Abstract
The use of human albumin is common in hepatology since international scientific societies support its administration to treat or prevent severe complications of cirrhosis, such as the prevention of post-paracentesis circulatory dysfunction after large-volume paracentesis and renal failure induced by spontaneous bacterial peritonitis, and the treatment of hepatorenal syndrome in association with vasoconstrictors. However, these indications are often disregarded, mainly because the high cost of human albumin leads health authorities and hospital administrations to restrict its use. On the other hand, physicians often prescribe human albumin in patients with advanced cirrhosis for indications that are not supported by solid scientific evidence and/or are still under investigation in clinical trials.
In order to implement appropriate prescription of human albumin and to avoid its futile use, the Italian Association for the Study of the Liver (AISF) and the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) nominated a panel of experts, who reviewed the available clinical literature and produced practical clinical recommendations for the use of human albumin in patients with cirrhosis.
1. Introduction
The clinical use of human albumin (HA) dates back to World War II when it was administered for fluid resuscitation. Since then, its administration has been extended to many other diseases since physicians commonly believe in its efficacy. However, apart from some clinical indications for which its use is supported by solid scientific evidence, in many other settings the efficacy of HA has been disproven by evidence-based medicine or is still under debate.
Hepatology is an area in which the use of HA is particularly common, since this treatment is currently employed to treat or prevent severe complications of cirrhosis. Randomised clinical trials and meta-analyses have demonstrated its efficacy in the prevention of post-paracentesis circulatory dysfunction (PPCD) after large-volume paracentesis and renal failure induced by spontaneous bacterial peritonitis (SBP), and in the treatment of hepatorenal syndrome (HRS) in association with vasoconstrictors. Although endorsed by international scientific societies1,2, these indications are often disregarded, even in specialised centres, mainly because the high cost of HA leads health authorities and hospital administrations to restrict its use. On the other hand, physicians often prescribe HA in patients with advanced cirrhosis for indications that are not supported by solid scientific evidence and/or are still under investigation in clinical trials. This inappropriate use may limit the availability for the patients in whom HA administration is supported by solid scientific evidence.
In order to implement appropriate prescription of HA and to avoid its futile use, the Italian Association for the Study of the Liver (AISF) and the Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) nominated a panel of experts, who reviewed the available clinical literature and produced practical clinical recommendations for the use of HA in patients with liver cirrhosis. The initial draft was revised by a second panel of experts identified by the two scientific societies, so that the final version resulted from the consensus of the two working groups.
The level of evidence and strength of recommendation were judged according to the Grading of Recommendations Assessment Development and Evaluation (GRADE) system3. The strength of the evidence has been classified into four levels: high (A), moderate (B), low (C), and very low (D) quality evidence, while that of the recommendation has been divided into two: strong (1) and weak (2) (Table I). Where no clear evidence exists, the recommendations are based on the consensus advice of the writing committee and the expert opinion(s) reported in the literature.
Table I.
Grading evidences and recommendations (adapted from the GRADE system).
| Quality of evidence | |
|---|---|
| A - High | Further research is very unlikely to change our confidence in the estimate of effect.
|
| B - Moderate | Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
|
| C - Low | Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
|
| D - Very low | Any estimate of effect is very uncertain. Expert opinion
|
|
| |
| Strenght of recommendation | |
|
| |
| 1 - Strong | Factors influencing the strength of the recommendation included the quality of evidence, presumed patient-important outcomes, and cost. |
| 2 - Weak | Variability in preferences and values, or more uncertainty: more likely a weak recommendation is warranted. Recommendation is made with less certainty: high cost or resource consumption |
2. The albumin molecule
2.1 Structure
HA is the main circulating protein in healthy individuals (3.5–5 g/dL), accounting for about 50% of the plasma proteins. It is a small protein (molecular weight: 66.5 kDa), consisting of a single chain of 585 amino acids organised in three repeated homologues domains (I, II, and III), each of which comprises two separate sub-domains (A and B). Of the 35 cysteine residues of the molecule, 34 are involved in internal disulphide bonds which stabilise the spatial conformation of the molecule, while the cysteine at position 34 (Cys-34) remains free in the reduced form, thus representing the major functional site of HA4.
2.2 Metabolism
HA is synthesised by hepatocytes and released into the circulation (about 10–15 grams every day). Its synthesis is stimulated by hormonal factors, such as insulin, cortisol and growth hormone, while pro-inflammatory mediators exert an inhibitory effect. Once produced, approximately 30–40% circulates in the blood stream, while the remaining leaves the vascular compartment at a rate of 5% per hour (transcapillary escape rate) and returns to it via the lymphatic system; the amount that returns to the vascular compartment is the same as the amount that leaves it. The circulatory half-life of HA is approximately 16–18 hours, while its total half-life varies from about 12 to 19 days in healthy young adults. HA is mainly degraded by the muscles, liver and kidneys, although many other tissues can participate in its catabolism4–6.
2.3 Properties
HA is the main modulator of fluid distribution in the various compartments of the body accounting for about 70–80% of the plasma oncotic pressure. Two-thirds of the oncotic capacity derives from the direct osmotic effect related to its molecular mass and high plasma concentration while one-third is the results of the Gibbs-Donnan effect, due to the negative net charge of the molecule that is consequently able to attract positively charged molecules (i.e. sodium and, therefore, water) into the bloodstream4–6.
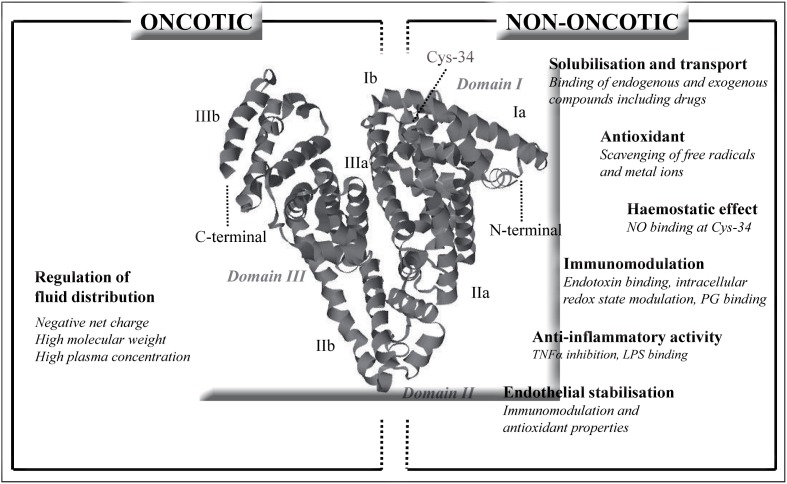
HA has many other biological properties that are unrelated to the regulation of fluid compartmentalisation (Figure 1). Some of these non-oncotic properties assume particular importance, such as scavenging and detoxification of reactive oxygen and nitrogen species, binding and transport of many hydrophobic endogenous molecules (e.g., cholesterol, fatty acids, bilirubin, thyroxine) and exogenous ones (e.g., drugs, including many antibiotics), preservation of the functional integrity of the microcirculation (e.g., endothelial stabilisation and platelet anti-aggregation), and modulation of the immune and inflammatory responses (e.g., binding of endotoxins, prostaglandins, and pro-inflammatory cytokines)4,5.
Figure 1.
Oncotic and non-oncotic properties of human albumin.
Cys-34: cysteine-34; LPS: lipopolysaccharides: NO: nitric oxide; PG: prostaglandins; TNFα: tumour necrosis factor-alpha.
3. Rationale for the use of human albumin in liver cirrhosisis
Hypoalbuminaemia is a typical feature of cirrhosis and is an independent adverse prognostic factor. Beside decreased hepatocyte synthesis, it results from dilution of the extracellular fluid protein content due to the total plasma volume expansion consequent to renal sodium and water retention, from increased catabolism probably accelerated by the damage of the molecule, and from the increased trans-capillary escape rate towards the extravascular space, at least in patients with refractory ascites4,5.
In addition to quantitative changes, HA undergoes structural and functional alterations that are probably favoured by the pro-inflammatory and pro-oxidant state of advanced cirrhosis6,7. Extensive post-transcriptional changes, involving several sites of the molecule, develop and increase in parallel with the disease severity7. Physiological functions of HA appear to be impaired, including the ability to chelate cobalt and binding and transport capacities8.
It can, therefore, be hypothesised that, in patients with cirrhosis, the global function of HA, resulting from both oncotic and non-oncotic properties, is not only related to its absolute concentration in the circulation, but also to the degree of preservation of its structural and functional integrity5–8.
