volume, with slightly more non-respondents from the South. Many of the questions asked about the social worker’s experience over the previous year, so responses may be subject to recall bias. Our study presents the perspective of social workers and not patients. Previous research has used focus groups and surveys of health care providers to learn about patient barriers in areas other than HCT [19, 20]. However, our study lays the foundation for more research that can be directed toward patients.
In conclusion, our study highlighted housing-associated barriers and potential solutions for patients undergoing HCT. Identification of barriers may reveal specific factors that can be modified to potentially improve access to HCT. Transplant centers and social workers can benefit from this knowledge by learning how other centers have addressed housing barriers and consider collaborative efforts on developing ways to overcome these barriers.
Acknowledgments
Our sincere thanks to the National Marrow Donor Program’s System Capacity Initiative Program for supporting this study. We also thank William Vaughn, MD (University of Alabama) for participating in the study protocol team, Linda Burns, MD (NMDP) for reviewing and providing comments on the draft manuscript, Diane W. Carr, MPH and Viengneesee Thao, MS (NMDP) for their help in reaching out to potential survey participants, and Tammy Payton (NMDP) for her coding of focus group responses. Finally, we thank the social workers who participated in the focus groups and survey.
Footnotes
Competing interests The authors declare that they have no competing interests.
Disclosure CIBMTR® (Center for International Blood and Marrow Transplant Research®) is a research collaboration between the National Marrow Donor Program®/Be The Match® and Medical College of Wisconsin. The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from AABB; Allos, Inc.; Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US, Inc.; Be the Match Foundation; Biogen IDEC; BioMarin Pharmaceutical, Inc.; Biovitrum AB; BloodCenter of Wisconsin; Blue Cross and Blue Shield Association; Bone Marrow Foundation; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; ClinImmune Labs; CTI Clinical Trial and Consulting Services; Eisai, Inc.; Genentech, Inc.; Genzyme Corporation; Histogenetics, Inc.; HKS Medical Information Systems; Hospira, Inc.; Kirin Brewery Co., Ltd.; The Leukemia & Lymphoma Society; Merck & Company; The Medical College of Wisconsin; Millennium Pharmaceuticals, Inc.; Miller Pharmacal Group; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Nature Publishing Group; Novartis Oncology; Oncology Nursing Society; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; Pall Life Sciences; Pfizer Inc; Schering Corporation; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, Inc.; StemSoft Software, Inc.; Sysmex America, Inc.; THERAKOS, Inc.; Vidacare Corporation; ViraCor Laboratories; ViroPharma, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institutes of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
The Health Services Research program is supported in part by Health Resources and Services Administration Contract No. HHSH234200637018C. The views expressed in this article do not reflect the official policy or position of the Health Resources and Services Administration or the National Marrow Donor Program/Be The Match®.
References
- 1.Pasquini MC, Wang Z. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR summary slides 2012 [Google Scholar]
- 2.Majhail NS, Murphy EA, Denzen EM, Stickney Ferguson S, Anasetti C, Bracey A, Burns L, Champlin R, Hubbard N, Markowitz M, Maziarz RT, Medoff E, Neumann J, Schmit-Pokorny K, Weisdorf DJ, Yolin Raley DS, Chell J, Snyder EL. The national marrow donor Program’s symposium on hematopoietic cell transplantation in 2020: a health care resource and infrastructure assessment. Biol Blood Marrow Transplant. 2012;18:172–182. doi: 10.1016/j.bbmt.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Majhail NS, Murphy EA, Omondi NA, Robinett P, Gajewski JL, LeMaistre CF, Confer D, Rizzo JD. Allogeneic transplant physician and center capacity in the United States. Biol Blood Marrow Transplant. 2011;17:956–961. doi: 10.1016/j.bbmt.