Abstract
Background
Hypothermic storage of red blood cells (RBCs) results in progressive deterioration of the rheological properties of the cells, which may reduce the efficacy of RBC transfusions. Recent studies have suggested that storing RBC units under anaerobic conditions may reduce this storage-induced deterioration.
Materials and methods
The aim of this study was to compare the rheological properties of conventionally and anaerobically stored RBC and provide a measure of the relationship between oxidative damage to stored RBC and their ability to perfuse microvascular networks. Three different microfluidic devices were used to measure the ability of both types of stored RBC to perfuse artificial microvascular networks. Flow rates of the RBC passing through the entire network (bulk perfusion) and the individual capillaries (capillary perfusion) of the devices were measured on days 2, 21, 42, and 63 of storage.
Results
The bulk perfusion rates for anaerobically stored RBC were significantly higher than for conventionally stored RBCs over the entire duration of storage for all devices (up to 10% on day 42; up to 14% on day 63). Capillary perfusion rates suggested that anaerobically stored RBC units contained significantly fewer non-deformable RBC capable of transiently plugging microfluidic device capillaries. The number of plugging events caused by these non-deformable RBC increased over the 63 days of hypothermic storage by nearly 16- to 21-fold for conventionally stored units, and by only about 3- to 6-fold for anaerobically stored units.
Discussion
The perfusion measurements suggest that anaerobically stored RBC retain a greater ability to perfuse networks of artificial capillaries compared to conventionally (aerobically) stored RBC. It is likely that anaerobic storage confers this positive effect on the bulk mechanical properties of stored RBC by significantly reducing the number of non-deformable cells present in the overall population of relatively well-preserved RBC.
Keywords: anaerobic, storage, rheology, microfluidic, deformability
Introduction
The transfusion of red blood cells (RBC) is the primary blood component therapy aimed at correcting the potential deficit in the oxygenation of tissues and vital end organs in severely anaemic patients. In order for RBC to deliver oxygen to the tissues, the cells must be able to deform continually while traversing vast networks of capillaries. The majority of RBC units used for transfusion are stored at 2–6 °C for up to 6 weeks in plastic blood bags that contain an anticoagulant-preservative solution1. The biochemical and mechanical properties of RBC deteriorate progressively in hypothermic storage, leading to a significant reduction in the ability of stored RBC to perfuse microvascular networks in vitro2,3, ex vivo4, and in vivo5–7. Significant research effort aimed at preventing this storage-induced deterioration has been focused on reducing the oxidative damage experienced by RBC during hypothermic storage by first de-oxygenating RBC and then storing them under oxygen-free (anaerobic) conditions8. Anaerobic storage of RBC has been shown to diminish the overall rate of storage lesion development (e.g. haemolysis, vesicle production and phosphatidylserine-exposure) and increase levels of ATP, 24-hour in vivo recovery rate and maintenance of 2,3-diphosphoglycerate levels beyond 3 weeks during storage and consequently extend the storage times by 50% (to 63 days)9–11. However, little is known about the effect of anaerobic storage on the mechanical properties of stored RBC8.
In this study, we hypothesised that anaerobic storage would significantly attenuate the oxygen-dependent damage to RBC mechanical properties and would, therefore, be better than conventional (aerobic) storage at preserving the ability of stored RBC to perfuse microvascular networks. We tested this hypothesis experimentally by quantifying perfusion of several artificial microvascular networks (AMVN) for RBC stored following the conventional procedure (aerobically) and stored using the experimental anaerobic storage system.
Materials and methods
Design of the capillary network devices
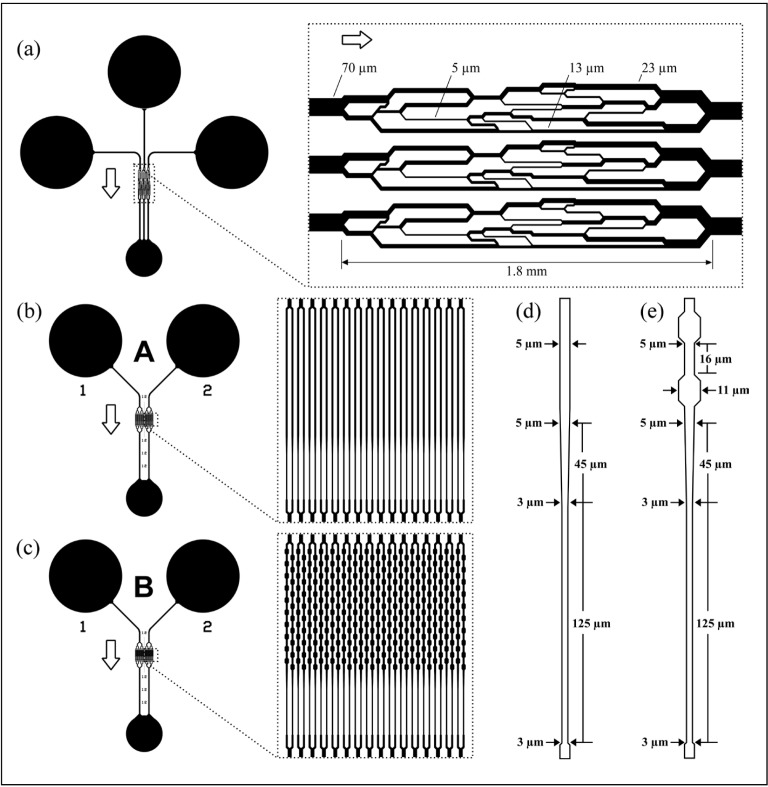
Figure 1 schematically illustrates the three microfluidic devices used in this study to compare the rheological properties of RBC stored in aerobic (conventional) and anaerobic environments. We refer to the device in Figure 1a as the AMVN2,12–14, the device in Figure 1b as “Device A”, and the device in Figure 1c as “Device B”. The design and fabrication of the AMVN device and the validation of the AMVN perfusion measurement have been described in detail previously elsewhere2,3,15. Briefly, the AMVN device comprised three identical network units connected to three separate inlets via the arterioles and one common outlet connected via the venules (Figure 1). Each network consisted of 5 μm deep interconnecting channels ranging in widths from 5 μm (capillaries) to 70 μm (arterioles, venules) (Figure 1a, inset). The AMVN perfusion measurement was previously validated via repeated measurements (n=15) of a single blood sample and was found to be reproducible within 2% between devices2. The