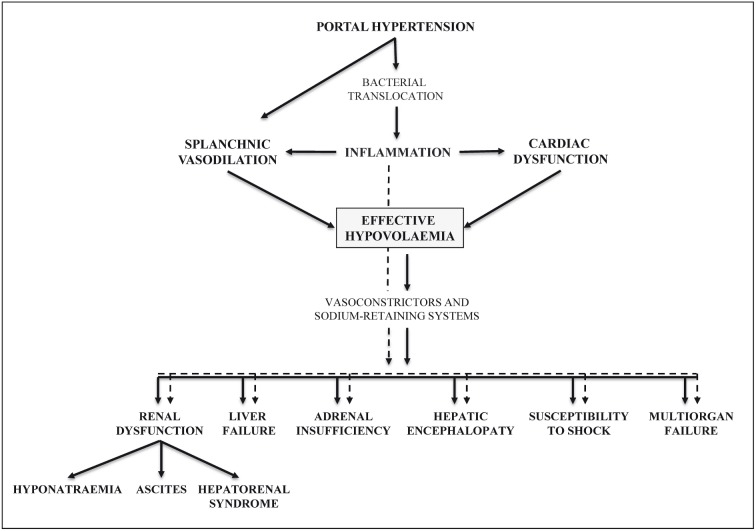
Advanced cirrhosis is characterised by two major systemic features: circulatory dysfunction and chronic inflammation. These alterations are strictly inter-related and cooperate to cause the multi-organ dysfunction and failure occurring in end-stage cirrhosis (Figure 2)5.
Figure 2.
Major pathophysiological events in advanced liver cirrhosis.
The cardiovascular alterations involve both the circulation and the heart9. The dominant haemodynamic change is a progressive reduction of effective arterial volaemia. Effective hypovolaemia mainly results from the fall of systemic vascular resistance, mostly in the splanchnic area, due to the increased production of vasoactive substances (e.g. nitric oxide, carbon monoxide, endocannabinoids), inducing vasodilation and hampering the response to vasoconstrictors. This evokes the compensatory activation of neuro-humoral systems able to promote vasoconstriction and renal retention of sodium and water, including the renin-angiotensin-aldosterone axis (RAA), sympathetic nervous system (SNS), and arginine-vasopressin (ADH). As a result, from a functional point of view, patients with decompensated cirrhosis are hypovolaemic and exhibit cardiovascular hyporeactivity, even though their cardiac output can be normal or elevated. However, a fall in cardiac output, leading to an exacerbation of effective hypovolaemia, is observed in the more advanced stages of the disease, indicating the occurrence of clinically relevant cardiac dysfunction. In the end-stage of the disease, the extreme effective hypovolaemia directly induces a further reduction in the perfusion of kidneys and other organs, thus generating ischaemic tissue damage until multi-organ failure develops. In this pathophysiological scenario, preservation of the effective blood volume is a primary goal in the management of these patients.
Patients with decompensated cirrhosis also have a chronic state of moderate systemic inflammation, which is related to the sustained stimulation of the immune system cells activated by the translocation of bacterial products (e.g., lipopolysaccharide, bacterial DNA) from the intestinal lumen to the circulation due to quantitative and qualitative changes in gut microbiota, impairment in the intestinal mucosal barrier, increased epithelial permeability, and impaired intestinal immunity10. In a vicious circle, the cytotoxic agents released during the inflammatory process contribute to produce splanchnic vasodilation and depress cardiac contractility, thus exacerbating circulatory dysfunction10. They can also contribute directly to organ dysfunction by inducing microvascular coagulation and cell damage10.
While systemic inflammation and oxidative stress are steadily moderate in patients with decompensated cirrhosis, they become rapidly severe in patients with acute-on-chronic liver failure6. Acute-on-chronic liver failure is a clinical syndrome characterised by acute development of organ failure (of liver, kidneys, brain, lungs, coagulation, and circulation) and a short-term, high risk of mortality, usually precipitated by bacterial infection, acute alcoholic hepatitis or other clinical events, even if a specific cause cannot be identified in a considerable number of cases11.
Due to its oncotic and non-oncotic properties, HA can exert a beneficial effect at different steps of the vicious circle that links circulatory dysfunction, inflammatory response, and oxidative stress in patients with decompensated cirrhosis.
4. Clinical use of albumin in cirrhosis
HA is given to patients with advanced cirrhosis with the purpose of antagonising the effective hypovolaemia, mainly because of its capacity to act as a potent plasma-expander by increasing cardiac output and refilling the dilated arterial vascular compartment, although some positive effects on circulatory function and cardiac contractility also appear to be mediated by its non-oncotic properties12,13.
International guidelines currently endorse the use of HA to manage clinical complications of cirrhosis characterised by extreme effective hypovolaemia: (i) prevention of PPCD; (ii) prevention of renal failure induced by SBP; and (iii) diagnosis and treatment of HRS, in association with vasoconstrictor drugs1,2. A consequent assumption is that the presence of hypoalbuminaemia should not be a pre-requisite for the administration of exogenous HA in these conditions.
Beside these universally accepted and evidence-based indications, the administration of HA has been proposed for other clinical conditions in patients with cirrhosis. Long-term treatment of ascites not responding to diuretic treatment is allowed in Italy by the Italian Medicines Agency (AIFA), even if solid scientific evidence supporting its use in this context has not been published as yet. Treatment of hyponatraemia is another example of HA use not based on evidence. Other clinical indications currently under investigation in patients with cirrhosis are treatment of bacterial infections other than SBP, hepatic encephalopathy, and septic shock (Table II).
Table II.
Summary of the panel’s indications for the use of HA in patients with cirrhosis.
| Clinical condition | Doses and schedules of administration | Indications for the use of HA | Quality of evidence and strenght of recommendation | |
|---|---|---|---|---|
| Prevention of PPCD | Paracentesis ≥5 litres | 6–8 g per litre of removed ascites | Mandatory in all patients | A1 |
| Paracentesis <5 litres | Preferred if concerns regarding use of synthetic colloids or crystalloids | B1 | ||
| Prevention of renal failure after SBP | High-risk patients | 1.5 g/kg at diagnosis + 1 g/kg on the 3rd day |
Mandatory in all patients | A1 |
| Low-risk patients* | Consider in individual patients | B1 | ||
| Diagnosis of HRS | 1 g/kg/die for 2 consecutive days |
To be used regularly | D1 | |
| Treatment of type I HRS (in association with vasoconstrictors) |
1 g/kg at diagnosis + 20–40 g/die until vasoconstrictors are stopped |
Mandatory in all patients | A1 | |
| Long-term treatment of ascites | To be defined | Consider in difficult-to-treat ascites | C1 | |
| Treatment of severe hyponatraemia | To be defined | Consider if no response to standard measures | D1 | |
| Prevention of renal failure after non-SBP bacterial infections | - | Not indicated at present | B1 | |
| Treatment of septic shock | To be defined | Consider in all patients | C1 | |
| Treatment of hepatic encephalopathy | - | Not indicated at present | B1 | |
Low-risk patients: serum albumin <4 mg/dL and serum creatinine <1 mg/dL.
HA: human albumin; PPCD: post-paracentesis circulatory dysfunction; HRS: hepatorenal syndrome; SBP: spontaneous bacterial peritonitis.
AISF-SIMTI recommendations
- In the specific setting of patients with advanced cirrhosis, the presence of hypoalbuminaemia should not be the guide to the prescription of HA (B1).
- As in other clinical settings, in patients with advanced cirrhosis, hypoalbuminaemia per se is not an indication for the prescription of HA (B1).
5. Evidence-based clinical indications
5.1 Prevention of post-paracentesis circulatory dysfunction
5.1.1 Clinical and pathophysiological background
Paracentesis is the current first-line treatment for patients with tense and refractory ascites1,2. In the majority of patients not receiving plasma expansion, the removal of large volumes of ascites induces the development of PPCD, which is diagnosed by a significant increase (>50%) in plasma renin activity 4–6 days after paracentesis14. PPCD is associated with a higher rate of recurrence of the ascites, dilutional hyponatraemia, renal impairment, re-hospitalisation, and poorer survival14–16.
By reducing intra-abdominal pressure, large-volume paracentesis boosts venous return to the heart. As a result, right atrial pressure decreases, while cardiac output and stroke volume increase. However, because of a paradoxically excessive drop of systemic vascular resistance, effective circulating volume declines further, leading to a reduction in arterial pressure. In the days after paracentesis, there is marked activation of the RAA axis and SNS, which persists in some cases for months14,15,17–19.
5.1.2 Albumin for preventing post-paracentesis circulatory dysfunction
The most effective method to prevent PPCD is to counteract effective hypovolaemia by using plasma-expanders. It was first demonstrated in the 1980s that infusion of HA after paracentesis improves circulatory dysfunction and prevents the occurrence of PPCD14.
Since the early 1990s, less costly alternatives, such as crystalloids and artificial colloid volume expanders, have been compared to HA15,20–26. When more than 5 L of ascites are removed, HA (6–8 g/L of ascites removed) has been found to more effective at preventing PPCD than the other plasma expanders in several studies15,20,25,26, although other investigations - all including very low numbers of patients - did not confirm this positive result21–24.
