Introduction
Patient Blood Management (PBM) is a holistic approach to the management of blood as a resource for each, single patient; it is a multimodal strategy that is implemented through the use of a set of techniques that can be applied in individual cases. Indeed, the overall outcome resulting from the implementation of PBM cannot be fully appreciated and explained simply by summing the effects of the single strategies and techniques used, since these can only produce the expected optimal outcome if used in combination1. PBM is, therefore, a patient-centred, multiprofessional, multidisciplinary and multimodal approach to the optimal management of anaemia and haemostasis (also during surgery), to limiting allogeneic transfusion needs in the peri-operative period, and to appropriate use of blood components and, when relevant, plasma-derived medicinal products2. The concept of PBM is not centred on a specific pathology or procedure, nor on a specific discipline or sector of medicine, but is aimed at managing a resource, “the patient’s blood”, shifting attention from the blood component to the patient who, therefore, acquires a central and pre-eminent role3,4.
PBM combines the dual purposes of improving the outcomes of patients and reducing costs, being based on the patient rather than on allogeneic blood as the resource. For this reason, PBM goes beyond the concept of appropriate use of blood components and plasma-derived medicinal products, since its purpose is to avoid or significantly reduce their use, managing, in good time, all the modifiable risk factors that can lead to a transfusion being required5. These aims can be achieved through the so-called “three pillars of PBM” (Table I)5, which are crucial for making the paradigmatic shift that characterises the innovative, patient-centred approach: (i) optimising the patient’s erythropoiesis; (ii) minimising bleeding; and (iii) optimising and exploiting an individual’s physiological reserve to tolerate anaemia5. Each of these three key points is a strategic response to clinical circumstances that can cause adverse outcomes and necessitate the use of allogeneic transfusion therapy, namely anaemia, blood loss and hypoxia, respectively.
Table I.
The three pillars of Patient Blood Management (modified from Hofmann A et al.5).
| Period | Pillar 1 | Pillar 2 | Pillar 3 |
|---|---|---|---|
| Optimisation of erythropoiesis | Minimisation of blood loss | Optimisation of tolerance of anaemia | |
| Pre-operative |
|
|
|
| Intra-operative |
|
|
|
| Post-operative |
|
|
|
PBM is, therefore, intended to guarantee all patients a series of personalised programmes, based on surgical requirements and the characteristics of the patients themselves, with the dual purposes of using allogeneic transfusion support appropriately and reducing the need for this resource. For this reason, PBM requires multidisciplinary and multimodal strategies to systematically identify, evaluate and manage anaemia (boosting, if necessary, individual physiological reserves) and to avoid or minimise blood losses.
It seems necessary to produce specific national standards. In fact, in the USA, PBM is the object of attention from the Association for Advancing Transfusions and Cellular Therapies (formerly known as the American Association of Blood Banks - AABB) which recently published the first edition of “Standards for a Patient Blood Management Program” precisely with the aim of supplying healthcare structures with solid elements for the standardisation of procedures and activities for implementing and/or optimising a PBM programme. The Society for the Advancement of Blood Management (SABM), also in the USA, has published a second edition of “Administrative and Clinical Standards for Patient Blood Management Programs”6 and the Joint Commission has published seven parameters for measuring the performance of healthcare structures in the field of PBM7.
Methodology for producing the recommendations for the implementation of a Patient Blood Management programme
The methodology used to prepare the recommendation grades is based on that described by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group8. According to the GRADE system, recommendations are classified by grade, expressed in Arabic numbers (1, 2), according to their strength, and in letters (A, B, C), according to the quality and type of evidence derived from the studies.
In detail:
- Grade 1: the authors are certain that the advantages for health clearly outweigh the disadvantages, in terms of both risk and financial cost. This is, therefore, a strong recommendation.
- Grade 2: the authors are less certain, the balance between advantages and disadvantages is less clear.
This is, therefore, a weak recommendation.
As far as concerns the quality and type of evidence drawn from the studies on which the recommendations are based, these can be classified into three levels:
- Grade A: high level. The evidence is derived from numerous, consistent randomised trials without major limitations. It is unlikely that future research will change the conclusions of these trials.
- Grade B: moderate level. The evidence is derived from randomised clinical trials, but with major limitations (such as inconsistent results, wide confidence intervals, methodological weaknesses). Grade B is also attributed to recommendations deriving from strong evidence from observational studies or case series (for example, treatment effects or the demonstration of a dose-response effect). Subsequent research could modify the conclusions of these trials.
- Grade C: low or very low level. The evidence is derived from the analysis of observational clinical studies with less consistent results, or from experts’ opinions/clinical experience. Future research is likely to change the conclusions reached.
In brief:
- Grade 1A: strong recommendation based on scientific evidence of high quality.
- Grade 1B: strong recommendation based on scientific evidence of moderate quality.
- Grade 1C: strong recommendation based on scientific evidence of low quality.
- Grade 2A: weak recommendation based on scientific evidence of high quality.
- Grade 2B: weak recommendation based on scientific evidence of moderate quality.
- Grade 2C: weak recommendation based on scientific evidence of low quality.
The clinical-organisational course of the patient undergoing elective major orthopaedic surgery
Pre-operative period and role of the pre-operative assessment
The pre-operative assessment is performed in order to obtain clinical and laboratory information supplementary to that acquired from the clinical history with the aim of9:
- confirming, or not, the planned diagnostic-therapeutic pathway (clinical management);
- identifying previously undetected disorders that must be treated before the operation or which make it necessary to modify the surgical or anaesthetic technique;
- limiting possible adverse outcomes or increasing the benefits for patient by modifying, if necessary, the clinical pathway;
- facilitating the evaluation of potential risks for the patient (including the possibility of informing the patient of a potentially increased risk);
- predicting possible post-operative complications;
- establishing the baseline reference parameters, which can be used for subsequent post-operative evaluations;
- considering the value of performing screening not related to the operation.
A pre-operative evaluation is always necessary when anaesthesia is planned.
Selective use of pre-operative tests, based on the history, clinical examination, and type and invasiveness of the surgical and anaesthesiological procedures, helps the management of the patient.
In order to shorten the time spent in hospital and optimise the planning of elective surgery, the pre-operative evaluation is performed in an out-patient setting (pre-admission), at an appropriate time before the operation (on average, 30 days before), and includes any additional clinical-diagnostic investigations needed10–12.
In all candidates for elective surgery, a multidisciplinary and multimodal approach must be used, based on an agreed programme of co-ordinated interventions, aimed at peri-operative management of the “patient’s blood” as a resource. These interventions start with the pre-operative optimisation of erythropoiesis, haemostasis and tolerance of anaemia.
Depending on the organisation in different hospitals or healthcare authorities, pre-operative management of the resource “patient’s blood” must guarantee a structured diagnostic-therapeutic pathway involving at least three specialists: a surgeon, an anaesthetist and a transfusion medicine specialist; these professionals collaborate in the setting of a multidisciplinary outpatient clinic (the Anaemia Clinic) which takes the role of case manager. The Anaemia Clinic must allow for, when necessary, the inclusion and collaboration of other medical staff, such as experts in haemostasis and thrombosis, haematologists, cardiologists or other specialists with expertise in identifying and treating, in a multidisciplinary and multimodal method, underlying disorders in candidates for elective surgery. Furthermore, the patient’s general practitioner should be kept informed and, when possible, involved.
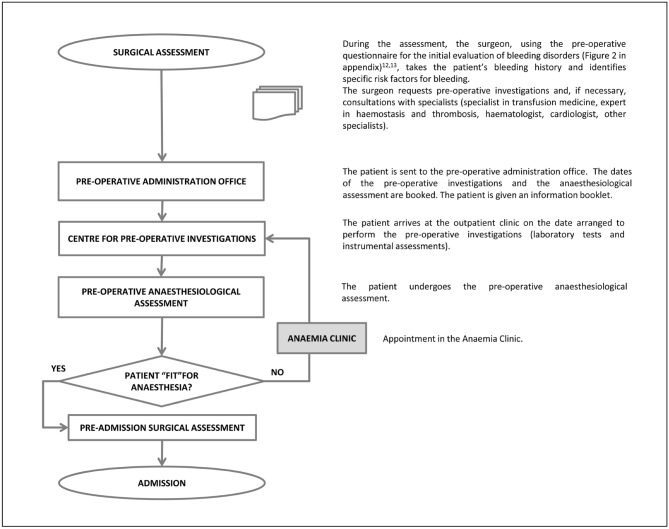
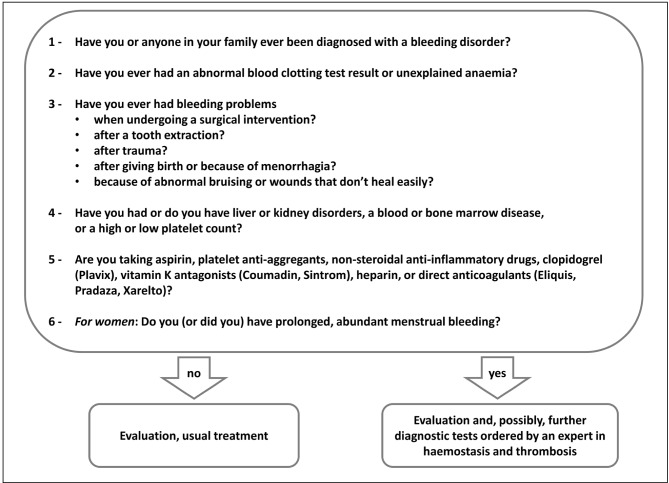
The pre-operative process starts in the surgical outpatient clinic (Figure 1). During this appointment the surgeon, having defined the indication for surgery, the grading of the intervention and its level of priority, takes the patient’s history, with the purpose, among others, of detecting risk factors for bleeding. To this aim it can be useful to administer a questionnaire for an initial evaluation of bleeding disorders (Figure 212,13), which can be followed by appropriate pre-operative investigations.
Figure 1.
Pre-operative flow-chart for patients undergoing elective major orthopaedic surgery and included in a Patient Blood Management programme.
Figure 2.
Initial evaluation of bleeding disorders (modified from Liumbruno GM et al.12, Nichols WL et al.13).
Subsequently the patient is sent to the pre-operative administration office which, in conformity with locally used procedures, enters the patient into a specific pre-operative course aimed at booking and performing the pre-operative investigations and anaesthesiological evaluation, as well as adding the patient to the waiting list (surgical register).
Once the prescribed investigations have been performed in a centre for pre-operative examinations, the patient is sent to the pre-operative anaesthesiology clinic where the anaesthetist examines the results of the pre-operative investigations and evaluates the patient’s clinical state; if the patient is considered fit for anaesthesia, the specialist defines the “risk class”14, obtains informed consent to the anaesthesia and prescribes the anaesthesiological pre-medication.
If the pre-operative investigations that have been carried out are not considered sufficient, the anaesthetist orders other investigations that will be performed, when possible, on the same day as ordered or, at any rate, as soon as possible (dedicated pre-operative pathway). Furthermore, the anaesthetist may consider that the patient is not fit for anaesthesia because of the presence of intercurrent or chronic disorders or anaemia, which require specific investigations and/or treatment. In this case, after consultation with the surgeon and/or other specialists of the Anaemia Clinic, the anaesthetist may decide to prescribe what is deemed useful, setting a new date for the anaesthesiological re-evaluation, through the centre for pre-operative investigations.
The specialists of the Anaemia Clinic (anaesthetist, surgeon, specialist in transfusion medicine, expert in haemostasis and thrombosis, haematologist, or other specialist with expertise in identifying and treating, in a multidisciplinary and multimodal method, underlying disorders in candidates for elective surgery), by adopting the strategies and techniques indicated in the three pillars of PBM (Table I)5, have the task of setting up the multidisciplinary programme of co-ordinated interventions, aimed at the peri-operative management of the resource “patient’s blood”. The three pillars of the PBM in the pre-operative period are:
Optimisation of erythropoiesis: detect anaemia; identify and treat its underlying causes; re-evaluate the patient, if necessary; treat iron deficiency and iron-deficiency anaemia, anaemia of chronic disease and functional iron deficiencies, so-called iron-restricted erythropoiesis; treat deficiencies of other haematinics.
Minimise blood losses: identify and manage bleeding risk, minimise iatrogenic bleeding, plan the procedure carefully and prepare well; organise pre-operative autologous blood donation in very selected cases.
Optimisation of the tolerance of anaemia: assess and optimise the patient’s physiological reserve to tolerate anaemia and risk factors; compare estimated blood loss with the individual patient’s tolerable blood loss; formulate a personalised blood management programme that includes patient-specific blood-conservation techniques; adopt restrictive blood transfusion thresholds.
Information about strategies included in local PBM programmes should be supplied to all patients, preferably before their admission to hospital, because this improves compliance with diagnostic-therapeutic care pathways adopted in the healthcare structure.
- It is recommended that the patient’s pre-operative evaluation, aimed at detecting any anaemia and optimising erythropoiesis, at identifying and managing bleeding risk as well as assessing and optimising the patient’s personal physiological tolerance of anaemia and risk factors, be carried out at least 30 days before the planned date of the operation, in order to allow more detailed investigations and/or arrange appropriate treatment [1C].
- It is recommended that a structured questionnaire, aimed at picking up risk factors for bleeding, is included when taking the clinical history of an adult candidate for elective (orthopaedic) surgery [1C].
- It is recommended that all adult patients who are candidates for elective major orthopaedic surgery for which a multidisciplinary programme of co-ordinated interventions has been established involving the adoption of pharmacological and non-pharmacological techniques aimed at optimising erythropoiesis, minimising blood losses and optimising tolerance of anaemia, before giving consent to one or more of the above-mentioned treatments, receive detailed information on their clinical state and strategies to limit allogeneic transfusion needs included in the local PBM programme; explanatory material prepared ad hoc by the hospital may be used for this purpose [1C].
Once the pre-operative process has been concluded with the patient deemed fit for anaesthesia, the patient undergoes the pre-admission surgical assessment, with the purposes of: a) re-evaluating the grading of the operation to be performed; b) re-assessing the patient’s general and specific conditions; c) planning the procedure; d) filling in the clinical records and single treatment chart with the pre-operative prescriptions (for example: specific therapies to optimise erythropoiesis, antibiotic prophylaxis, anti-thrombotic prophylaxis); e) collecting the informed consent to the intervention.
Having reached this point of the diagnostic-therapeutic care pathway, the patient is ready to be admitted to hospital on the planned day.
Intra-operative period
Once the patient has completed the pre-operative preparation for anaesthesia and the operation, on the planned day the patient is admitted to the surgical ward, where he or she is greeted by a nurse. The nursing staff fill in the nursing records with the planned assessment forms (for example: pain detection, detection of pressure ulcers) and check that the patient has been suitably prepared with respect to the pre-operative instructions provided.
Subsequently, the surgeon (and anaesthetist, if necessary), having checked the correct planning of the operation after optimisation of erythropoiesis and the use of other strategies and techniques indicated in the pre-operative period in the three pillars of the PBM (Table I)5, examines, and, if necessary, updates the clinical documentation, the single treatment chart and the informed consent to the procedure; the same surgeon marks the site of surgery.
Before entering the operating theatre, the nurse checks the anaesthesiological instructions, administers and records the treatments prescribed (general, antibiotic, pre-anaesthesia), takes any blood samples required and informs the patient about the surgical procedure; the nurse also checks that the patient is not wearing any jewellery or removable prostheses, checks the patient’s personal hygiene (if necessary, inviting the patient to wash again before the operation), shaves the patient, when required, and cleans the area of skin involved by the operation with antiseptics.
The patient is transported to the induction/recovery room, where the surgical team (anaesthetist and surgeon) re-evaluates him or her, “signs in” the patient (using the operating theatre check-list), informs the patient, if collaborative, about the procedures that will be performed and introduces one or more devices for intravenous access. The nurse monitors the patient’s vital parameters and administers antibiotic prophylaxis; all the activities are recorded on the nursing form and on the single treatment chart
In the operating theatre the same team carries out the “time out” checks (operating theatre check-list), performs the operation and provides anaesthesia, optimises the macrocirculation, maintains homeostasis and takes samples for any intra-operative blood-chemistry tests.
In this stage the three pillars of the PBM involve the following.
Optimisation of erythropoiesis: check appropriate timing of the surgery after optimisation of erythropoiesis.
Minimisation of blood loss: ensure meticulous haemostasis and use appropriate surgical techniques; adopt blood-sparing strategies; use blood-conserving anaesthetic techniques; use autologous blood transfusion, if foreseen by the personalised PBM plan drawn up by the case manager of the Anaemia Clinic; use pharmacological strategies and haemostatic agents; use point-of-care (POC) tests.
Optimisation of tolerance of anaemia: optimise cardiac output; optimise ventilation and oxygenation; adopt restrictive transfusion thresholds.
At the end of the intervention, the surgical team carries out the “sign out” controls (operating theatre check-list) and the patient is then transferred to the induction/recovery room for post-operative observation. Here the patient is re-assessed and monitored until his or her transfer to the ward; the same team prescribes the post-operative controls and treatments that must be carried out in the ward in the first 24 hours following the operation and which also have the purpose of correctly implementing the PBM strategies included in the multidisciplinary programme of co-ordinated interventions.
Post-operative period
After the operation, the patient can be transferred to the surgical ward from which he or she came, or depending on needs and clinical conditions, can be admitted to an intensive or sub-intensive care facility.
The post-operative controls and treatments for the 24 hours following the surgical intervention are prescribed by the anaesthetist and surgeon during the period that the patient regains consciousness.
During the post-operative period, besides the management of any urgent or emergency clinical situations, the co-ordinated interventions foreseen by the multidisciplinary programme, aimed at implementing the techniques and strategies included in the three pillars of PBM and reported here, must be carried out.
Optimisation of erythropoiesis: stimulate erythropoiesis, if necessary; consider drug interactions that can cause or enhance post-operative anaemia.
Minimisation of blood losses: ensure careful monitoring of the patient and management of post-operative bleeding; guarantee fast rewarming/maintenance of normothermia (except when there is a specific indication for hypothermia); use autologous transfusion techniques if foreseen by the personalised PBM drawn up by the case manager of the Anaemia Clinic; minimise iatrogenic bleeding; manage haemostasis and anticoagulation; administer prophylaxis against upper gastrointestinal tract bleeding; give prophylaxis against infections and treat any that occur.
Optimisation of the tolerance of anaemia: optimise the tolerance of anaemia; maximise oxygen delivery; minimise oxygen consumption; adopt restrictive transfusion thresholds.
The above-mentioned strategies must be implemented throughout the post-operative period until the patient’s clinical conditions have normalised, which is when the feasibility of the patient’s discharge from hospital is evaluated. On discharge the ward doctor prepares the discharge letter and gives it to the patient. The discharge letter contains a description of the salient diagnostic-therapeutic interventions performed, information on the domiciliary management of the surgical wound and any drains, as well as the pharmacological treatment (medication reconciliation).
Healing of the surgical wound and correct compliance with the prescribed domiciliary drug treatment must be checked at each outpatient follow-up until conclusion of the post-discharge process (30 days if the patient has not had a prosthesis implanted, 1 year if prosthetic material has been implanted).
The three pillars of Patient Blood Management
Optimisation of erythropoiesis
Pre-operative period
Detection of anaemia
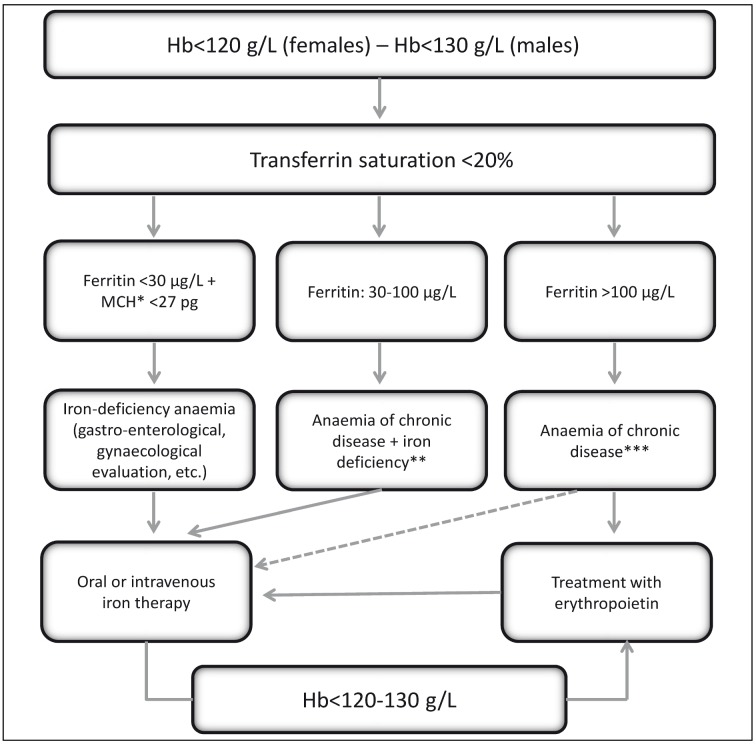
The prevalence of pre-operative anaemia in surgical patients varies greatly, ranging from 5% (among geriatric patients with hip fracture) to 75.8% (among patients with stage D colon cancer according to Dukes’ classification)15; among candidates for orthopaedic surgery associated with moderate to substantial peri-operative bleeding, such as elective joint replacement (of the hip or knee) or urgent surgery (of the hip), the prevalence of pre-operative anaemia ranges from 24±9% to 44±9%, respectively16. Epidemiological studies on anaemia in patients undergoing elective hip or knee replacement surgery showed that the anaemia is hypochromic and microcytic in between 23 and 70% of cases16. Figure 3 presents a simple algorithm for the diagnosis of iron-deficiency anaemia17–19. Among the same patients the prevalence of vitamin B12 deficiency is about 12% and that of folate deficiency about 3%20,21. The other forms of anaemia are due to inflammatory disease, chronic kidney disease or an unknown cause22,23.
Figure 3.
Algorithm for the diagnosis of iron-deficiency anaemia (modified from Muñoz M et al.17–19).
Hb: haemoglobin; MCH: mean corpuscular haemoglobin; The dashed arrow indicates the need to measure the ferritin level; *: The mean corpuscular volume (MCV) is a relatively late indicator of iron deficiency in patients without active bleeding; in the presence of a low MCV the differential diagnosis from thalassaemia must be made; the MCV can be normal in the presence of vitamin B12 or folate deficiency, post-haemorrhagic reticulocytosis, initial response to oral iron therapy, alcohol intake or myelodysplasia. ** Additional laboratory tests: reticulocyte count; creatinine; C-reactive protein. *** Additional laboratory tests to evaluate the iron deficiency: ratio between soluble transferrin receptor (sTfR) and logarithm of ferritin; hypochromic red blood cells; haemoglobin content in reticulocytes.
Pre-operative anaemia, even if mild, in patients who are candidates for major surgery (not cardiac) is independently associated with a higher risk of morbidity and mortality at 30 days24. Furthermore, a recent, retrospective study demonstrated that, in the peri-operative period of patients undergoing heart surgery, the sum of two risk factors, that is anaemia (haematocrit <25%) and transfusion therapy with red cell concentrates, had a greater, and statistically significant, effect on post-operative morbidity and mortality25. Anaemia is, therefore, a contraindication to performing elective surgery.
It is recommended that elective major surgery is not performed in patients who are found to have anaemia until the anaemia has been correctly classified and treated [1B].
Anaemia is defined according to threshold values of haemoglobin (Hb) indicated by the World Health Organization (WHO)26: children up 5 years: 110 g/L; children between 5 and 12 years old: 115 g/L; children between 12 and 15 years: 120 g/L; pregnant women: 110 g/L; women who are not pregnant (aged 15 years old or more): 120 g/L; men (aged 15 years or more): 130 g/L.
It is recommended that the pre-operative assessment of the patient, aimed at detecting any anaemia and optimising erythropoiesis, is performed at least 30 days before the planned date of the operation, in order to enable more detailed diagnostic investigations and/or plan appropriate treatment [1C]6,11,12.
It is recommended that, if a state of anaemia is detected, the subsequent laboratory tests are directed at identifying iron deficiency or other nutritional deficiencies (folic acid and/or vitamin B12), chronic kidney disease and/or chronic inflammatory disorders [1C].
It is recommended that the detection and treatment of anaemia, and any further related clinical-diagnostic investigations, are included within a global PBM strategy become a standard of care for all candidates for elective surgery, especially if the risk of peri-operative bleeding is substantial [1C].
Stimulation of erythropoiesis
These recommendations concern the management of oral and intravenous iron therapy, given the paucity of studies on the use of other haematinics in the peri-operative setting27.
Since the pre-operative Hb value is the main, independent risk factor for requiring transfusion support with packed red cells, it is recommended that any nutritional deficiencies (iron, vitamin B12, folate), once detected, are treated with haematinics [1C]11.
It is suggested that the target Hb value before elective major orthopaedic surgery is at least within the normal range according to the previously cited WHO criteria [2C]11.
Oral iron therapy
In adult patients with iron-deficiency anaemia who are candidates for elective hip or knee replacement surgery, oral iron therapy, particularly if combined with restrictive transfusion protocols, has been found to be effective in the treatment of pre-operative anaemia, in limiting transfusion needs and, in some cases, also reducing the duration of time spent in hospital28–30.
It is suggested that oral iron therapy is used for the treatment of pre-operative iron-deficiency and to minimise transfusion requirements in adult patients who are candidates for elective major orthopaedic surgery [2B].
Intravenous iron therapy
The pre-operative use of intravenous iron to treat adult patients with iron-deficiency anaemia who are candidates for major surgery, including orthopaedic surgery, has been shown to be effective in correcting anaemia and limiting transfusion requirements31–33.
A recent randomised, multicentre study demonstrated the superiority (and safety) of infusion of iron carboxymaltose with respect to iron therapy per os in the correction of iron-deficiency anaemia in patients with a poor response to oral therapy34.
It is suggested that intravenous iron therapy is used for the treatment of pre-operative iron-deficiency anaemia and to limit transfusion requirements in adult patients who are candidates for elective major orthopaedic surgery [2A].
Post-operative period
Detection of anaemia
Anaemia develops in 74% of patients during hospitalisation (including those admitted with normal Hb values) and, besides causing a substantial use of resources, in part because of the prolongation of the time spent in hospital, is associated with significant increases in mortality35 and morbidity11. The prevalence of anaemia in the post-operative period in patients who have undergone elective or urgent major orthopaedic surgery is much higher, being 51% and 87%, respectively16. Furthermore, anaemia, which can be present in as many as 90% of surgical patients15, although caused mainly by peri-operative bleeding, can be worsened by repeated withdrawal of blood for laboratory tests and by ineffective erythropoiesis due to the inflammatory response induced by the surgical procedure itself36,37. This inflammation reduces the availability of iron for erythropoiesis through inhibition of its intestinal absorption and a reduction in hepcidin-mediated mobilisation of iron stores23,38–40.
The best strategy to prevent post-operative anaemia and avoid transfusions is to detect and correct any anaemia present in the pre-operative period, when possible11.
However, most adults in good health with normal baseline levels of Hb do not normally require transfusion therapy with packed red cells following a surgical intervention provided that the loss of blood during the intervention is less than 1,000 mL and the intravascular volume is maintained with crystalloids or colloids12,41.
Stimulation of erythropoiesis
Oral iron therapy
As already mentioned, the ineffective erythropoiesis caused by the post-operative inflammatory response36,37, which inhibits intestinal absorption of iron and reduces the mobilisation of iron stores, makes oral iron therapy unfeasible in the post-operative period23,38–40.
Indeed, various randomised studies have demonstrated that oral iron therapy in patients undergoing elective or urgent orthopaedic surgery is not only not effective at correcting the post-operative anaemia and reducing transfusion requirements, but is also often associated with the development of adverse events42–44.
Oral iron therapy is not recommended for the treatment of post-operative anaemia and minimisation of transfusion needs in patients undergoing elective or urgent major orthopaedic surgery [1B].
Intravenous iron therapy
Intravenous administration of iron in the post-operative period, in patients who have undergone lower limb arthroplasty45,46 or correction of scoliosis47, is effective at correcting anaemia and limiting transfusion needs.
It is suggested that intravenous iron therapy is used for the treatment of post-operative anaemia and to limit transfusion needs in patients undergoing elective major orthopaedic surgery [2C].
Short-term management in the peri-operative period
Stimulation of erythropoiesis
Iron therapy
In orthopaedic patients with hip fractures, the short-term use of intravenous iron and adoption of restrictive transfusion strategies were demonstrated to be effective in limiting transfusion requirements, particularly in non-anaemic patients48–50; other studies, in anaemic patients, showed that intravenous iron was more effective if combined with recombinant human erythropoietin51,52.