architecture of Device A and Device B was identical (Figure 1b, 1c) except for the shape of their pipette-like capillary elements (compare Figure 1d and 1e). Each of these devices consisted of two identical network units with two independent inlets and one common outlet. Each network unit consisted of an arteriole channel connecting the inlet to a parallel array of 32 capillaries (Figure 1b, c, insets) via a series of bifurcating channels; each capillary array was drained through a series of channels converging into a single venule. Each capillary of Device A (Figure 1b) comprised a straight portion, 330 μm long and 5 μm wide, followed by a 45 μm long portion that tapered gradually in width from 5 μm to 3 μm and further extended to a length of 125 μm on the venule side (Figure 1d). Each capillary of Device B (Figure 1c) comprised a 330 μm long portion with a series of ten repeating constrictions (5 μm) and expansions (11 μm) of its width, followed by a 45 μm long portion that tapered gradually in width from 5 μm to 3 μm and extended further to a length of 125 μm on the venule side (Figure 1e). The depth of all the channels was 5 μm throughout. All microfluidic devices used in this study were fabricated via soft lithography as described previously in detail2,14.
Figure 1.
Schematic illustration of the microfluidic devices used for measuring the mechanical properties of stored RBC.
(a) The AMVN device consisted of three network units, where each network unit contained microchannels of various widths ranging from 70 μm (arteriole, venule) to 5 μm (capillaries). Each device consisted of three identical network units with three independent inlets and a common outlet. (b) Device A consisted of two identical network units with two independent inlets and a common outlet. Each network unit consisted of an arteriole channel connecting the inlet to a parallel array of 32 capillaries (insert) through a series of bifurcating channels; each capillary array was drained through a series of channels converging into a single venule. (c) The design of Device B was identical to Device A except for the shape of the pipette-like capillary elements. (d) Design of individual capillaries of Device A. (e) Design of individual capillaries of Device B. The depth of all channels was 5 μm throughout; arrows indicate the direction of blood flow in the device.
RBC: red blood cell; AMVN: artificial microvascular networks.
Preparation of red blood cell samples
All RBC samples used in this study were obtained from a randomised, dual-arm cross-over clinical study conducted at the Dartmouth-Hitchcock Medical Center with the main purpose of comparing RBC units stored conventionally (aerobically) and anaerobically for in vitro parameters (pO2, pH, ATP, 2,3-diphosphoglycerate, haemolysis, glucose, lactate, Na+, K+ and RBC morphology) and for the 24-hour in vivo RBC recovery on day 21 and day 42 of storage, measured using the standard double-labelling (51Cr, 99mTc) method16. In that study, healthy consenting volunteers (n=13) donated blood on two separate occasions (separated by at least 9 weeks). For each subject, the first donated unit was randomly assigned to be stored conventionally (control arm) or anaerobically (test arm), and the second donated unit was allocated to the other arm (thus creating 13 matched pairs). Whole blood was collected into citrate phosphate double dextrose (CP2D) anticoagulant solution (Pall Medical, Covina, CA, USA). The blood was centrifuged (at 2,000 g for 3 minutes) to remove the supernatant, and a RBC storage solution was added to each unit. RBC units stored conventionally (control, C) received 100 mL of AS-3 additive solution (Nutricel, Pall Medical), and RBC units stored anaerobically (test, T) received 200 mL of experimental additive solution OFAS3 (New Health Sciences, Bethesda, MD, USA)11. All units were leucoreduced by filtration (RC2D, Pall Medical). Oxygen and carbon dioxide levels of the test units (RBC to be stored anaerobically) were reduced by passing RBC through a prototype disposable hollow-fibre O2/CO2 depletion device under gravity. The prototype device consisted of a bundle of 150 μm inner diameter microporous hollow fibres (Celgard, 3M, Charlotte, NC, USA). The hollow fibre bundle was wrapped around a core of oxygen sorbent packets (ZB, Mitsubishi Gas Chemical Co., Tokyo, Japan) and encased in a clear polycarbonate cartridge. RBC suspension flowed in the lumen of the hollow fibres at a rate of ~300 mL/min into a standard PVC storage bag over-wrapped with an oxygen-barrier bag (Rollprint Z, Addison, IL, USA) containing oxygen sorbent packets (ZB, Mitsubishi Gas Chemical Co.) Both anaerobic (test) and conventional (control) units were placed in 2–6 ºC storage within 8 hours of collection. All RBC units were sampled on day 2 (post-processing) and on days 21, 42 and 63 of storage, and 2 mL of each sample were packaged into waterproof, thermally isolated containers (filled with a mixture of water and ice, and/or thermal packs), and shipped on the same day via an overnight courier (FedEx Corporation, Memphis, TN, USA) to Tulane University (New Orleans, LA, USA) where the mechanical properties of the RBC were measured.