In contrast, when less than 5 L of ascites are removed, the incidence of PPCD is low and dextran-70 (8 g/L of ascites removed), polygeline (150 mL/L of ascites removed) or saline (150 mL/L of ascites removed) show an efficacy similar to that of HA15,25. However, polygeline is no longer used in many countries because of the potential risk of transmission of prions1, while there are concerns about the possibility that dextrans and starches may induce renal failure27 and accumulate in the liver28. Furthermore, infusion of large volumes of saline should be avoided in fluid-overloaded patients with ascites and/or peripheral oedema.
More recently, the use of vasoconstrictors, including vasopressin, terlipressin, and midodrine, has been compared to the administration of HA after paracentesis29–33. However, the results of the few randomised clinical trials are variable, probably because of their very limited sample size, so that the clinical utility of vasopressors after paracentesis cannot be ascertained.
Despite this greater efficacy in preventing PPCD, randomised trials have not shown differences in the survival of patients receiving HA compared with that of patients given alternative treatments15,25,34. However, a recent meta-analysis indicates that HA after large-volume paracentesis is significantly more effective than other treatments at reducing not only PPCD and hyponatraemia, but also mortality35. Effect sizes were substantial, with HA lowering the odds of PPCD by 66%, hyponatraemia by 42%, and death by 36%. In all eligible trials included, the average volume of ascites removed was greater than 5 L and no major differences were apparent according to whether volumes of ascites removed were 5.5–8.0 L or more than 8 L. In the majority of studies included, the dose of HA administered per litre of ascites removed was 8 g, although 5 or 6 g were also infused in a minority of trials.
Only one randomised trial considering HA infusion after large-volume paracentesis compared standard (8 g/L of ascites removed) versus reduced (4 g/L of ascites removed) doses. The incidence of PPCD, hyponatraemia, and renal failure were low, but similar in the two groups. Although, if confirmed, these results could support a reduction of the costs related to paracentesis, the small sample size again precludes definitive conclusions36.
Finally, a recent health economic analysis suggested that HA is more cost-effective than alternative but cheaper plasma volume expanders since its administration is associated with a lower number of liver-related complications within the first 30 days34.
AISF-SIMTI recommendations on the use of albumin to prevent post-paracentesis circulatory dysfunction
- HA should be administered after large-volume paracentesis exceeding 5 L at the dose of 6–8 g/L of ascites removed, since it reduces the incidence of PPCD and improves patients’ clinical outcome (A1).
- When the amount of ascites removed exceeds 5 L, the use of alternative plasma expanders is not recommended because they are less effective in the prevention of PPCD (A1). The combined use of HA and other plasma expanders in order to reduce the dose of HA is also not recommended (D1).
- When the amount of ascites removed is less than 5 L, HA can be used if there are concerns regarding the administration of crystalloids or synthetic colloids (volume overload, renal failure, coagulopathy) (B1).
- The use of vasoconstrictors instead of HA or the use of reduced doses of HA should be limited to controlled clinical trials (C1).
- Although there are no studies on the modalities of albumin administration, it seems advisable to infuse HA relatively slowly to avoid possible cardiac overload due to the existence of a latent cirrhotic cardiomyopathy, starting during the presumed final part of the paracentesis or at the end of paracentesis when the volume of ascites removed is known and the paracentesis-induced increase in cardiac output begins to return to baseline (D2).
5.2 Prevention of renal failure after spontaneous bacterial peritonitis
5.2.1 Clinical and pathophysiological background
SBP is a life-threatening infection of ascitic fluid that occurs frequently in patients with advanced cirrhosis and requires prompt recognition and treatment. The diagnosis is based on the presence of >250 polymorphonuclear cells/mm3 of ascites, in the absence of an intra-abdominal source of infection or malignancy37. About one-third of patients with SBP develop renal impairment, which is often progressive irrespectively of resolution of the infection and is an independent predictor of in-hospital mortality38,39, as 42% of patients with this complication will die, while the mortality of those who do not develop renal impairment is only 7%38.
The high incidence of renal failure after SBP is caused by the abrupt deterioration of circulatory function, involving both vascular tone and cardiac function, and is mediated by the marked activation of the pro-inflammatory and vasoactive systems. While systemic vascular resistance remains substantially unchanged, possibly as a result of a striking compensatory activation of vasoconstrictor systems, cardiac output declines significantly as a consequence of chronotropic incompetence and reduced myocardial contractility. These events ultimately lead to a marked reduction of effective volaemia and arterial pressure, thus impairing renal and, more in general, organ perfusion40.
5.2.2 Albumin for preventing renal failure after spontaneous bacterial peritonitis
Administration of HA, but not hydroxyethyl starch, improves circulatory dysfunction in patients with SBP12. Along with parameters reflecting plasma volume expansion, other haemodynamic effects can be explained only recalling the non-oncotic properties of the molecule. In fact, the striking rise in systemic vascular resistance seen after HA, but not after hydroxyethyl starch infusion, and the concomitant significant reduction of the circulating levels of von Willebrand-related antigen, factor VIII, and nitric oxide metabolites, indicate that HA counteracts endothelial activation12. Furthermore, the improvement of stroke index supports a direct effect of HA on cardiac function12, as also suggested by the experimental finding in rats with cirrhosis and ascites that plasma volume expansion with HA, but not with hydroxyethyl starch, ameliorates left ventricular function ex vivo13.
The first controlled clinical trial assessing the effect of HA administration in patients with cirrhosis and SBP showed that such treatment, associated with antibiotic therapy, significantly reduced the incidence of renal failure and the in-hospital and 3-month mortality rates41. In more detail, 126 patients were randomised to receive either HA 1.5 g/kg/body weight (b.w.) at diagnosis and 1 g/kg b.w. on day 3 in addition to cefotaxime or cefotaxime alone. Although infection resolution rates were similar in the two groups, the incidence of renal impairment dropped from 33% in patients receiving only cefotaxime to 10% in those treated with cefotaxime plus HA. Baseline independent predictors of the development of renal impairment included serum bilirubin and creatinine, and treatment with cefotaxime alone. Confirming that the occurrence of renal failure in the setting of SBP carries a highly adverse prognostic factor, 29% of patients in the cefotaxime group died in hospital and 41% within 3 months. These figures were strikingly reduced by HA administration, as in-hospital and 3-months mortality rates were 10% and 22%, respectively.
Post-hoc analysis showed that the incidence of renal impairment was higher among those with a baseline serum bilirubin ≥4 mg/dL (48% in the cefotaxime group and 12% in cefotaxime plus HA group) or serum creatinine ≥1 mg/dL (32% and 14%, respectively) than in patients with serum bilirubin <4 mg/dL and serum creatinine < 1 mg/dL (7% and 0%, respectively).
This observation raises the question of whether all patients with SBP need HA administration. In a preliminary, small study, patients with SBP at low-risk of renal impairment (defined as bilirubin <4 mg/dL and serum creatinine <1 mg/dL) received only antibiotic treatment and none developed renal impairment or died42. In a more recent retrospective study43, episodes of “low-risk” SBP were associated with a much lower incidence of renal failure as well as lower in-hospital and 3-month mortality rates compared with “high-risk” episodes (4.7%, 3.1% and 7% vs 25.6%, 38.2% and 47%, respectively). Among the latter, those treated with HA had a lower in-hospital mortality than those treated only with antibiotics (28.8% vs 46.8%) and a higher probability of survival at 3 months (62% vs 45%). Thus, HA administration clearly improves the survival of patients with high-risk SBP, but it does not seem to be necessary for patients with low-risk SBP. However, this conclusion awaits confirmation in a randomised, prospective study.
Similarly, confirmation is also needed for the results of a pilot study that assessed whether a lower dose of HA could be used44. In the pilot study, a reduced dose regimen (1.0 g/kg b.w. at diagnosis and 0.5 g/kg b.w. on day 3) appeared to be as effective as the standard regimen in preventing renal failure in a group of cirrhotic patients with SBP including 77% at “high-risk”. In-hospital (27% vs 21%) and 3-month mortality (36% vs 37%) rates also did not differ between patients receiving the reduced or the standard HA dose, respectively.
Finally, a recent meta-analysis of randomised trials substantially confirmed that HA infusion prevents renal impairment and reduces mortality among patients with SBP45. However, there is still limited evidence available on the outcomes of patients with low-risk SBP who do not receive HA as well as on the responsiveness of low-risk patients to HA infusion.
AISF-SIMTI recommendations on the use of albumin to prevent renal failure after spontaneous bacterial peritonitis
- HA (1.5 g/kg/b.w. at diagnosis and 1 g/kg/b.w. on day 3) should be administered, in association with antibiotic therapy, in cirrhotic patients with SBP since this approach reduces the incidence of renal failure and improves survival (A1).
- Patients with baseline serum bilirubin <4 mg/dL and serum creatinine <1 mg/dL have a low risk of developing renal failure after SBP. In this group of patients the benefit of HA is unclear and the decision to administer HA should be individualised (B1).
- The use of crystalloids and synthetic colloids instead of HA or in association to HA is not recommended (D1).
- The use of reduced doses of HA should be limited to controlled clinical trials (Level C1).