Similar results were obtained in patients undergoing knee replacement surgery53. Recently a large, observational study in patients who underwent elective lower limb arthroplasty or urgent surgery for hip fracture further confirmed the above described results: short-term infusions of iron were found to be effective with or without concomitant treatment with erythropoietin54.
Short-term treatment with intravenous iron is suggested in order to minimise transfusion requirements in adult patients undergoing elective major orthopaedic surgery who are at risk of severe anaemia in the post-operative period [2B].
Dose of iron therapy
Oral iron
A recently published randomised clinical trial in critically ill patients with iron-deficiency anaemia demonstrated that 65 mg of elemental iron/die per os are effective at reducing transfusion requirements.
A dose of 100 mg of elemental iron/die for 2–6 weeks prior to the surgical intervention is recommended for the treatment of pre-operative anaemia [1C]55.
Intravenous iron
It is suggested that the dose of intravenous iron needed to replenish iron stores is calculated using Ganzoni’s formula19,56: “total iron requirements (mg) = [desired Hb – actual Hb (mg/dL)] × weight (kg) × 0.24 + 500 mg (for iron stores)” [2C].
The therapeutic regimen to adopt varies depending on the formulation of iron used.
It is suggested administering 200 mg of elemental iron intravenously for every 500 mL of blood lost [2C]27.
Safety of intravenous iron therapy
Numerous studies performed in thousands of patients in different clinical settings have demonstrated the safety of intravenous iron therapy57,58. The European Medicines Agency (EMA) recently published “New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines”59. A year later, in 2014, Rampton et al. published guidelines for the management of hypersensitivity reactions to intravenous iron60.
According to the Committee for Medicinal Products for Human Use (CHMP) of the EMA: a) intravenous iron-based medicines should be used when oral iron cannot be used or does not work, particularly in patients in dialysis, in the peri-operative period, or in the presence of disorders of gastrointestinal absorption; b) the benefits of intravenous iron outweigh its risks, provided that appropriate measures are taken to minimise the possibility of allergic reactions; c) the data on the risk of hypersensitivity come mainly from spontaneous post-marketing notifications and the total number of deaths or life-threatening events is low; d) these data cannot be used to pick up any differences in the safety profile of the various iron-containing preparations.
The CHMP of the EMA has also produced the following recommendations for healthcare staff, with the aim of improving patients’ safety.
Intravenous iron must only be administered when “staff trained to evaluate and manage anaphylactic and anaphylactoid reactions” and “resuscitation facilities” are immediately available.
A test dose is no longer recommended.
If a hypersensitivity reaction occurs, “healthcare professionals should immediately stop the iron administration and consider appropriate treatment for the hypersensitivity reaction”.
“Patients should be closely observed for signs and symptoms of hypersensitivity reactions during and for at least 30 minutes following each injection of an intravenous iron medicine.”
Intravenous iron is contraindicated in patients with hypersensitivity to the active substance or excipients or to other iron-containing products administered parenterally.
The risk of hypersensitivity is increased “in patients with known allergies or immune or inflammatory conditions and in patients with a history of severe asthma, eczema or other atopic allergy.”
“Intravenous iron products should not be used during pregnancy unless clearly necessary” and their use “should be confined to the second or third trimester, provided the benefits of treatment clearly outweigh the potential serious risks to the foetus such as anoxia and foetal distress.”
As far as concerns the safety of intravenous iron, Auerbach and Macdougall state that, based on all retrospective and prospective studies, provided that high molecular weight iron dextran is avoided, which is no longer on the market, all the other preparations are safe and probably much safer than most doctors perceive58,61.
In contrast, as far as concerns the risk of infections the data currently available in the literature do not allow definitive conclusions to be drawn and, for this reason, it is suggested that intravenous iron therapy is avoided in patients with acute infections [2C]27,57.
It is suggested that intravenous iron is not administered to patients with ferritin values >300–500 ng/mL and with a transferrin saturation >50% [2C]27.
Use of erythropoietin in the peri-operative period
There are two possible strategies for the use of erythropoietin in the peri-operative period12: the erythropoietin can be administered to optimise autologous donation, in the very few cases in which autologous donation is indicated, or it can be used in patients who are candidates for elective surgery who cannot complete a predeposit programme, in the very few cases in which such a programme is indicated. However, the prescription of erythropoietin α, β and z is currently paid for by the National Health Service if used as a treatment to increase the amount of autologous blood in the setting of a pre-operative deposit programme, with the limitations set out in the technical data sheet. Erythropoietin α can also be charged to the National Health Service when prescribed to reduce allogeneic transfusions in adults patients who are candidates for elective major orthopaedic surgery considered to involve a high risk of complications requiring transfusion, for which a pre-operative autologous blood donation programme is not available.
Erythropoietin has been found to be effective in limiting transfusion requirements in candidates for lower limb elective joint replacement surgery62–64, although the costs are unacceptable65.
It is suggested that erythropoietin is administered to adult candidates for elective major orthopaedic surgery who undergo a pre-deposit programme in which the donation of at least three units of whole blood is planned or for which a pre-deposit programme is not available and it is expected that the blood loss will be greater than 1,000 mL [2B].
In order to avoid a “functional deficiency” of iron during treatment with erythropoietin, it is suggested that intravenous iron is administered [2B]12,66–69.
Minimisation of blood loss
Pre-operative period
Pre-deposit
Pre-deposit autologous blood transfusion consists in collecting units of blood from the patient (pre-deposit), storing them (without fractionation) and using them exclusively for the patient-donor. It has been widely shown that pre-depositing blood increases the risk of requiring transfusion therapy, including allogeneic transfusions70.
In adult candidates for elective major orthopaedic surgery, it is recommended that the practice of pre-deposit is limited to subjects with rare red blood cell groups or complex alloimmunisation, for whom it is impossible to find compatible blood components [1A]12,69.
In any case, there is no need for autologous blood collection if the patient’s basal Hb is such that, considering peri-operative losses, a stable, post-operative Hb of 100 g/L or more can be expected.
Contraindications to the collection of autologous blood are:
- Hb values lower than the threshold values indicated by the WHO to define anaemia [children up 5 years: 110 g/L; children between 5 and 12 years old: 115 g/L; children between 12 and 15 years: 120 g/L; pregnant women: 110 g/L; women who are not pregnant (aged 15 years old or more): 120 g/L; men (aged 15 years or more): 130 g/L]26;
- severe heart disease;
- positivity for one of the following tests, the results of which must be known before starting the autologous pre-deposit programme: HBsAg, anti-HCV antibodies, anti-HIV 1–2 antibodies;
- epilepsy;
- ongoing bacteraemia.
However, even in the presence of criteria for exclusion from autologous blood collection, a patient can, exceptionally, be accepted if the indications are appropriate and there are specific, documented clinical circumstances motivating the recourse to autologous donation.
It is recommended that the interval between collecting one unit of autologous blood and the next is at least 7 days and that, in all cases, the last unit is collected at least 7 days before the planned operation [1C]71–76.
Identifying and managing the risk of bleeding
The second pillar of PBM includes all the strategies to minimise bleeding and preserve the individual blood reserves.
These strategies are applied right from the pre-operative stage, through the definition of parameters to stratify bleeding risk, that is, a detailed history and thorough clinical examination. Much care must be given to the patient’s drug history, since the number of patients being treated with antiplatelet agents, anticoagulants, anti-inflammatory drugs and antidepressants or herbal products with antiplatelet effects is increasing continuously.
Recent guidelines (British, Australian and Italian)12,77–79 recommend the use of structured questionnaires to reduce bleeding and preserve individual blood reserves through the identification of patients at risk of bleeding and for a potential quantification of the bleeding risk in patients with congenital clotting disorders80.
In fact, in the context of pre-operative screening, a standardised questionnaire covering the patient’s clinical and drug history seems to be superior to an assessment based only on the results of common laboratory tests [activated partial thromboplastin time (aPTT), prothrombin time /International Normalized Ratio (PT/INR), platelet count]81.
The second important element is the physical examination of the patient, which is aimed at detecting any signs of cutaneous bleeding (petechiae, ecchymoses, haematomas) which could suggest the presence of liver disease, a congenital clotting disorder or platelet disorder82.
Although various guidelines recommend the use of standard laboratory tests (aPTT, PT, platelet count) in the pre-operative period to define the bleeding risk77,79,83, a systematic review demonstrated that altered coagulation screening tests in the pre-operative period are not predictive of intra- or post-operative bleeding84. However, given their low cost and for reasons of prudence, the guidelines of the Italian Society for the Study of Haemostasis and Thrombosis (SISET) suggest that these tests are performed79.
Low plasma levels of fibrinogen seem to be predictive of an increased risk of intra-operative bleeding in heart surgery85.
Results of clotting tests performed in the pre-operative period with point-of-care (POC) instruments do not predict bleeding during or after surgery86,87, whereas monitoring haemostasis by POC instruments during the intra-operative phase is useful for assessing the causes of bleeding.
Although there are reports on possible correlations between some haemostasis-related genetic polymorphisms or mutations and an increased risk of surgical bleeding, at present it is not possible to assign a clear predictive value to genetic tests88.
Platelet function can be assessed through the use of several analysers (PFA 100/200, CPA Impact-R, MEA Multiplate, PlateletWorks, VerifyNow), which have a varied sensitivity for the different antiplatelet drugs. However, given the variability and poor standardisation of tests to determine platelet function, the SISET guidelines do not recommend their routine use prior to surgery79. However, the guidelines of the European Society of Anaesthesiology suggest evaluating platelet function in the case of a positive history of bleeding and in the case of known alterations in platelet function because of congenital disorders or medication use88.
Correct pre-operative management of the bleeding risk in surgical patients involves appropriate interventions to acquired factors (drugs, diseases) or congenital conditions predisposing to bleeding.
It is recommended that a careful personal and family history is taken from the patient in order to pick up any bleeding risk, information about ongoing medication use or the consumption of any over-the-counter or herbal products, because this assessment is considered more informative of peri-operative bleeding risk than isolated evaluation of results of pre-operative screening coagulation tests [1C].
It is suggested that the platelet count, PT and aPTT are determined before every surgical intervention or invasive procedure that carries a risk of bleeding [2C].
In the case of a positive history of bleeding, it is suggested that an expert in haemostasis is consulted in order to diagnose possible bleeding syndromes [2C].
Minimising iatrogenic blood loss and planning the procedure
Management of antiplatelet treatment
It is known that cyclo-oxygenase-2-selective non-steroidal anti-inflammatory drugs are not responsible for increased bleeding during total knee or hip replacement89–91 and, for this reason, it is not necessary that the use of these drugs is suspended before elective lower limb arthroplasty. In contrast, ibuprofen, diclofenac and indomethacin significantly increase blood loss during total knee replacement surgery92, and so the use of these drugs should be suspended.
Monotherapy with aspirin (ASA) or clopidogrel does not need to be stopped before urgent orthopaedic surgery nor is it necessary to delay surgery in patients taking these drugs93,94.
According to a recent Italian intersociety consensus statement95 ASA, if taken for primary prevention, should be suspended 7 days before elective arthroplasties whereas it should be stopped on admission to hospital in the case of fracture of the femoral neck. If it is being taken for secondary prevention (i.e., in a patient who has had a previous cardiovascular event), it should be continued in the peri-operative period at a dose of 75–100 mg/die.
It is suggested that cyclo-oxygenase-2-selective non-steroidal anti-inflammatory drugs are not suspended prior to elective lower limb joint replacement surgery [2B].
It is suggested that ibuprofen, diclofenac and indomethacin are suspended prior to elective lower limb joint replacement surgery [2B].
It is recommended that ASA monotherapy, being taken for secondary prevention, is not suspended before elective lower limb joint replacement surgery [1B].
Ticlopidine and clopidogrel belong to the family of thienopyridines, being first- and second-generation examples, respectively; both inhibit ADP-induced platelet activation by binding to the P2Y12 receptor. Prasugrel is a third-generation thienopyridine, which must be converted to its active metabolite before binding to the P2Y12 platelet receptor. Thienopyridines have much stronger antiplatelet activity than ASA.
Various studies have described peri-operative bleeding complications in association with the use of clopidogrel and the risk of bleeding can increase when clopidogrel is given together with ASA. At present, there are no data available on the use of prasugrel in the peri-operative period; the platelet inhibition induced by this drug lasts at least 7 days88.
Ticagrelor, another antiplatelet agent, unlike the thienopyridines, has a direct effect on the P2Y12 receptor, without requiring biotransformation by the P450 cytochrome; it has a rapid onset of action and the platelet inhibition decreases to 10% after about 4.5 days96.
Since clopidogrel and prasugrel are responsible for peri-operative bleeding, it is recommended that these drugs are suspended 5 and 7 days, respectively, before surgery, in cases of increased bleeding risk [1C].
It is suggested that ticagrelor is suspended 5 days pre-operatively [2C].
Patients with acute coronary syndrome or those undergoing angioplasty benefit from dual antiplatelet therapy with a combination of ASA and another platelet anti-aggregant drug (thienopyridine or ticagrelor), even if this increases the risk of bleeding complications97.
In the case of urgent or emergency surgery, it is recommended that the decision regarding the continuation of treatment with antiplatelet drugs in the peri-operative period is the result of a multidisciplinary evaluation [1C].
It is suggested that urgent or emergency surgical interventions are performed, maintaining dual antiplatelet therapy (ASA/clopidogrel; ASA/prasugrel; ASA/ticagrelor) or at least ASA, when the bleeding risk is high [2C].
Oral dual antiplatelet therapy is necessary for patients with stents, in whom the suspension of one or both of the anti-aggregant drugs, particularly in the first months after the procedure, carries a significant risk of thrombosis of the stent, which is a potentially fatal event98. The growing number of coronary artery revascularisation procedures carried out each year is inevitably leading to an increase in the number of patients with coronary artery stents who must undergo surgical interventions. The management of antiplatelet therapy in these patients is often arbitrary, despite it being a critical factor in the prevention of ischaemic-haemorrhagic complications.
It is recommended that elective orthopaedic surgery is not performed during the first 3 months after implantation of a metal stent or during the first 12 months after implantation of a drug-eluting stent [1C].
Management of anticoagulant therapy
Vitamin K antagonists (VKA) are used for the prophylaxis or treatment of thromboembolic events, particularly in patients with mechanical heart valves, atrial fibrillation or a past history of venous thromboembolism. It is not always necessary to suspend the use of these drugs and the decision depends on the type and site of the operation or invasive procedure88,99,100.
In patients with a low/medium risk of thromboembolism, it is suggested that VKA treatment is suspended 5 days before the planned elective joint replacement surgery and bridging therapy is established [administering low molecular weight heparin (LMWH) at prophylactic doses] in the following way: last dose of VKA on day −5; first subcutaneous dose of LMWH once a day, starting on day −4 if the patient was being treated with acenocoumarol, and starting on day – 3 if the patient was being treated with warfarin [2C].
In patients at high risk of thromboembolism [i.e., with atrial fibrillation and a CHADS2 (Congestive heart failure, Hypertension, Age ≥75 years, Diabetes mellitus, prior Stroke or transient ischaemic attack or thromboembolism) score >2; with recurrent venous thromboembolism treated for less than 3 months; with a mechanical heart valve] it is recommended that bridging therapy (LMWH at therapeutic doses) is given in the following manner: last dose of the VKA on day −5; first subcutaneous dose of LMWH twice daily starting from day −4 if the patient was being treated with acenocoumarol, and starting from day −3 if the patient was being treated with warfarin [1C].
An Italian study demonstrated the efficacy and safety of using reduced therapeutic doses (65–70 IU/kg twice daily) instead of the classical bridging therapy with 100 IU/kg twice daily100.
It is suggested that the last dose of LMWH is administered 12 hours before a planned operation and/or invasive procedure, except when a full dose of anticoagulant is being used, in which case an interval of 24 hours is suggested [2C].
The new oral anticoagulants
Dabigatran etexilate is an oral inhibitor of thrombin. It has a half-life of 14–17 hours, is eliminated mostly (80%) in the urine, does not interact with food and does not necessitate blood tests for monitoring. It is indicated for the prevention of venous thromboembolism in major orthopaedic surgery, for the prevention of emboli in patients with atrial fibrillation and in the treatment and secondary prevention of deep vein thrombosis and pulmonary embolism101–105.
Rivaroxaban and apixaban are oral inhibitors of activated factor X (FXa). They have half-lives of 9–12 hours and 10–15 hours, respectively; rivaroxaban is eliminated mainly through the kidneys (although only half in an active form), while apixaban is eliminated partly through the hepatic route and partly in the urine. Both are indicated for the prevention of venous thromboembolism in major orthopaedic surgery, for the prevention of emboli in patients with atrial fibrillation and for secondary prevention of deep vein thrombosis and pulmonary embolism106–113.
The management of new oral anticoagulant (NOAC) use during surgical interventions is based largely on the consensus of opinions of experts, although the experts have produced contrasting advice, particularly with regards to the indication for and duration of bridging therapy with parenteral anticoagulants, which is proposed by some experts also for patients being treated with NOAC. Indeed, this is the strategy suggested by the recent guidelines from the European Society of Anaesthesiology88. Some scientific societies have suggested indications for suspending or not suspending treatment with NOAC114. Based on the pharmacokinetic and pharmacodynamics characteristics of NOAC, a temporary (short-term) suspension of these drugs is possible without requiring bridging therapy, which would expose the patient to a higher risk of bleeding, as demonstrated by recent registry data115. For this reason the following recommendations and suggestions reflect the literature data and recent guidelines of the European Heart Rhythm Association (EHRA), which do not include the use of bridging therapy114,116–118.
Table II shows the stratification of bleeding risk in relationship to invasive procedures or surgical interventions: the bleeding risk is defined as “clinically unimportant”, “low” or “high”114.
Table II.
Classification of elective surgical interventions divided according to bleeding risk. (Modified from Heidbuchel H et al.114)
Interventions with a clinically unimportant risk of bleeding:
|
Interventions with a low risk of bleeding:
|
Interventions with a high risk of bleeding:
|
It is suggested that NOAC (dabigatran, rivaroxaban, apixaban) are not suspended and that the operation is performed 12–24 hours (depending on whether the drug is administered, respectively, once or twice a day) after the last dose in the case of: dermatological surgery, dental procedures, gastroscopy and colonoscopy (without biopsies), ocular interventions (particularly of the anterior chamber, such as cataract surgery) and operations involving a clinically unimportant risk of bleeding (see Table II) [2C]114.
It is suggested that NOAC are suspended 24 hours before elective surgery that carries a low risk of bleeding, in patients with normal renal function [creatinine clearance (CrCl) ≥80 mL/minute] [2C].
It is suggested that NOAC are suspended 48 hours before elective surgery that carries a high risk of bleeding in patients with normal renal function (CrCl ≥80 mL/minute) [2C].
In patients with impaired renal function, the suspension of the NOAC should be graduated according to the type of drug and the CrCl, as indicated in Table III114.
Table III.
When to suspend treatment with new oral anticoagulants before surgery on the basis of the patient’s renal function (determined by creatinine clearance [CrCl]) and bleeding risk (low or high, see Table II) associated with the surgical procedure. (Modified from Heidbuchel H et al.114)
| CrCl (mL/minute) | Bleeding risk associated with the surgical procedure | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| New oral anticoagulants | ||||||
|
| ||||||
| Dabigatran | Apixaban | Rivaroxaban | ||||
|
|
|
|
||||
| Low | High | Low | High | Low | High | |
| ≥80 | ≥24 h | ≥48 h | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| 50–80 | ≥36 h | ≥72 h | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| 30–50 | ≥48 h | ≥96 h | ≥24 h | ≥48 h | ≥24 h | ≥48 h |
| 15–30 | NI | NI | ≥36 h | ≥48 h | ≥36 h | ≥48 h |
| <15 | NI | |||||
CrCl: creatinine clearance; h: hours; NI: use of drug not indicated.
It is suggested that rivaroxaban and apixaban are suspended 36 or 48 hours before surgery with a low or high bleeding risk, respectively, in patients with a CrCl between 15–30 mL/minute; that dabigatran is suspended 36 or 72 hours before surgery with a low or high bleeding risk, respectively, in patients with a CrCl between 50–80 mL/minute; and that dabigatran is suspended 48 or 96 hours before surgery with a low or high risk of bleeding respectively, in patients with a CrCl between 30–50 mL/minute [2C].
New oral anticoagulants and laboratory tests
The NOAC do not require routine monitoring through coagulation tests; in any case, global tests, such as the PT and aPTT, are not useful for quantifying the anticoagulant effect of these new drugs and other quantitative tests are not yet available for routine use in all hospitals. Furthermore, POC instruments to determine the INR should not be used in patients being treated with NOAC. However, in emergencies (severe haemorrhage, thrombotic events, urgent surgery, liver or kidney failure, suspected overdose or pharmacological interactions) it may be necessary to quantify and have a rough idea of the anticoagulant effect of NOAC through the available coagulation tests; in these cases it is extremely important to know precisely when the drug was taken with respect to the blood sample, since the maximum effect of NOAC occurs at the time of their peak plasma concentration, which is reached 1–3 hours after drug intake114. Thus, for a correct clinical interpretation of the laboratory data, it is essential to know the type of drug taken, the dose and the time the last dose was taken.
At present, there is still no definitive information on whether assays prior to taking the next dose (trough concentration) or at the time of the peak plasma concentration are better indicated. Analyses carried out in the USA by the Food and Drug Administration (FDA) on the phase III studies “Re-LY” and “ROCKET AF” showed that the trough drug level was correlated with clinical events. On the basis of this evidence, it has been proposed that controls should be performed before the patient takes the next dose of the drug (trough concentration).
Both first and second level clotting tests are variably affected by the different anticoagulant drugs. In general, it can be stated that the PT and chromogenic tests for assaying anti-FXa activity are more influenced by anti-FXa drugs (rivaroxaban, apixaban and edoxaban), whereas the aPTT, thrombin time, ecarin clotting time and ecarin chromogenic assay are more affected by drugs that inhibit thrombin (dabigatran).
Two coagulation tests for evaluating the anticoagulant effect of the direct inhibitors of thrombin, such as dabigatran, have so far been identified in the literature: the aPTT and the diluted thrombin time. Alterations of these tests can be indicative of an increased bleeding risk119,120. While the aPTT only provides a qualitative assessment and there is great variability between the different reagents on the market, the diluted thrombin time is able to provide a quantitative evaluation of the level of dabigatran present in the circulation and, considering its relative simplicity, should be used.
The inhibitors of FXa, such as rivaroxaban, cause a prolongation of the PT although this provides only approximate information, given the wide variability of results depending on the type of reagent used121.
An anti-FXa chromogenic test, using calibrators that are readily available on the market, has recently been developed. It is, however, important to clarify that, at present, these new tests (diluted thrombin time, for dabigatran, and anti-FXa activity assay, for the inhibitors of FXa) can provide plain information on the absence of the anticoagulant in the circulation, while there is not a clear, defined relationship between the circulating level and risk of bleeding or thrombosis. This aspect must be studied in the coming years.
At present we know that the concentrations of the different oral anticoagulants can be measured using tests that are specific and sensitive for the individual compounds: these tests are simple, cheap and can be performed in all laboratories. We also know that information regarding the concentration of the anticoagulant drugs in the blood is useful for managing patients who are being assessed for particular clinical situations, such as urgent or elective surgery, invasive procedures, and haemorrhagic or thromboembolic complications. Furthermore, in the same way as for VKA, the results of these tests could be useful in the near future for determining threshold levels of anticoagulation above which surgery, invasive procedures and thrombolysis are contraindicated. Currently, one of the most important practical problems is the limited number of Italian laboratories able to carry out these specific tests; however, the growing use of NOAC and the consequent increase in the clinical needs of treated patients should lead to a progressively greater use of these tests in various different hospital settings.
No evidence-based recommendations can be made on the use of laboratory tests in the pre-operative evaluation of the anticoagulant effect of the NOAC.
The management of patients with comorbidities related to altered haemostasis
Patients with endocrine, metabolic or systemic diseases, such as FX deficiency in amyloidosis, can have altered haemostasis with a tendency to bleeding, similar to that in patients with congenital deficiencies of clotting factors122,123. The treatment strategy for these coagulopathies is frequently unclear.
It is suggested that the treatment of patients with altered haemostasis related to systemic, metabolic or endocrine diseases is established through consultation with an expert in haemostasis and thrombosis [2C].
Other drugs, besides antiplatelet agents and anticoagulants, such as selective serotonin reuptake inhibitors, valproic acid and Ginkgo biloba, can interfere with haemostasis, predisposing to bleeding.
Selective serotonin reuptake inhibitors have been associated with an increased tendency to bleed, due to depletion of serotonin from platelets124; however, the transfusion requirements of patients being treated with selective serotonin reuptake inhibitors who underwent major orthopaedic surgery or heart surgery were not increased125,126.
In some cases valproic acid (an anti-epileptic drug) can lead to a reduction in the levels of some clotting factors [FVII, FVIII, FXIII, von Willebrand factor (vWF), fibrinogen], platelets, protein C and antithrombin127; however, these changes did not cause bleeding complications128,129.
As far as concerns the extract of Ginkgo biloba, a meta-analysis of 18 randomised, controlled trials did not show an increase in bleeding associated with daily, oral intake of this medicinal plant130.
It is suggested that treatment with selective serotonin release inhibitors is not routinely suspended in patients undergoing surgery [2B].
When selective serotonin reuptake inhibitors are used together with antiplatelet agents, it is suggested that the patient is assessed individually to define the strategy to adopt prior to surgery [2C].
In the case of surgery, it is suggested considering, on an individual basis and with specialist advice, the suspension of treatment with valproic acid, because this drug can promote bleeding [2C].
It is recommended that the use of Ginko biloba extract is not suspended in the case of surgical interventions [1B].
Management of the patient with a congenital bleeding disorder
Defects of primary haemostasis
The most common congenital bleeding disorder is certainly that of hereditary deficiency of vWF, which has prevalence of 0.6–1.3% in the population13,131. The disease is due to either a lack of vWF or dysfunction of the protein and is classified into three types: type 1: partial quantitative defect; type 2: qualitative defect, of which there are four variants: 2A, 2B, 2M, and 2N; and type 3: total lack of vWF. The acquired form of von Willebrand’s disease (vWD) is the result of autoimmune and/or neoplastic disorders, or has a drug-based aetiology132.
The bleeding tendency is a consequence of altered platelet adhesion because of a lack or dysfunction of vWF and/or reduced levels of FVIII13,131,132.
Given that the laboratory diagnosis of vWD is complex, the laboratory tests must always be guided by the patient’s clinical history and physical examination. Additionally, there are questionnaires and bleeding scores that have been specifically designed to evaluate and quantify bleeding risk133–135.
The prevention and management of bleeding in vWD is based on three treatment strategies: administration of desmopressin, which induces mobilisation of vWF from endothelial storage sites (stimulation therapy); administration of plasma-derived concentrates of vWF or vWF-rich FVIII (replacement therapy); administration of antifibrinolytics or platelet transfusions (haemostatic therapy). There are numerous national and international guidelines on this subject13,131,136–140.
It is recommended that patients with vWD are managed in the pre-operative period in collaboration with an expert in haemostasis and thrombosis [1C].
It is recommended that criteria are used to define and evaluate bleeding risk in patients with vWD [1C].
It is recommended that patients with vWD who are to undergo minor surgery and are expected to have mild bleeding are given desmopressin, after a trial test, at doses explained in specific guidelinese (0.3 μg/kg diluted in 50 mL of saline solution and infused slowly, over more than 30 minutes) [1C]136–140.
It is recommended that patients with vWD who are to undergo major surgery and are expected to have clinically relevant bleeding are given replacement therapy with plasma-derived vWF or vWF-rich FVIII, using the treatment regimens explained in the specific guidelines [1C].
In patients with vWD who are to undergo major surgery, it is suggested that antifibrinolytics, as an adjuvant to more specific treatments, and platelet transfusions are used only in the case of failure of other treatments [2C].
In the context of disorders of primary haemostasis, platelet dysfunction is a diagnostic challenge. There does not seem to be a correlation between the severity of bleeding, the degree of vWF/platelet dysfunction and diagnostic laboratory tests, so that disorders of platelet function constitute a risk factor for bleeding rather than an unequivocal cause.
The use of platelet function tests with the PFA 100/200 cannot be recommended in patients with defects of primary haemostasis because of the poor sensitivity of the tests as well as false positive and false negative results141–143.
The most common and least severe platelet disorders respond well to desmopressin, which shortens the bleeding time, whether used as prophylaxis or for the treatment of bleeding144–146. When used, the standard dose is 0.3 μg/kg, diluted in 50 mL of saline solution and infused slowly, over more than 30 minutes144.