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denzen EM, Majhail NS, Stickney Ferguson S, Anasetti C, Bracey A, Burns L, Champlin R, Chell J, Leather H, Lill M, Maziarz RT, Medoff E, Neumann J, Schmit-Pokorny K, Snyder EL, Wiggins L, Yolin Raley DS, Murphy EA. Hematopoietic cell transplantation in 2020: summary of year 2 recommendations of the National Marrow Donor Program’s system capacity initiative. Biol Blood Marrow Transplant. 2013;19:4–11. doi: 10.1016/j.bbmt.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, Hartzman R, Rizzo JD, Horowitz M, Confer D, Maiers M. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014;371:339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majhail NS, Rizzo JD, Hahn T, Lee SJ, McCarthy PL, Ammi M, Denzen E, Drexler R, Flesch S, James H, Omondi N, Pedersen TL, Murphy E, Pederson K. Pilot study of patient and caregiver out-of-pocket costs of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2012 doi: 10.1038/bmt.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khera N, Chang Y-H, Hashmi S, Slack J, Beebe T, Roy V, Noel P, Fauble V, Sproat L, Tilburt J, Leis JF, Mikhael J. Financial burden in recipients of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:1375–1381. doi: 10.1016/j.bbmt.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Nassar KE, Kim HT, Blossom J, Ho VT, Soiffer RJ, Cutler CS, Alyea EP, Koreth J, Antin JH, Armand P. The impact of geographic proximity to transplant center on outcomes after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:708–715. doi: 10.1016/j.bbmt.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore HK, Burton Santibañez ME, Denzen EM, Carr DW, Murphy EA. Barriers to accessing health care for hematopoietic cell transplantation recipients living in rural areas: perspectives from healthcare providers. Clin J Oncol Nurs. 2013;17:405–411. doi: 10.1188/13.CJON.405-411. [DOI] [PubMed] [Google Scholar]
- 10.Martin-McDonald K, Rogers-Clark C, Hegney D, McCarthy A, Pearce S. Experiences of regional and rural people with cancer being treated with radiotherapy in a metropolitan centre. Int J Nurs Pract. 2003;9:176–182. doi: 10.1046/j.1440-172x.2003.00421.x. [DOI] [PubMed] [Google Scholar]
- 11.Untalan FF, Woodruff K, Liao M, Hardy C. Stressors associated with pacific islands children diagnosed with cancer and severe blood disorders. J Pediatr Oncol Nurs. 2004;21:40–50. doi: 10.1177/1043454203262002. [DOI] [PubMed] [Google Scholar]
- 12.Preussler JM, Farnia SH, Denzen EM, Majhail NS. Variation in medicaid coverage for hematopoietic cell transplantation. J Oncol Pract. 2014;10:e196–200. doi: 10.1200/JOP.2013.001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaughn SR, Schumm JS, Sinagub JM. Focus group interviews in education and psychology. 1. Sage Publications Inc; Thousand Oaks, CA: 1996. [Google Scholar]
- 14.Stewart DW, Shamdasani PN, Rook D. Focus groups: theory and practice. 2. Sage Publications Inc; Thousand Oaks, CA: 2006. [Google Scholar]
- 15.MacQueen KM, McLellan E, Kay K, Milstein B. Codebook development for team-based qualitative analysis. Field Methods. 1998;10:31–36. doi: 10.1177/1525822X980100020301. [DOI] [Google Scholar]
- 16.Carey JW, Morgan M, Oxtoby MJ. Intercoder agreement in analysis of responses to open-ended interview questions: examples from tuberculosis research. Field Methods. 1996;8:1–5. doi: 10.1177/1525822X960080030101. [DOI] [Google Scholar]
- 17.Ronald McDonald House. [Accessed 30 Jan 2015]; http://www.rmhc.org/ronald-mcdonald-house.
- 18.Support Programs & Services. [Accessed 30 Jan 2015];Hope Lodge. http://www.cancer.org/treatment/supportprogramsservices/hopelodge/index.
- 19.Burg MA, Zebrack B, Walsh K, Maramaldi P, Lim J-W, Smolinski KM, Lawson K. Barriers to accessing quality health care for cancer patients: a survey of members of the association of oncology social work. Soc Work Health Care. 2010;49:38–52. doi: 10.1080/00981380903018470. [DOI] [PubMed] [Google Scholar]
- 20.Simmons VN, Jiménez JC, Castro E, Litvin EB, Gwede CK, Vadaparampil ST, Mclntyre J, Meade CD, Brandon TH, Quinn GP. Initial efforts in community engagement with health care providers: perceptions of barriers to care for cancer patients in Puerto Rico. P R Health Sci J. 2011;30:28–34. [PMC free article] [PubMed] [Google Scholar]