5.3 Diagnosis and treatment of hepatorenal syndrome
5.3.1 Clinical and pathophysiological background
HRS is defined as the occurrence of renal failure in patients with advanced liver disease without another, identifiable cause46–48. Type 1 HRS is a rapidly progressive acute renal failure that frequently develops in temporal relationship with a precipitating factor, such as a bacterial infection. Its prognosis is dismal since the median survival without treatment is approximately 2 weeks. Type 2 HRS occurs in patients with refractory ascites and is characterised by a moderate, slowly progressive degree of functional renal failure, often with avid sodium retention. Patients with type 2 HRS may eventually develop type 1 HRS either spontaneously or following a precipitating event, such as SBP46,47.
Marked renal arterial vasoconstriction is the main pathophysiological feature of type 1 HRS. It develops in the context of a severe reduction of the effective circulating volume, which is related both to splanchnic arterial vasodilation and to an inadequate cardiac output, leading to extreme over-activation of the endogenous vasoconstrictor and sodium-retaining systems (RAA, SNS, and ADH)47,49.
5.3.2 Albumin for the diagnosis
The diagnosis of HRS is one of exclusion. Besides excluding organic causes, the diagnosis is based on the lack of response to volume expansion in order to differentiate HRS from other forms of functional renal failure46–48. No studies have been performed to establish whether plasma volume expansion should be performed with HA rather than saline. Members of the panel of the International Club of Ascites have agreed that HA causes a greater and more sustained expansion than saline47,48, as it can be assumed by the studies on PPCD showing the superiority of HA over saline or synthetic colloids.
5.3.3 Albumin for the treatment of type 1 hepatorenal syndrome
Most of the existing information on treatment of HRS is related to patients with type 1 HRS. Therefore, all the following comments refer to type 1 HRS unless otherwise specified. Once diagnosed, treatment should be started early in order to prevent the progression of renal failure.
The most effective treatment of HRS currently available is the administration of vasoconstrictor drugs together with HA. Terlipressin, a vasopressin analogue acting on V1 receptors, mainly located in the splanchnic area, is the most studied vasoconstrictor. It improves the markedly impaired circulatory function by causing vasoconstriction of the extremely dilated splanchnic vascular bed, thus increasing mean arterial pressure and renal perfusion50. In most studies, terlipressin was given in combination with HA in order to further improve the effective circulating volume. Interestingly, when terlipressin has been used without HA, treatment was considerably less effective51.
Several randomised52–56 and non-randomised51,57,58 clinical trials have shown that terlipressin and HA improve renal function and that full reversal of type 1 HRS is obtained in up to 40–50% of patients. Although responders survive longer than non-responders, the overall survival was not significantly superior in patients receiving terlipressin plus HA than in those treated with HA alone or placebo52,53,57. Nevertheless, a recent meta-analysis showed that the combined treatment is also able to improve short-term survival59.
HA is given at the initial dose of 1 g/kg.b.w. on day 1, followed by 20–40 g/day according to the central venous pressure and/or evidence of volume overload until terlipressin is stopped52,53.
A few studies have evaluated other vasoconstrictors for the management of type 1 HRS, such as noradrenaline, which showed a similar efficacy as terlipressin60,61, and midodrine plus octreotide, a combination that was less effective than terlipressin62. With both treatment approaches, HA is given at the doses described for use with terlipressin60–62.
Information on the use of vasoconstrictors plus HA in patients with type 2 HRS is really limited. Although the combined treatment improves renal function63,64, the rate of HRS recurrence after treatment withdrawal appears to be very high.
AISF-SIMTI recommendations on the use of albumin in the diagnosis and treatment of hepatorenal syndrome
- HA administration (1 g/kg/b.w. for two consecutive days) should be used to expand plasma volume for the differential diagnosis of HRS (D1).
- HA should be given with terlipressin in patients with type 1 HRS at the dose of 1 g/kg/b.w. on day 1 followed by 20–40 g per day until terlipressin is withdrawn (A1). When possible, the HA dose should be titrated according to the level of the central venous pressure. Alternatively, HA should be reduced or stopped in the presence of clinical evidence of volume overload and/or pulmonary oedema (A1).
- HA should be given with other vasoconstrictors (noradrenaline or midodrine plus octreotide) in patients with type 1 HRS at the same doses as used with terlipressin (A1).
- If patients with type 2 HRS are treated with vasoconstrictors, HA should be added according to the dosages used in type 1 HRS (B1).
6. Non-evidence-based clinical indications
6.1 Long-term treatment of cirrhotic ascites
6.1.1 Clinical and pathophysiological background
Ascites is the most frequent complication of liver cirrhosis, occurring in more than 50% of patients within 10 years of the diagnosis, and is associated with a significant worsening of the prognosis65,66. Medical treatment of uncomplicated ascites is based on diuretics associated to a mild reduction of dietary sodium intake65,66. Approximately 10% of patients per year develop refractory ascites, as defined by the International Club of Ascites, either because of a lack of response to medical treatment or because of the onset of diuretic-induced complications that preclude the use of an effective dosage of these drugs65,67. Refractory ascites is associated with an increased incidence of severe complications of cirrhosis, such as HRS, hyponatraemia, SBP, and umbilical hernia rupture and strangulation. Thus, the overall probability of survival of patients with refractory ascites is very poor, being approximately 30% at 2 years65–67.
Renal sodium retention and ascites formation are consequences of effective hypovolaemia, resulting from arteriolar vasodilation mainly in the splanchnic area, which promotes vasoconstriction and renal retention of sodium and water through the compensatory activation of neuro-humoral systems (RAA, SNS, ADH) and, in the more advanced stage of cirrhosis, through reduced renal perfusion66.
Thus, preservation of the central blood volume represents a potential target in the management of ascites.
6.1.2 Albumin for the long-term treatment of ascites
The use of HA for the treatment of ascites is allowed within the Italian National Health Service, but reimbursement for out-of-hospital prescriptions is limited to patients with ascites not responding to standard diuretic therapy (Note 15 of the Italian Medicines Agency). However, the long-term administration of HA to treat ascites is still debated, because of the lack of definitive scientific evidence supporting its clinical benefit.
Apart from a few reports of uncontrolled small studies and sporadic cases from more than a half century ago, only two randomised clinical trials from an Italian group have been published so far68,69. In 1999, two groups of hospitalized patients with cirrhosis and ascites were randomised to receive diuretics, given in a stepwise response-guided schedule, with or without daily infusion of 12.5 g HA during hospitalisation (median duration >20 days) followed by 25 g/week after discharge (median follow-up >20 months)68. The treatment with diuretics plus HA was overall more effective than diuretics alone in resolving ascites during hospitalisation and reducing the time the patients stayed in hospital. However, these results were achieved only in the subset of patients receiving low-dose diuretics (25 mg/day furosemide and 200 mg/day potassium canrenoate), while the advantage was lost when the anti-aldosteronic drug was used alone or when higher doses of diuretics were needed. No effect on survival after discharge was seen68. Despite the positive results, it is hard to transfer these regimens into current clinical practice in which these patients are managed as outpatients.
In a subsequent study69, a cohort of 100 patients was randomised to receive chronic treatment with either diuretics alone or diuretics plus HA (25 g/week for the first year, then 25 g every 2 weeks) (median follow >84 months). The recurrence rate of moderate to severe ascites and mortality were significantly lower in patients who received the supplemental HA. However, the relatively small sample size prevents firm conclusions from being reached.
No other controlled clinical trials have been performed so far to evaluate the effectiveness of prolonged HA administration in the treatment of cirrhosis and ascites. The lack of confirmatory randomised studies, together with the high cost of this therapeutic strategy, explain why long-term chronic HA infusion is not endorsed by international guidelines1,2 and it is, therefore, not usually included among therapeutic options for difficult-to-treat ascites in countries other than Italy.
A definite solution to this controversial issue will be provided by an open-label, multicentre, randomised, no-profit clinical trial, currently underway in Italy. This trial, supported by the Italian Medicines Agency, is comparing the effectiveness of long-term weekly administration of HA (40 g twice a week for the first 2 weeks and 40 g once a week for a maximum of 18 months independently of the serum albumin level) in 420 patients with cirrhosis and uncomplicated ascites receiving at least 200 mg per day of an anti-aldosteronic drug and 25 furosemide per day (ANSWER study, NCT 01288794, www.clinicaltrials.gov). The preliminary results from 386 patients were recently presented in abstract form70, showing that patients treated with diuretics plus HA required significantly fewer paracenteses and had lower incidences of refractory ascites as well as SBP, renal impairment, and hepatic encephalopathy.
Thus, although increasing evidence supports the benefit of HA in improving the management of ascites and the clinical outcome of patients with decompensated cirrhosis, the final results of the ANSWER study are eagerly awaited to confirm the efficacy of long-term administration of HA for this indication.
AISF-SIMTI recommendations on the long-term use of albumin to treat ascites
- Long-term HA can be effective in the treatment of ascites in association with diuretics (C1). The efficacy, dosage and timing of HA administration need to be defined by adequately-powered randomised controlled trials.