Upon arrival, each RBC sample was placed in a blood bank refrigerator (Jewett BBR6-1B18, Thermo Fisher Scientific, Asheville, NC, USA) and stored at 2–6 °C until use (samples that arrived warm were omitted from analysis). To measure the mechanical properties of the RBC, a sample was taken out of the refrigerator and mixed gently (by inversion). Standard haematological parameters were determined with a haematology analyser (Medonic M-Series, Boule Medical AB, Stockholm, Sweden). The average haematocrit of the RBC samples from units stored conventionally was 44%, while that of units stored anaerobically was 37%. The haematocrit of all the samples was adjusted to 40% either by adding a calculated volume of normal saline, or by removing the supernatant (via gentle centrifugation at 800 g for 5 minutes). After the adjustment, the sample was placed on a tube rotator (Barnstead Thermolyne, Dubuque, IA, USA) to maintain the uniformity of the haematocrit in the sample.
Measurement of the network and capillary perfusion
The experimental set up and procedures used in this study for measuring the network and capillary perfusion have been described in detail previously2,15. In brief, images of the downstream portion of the microchannel were captured using a bright-field microscope equipped with a high-speed digital camera and analysed offline using a custom image algorithm which determined the average velocity of the RBC2. To assure integrity of the data collected in this study, we implemented the previously described quality control measures2; devices that did not pass the quality control were discarded (<10%). Bulk flow rates for each RBC sample were measured using three or four AMVN devices (3 measurements per device), one Device A and one Device B (2 measurements per device). To measure the capillary flow rates, we used a single network of 32 capillaries in one Device A and one Device B. All measurements were made at a driving pressure differential of 20 cmH2O. The driving pressure of the system was set by adjusting the height difference (hydrostatic pressure) between the inlets of the microfluidic device and a water reservoir attached to the device outlet by flexible tubing. Data obtained using defective devices or using samples that were damaged in shipping were omitted from the analysis. All reported p-values were determined using the paired two-tailed, unequal variance t-test.
Results
Network perfusion rates
Overall, we observed a marked reduction in the network perfusion rate over the duration of storage for both conventionally stored RBC units and anaerobically stored RBC units in all three microfluidic devices. The network perfusion rate for RBC stored anaerobically was consistently higher than for their conventionally stored counterparts throughout the whole duration of storage (Figure 2). Figure 2a shows the change in the network perfusion rate for both conventionally and anaerobically stored RBC samples over the duration of storage, as measured with the AMVN. The perfusion rate for conventionally stored RBC declined 10% from day 2 to day 42 (p<0.05) and 14% by day 63 (p<0.05). For the anaerobically stored RBC, the perfusion rate declined 10% by day 21 (p<0.05), 14% by day 42 (p<0.05), and 14% by day 63 (p<0.05). The perfusion rate for anaerobically stored RBCs on day 63 was 6% higher than for conventionally stored RBC of the same nominal age (p<0.05). Figure 2b shows the change in the perfusion rate for both the conventionally and anaerobically stored RBC samples over the duration of storage, as measured with Device A. The perfusion rate for RBC stored conventionally declined 17% from day 2 to day 21 (p<0.05), 27% by day 42 (p<0.05), and 33% by day 63 (p<0.05). For the anaerobically stored RBC, the perfusion rate declined 15% from day 2 to day 21 (p<0.05), 22% by day 42 (p<0.05), and 23% by day 63 (p<0.05). The perfusion rate for anaerobically stored RBC on day 63 was 14% higher than for conventionally stored RBC of the same nominal age (p<0.05), and 7% higher than for conventionally stored RBC on day 42 (p<0.05). Figure 2c shows the change in the perfusion rate for the conventionally and anaerobically stored RBC samples over the duration of storage, as measured with Device B. The perfusion rate for conventionally stored RBC declined 15% from day 2 to day 21 (p<0.05), 26% by day 42 (p<0.05), and 28% by day 63 (p<0.05). For the anaerobically stored RBC, the perfusion rate declined 15% from day 2 to day 21 (p<0.05), 19% by day 42 (p<0.05), and 21% by day 63 (p<0.05). In comparison to the conventionally stored RBC of the same nominal age, the perfusion rate for anaerobically stored RBC was higher by 10% on day 42 (p<0.05) and by 11% on day 63 (p<0.05). In addition, the perfusion rate for anaerobically stored RBC on day 63 was 8% higher than for conventionally stored RBC on day 42. Statistically significant differences between anaerobically and conventionally stored RBC were observed for all devices on Day 63, as well for Device B on Day 42 (indicated by [*] in Figure 2).
Figure 2.