Antifibrinolytics are useful as an adjunctive therapy in platelet disorders; small bleeds, such as those occurring in dental surgery, can respond well to these agents, even when used alone. The use of antifibrinolytic agents for the treatment of hereditary platelet disorders is not, however, based on evidence, although tranexamic acid (TXA) can partially correct the effects of clopidogrel on primary haemostasis142,144,147,148.
In patients with hereditary disorders of platelet function, it is suggested that desmopressin is used for the prevention and control of bleeding, and that TXA is used as an adjuvant [2C].
The best known congenital platelet disorders are Glanzmann’s thrombasthenia and Bernard-Soulier syndrome. These are characterised, respectively, by defective platelet aggregation and adhesion, due to alterations in platelet membrane glycoproteins (GP IIb/IIIa in Glanzmann’s thrombasthenia and GP Ib-IX in Bernard-Soulier syndrome).
In most patients with these disorders, mild mucocutaneous bleeding responds to treatment with antifibrinolytic agents. However, the use of recombinant activated factor VII (rFVIIa) is indicated for surgery and invasive procedures (including dental extractions, sometimes), even if the technical data sheet states that it is indicated only for those patients with Glanzmann’s thrombasthenia who have antibodies to GPIIb/IIIa and/or human leucocyte antigens or have become refractory to platelet transfusions149,150.
When used, rFVIIa should be administered at a dose of 90 μg/kg, immediately before the intervention, repeated every 2 hours for the first 12 hours and, subsequently, every 3–4 hours until the bleeding risk has disappeared151.
However, there are no universally accepted doses of rFVIIa defined for the various different settings of use. rFVIIa is not indicated in other forms of platelet disorders, for which platelet transfusions are necessary.
It is recommended considering the use of rFVIIa in patients with Glanzmann’s thrombasthenia who are to undergo surgical interventions [1C].
In patients with Glanzmann’s thrombasthenia or Bernard-Soulier syndrome undergoing elective, major surgery, it is suggested that platelet concentrates are transfused when other therapeutic options, including rFVIIa, do not guarantee a therapeutic effect [2C]142,145.
It is suggested that the first doses of platelets are administered immediately before the intervention and further doses given after it, depending on clinical need [2C].
In cases of urgent surgery, single-unit platelet concentrates can be given, despite the awareness of the high risk of alloimmunisation and the consequent, subsequent limitation of response to this treatment145.
As far as concerns treatment, the hereditary platelet disorders are considered moderate platelet disorders and, in the absence of platelet dysfunction, should be treated on the basis of the platelet count. Guidelines on platelet transfusions suggest a threshold of 50×109 platelets/L for major surgery or invasive procedures152–155 [liver biopsy, laparotomy, diagnostic lumbar puncture, insertion of central venous catheters (recent North-American guidelines suggest a threshold of 20×109 platelets/L for this last procedure)]155 and a threshold of 100×109 platelets/L for neurosurgical or ophthalmological interventions152–154. However, there is still insufficient evidence to recommend a threshold for prophylactic transfusions in the peri-operative period in patients with hereditary platelet disorders.
It is recommended that platelet transfusion are not used routinely in patients with hereditary platelet disorders [1C].
In patients with hereditary platelet disorders no evidence-based recommendations can be made concerning the threshold to adopt in the peri-operative period for prophylactic transfusion therapy with platelet concentrates.
Defects of haemostasis related to clotting factor deficiencies
Congenital deficiency of FVIII and FIX in the plasma causes haemophilia A and haemophilia B, respectively.
The prevalence of haemophilia A in the population is 1:10,000, while that of haemophilia B is 1:60,000. The clinical manifestations of haemophilia are spontaneous bleeding, mainly within joints, and excessive bleeding in the case of trauma and/or surgical interventions. The severity of the bleeding is related to the extent of the factor deficiency. Both forms of haemophilia are classified as mild, moderate or severe, depending on the level of FVIII or FIX present.
In the severe forms of haemophilia, replacement therapy can lead to the development of antibodies against FVIII or FIX, known as “inhibitors”, a critical circumstance that requires various therapeutic strategies.
Anti-FVIII autoantibodies cause the bleeding disorder known as acquired haemophilia, which is rare condition characterised by a predisposition to potentially dangerous bleeding. This disorder is usually associated with cancer, autoimmune disorders, drugs or pregnancy.
The treatment of haemophilia is essentially replacement therapy with plasma-derived or recombinant concentrates of the deficient clotting factor (FVIII or FIX). Mild haemophilia A can also be treated with desmopressin and TXA, instead of replacement of the deficient factor with concentrates.
Despite the wide variability in the doses of concentrates used for the prophylaxis or treatment of bleeding, in the case of surgery, the World Federation of Haemophilia recommends that the levels of the deficient factor are 80–100% in the pre-operative period156; it also recommends that the levels are maintained around 60–80% in the first 3 days after the operation, around 40–60% for the next 3 days and around 30–50% in the second week after surgery.
In the case of haemophilia B, the recommended factor levels are slightly lower: 60–80%, 40–60%, 30–50% and 20–40%, respectively140,156–162.
In conclusion, the recommendations for the management of patients with haemophilia or other congenital bleeding disorders are the following.
Collaboration with an expert in haemostasis and thrombosis is recommended during the planning of the surgical intervention [1C].
It is recommended that adequate replacement therapy is given during the peri-operative period [1C].
It is suggested that guidelines on how to perform replacement therapy (target level of deficient factor and duration of treatment) are followed for patients who are candidates for elective surgery [2C].
With regards to replacement therapy in the peri-operative period, it is recommended that either recombinant or plasma-derived concentrate is used [1C].
In the presence of inhibitor, treatment with rFVIIa or activated prothrombin complex concentrate (PCC), i.e. a factor eight inhibitor bypassing agent (FEIBA), is suggested [2C].
A recent, prospective study carried out on 24 haemophilic patients undergoing orthopaedic surgery showed the presence of subclinical deep vein thromboses in over 10% of cases. On the basis of these results, antithrombotic prophylaxis could also be indicated in haemophilic patients, in individual cases. Many Haemophilia Centres in Europe use pharmacological antithrombotic prophylaxis after orthopaedic surgery163.
For haemophiliacs undergoing major surgery, it is suggested that peri-operative thromboprophylaxis is individualised [2C].
Congenital deficiencies of clotting factors other than FVIII and FIX are very rare and have a prevalence of between 1:500,000 and 1:2,000,000164; the prevalence of autosomal dominant FXI deficiency is 1:30,000, but the most common of these deficiencies is that of FVII.
The levels of evidence on treatment of these deficiencies are low (descriptive studies and experts’ opinions) and data on pre-operative prophylactic therapy are scarce.
In cases of major surgery in patients with FVII deficiency, the proposed threshold for replacement therapy with FVII concentrate is 10% of the normal plasma level164–166. Above this level replacement therapy does not seem to be necessary, as demonstrated by a retrospective analysis of surgical procedures conducted without giving such therapy and during which the frequency of bleeding events was 15%167.
rFVIIa is the treatment of choice for congenital FVII deficiency; if this is not available, a plasma-derived concentrate of FVII is preferable to PCC, because of the potential prothrombotic effect of the latter140,164.
In congenital FVII deficiency, as an alternative to rFVIIa, it is suggested that a plasma-derived concentrate of FVII is used at the dose of 10–40 IU/kg [2C]164.
In patient with congenital FVII deficiency who are to undergo major surgery, it is suggested using a dose of rFVIIa of 15–30 μg/kg every 4–6 hours, usually giving at least three doses [2B]164.
In cases of congenital deficiency of fibrinogen or FXIII the British guidelines recommend using specific concentrates164. Replacement therapy is indicated in hypofibrinogenaemia when the concentration of fibrinogen is <1 g/L or <20–30% of normal. However, the possible risk of thrombosis must be kept in mind when using plasma-derived concentrates.
Fresh-frozen plasma, preferably industry-produced and virus-inactivated, is the only therapeutic option in patients with congenital FV or FXI deficiency164. There is a plasma-derived concentrate of FXI on the market, but it is not currently available in Italy. Patients with FXI deficiency and inhibitors have been treated with success, on the occasion of surgery, with low doses of rFVIIa (33–47 μg/kg)168; contemporaneous administration of TXA has also been demonstrated to be effective in controlling bleeding164.
PCC are the reference concentrates for deficiencies of FII and FX, for which specific concentrates are not available164,169,170.
In patients with other clotting factor deficiencies, no evidence-based recommendations can be made on the peri-operative use of rFVIIa, desmopressin or TXA.
Management of patients with acquired platelet disorders
To reduce the risk of bleeding associated with major surgery or invasive procedures in patients with acquired platelet disorders, it is suggested that a prophylactic transfusion of platelet concentrates is given if the platelet count is below the threshold of 50×109 platelets/L [2C]152–155.
It is suggested that the first doses of platelets are given immediately before the operation and further doses after it, depending on the clinical need [2C].
Intra-operative period
Autologous transfusion techniques
Acute normovolaemic haemodilution
Acute normovolaemic haemodilution is a type of autologous transfusion introduced in the 1970s171–173. It consists in removing at least three or four units of autologous blood, maintaining isovolaemia, immediately before elective surgery174. Acute normovolaemic haemodilution is usually performed after induction of anaesthesia175, just before the surgical incision176. The circulating blood volume is maintained by infusing crystalloids, administering 2–3 mL for every mL of blood removed, or colloids, in a ratio of 1:1 with the volume of blood removed174.
The efficacy of acute normovolaemic haemodilution in reducing the need for allogeneic red blood cell transfusion is, however, doubtful177. Various studies, including prospective, randomised trials, showed that it could reduce recourse to allogeneic transfusion therapy in patients undergoing elective heart surgery, orthopaedic operations (knee replacement), abdominal, vascular, urological, maxillofacial, or liver surgery and in patients undergoing surgery because of burns178–189. However, other studies did not find any substantial benefit or even found an increased need for allogeneic transfusions190–192.
The lack of real benefits from this procedure and the increased risk of requiring transfusion therapy, including allogeneic transfusions, were demonstrated by some meta-analyses193–196 and confirmed by a recent study evaluating the efficacy of autologous transfusion techniques, based on mathematical models76. Other pharmacological strategies for conserving blood, such as the use of TXA, have been found to be more effective than acute normovolaemic haemodilution in limiting the use of allogeneic red blood cell transfusions195,197. Finally, the reduction of overall bleeding is minimal, there is an increase in intra-operative bleeding (heart, liver and thoracic surgery), the relative risk of re-operation because of bleeding is increased and there is a lack of information on the safety of the procedure195,198,199.
The routine use of acute normovolaemic haemodilution as a (single) technique to reduce allogeneic transfusion requirements is not recommended [1A].
Intra-operative blood recovery
Intra-operative blood recovery is a method of saving blood in which blood lost in the surgical field is re-used. This blood is aspirated and anticoagulated before passing into a collection reservoir and from there, through microaggregate filters of varied diameter, into the bowl of a specific cell separator, to be concentrated by centrifugation and then washed with physiological saline before being re-infused back into the patient200.
Intra-operative blood recovery is indicated in many types of elective and urgent surgery, when the blood loss is expected to be at least 1,000 mL or ≥20% of the patient’s circulatory volume200,201.
The meta-analyses currently available show that intra-operative blood recovery significantly reduces the need for allogeneic red blood cell transfusions in patients undergoing elective surgery202–204. A study published in 2013 evaluating the efficacy of autologous blood transfusion techniques, based on mathematical models, indicated that this is the most effective and efficient of such tecniques76. However, a recent randomised, controlled trial, carried out in the specific setting of elective lower limb joint replacement surgery in patients with pre-operative Hb concentrations above 130 g/L, found that intra-operative blood recovery, like post-operative blood recovery, not only did not reduce transfusion needs, but increased costs205.
It is recommended that intra-operative blood recovery is used in major orthopaedic surgery, including vertebral column surgery206–212, only in cases in which blood loss is expected to be at least 1,000 mL or in any case ≥20% of the patient’s circulatory volume despite adopting multimodal strategies, including the use of blood-conserving techniques (pharmacological, surgical and anaesthesiological), and taking into account both the characteristics of the individual patient (bleeding risk, multiple alloimmunisation) and the experience of the surgical and anaesthetic teams [1B]199.
Blood-saving surgical techniques
The greater the expected blood loss, the stronger the advice to use multiple mehtods, appropriate for the clinical circumstances, to reduce bleeding. The use of appropriate combinations of methods has a synergistic effect on reducing blood losses. Meticulous haemostasis and surgical technique are essential. The haemostasis must be achieved through a combination of methods, starting from the surgical approach, which must have the least traumatic impact possible, with well-planned surgical exposure, arriving through the least vascularised tissue planes and with atraumatic management of the tissues213. Furthermore, immediately before the surgical incision, a dose of local anaesthetic and adrenaline can be given at the incision site in order to obtain rapid, localised vasoconstriction. The duration of the intervention must be minimised and, in cases of complex procedures in which repeated surgery is necessary, particularly in patients with multiple trauma, surgery should be fractionated and conducted in stages214.
In the intra-operative phase the surgeon has various instruments available to achieve perfect haemostasis, such as monopolar or bipolar electrocautery devices, ultrasound cautery probes and argon coagulators; these last two are not commonly used in orthopaedic surgery and are mainly reserved for very selected cases, in particular patients with cancer215.
As far as concerns knee arthroplasty, although special surgical techniques and instruments, which avoid violation of both the tibial and femoral intramedullary canals and, therefore, minimise blood losses, were introduced only recently, their use has become consolidated216. However, given the notable pre-operative organisation and greater consumption of resources associated with the sue of these “custom-fit” methods, they are reserved for selected cases; their routine use is also avoided because, at present, there are no studies demonstrating their long-term efficacy in terms of functional outcomes and, furthermore, a recent meta-analysis did not show that they produce an overall improvement in surgical efficiency or that they are cost-effective217.
A survey by the American Association of Hip and Knee Surgeons found that a tourniquet is often used during total knee replacement: the tourniquet must be applied to the root of the limb to be operated only in certain stages of the surgical procedure and after complete “squeezing” of the limb218. However, some studies have associated tourniquet use with complications such as cutaneous or muscular damage, rhabdomyolysis, neurological damage, post-operative stiffness, deep vein thrombosis and pulmonary embolism219,220. Furthermore, a recent meta-analysis provided some evidence that the use of a tourniquet can increase post-operative complications and reduce the range of movements in the early post-operative period, that it does not reduce total blood loss significantly or the need for transfusion therapy, but only decreases intra-operative bleeding221. In contrast, another meta-analysis showed that application of a tourniquet could reduce total blood losses, lower the incidence of transfusions and shorten the duration of the operation, although the same analysis indicated some possible disadvantages and complications associated with tourniquet use222.
With the purpose of containing intra-operative bleeding effectively during elective arthroplasties, it is suggested that combinations of surgical techniques and instruments (considered appropriate for their synergistic effect on reducing blood losses also on the basis of the surgical team’s experience) are used in order to minimise trauma to tissues and vessels and promote local haemostasis, which can also be aided by local administration of vasoconstrictive drugs [2C].
Given the possible post-operative complications and the controversial effect on reducing transfusion needs, no evidence-based recommendations can be made concerning the routine use of a tourniquet as a blood-conserving technique in elective prosthetic joint surgery.
Infusion fluids
The correction of bleeding-induced hypovolaemia by infusion of crystalloids and/or colloids is a priority in the management of patients with acute or subacute bleeding and is the first alternative to allogeneic transfusion, because acute hypovolaemia is less well tolerated than anaemia27. Adequate restoration of the circulatory volume and, consequently, the cardiac output, enables oxygen transport to the tissues to be maintained. However, the best fluid replacement strategy in patients with critical bleeding is still the subject of debate223.
Crystalloid and non-protein-containing colloid solutions are the treatment of first choice199,224. Isotonic 0.9% saline solution, Ringer’s solution and other balanced saline solutions, such as Ringer’s lactate, are those most commonly used at the moment. They are cheap and do not alter haemostasis or renal function27. However, the choice of fluids to administer intravenously during surgical procedures and in critically ill patients is dictated more by clinical practice than by solid evidence225. The debate on the choice of fluid has concentrated mainly on evaluating differences in outcomes depending on whether crystalloid or colloid solutions are used. Recently, however, there has been interest in differences in outcomes in relation to the chlorine content in crystalloid solutions. In fact, new notions on the conventional Starling’s model, regarding microvascular fluid exchange, indicate that the efficacy of colloids in restoring and maintaining depleted intravascular volume is only moderately greater than that of crystalloids. Various very recent randomised controlled trials have demonstrated that the modest improvements in certain physiological end-points, obtained in the short-term with colloids, do not translate into a better outcome for the individual patient. Furthermore, there is substantial evidence that some types of fluids (solutions of hydroxyethyl starch and of albumin) can even have a negative effect on some outcome markers when administered to selected populations of patients.
The most commonly used colloids are the protein-free ones, such as solutions of hydroxyethyl starch or gelatine, and solutions of human albumin (natural colloid)27. Given their low molecular weight, gelatine solutions have a short intravascular half-life (2–3 hours) and a limited capacity to expand plasma (70–80%). Solutions of 6% hydroxyethyl starch have a longer half-life (6–8 hours) and a greater capacity to expand the plasms (80–120%) and are, therefore, the colloids most widely used at present for expanding blood volume; however, when used as volume expanders to correct acute hypovolaemia in patients with haemorrhagic shock, they can increase the risk of mortality and renal failure226. Furthermore, massive use of high molecular weight hydroxyethyl starch solutions can cause alterations of haemostasis characterised by impaired platelet function88,199. Finally, following a review, performed for safety reasons, of the indications for the use of hydroxyethyl starch solutions, restrictions have been applied to the duration of treatment, which must be limited to the initial phase of volume restoration, for a maximum of 24 hours. Furthermore, the following monitoring is expected: a) continuous haemodynamic monitoring; b) electrolytes and fluid balance; c) renal function, for at least 90 days; d) clotting parameters, in the case of repeated administrations227.
Albumin 5% has the capacity to expand the plasma volume by 75% of the volume infused27; its clinical effects, in terms of morbidity and mortality at 28 days, are the same as those of isotonic saline 0.9% when used to restore circulatory volume in patients in intensive care228. For this reason, solutions of albumin 5% should be used as second-line treatment when crystalloid or non-protein colloid solutions have already been used at maximum doses, without having produced an adequate clinical response, and when non-protein colloids are contraindicated199.
In order to correct bleeding-induced hypovolaemia, as a pharmacological alternative to improve oxygen transport, it is recommended that crystalloid or protein-free colloid solutions are used as the treatment of first choice, with albumin 5% as second-line therapy, when crystalloid or non-protein colloid solutions have already been used at maximum doses, without having produced an adequate clinical response, and when non-protein colloids are contraindicated [1A].
It is recommended that hydroxyethyl starch solutions are not used to correct acute hypovolaemia in bleeding patients because of the increased risk of mortality and renal failure [1B].
It is recommended that high molecular weight hydroxyethyl starch solutions are not used, in order to avoid haemostatic alterations characterised by impaired platelet function [1B].
Patients with moderate bleeding
Patients with limited or moderate bleeding (<30% of circulatory volume or <1,300 mL), who are not at risk of further bleeding, can be treated with infusions of crystalloid solutions27,199,229, reserving the non-protein colloids for subjects with haemodynamic instability199,224.
With the purpose of limiting transfusion support in patients with moderate bleeding, it is recommended that the patient’s circulatory volume is first restored using crystalloid or non-protein colloid solutions [1C].
Patients with severe bleeding
Patients with severe bleeding (30–40% of circulatory volume) can initially be treated with crystalloid solutions27,199,229. Small volumes of Ringer’s lactate can help to maintain a systolic pressure of 80–90 mmHg (controlled hypotension)230. However, after the initial use of moderate volumes of crystalloids, it is justifiable to add non-protein colloids or vasoactive drugs. Once the circulatory volume has been restored, the possible need for transfusion can be evaluated based on laboratory parameters and estimated blood losses27,199.
With the purpose of limiting transfusion support in patients with severe bleeding, it is recommended that the patient’s circulatory volume is first restored using crystalloid or non-protein colloid solutions [1C].
Patients with life-threatening bleeding
Patients with life-threatening bleeding (>40% of circulatory volume) who do not respond to initial treatment with 2 L of fluids, have instable haemodynamics or are bleeding at a rate ≥50 mL/minute, require transfusion of red cell concentrates, which can be a potentially life-saving therapy27,199.
Massive transfusion protocols based on the use of fixed blood component ratios, derived from experience in the military setting, are not currently supported by solid evidence and have important limitations caused by a survival bias27,231. For this reason, until new evidence is available, the traditional therapeutic approach, based on restoring circulatory volume, monitoring haemostasis and other laboratory parameters and providing transfusion support with the appropriate blood component, should be considered valid for the majority of bleeding patients27,199.
The use of POC instruments for global monitoring of haemostasis of whole blood can facilitate an individualised approach to the required transfusion support and, in some cases, enable the use of fresh-frozen plasma to be reduced27,231.
For specific recommendations on the use of POC instruments, refer to the section dedicated to this subject.
With the purpose of limiting transfusion support in patients with life-threatening bleeding, it is suggested that the patient’s circulatory volume is initially expanded with crystalloid or non-protein colloid solutions, followed by transfusion therapy with blood components and, if necessary, with plasma-derived medicinal products [2B].
Dosing infusion fluids
The starting dose of crystalloids (preferably Ringer’s lactate solution), to infuse at the rate of 60–80 mL/kg/hour to maintain a systolic pressure of about 80–90 mmHg, is 3 mL per mL of blood loss27,230.
The initial dose of colloids is 1 mL per mL of blood loss27,230.
Haemostatic agents for topical use
The haemostatic agents for topical use include blood components, such as fibrin glue, and medical devices.
Fibrin glue is a blood component for topical use that has been exploited in surgery for more than 20 years199; its main components are fibrinogen, FXIII, thrombin (and calcium chloride with or without antifibrinolytics) which can be applied contemporaneously or in succession on the surfaces to be treated. The application of fibrin glue reproduces, in situ, the final stage of the coagulation cascade mediating the activation of fibrinogen by thrombin199. Given its haemostatic potential, this blood component for topical applications has been used with the aim of reducing allogeneic transfusion requirements in various surgical settings, despite its high cost. However, its efficacy in controlling bleeding was found to be greatest in orthopaedic surgery232.
In contrast, medical devices act through a mechanical or physical effect. Examples include cellulose or gelatine granules which, when saturated with blood, swell until forming a mass which reduces bleeding by mechanical compression.
The various haemostatic agents for topical use have overlapping clinical indications. Some products are indicated to facilitate haemostasis (Floseal®, Sivek®, Tabotamp®, and Curaspon Standard®), others, including fibrin glue, are also indicated to seal tissues and support sutures (Beriplast®, Quixil®, Tachosil®, Tisseel®, Tissucol®, Coseal®, and Glubran®)233. However, some issues have emerged from the analysis of the efficacy and safety of these products, mainly because of the lack of solid evidence derived from well-performed, randomised studies234.
It is suggested that fibrin glue is used to promote local haemostasis and as a complementary method to limit intra-operative use of blood, on the basis of local protocols that take into consideration the characteristics of the individual patient (bleeding risk, multiple alloimmunisation), the type of operation, the experience of the surgical and anaesthetic teams, as well as the possibility of combining this blood component with other blood-saving strategies, taking into account the cost-efficacy ratio [2B].
No evidence-based recommendations can be made on the routine use of haemostatic medical devices for topical use in elective orthopaedic surgery.
Blood-conserving anaesthesiological techniques
Various studies have demonstrated that neuroaxial anaesthesia (epidural and subarachnoid) can be associated with a significant reduction in bleeding, especially if used during orthopaedic operations,235,236. The data are not, however, unanimous because a meta-analysis concerning patients undergoing total knee replacement did not show significant differences depending on whether neuroaxial or general anaesthesia was used237. Nevertheless, it does seem that neuroaxial anaesthesia can reduce transfusion requirements because of the relative systemic hypotension associated with the technique which is due to sympathetic nervous system blockade and a consequent reduction in venous tone. This effect is, however, variable and its magnitude is not always predictable. In fact, there is not a good correlation between the dose of anaesthetic administered and the effect on blood pressure, because of the variations in blood pressure induced by the different level of nervous blockade obtained238.
Besides neuroaxial anaesthesia, lumbar plexus blockade was also associated with a reduction of both intra-operative blood loss (22%, 310 mL compared to 617 mL) and total blood loss (45%, 712 mL compared to 1,074 mL), when used in patients undergoing prosthetic hip surgery239,240.
As far as concerns general anaesthesia, the use of completely intravenous propofol-based anaesthesia is associated with a decrease in blood loss in spinal surgery241.
However, studies comparing blood losses related to various different anaesthesiological techniques in orthopaedic surgery should be interpreted with care, since most were conducted in a period in which more liberal transfusion practices were adopted compared to those currently in use. Furthermore, it is possible that additional blood-sparing techniques (such as peri-operative blood recovery) can reduce or even annul the advantages of using neuroaxial techniques of anaesthesia. Nevertheless, despite these limitations, it can be expected that these latter may reduce surgical bleeding, at least in selected subgroups of patients.
As far as concerns body temperature, it is now well established that this is related to the degree of transfusion support. Hypothermia during surgical procedures is produced by a combination of different factors which participate in the loss of body heat (low temperature in the operating theatre, administration of fluids that have not been warmed, changes in the mechanisms of thermoregulation induced by the anaesthesia and evaporation from body cavities in the case of abdominal or thoracic surgery). A drop, even moderate, in body temperature is able to affect the physiological mechanisms of haemostasis, modifying platelet function and inhibiting the temperature-dependent enzyme reactions of coagulation. It has been shown that even mild hypothermia (a decrease of <1 °C in the body temperature) can increase blood losses by as much as 16%, with a related increase in the risk of receiving transfusion therapy (22%)242–244.
The choice of anaesthetic technique in orthopaedic surgery must take into account the potential benefit of regional techniques in limiting transfusion requirements; anaesthetists should be aware that regional anaesthesia in spontaneously ventilated subjects may have advantages in terms of transfusion requirements (by decreasing blood loss).
It is suggested that loco-regional anaesthetic techniques are used, depending on the experience of the anaesthetic team, with the aim of contributing to limiting intra-operative blood loss [2C].
It is essential that body temperature is monitored during surgery.
It is recommended that hypothermia is prevented, and treated if it occurs, by pre-warming infusion fluids and warming the patient; the purpose is to limit intra-operative bleeding, as well as for obvious reasons of comfort [1C].
Pharmacological techniques and haemostatic agents
Drugs to reduce surgical bleeding and transfusion needs in surgery can be used both in prevention and correction of any disorders of haemostasis in the peri-operative period. Recently the indications for the use of individual clotting factor concentrates (fibrinogen, rFVIIa) or combinations (PCC) have been extended to surgical patients with life-threatening bleeding, although solid confirmation of the evidence is still required88.
Recent data confirm the central role of fibrinogen in the formation of stable clots. In this regard, there are emerging data from both randomised and prospective cohort studies, carried out in various clinical settings, indicating that optimisation of coagulation through administration of fibrinogen concentrate is associated with reductions in peri-operative bleeding and transfusion support245–247.
A recent meta-analysis showed the clinical and statistical heterogeneity of the trials included and highlighted that it was impossible to classify the trials as being “at low risk of bias”; the meta-analysis confirmed the statistically significant reduction in allogeneic transfusion support but did not show any effect on the other pre-defined outcomes, including mortality and bleeding248. However, an update of the abovementioned meta-analysis249 did find a statistically significant reduction of bleeding, mainly in elective heart surgery, while reiterating the lack of demonstrated efficacy on mortality and the need for caution when extrapolating results to other settings such as trauma and obstetrics. In fact, the increasing use of fibrinogen concentrates is supported mainly by observational studies with substantial methodological limitations which do not currently justify the routine administration of these concentrates.
The usefulness of determining the pre-operative concentration of plasma fibrinogen, according to the Clauss method, has been highlighted250,251. The liver produces from 2 to 5 grams/day of fibrinogen and guarantees mean plasma levels between 2 and 4.5 g/L252.
Fibrinogen concentrate has been demonstrated to have a better cost/benefit ratio in clinical use when the indication comes from monitoring coagulation through thromboelastographic methods88,199,253.
In patients with congenital deficiency of fibrinogen, levels of fibrinogen in the blood below 1 g/L are considered critical164,166,254.