6.2 Treatment of hyponatraemia
6.2.1 Clinical and pathophysiological background
Hyponatraemia is a common finding in patients with cirrhosis and is characterised by low serum sodium levels (<135 mmol/L) usually associated with the presence of ascites, often refractory, and peripheral oedema. It is caused mainly by impaired solute-free water excretion secondary to hypersecretion of ADH, which is stimulated by effective hypovolaemia and results in a disproportionate retention of water relative to sodium retention71,72.
Besides the fact that serum sodium concentration is an important marker of prognosis in cirrhosis71,73, hyponatremia, mostly when severe (<125 mmol/L), can induce neurological complications per se or precipitate hepatic encephalopathy and is associated with reduced survival after liver transplantation74,75.
6.2.2 Albumin for the treatment of hyponatraemia
It is generally considered that management of hyponatremia should be started when the serum sodium concentration is lower than 130 mmol/L and includes fluid restriction, withdrawal of diuretics, and infusion of hypertonic saline solution although this latter approach is still controversial1.
Based on a strong pathophysiological rationale to use HA, that is blunting the non-osmotic hypersecretion of ADH through the improvement of effective hypovolaemia, many hepatologists consider HA an effective treatment for hyponatraemia. However, no randomised trials directly assessing the efficacy of HA have been published in extenso. Apart from a positive report on a few isolated cases76, the results of a small randomised trial, published in 2007 only in abstract form, showed that the administration of HA improves serum sodium concentration77.
AISF-SIMTI recommendations on the use of albumin to treat hyponatraemia
- Based on the pathophysiological background, HA might be effective to correct severe hyponatraemia not responding to standard measures, particularly in patients with symptoms related to hyponatraemia or waiting for liver transplantation (D1).
7. Clinical indications under investigations
7.1 Prevention of renal failure after bacterial infections other than spontaneous bacterial peritonitis
7.1.1 Clinical and pathophysiological background
Renal failure also frequently occurs in patients with bacterial infections other than SBP. In one study that enrolled 106 consecutive patients with cirrhosis and sepsis unrelated to SBP78, renal impairment developed in 27% of cases compared with only 8% in those without sepsis. The infections that most often led to renal failure were culture-negative sepsis (66%) and spontaneous bacteraemia (45%), followed by cellulitis, pneumonia and urinary tract infections. Unlike the situation with SBP, however, reversible renal impairment prevailed (76%), with the proportion not being substantially different from that found in patients without sepsis (62%).
As in SBP, the development of renal impairment strongly influences the outcome of patients with non-SBP bacterial infections, as 43% of these patients who developed renal impairment died in hospital79 and 66% within 3 months78, which are far higher percentages than those in patients who did not develop renal impairment (7% and 13%, respectively).
7.1.2 Albumin for the treatment of bacterial infections other than spontaneous bacterial peritonitis
Whether HA administration can be beneficial for non-SBP bacterial infections is still under investigation. The first published randomised trial on this issue80 enrolled 110 patients affected by pneumonia, urinary tract or skin infections, or culture-positive bacteraemia; however, in 20% of patients, infection was only suspected. The patients were randomised to receive appropriate antibiotic treatment alone or antibiotic treatment plus HA at the “standard” dose regimen used for SBP. Although both renal function (evaluated by serum creatinine and estimated glomerular filtration rate) and circulatory function (assessed by plasma renin activity, plasma aldosterone concentration, noradrenaline level, and mean arterial pressure) improved in patients receiving HA, the occurrence of type 1 HRS and the 3-month mortality rate did not differ between the two groups. However, multivariate analysis showed that treatment with HA was an independent predictive factor of survival when groups were adjusted according to the other predictors of survival, with a 3.4 relative risk of death for patients receiving only antibiotics.
A more recent French multicentre, randomised trial81 enrolled 193 cirrhotic patients with a Child-Pugh score greater than 8 and sepsis unrelated to SBP to receive antibiotics plus HA (1.5 g/kg on day 1 and 1 g/kg on day 3) or antibiotics alone. HA infusion delayed the occurrence of renal failure (mean time to onset: 29±22 vs 12±9 days, p=0.018), but the 3-month survival and renal failure rate were similar (70.2 vs 78.3% and 14.3 vs 13.5%, respectively). Post-hoc analysis showed a trend toward a better survival in patients with ascites and severe circulatory dysfunction. Interestingly, pulmonary oedema developed in about 8% of patients receiving HA.
Thus, HA administration appears to produce some benefit only in selected patients with bacterial infections, i.e. those patients at high risk of renal failure and death, but further studies are needed to confirm this conclusion. An ongoing large, multicentre European randomised trial (INFECIR-2 study, ClinicalTrials.gov, Identifier: NTC02034279) has been started by the EASL Chronic Liver Failure Consortium with the aim of assessing the effect of HA administration in high-risk patients with non-SBP bacterial infections, as defined by the presence of liver and kidney impairment, parameters of systemic inflammatory response syndrome (SIRS), and type of infection.
AISF-SIMTI recommendations on the use of albumin for the prevention of renal failure after bacterial infections other than spontaneous bacterial peritonitis
- HA administration, in association with antibiotics, is not currently indicated in patients with cirrhosis and bacterial infections other than SBP (B1).
7.2 Treatment of septic shock
Patients with septic shock have shown improved survival with early goal-directed therapy, based on the combination of empirical antibiotics, vasoconstrictors, and volume replacement82. Regarding this last, there are not specific studies evaluating the administration of HA in cirrhotic patients with septic shock. However, some data can be extrapolated from large randomised trials comparing crystalloids with HA in the general population. In a subgroup of 1,218 patients with severe sepsis enrolled in the SAFE study, multivariate analysis revealed that those receiving HA had a lower risk of death at day 28 as compared to those receiving saline (adjusted odd ratio: 0.71)83. Furthermore, a meta-analysis comparing HA and crystalloids in septic patients showed a survival benefit with HA84. This positive result was very recently challenged by a randomised trial (the ALBIOS study), including nearly 2,000 patients with severe sepsis enrolled in about 100 Italian Intensive Care Units, showing that HA administration did not improve 28-day and 90-day survival rates compared to those achieved with administration of crystalloids. However, according to a subgroup post-hoc analysis, a benefit of HA was found in patients with septic shock85.
Although only a few patients with cirrhosis were enrolled in these trials and the results should not, therefore, be transferred automatically to the setting of cirrhosis, some considerations favour the use of HA in patients with cirrhosis and septic shock. First, expansion with saline or Ringer’s lactate solution requires infusion of high volumes of fluids, which can be detrimental because they can worsen ascites and oedema. Moreover, the use of low-molecular-weight hydroxyethyl starch solutions raises concerns because of an increased risk of kidney and liver injury27,28. Finally, a specific benefit of HA may also derive from the non-oncotic properties of the molecule since these can antagonise some of the pathophysiological mechanisms related to septic shock6.
AISF-SIMTI recommendations on the use of albumin in the treatment of septic shock in patients with cirrhosis
- HA solutions might be effective and safe in cirrhotic patients with septic shock (C1).
7.3 Treatment of hepatic encephalopathy
7.3.1 Clinical and pathophysiological background
Hepatic encephalopathy is characterised by an acute change in mental status, frequently induced by a precipitating event, such as constipation, severe infection, gastrointestinal bleeding, renal failure or acute liver injury. It is a major complication of cirrhosis, being associated with high mortality, poor quality of life, and a high risk of recurrence85. The underlying pathophysiological mechanisms include over-exposure of the brain to plasma ammonia and other toxins escaping from the splanchnic circulation and the activation of inflammatory mediators, including oxidative stress, which induce swelling of astrocytes and disturb neurotransmission85. The treatment of hepatic encephalopathy is based mainly on correcting precipitating factors and reducing ammonia absorption86.
7.3.2 Albumin for the treatment of hepatic encephalopathy
Administration of HA may reduce oxidative stress-mediated injury and improve hepatic encephalopathy. However, so far, only one randomised clinical trial on this issue has been published: in this trial, the efficacy of HA was assessed in 56 patients with episodic grade II–IV hepatic encephalopathy, stratified according the severity of the encephalopathy87. The addition of HA (1.5 g/kg on the first day and 1 g/kg after 48 h) to the current standard management did not improve the resolution of the encephalopathy during hospitalisation, although treatment with HA was found to be an independent predictor of 90-day transplant-free survival.
Very recently, a small study found no differences in the incidence of overt hepatic encephalopathy after placement of a transjugular intrahepatic porto-systemic shunt (TIPS) between a group of patients treated with HA and historical controls during the first month (34 vs 31 %) or during the follow-up (39 vs 48 %)88.
AISF-SIMTI recommendations on the use of albumin in the treatment of hepatic encephalopathy
- Administration of HA is not currently indicated for the treatment of hepatic encephalopathy (B1).