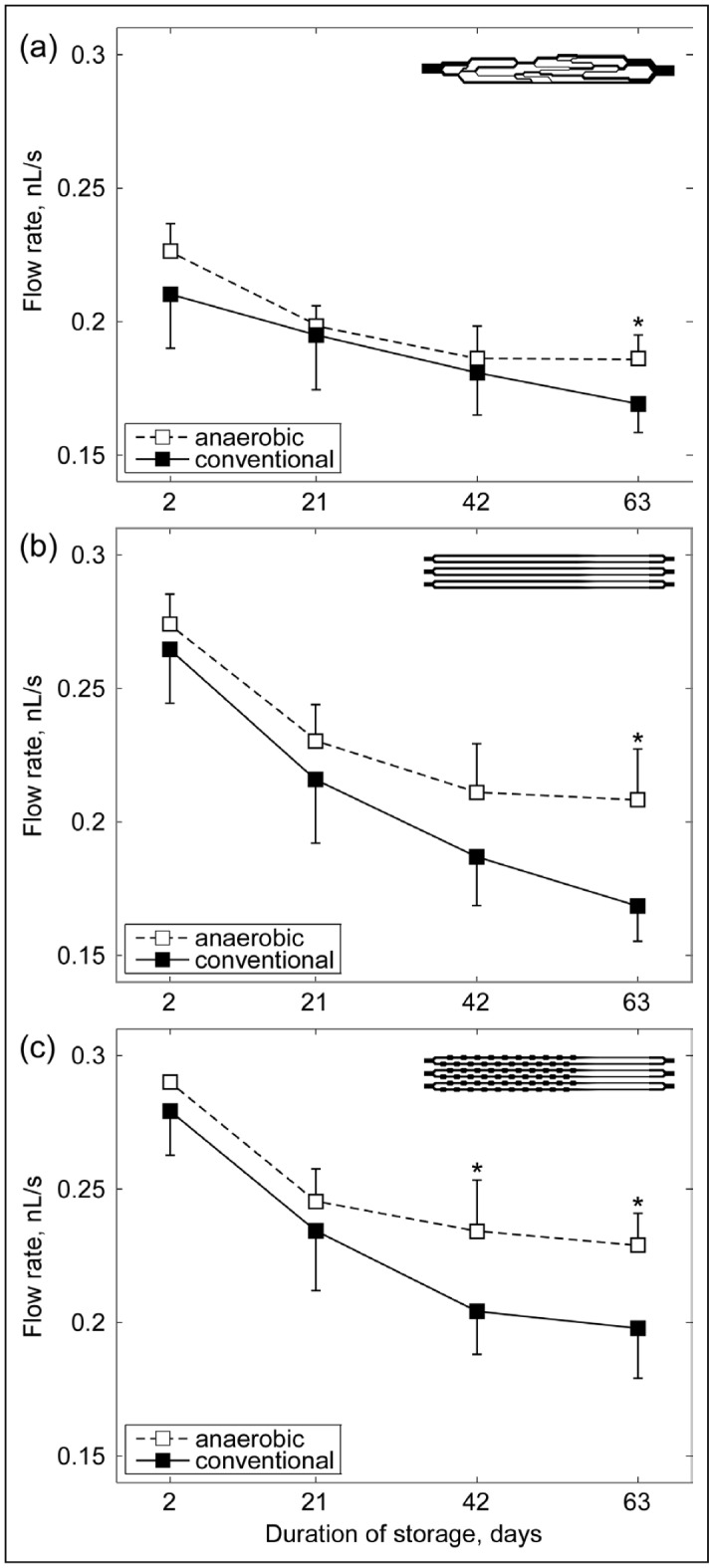
The effect of aerobic and anaerobic storage conditions on the mechanical properties of stored RBC, as reflected by their ability to perfuse the microfluidic devices.
Device perfusion for matched pairs of blood units was measured by quantifying the rate of blood flow in venules of (a) the AMVN device, (b) Device A containing straight “pipette” elements, and (c) Device B containing pipette elements with repeated constrictions and expansions. The driving pressure differential was −20 cmH2O. Values are means ± SD. Statistically significant differences (p<0.05) between anaerobic and conventional (aerobic) storage of the same duration are indicated by (*).
RBC: red blood cell; AMVN: artificial microvascular networks; SD: standard deviation.
Capillary flow rates
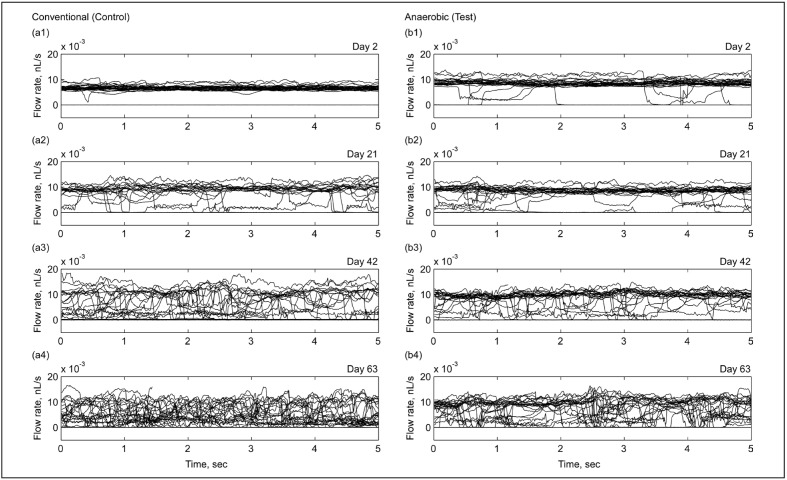
We measured the flow rate in each of the 32 capillaries forming a single network unit in Device A and Device B, using the same methodology as for determining the overall network perfusion rate. Figure 3 shows the representative traces of the capillary flow rates in Device A for conventionally (Figure 3a1–4) and anaerobically (Figure 3b1–4) stored RBC on days 2, 21, 42 and 63 of storage (we observed similar dynamics for Device B). On day 2, the capillary flow rates for both samples were relatively uniform, clustering around a baseline level common to all capillaries (Figure 3a1,b1). The rates of flow in the capillaries for conventionally (aerobically) stored RBC clustered around a mean of 6.4 pL/s (Figure 3a1), and for anaerobically stored RBC, around a mean of 8.3 pL/s (Figure 3b1). However, the rate of flow in some capillaries was significantly lower, and even stopped for a brief period of time or permanently (for example, see Figure 3b1). We observed a marked increase in the number of individual capillaries experiencing these flow rate fluctuations later in storage with capillary flow traces switching between a “high-flow” state and a “no-flow” state caused by transient or permanent plugging (Figure 3a2–3, b2–3). With storage, the clustering of capillary flow traces around the baseline “high-flow” state became progressively less defined for the anaerobically stored sample (Figure 3b4), and nearly disappeared for the conventionally stored sample (Figure 3a4). On day 63, a fraction of capillaries were able to maintain their operation in the normal, “high-flow” state (55% for anaerobic vs 37% for conventional RBC).