In contrast, in patients with acquired hypofibrinogenaemia, the critical levels of fibrinogen, in the presence of massive bleeding, are more controversial and supported mainly by guidelines rather than evidence deriving from randomised studies248,249,255. In fact, two guidelines published in 2013 indicate that trigger levels of fibrinogenaemia in the presence of massive haemorrhage are <1.5–2 g/L88,256, while five earlier recommendations, published between 2006 and 2011, indicate levels <0.8–1 g/L199,257–260. Similarly, recommended target levels of plasma fibrinogen are >1 g/L261 or between 1.5 and 2 g/L259.
Plasma-derived fibrinogen concentrates have a consolidated and privileged role, with respect to fresh-frozen plasma and cryoprecipitate, in the treatment of bleeding related to a congenital deficiency of this clotting factor164,166,254. The use of this plasma-derived medicine is also increasing in the setting of bleeding due to acquired deficiencies, in part because of the numerous advantages it has compared to the use of allogeneic blood components. In fact, fibrinogen concentrate is safer, not only with regards to infection risk, and there are no problems of ABO incompatibility with the recipient; furthermore, fibrinogen concentrate enables replacement therapy to be delivered with much smaller volumes (200 mL per 4 grams of fibrinogen) compared to fresh-frozen plasma or cryoprecipitate255.
Data on the use of fibrinogen in elective orthopaedic surgery are very limited. The effect of fibrinogen concentrate was analysed retrospectively in a cohort of nine paediatric patients with craniosynostosis262 and prospectively in 66 patients treated with crystalloids or colloids during major orthopaedic surgery263. In both studies, the administration of fibrinogen had favourable effects on the dynamics of clot formation and on clot strength.
For specific recommendations on the use of fibrinogen, refer to the paragraph on POC instruments
In recent years there has been an increase in off-label use of rFVIIa for the treatment of critical bleeding in surgical patients264,265. However, a systematic Cochrane review recommends that rFVIIa is used exclusively in the context of clinical trials266. When used, the suggested dose is 90–120 mg/kg, repeated if necessary, after correction of some abnormalities, such as hypofibrinogenaemia, thrombocytopenia, hypothermia, acidosis and hyperfibrinolysis88.
For specific recommendations on the use of rFVIIa, refer to the paragraph on POC instruments
The main indication for the use of non-activated PCC is neutralisation of the anticoagulant effect in patients receiving oral treatment with VKA or NOAC, while activated PCC (FEIBA) are used in the treatment of patients with inhibitors of clotting factors and, recently, have also been suggested for use in patients being treated with NOAC. Thus, PCC, activated or not, are used both in cases of severe bleeding and in cases of urgent surgery. These plasma-derived medicinal products have, in fact, been demonstrated to be useful in preventing or treating bleeding in the presence of peri-operative coagulopathy88.
For specific recommendations on the use of PCC refer to the section on monitoring patients and management of post-operative bleeding
Fibrinolysis is an important cause of bleeding in surgical patients. There are some types of surgery (total knee replacement with a tourniquet, cardiopulmonary bypass, liver transplantation) which are more frequently associated with fibrinolysis and, therefore, benefit more than others from the use of TXA. Systematic reviews of randomised controlled trials indicate that the use of TXA has a significant impact, reducing transfusion support, particularly in cardiac and orthopaedic surgery, and indicate that the use of this drug is effectively associated with reductions in both the number of patients transfused and in their transfusion requirements88,267,268. As far as concerns the dose of TXA in major orthopaedic surgery, a meta-analysis showed that the reduction in the risk of receiving red blood cell transfusions is independent of the total dose of TXA administered269. Similarly, the timing of the administration of TXA, that is, at induction of anaesthesia rather than before release of the tourniquet, does not seem to affect the efficacy or safety of the treatment267.
It is suggested that intravenous TXA is administered in hip and knee replacement surgery (as well as in major surgery of the spinal column) [2A]270–278.
The most frequently used doses of TXA are as follows: total hip or knee replacement surgery, a starting dose of 10–15 mg/kg before surgery, followed or not by an infusion of 1 mg/kg/hour for 4–6 hours or repetition of the initial dose in the post-operative period; surgery to the spine, a starting dose of 20–100 mg/kg, followed by an infusion of 10 mg/kg/hour for 4–6 hours27.
Since there are reports of a state of hypercoagulability being induced in some patients (subjects with a past history of thromboembolic events, those aged over 60 years, females, those undergoing hip trauma surgery or cancer surgery), it is suggested that the risks and benefits of TXA are analysed carefully before its use [2C]279.
TXA applied topically, also in combination with intravenous administration280, has been found to be safe and effective in containing transfusion requirements in elective hip and knee surgery281–290 and does not cause adverse biomechanical effects on the prostheses used291.
It is suggested that TXA is used topically, also in combination with intravenously administered TXA, in patients undergoing elective hip or knee arthroplasty [2B].
It is suggested that TXA is used only by the topical route in patients undergoing elective hip or knee arthroplasty who have risk factors for hypercoagulability according to the pre-operative history [2B].
Point-of-care testing
Since the decision to transfuse red cell concentrates in the peri-operative period is based in part on the patient’s Hb concentration, the availability of rapid, reliable measurements of this parameter, with POC instruments, increases the safety of transfusion and optimises this therapeutic intervention88,292.
It is recommended that POC instruments acquired and used measure the concentration of Hb and the haematocrit without needing dilution of whole blood in the pre-analytic phase [1C].
The POC instruments for monitoring haemostasis use whole blood292 and can be employed not only in transfusion facilities and laboratories, but also in the Accident and Emergency Department and operating theatre, guaranteeing shorter performance times than those of standard laboratory tests88.
The main POC instruments that can be used for global monitoring of haemostasis are the thromboelastograph (TEG) and the thromboelastometer (ROTEM). These rapidly produce results in both numerical and graphical form and are able to detect the anticoagulant effect of acidosis or hypothermia and hyperthermia, since they can be used in a range of body temperatures between 22 °C and 42 °C; furthermore, they can pick up and quantify thrombocytopenia, clotting factor deficiencies, the effect of heparin, hypofibrinogenaemia and hyperfibrinolysis293.
Although a recent systematic Cochrane review showed that the use of TEG and ROTEM to monitor haemostasis in patients with massive bleeding does not have significant advantages in terms of morbidity and mortality compared with the use of standard laboratory tests294, these POC instruments are widely used in elective surgery, in heart surgery and, in particular, in liver transplantation and in elective major orthopaedic surgery to guide replacement therapy with clotting factors (with plasma or fibrinogen concentrate)27,245,246,295–299, or to monitor hyperfibrinolysis and to evaluate the possible use of TXA300.
One limitation of the TEG is its incapacity to evaluate platelet function adequately27,301.
It is suggested that POC instruments (TEG and ROTEM) are used to monitor overall haemostasis with the purpose of guiding clotting factor replacement therapy and to limit the use of transfusion support with blood components in elective major orthopaedic surgery with a high risk of bleeding or in the presence of major bleeding [2A].
It is suggested that the concentration of fibrinogen is measured pre-operatively using the Clauss method [2C]199,250.
In the presence of massive bleeding during elective major orthopaedic surgery and in association with the correction of the triggering cause, it is suggested that severe hypofibrinogenaemia (<1 g/L) which persists despite treatment with fresh-frozen plasma should be treated with fibrinogen concentrate (or, if not available, with cryoprecipitate) [2C].
In the same conditions and during massive transfusion, it is suggested that treatment with fibrinogen concentrate is considered when the level of fibrinogenaemia is <1.5 g/L, to prevent the fibrinogen level from falling below 1 g/L, the critical threshold for haemostasis [2C].
It is suggested that the administration of fibrinogen is privileged over that of fresh-frozen plasma (or cryoprecipitate) when there are contraindications to volume overloading [2C].
It is suggested that an initial dose of 25–50 mg/kg of fibrinogen concentrate is administered [2C].
rFVIIa is indicated for the treatment of patients with haemophilia with inhibitors, those with congenital deficiency of FVII and patients with Glanzmann’s thrombasthenia. However, it has also been widely used to control bleeding in off-label settings, such as trauma, heart surgery, liver surgery, post-partum and cerebral haemorrhage.
Although rFVIIa has been demonstrated to have variable effects on morbidity and transfusion requirements, a definitive effect on mortality has not been observed and, for this reason, there is not a clear indication for its use, particularly in elective surgery256,265,302–304. Furthermore, there have been recent reports of side effects, especially arterial and venous thromboembolic events, which have limited or even contraindicated its use, as in the case of cerebral haemorrhage304,305. Exceptionally, the use of rFVIIa can be considered in patients with life-threatening haemorrhage and after the failure of conventional haemostatic treatments27.
A good outcome and effective response to the use of rFVIIa do, however, require the prior restoration and control of some conditions: adequate levels of platelets and plasma fibrinogen, almost physiological levels of pH and body temperature and correction of hypocalcaemia88,256.
Off-label administration of rFVIIa (90 μg/kg) is suggested for the treatment of bleeding uncontrolled by conventional surgical or radiological treatment and/or in the case of failure of haemostatic therapy [2C].
Post-operative period
Autologous transfusion techniques
Post-operative blood recovery
Post-operative blood recovery consists in collecting the blood that the patient loses through surgical drains into a specific container and subsequently re-infusing it into the patient306. This procedure can make use of two systems: “no wash” and “wash”.
With the “no wash system” the blood is transferred from the container connected to the drains to the infusion set and is re-infused without undergoing further treatment. There is an integrated double filtration system with a first filter of 100–200 μ, for fibrin and macroaggregates, and a second filter of 40 μ for microaggregates. Anticoagulants are not necessary because this blood does not contain fibrinogen. This system involves simple, economic and easy-to-use methods.
The “wash system” involves the use of specific equipment which centrifuges the blood collected, eliminates the supernatant, washes the red blood cells and resuspends them in saline solution. This system is more demanding and staff require careful training in its use.
The main controversies regarding the use of unwashed blood concern the volume of blood that can be recovered, which is often less than a therapeutic dose, the quality and safety of the blood, because of the numerous contaminants that it contains, and the lack of adoption of transfusion triggers to decide when to transfuse the patient307–310.
However, although intuitively washing could reduce the presence of contaminants in recovered blood and the possible complications in recipients associated with their administration308, it should be remembered that, at present, there are no direct comparisons between blood products obtained with the two methods and the outcomes associated with their use309. For this reason, further studies are needed to evaluate the safety of post-operative blood recovery techniques that do not include washing of the recovered blood311.
Post-operative blood recovery, both with and without washing, has varied efficacy in reducing the amount of transfusion support with allogeneic red blood cells both in elective joint prosthesis surgery and in spinal surgery212,312–316.
Numerous meta-analyses have demonstrated the efficacy of TXA, also when applied topically289, in limiting post-operative bleeding in patients undergoing major orthopaedic surgery269–277,289,290,317,318: TXA could, therefore, further reduce the role of post-operative blood recovery techniques.
Furthermore, a recent randomised controlled trial demonstrated, in patients undergoing elective lower limb arthroplasty with pre-operative Hb above 130 g/L, that not only were intra-operative and post-operative blood recovery unable to decrease transfusion requirements, but they actually increased costs205.
It is recommended that post-operative blood recovery is used in elective major orthopaedic surgery (hip arthroplasty, knee arthroplasty, spinal surgery) only in the case in which it is expected that the post-operative blood loss will be ≥10% of the patient’s circulatory volume despite implementing multimodal strategies, including integrated use of other blood-conserving techniques (pharmacological, surgical and anaesthesiological), also taking into account the characteristics of the individual patient (bleeding risk, multiple alloimmunisation) [1B].
It is suggested that the use of “washed” blood should be preferred (both in orthopaedic surgery and in other settings) [2B]306.
When using systems that do not include washing, it is suggested that the concentration of free Hb is determined before re-infusing the unwashed blood, in order to check that the degree of haemolysis is less than 0.8% of the red cell mass contained in the product transfused into the patient [2C]306,319–321.
Control of body temperature and prevention of stress ulcers
For the reasons already set out, patients must maintain a body temperature suitable for optimal haemostatic function also in the post-operative period. In this period a patient’s body temperature may be low because of ineffective treatment of a possible intra-operative hypothermia, because of the prolongation of thermomodulatory effects of anaesthetics or because of admission in poorly heated rooms322. It is, therefore, essential that the patient’s body temperature continues to be monitored also in the period following any surgical procedure and that all methods and strategies useful for preventing hypothermia (blankets, warming fluids administered intravenously, etc.) are implemented.
It is recommended that the patient’s body temperature is monitored also in the post-operative period, implementing all strategies aimed at preventing hypothermia [1C].
Classically, all patients subjected to a state of stress (including that related to a surgical intervention) are at risk of upper gastrointestinal tract bleeding. Decades of research have demonstrated the advantages of pharmacological prophylaxis of this complication; such prophylaxis lowers the incidence of gastrointestinal bleeding deriving from the development of stress-induced gastritis and is based on the use of various categories of drugs: proton pump inhibitors, antagonists of histamine H2 receptors and sucralfate. In critically ill patients, proton pump inhibitors seem to be more effective than the antagonists of histamine H2 receptors in preventing clinically important bleeding from the upper gastrointestinal tract. The robustness of this conclusion is, however, limited by the poor methodological quality of the studies available, by differences between them and by possible publication bias323. Recent observational studies do, however, suggest that bleeding from stress ulcers is very rare. Furthermore, the risk of bleeding does not seem to be modified significantly by the use of acid-suppressing therapy which could even cause a potential increase in the risk of pneumonia and infections by Clostridium difficilis because of excessive alkalinisation of the gastric secretions324,325.
Because it is considered that the mechanism responsible for stress-induced gastritis is a decrease in blood flow in the mucosa, with consequent tissue ischaemia, it is thought that early recovery of central alimentation, which improves mucosal blood flow, can lower the risk of occult or clinically evident bleeding. However, the clinical studies that have evaluated the efficacy of this strategy compared with acid-suppressing drugs have yielded variable results and, so far, randomised controlled trials have not been performed326.
It is suggested that prophylaxis of stress-induced gastrointestinal tract ulcers is not given. This suggestion does not apply to patients in the intensive care unit and those already with local disorders for which prophylaxis is specifically indicated [2C].
Careful monitoring of the patient and management of post-operative bleeding
Anticoagulant treatment and pharmacological interactions
In the post-operative period, re-introduction of anticoagulant therapy or the start of anticoagulant prophylaxis, dictated by the need to protect the patient from the risk of thromboembolism compels an evaluation of bleeding risk.
There are two main mechanisms by which anticoagulant drugs are metabolised and eliminated from the body: the cytochrome system (CYP450) and the P-glycoprotein transport system327. All the drugs or substances that activate or inhibit these systems can have repercussions on the anticoagulant effect which will be inhibited or potentiated.
At present, few interactions between NOAC and food are known. Table IV327 reports the possible interactions between NOAC and other drugs; these interactions are based on the mechanisms of metabolism and it is important to know them because elderly patients are ever more frequently taking multiple drugs concurrently over the long term327.
Table IV.
Interactions between new oral anticoagulants and other drugs (modified from Pengo V et al.327).
| Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|
| Glycoprotein-P inhibitors: amiodarone, phenothiazine, thioxanthenes, carboxylic acid, azole antifungal agents, verapamil, antimalarial drugs, cyclosporine | Yes | Yes | Yes |
| Glycoprotein-P inducers: dexamethasone, rifampicin, hypericum* | Yes | Yes | Yes |
| Cytochrome CYP3A4 inhibitors: phenothiazine, carboxylic acid, azole antifungal agents, verapamil, erythromycin, telithromycin, nefazodone, antimalarial drugs, cyclosporine, thioxanthenes | No | Yes | Yes |
| Cytochrome CYP3A4 inducers: carbamazepine, efavirenz, nevirapine, phenytoin, phenobarbital, rifabutin, rifapentin, hypericum*, alcohol, eucalyptol | No | Yes | Yes |
| Non-steroidal anti-inflammatory drugs: aspirin, naproxen, diclofenac | Yes | Yes | Yes |
| Anti-platelet drug: clopidogrel | Yes | Yes | Yes |
or St. John’s wort, Hypericum perforatum.
Many of the numerous interactions between warfarin and other drugs, or food, are due to the former’s almost exclusive hepatic clearance, mediated by CYP450, in particular the isoform CYP2C9. In contrast, dabigatran seems to be metabolised in the plasma and liver without the intervention of CYP450, but is a substrate for P-glycoprotein and, for this reason, is susceptible to the effects of inhibitors or activators of P-glycoprotein. Rivaroxaban and apixaban are metabolised by the CYP3A4 isoform of CYP450 and are also substrates for P-glycoprotein328, thus being sensitive to inhibitors and inducers of both systems. However, the demonstrated pharmacological interactions only make dose adjustments of NOAC necessary in some cases. Other possible interactions are caused by the association with non-steroidal anti-inflammatory drugs and platelet anti-aggregants329.
Monitoring the patient and post-operative bleeding
In the post-operative period the patient may be subject to both thromboembolic and haemorrhagic complications.
In the post-operative period, it is recommended that antiplatelet therapy is restarted as early as possible, to prevent platelet activation and possible thrombotic complications [1C].
It is suggested that the first post-operative dose of clopidogrel or prasugrel is administered no more than 24 hours after the end of the operation [2C].
It is suggested that this first post-operative dose is not a loading dose [2C].
Although the use of transfusion targets, determined by coagulation tests or POC tests, are used to guide treatment with blood components, these tests are not useful for monitoring thrombotic risk330–333. The evidence for post-operative use of platelet function tests (PFA-200, MEA) is very limited334,335.
Some studies have shown that desmopressin can improve altered platelet function in volunteers treated with aspirin or clopidogrel336,337; however, its use in acquired bleeding disorders is not supported by solid evidence.
Despite the lack of studies on the capacity of transfused platelets to overcome the effect of treatment with clopidogrel or prasugrel, the management of important bleeding in patients being treated with antiplatelet drugs is based on the transfusion of platelets338. Likewise, studies have not been performed on the efficacy of platelet transfusions in patients being treated with ticagrelor; the presence of this drug in the plasma makes the transfusion of platelets ineffective when the drug has been administered in the preceding 12 hours88.
It is suggested that platelets are transfused in the case of intra-operative or post-operative bleeding, clearly related to the intake of antiplatelet agents (aspirin, clopidogrel, prasugrel) [2C].
Unfractionated heparin (UFH) and LMWH are used in bridging therapy, in peri-operative thromboprophylaxis, in the treatment of venous thromboembolism, in disseminated intravascular coagulation and for anticoagulation in haemodialysis and heart surgery.
The aPTT and measurement of anti-FXa activity are able to determine the anticoagulant effect of UFH and LMWH, respectively, even if these tests are only useful in particular circumstances in the context of surgery.
The anticoagulant effect of heparins can be corrected rapidly by the intravenous administration of protamine sulphate (1 mg of protamine neutralises 100 IU of UFH or 100 units of anti-FXa of LMWH)339; however, the correction induced by protamine is less efficient in the case of subcutaneous administration of UFH or LMWH and it may, therefore, be necessary to prolong the intravenous infusion of protamine or give a second dose.
It is recommended that bleeding associated with the intravenous administration of UFH is treated with an intravenous infusion of protamine sulphate at a dose of 1 mg per 100 IU of UFH administered in the preceding 2–3 hours [1A].
It is suggested that bleeding associated with subcutaneous administration of UFH that does not respond to intravenous protamine sulphate (1 mg per 100 IU of UFH) is treated with a continuous intravenous infusion of protamine sulphate at a dose guided by the aPTT [2C].
It is suggested that bleeding associated with subcutaneous administration of LMWH is treated with intravenous protamine sulphate at a dose of 1 mg per 100 units of anti-FXa of LMWH administered [2C].
If there is no response, it is suggested that a second dose of protamine sulphate is given (0.5 mg per 100 units of anti-FXa of LMWH administered) [2C].
Fondaparinux is a synthetic analogue of the polysaccharide sequence present in UFH and LMWH with a selective action on FXa, which is inactivated after binding to antithrombin. It is used in the prophylaxis and treatment of thromboembolic diseases and myocardial infarction. There is no antidote for fondaparinux; on the basis of in vitro studies, it has been proposed that rFVIIa is given to control severe bleeding caused by the administration of fondaparinux339,340.
It is suggested that bleeding related to the subcutaneous administration of fondaparinux is treated with rFVIIa (off-label use) [2C].
Pharmacological VKA, such as acenocoumarol (sintrom) and warfarin (coumadin) and others not yet available in Italy, are used for the prophylaxis or treatment of ischaemic and thrombotic events in patients with mechanical prosthetic heart values, in atrial fibrillation and in thromboembolic disease. With an increase in the number of elderly subjects and of disorders associated with thromboembolism, treatment with VKA is becoming ever more widespread and thus the bleeding complications of these drugs are becoming more frequent.
The anticoagulant effect of VKA can be rapidly reversed by the prompt use of PCC341–347, although there are no randomised controlled trials supporting their use in a setting other than haemophilia and the therapeutic dose has not been standardised because of the differences between the individual products256.
The therapeutic anticoagulant effect of VKA is evaluated by measuring the INR which, before a surgical interventions, should be 1.5 or less. To achieve such a level, the Federation of Centres for the Diagnosis of Thrombosis and Monitoring of Antithrombotic Therapy argues for rapid neutralisation of VKA by intravenous administration of vitamin K and PCC, at a dose regulated on the basis of the pre-operative value of the INR348.
Since the use of PCC exposes treated patients to an increased risk of arterial and venous thrombosis, thromboprophylaxis should be resumed as soon as the bleeding has been controlled.
In patients receiving oral anticoagulant treatment with VKA who must undergo urgent surgery or who develop bleeding complications in the post-operative period, it is recommended that the anticoagulant therapy is interrupted immediately, that 10–20 mg of vitamin K1 is administered as soon as possible by slow intravenous infusion (15–30 minutes) and that PCC is infused slowly at the following doses: 20 IU/kg, if the INR is <2; 30 IU/kg, if the INR is between 2 and 3.9; 40 IU/kg, if the INR is between 4 and 5.9; and 60 IU/kg, if the INR is >6 [1B].
In recent years, NOAC (dabigatran, rivaroxaban, apixaban) are becoming increasingly used for the prophylaxis and treatment of thromboembolic events. The current lack of valid laboratory tests able to measure their effective anticoagulant action makes therapeutic monitoring futile, whereas such monitoring is essential for VKA.
However, there is a specific test to measure the anti-FXa activity of the new anticoagulants with an anti-FXa effect (rivaroxaban, apixaban)349 and there is also a method to evaluate the inhibitory effect of the drugs with an anti-thrombin mechanism of action (dabigatran): the diluted thrombin time119. However, an accepted protocol for their peri-operative use has not yet been established.
No evidence-based recommendations can be made on the use of laboratory tests in the post-operative evaluation of the anticoagulant effect of NOAC.
Whereas there is an antidote for VKA, there is not a specific antidote to neutralise the anticoagulant effect of NOAC, whether their mechanism of action is anti-FXa or anti-thrombin339.
Clinical experience is very limited and the paucity of published studies does not allow specific recommendations to be made. However, it has recently been demonstrated, in healthy volunteers, that the effect of these drugs on the tests of inhibition of FXa can be quickly and completely corrected by high doses of PCC (50 IU/kg); in contrast, this same dose does not correct the prolongation of the aPTT and thrombin time induced by dabigatran350.
In the case of direct inhibitors of thrombin (dabigatran), the anticoagulant effect has been corrected by dialysis or the administration of activated concentrates of PCC (FEIBA), although the guidelines of the European Heart Rhythm Association suggest using PCC, as for other NOAC114. There is little evidence in favour of the use of rFVIIa114,351,352.
In patients being treated or suspected of being treated with oral anti-FXa agents, such as rivaroxaban or apixaban, it is suggested that anti-FXa activity is determined, where this test is available [2C].
In the presence of important bleeding, it is suggested that the effect of rivaroxaban or apixaban is neutralised with high doses of PCC (25–50 IU/kg) or with activated PCC (50 U/kg, repeated if necessary) or rFVIIa (90 μg/kg) [2C].
In the presence of important bleeding in patients being treated with oral direct inhibitors of thrombin, such as dabigatran, it is suggested that high doses of PCC (25–50 IU/kg) are administered, or activated PCC (50 U/kg, repeated if necessary) or rFVIIa (90 μg/kg) [2B].
The more common and less severe platelet disorders respond well to desmopressin, which shortens the bleeding time, whether used as prophylaxis or for the treatment of bleeding144–146. When used, the standard dose is 0.3 μg/kg, diluted in 50 mL of saline solution and infused slowly, over more than 30 minutes144. The use of antifibrinolytic drugs in inherited platelet disorders is not evidence-based.
In patients with inherited disorders of platelet function, it is suggested that desmopressin is used for the prevention and control of post-operative bleeding, and that TXA is used as an adjuvant [2C].
rFVIIa is indicated for the treatment of bleeding only in patients with Glanzmann’s thrombasthenia, whereas it is not indicated for any other congenital or acquired platelet disorders. When used, the proposed dose is 90 μg/kg, repeated every 2 hours for 12 hours and, subsequently, every 3–4 hours until the bleeding stops142.
It is recommended that rFVIIa is used in the treatment of bleeding in patients with Glanzmann’s thrombasthenia [1C].
Patients with thrombocytopenia resemble subjects with modest platelet disorders as far as concerns the treatment of post-operative bleeding complications153 and should be treated on the basis of the platelet count and concomitant clinical conditions152–155,306,353,354.
In patients with platelet disorders with a platelet count between 50 and 100×109/L, it is suggested that transfusion of platelet concentrates is considered if there is a high bleeding risk or a risk of bleeding in critical sites such as the eye or brain [2C]150,152,306.
In the case of congenital functional platelet deficiencies, it is suggested that transfusion of platelet concentrates is considered, independently of the platelet count, in the presence of peri-operative bleeding not related to the surgery or to other clotting disorders [2C]150,152,306.
Among the bleeding disorders due to a congenital or acquired deficiency of clotting factors, the lack of VWF is undoubtedly the most widespread and the treatment, in the case of post-operative bleeding, is as recommended below13,131,133,136,137.
It is recommended that the post-operative management of the patient with vWD is conducted in collaboration with an expert in haemostasis and thrombosis [1C].
In the presence of mild, post-operative bleeding in a patient with vWD, it is recommended that desmopressin is used, after a trial test, at doses described in specific guidelines (0.3 μg/kg, diluted in 50 mL of saline solution and infused slowly, over more than 30 minutes) [1C]136–140.
In vWD patients with severe post-operative bleeding it is recommended that replacement therapy with plasma-derived vWF or vWF-rich FVIII is given, according to the treatment regimens set out in specific guidelines [1C].
In vWD patients undergoing major surgery, it is suggested administering antifibrinolytics in the post-operative period, as an adjuvant to more specific treatment, and platelet transfusions, only in the case of failure of other treatments [2C].
It is recommended that the post-operative management of patients with haemophilia or other congenital bleeding disorders is conducted in collaboration with an expert in haemostasis and thrombosis [1C].
Personalised thromboprophylaxis is suggested in the post-operative period in haemophilic patients undergoing major surgery [2C]163.
For patients with deficiencies of FVIII (haemophilia A) or FIX (haemophilia B) without inhibitors, the recommendations for post-operative management are reported below140,156,158,160.
It is recommended that appropriate replacement therapy is given [1C].
It is suggested that published guidelines are followed when giving replacement therapy (target of the missing factor and duration of treatment) [2C].
For replacement treatment in the post-operative period, it is recommended that either recombinant or plasma-derived concentrates are used [1C].
The suggested treatment in patients with inhibitors is rFVIIa or activated PCC (FEIBA) [2C].
rFVIIa is the treatment of choice for congenital deficiency of FVII; if this is not available, plasma concentrate of FVII is preferred over PCC, because of the potential prothrombotic effect of these latter140,164.
In the post-operative period in patients with congenital deficiency of FVII, it is suggested that plasma concentrate of FVII, at a dose of 10–40 IU/kg, is used as an alternative to rFVIIa [2C]164.
In the post-operative period in patients with congenital deficiency of FVII undergoing major surgery, it is suggested that rFVIIa, at a dose of 15–30 μg/kg every 4–6 hours, is used, usually for a minimum of three doses [2B]164.
In cases of congenital deficiency of fibrinogen and FXIII, the British guidelines recommend the use of specific concentrates164. In hypofibrinogenaemia, replacement therapy is indicated for fibrinogen levels <1 g/L or <20–30% of normal. However, a possible risk of thrombosis must be kept in mind when plasma fibrinogen concentrates are used.
In the case of a congenital lack of FV and FXI, fresh-frozen plasma, preferably a industry-produced, virus-inactivated product, is the only therapeutic option, also in the post-operative period164. Patients with FXI deficiency and inhibitors undergoing surgery can be treated successfully with low doses of rFVIIa (33–47 μg/kg)168; the contemporary administration of TXA was found to be effective in controlling bleeding164.