8. The prescription of albumin in Italy
Studies and surveys in different countries have consistently reported that many HA prescriptions, ranging from 40% up to 90%, are not supported by clinical evidence, guidelines or practical recommendations89–92. Most of the inappropriate prescriptions derive from the use for nutritional interventions or for correcting hypoalbuminaemia per se (without hypovolaemia), which still occurs in many clinical areas (e.g. surgery, internal medicine, geriatrics, oncology), despite the existence of solid data against a real benefit. Other clinical uses for HA administration not supported by solid scientific evidence are nephrotic syndrome, pancreatitis, abdominal surgery, acute respiratory distress syndrome, cerebral ischaemia, and enteric disease89–92.
The Italian consumption of HA from 2007 to 2011 was analysed in the first Italian report on the prescription of plasma-derived medicinal product93,94.
The total demand for HA remained substantially stable in the above 5-year period, decreasing only minimally (−1%) from 36,652,396 g in 2007 to 36,442,660 g in 2011. A similar trend (−3%) was maintained when HA consumption was standardised per population, going from 620 g per 1,000 residents in 2007 to 601 g per 1,000 residents in 201195. This trend was evident in all the Italian regions, with the exception of Valle d’Aosta, Umbria, and Lombardy, where the standardied consumption increased considerably (+57%, +54%, and +25%, respectively).
The differences in the amount of prescribed HA among the Italian regions are quite remarkable. The five regions with the highest HA standardised consumption were Sardinia, Puglia, Campania, Calabria, and Lazio, with levels of 103%, 53%, 23%, 21%, and 17% above the Italian mean, respectively. It is important to note that these five regions account for 28% of the total Italian consumption although their combined population is 19.1% of the total Italian population. On the other hand, the five-lowest standardised HA demands were recorded in Bolzano, Trento, Friuli-Venezia Giulia, Marche, and Piedmont, being 62%, 61%, 54%, 39%, and 38% lower than the national mean level, respectively96.
HA consumption also appears to be greatly influenced by the channel of HA distribution (pharmacies open to the public, public healthcare system, private healthcare facilities)94.
Most HA was distributed through the public healthcare system with a mean national amount of 422 g per 1,000 residents. This channel of distribution was particularly predominant in Sardinia and Tuscany (157% and 49% above the mean national level, respectively).
The mean national level of HA distributed by pharmacies open to the public was 83 g per 1,000 residents in 2011. However, this channel of distribution was much more substantial in Campania, Puglia and Calabria, accounting for 270, 262, and 231 g per 1,000 residents, respectively. These three regions showed really remarkable deviations above the national mean level of 324%, 314%, and 277%, respectively. Furthermore, these are among the Italian regions with the highest standardised HA consumption.
As far as the distribution by private healthcare facilities is concerned, the mean national value in 2011 was 67 g per 1,000 residents. Conspicuous use was reported in Lazio, Molise and Lombardy, where private hospitals account for a significant part of the healthcare organisation; in these three regions the deviation above the national mean value was 153%, 128%, and 68%, respectively.
Finally, benchmarking national data shows that the 2011 standardised HA consumption per 1000 residents was several times higher in Italy (601 g) than in other European countries of comparable socio-economic level, such as France (238 g), Germany (148 g), and the United Kingdom (82 g)96.
Unfortunately, this report lacks data regarding the clinical indications for the HA prescriptions, thus precluding an analysis of appropriateness. However, although the very high prevalence of cirrhosis, particularly in the Southern regions, may at least in part explain the above described differences, all these data together suggest that there is substantial room for improving the appropriate use of HA.
Besides the large proportion of inappropriate use, the high cost, theoretical risk of disease transmission and existence of more economic alternatives of comparable efficacy have prompted interventions aiming at rationalising and rendering more appropriate the use of HA89–91. In this regard, a recent report from an Italian academic hospital showed that the enforcement of in-hospital guidelines produced two major consequences: first, the increasing trend observed before implementation of the recommendations was interrupted and HA consumption dropped about 15% remaining stable in the following years; second, a more liberal use of HA was guaranteed for indications supported by solid scientific data, while its futile administration in settings in which there is a lack of clinical evidence of efficacy was avoided. This more appropriate prescription of HA was achieved while maintaining health care expenditure under control97.
Acknowledgements
The Writing Committee would like to express its deep gratitude to the Collegues who reviewed the manuscript: Carlo Alessandria (Division of Gastroenterology and Hepatology, AOU Città della Salute e della Scienza, University of Turin), Oliviero Riggio (Department of Clinical Medicine, “Sapienza” University of Rome), and Francesco Salerno (Department of Internal Medicine, Policlinico IRCCS San Donato, University of Milan) as AISF experts; Pierluigi Berti (Immunohaematology and Transfusion Service, Aosta), Giuseppina Facco (Italian National Blood Centre, National Institute of Health, Rome and Immunohaemathology and Transfusion Medicine Unit, AOU “Città della Salute e della Scienza”, Turin), and Francesco Fiorin (Transfusion Service, San Donà di Piave) as SIMTI experts.
Footnotes
Disclosure of conflicts of interest
PC has been a speaker for Grifol and Baxalta and a scientific consultant for Kedrion; PA has been a speaker for Baxalta; DP has been a speaker for Grifols; MB has been a speaker for Baxalta and PPTA Europe, and consultant for CLS Behring GmBH.
GML, FB, PP, and CV declare no conflict of interest.
This article is being published jointly in “Digestive and Liver Disease”.
References
- 1.European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases. Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651–3. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 3.The GRADE system. [Accessed on 05/09/2015]. Available at: www.gradeworkinggroup.org/
- 4.Fanali G, di Masi A, Trezza V, et al. Human serum albumin: from bench to bedside. Mol Aspects Med. 2012;33:209–90. doi: 10.1016/j.mam.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Martinez R, Caraceni P, Bernardi M, et al. Albumin: pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836–46. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo V, García-Martinez R, Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 7.Domenicali M, Baldassarre M, Giannone FA, et al. Post-transcriptional changes of serum albumin: clinical and prognostic significance in hospitalized patients with cirrhosis. Hepatology. 2014;60:1851–60. doi: 10.1002/hep.27322. [DOI] [PubMed] [Google Scholar]
- 8.Jalan R, Schnurr K, Mookerjee RP, et al. Alteration in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology. 2009;50:555–64. doi: 10.1002/hep.22913. [DOI] [PubMed] [Google Scholar]
- 9.Møller S, Henriksen JH. Cardiovascular complications of cirrhosis. Postgrad Med J. 2009;85:44–54. doi: 10.1136/gut.2006.112177. [DOI] [PubMed] [Google Scholar]
- 10.Bernardi M, Moreau R, Angeli P, et al. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63:1272–84. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–37. doi: 10.1053/j.gastro.2013.02.042. [DOI] [PubMed] [Google Scholar]
- 12.Fernández J, Monteagudo J, Bargallo X, et al. A randomized unblinded pilot study comparing albumin vs. hydroxyethyl starch in spontaneous bacterial peritonitis. Hepatology. 2005;42:627–34. doi: 10.1002/hep.20829. [DOI] [PubMed] [Google Scholar]
- 13.Bortoluzzi A, Ceolotto G, Gola E, et al. Positive cardiac inotropic effect of albumin infusion in rodents with cirrhosis and ascites: molecular mechanisms. Hepatology. 2013;57:266–76. doi: 10.1002/hep.26021. [DOI] [PubMed] [Google Scholar]
- 14.Ginès P, Tito LV, Arroyo V, et al. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–502. doi: 10.1016/0016-5085(88)90691-9. [DOI] [PubMed] [Google Scholar]
- 15.Ginès A, Fernandez-Esparrach G, Monescillo A, et al. Randomized controlled trial comparing albumin, dextran-70 and polygelin in cirrhotic patients with ascites treated by paracentesis. Gastroenterology. 1996;111:1002–10. doi: 10.1016/s0016-5085(96)70068-9. [DOI] [PubMed] [Google Scholar]
- 16.Solà R, Vila MC, Andreu M, et al. Total paracentesis with dextran 40 vs. diuretics in the treatment of ascites in cirrhosis: a randomized controlled study. J Hepatol. 1994;20:282–8. doi: 10.1016/s0168-8278(05)80070-4. [DOI] [PubMed] [Google Scholar]