Figure 3.
Representative traces of flow rates in individual capillaries of a single network in Device A (each graph shows 32 traces) for RBC stored conventionally (a1–4) and anaerobically (b1–4) on days 2 (a1, b1), 21 (a2, b2), 42 (a3, b3), and 63 (a4, b4).
RBC: red blood cell.
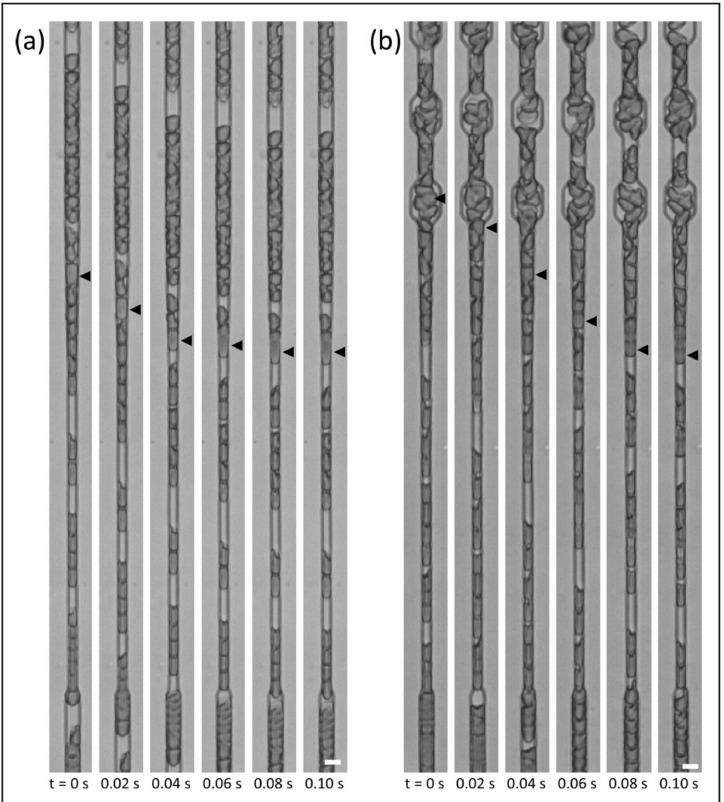
Capillary plugging by poorly deformable red blood cells
Figure 4 illustrates the transient plugging of individual capillaries by a single poorly deformable RBC (on day 42 of conventional storage) - the source of dynamic fluctuations of the rate of flow in the capillaries of Device A and Device B (Figure 3). To quantify the differences observed in the dynamics of capillary flow between the conventionally and anaerobically stored samples, we measured the average “number of plugging events” (NPE) occurring within the capillary networks of Device A and Device B. To determine the average NPE, we collected 5-second-long traces of blood flow in capillaries (Figure 3), counted the number of times the flow rate in each capillary dropped below the baseline “cut off” value (defined as mean flow rate in all capillaries of the network for the day 2 sample, minus two standard deviations), and then averaged the total NPE across all samples of the same storage duration.
Figure 4.
Transient plugging of the microfabricated pipette-like capillaries by poorly deformable stored RBC.
(a) Plugging of a Device A capillary and (b) plugging of a Device B capillary by an intact RBC from a sample stored conventionally for 42 days. Scale bars are 5 μm.
RBC: red blood cell.
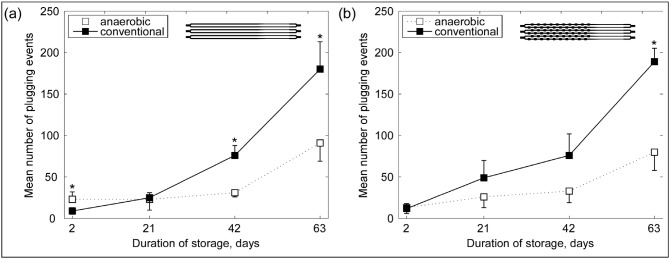
Figure 5 shows the change in average NPE for RBC stored conventionally and anaerobically throughout storage. In Device A, the average NPE for conventionally stored RBC increased 3-fold by day 21 (p<0.05), 9-fold by day 42 (p<0.001), and 21-fold by day 63 (p<0.05), while for RBC stored anaerobically the average NPE stayed unchanged for the first 42 days of storage and then increased 3-fold by day 63 (p<0.01) (Figure 5a). In Device B, the average NPE for conventionally stored RBC increased 4-fold by day 21, 6-fold by day 42 (p<0.05) and 16-fold by day 63 (p<0.01), while for anaerobically stored RBC the average NPE increased 2-fold by day 21, remained relatively unchanged until day 42, and then increased 6-fold by day 63 (p<0.01) (Figure 5b). In both devices, conventionally stored RBC had an average NPE about 2-fold higher than anaerobically stored RBC of the same nominal age at day 42 and day 63 of storage (Figure 5).