PCC are the reference concentrates for the treatment of FII and FX deficiencies, for which specific concentrates are not available164,169,170.
In patients with other rare clotting factor deficiencies no evidence-based recommendations can be made on the peri-operative use of rFVIIa, desmopressin or TXA.
Management of the patient with acquired thrombocytopenia
It is suggested that the decision to transfuse platelet concentrates in the post-operative period is not based exclusively on a low platelet count, but also on the clinical conditions of the patient (in particular, body temperature >38.5 °C, plasma clotting disorders, recent bleeding, neurological deficits) [2C]150,152,306.
Platelet transfusion is rarely indicated in the surgical patient with normal platelet function in whom the platelet count is greater than 100×109/L; such a transfusion is suggested, however, when the count is less than 50×109/L and there is excessive bleeding [2C]150,152,306.
In the case of intermediate values (platelet count between 50×109/L and 100×109/L) it is suggested that platelet transfusions are considered in specific situations such as secondary platelet dysfunction or when there is a risk of bleeding in critical sites, such as the eye and brain [2C]150,152,306.
In the case of an acquired deficit of platelet function (for example: anti-aggregant drugs, cardiopulmonary by-pass) it is suggested that platelet transfusions are administered, independently of the platelet count, in the presence of peri-operative bleeding not related to the surgery or to other clotting disorders [2C]150,152,306.
In the case of acute disseminated intravascular coagulation, in the presence of major bleeding and thrombocytopenia, it is suggested that the platelet count is maintained around 50×109/L [2C]306.
In patients with disseminated intravascular coagulation who are not bleeding, prophylactic transfusion of platelet concentrates is suggested in cases in which the thrombocytopenia and stratification of bleeding risk indicate a high probability of bleeding [2C]306.
It is suggested that prophylactic platelet transfusion is not used routinely when the thrombocytopenia is due to increased platelet destruction (heparin-induced thrombocytopenia, autoimmune thrombocytopenia, thrombotic thrombocytopenic purpura) because it is ineffective in these cases [2C]152,306.
In anaemic, thrombocytopenic patients (platelet count ≤20×109/L) without active bleeding, it is suggested that the haematocrit is increased to about 30% to reduce the bleeding risk [2B]306.
Optimising tolerance of anaemia
Pre-operative period
Evaluation and optimisation of the individual physiological reserve for tolerance of anaemia and risk factors
Since the decrease in availability of oxygen in an acute episode of anaemia is initially compensated for by an increase in cardiac output, the compensatory cardiac reserve could be used to determine the tolerance to anaemia. However, although evaluating cardio-respiratory functional reserve in patients who are candidates for surgery is part of daily clinical practice for anaesthetists, estimating tolerance of anaemia in an individual patient remains a challenge that has not been completely won. In fact, there are not yet methods or investigations that enable the tolerance of anaemia of a given patient to be predicted a priori355. There are, however, various, detailed guidelines to which the reader is referred356–359.
In any case, it is worth remembering some key concepts here:
- pre-operative evaluation of the cardiac reserve is performed primarily to identify those patients for whom cardiac stress related to the operation and to the peri-operative period could represent a particular risk for morbidity or mortality which would be associated with the risk already present due to the patient’s underlying condition. From this point of view, the tolerance of anaemia is deduced from the real state of compensation and the cardiac reserve, determined by various tests355.
- There is little evidence from well-conducted, randomised trials and many recommendations are based mainly on experts’ opinions359.
- In the last 5–10 years, various steps forward have been made. Greater emphasis is now given to stratification of clinical risk rather than to routine cardiac testing360.
- The same considerations apply to the pre-operative evaluation of respiratory function and, given the increase in the current day population of overweight subjects, also to the metabolic evaluation361.
In all patients potentially at risk of acute peri-operative anaemia, it is recommended that the cardio-respiratory reserve is evaluated during the pre-operative assessment, using specific diagnostic protocols and flow-charts based on the best, periodically updated indications [1C].
Adoption of restrictive transfusion thresholds
Most of the currently available guidelines recommend basing decisions on transfusion therapy with red blood cells on both the patient’s Hb values as well as symptoms of anaemia (chest pain, congestive heart failure, tachycardia not responsive to the administration of fluid or postural hypotension)12,199,306,362–366. Although the search for physiological transfusion triggers that are most indicative of the state of tissue oxygenation and the presence of ischaemia continues to be a subject of research, at present there are no data from clinical studies that support the use of such measurements, which have, in any case, not yet been validated in clinical practice367. The Hb concentration does, therefore, remain the most widely used transfusion trigger, together with clinical assessment of the patient.
Following the Transfusion Requirements in Critical Care (TRICC) clinical trial368, published in 1999, various studies showed that adopting a restrictive transfusion threshold (Hb=70–80 g/L) guarantees patients’ safety while reducing transfusion use.
In particular, in orthopaedic patients, the recent transfusion trigger trial for Functional Outcomes in Cardiovascular patients Undergoing Surgical hip fracture repair (FOCUS) demonstrated, in elderly patients at high cardiovascular risk, that a liberal strategy (Hb transfusion threshold=100 g/L) did not reduce mortality, the rate of inability to walk autonomously at 60 days after surgery or even morbidity during the admission, in comparison with a restrictive strategy (Hb transfusion threshold >80 g/L or symptomatic anaemia)369. These results were recently further supported by a post-hoc analysis of a randomised study of patients undergoing lower limb prosthetic surgery370 and by a meta-analysis371.
The greater efficacy of a restrictive transfusion threshold (Hb<70 g/L) compared to the liberal one (Hb<90 g/L) was also supported by a significantly lower mortality in patients with gastrointestinal bleeding372.
Another randomised study demonstrated that a restrictive strategy (haematocrit >24%) and a liberal one (haematocrit >30%) were equally safe in patients undergoing heart surgery373.
On the other hand, a recent pilot study in patients with acute coronary syndrome showed that the liberal transfusion strategy was associated with a better outcome in this particular setting374.
It is recommended that a restrictive transfusion threshold (Hb 70–80 g/L) is adopted in all hospitalised, clinically stable patients [1A].
It is suggested that a restrictive transfusion threshold is adopted in hospitalised patients with previous cardiovascular disorders [2B].
It is suggested that transfusion therapy is considered in hospitalised patients with previous cardiovascular disease if they are symptomatic or have a Hb≤80 g/L [2B].
It is suggested that hospitalised patients with acute coronary syndrome who are haemodynamically stable undergo a careful clinical evaluation, aimed at determining a personalised transfusion threshold [2C].
Intra-operative period
Optimising cardiac output. Optimising ventilation and oxygenation
The availability of oxygen in the tissues is the product of the cardiac output and the arterial oxygen content. For this reason, from a physiological point of view, the availability of oxygen in tissues can be stabilised by manipulating the two factors involved.
There is currently more and better clinical evidence regarding optimisation of haemodynamic function (or goal-directed therapy [GDT])375,376. However, two recent, randomised studies in surgical patients at high risk did not show a significant impact of GDT on outcome377,378. The physiological rationale underlying GDT is that bringing a patient’s cardiovascular function to an optimal level, through a specific, codified therapeutic approach, ensures the best oxygen supply to tissues in that individual patient. GDT is, therefore, mainly based on the intravenous administration of fluids, but also cardio-active drugs, in doses such as to bring the individual patient to the part most efficient of his or her cardiac performance, as expressed by the Maestrini-Starling law379,380. This objective can only be achieved if systems for haemodynamic monitoring are used that evaluate cardiac output rather than the so-called “filling pressure”, heart rate or mean systemic blood pressure. In fact, these last parameters have now been considered for some years as insensitive indicators of hypovolaemia or changes in cardiac output381. Cardiac output has traditionally been evaluated by an invasively introduced Swan-Ganz catheter. More recently, less invasive, reliable monitoring instruments have become available (particularly in circumstances of totally assisted pulmonary ventilation)382–384.
It has been amply demonstrated that the management of circulatory volume according to the principles of GDT also enables better dosing of fluid therapy, avoiding the complications related to the administration of too much or too little fluids, thus positively affecting the patients’ outcome375,385–388.
Prompt intra-operative management of haemodynamics, according to the principles of GDT, is recommended [1B].
It is recommended that hypervolaemia and hypovolaemia are avoided [1B].
It is recommended privileging systems that monitor blood flow in order to guide fluid therapy [1B].
In cases in which the patient is undergoing mechanical ventilation, the intra-operative availability of oxygen in the tissues could also be improved by increasing the inspired fraction of oxygen (FiO2), since this is able to increase the arterial partial pressure of oxygen proportionally. Furthermore, because of the great difference in the partial pressure of oxygen between blood and tissues, in conditions of marked anaemia, an increase in the amount of oxygen physically dissolved in the blood, which is obtained by ventilation with high FiO2, can improve tissue oxygen availability. In this regard, it has been demonstrated in animal models that hyperoxic ventilation increases the rate of survival and tolerance of anaemia by guaranteeing tissue oxygenation even at very low levels of Hb389–392. Thus, hyperoxic ventilation can be considered for patients with acute anaemia undergoing general anaesthesia. However, at present there is a lack of large clinical studies demonstrating that this method does actually improve tissue oxygenation during acute, severe anaemia. Furthermore, there are not yet controlled studies clarifying whether ventilation with high concentrations of oxygen can really improve survival in cases of acute anaemia that continues for prolonged periods. Finally, it should be remembered that the side effects of prolonged hyperoxic ventilation (arteriolar vasoconstriction, generation of oxygen radicals and formation of atelectasis) can outweigh the positive effects on oxygen transport and tissue oxygenation355. However, considering that the exposure is limited in time (intra-operative period) it is reasonable to expect that the damage known to occur with prolonged hyperoxia does not develop in surgical patients treated with this method393.
In the case of acute intra-operative anaemia in patients undergoing general anaesthesia and mechanical ventilation, it is suggested that hyperoxic pulmonary ventilation is administered (FiO2=1). This technique, especially if used in combination with normovolaemic haemodilution, can be useful, at least to enable greater availability of tissue oxygen until the bleeding is controlled [2C].
Post-operative period
Optimising the tolerance of anaemia. Maximising oxygen delivery. Minimising oxygen consumption
The considerations on maximising oxygenation in the intra-operative period are essentially valid also in the post-operative period. However, it is worth emphasising that the techniques used necessitate monitoring and a level of knowledge and specific skills that strongly suggest that the post-operative management of patients at risk is performed in appropriately equipped healthcare settings (recovery room, intensive care unit, sub-intensive care unit)394,395.
Indeed, one aspect worth highlighting is that post-operative complications, including those due to poor tolerance of anaemia, if not promptly identified and treated adequately, can have a substantial impact on both the patient and the whole healthcare system, since they can be potentially disastrous for the former and associated with prolonged admissions, with increased costs, for the latter. In this regard, recent data suggest that these costs could be due not only to the short-term management of the patient, but also to the long-term management, because of repeated hospital admissions for recurrences and the development of a state of chronic disease396–398.
In the case of patients with a reduced physiological reserve, which may cause a peri-operative reduction in tolerance of anaemia, it is recommended that post-operative observation is planned for an appropriate period (the duration of which varies according to the attendant clinician) in settings of variable intensity of care depending on the patients’ requirements. In these structures it should be possible to monitor and, if necessary, support the physiological variables involved in the process of adaptation to anaemia [1C].
It has been known for many years that there is a relationship between post-operative pain, stress and oxygen consumption399. Furthermore, acute post-operative pain, if not adequately treated, causes a series of reflexes that alter physiological homeostasis, such as the secretion of catabolic hormones, with significant haemodynamic, renal and immune repercussions. Finally, post-operative pain causes important respiratory alterations, inducing a restrictive syndrome. In particular the activity of the diaphragm and other respiratory muscles is reduced after abdominal surgery. The logical consequences of this are a change in the ventilation/perfusion ratio and a greater probability of developing hypercapnia, hypoxia, atelectasis and inflammatory lung phenomena400,401. Consequently, pain is nowadays considered a complication of surgery, rather than a simple symptom, and does, therefore, require appropriate treatment according to well-defined protocols402,403.
Epidural analgesia is a consolidated technique that, for several years, has been considered the gold standard in the management of post-operative pain, particularly in the elderly and in patients with impaired respiratory function404–407. However, recent data show that the benefits of this technique are not as great as thought and that the advantages with regard to decreasing the incidence of cardiovascular and respiratory complications are probably limited to high-risk patients undergoing abdominal or thoracic surgery who receive thoracic epidural analgesia only with local anaesthetics. Furthermore, there is recent evidence showing that less invasive, regional analgesic techniques (paravertebral block for thoracotomy, femoral block for hip and knee surgery, local infiltrations - possibly continuous - of the wound, local analgesic infiltration in lower limb surgery) are as effective as epidural analgesia. For this reason, the number of indications for epidural analgesia seems to be decreasing408–413. In orthopaedic limb surgery, the use of continuous peripheral nerve blocks has been shown to be as effective as peridural analgesia414–417.
In order to optimise the treatment of pain, it is recommended that the level of post-operative pain (at rest and not) is evaluated periodically, using an international scales: a numerical rating scale, a visual analogue scale or a verbal rating scale. This information must be recorded in the patient’s clinical records [1A].
Given their greater efficacy, it is recommended that epidural techniques of analgesia are used rather than systemic opioids [1A].
In limb surgery, it is recommended that continuous peripheral nerve blocks are used [1A].
Footnotes
The Authors declare no conflict of interest.
References
- 1.Department of Health, Victoria, Australia. Patient Blood Management. [Accessed on 03/12/2014]. Available at: http://www.health.vic.gov.au/bloodmatters/management/index.htm.
- 2.Goodnough LT, Shander A. Current status of pharmacologic therapies in patient blood management. Anesth Analg. 2013;116:15–34. doi: 10.1213/ANE.0b013e318273f4ae. [DOI] [PubMed] [Google Scholar]
- 3.Shander A. Introduction. Best Pract Res Clin Anaesthesiol. 2013;27:1–3. doi: 10.1016/j.bpa.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Liumbruno GM, Vaglio S, Grazzini G, et al. Patient blood management: a fresh look at a fresh approach to blood transfusion. Minerva Anestesiol. 2015;81:1127–37. [PubMed] [Google Scholar]
- 5.Hofmann A, Farmer S, Shander A. Five drivers shifting the paradigm from product-focused transfusion practice to patient blood management. Oncologist. 2011;16(Suppl 3):3–11. doi: 10.1634/theoncologist.2011-S3-3. [DOI] [PubMed] [Google Scholar]
- 6.SABM. Administrative and Clinical Standards for Patient Blood Management Programs. 2nd edition. [Accessed on 03/12/2014]. Available at: http://www.sabm.org/publications.
- 7.De Leon EM, Szallasi A. “Transfusion indication RBC (PBM-02)”: gap analysis of a Joint Commission Patient Blood Management Performance Measure at a community hospital. Blood Transfus. 2014;12(Suppl 1):s187–90. doi: 10.2450/2012.0088-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GRADE working group. [Accessed on 03/12/2014]. Available at: http://www.gradeworkinggroup.org/index.htm.
- 9.Agenzia per i Servizi Sanitari Regionali. Valutazione preoperatoria del paziente da sottoporre a chirurgia elettiva. Linee guida nazionali di riferimento, Luglio 2005. [Accessed on 03/12/2014]. Available at: http://www.agenas.it/images/agenas/pnlg/chirurgia_elettiva.pdf.
- 10.Goodnough LT, Shander A, Spivak JL, et al. Detection, evaluation, and management of anemia in the elective surgical patient. Anesth Analg. 2005;101:1858–61. doi: 10.1213/01.ANE.0000184124.29397.EB. [DOI] [PubMed] [Google Scholar]
- 11.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the transfusion management of patients in the peri-operative period. I. The pre-operative period. Blood Transfus. 2011;9:19–40. doi: 10.2450/2010.0074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols WL, Hultin MB, James AH, et al. von Willebrand disease (vWD): evidence-based diagnosis and management guidelines, the National Heart, Lung and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14:171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- 14.Owens WD, Felts JA, Spitznagel EL., Jr ASA physical status classifications: a study of consistency of ratings. Anesthesiology. 1978;49:239–43. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Shander A, Knight K, Thurer R, et al. Prevalence and outcomes of anemia in surgery: a systematic review of the literature. Am J Med. 2004;116(Suppl 7A):58S–69S. doi: 10.1016/j.amjmed.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology. 2010;113:482–95. doi: 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 17.Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part I: Molecular basis of iron homoeostasis. J Clin Pathol. 2011;64:281–6. doi: 10.1136/jcp.2010.079046. [DOI] [PubMed] [Google Scholar]
- 18.Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. J Clin Pathol. 2011;64:287–96. doi: 10.1136/jcp.2010.086991. [DOI] [PubMed] [Google Scholar]
- 19.Muñoz M, García-Erce JA, Cuenca J, et al. AWGE (Spanish Anaemia Working Group) On the role of iron therapy for reducing allogeneic blood transfusion in orthopaedic surgery. Blood Transfus. 2012;10:8–22. doi: 10.2450/2011.0061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisbe E, Castillo J, Sáez M, et al. Prevalence of preoperative anemia and hematinic deficiencies in patients scheduled for elective major orthopedic surgery. Transfus Alternat Transfus Med. 2008;10:166–73. [Google Scholar]
- 21.Saleh E, McClelland DB, Hay A, et al. Prevalence of anaemia before major joint arthroplasty and the potential impact of preoperative investigation and correction on perioperative blood transfusions. Br J Anaesth. 2007;99:801–8. doi: 10.1093/bja/aem299. [DOI] [PubMed] [Google Scholar]
- 22.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. Prevalence of anemia in persons 65 years and older in the United States: evidence for a high rate of unexplained anemia. Blood. 2004;104:2263–8. doi: 10.1182/blood-2004-05-1812. [DOI] [PubMed] [Google Scholar]
- 23.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–23. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 24.Musallam KM, Tamim HM, Richards T, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378:1396–407. doi: 10.1016/S0140-6736(11)61381-0. [DOI] [PubMed] [Google Scholar]
- 25.Loor G, Rajeswaran J, Li L, et al. The least of 3 evils: exposure to red blood cell transfusion, anemia, or both? J Thorac Cardiovasc Surg. 2013;146:1480–7. doi: 10.1016/j.jtcvs.2013.06.033. [DOI] [PubMed] [Google Scholar]
- 26.de Benoist B, McLean E, Egli I, Cogswell M. WHO Global Database on Anaemia. Geneva, Switzerland: World Health Organization Press; 2008. [Accessed on 05/01/2015]. Worldwide Prevalence of Anaemia 1993–2005. Available at: http://whqlibdoc.who.int/publications/2008/9789241596657_eng.pdf. [Google Scholar]
- 27.Leal-Noval SR, Muñoz M, Asuero M, et al. Spanish Consensus Statement on alternatives to allogeneic blood transfusion: the 2013 update of the “Seville Document”. Blood Transfus. 2013;11:585–610. doi: 10.2450/2013.0029-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews CM, Lane DW, Bradley JG. Iron pre-load for major joint replacement. Transfus Med. 1997;7:281–6. doi: 10.1046/j.1365-3148.1997.d01-42.x. [DOI] [PubMed] [Google Scholar]
- 29.Cuenca J, Garcia-Erce JA, Martinez F, et al. Preoperative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Int J Surg. 2007;5:89–94. doi: 10.1016/j.ijsu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Rogers BA, Cowie A, Alcock C, Rosson JW. Identification and treatment of anaemia in patients awaiting hip replacement. Ann R Coll Surg Engl. 2008;90:504–7. doi: 10.1308/003588408X301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bisbe E, García-Erce JA, Díez-Lobo AI, Muñoz M. A multicentre comparative study on the efficacy of intravenous ferric carboxymaltose and iron sucrose for correcting preoperative anaemia in patients undergoing major elective surgery. Br J Anaesth. 2011;107:477–8. doi: 10.1093/bja/aer242. [DOI] [PubMed] [Google Scholar]
- 32.Theusinger OM, Leyvraz PF, Schanz U, et al. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: efficacy and limits: a prospective study. Anesthesiology. 2007;107:923–7. doi: 10.1097/01.anes.0000291441.10704.82. [DOI] [PubMed] [Google Scholar]
- 33.Beris P, Muñoz M, Garcia-Erce JA, et al. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 34.Onken JE, Bregman DB, Harrington RA, et al. A multicenter, randomized, active-controlled study to investigate the efficacy and safety of intravenous ferric carboxymaltose in patients with iron deficiency anemia. Transfusion. 2014;54:306–15. doi: 10.1111/trf.12289. [DOI] [PubMed] [Google Scholar]
- 35.Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: prevalence, outcomes, and healthcare implications. J Hosp Med. 2013;8:506–12. doi: 10.1002/jhm.2061. [DOI] [PubMed] [Google Scholar]
- 36.Shander A. Anemia in the critically ill. Crit Care Clin. 2004;20:159–78. doi: 10.1016/j.ccc.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Napolitano LM. Scope of the problem: epidemiology of anemia and use of blood transfusions in critical care. Crit Care. 2004;8(Suppl 2):S1–8. doi: 10.1186/cc2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Iperen CE, Kraaijenhagen RJ, Biesma DH, et al. Iron metabolism and erythropoiesis after surgery. Br J Surg. 1998;85:41–5. doi: 10.1046/j.1365-2168.1998.00571.x. [DOI] [PubMed] [Google Scholar]
- 39.van Iperen CE, van de Wiel A, Marx JJ. Acute event-related anaemia. Br J Haematol. 2001;115:739–43. doi: 10.1046/j.1365-2141.2001.03167.x. [DOI] [PubMed] [Google Scholar]
- 40.Means RT. Hepcidin and cytokines in anaemia. Hematology. 2004;9:357–62. doi: 10.1080/10245330400018540. [DOI] [PubMed] [Google Scholar]
- 41.Gottschall JL. Blood Transfusion Therapy: A Physician’s Handbook. 8th ed. Bethesda, MD: AABB; 2005. [Google Scholar]
- 42.Mundy GM, Birtwistle SJ, Power RA. The effect of iron supplementation on the level of Hb after lower limb arthroplasty. J Bone Joint Surg Br. 2005;87-B:213–7. doi: 10.1302/0301-620x.87b2.15122. [DOI] [PubMed] [Google Scholar]
- 43.Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92-A:265–9. doi: 10.2106/JBJS.I.00883. [DOI] [PubMed] [Google Scholar]
- 44.Weatherall M, Maling TJ. Oral iron therapy for anaemia after orthopaedic surgery: randomized clinical trial. ANZ J Surg. 2004;74:1049–51. doi: 10.1111/j.1445-1433.2004.03265.x. [DOI] [PubMed] [Google Scholar]
- 45.Muñoz M, Naveira E, Seara J, et al. Role of parenteral iron in transfusion requirements after total hip replacement. A pilot study. Transfus Med. 2006;16:137–42. doi: 10.1111/j.1365-3148.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 46.Muñoz M, Naveira E, Seara J, Cordero J. Effects of postoperative intravenous iron on transfusion requirements after lower limb arthroplasty. Br J Anaesth. 2012;108:532–4. doi: 10.1093/bja/aes012. [DOI] [PubMed] [Google Scholar]
- 47.Berniére J, Dehullu JP, Gall O, Murat I. Intravenous iron in the treatment of postoperative anemia in surgery of the spine in infants and adolescents. Rev Chir Orthop Reparatrice Appar Mot. 1998;84:319–22. [PubMed] [Google Scholar]
- 48.Cuenca J, Garcia-Erce JA, Muñoz M, et al. Patients with pertrochanteric hip fracture may benefit from preoperative intravenous iron therapy: a pilot study. Transfusion. 2004;44:1447–52. doi: 10.1111/j.1537-2995.2004.04088.x. [DOI] [PubMed] [Google Scholar]
- 49.Cuenca J, Garcia-Erce JA, Martinez AA, et al. Role of parenteral iron in the management of anaemia in the elderly patient undergoing displaced subcapital hip fracture repair: preliminary data. Arch Orthop Trauma Surg. 2005;125:342–7. doi: 10.1007/s00402-005-0809-3. [DOI] [PubMed] [Google Scholar]
- 50.Serrano-Trenas JA, Font-Ugalde P, Muñoz-Cabello L, et al. Role of perioperative intravenous iron therapy in elderly hip fracture patients. A single center randomized controlled trial. Transfusion. 2010;51:97–104. doi: 10.1111/j.1537-2995.2010.02769.x. [DOI] [PubMed] [Google Scholar]
- 51.García-Erce JA, Cuenca J, Muñoz M, et al. Perioperative stimulation of erythropoiesis with intravenous iron and erythropoietin reduces transfusion requirements in patients with hip fracture. A prospective observational study. Vox Sang. 2005;88:235–43. doi: 10.1111/j.1423-0410.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 52.García-Erce JA, Cuenca J, Haman-Alcober S, et al. Efficacy of preoperative recombinant human erythropoietin administration for reducing transfusion requirements in patients undergoing surgery for hip fracture repair. An observational cohort study. Vox Sang. 2009;97:260–7. doi: 10.1111/j.1423-0410.2009.01200.x. [DOI] [PubMed] [Google Scholar]
- 53.Cuenca J, Garcia-Erce JA, Martinez F, et al. Perioperative intravenous iron, with or without erythropoietin, plus restrictive transfusion protocol reduce the need for allogenic blood after knee replacement surgery. Transfusion. 2006;46:1112–9. doi: 10.1111/j.1537-2995.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 54.Muñoz M, Gómez-Ramírez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54:289–99. doi: 10.1111/trf.12195. [DOI] [PubMed] [Google Scholar]
- 55.Pieracci FM, Henderson P, Rodney JR, et al. Randomized, double-blind, placebo-controlled trial of effects of enteral iron supplementation on anemia and risk of infection during surgical critical illness. Surg Infect (Larchmt) 2009;10:9–19. doi: 10.1089/sur.2008.043. [DOI] [PubMed] [Google Scholar]
- 56.Ganzoni AM. Intravenous iron-dextran: therapeutic and experimental possibilities. Schweiz Med Wochenschr. 1970;100:301–3. [PubMed] [Google Scholar]
- 57.Auerbach M, Ballard H. Clinical use of intravenous iron: administration, efficacy, and safety. Hematology Am Soc Hematol Educ Program. 2010;2010:338–47. doi: 10.1182/asheducation-2010.1.338. [DOI] [PubMed] [Google Scholar]
- 58.Auerbach M, Macdougall I. Safety of intravenous iron formulations: facts and folklore. Blood Transfus. 2014;12:296–300. doi: 10.2450/2014.0094-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.European Medicines Agency. New recommendations to manage risk of allergic reactions with intravenous iron-containing medicines. Jun 28, 2013. [Accessed on 05/01/2015]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2013/06/WC500144874.pdf.