- 17.Panos MZ, Moore K, Vlavianos P, et al. Single, total paracentesis for tense ascites: sequential hemodynamic changes and right atrial size. Hepatology. 1990;11:662–7. doi: 10.1002/hep.1840110420. [DOI] [PubMed] [Google Scholar]
- 18.Pozzi M, Osculati G, Boari G, et al. Time course of circulatory and humoral effects of rapid total paracentesis in cirrhotic patients with tense, refractory ascites. Gastroenterology. 1994;106:709–19. doi: 10.1016/0016-5085(94)90706-4. [DOI] [PubMed] [Google Scholar]
- 19.Ruiz del Arbol L, Monescillo A, Jimenez W, et al. Paracentesis-induced circulatory dysfunction: mechanism and effect on hepatic hemodynamics in cirrhosis. Gastroenterology. 1997;113:579–86. doi: 10.1053/gast.1997.v113.pm9247479. [DOI] [PubMed] [Google Scholar]
- 20.Planas R, Ginès P, Arroyo V, et al. Dextran-70 versus albumin as plasma expanders in cirrhotic patients with tense ascites treated with total paracentesis. Results of a randomized study. Gastroenterology. 1990;99:1736–44. doi: 10.1016/0016-5085(90)90481-f. [DOI] [PubMed] [Google Scholar]
- 21.Salerno F, Badalamenti S, Lorenzano E, et al. Randomized comparative study of hemaccel vs. albumin infusion after total paracentesis in cirrhotic patients with refractory ascites. Hepatology. 1991;13:707–13. [PubMed] [Google Scholar]
- 22.Fassio E, Terg R, Landeira G, et al. Paracentesis with dextran 70 vs. paracentesis with albumin in cirrhosis with tense ascites. Results of a randomized study. J Hepatol. 1992;14:310–6. doi: 10.1016/0168-8278(92)90176-p. [DOI] [PubMed] [Google Scholar]
- 23.Altman C, Bernard B, Roulot D, et al. Randomized comparative multicenter study of hydroxyethyl starch versus albumin as a plasma expander in cirrhotic patients with tense ascites treated with paracentesis. Eur J Gastroenterol Hepatol. 1998;10:5–10. doi: 10.1097/00042737-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 24.García-Compeán D, Blanc P, Larrey D, et al. Treatment of cirrhotic tense ascites with dextran-40 versus albumin associated with large volume paracentesis: a randomized controlled trial. Ann Hepatol. 2002;1:29–35. [PubMed] [Google Scholar]
- 25.Sola-Vera J, Miñana J, Ricart E, et al. Randomized trial comparing albumin and saline in the prevention of paracentesis-induced circulatory dysfunction in cirrhotic patients with ascites. Hepatology. 2003;37:1147–53. doi: 10.1053/jhep.2003.50169. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Khalek EE, Arif SE. Randomized trial comparing human albumin and hydroxyethyl starch 6% as plasma expanders for treatment of patients with liver cirrhosis and tense ascites following large volume paracentesis. Arab J Gastroenterol. 2010;11:24–9. [Google Scholar]
- 27.Brunkhorst FM, Angel C, Bloos F, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med. 2008;358:125–39. doi: 10.1056/NEJMoa070716. [DOI] [PubMed] [Google Scholar]
- 28.Christidis C, Mal F, Ramos J, et al. Worsening of hepatic dysfunction as a consequence of repeated hydroxyethylstarch infusions. J Hepatol. 2001;35:726–32. doi: 10.1016/s0168-8278(01)00200-8. [DOI] [PubMed] [Google Scholar]
- 29.Moreau R, Asselah T, Condat B, et al. Comparison of the effect of terlipressin and albumin on arterial blood volume in patients with cirrhosis and tense ascites treated by paracentesis: a randomised pilot study. Gut. 2002;50:90–4. doi: 10.1136/gut.50.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh V, Kumar B, Nain CK, et al. Noradrenaline and albumin in paracentesis-induced circulatory dysfunction in cirrhosis: a randomized pilot study. J Intern Med. 2006;260:62–8. doi: 10.1111/j.1365-2796.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- 31.Singh V, Kumar R, Nain CK, et al. Terlipressin versus albumin in paracentesis-induced circulatory dysfunction in cirrhosis: a randomized study. J Gastroenterol Hepatol. 2006;21:303–7. doi: 10.1111/j.1440-1746.2006.04182.x. [DOI] [PubMed] [Google Scholar]
- 32.Appenrodt B, Wolf A, Grunhage F, et al. Prevention of paracentesis-induced circulatory dysfunction: midodrine vs albumin. A randomized pilot study. Liver Int. 2008;28:1019–25. doi: 10.1111/j.1478-3231.2008.01734.x. [DOI] [PubMed] [Google Scholar]
- 33.Singh V, Dheerendra PC, Singh B, et al. Midodrine versus albumin in the prevention of paracentesis-induced circulatory dysfunction in cirrhotics: a randomized pilot study. Am J Gastroenterol. 2008;103:1399–405. doi: 10.1111/j.1572-0241.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- 34.Moreau R, Valla DC, Durand-Zaleski I, et al. Comparison of outcome in patients with cirrhosis and ascites following treatment with albumin or a synthetic colloid: a randomised controlled pilot trail. Liver Int. 2006;26:46–54. doi: 10.1111/j.1478-3231.2005.01188.x. [DOI] [PubMed] [Google Scholar]
- 35.Bernardi M, Caraceni P, Navickis RJ, Wilkes MM. Albumin infusion in patients undergoing large-volume paracentesis: a meta-analysis of randomized trials. Hepatology. 2012;55:1172–81. doi: 10.1002/hep.24786. [DOI] [PubMed] [Google Scholar]
- 36.Alessandria C, Elia C, Mezzabotta, et al. Prevention of paracentesis-induced circulatory dysfunction in cirrhosis: standard vs half albumin doses. A prospective, randomized, unblinded pilot study. Dig Liver Dis. 2011;43:881–6. doi: 10.1016/j.dld.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 37.Rimola A, García-Tsao G, Navasa M, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. J Hepatol. 2000;32:142–53. doi: 10.1016/s0168-8278(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 38.Follo A, Llovet JM, Navasa M, et al. Renal impairment after spontaneous bacterial peritonitis in cirrhosis: incidence, clinical course, predictive factors and prognosis. Hepatology. 1994;20:1495–501. doi: 10.1002/hep.1840200619. [DOI] [PubMed] [Google Scholar]
- 39.Tandon P, Garcia-Tsao G. Renal dysfunction is the most important independent predictor of mortality in cirrhotic patients with spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2011;9:260–5. doi: 10.1016/j.cgh.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-del-Arbol L, Urman J, Fernández J, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–8. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 41.Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–9. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 42.Sigal SH, Stanca CM, Fernandez J, et al. Restricted use of albumin for spontaneous bacterial peritonitis. Gut. 2007;56:597–9. doi: 10.1136/gut.2006.113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poca M, Concepción M, Casas M, et al. Role of albumin treatment in patients with spontaneous bacterial peritonitis. Clin Gastroenterol Hepatol. 2012;10:309–15. doi: 10.1016/j.cgh.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 44.De Araujo A, De Barros Lopes A, Rossi G, et al. Low-dose albumin in the treatment of spontaneous bacterial peritonitis: should we change the standard treatment? Gut. 2012;61:1371–2. doi: 10.1136/gutjnl-2011-301739. [DOI] [PubMed] [Google Scholar]
- 45.Salerno F, Navickis RJ, Wilkes MM. Albumin infusion improves outcomes of patients with spontaneous bacterial peritonitis: a meta-analysis of randomized trials. Clin Gastroenterol Hepatol. 2013;11:123–30. doi: 10.1016/j.cgh.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Arroyo V, Ginès P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–76. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 47.Salerno F, Gerbes A, Ginès P, et al. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut. 2007;56:1310–8. doi: 10.1136/gut.2006.107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–7. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 49.Ginès P, Schrier RW. Renal failure in cirrhosis. N Engl J Med. 2009;361:1279–90. doi: 10.1056/NEJMra0809139. [DOI] [PubMed] [Google Scholar]
- 50.Moreau R, Lebrec D. The use of vasoconstrictors in patients with cirrhosis: type 1 HRS and beyond. Hepatology. 2006;43:385–94. doi: 10.1002/hep.21094. [DOI] [PubMed] [Google Scholar]
- 51.Ortega R, Ginès P, Uriz J, et al. Terlipressin therapy with and without albumin for patients with hepatorenal syndrome: results of a prospective, nonrandomized study. Hepatology. 2002;36:941–8. doi: 10.1053/jhep.2002.35819. [DOI] [PubMed] [Google Scholar]
- 52.Martín-Llahí M, Pépin M-N, Guevara M, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–9. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 53.Sanyal AJ, Boyer T, Garcia-Tsao G, et al. A randomized, prospective, double-blind, placebo-controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology. 2008;134:1360–8. doi: 10.1053/j.gastro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neri S, Pulvirenti D, Malaguarnera M, et al. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Dig Dis Sci. 2008;53:830–5. doi: 10.1007/s10620-007-9919-9. [DOI] [PubMed] [Google Scholar]
- 55.Solanki P, Chawla A, Garg R, et al. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective randomized placebo-controlled clinical trial. J Gastroenterol Hepatol. 2003;18:152–6. doi: 10.1046/j.1440-1746.2003.02934.x. [DOI] [PubMed] [Google Scholar]