Figure 5.
RBC from the same donor stored conventionally and anaerobically were passed through the networks of (a) Device A and (b) Device B.
Total number of “plugging events” that occurred in the 32 capillaries of a single network during a 5-second interval were counted at a pressure differential of −20 cmH2O for each sample and then averaged across units of the same nominal age. A “plugging event” is defined by counting the number of times RBC plugged (usually transiently) the capillaries of a single network; more specifically, the number of times the flow rates of the RBC in the capillaries dropped below the baseline mean minus 2SD of the corresponding day 2 sample. Note the increase in the mean number of plugging events as the duration of storage increases for both samples. On average, RBC stored conventionally displayed more plugging events. Values shown are means ± SD. Statistically significant differences (p<0.05) between aerobic and anaerobic storage of the same duration are indicated by (*).
RBC: red blood cell; SD: standard deviation.
Discussion
An extensive body of in vitro studies unequivocally shows RBC degradation (storage lesions) occurring during conventional storage. Reducing or preventing this degradation is expected to increase the efficacy of transfusions (more oxygen delivery to peripheral tissues immediately after transfusion) and to reduce negative side effects. Numerous emerging omics studies document the development of storage lesions at the molecular level17–25. Most notably, D’Amici’s group and others have shown that oxidative damage occurs as early as in the first 7–14 days of RBC storage, and that the damage can be significantly reduced if RBC are stored under anaerobic conditions26–28. Furthermore, gamma-irradiation and pathogen inactivation systems have been shown to induce, via production of reactive oxygen species, significant RBC oxidation that has resulted in reduced RBC survival in vivo29–34. The causality between the oxidation of RBC membrane/cytoskeleton and a reduction in RBC deformability has been well established in in vitro studies, as well as in the examination of RBC from various haemoglobinopathies35–40.
Although previous studies of anaerobically stored RBC have documented a significantly reduced accumulation of storage lesions compared to that of blood stored conventionally for the same period2,8,11,16,41–44, the deformability of RBC stored under anaerobic conditions has never been previously investigated. This study builds on our previous work in which we validated the measurement of AMVN perfusion as a metric of RBC deformability, in a side-by-side comparison with the conventional metrics of RBC deformability (micropore filtration and ektacytometry) using RBC modified by exposure to glutaraldehyde and diamide of varying concentrations15, and demonstrated the sensitivity of the AMVN perfusion measurement to storage-induced deterioration of RBC mechanical properties for conventional (aerobic) storage2,3. Specifically for this study, we also developed two alternative AMVN devices with capillary microchannels designed to be particularly sensitive to the passage of individual poorly-deformable RBC that may be present in a sample. We used these alternative AMVN devices to gain a better understanding of how the changes in deformability of a relatively small sub-population of cells affected the overall ability of stored RBC to perfuse the microvasculature, both at the level of the network and at the level of individual capillaries.
We found that the ability of stored RBC to perfuse an artificial microvascular network decreased progressively with the duration of storage both for RBC stored conventionally and for those stored anaerobically. However, throughout the entire duration of storage, the network perfusion for anaerobically stored RBC was consistently higher than that for RBC stored conventionally. The number of capillary plugging events increased more dramatically for RBC that had been stored conventionally than for those stored anaerobically, with the most significant differences observed on day 42 and day 63 of storage. Our results demonstrate that anaerobic conditions may diminish the deleterious effects of hypothermic storage on the ability of stored RBC to perfuse microvascular networks, and suggest that this benefit may be due to a significant reduction in the number of poorly-deformable RBC that are capable of transiently plugging the narrowest capillaries. This novel insight into the effect of anaerobic storage on the mechanical properties of RBC may potentially have significant implications for improving the safety and efficacy of stored blood, and its mechanism should, therefore, be carefully investigated in future studies. In addition, the microfluidic devices developed in this study should be useful for testing the effects of various storage conditions, additive solutions, and rejuvenation strategies on the mechanical properties of stored RBC.
Conclusions
We evaluated the rheological properties of RBC from units that had been stored either aerobically (conventionally) or anaerobically (oxygen-free) using three different microfluidic devices designed to test the ability of RBC to perfuse different types of capillary networks under nearly-physiological flow conditions. The devices proved sensitive to the storage-induced decline in RBC deformability. Our results suggest that anaerobic storage preserves the ability of stored RBC to perfuse networks of artificial capillaries significantly better than conventional (aerobic) storage. We further show that anaerobic storage is capable of significantly reducing the number of the least deformable cells (those capable of at least transiently plugging capillaries) present in the overall population of relatively well-preserved RBC. Since the quality of transfused blood ultimately depends upon its ability to readily traverse capillaries in vivo, anaerobic storage may therefore have the potential to significantly improve the safety and efficacy of stored blood.
Footnotes
Authorship contributions
SSS, LJD and TY designed the study. LJD provided the blood samples. JMB performed the experiments. XY and NZP developed the image analysis code. JMB and SSS analysed and interpreted the data, and wrote the manuscript. All Authors critically reviewed, edited and approved the manuscript.
Funding and resources
This work was supported in part by an award from the National Blood Foundation (PI: Shevkoplyas), a 2012 NIH Director’s Transformative Research Award (NHLBI R01HL117329, PI: Shevkoplyas) and a Phase II SBIR grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health (2-R44-HL-088848-02, PI: Yoshida).