- 60.Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica. 2014;99:1671–6. doi: 10.3324/haematol.2014.111492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muñoz M, Gómez-Ramírez S, Liumbruno GM, Grazzini G. Intravenous iron and safety: is the end of the debate on the horizon? Blood Transfus. 2014;2:287–9. doi: 10.2450/2014.0144-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laupacis A, Fergusson D. Erythropoietin to minimize perioperative blood transfusion: a systematic review of randomized trials. Transfus Med. 1998;8:309–17. doi: 10.1046/j.1365-3148.1998.00171.x. [DOI] [PubMed] [Google Scholar]
- 63.Feagan BG, Wong CJ, Kirkley A, et al. Erythropoietin with iron supplementation to prevent allogeneic blood transfusion in total hip joint arthroplasty. A randomized, controlled trial. Ann Intern Med. 2000;133:845–54. doi: 10.7326/0003-4819-133-11-200012050-00008. [DOI] [PubMed] [Google Scholar]
- 64.Weber EWG, Slappendel R, Hémon Y, et al. Effects of epoetin alfa on blood transfusions and postoperative recovery in orthopaedic surgery: the European Epoetin Alfa Surgery Trial (EEST) Eur J Anaesthesiol. 2005;22:249–57. doi: 10.1017/s0265021505000426. [DOI] [PubMed] [Google Scholar]
- 65.So-Osman C, Nelissen RG, Koopman-van Gemert AW, et al. Patient blood management in elective total hip- and knee-replacement surgery (part 1): a randomized controlled trial on erythropoietin and blood salvage as transfusion alternatives using a restrictive transfusion policy in erythropoietin-eligible patients. Anesthesiology. 2014;120:839–51. doi: 10.1097/ALN.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 66.Finch CA, Huebers H. Perspectives in iron metabolism. N Engl J Med. 1982;306:1520–8. doi: 10.1056/NEJM198206243062504. [DOI] [PubMed] [Google Scholar]
- 67.Auerbach M, Goodnough LT, Shander A. Iron: the new advances in therapy. Best Pract Res Clin Anaesthesiol. 2013;27:131–40. doi: 10.1016/j.bpa.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Goodnough LT. Iron deficiency syndromes and iron-restricted erythropoiesis (CME) Transfusion. 2012;52:1584–92. doi: 10.1111/j.1537-2995.2011.03495.x. [DOI] [PubMed] [Google Scholar]
- 69.British Committee for Standards in Haematology, Transfusion Task Force. Boulton FE, James V. Guidelines for policies on alternatives to allogeneic blood transfusion. 1. Predeposit autologous blood donation and transfusion. Transfus Med. 2007;17:354–65. doi: 10.1111/j.1365-3148.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 70.Henry DA, Carless PA, Moxey AJ, et al. Pre-operative autologous donation for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2002;2:CD003602A. doi: 10.1002/14651858.CD003602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Singbartl G. Pre-operative autologous blood donation: clinical parameters and efficacy. Blood Transfus. 2011;9:10–8. doi: 10.2450/2010.0088-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pottgiesser T, Specker W, Umhau M, et al. Recovery of haemoglobin mass after blood donation. Transfusion. 2008;48:1390–7. doi: 10.1111/j.1537-2995.2008.01719.x. [DOI] [PubMed] [Google Scholar]
- 73.Singbartl G. Preoperative autologous blood donation - part I. Only two clinical parameters determine efficacy of the autologous predeposit. Minerva Anestesiol. 2007;73:143–51. [PubMed] [Google Scholar]
- 74.Singbartl G, Malgorzata S, Quoss A. Preoperative autologous blood donation - part II. Adapting the predeposit concept to the physiological basics of erythropoiesis improves its efficacy. Minerva Anestesiol. 2007;73:153–60. [PubMed] [Google Scholar]
- 75.Singbartl G, Schreiber J, Singbartl K. Preoperative autologous blood donation versus intraoperative blood salvage: intraindividual analyses and modeling of efficacy in 1103 patients. Transfusion. 2009;49:2374–83. doi: 10.1111/j.1537-2995.2009.02291.x. [DOI] [PubMed] [Google Scholar]
- 76.Singbartl G, Held AL, Singbartl K. Ranking the effectiveness of autologous blood conservation measures through validated modeling of independent clinical data. Transfusion. 2013;53:3060–79. doi: 10.1111/trf.12233. [DOI] [PubMed] [Google Scholar]
- 77.Chee YL, Crawford JC, Watson HG, Greaves M. Guidelines on the assessment of bleeding risk prior to surgery or invasive procedures. British Committee for Standards in Haematology. Br J Haematol. 2008;140:496–504. doi: 10.1111/j.1365-2141.2007.06968.x. [DOI] [PubMed] [Google Scholar]
- 78.Fries D, Innerhofer P, Perger P, et al. Coagulation management in trauma-related massive bleeding. Recommendations of the Task Force for Coagulation (AGPG) of the Austrian Society of Anesthesiology, Resuscitation and Intensive Care Medicine (OGARI) Anasthesiol Intensivmed Notfallmed Schmerzther. 2010;45:552–61. doi: 10.1055/s-0030-1265746. [DOI] [PubMed] [Google Scholar]
- 79.Cosmi B, Alatri A, Cattaneo M, et al. Assessment of the risk of bleeding in patients undergoing surgery or invasive procedures: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) Thromb Res. 2009;124:e6–12. doi: 10.1016/j.thromres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 80.Tosetto A, Castaman G, Rodeghiero F. Bleeding scores in inherited bleeding disorders: clinical or research tools? Haemophilia. 2008;14:415–22. doi: 10.1111/j.1365-2516.2007.01648.x. [DOI] [PubMed] [Google Scholar]
- 81.Koscielny J, Ziemer S, Radtke H, et al. A practical concept for preoperative identification of patients with impaired primary hemostasis. Clin Appl Thromb Hemost. 2004;10:195–204. doi: 10.1177/107602960401000301. [DOI] [PubMed] [Google Scholar]
- 82.Watson HG, Greaves M. Can we predict bleeding? Semin Thromb Hemost. 2008;34:97–103. doi: 10.1055/s-2008-1066028. [DOI] [PubMed] [Google Scholar]
- 83.National Institute for Health and Clinical Excellence (NICE) Preoperative tests (CG3): The use of routine preoperative tests for elective surgery. Jun, 2003. [Accessed on 05/01/2015]. Available at: http://www.nice.org.uk/Guidance/CG3.
- 84.Segal JB, Dzik WH Transfusion Medicine/Hemostasis Clinical Trials Network. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45:1413–25. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 85.Ucar HI, Oc M, Tok M, et al. Preoperative fibrinogen levels as a predictor of postoperative bleeding after open heart surgery. Heart Surg Forum. 2007;10:E392–6. doi: 10.1532/HSF98.20071065. [DOI] [PubMed] [Google Scholar]
- 86.Coakley M, Evans C, Collins P, Hall JE. Predicting blood loss using novel thromboelastometry assays in cardiac surgery. Anaesthesia. 2010;65:99–100. [Google Scholar]
- 87.Reinhofer M, Brauer M, Franke U, et al. The value of rotation thromboelastometry to monitor disturbed perioperative haemostasis and bleeding risk in patients with cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2008;19:212–9. doi: 10.1097/MBC.0b013e3282f3f9d4. [DOI] [PubMed] [Google Scholar]
- 88.Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding. Eur J Anaesthesiology. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 89.Meunier A, Lisander B, Good L. Effects of celecoxib on blood loss, pain, and recovery of function after total knee replacement: a randomized placebo controlled trial. Acta Orthop. 2007;78:661–7. doi: 10.1080/17453670710014365. [DOI] [PubMed] [Google Scholar]
- 90.Weber EW, Slappendel R, Durieux ME, et al. COX 2 selectivity of non-steroidal anti-inflammatory drugs and perioperative blood loss in hip surgery. A randomized comparison of indomethacin and meloxicam. Eur J Anaesthesiol. 2003;20:963–6. doi: 10.1017/s0265021503001558. [DOI] [PubMed] [Google Scholar]
- 91.Li W, Lian YY, Yue WJ, et al. Experimental study of COX-2 selective and traditional non-steroidal anti-inflammatory drugs in total hip replacement. J Int Med Res. 2009;37:472–8. doi: 10.1177/147323000903700223. [DOI] [PubMed] [Google Scholar]
- 92.Slappendel R, Weber EW, Benraad B, et al. Does ibuprofen increase perioperative blood loss during hip arthroplasty? Eur J Anaesthesiol. 2002;19:829–31. doi: 10.1017/s0265021502001345. [DOI] [PubMed] [Google Scholar]
- 93.Anekstein Y, Tamir E, Halperin N, Mirovsky Y. Aspirin therapy and bleeding during proximal femoral fracture surgery. Clin Orthop Relat Res. 2004;418:205–8. doi: 10.1097/00003086-200401000-00034. [DOI] [PubMed] [Google Scholar]
- 94.Lavelle WF, Demers Lavelle EA, Uhl R. Operative delay for orthopedic patients on clopidogrel (plavix): a complete lack of consensus. J Trauma. 2008;64:996–1000. doi: 10.1097/TA.0b013e3180485d23. [DOI] [PubMed] [Google Scholar]
- 95.Randelli F, Biggi F, Della Rocca G, et al. Inter-society consensus statement on antithrombotic prophylaxis in hip and knee replacement and in femoral neck fracture surgery. J Orthop Traumatol. 2011;12:69–76. doi: 10.1007/s10195-010-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gurbel PA, Bliden KP, Butler K, et al. Randomized double-blind assessment of the ONSET and OFFSET of the antiplatelet effects of ticagrelor versus clopidogrel in patients with stable coronary artery disease: the ONSET/OFFSET study. Circulation. 2009;120:2577–85. doi: 10.1161/CIRCULATIONAHA.109.912550. [DOI] [PubMed] [Google Scholar]
- 97.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–15. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 98.Rossini R, Bramucci E, Castiglioni B, et al. Società Italiana di Cardiologia Invasiva (GISE); Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO) Coronary stenting and surgery: perioperative management of antiplatelet therapy in patients undergoing surgery after coronary stent implantation. G Ital Cardiol (Rome) 2012;13:528–51. doi: 10.1714/1114.12251. [DOI] [PubMed] [Google Scholar]
- 99.Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141(2 Suppl):e326S–50S. doi: 10.1378/chest.11-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pengo V, Cucchini U, Denas G, et al. Standardized low-molecular-weight heparin bridging regimen in outpatients on oral anticoagulants undergoing invasive procedure or surgery: an inception cohort management study; Italian Federation of Centers for the Diagnosis of Thrombosis and Management of Antithrombotic Therapies (FCSA) Circulation. 2009;119:2920–7. doi: 10.1161/CIRCULATIONAHA.108.823211. [DOI] [PubMed] [Google Scholar]
- 101.Eriksson BI, Dahl OE, Rosencher N, et al. Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, double-blind, non-inferiority trial. Lancet. 2007;370:949–56. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 102.Eriksson BI, Dahl OE, Rosencher N, et al. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the RE-MODEL randomized trial. J Thromb Haemost. 2007;5:2178–85. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 103.The RE-MOBILIZE Writing Committee. The oral thrombin inhibitor dabigatran etexilate vs. the North American enoxaparin regimen for the prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9. doi: 10.1016/j.arth.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 104.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–51. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 105.Schulman S, Kearon C, Kakkar AK, et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–52. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 106.Lassen MR, Ageno W, Borris LC, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–86. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 107.Eriksson BI, Borris LC, Friedman RJ, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–75. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 108.Xu Q. Xarelto (Rivaroxaban): Cardiovascular and Renal Drugs Advisory Committee Meeting. Mar 19, 2009. [Accessed on 05/01/2015]. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/CardiovascularandRenalDrugsAdvisoryCommittee/UCM143660.pdf.
- 109.EINSTEIN Investigators. Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 110.Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 111.Lassen MR, Raskob GE, Gallus A, et al. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375:807–15. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 112.Lassen MR, Gallus A, Raskob GE, et al. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–98. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 113.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 114.Heidbuchel H, Verhamme P, Alings M, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15:625–51. doi: 10.1093/europace/eut083. [DOI] [PubMed] [Google Scholar]
- 115.Beyer-Westendorf J, Gelbricht V, Förster K, et al. Peri-interventional management of novel oral anticoagulants in daily care: results from the prospective Dresden NOAC registry. Eur Heart J. 2014;35:1888–96. doi: 10.1093/eurheartj/eht557. [DOI] [PubMed] [Google Scholar]
- 116.Pernod G, Albaladejo P, Godier A, et al. Management of major bleeding complications and emergency surgery in patients on long-term treatment with direct oral anticoagulants, thrombin or factor-Xa inhibitors: proposals of the working group on perioperative haemostasis (GIHP) Arch Cardiovasc Dis. 2013;106:382–93. doi: 10.1016/j.acvd.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 117.Healey JS, Eikelboom J, Douketis J, et al. Procedural bleeding and thromboembolic events with dabigatran compared to warfarin: results from the RE-LY randomized trial. Circulation. 2012;126:343–8. doi: 10.1161/CIRCULATIONAHA.111.090464. [DOI] [PubMed] [Google Scholar]
- 118.Sie P, Samama CM, Godier A, et al. Surgery and invasive procedures in patients on long-term treatment with direct oral anticoagulants: thrombin or factor-Xa inhibitors. Recommendations of the Working Group on Perioperative Haemostasis and French Study Group on Thrombosis and Haemostasis. Arch Cardiovasc Dis. 2011;104:669–76. doi: 10.1016/j.acvd.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 119.van Ryn J, Stangier J, Haertter S, et al. Dabigatran etexilate - a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–27. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 120.Huisman MV, Lip GY, Diener HC, et al. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107:838–47. doi: 10.1160/TH11-10-0718. [DOI] [PubMed] [Google Scholar]
- 121.Mueck W, Lensing AW, Agnelli G, et al. Rivaroxaban: population pharmacokinetic analyses in patients treated for acute deep-vein thrombosis and exposure simulations in patients with atrial fibrillation treated for stroke prevention. Clin Pharmacokinet. 2011;50:675–86. doi: 10.2165/11595320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 122.Franchini M, Lippi G, Manzato F, et al. Hemostatic abnormalities in endocrine and metabolic disorders. Eur J Endocrinol. 2010;162:439–51. doi: 10.1530/EJE-09-0958. [DOI] [PubMed] [Google Scholar]
- 123.Franchini M. Hemostasis and thyroid diseases revisited. J Endocrinol Invest. 2004;27:886–92. doi: 10.1007/BF03346287. [DOI] [PubMed] [Google Scholar]
- 124.McCloskey DJ, Postolache TT, Vittone BJ, et al. Selective serotonin reuptake inhibitors: measurement of effect on platelet function. Transl Res. 2008;151:168–72. doi: 10.1016/j.trsl.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Movig KL, Janssen MW, de Waal Malefijt J, et al. Relationship of serotonergic antidepressants and need for blood transfusion in orthopedic surgical patients. Arch Intern Med. 2003;163:2354–8. doi: 10.1001/archinte.163.19.2354. [DOI] [PubMed] [Google Scholar]
- 126.Andreasen JJ, Riis A, Hjortdal VE, et al. Effect of selective serotonin reuptake inhibitors on requirement for allogeneic red blood cell transfusion following coronary artery bypass surgery. Am J Cardiovasc Drugs. 2006;6:243–50. doi: 10.2165/00129784-200606040-00004. [DOI] [PubMed] [Google Scholar]
- 127.Kose G, Arhan E, Unal B, et al. Valproate associated coagulopathies in children during short-term treatment. J Child Neurol. 2009;24:1493–8. doi: 10.1177/0883073808331084. [DOI] [PubMed] [Google Scholar]
- 128.Schädlich D, Friebel D, Schallner J, et al. Evaluation of haemostasis in children treated with valproic acid. Hamostaseologie. 2010;30(Suppl 1):S132–7. [PubMed] [Google Scholar]
- 129.Manohar C, Avitsian R, Lozano S, et al. The effect of antiepileptic drugs on coagulation and bleeding in the perioperative period of epilepsy surgery: the Cleveland Clinic experience. J Clin Neurosci. 2011;18:1180–4. doi: 10.1016/j.jocn.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 130.Kellermann AJ, Kloft C. Is there a risk of bleeding associated with standardized Ginkgo biloba extract therapy? A systematic review and meta-analysis. Pharmacotherapy. 2011;31:490–502. doi: 10.1592/phco.31.5.490. [DOI] [PubMed] [Google Scholar]
- 131.Mannucci PM. Treatment of von Willebrand’s disease. N Engl J Med. 2004;351:683–94. doi: 10.1056/NEJMra040403. [DOI] [PubMed] [Google Scholar]
- 132.Franchini M, Lippi G. Acquired von Willebrand syndrome: an update. Am J Hematol. 2007;82:368–75. doi: 10.1002/ajh.20830. [DOI] [PubMed] [Google Scholar]
- 133.Rodeghiero F, Castaman G, Tosetto A. How I treat von Willebrand disease. Blood. 2009;114:1158–65. doi: 10.1182/blood-2009-01-153296. [DOI] [PubMed] [Google Scholar]
- 134.Tosetto A, Rodeghiero F, Castaman G, et al. A comparison between two semi quantitative bleeding scales for the diagnosis and assessment of bleeding severity in type 1 von Willebrand disease. Haemophilia. 2011;17:165–6. doi: 10.1111/j.1365-2516.2010.02381.x. [DOI] [PubMed] [Google Scholar]
- 135.Tosetto A, Castaman G, Plug I, Rodeghiero F, Eikenboom J. Prospective evaluation of the clinical utility of quantitative bleeding severity assessment in patients referred for hemostatic evaluation. J Thromb Haemost. 2011;9:1143–8. doi: 10.1111/j.1538-7836.2011.04265.x. [DOI] [PubMed] [Google Scholar]
- 136.Mannucci PM, Franchini M, Castaman G, Federici AB. Italian Association of Hemophilia Centers. Evidence-based recommendations on the treatment of von Willebrand disease in Italy. Blood Transfus. 2009;7:117–26. doi: 10.2450/2008.0052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pasi KJ, Collins PW, Keeling DM, et al. Management of von Willebrand disease: a guideline from the UK Haemophilia Centre Doctors’ Organization. Haemophilia. 2004;10:218–31. doi: 10.1111/j.1365-2516.2004.00886.x. [DOI] [PubMed] [Google Scholar]
- 138.Michiels JJ, van Vliet HH, Berneman Z, et al. Managing patients with von Willebrand disease type 1, 2 and 3 with desmopressin and von Willebrand factor-factor VIII concentrate in surgical settings. Acta Haematol. 2009;121:167–76. doi: 10.1159/000214857. [DOI] [PubMed] [Google Scholar]
- 139.Castaman G, Lethagen S, Federici AB, et al. Response to desmopressin is influenced by the genotype and phenotype in type 1 von Willebrand disease (VWD): results from the European Study MCMDM-1VWD. Blood. 2008;111:3531–9. doi: 10.1182/blood-2007-08-109231. [DOI] [PubMed] [Google Scholar]
- 140.Keeling D, Tait C, Makris M. Guideline on the selection and use of therapeutic products to treat haemophilia and other hereditary bleeding disorders. A United Kingdom Haemophilia Center Doctors’ Organisation (UKHCDO) guideline approved by the British Committee for Standards in Haematology. Haemophilia. 2008;14:671–84. doi: 10.1111/j.1365-2516.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- 141.Quiroga T, Goycoolea M, Panes O, et al. High prevalence of bleeders of unknown cause among patients with inherited mucocutaneous bleeding. A prospective study of 280 patients and 299 controls. Haematologica. 2007;92:357–65. doi: 10.3324/haematol.10816. [DOI] [PubMed] [Google Scholar]
- 142.Bolton-Maggs PH, Chalmers EA, Collins PW, et al. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. Br J Haematol. 2006;135:603–33. doi: 10.1111/j.1365-2141.2006.06343.x. [DOI] [PubMed] [Google Scholar]
- 143.Podda GM, Bucciarelli P, Lussana F, et al. Usefulness of PFA-100 testing in the diagnostic screening of patients with suspected abnormalities of hemostasis: comparison with the bleeding time. J Thromb Haemost. 2007;5:2393–8. doi: 10.1111/j.1538-7836.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 144.Coppola A, Di Minno G. Desmopressin in inherited disorders of platelet function. Haemophilia. 2008;14(Suppl 1):31–9. doi: 10.1111/j.1365-2516.2007.01607.x. [DOI] [PubMed] [Google Scholar]
- 145.Peyvandi F, Cattaneo M, Inbal A, et al. Rare bleeding disorders. Haemophilia. 2008;14(Suppl 3):202–10. doi: 10.1111/j.1365-2516.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 146.Rao AK, Ghosh S, Sun L, et al. Mechanisms of platelet dysfunction and response to DDAVP in patients with congenital platelet function defects. A double-blind placebo-controlled trial. Thromb Haemost. 1995;74:1071–8. [PubMed] [Google Scholar]
- 147.Alamelu J, Liesner R. Modern management of severe platelet function disorders. Br J Haematol. 2010;149:813–23. doi: 10.1111/j.1365-2141.2010.08191.x. [DOI] [PubMed] [Google Scholar]
- 148.Weber CF, Görlinger K, Byhahn C, et al. Tranexamic acid partially improves platelet function in patients treated with dual antiplatelet therapy. Eur J Anaesthesiol. 2011;28:57–62. doi: 10.1097/EJA.0b013e32834050ab. [DOI] [PubMed] [Google Scholar]
- 149.Hennewig U, Laws HJ, Eisert S, Gobel U. Bleeding and surgery in children with Glanzmann thrombasthenia with and without the use of recombinant factor VII a. Klin Padiatr. 2005;217:365–70. doi: 10.1055/s-2005-872523. [DOI] [PubMed] [Google Scholar]
- 150.Tosetto A, Balduini CL, Cattaneo M, et al. Management of bleeding and of invasive procedures in patients with platelet disorders and/or thrombocytopenia: Guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) Thromb Res. 2009;124:e13–e18. doi: 10.1016/j.thromres.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 151.Poon MC, D’Oiron R, Von Depka M, et al. Prophylactic and therapeutic recombinant factor VIIa administration to patients with Glanzmann’s thrombasthenia: results of an international survey. J Thromb Haemost. 2004;2:1096–103. doi: 10.1111/j.1538-7836.2004.00767.x. [DOI] [PubMed] [Google Scholar]
- 152.British Committee for Standards in Haematology Blood Transfusion Task Force. Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122:10–23. doi: 10.1111/j.1365-2141.2010.08444.x. [DOI] [PubMed] [Google Scholar]
- 153.Blumberg N, Heal JM, Phillips GL. Platelet transfusions: trigger, dose, benefits, and risks. F1000 Med Rep. 2010;2:5. doi: 10.3410/M2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of plasma and platelets. Blood Transfus. 2009;7:132–50. doi: 10.2450/2009.0005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kaufman RM, Djulbegovic B, Gernsheimer T, et al. Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2015;162:205–13. doi: 10.7326/M14-1589. [DOI] [PubMed] [Google Scholar]
- 156.Srivastava A, Brewer AK, Mauser-Bunschoten EP, et al. Treatment Guidelines Working Group on Behalf of The World Federation Of Hemophilia. Guidelines for the management of hemophilia. Haemophilia. 2013;19:e1–47. doi: 10.1111/j.1365-2516.2012.02909.x. [DOI] [PubMed] [Google Scholar]
- 157.Mannucci PM, Tuddenham EG. The hemophilias - from royal genes to gene therapy. N Engl J Med. 2001;344:1773–9. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 158.White GC, 2nd, Rosendaal F, Aledort LM, et al. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85:560. [PubMed] [Google Scholar]
- 159.Franchini M, Zaffanello M, Lippi G. Acquired hemophilia in pediatrics: a systematic review. Pediatr Blood Cancer. 2010;55:606–11. doi: 10.1002/pbc.22657. [DOI] [PubMed] [Google Scholar]
- 160.Hermans C, Altisent C, Batorova A, et al. Replacement therapy for invasive procedures in patients with haemophilia: literature review, European survey and recommendations. Haemophilia. 2009;15:639–58. doi: 10.1111/j.1365-2516.2008.01950.x. [DOI] [PubMed] [Google Scholar]
- 161.Batlle J, Villar A, Liras A, et al. Consensus opinion for the selection and use of therapeutic products for the treatment of haemophilia in Spain. Blood Coagul Fibrinolysis. 2008;19:333–40. doi: 10.1097/MBC.0b013e328300c814. [DOI] [PubMed] [Google Scholar]
- 162.Santagostino E, Mannucci PM, Bianchi Bonomi A. Guidelines on replacement therapy for haemophilia and inherited coagulation disorders in Italy. Haemophilia. 2000;6:1–10. doi: 10.1046/j.1365-2516.2000.00361.x. [DOI] [PubMed] [Google Scholar]
- 163.Hermans C, Hammer F, Lobet S, Lambert C. Subclinical deep venous thrombosis observed in 10% of hemophilic patients undergoing major orthopedic surgery. J Thromb Haemost. 2010;8:1138–40. doi: 10.1111/j.1538-7836.2010.03829.x. [DOI] [PubMed] [Google Scholar]
- 164.Mumford AD, Ackroyd S, Alikhan R, et al. BCSH Committee. Guideline for the diagnosis and management of the rare coagulation disorders: a United Kingdom Haemophilia Centre Doctors’ Organization guideline on behalf of the British Committee for Standards in Haematology. Br J Haematol. 2014;167:304–26. doi: 10.1111/bjh.13058. [DOI] [PubMed] [Google Scholar]
- 165.Castaman G. Prophylaxis of bleeding episodes and surgical interventions in patients with rare inherited coagulation disorders. Blood Transfus. 2008;6(Suppl 2):s39–44. doi: 10.2450/2008.0036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bolton-Maggs PH, Perry DJ, Chalmers EA, et al. The rare coagulation disorders-review with guidelines for management from the United Kingdom Haemophilia Centre Doctors’ Organisation. Haemophilia. 2004;10:593–628. doi: 10.1111/j.1365-2516.2004.00944.x. [DOI] [PubMed] [Google Scholar]
- 167.Benlakhal F, Mura T, Schved JF, Giansily-Blaizot M French Study Group of Factor VII Deficiency. A retrospective analysis of 157 surgical procedures performed without replacement therapy in 83 unrelated factor VII-deficient patients. J Thromb Haemost. 2011;9:1149–56. doi: 10.1111/j.1538-7836.2011.04291.x. [DOI] [PubMed] [Google Scholar]
- 168.Kenet G, Lubetsky A, Luboshitz J, et al. Lower doses of rFVIIa therapy are safe and effective for surgical interventions in patients with severe FXI deficiency and inhibitors. Haemophilia. 2009;15:1065–73. doi: 10.1111/j.1365-2516.2009.02043.x. [DOI] [PubMed] [Google Scholar]
- 169.Barillari G, Pasca S, Gonano N, Daminato R. Prothrombin complex concentrate such as therapy and prophylaxis in factor X-deficient patient (Friuli variant) Clin Appl Thromb Hemost. 2011;17:332–6. doi: 10.1177/1076029610365331. [DOI] [PubMed] [Google Scholar]
- 170.van Veen JJ, Hampton KK, Maclean R, et al. Blood product support for delivery in severe factor X deficiency: the use of thrombin generation to guide therapy. Blood Transfus. 2007;5:204–9. doi: 10.2450/2007.0023-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Messmer K, Lewis DH, Sunder-Plassmann L, et al. Acute normovolemic hemodilution. Changes of central hemodynamics and microcirculatory flow in skeletal muscle. Eur Surg Res. 1972;4:55–70. doi: 10.1159/000127600. [DOI] [PubMed] [Google Scholar]
- 172.Laks H, O’Connor NE, Pilon RN, et al. Acute normovolemic hemodilution: effects on hemodynamics, oxygen transport, and lung water in anesthetized man. Surg Forum. 1973;24:201–2. [PubMed] [Google Scholar]
- 173.Bauer H, Pichlmaier H, Ott E, et al. Autotransfusion through acute, preoperative hemodilution: 1st clinical experiences. Langenbecks Arch Chir. 1974;(Suppl):185–9. [PubMed] [Google Scholar]
- 174.Monk TG. Acute normovolemic hemodilution. Anesthesiology Clin N Am. 2005;23:271–81. doi: 10.1016/j.atc.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 175.Kreimeier U, Messmer K. Perioperative hemodilution. Transfus Apher Sci. 2002;27:59–72. doi: 10.1016/s1473-0502(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 176.Shander A, Goodnough LT. Objectives and limitations of bloodless medical care. Curr Opin Hematol. 2006;13:462–70. doi: 10.1097/01.moh.0000245692.32085.bd. [DOI] [PubMed] [Google Scholar]
- 177.Cardone D, Klein AA. Perioperative blood conservation. Eur J Anaesthesiol. 2009;26:722–9. doi: 10.1097/EJA.0b013e32832c5280. [DOI] [PubMed] [Google Scholar]
- 178.Kochamba GS, Pfeffer TA, Sintek Cf, et al. Intraoperative autotransfusion reduces blood loss after cardiopulmonary bypass. Ann Thorac Surg. 1996;61:900–3. doi: 10.1016/0003-4975(95)01155-2. [DOI] [PubMed] [Google Scholar]
- 179.Helm RE, Klemperer JD, Rosengart TK, et al. Intraoperative autologous blood donation preserves red cell mass but does not decrease postoperative bleeding. Ann Thorac Surg. 1996;62:1431–41. doi: 10.1016/0003-4975(96)00755-2. [DOI] [PubMed] [Google Scholar]
- 180.Olsfanger D, Fredman B, Goldstein B, et al. Acute normovolaemic haemodilution decreases postoperative allogeneic blood transfusion after total knee replacement. Br J Anaesth. 1997;79:317–21. doi: 10.1093/bja/79.3.317. [DOI] [PubMed] [Google Scholar]
- 181.Terada N, Arai Y, Matsuta Y, et al. Acute normovolemic hemodilution for radical prostatectomy: can it replace preoperative autologous blood transfusion? Int J Urol. 2001;8:149–52. doi: 10.1046/j.1442-2042.2001.00272.x. [DOI] [PubMed] [Google Scholar]
- 182.Matot I, Scheinin O, Jurim O, et al. Effectiveness of acute normovolaemic haemodilution to minimize allogeneic blood transfusion in major liver resections. Anesthesiology. 2002;97:794–800. doi: 10.1097/00000542-200210000-00008. [DOI] [PubMed] [Google Scholar]
- 183.Wong JC, Torella F, Haynes SL, et al. Autologous versus allogeneic transfusion in aortic surgery: a multicenter randomized clinical trial. Ann Surg. 2002;235:145–51. doi: 10.1097/00000658-200201000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Habler O, Schwenzer K, Zimmer K, et al. Effects of standardized acute normovolemic hemodilution on intraoperative allogeneic blood transfusion in patients undergoing major maxillofacial surgery. Int J Oral Maxillofac Surg. 2004;33:467–75. doi: 10.1016/j.ijom.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 185.Bennet J, Haynes S, Torella F, et al. Acute normovolemic hemodilution in moderate blood loss surgery: a randomized controlled trial. Transfusion. 2006;46:1097–103. doi: 10.1111/j.1537-2995.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 186.Takayanagi A, Masumori N, Kobayashi K, et al. Acute normovolemic hemodilution for radical retropubic prostatectomy and radical cystectomy. Urology. 2008;72:401–5. doi: 10.1016/j.urology.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 187.Imai R, Matsumura H, Uchida R, Watanabe K. Perioperative hemodilutional autologous blood transfusion in burn surgery. Int J Care Injured. 2008;39:57–60. doi: 10.1016/j.injury.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 188.Parkin IR, Chiu GA, Schwarz PA, Hodder SC. Acute perioperative normovolaemic haemodilution in major maxillofacial surgery. Br J Oral Maxillofac Surg. 2008;46:387–90. doi: 10.1016/j.bjoms.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 189.Jarnagin WR, Gonen M, Maithel SK, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg. 2008;248:360–9. doi: 10.1097/SLA.0b013e318184db08. [DOI] [PubMed] [Google Scholar]