- 56.Yang YZ, Dan ZL, Liu NZ, et al. Efficacy of terlipressin in treatment of liver cirrhosis with hepatorenal syndrome. J Intern Intens Med. 2001;7:123–5. [Google Scholar]
- 57.Salerno F, Cazzaniga M, Merli M, et al. Diagnosis, treatment and survival of patients with hepatorenal syndrome: a survey on daily medical practice. J Hepatol. 2011;55:1241–8. doi: 10.1016/j.jhep.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Rodríguez E, Elia C, Solà E, et al. Terlipressin and albumin for type-1 hepatorenal syndrome associated with sepsis. J Hepatol. 2014;60:955–61. doi: 10.1016/j.jhep.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 59.Gluud LL, Christensen K, Christensen E, Krag A. Terlipressin for hepatorenal syndrome. Cochrane Database Syst Rev. 2012;9:CD005162. doi: 10.1002/14651858.CD005162.pub3. [DOI] [PubMed] [Google Scholar]
- 60.Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007;47:499–505. doi: 10.1016/j.jhep.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 61.Singh V, Ghosh S, Singh B, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. J Hepatol. 2012;56:1293–8. doi: 10.1016/j.jhep.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 62.Cavallin M, Kamath PS, Merli M, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology. 2015;62:567–74. doi: 10.1002/hep.27709. [DOI] [PubMed] [Google Scholar]
- 63.Alessandria C, Venon WD, Marzano A, et al. Renal failure in cirrhotic patients: role of terlipressin in clinical approach to hepatorenal syndrome type 2. Eur J Gastroenterol Hepatol. 2002;14:1363–8. doi: 10.1097/00042737-200212000-00013. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh S, Choudhary NS, Sharma AK, et al. Noradrenaline vs terlipressin in the treatment of type 2 hepatorenal syndrome: a randomized pilot study. Liver Int. 2013;33:1187–93. doi: 10.1111/liv.12179. [DOI] [PubMed] [Google Scholar]
- 65.Moore KP, Wong F, Gines P, et al. The management of ascites in cirrhosis: report on the Consensus Conference of the International Ascites Club. Hepatology. 2003;38:258–66. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 66.Gines P, Cardenas A, Arroyo V, Rodes J. Management of cirrhosis and ascites. N Eng J Med. 2004;350:1646–54. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 67.Arroyo V, Gines P, Gerbes AL, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. Hepatology. 1996;23:164–76. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 68.Gentilini P, Casini-Raggi V, Di Fiore G, et al. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. J Hepatol. 1999;30:639–45. doi: 10.1016/s0168-8278(99)80194-9. [DOI] [PubMed] [Google Scholar]
- 69.Romanelli RG, La Villa G, Barletta G, et al. Long-term albumin infusion improves survival in patients with cirrhosis and ascites: an unblinded randomized trial. World J Gastroenterol. 2006;12:1403–7. doi: 10.3748/wjg.v12.i9.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernardi M, Riggio O, Angeli P, et al. Long-term use of human albumin for the treatment of ascites in patients with hepatic cirrhosis: the interim analysis of the ANSWER study. Dig Liv Dis. 2015;47(Suppl 1):e6. [Google Scholar]
- 71.Ginès P, Berl T, Bernardi M, et al. Hyponatremia in cirrhosis: from pathogenesis to treatment. Hepatology. 1998;28:851–64. doi: 10.1002/hep.510280337. [DOI] [PubMed] [Google Scholar]
- 72.Angeli P, Wong F, Watson H, Gines P. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology. 2006;44:1535–42. doi: 10.1002/hep.21412. [DOI] [PubMed] [Google Scholar]
- 73.Kim WR, Biggins SW, Krmers WK, et al. Hyponatremia and mortality among patients on the liver transplant waiting list. N Engl J Med. 2008;359:1018–26. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guevara M, Baccaro ME, Ríos J, et al. Risk factors for hepatic encephalopathy in patients with cirrhosis and refractory ascites: relevance of serum sodium concentration. Liver Int. 2010;30:1137–42. doi: 10.1111/j.1478-3231.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 75.Yun BC, Kim WR, Benson JT, et al. Impact of pretransplant hyponatremia on outcome following liver transplantation. Hepatology. 2009;49:1610–5. doi: 10.1002/hep.22846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCormick PA, Mistry P, Kaye G, et al. Intravenous albumin infusion is an effective therapy for hyponatraemia in cirrhotic patients with ascites. Gut. 1990;31:204–7. doi: 10.1136/gut.31.2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jalan R, Mookerjee R, Cheshire L, et al. Albumin infusion for severe hyponatremia in patients with refractory ascites: a randomized clinical trail. J Hepatol. 2007;46:232A. [Abstract] [Google Scholar]
- 78.Terra C, Guevara M, Torre A, et al. Renal failure in patients with cirrhosis and sepsis unrelated to spontaneous bacterial peritonitis: value of MELD score. Gastroenterology. 2005;129:1944–53. doi: 10.1053/j.gastro.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 79.Fasolato S, Angeli P, Dallagnese L, et al. Renal failure and bacterial infections in patients with cirrhosis: epidemiology and clinical features. Hepatology. 2007;45:223–9. doi: 10.1002/hep.21443. [DOI] [PubMed] [Google Scholar]
- 80.Guevara M, Terra C, Nazar A, et al. Albumin for bacterial infections other than spontaneous bacterial peritonitis in cirrhosis. A randomized, controlled study. J Hepatol. 2013;57:759–65. doi: 10.1016/j.jhep.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 81.Thévenot T, Bureau C, Oberti F, et al. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol. 2015;62:822–30. doi: 10.1016/j.jhep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 82.Jones AE, Puskarich MA. The Surviving Sepsis Campaign guidelines 2012: update for emergency physicians. Ann Emerg Med. 2014;63:35–47. doi: 10.1016/j.annemergmed.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 83.SAFE Study Investigators. Finfer S, McEvoy S, et al. Impact of albumin compared to saline on organ function and mortality of patients with severe sepsis. Intensive Care Med. 2011;37:86–96. doi: 10.1007/s00134-010-2039-6. [DOI] [PubMed] [Google Scholar]
- 84.Delaney A, Dan A, McCaffrey J, Finfer S. The role of albumin as a resuscitation fluid for patients with sepsis: A systematic review and meta-analysis. Crit Care Med. 2011;39:386–91. doi: 10.1097/CCM.0b013e3181ffe217. [DOI] [PubMed] [Google Scholar]
- 85.Caironi P, Tognoni G, Masson S, et al. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–21. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 86.Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–35. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 87.Simón-Talero M, García-Martínez R, et al. Effects of intravenous albumin in patients with cirrhosis and episodic hepatic encephalopathy: a randomized double-blind study. J Hepatol. 2013;59:1184–92. doi: 10.1016/j.jhep.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 88.Riggio O, Nardelli S, Pasquale C, et al. No effect of albumin infusion on the prevention of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Metab Brain. 2015 doi: 10.1007/s11011-015-9713-x.. [DOI] [PubMed] [Google Scholar]
- 89.Vargas E, De Miguel V, Portolés A, et al. Use of albumin in two Spanish university hospitals. Eur J Clin Pharmacol. 1997;52:465–70. doi: 10.1007/s002280050320. [DOI] [PubMed] [Google Scholar]
- 90.Debrix I, Combeau D, Stephan F, Benomar A, Becker A. Clinical practice guidelines for the use of albumin: results of a drug use evaluation in a Paris hospital. Tenon Hospital Paris. Pharm World Sci. 1999;21:11–6. doi: 10.1023/a:1008635005398. [DOI] [PubMed] [Google Scholar]
- 91.Tarín Remohí MJ, Sánchez Arcos A, Santos Ramos B, et al. Costs related to inappropriate use of albumin in Spain. Ann Pharmacother. 2000;34:1198–205. doi: 10.1345/aph.19385. [DOI] [PubMed] [Google Scholar]
- 92.Tanzi M, Gardner M, Megellas M, et al. Evaluation of the appropriate use of albumin in adult and pediatric patients. Am J Health Syst Pharm. 2003;60:1330–5. doi: 10.1093/ajhp/60.13.1330. [DOI] [PubMed] [Google Scholar]
- 93.Calizzani G, Lanzoni M, Candura F, et al. Anni 2007–2011. Roma: Istituto Superiore di Sanità; 2012. [Accessed on 31/10/2013]. Analisi della domanda dei principali medicinali plasmaderivati in Italia. Rapporti ISTISAN 12/53. Available at: http://www.iss.it/binary/publ/cont/dodici53web.pdf. [Google Scholar]
- 94.Lanzoni M, Biffoli C, Candura F, et al. Plasma-derived medicinal products in Italy: information sources and flows. Blood Transfus. 2013;11(Suppl 4):3–7. doi: 10.2450/2013.004s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vaglio S, Calizzani G, Lanzoni M, et al. The demand for human albumin in Italy. Blood Transfus. 2013;11(Suppl 4):26–32. doi: 10.2450/2013.006s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vaglio S, Calizzani G, Grazzini G, et al. Italian albumin usage (or misusage?) Eur J Intern Med. 2014;25:e31–2. doi: 10.1016/j.ejim.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 97.Mirici-Cappa F, Caraceni P, Domenicali M, et al. How albumin administration for cirrhosis impacts on hospital albumin consumption and expenditure. World J Gastroenterol. 2011;17:3479–86. doi: 10.3748/wjg.v17.i30.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]