Disclosure of conflicts of interest
New Health Sciences, Inc. is commercialising the anaerobic storage system and the artificial microvascular network technologies described in this article. TY is a Director of Research & Development at New Health Sciences Inc. LJD is a member of the Scientific Advisory board of New Health Sciences Inc. LJD and SSS have received research support from New Health Sciences, Inc. LJD, NZP and SSS have received compensation as consultants for New Health Sciences Inc. LJD, SSS, JMB and TY are inventors of some of the technology described in the article. XY declares no conflict of interest.
References
- 1.Roback JD, Combs MR, Grossman BJ, Hillyer CD, editors. Technical Manual. 16th ed. Bethesda, MD: AABB; 2008. [Google Scholar]
- 2.Burns JM, Yang X, Forouzan O, et al. Artificial microvascular network: a new tool for measuring rheologic properties of stored red blood cells. Transfusion. 2012;52:1010–23. doi: 10.1111/j.1537-2995.2011.03418.x. [DOI] [PubMed] [Google Scholar]
- 3.Reinhart WH, Piety NZ, Deuel JW, et al. Washing stored red blood cells in an albumin solution improves their morphologic and hemorheologic properties. Transfusion. 2015;55:1872–81. doi: 10.1111/trf.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Safeukui I, Buffet PA, Deplaine G, et al. Quantitative assessment of sensing and sequestration of spherocytic erythrocytes by the human spleen. Blood. 2012;120:424–30. doi: 10.1182/blood-2012-01-404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simchon S, Jan KM, Chien S. Influence of reduced red cell deformability on regional blood flow. Am J Physiol. 1987;253:H898–903. doi: 10.1152/ajpheart.1987.253.4.H898. [DOI] [PubMed] [Google Scholar]
- 6.Turner G. Cerebral malaria. Brain Pathol. 1997;7:569–82. doi: 10.1111/j.1750-3639.1997.tb01075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai AG, Cabrales P, Intaglietta M. Microvascular perfusion upon exchange transfusion with stored red blood cells in normovolemic anemic conditions. Transfusion. 2004;44:1626–34. doi: 10.1111/j.0041-1132.2004.04128.x. [DOI] [PubMed] [Google Scholar]
- 8.Yoshida T, Shevkoplyas SS. Anaerobic storage of red blood cells. Blood Transfus. 2010;8:220–36. doi: 10.2450/2010.0022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshida T, AuBuchon JP, Tryzelaar L, et al. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 2007;92:22–31. doi: 10.1111/j.1423-0410.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 10.Yoshida T, AuBuchon JP, Dumont LJ, et al. The effects of additive solution pH and metabolic rejuvenation on anaerobic storage of red cells. Transfusion. 2008;48:2096–105. doi: 10.1111/j.1537-2995.2008.01812.x. [DOI] [PubMed] [Google Scholar]
- 11.Dumont LJ, Yoshida T, AuBuchon JP. Anaerobic storage of red blood cells in a novel additive solution improves in vivo recovery. Transfusion. 2009;49:458–64. doi: 10.1111/j.1537-2995.2008.02038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shevkoplyas SS, Yoshida T, Gifford SC, Bitensky MW. Direct measurement of the impact of impaired erythrocyte deformability on microvascular network perfusion in a microfluidic device. Lab Chip. 2006;6:914–20. doi: 10.1039/b601554a. [DOI] [PubMed] [Google Scholar]
- 13.Shevkoplyas SS, Gifford SC, Yoshida T, Bitensky MW. Prototype of an in vitro model of the microcirculation. Microvasc Res. 2003;65:132–6. doi: 10.1016/s0026-2862(02)00034-1. [DOI] [PubMed] [Google Scholar]
- 14.Forouzan O, Yang X, Sosa JM, et al. Spontaneous oscillations of capillary blood flow in artificial microvascular networks. Microvasc Res. 2012;84:123–32. doi: 10.1016/j.mvr.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Sosa JM, Nielsen ND, Vignes SM, et al. The relationship between red blood cell deformability metrics and perfusion of an artificial microvascular network. Clin Hemorheol Microcirc. 2014;57:275–89. doi: 10.3233/CH-131719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumont DF, Szczepiorkowski ZM, Siegel AH, et al. Randomized cross-over in vitro and in vivo evaluation of a prototype anaerobic onditioning and storage system vs. standard aerobic storage. Vox Sang. 2012;103:123. [Google Scholar]
- 17.Liumbruno G, D’Alessandro A, Grazzini G, Zolla L. Blood-related proteomics. J Proteomics. 2009;73:483–507. doi: 10.1016/j.jprot.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Anniss AM, Glenister KM, Killian JJ, Sparrow RL. Proteomic analysis of supernatants of stored red blood cell products. Transfusion. 2005;45:1426–33. doi: 10.1111/j.1537-2995.2005.00547.x. [DOI] [PubMed] [Google Scholar]
- 19.Bosman GJ, Lasonder E, Luten M, et al. The proteome of red cell membranes and vesicles during storage in blood bank conditions. Transfusion. 2008;48:827–35. doi: 10.1111/j.1537-2995.2007.01630.x. [DOI] [PubMed] [Google Scholar]
- 20.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97:107–15. doi: 10.3324/haematol.2011.051789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J Proteomics. 2012;76:168–80. doi: 10.1016/j.jprot.2012.03.012. Spec No. [DOI] [PubMed] [Google Scholar]