- 190.Kahraman S, Altunkaya H, Celebioglu B, et al. The effect of acute normovolemic hemodilution on homologous blood requirements and total estimated red blood cell volume lost. Acta Anaesthesiol Scand. 1997;41:614–7. doi: 10.1111/j.1399-6576.1997.tb04752.x. [DOI] [PubMed] [Google Scholar]
- 191.Hohn L, Schweizer A, Licker M, Morel DR. Absence of beneficial effect of acute normovolemic hemodilution combined with aprotinin on allogeneic blood transfusion requirements in cardiac surgery. Anesthesiology. 2002;96:276–92. doi: 10.1097/00000542-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 192.Svenmarker S, Engstrom KG. The inflammatory response to recycled pericardial suction blood and the influence of cell-saving. Scand Cardiovasc J. 2003;37:158–64. doi: 10.1080/14017430310001465. [DOI] [PubMed] [Google Scholar]
- 193.Bryson GL, Laupacis A, Wells GA. Does acute normovolemic hemodilution reduce perioperative allogeneic transfusion? A meta-analysis. The International Study of Perioperative Transfusion. Anesth Analg. 1998;86:9–15. doi: 10.1213/00000539-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 194.Gillon J, Desmond M, Thomas MJ. Acute normovolemic haemodilution. Transfus Med. 1999;9:259–64. doi: 10.1046/j.1365-3148.1999.00205.x. [DOI] [PubMed] [Google Scholar]
- 195.Segal JB, Blasco-Colmenares E, Norris EJ, Guallar E. Preoperative acute normovolemic hemodilution: a meta-analysis. Transfusion. 2004;44:632–44. doi: 10.1111/j.1537-2995.2004.03353.x. [DOI] [PubMed] [Google Scholar]
- 196.Carless P, Moxey A, O’Connell DO, Henry D. Autologous transfusion techniques: a systematic review of their efficacy. Transfus Med. 2004;14:123–44. doi: 10.1111/j.0958-7578.2004.0489.x. [DOI] [PubMed] [Google Scholar]
- 197.Zohar E, Fredman B, Ellis M, et al. A comparative study of the postoperative allogeneic blood sparing effect of tranexamic acid versus acute normovolemic hemodilution after total knee replacement. Anesth Analg. 1999;89:1382–7. doi: 10.1097/00000539-199912000-00010. [DOI] [PubMed] [Google Scholar]
- 198.Davies L, Brown TJ, Haynes S, et al. Cost-effectiveness of cell salvage and alternative methods of minimizing perioperative allogeneic blood transfusion: a systematic review and economic model. Health Technol Assess. 2006;10:1–210. doi: 10.3310/hta10440. [DOI] [PubMed] [Google Scholar]
- 199.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the transfusion management of patients in the peri-operative period. II. The intra-operative period. Blood Transfus. 2011;9:189–217. doi: 10.2450/2011.0075-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Esper SA, Waters JH. Intra-operative cell salvage: a fresh look at the indications and contraindications. Blood Transfus. 2011;9:139–47. doi: 10.2450/2011.0081-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Pape A, Habler O. Alternatives to allogeneic blood transfusions. Best Pract Res Clin Anaesthesiol. 2007;21:221–39. doi: 10.1016/j.bpa.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 202.Huet C, Salmi LR, Fergusson D, et al. A meta analysis of the effectiveness of cell salvage to minimize perioperative allogeneic blood transfusion in cardiac and orthopedic surgery. Anesth Analg. 1999;89:861–78. doi: 10.1097/00000539-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 203.Carless P, Moxey A, O’Connell DO, Henry D. Autologous transfusion techniques: a systematic review of their efficacy. Transfus Med. 2004;14:123–44. doi: 10.1111/j.0958-7578.2004.0489.x. [DOI] [PubMed] [Google Scholar]
- 204.Carless PA, Henry DA, Moxey AJ, et al. Cell salvage for minimizing perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2010;4:CD001888. doi: 10.1002/14651858.CD001888.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.So-Osman C, Nelissen RG, Koopman-van Gemert AW, et al. Patient blood management in elective total hip- and knee-replacement surgery (part 2): a randomized controlled trial on blood salvage as transfusion alternative using a restrictive transfusion policy in patients with a preoperative hemoglobin above 13 g/dl. Anesthesiology. 2014;120:839–51. doi: 10.1097/ALN.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 206.Tenholder M, Cushner FD. Intraoperative blood management in joint replacement surgery. Orthopedics. 2004;27(6 Suppl):s663–8. doi: 10.3928/0147-7447-20040602-07. [DOI] [PubMed] [Google Scholar]
- 207.Tobias JD. Strategies for minimizing blood loss in orthopedic surgery. Semin Hematol. 2004;41(1 Suppl 1):145–56. doi: 10.1053/j.seminhematol.2003.11.025. [DOI] [PubMed] [Google Scholar]
- 208.Phillips SJ, Chavan R, Porter ML, et al. Does salvage and tranexamic acid reduce the need for blood transfusion in revision hip surgery? J Bone Joint Surg Br. 2006;88-B:1141–2. doi: 10.1302/0301-620X.88B9.17605. [DOI] [PubMed] [Google Scholar]
- 209.Bridgens JP, Evans CR, Dobson PM, Hamer AJ. Intraoperative red blood-cell salvage in revision hip surgery. A case-matched study. J Bone Joint Surg Am. 2007;89:270–5. doi: 10.2106/JBJS.F.00492. [DOI] [PubMed] [Google Scholar]
- 210.Bess RS, Lenke LG. Blood loss minimization and blood salvage techniques for complex spinal surgery. Neurosurg Clin N Am. 2006;17:227–34. doi: 10.1016/j.nec.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 211.Gause PR, Siska PA, Westrick ER, et al. Efficacy of intraoperative cell saver in decreasing postoperative blood transfusions in instrumented posterior lumbar fusion patients. Spine (Phila Pa 1976) 2008;33:571–5. doi: 10.1097/BRS.0b013e3181657cc1. [DOI] [PubMed] [Google Scholar]
- 212.Mirza AH, Aldlyami E, Bhimarasetty C, et al. The role of perioperative cell salvage in instrumented anterior correction of thoracolumbar scoliosis: a case-controlled study. Acta Orthop Belg. 2009;75:87–93. [PubMed] [Google Scholar]
- 213.Lemos MJ, Healy WL. Blood transfusion in orthopaedic operations. J Bone Joint Surg Am. 1996;78:1260–70. doi: 10.2106/00004623-199608000-00019. [DOI] [PubMed] [Google Scholar]
- 214.Bowen JR, Angus PD, Huxster RR, MacEwen GD. Posterior spinal fusion without blood replacement in Jehovah’s Witnesses. Clin Orthop Relat Res. 1985;198:284–8. [PubMed] [Google Scholar]
- 215.Takeda N, Kobayashi T, Tandai S, et al. Treatment of giant cell tumors in the sacrum and spine with curettage and argon beam coagulator. J Orthop Sci. 2009;14:210–4. doi: 10.1007/s00776-008-1299-2. [DOI] [PubMed] [Google Scholar]
- 216.Bonicoli E, Andreani L, Parchi P, et al. Custom-fit total knee arthroplasty: our initial experience with 30 Knees. Eur J Orthop Surg Traumatol. 2014;24:1249–54. doi: 10.1007/s00590-013-1304-0. [DOI] [PubMed] [Google Scholar]
- 217.Sassoon A, Nam D, Nunley R, Barrack R. Systematic review of patient-specific instrumentation in total knee arthroplasty: new but not improved. Clin Orthop Relat Res. 2015;473:151–8. doi: 10.1007/s11999-014-3804-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Berry DJ, Bozic KJ. Current practice patterns in primary hip and knee arthroplasty among members of the American Association of Hip and Knee Surgeons. J Arthroplasty. 2010;25(6 Suppl):2–4. doi: 10.1016/j.arth.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 219.Hernandez AJ, Almeida AM, Fávaro E, Sguizzato GT. The influence of tourniquet use and operative time on the incidence of deep vein thrombosis in total knee arthroplasty. Clinics (Sao Paulo) 2012;67:1053–7. doi: 10.6061/clinics/2012(09)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Palmer SH, Graham G. Tourniquet-induced rhabdomyolysis after total knee replacement. Ann R Coll Surg Engl. 1994;76:416–7. [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang W, Li N, Chen S, et al. The effects of a tourniquet used in total knee arthroplasty: a meta-analysis. J Orthop Surg Res. 2014;9:13. doi: 10.1186/1749-799X-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jiang FZ, Zhong HM, Hong YC, Zhao GF. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci. 2015;20:110–23. doi: 10.1007/s00776-014-0664-6. [DOI] [PubMed] [Google Scholar]
- 223.Kwan I, Bunn F, Chinnock P, Roberts I. Timing and volume of fluid administration for patients with bleeding. Cochrane Database Syst Rev. 2014;3:CD002245. doi: 10.1002/14651858.CD002245.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Perel P, Roberts I, Ker K. Colloids versus crystalloids for fluid resuscitation in critically ill patients. Cochrane Database Syst Rev. 2013;2:CD000567. doi: 10.1002/14651858.CD000567.pub6. [DOI] [PubMed] [Google Scholar]
- 225.Raghunathan K, Murray PT, Beattie WS, et al. ADQI XII Investigators Group. Choice of fluid in acute illness: what should be given? An international consensus. Br J Anaesth. 2014;113:772–83. doi: 10.1093/bja/aeu301. [DOI] [PubMed] [Google Scholar]
- 226.Zarychanski R, Abou-Setta AM, Turgeon AF, et al. Association of hydroxyethyl starch administration with mortality and acute kidney injury in critically ill patients requiring volume resuscitation: a systematic review and meta-analysis. JAMA. 2013;309:678–88. doi: 10.1001/jama.2013.430. [DOI] [PubMed] [Google Scholar]
- 227.Agenzia Italiana del Farmaco (AIFA) Nota informativa importante concordata con l’Agenzia Europea dei Medicinali (EMA) e l’agenzia Italiana del Farmaco (AIFA) Restrizione d’uso di HES (medicinali contenenti amido idrossietilico) Amidolite, Volulyte, HAES-STERIL, Voluven, Hyperhaes, Vonten e Plasmavolume. [Accessed on 22/01/2015]. Available at: http://www.agenziafarmaco.gov.it/sites/default/files/IT_DHPC_HES_common.pdf.
- 228.Finfer S, Bellomo R, Boyce N, et al. SAFE Study Investigators. A comparison of albumin and saline for fluid resuscitation in the intensive care unit. N Engl J Med. 2004;350:2247–56. doi: 10.1056/NEJMoa040232. [DOI] [PubMed] [Google Scholar]
- 229.Søreide E, Deakin CD. Pre-hospital fluid therapy in the critically injured patient: a clinical update. Injury. 2005;36:1001–10. doi: 10.1016/j.injury.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 230.Santry HP, Alam HB. Fluid resuscitation: past, present, and the future. Shock. 2010;33:229–41. doi: 10.1097/SHK.0b013e3181c30f0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Waters JH. Role of the massive transfusion protocol in the management of haemorrhagic shock. Br J Anaesth. 2014;113(Suppl 2):ii3–8. doi: 10.1093/bja/aeu379. [DOI] [PubMed] [Google Scholar]
- 232.Carless PA, Henry DA, Anthony DM. Fibrin sealant use for minimising peri-operative allogeneic blood transfusion. Cochrane Database Syst Rev. 2003;2:CD004171. doi: 10.1002/14651858.CD004171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sileshi B, Achneck HE, Lawson JH. Management of surgical hemostasis: topical agents. Vascular. 2008;16(Suppl 1):S22–8. [PubMed] [Google Scholar]
- 234.Achneck HE, Sileshi B, Jamiolkowski RM, et al. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251:217–28. doi: 10.1097/SLA.0b013e3181c3bcca. [DOI] [PubMed] [Google Scholar]
- 235.Mauermann W, Shilling AM, Zuo Z. A comparison of neuraxial block versus general anesthesia for elective total hip replacement: a meta-analysis. Anesth Analg. 2006;120:1018–27. doi: 10.1213/01.ane.0000237267.75543.59. [DOI] [PubMed] [Google Scholar]
- 236.Richman JM, Rowlison AJ, Maine DN, et al. Does neuraxial anesthesia reduce intraoperative blood loss? A meta-analysis. J Clin Anesth. 2006;18:427–35. doi: 10.1016/j.jclinane.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 237.Macfarlane AJ, Prasad GA, Chan VW, et al. Does regional anesthesia improve outcome after total knee arthroplasty? Clin Orthop Relat Res. 2009;467:2379–402. doi: 10.1007/s11999-008-0666-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Jellish WS, Shea JF. Spinal anaesthesia for spinal surgery. Best Pract Res Clin Anaesthesiol. 2003;17:323–34. doi: 10.1016/s1521-6896(02)00115-5. [DOI] [PubMed] [Google Scholar]
- 239.Stevens RD, Van Gessel E, Flory N, et al. Lumbar plexus block reduces pain and blood loss associated with total hip arthroplasty. Anesthesiology. 2000;93:115–21. doi: 10.1097/00000542-200007000-00021. [DOI] [PubMed] [Google Scholar]
- 240.Twyman R, Kirwan T, Fennelly M. Blood loss reduced during hip arthroplasty by lumbar plexus block. J Bone Joint Surg Br. 1990;72:770–1. doi: 10.1302/0301-620X.72B5.2211752. [DOI] [PubMed] [Google Scholar]
- 241.Albertin A, La Colla L, Gandolfi A, et al. Greater peripheral blood flow but less bleeding with propofol versus sevoflurane during spine surgery: a possible physiologic model? Spine. 2008;33:2017–22. doi: 10.1097/BRS.0b013e31817e0405. [DOI] [PubMed] [Google Scholar]
- 242.Rajagopalan S, Mascha E, Na J, et al. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology. 2008;108:71–7. doi: 10.1097/01.anes.0000296719.73450.52. [DOI] [PubMed] [Google Scholar]
- 243.Akca O, Sessler DI. Thermal management and blood loss during hip arthroplasty. Minerva Anestesiol. 2002;68:182–5. [PubMed] [Google Scholar]
- 244.Schmied H, Schiferer A, Sessler DI, et al. The effects of red-cells scavenging, hemodilution, and active warming on allogenic blood requirements in patients undergoing hip or knee arthroplasty. Anesth Analg. 1998;86:389–91. doi: 10.1097/00000539-199802000-00032. [DOI] [PubMed] [Google Scholar]
- 245.Rahe-Meyer N, Pichlmaier M, Haverich A, et al. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br J Anaesth. 2009;102:785–92. doi: 10.1093/bja/aep089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rahe-Meyer N, Solomon C, Winterhalter M, et al. Thromboelastometry guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138:694–702. doi: 10.1016/j.jtcvs.2008.11.065. [DOI] [PubMed] [Google Scholar]
- 247.Fenger-Eriksen C, Jensen TM, Kristensen BS, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost. 2009;7:795–802. doi: 10.1111/j.1538-7836.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- 248.Wikkelsø A, Lunde J, Johansen M, et al. Fibrinogen concentrate in bleeding patients. Cochrane Database Syst Rev. 2013;8:CD008864. doi: 10.1002/14651858.CD008864.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lunde J, Stensballe J, Wikkelsø A, et al. Fibrinogen concentrate for bleeding - a systematic review. Acta Anaesthesiol Scand. 2014;58:1061–74. doi: 10.1111/aas.12370. [DOI] [PubMed] [Google Scholar]
- 250.Mackie IJ, Kitchen S, Machin SJ, Lowe GD Haemostasis and Thrombosis Task Force of the British Committee for Standards in Haematology. Guidelines on fibrinogen assays. Br J Haematol. 2003;121:396–404. doi: 10.1046/j.1365-2141.2003.04256.x. [DOI] [PubMed] [Google Scholar]
- 251.Eckman MH, Erban JK, Singh SK, Kao GS. Screening for the risk for bleeding or thrombosis. Ann Intern Med. 2003;138:W15–24. doi: 10.7326/0003-4819-138-3-200302040-00011-w1. [DOI] [PubMed] [Google Scholar]
- 252.Kreuz W, Meili E, Peter-Salonen K, et al. Pharmacokinetic properties of a pasteurised fibrinogen concentrate. Transfus Apher Sci. 2005;32:239–46. doi: 10.1016/j.transci.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 253.Ogawa S, Szlam F, Chen EP, et al. A comparative evaluation of rotation thromboelastometry and standard coagulation tests in hemodilution induced coagulation changes after cardiac surgery. Transfusion. 2012;52:14–22. doi: 10.1111/j.1537-2995.2011.03241.x. [DOI] [PubMed] [Google Scholar]
- 254.Bornikova L, Peyvandi F, Allen G, et al. Fibrinogen replacement therapy for congenital fibrinogen deficiency. J Thromb Haemost. 2011;9:1687–704. doi: 10.1111/j.1538-7836.2011.04424.x. [DOI] [PubMed] [Google Scholar]
- 255.Aubron C, Reade MC, Fraser JF, Cooper DJ. Efficacy and safety of fibrinogen concentrate in trauma patients: a systematic review. J Crit Care. 2014;29:471.e11–7. doi: 10.1016/j.jcrc.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 256.Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care. 2013;17:R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Stainsby D, MacLennan S, Thomas D, et al. Guidelines on the management of massive blood loss. Br J Haematol. 2006;135:634–41. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- 258.American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 259.German Medical Association. Cross-sectional guidelines for therapy with blood components and plasma derivatives. 2009. [Accessed on 05/01/2015]. Available at: http://www.bundesaerztekammer.de/downloads/Querschnittsleitlinie_Gesamtdokument-englisch_07032011.pdf.
- 260.Association of Anaesthetists of Great Britain and Ireland. Thomas D, Wee M, Clyburn P, et al. Blood transfusion and the anaesthetist: management of massive haemorrhage. Anaesthesia. 2010;65:1153–61. doi: 10.1111/j.1365-2044.2010.06538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gaarder C, Naess PA, Frischknecht Christensen E, et al. Scandinavian guidelines: “The massively bleeding patient”. Scand J Surg. 2008;97:15–36. doi: 10.1177/145749690809700104. [DOI] [PubMed] [Google Scholar]
- 262.Haas T, Fries D, Velik-Salchner C, et al. Fibrinogen in craniosynostosis surgery. Anesth Analg. 2008;106:725–31. doi: 10.1213/ane.0b013e318163fb26. [DOI] [PubMed] [Google Scholar]
- 263.Mittermayr M, Streif W, Haas T, et al. Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesth Analg. 2007;105:905–17. doi: 10.1213/01.ane.0000280481.18570.27. [DOI] [PubMed] [Google Scholar]
- 264.Ranucci M, Isgrò G, Soro G, et al. Efficacy and safety of recombinant activated factor VII in major surgical procedures. Arch Surg. 2008;143:296–304. doi: 10.1001/archsurg.2007.66. [DOI] [PubMed] [Google Scholar]
- 265.Zangrillo A, Mizzi A, Biondi-Zoccai G, et al. Recombinant activated factor VII in cardiac surgery: a meta-analysis. J Cardiothor Vasc Anesth. 2009;23:34–40. doi: 10.1053/j.jvca.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 266.Stanworth SJ, Birchall J, Doree CJ, Hyde C. Recombinant factor VIIa for the prevention and treatment of bleeding in patients without haemophilia. Cochrane Database Syst Rev. 2007;2:CD005011. doi: 10.1002/14651858.CD005011.pub2. [DOI] [PubMed] [Google Scholar]
- 267.Ozier Y. Pharmacological agents: antifibrinolytics and desmopressin. Best Pract Res Clin Anaesthesiol. 2010;24:107–19. doi: 10.1016/j.bpa.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 268.Henry DA, Carless PA, Moxey AJ, et al. Anti-fibrinolytic use for minimising perioperative allogeneic blood transfusion. Cochrane Database Syst Rev. 2011;3:CD001886. doi: 10.1002/14651858.CD001886.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Cid J, Lozano M. Tranexamic acid reduces allogeneic red cell transfusions in patients undergoing total knee arthroplasty: results of a meta-analysis of randomized controlled trials. Transfusion. 2005;45:1302–7. doi: 10.1111/j.1537-2995.2005.00204.x. [DOI] [PubMed] [Google Scholar]
- 270.Yang ZG, Chen WP, Wu LD. Effectiveness and safety of tranexamic acid in reducing blood loss in total knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2012;94:1153–9. doi: 10.2106/JBJS.K.00873. [DOI] [PubMed] [Google Scholar]
- 271.Zhang H, Chen J, Chen F, Que W. The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2012;20:1742–52. doi: 10.1007/s00167-011-1754-z. [DOI] [PubMed] [Google Scholar]
- 272.Alshryda S, Sarda P, Sukeik M, et al. Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br. 2011;93:1577–85. doi: 10.1302/0301-620X.93B12.26989. [DOI] [PubMed] [Google Scholar]
- 273.Yang B, Li H, Wang D, et al. Systematic review and meta-analysis of perioperative intravenous tranexamic acid use in spinal surgery. PLoS One. 2013;8:e55436. doi: 10.1371/journal.pone.0055436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Yuan C, Zhang H, He S. Efficacy and safety of using antifibrinolytic agents in spine surgery: a meta-analysis. PLoS One. 2013;8:e82063. doi: 10.1371/journal.pone.0082063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Sukeik M, Alshryda S, Haddad FS, Mason JM. Systematic review and meta-analysis of the use of tranexamic acid in total hip replacement. J Bone Joint Surg Br. 2011;93:39–46. doi: 10.1302/0301-620X.93B1.24984. [DOI] [PubMed] [Google Scholar]
- 276.Zhou XD, Tao LJ, Li J, Wu LD. Do we really need tranexamic acid in total hip arthroplasty? A meta-analysis of nineteen randomized controlled trials. Arch Orthop Trauma Surg. 2013;133:1017–27. doi: 10.1007/s00402-013-1761-2. [DOI] [PubMed] [Google Scholar]
- 277.Gandhi R, Evans HM, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184. doi: 10.1186/1756-0500-6-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Poeran J, Rasul R, Suzuki S, et al. Tranexamic acid use and postoperative outcomes in patients undergoing total hip or knee arthroplasty in the United States: retrospective analysis of effectiveness and safety. BMJ. 2014;349:g4829. doi: 10.1136/bmj.g4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Ortmann E, Besser MW, Klein AA. Antifibrinolytic agents in current anaesthetic practice. Br J Anaesth. 2013;111:549–63. doi: 10.1093/bja/aet154. [DOI] [PubMed] [Google Scholar]
- 280.Huang Z, Ma J, Shen B, Pei F. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29:2342–6. doi: 10.1016/j.arth.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 281.Konig G, Hamlin BR, Waters JH. Topical tranexamic acid reduces blood loss and transfusion rates in total hip and total knee arthroplasty. J Arthroplasty. 2013;28:1473–6. doi: 10.1016/j.arth.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Alshryda S, Mason J, Sarda P, et al. Topical (intra-articular) tranexamic acid reduces blood loss and transfusion rates following total hip replacement: a randomized controlled trial (TRANX-H) J Bone Joint Surg Am. 2013;95:1969–74. doi: 10.2106/JBJS.L.00908. [DOI] [PubMed] [Google Scholar]
- 283.Gilbody J, Dhotar HS, Perruccio AV, Davey JR. Topical tranexamic acid reduces transfusion rates in total hip and knee arthroplasty. J Arthroplasty. 2014;29:681–4. doi: 10.1016/j.arth.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 284.Martin JG, Cassatt KB, Kincaid-Cinnamon KA, et al. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty. 2014;29:889–94. doi: 10.1016/j.arth.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 285.Tuttle JR, Ritterman SA, Cassidy DB, et al. Cost benefit analysis of topical tranexamic acid in primary total hip and knee arthroplasty. J Arthroplasty. 2014;29:1512–5. doi: 10.1016/j.arth.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 286.Patel JN, Spanyer JM, Smith LS, et al. Comparison of intravenous versus topical tranexamic acid in total knee arthroplasty: a prospective randomized study. J Arthroplasty. 2014;29:1528–31. doi: 10.1016/j.arth.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 287.Yue C, Kang P, Yang P, et al. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty. 2014;29:2452–6. doi: 10.1016/j.arth.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 288.Chang CH, Chang Y, Chen DW, et al. Topical tranexamic acid reduces blood loss and transfusion rates associated with primary total hip arthroplasty. Clin Orthop Relat Res. 2014;472:1552–7. doi: 10.1007/s11999-013-3446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Alshryda S, Sukeik M, Sarda P, et al. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J. 2014;96-B(8):1005–15. doi: 10.1302/0301-620X.96B8.33745. [DOI] [PubMed] [Google Scholar]
- 290.Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee. 2014;21:987–93. doi: 10.1016/j.knee.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 291.Alshryda S, Mason JM, Sarda P, et al. The effect of tranexamic acid on artificial joint materials: a biomechanical study (the bioTRANX study) J Orthop Traumatol. 2015;16:27–34. doi: 10.1007/s10195-014-0312-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Briggs C, Guthrie D, Hyde K, et al. British Committee for Standards in Haematology General Haematology Task Force. Guidelines for point-of-care testing: haematology. Br J Haematol. 2008;142:904–15. doi: 10.1111/j.1365-2141.2008.07274.x. [DOI] [PubMed] [Google Scholar]
- 293.Luddington RJ. Thrombelastography/thromboelastometry. Clin Lab Haematol. 2005;27:81–90. doi: 10.1111/j.1365-2257.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- 294.Afshari A, Wikkelsø A, Brok J, et al. Thrombelastography (TEG) or thromboelastometry (ROTEM) to monitor haemotherapy versus usual care in patients with massive transfusion. Cochrane Database Syst Rev. 2011;3:CD007871. doi: 10.1002/14651858.CD007871.pub2. [DOI] [PubMed] [Google Scholar]
- 295.Schöchl H, Nienaber U, Maegele M, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15:R83. doi: 10.1186/cc10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Weber CF, Görlinger K, Meininger D, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117:531–47. doi: 10.1097/ALN.0b013e318264c644. [DOI] [PubMed] [Google Scholar]
- 297.Görlinger K, Dirkmann D, Hanke AA, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115:1179–91. doi: 10.1097/ALN.0b013e31823497dd. [DOI] [PubMed] [Google Scholar]
- 298.Fuchs RJ, Levin J, Tadel M, Merritt W. Perioperative coagulation management in a patient with afibrinogenemia undergoing liver transplantation. Liver Transpl. 2007;13:752–6. doi: 10.1002/lt.21164. [DOI] [PubMed] [Google Scholar]
- 299.Wang SC, Shieh JF, Chang KY, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42:2590–3. doi: 10.1016/j.transproceed.2010.05.144. [DOI] [PubMed] [Google Scholar]
- 300.Trzebicki J, Flakiewicz E, Kosieradzki M, et al. The use of thromboelastometry in the assessment of hemostasis during orthotopic liver transplantation reduces the demand for blood products. Ann Transplant. 2010;15:19–24. [PubMed] [Google Scholar]
- 301.Kozek-Langeneckerr SA. Perioperative coagulation monitoring. Best Pract Res Clin Anaesth. 2010;24:27–40. doi: 10.1016/j.bpa.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 302.Aguado Romeo MJ, Molina Linde JM, Villegas Portero R. Appropriate use of recombinant activated factor VII in non-haemophilic patients. Executive summary. Sevilla: Agencia de Evaluación de Tecnologías Sanitarias; 2007. [Accessed on 05/01/2015]. Available at: http://aunets.isciii.es/ficherosproductos/sinproyecto/656_AETSA_2007-04.pdf. [Google Scholar]
- 303.Gill R, Herbertson M, Vuylsteke A, et al. Safety and efficacy of recombinant activated factor VII: a randomized placebo controlled trial in the setting of bleeding after cardiac surgery. Circulation. 2009;120:21–7. doi: 10.1161/CIRCULATIONAHA.108.834275. [DOI] [PubMed] [Google Scholar]
- 304.Mayer SA, Brun NC, Begtrup K, et al. FAST Trial Investigators. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358:2127–37. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 305.Levy M, Levy J, Andersen H, Truloff D. Safety of recombinant activated factor VII in randomized clinical trials. N Engl J Med. 2010;363:1791–800. doi: 10.1056/NEJMoa1006221. [DOI] [PubMed] [Google Scholar]
- 306.Liumbruno GM, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology Working Party. Recommendations for the transfusion management of patients in the peri-operative period. III. The post-operative period. Blood Transfus. 2011;9:320–35. doi: 10.2450/2011.0076-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Hansen E, Pawlik M. Reasons against the retransfusion of unwashed wound blood. Transfusion. 2004;44(12 Suppl):45S–53S. doi: 10.1111/j.0041-1132.2004.04179.x. [DOI] [PubMed] [Google Scholar]
- 308.Liumbruno GM, Waters JH. Unwashed shed blood: should we transfuse it? Blood Transfus. 2011;9:241–5. doi: 10.2450/2011.0109-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Liumbruno GM, Grazzini G, Rafanelli D. Post-operative blood salvage in patient blood management: is it really cost-effective and safe? Blood Transfus. 2013;11:175–7. doi: 10.2450/2013.0001-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Muñoz M, Ariza D, Campos A, et al. The cost of post-operative shed blood salvage after total knee arthroplasty: an analysis of 1,093 consecutive procedures. Blood Transfus. 2013;11:260–71. doi: 10.2450/2012.0139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.National Blood Authority. Patient Blood Management Guideline: Module 2 - Perioperative. Canberra, Australia: National Blood Authority; 2012. [Accessed on 19/01/2014]. Available at: http://www.nba.gov.au/guidelines/module2/po-v1a.pdf. [Google Scholar]
- 312.Thomas D, Wareham K, Cohen D, Hutchings H. Autologous blood transfusion in total knee replacement surgery. Br J Anaesth. 2001;86:669–73. doi: 10.1093/bja/86.5.669. [DOI] [PubMed] [Google Scholar]
- 313.Muñoz M, Ariza D, Florez A, Campos A. Reinfusion drains reduce postoperative transfusion requirements after primary total knee replacement surgery. Transfus Med. 2008;18:269–71. doi: 10.1111/j.1365-3148.2008.00867.x. [DOI] [PubMed] [Google Scholar]
- 314.Mirza SB, Campion J, Dixon JH, Panesar SS. Efficacy and economics of postoperative blood salvage in patients undergoing elective total hip replacement. Ann R Coll Surg Engl. 2007;89:777–84. doi: 10.1308/003588407X209310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Trujillo MM, Carrero A, Muñoz M. The utility of the perioperative autologous transfusion system OrthoPAT in total hip replacement surgery: a prospective study. Arch Orthop Trauma Surg. 2008;128:1031–8. doi: 10.1007/s00402-007-0440-6. [DOI] [PubMed] [Google Scholar]
- 316.Sebastián C, Romero R, Olalla E, et al. Postoperative blood salvage and reinfusion in spinal surgery: blood quality, effectiveness and impact on patient blood parameters. Eur Spine J. 2000;9:458–65. doi: 10.1007/s005860000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Tan J, Chen H, Liu Q, et al. A meta-analysis of the effectiveness and safety of using tranexamic acid in primary unilateral total knee arthroplasty. J Surg Res. 2013;184:880–7. doi: 10.1016/j.jss.2013.03.099. [DOI] [PubMed] [Google Scholar]
- 318.Huang F, Wu D, Ma G, et al. The use of tranexamic acid to reduce blood loss and transfusion in major orthopedic surgery: a meta-analysis. J Surg Res. 2014;186:318–27. doi: 10.1016/j.jss.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 319.Decreto del Ministro della Salute 3 Marzo 2005. Caratteristiche e modalità per la donazione di sangue e di emocomponenti. Gazzetta Ufficiale della Repubblica Italiana, n. 85 del 13 aprile 2005.