- 22.Roback JD, Josephson CD, Waller EK, et al. Metabolomics of AS-1 RBC storage. Transfus Med Rev. 2014;28:41–55. doi: 10.1016/j.tmrv.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion. 2015;55:1155–68. doi: 10.1111/trf.12975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion. 2015;55:205–19. doi: 10.1111/trf.12804. [DOI] [PubMed] [Google Scholar]
- 25.D’Alessandro A, Hansen KC, Silliman CC, et al. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang. 2015;108:131–40. doi: 10.1111/vox.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Amici GM, Rinalducci S, Zolla L. Proteomic analysis of RBC membrane protein degradation during blood storage. J Proteome Res. 2007;6:3242–55. doi: 10.1021/pr070179d. [DOI] [PubMed] [Google Scholar]
- 27.Rinalducci S, D’Amici GM, Blasi B, et al. Peroxiredoxin-2 as a candidate biomarker to test oxidative stress levels of stored red blood cells under blood bank conditions. Transfusion. 2011;51:1439–49. doi: 10.1111/j.1537-2995.2010.03032.x. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessandro A, Gevi F, Zolla L. Red blood cell metabolism under prolonged anaerobic storage. Mol Biosyst. 2013;9:1196–209. doi: 10.1039/c3mb25575a. [DOI] [PubMed] [Google Scholar]
- 29.Moroff G, Holme S, AuBuchon JP, et al. Viability and in vitro properties of AS-1 red cells after gamma irradiation. Transfusion. 1999;39:128–34. doi: 10.1046/j.1537-2995.1999.39299154725.x. [DOI] [PubMed] [Google Scholar]
- 30.Cancelas JA, Dumont LJ, Rugg N, et al. Stored red blood cell viability is maintained after treatment with a second-generation S-303 pathogen inactivation process. Transfusion. 2011;51:2367–76. doi: 10.1111/j.1537-2995.2011.03163.x. [DOI] [PubMed] [Google Scholar]
- 31.Cancelas JA, Rugg N, Fletcher D, et al. In vivo viability of stored red blood cells derived from riboflavin plus ultraviolet light-treated whole blood. Transfusion. 2011;51:1460–8. doi: 10.1111/j.1537-2995.2010.03027.x. [DOI] [PubMed] [Google Scholar]
- 32.Patel RM, Roback JD, Uppal K, et al. Metabolomics profile comparisons of irradiated and nonirradiated stored donor red blood cells. Transfusion. 2015;55:544–52. doi: 10.1111/trf.12884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seghatchian J, Putter JS. Pathogen inactivation of whole blood and red cell components: an overview of concept, design, developments, criteria of acceptability and storage lesion. Transfus Apher Sci. 2013;49:357–63. doi: 10.1016/j.transci.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Wagner SJ. Developing pathogen reduction technologies for RBC suspensions. Vox Sang. 2011;100:112–21. doi: 10.1111/j.1423-0410.2010.01386.x. [DOI] [PubMed] [Google Scholar]
- 35.Aydogan S, Yapislar H, Artis S, Aydogan B. Impaired erythrocytes deformability in H(2)O(2)-induced oxidative stress: protective effect of L-carnosine. Clin Hemorheol Microcirc. 2008;39:93–8. [PubMed] [Google Scholar]
- 36.Browne P, Shalev O, Hebbel RP. The molecular pathobiology of cell membrane iron: the sickle red cell as a model. Free Radic Biol Med. 1998;24:1040–8. doi: 10.1016/s0891-5849(97)00391-2. [DOI] [PubMed] [Google Scholar]
- 37.Hebbel RP, Leung A, Mohandas N. Oxidation-induced changes in microrheologic properties of the red blood cell membrane. Blood. 1990;76:1015–20. [PubMed] [Google Scholar]
- 38.Kwan JM, Guo Q, Kyluik-Price DL, et al. Microfluidic analysis of cellular deformability of normal and oxidatively damaged red blood cells. Am J Hematol. 2013;88:682–9. doi: 10.1002/ajh.23476. [DOI] [PubMed] [Google Scholar]
- 39.Scott MD, van den Berg JJ, Repka T, et al. Effect of excess alpha-hemoglobin chains on cellular and membrane oxidation in model beta-thalassemic erythrocytes. J Clin Invest. 1993;91:1706–12. doi: 10.1172/JCI116380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kanias T, Acker JP. Biopreservation of red blood cells--the struggle with hemoglobin oxidation. FEBS J. 2010;277:343–56. doi: 10.1111/j.1742-4658.2009.07472.x. [DOI] [PubMed] [Google Scholar]
- 41.van Buskirk CM, Karon BI, Emery RL, et al. Comparison of cytokine, cell-free hemoglobin, and isoprostane accumulations in packed red blood cells during novel anaerobic and conventional cold storage. Transfusion. 2014;54S:SP53. [Google Scholar]
- 42.van Buskirk CM, Tarara JE, Jy WJ, Yoshida T. Comparison of microparticles production in packed red blood cells stored under anaerobic and conventional cold storage condition. Vox Sang. 2013;105(S1):150. [Google Scholar]
- 43.Yoshida T, Vernucci P, Vassallo RR, et al. Reduction of microparticle generation during anaerobic storage of red blood cells. Transfusion. 2012;52:83A. [Google Scholar]
- 44.D’Amici GM, Mirasole C, D’Alessandro A, et al. Red blood cell storage in SAGM and AS3: a comparison through the membrane two-dimensional electrophoresis proteome. Blood Transfus. 2012;10(Suppl 2):s46–54. doi: 10.2450/2012.008S. [DOI] [PMC free article] [PubMed] [Google Scholar]