- 320.Società Italiana di Medicina Trasfusionale e Immunoematologia (SIMTI) Standards of Transfusion Medicine. 2nd ed. Milan: SIMTI Servizi srl; 2010. [Accessed on 19/01/2014]. Available at: http://www.simti.it/pdf/Standard_EN_protetto.pdf. [Google Scholar]
- 321.Council of Europe. Guide to the Preparation, Use and Quality Assurance of Blood Components Recommendation No R (95) 15 on the Preparation, Use and Quality Assurance of Blood Components. 17th ed. Strasbourg: Council of Europe Publishing; 2013. [Google Scholar]
- 322.Horosz B, Malec-Milewska M. Inadvertent intraoperative hypothermia. Anaesthesiol Intensive Ther. 2013;45:38–43. doi: 10.5603/AIT.2013.0009. [DOI] [PubMed] [Google Scholar]
- 323.Alhazzani W, Alenezi F, Jaeschke RZ, et al. Proton pump inhibitors versus histamine 2 receptor antagonists for stress ulcer prophylaxis in critically ill patients: a systematic review and meta-analysis. Crit Care Med. 2013;41:693–705. doi: 10.1097/CCM.0b013e3182758734. [DOI] [PubMed] [Google Scholar]
- 324.Pilkington KB, Wagstaff MJ, Greenwood JE. Prevention of gastrointestinal bleeding due to stress ulceration: a review of current literature. Anaesth Intensive Care. 2012;40:253–9. doi: 10.1177/0310057X1204000207. [DOI] [PubMed] [Google Scholar]
- 325.Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med. 2010;38:2222–8. doi: 10.1097/CCM.0b013e3181f17adf. [DOI] [PubMed] [Google Scholar]
- 326.Hurt RT, Frazier TH, McClave SA, et al. Stress prophylaxis in intensive care unit patients and the role of enteral nutrition. JPEN J Parenter Enteral Nutr. 2012;36:721–31. doi: 10.1177/0148607112436978. [DOI] [PubMed] [Google Scholar]
- 327.Pengo V, Crippa L, Falanga A, et al. Italian Federation of Thrombosis Centers. Questions and answers on the use of dabigatran and perspectives on the use of other new oral anticoagulants in patients with atrial fibrillation. A consensus document of the Italian Federation of Thrombosis Centers (FCSA) Thromb Haemost. 2011;106:868–76. doi: 10.1160/TH11-05-0358. [DOI] [PubMed] [Google Scholar]
- 328.Garcia D, Libby E, Crowther MA. The new oral anticoagulants. Blood. 2010;115:15–20. doi: 10.1182/blood-2009-09-241851. [DOI] [PubMed] [Google Scholar]
- 329.Walenga JM, Adiguzel C. Drug and dietary interactions of the new and emerging oral anticoagulants. Int J Clin Pract. 2010;64:956–67. doi: 10.1111/j.1742-1241.2009.02286.x. [DOI] [PubMed] [Google Scholar]
- 330.Ak K, Isbir CS, Tetik S, et al. Thromboelastography-based transfusion algorithm reduces blood product use after elective CABG: a prospective randomized study. J Card Surg. 2009;24:404–10. doi: 10.1111/j.1540-8191.2009.00840.x. [DOI] [PubMed] [Google Scholar]
- 331.Girdauskas E, Kempfert J, Kuntze T, et al. Thromboelastometrically guided transfusion protocol during aortic surgery with circulatory arrest: a prospective, randomized trial. J Thorac Cardiovasc Surg. 2010;140:1117–24.e2. doi: 10.1016/j.jtcvs.2010.04.043. [DOI] [PubMed] [Google Scholar]
- 332.Despotis G, Eby C, Lublin DM. A review of transfusion risks and optimal management of perioperative bleeding with cardiac surgery. Transfusion. 2008;48(1 Suppl):2S–30S. doi: 10.1111/j.1537-2995.2007.01573.x. [DOI] [PubMed] [Google Scholar]
- 333.Verlicchi F, Facco G, Macrì M, et al. Blood transfusion practice: a nationwide survey in Italy. Blood Transfus. 2011;9:430–5. doi: 10.2450/2011.0023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Salama ME, Raman S, Drew MJ, et al. Platelet function testing to assess effectiveness of platelet transfusion therapy. Transfus Apher Sci. 2004;30:93–100. doi: 10.1016/j.transci.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 335.Rahe-Meyer N, Winterhalter M, Boden A, et al. Platelet concentrates transfusion in cardiac surgery and platelet function assessment by multiple electrode aggregometry. Acta Anaesthesiol Scand. 2009;53:168–75. doi: 10.1111/j.1399-6576.2008.01845.x. [DOI] [PubMed] [Google Scholar]
- 336.Reiter RA, Mayr F, Blazicek H, et al. Desmopressin antagonizes the in vitro platelet dysfunction induced by GPIIb/IIIa inhibitors and aspirin. Blood. 2003;102:4594–9. doi: 10.1182/blood-2002-11-3566. [DOI] [PubMed] [Google Scholar]
- 337.Leithauser B, Zielske D, Seyfert UT, Jung F. Effects of desmopressin on platelet membrane glycoproteins and platelet aggregation in volunteers on clopidogrel. Clin Hemorheol Microcirc. 2008;39:293–302. [PubMed] [Google Scholar]
- 338.Levi M, Eerenberg E, Kamphuisen PW. Bleeding risk and reversal strategies for old and new anticoagulants and antiplatelet agents. J Thromb Haemost. 2011;9:1705–12. doi: 10.1111/j.1538-7836.2011.04432.x. [DOI] [PubMed] [Google Scholar]
- 339.Kortchinsky T, Vigué B, Samama CM. Reversal for heparins and new anticoagulant treatments. Ann Fr Anesth Reanim. 2013;32:37–49. doi: 10.1016/j.annfar.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 340.Gerotziafas GT, Depasse F, Chakroun T, et al. Recombinant factor VIIa partially reverses the inhibitory effect of fondaparinux on thrombin generation after tissue factor activation in platelet rich plasma and whole blood. Thromb Haemost. 2004;91:531–7. doi: 10.1160/TH03-07-0483. [DOI] [PubMed] [Google Scholar]
- 341.Masotti L, Di Napoli M, Godoy DA, et al. The practical management of intracerebral hemorrhage associated with oral anticoagulant therapy. Int J Stroke. 2011;6:228–40. doi: 10.1111/j.1747-4949.2011.00595.x. [DOI] [PubMed] [Google Scholar]
- 342.Cartmill M, Dolan G, Byrne JL, Byrne PO. Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. Br J Neurosurg. 2000;14:458–61. doi: 10.1080/02688690050175265. [DOI] [PubMed] [Google Scholar]
- 343.Konig SA, Schick U, Dohnert J, et al. Coagulopathy and outcome in patients with chronic subdural haematoma. Acta Neurol Scand. 2003;107:110–6. doi: 10.1034/j.1600-0404.2003.01340.x. [DOI] [PubMed] [Google Scholar]
- 344.Baglin TP, Keeling DM, Watson HG. Guidelines on oral anticoagulation (warfarin): third edition - 2005 update. Br J Haematol. 2006;132:277–85. doi: 10.1111/j.1365-2141.2005.05856.x. [DOI] [PubMed] [Google Scholar]
- 345.Chapman SA, Irwin ED, Beal AL, et al. Prothrombin complex concentrate versus standard therapies for INR reversal in trauma patients receiving warfarin. Ann Pharmacother. 2011;45:869–75. doi: 10.1345/aph.1P605. [DOI] [PubMed] [Google Scholar]
- 346.Sarode R, Matevosyan K, Bhagat R, et al. Rapid warfarin reversal: a 3-factor prothrombin complex concentrate and recombinant factor VIIa cocktail for intracerebral hemorrhage. J Neurosurg. 2012;116:491–7. doi: 10.3171/2011.11.JNS11836. [DOI] [PubMed] [Google Scholar]
- 347.Imberti D, Barillari G, Biasioli C, et al. Emergency reversal of anticoagulation with a three-factor prothrombin complex concentrate in patients with intracranial hemorrhage. Blood Transfus. 2011;9:148–55. doi: 10.2450/2011.0065-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Prisco D, Baudo F, Moia M, et al. Raccomandazioni della FCSA. FCSA; Novembre. 2005. [Accessed on 05/01/2015]. Terapia Anticoagulante Orale, Chirurgia e Manovre Invasive. Available at: http://www.fcsa.it/Content/DocumentiRaccomandazioni/FCSAchirurgiaDefinitiva.pdf. [Google Scholar]
- 349.Samama MM, Martinoli JL, LeFlem L, et al. Assessment of laboratory assays to measure rivaroxaban - an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815–25. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 350.Eerenberg ES, Kamphuisen PW, Sijpkens MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–9. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 351.Kaatz S, Kouides PA, Garcia DA, et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am J Hematol. 2012;87(Suppl 1):S141–5. doi: 10.1002/ajh.23202. [DOI] [PubMed] [Google Scholar]
- 352.Marlu R, Hodaj E, Paris A, et al. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb Haemost. 2012;108:217–24. doi: 10.1160/TH12-03-0179. [DOI] [PubMed] [Google Scholar]
- 353.Greinacher A, Kiefel V, Kluter H, et al. [Recommendations for platelet transfusion by the Joint Thrombocyte Working Party of the German Societies of Transfusion Medicine and Immunohaematology (DGTI), Thrombosis and Haemostasis Research (GTH), and Haematology and Oncology (DGHO)]. Dtsch Med Wochenschr. 2006;131:2675–9. doi: 10.1055/s-2006-956275. [In German.] [DOI] [PubMed] [Google Scholar]
- 354.Slichter SJ. Evidence-based platelet transfusion guidelines. Hematology Am Soc Hematol Educ Program. 2007:172–8. doi: 10.1182/asheducation-2007.1.172. [DOI] [PubMed] [Google Scholar]
- 355.Meier J, Gombotz H. Pillar III - Optimisation of anaemia tolerance. Best Prac Res Clin Anaesth. 2013;27:111–9. doi: 10.1016/j.bpa.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 356.van Gelder FE, de Graaff JC, van Wolfswinkel L, van Klei WA. Preoperative testing in noncardiac surgery patients: a survey amongst European anaesthesiologists. Eur J Anaesthesiol. 2012;29:465–70. doi: 10.1097/EJA.0b013e32835423f0. [DOI] [PubMed] [Google Scholar]
- 357.De Hert S, Imberger G, Carlisle J, et al. Task Force on Preoperative Evaluation of the Adult Noncardiac Surgery Patient of the European Society of Anaesthesiology. Preoperative evaluation of the adult patient undergoing non-cardiac surgery: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2011;28:684–722. doi: 10.1097/EJA.0b013e3283499e3b. [DOI] [PubMed] [Google Scholar]
- 358.Sens N, Payan A, Sztark F, et al. Preoperative cardiac-risk assessment for non-cardiac surgery: The French RICARDO survey. Ann Fr Anesth Reanim. 2013;32:676–83. doi: 10.1016/j.annfar.2013.07.807. [DOI] [PubMed] [Google Scholar]
- 359.Pannell LM, Reyes EM, Underwood SR. Cardiac risk assessment before non-cardiac surgery. Eur Heart J Cardiovasc Imaging. 2013;14:316–22. doi: 10.1093/ehjci/jes288. [DOI] [PubMed] [Google Scholar]
- 360.Schiefermueller J, Myerson S, Handa AI. Preoperative assessment and perioperative management of cardiovascular risk. Angiology. 2013;64:146–50. doi: 10.1177/0003319712440874. [DOI] [PubMed] [Google Scholar]
- 361.Czoski-Murray C, Lloyd Jones M, McCabe C, et al. What is the value of routinely testing full blood count, electrolytes and urea, and pulmonary function tests before elective surgery in patients with no apparent clinical indication and in subgroups of patients with common comorbidities: a systematic review of the clinical and cost-effective literature. Health Technol Assess. 2012;16:1–159. doi: 10.3310/hta16500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Ferraris VA, Ferraris SP, Saha SP, et al. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83(5 Suppl):S27–86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 363.Liumbruno G, Bennardello F, Lattanzio A, et al. Recommendations for the transfusion of red blood cells. Blood Transfus. 2009;7:49–64. doi: 10.2450/2008.0020-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Napolitano LM, Kurek S, Luchette FA, et al. American College of Critical Care Medicine of the Society of Critical Care Medicine; Eastern Association for the Surgery of Trauma Practice Management Workgroup. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–57. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 365.Carson JL, Grossman BJ, Kleinman S, et al. Clinical Transfusion Medicine Committee of the AABB. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 366.Shander A, Gross I, Hill S, et al. College of American Pathologists; American Society of Anesthesiologists; Society of Thoracic Surgeons and Society of Cardiovascular Anesthesiologists; Society of Critical Care Medicine; Italian Society of Transfusion Medicine and Immunohaematology; American Association of Blood Banks. A new perspective on best transfusion practices. Blood Transfus. 2013;11:193–202. doi: 10.2450/2012.0195-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367.Hogshire L, Carson JL. Red blood cell transfusion: what is the evidence when to transfuse? Curr Opin Hematol. 2013;20:546–51. doi: 10.1097/MOH.0b013e32836508bd. [DOI] [PubMed] [Google Scholar]
- 368.Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 369.Carson JL, Terrin ML, Noveck H, et al. FOCUS Investigators. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.So-Osman C, Nelissen R, Brand R, et al. The impact of a restrictive transfusion trigger on post-operative complication rate and well-being following elective orthopaedic surgery: a post-hoc analysis of a randomised study. Blood Transfus. 2013;11:289–95. doi: 10.2450/2013.0172-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Villanueva C, Colomo A, Bosch A, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 373.Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 374.Carson JL, Brooks MM, Abbott JD, et al. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964–971.e1. doi: 10.1016/j.ahj.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Cecconi M, Corredor C, Arulkumaran N, et al. Clinical review: Goal-directed therapy - what is the evidence in surgical patients? The effect on different risk groups. Crit Care. 2013;17:209. doi: 10.1186/cc11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Cecconi M, Sutton L, Rhodes A. A cost-effectiveness analysis of postoperative goal-directed therapy for high-risk surgical patients. Crit Care Med. 2014;42:1194–203. doi: 10.1097/CCM.0000000000000164. [DOI] [PubMed] [Google Scholar]
- 377.Pestaña D, Espinosa E, Eden A, et al. Perioperative goal-directed hemodynamic optimization using noninvasive cardiac output monitoring in major abdominal surgery: a prospective, randomized, multicenter, pragmatic trial: POEMAS Study (PeriOperative goal-directed thErapy in Major Abdominal Surgery) Anesth Analg. 2014;119:579–87. doi: 10.1213/ANE.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 378.Pearse RM, Harrison DA, MacDonald N, et al. OPTIMISE Study Group. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA. 2014;311:2181–90. doi: 10.1001/jama.2014.5305. [DOI] [PubMed] [Google Scholar]
- 379.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 380.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 381.Pierrakos C, Velissaris D, Scolletta S, et al. Can changes in arterial pressure be used to detect changes in cardiac index during fluid challenge in patients with septic shock? Intensive Care Med. 2012;38:422–8. doi: 10.1007/s00134-011-2457-0. [DOI] [PubMed] [Google Scholar]
- 382.Cove ME, Pinsky MR. Perioperative hemodynamic monitoring. Best Pract Res Clin Anaesthesiol. 2012;26:453–62. doi: 10.1016/j.bpa.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 383.Geisen M, Rhodes A, Cecconi M. Less-invasive approaches to perioperative haemodynamic optimization. Curr Opin Crit Care. 2012;18:377–84. doi: 10.1097/MCC.0b013e328355894f. [DOI] [PubMed] [Google Scholar]
- 384.Manoach S, Weingart SD, Charchaflieh J. The evolution and current use of invasive hemodynamic monitoring for predicting volume responsiveness during resuscitation, perioperative, and critical care. J Clin Anesth. 2012;24:242–50. doi: 10.1016/j.jclinane.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 385.Doherty M, Buggy DJ. Intraoperative fluids: how much is too much? Br J Anaesth. 2012;109:69–79. doi: 10.1093/bja/aes171. [DOI] [PubMed] [Google Scholar]
- 386.Bundgaard-Nielsen M, Secher NH, Kehlet H. ‘Liberal’ vs. ‘restrictive’ perioperative fluid therapy: a critical assessment of the evidence. Acta Anaesthesiol Scand. 2009;53:843–51. doi: 10.1111/j.1399-6576.2009.02029.x. [DOI] [PubMed] [Google Scholar]
- 387.Rhodes A, Cecconi M, Hamilton M, et al. Goal-directed therapy in high-risk surgical patients: a 15-year follow-up study. Intensive Care Med. 2010;36:1327–32. doi: 10.1007/s00134-010-1869-6. [DOI] [PubMed] [Google Scholar]
- 388.Gurgel ST, do Nascimento P., Jr Maintaining tissue perfusion in high-risk surgical patients: a systematic review of randomized clinical trials. Anesth Analg. 2011;112:1384–91. doi: 10.1213/ANE.0b013e3182055384. [DOI] [PubMed] [Google Scholar]
- 389.Meier J, Wölkhammer S, Kemming GI, et al. Hyperoxic ventilation reduces 6-hour mortality at the critical hemoglobin concentration. Anesthesiology. 2004;100:70–6. doi: 10.1097/00000542-200401000-00014. [DOI] [PubMed] [Google Scholar]
- 390.Pape A, Meier J, Kertscho H, et al. Hyperoxic ventilation increases the tolerance of acute normovolemic anemia in anesthetized pigs. Crit Care Med. 2006;34:1475–82. doi: 10.1097/01.CCM.0000215826.45839.36. [DOI] [PubMed] [Google Scholar]
- 391.Kemming GI, Meisner FG, Kleen M, et al. Hyperoxic ventilation at the critical haematocrit. Resuscitation. 2003;56:289–97. doi: 10.1016/s0300-9572(02)00408-2. [DOI] [PubMed] [Google Scholar]
- 392.Kleen M, Habler O, Hutter J, et al. Hemodilution and hyperoxia locally change distribution of regional pulmonary perfusion in dogs. Am J Physiol. 1998;274:H520–8. doi: 10.1152/ajpheart.1998.274.2.H520. [DOI] [PubMed] [Google Scholar]
- 393.Kallet RH, Matthay MA. Hyperoxic acute lung injury. Respir Care. 2013;58:123–41. doi: 10.4187/respcare.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Ehrenfeld JM, Dexter F, Rothman BS, et al. Lack of utility of a decision support system to mitigate delays in admission from the operating room to the postanesthesia care unit. Anesth Analg. 2013;117:1444–52. doi: 10.1213/ANE.0b013e3182a8b0bd. [DOI] [PubMed] [Google Scholar]
- 395.Lalani SB, Ali F, Kanji Z. Prolonged-stay patients in the PACU: a review of the literature. J Perianesth Nurs. 2013;28:151–5. doi: 10.1016/j.jopan.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 396.Nathan DP, Brinster CJ, Jackson BM, et al. Predictors of decreased short- and long-term survival following open abdominal aortic aneurysm repair in a large contemporary series. J Vasc Surg. 2011;54:1237–43. doi: 10.1016/j.jvs.2011.05.028. [DOI] [PubMed] [Google Scholar]
- 397.Kamphues C, Bova R, Schricke D, et al. Postoperative complications deteriorate long-term outcome in pancreatic cancer patients. Ann Surg Oncol. 2011;19:856–63. doi: 10.1245/s10434-011-2041-4. [DOI] [PubMed] [Google Scholar]
- 398.Khuri SF, Henderson WG, DePalma RG, et al. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242:326–41. doi: 10.1097/01.sla.0000179621.33268.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Kehlet H. Surgical stress: the role of pain and analgesia. Br J Anaesth. 1989;63:189–95. doi: 10.1093/bja/63.2.189. [DOI] [PubMed] [Google Scholar]
- 400.Ballantyne JC, Carr DB, de Ferranti S, et al. The comparative effects of postoperative analgesic therapies on pulmonary outcome: cumulative meta-analyses of randomized, controlled trials. Anesth Analg. 1998;86:598–612. doi: 10.1097/00000539-199803000-00032. [DOI] [PubMed] [Google Scholar]
- 401.Tiengo M, Benedetti C. Fisiopatologia e terapia del dolore. Milano: Masson; 1996. pp. 536–38. [Google Scholar]
- 402.Savoia G, Alampi D, Amantea B, et al. Postoperative pain treatment SIAARTI Recommendations 2010. Short version. Minerva Anestesiol. 2010;76:657–67. [PubMed] [Google Scholar]
- 403.Allegri M, Clark MR, De Andrés J, Jensen TS. Acute and chronic pain: where we are and where we have to go. Minerva Anestesiol. 2012;78:222–35. [PubMed] [Google Scholar]
- 404.Curatolo M. Adding regional analgesia to general anaesthesia: increase of risk or improved outcome? Eur J Anaesthesiol. 2010;27:586–91. doi: 10.1097/EJA.0b013e32833963c8. [DOI] [PubMed] [Google Scholar]
- 405.Block BM, Liu SS, Rowlingson AJ, et al. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA. 2003;290:2455–63. doi: 10.1001/jama.290.18.2455. [DOI] [PubMed] [Google Scholar]
- 406.Holte K, Kehlet H. Effect of postoperative epidural analgesia on surgical outcome. Minerva Anestesiol. 2002;68:157–61. [PubMed] [Google Scholar]
- 407.Panaretou V, Toufektzian L, Siafaka I, et al. Postoperative pulmonary function after open abdominal aortic aneurysm repair in patients with chronic obstructive pulmonary disease: epidural versus intravenous analgesia. Ann Vasc Surg. 2012;26:149–55. doi: 10.1016/j.avsg.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 408.Rawal N. Epidural technique for postoperative pain: gold standard no more? Reg Anesth Pain Med. 2012;37:310–7. doi: 10.1097/AAP.0b013e31825735c6. [DOI] [PubMed] [Google Scholar]
- 409.Pöpping DM, Zahn PK, Van Aken HK, et al. Effectiveness and safety of postoperative pain management: a survey of 18.925 consecutive patients between 1998 and 2006 (2nd revision): a database analysis of prospectively raised data. Br J Anaesth. 2008;101:832–40. doi: 10.1093/bja/aen300. [DOI] [PubMed] [Google Scholar]
- 410.Singelyn FJ, Deyaert M, Joris D, et al. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 411.Lirk P, Hollmann M. Outcome after regional anesthesia: weighing risks and benefits. Minerva Anestesiol. 2014;80:610–8. [PubMed] [Google Scholar]
- 412.Cwik J. Postoperative considerations of neuraxial anesthesia. Anesthesiol Clin. 2012;30:433–43. doi: 10.1016/j.anclin.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 413.Hanna MN, Murphy JD, Kumar K, Wu CL. Regional techniques and outcome: what is the evidence? Curr Opin Anaesthesiol. 2009;22:672–7. doi: 10.1097/ACO.0b013e32832f330a. [DOI] [PubMed] [Google Scholar]
- 414.Zaric D, Boysen K, Christiansen C, et al. A comparison of epidural analgesia with combined continuous femoral-sciatic nerve blocks after total knee replacement. Anesth Analg. 2006;102:1240–6. doi: 10.1213/01.ane.0000198561.03742.50. [DOI] [PubMed] [Google Scholar]
- 415.Horlocker TT, Kopp SL, Pagnano MW, Hebl JR. Analgesia for total hip and knee arthroplasty: a multimodal pathway featuring peripheral nerve block. J Am Acad Orthop Surg. 2006;14:126–35. doi: 10.5435/00124635-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 416.Koscielniak-Nielsen ZJ, Dahl JB. Ultrasound-guided peripheral nerve blockade of the upper extremity. Curr Opin Anaesthesiol. 2012;25:253–9. doi: 10.1097/ACO.0b013e32835069c2. [DOI] [PubMed] [Google Scholar]
- 417.Chelly JE, Ghisi D, Fanelli A. Continuous peripheral nerve blocks in acute pain management. Br J Anaesth. 2010;105:S86–96. doi: 10.1093/bja/aeq322. [DOI] [PubMed] [Google Scholar]