Abstract
Infants with respiratory distress syndrome (RDS) may suffer from severe hypoxia, asphyxia. In this study, we aimed to evaluate serum ischemia-modified albumin (IMA) level as a diagnostic marker for hypoxia in preterm infants with RDS. Thirty-seven premature newborns with RDS were allocated as the study group and 42 healthy preterm neonates were selected as the control group. IMA was measured as absorbance unit (ABSU) in human serum with colorimetric assay method which is based on reduction in albumin cobalt binding. IMA levels were significantly higher in neonates with RDS as compared to the control group (P < 0.001). Cut-off value of IMA (ABSU) was 0.72, the sensitivity level was 91.9 %, the specificity was 78.6 %, positive predictive value was 79.1 % and negative predictive value was 91.7 % at RDS. Area under curve values was 0.93 (P < 0.001; 95 % CI, 0.88–0.98) in the receiver operating characteristic curve. We concluded that elevated blood IMA levels might be accepted as a useful marker for hypoxia in newborn with RDS.
Keywords: Prematurity, Newborn, Respiratory distress syndrome, Ischemia modified albumin
Introduction
Respiratory distress syndrome (RDS) or also known as the hyaline membrane disease is the most common disease resulting in severe morbidities and mortality in premature infants [1]. Incidence of RDS increases with decreasing gestational age and birth weight. EuroNeo Net figures for 2010 show an incidence of 92 % at 24–25 weeks of gestation, 88 % at 26–27 weeks, 76 % at 28–29 weeks and 57 % at 30–31 weeks [1, 2]. Prematurity is the main cause of RDS [3]. The basic physiopathologies of RDS are mulifactorial including impaired gas exchange, decreased static compliance, and alveolar collapse due to the lack of surfactant, pulmonary edema caused by disruption of alveolo-capilar membrane integrity and finally lung injury [1, 4]. Clinically, RDS presents with early respiratory distress comprising cyanosis, grunting, retractions and tachypnea that those advance rapidly from birth. Decreased pulmonary ventilation and perfusion hypoxia, hypercapnia, and acidosis occur during the course of RDS. Therefore, management of infants with RDS should be instituted promptly in a disciplined manner early after birth. Infants with RDS may suffer from hypoxia, asphyxia, and hypoxia-ischemia may cause multiple organ damage that may be leading causes of increased morbidity and mortality in infants with RDS [2, 5]. Different biochemical and biophysical tests have been investigated for the prediction of infants at risk for hypoxia and prevention of this damage, however many of them have remained limited [6, 7].
Ischemia modified albumin (IMA) is a modification of human serum albumin (HSA). N-terminal amino acids of HSA temporarily bind to transitional metals such as cobalt, nickel and copper. Hypoxia, acidosis or ischemia leads to a change on this region and reduce the binding capacity of HSA to these metals. The resulting molecule is called IMA [8, 9]. IMA rapidly increases within 5–10 min after the ischemic event and remains high for 30 min. It returns to baseline 12 h after the ischemia event, but if the ischemic event persists, it continues to rise [8]. In recent clinical studies, it has been found that IMA is a new biochemical marker for the early diagnosis of myocardial ischemic events and cerebrovascular accidents in adults [7–9]. Furthermore, few studies have determined that cord blood levels of IMA are higher in perinatal asfixia and complicated deliveries thought to be associated with fetal distress, hypoxia and oxidative stress in newborns. Additionally, some clinical and experimental studies have recently indicated elevated levels of IMA in ischemic and hypoxic conditions such as necrotizing enterocolitis (NEC) in newborns [10–13]. In these considerations, the primary aim of this study was to evaluate the diagnostic significance of serum IMA level in newborns with RDS which is a leading cause of hypoxia particularly in preterm infants.
Methodology
This study was carried out on premature infants born less than 34 weeks with diagnosed RDS between January 1st 2013 and June 1st 2013. Local ethical committees approved the study protocol, and informed consents were obtained from the parents. RDS was diagnosed according to the criteria involving severe respiratory distress, reticulogranular appearance on chest X-rays [14]. The inclusion criteria for the study group were preterm birth less than 34 weeks by singleton pregnancies, and diagnosed as RDS. Gestational age was correctly determined by the last menstrual period and/or ultrasonographic examination prior to 20 weeks of gestation. The exclusion criteria were chorioamnionitis, abnormal fetal karyotype, smoking, neonatal hipoglisemi, neonatal hypoalbuminemia (serum albumin < 2.5 g/dL) and elevated levels of C-reactive protein (hs-CRP) in newborns (>1 mg/L), major congenital somatic anomalies, severe congenital heart disease, intrauterine growth restriction, placental abruption, and antenatal heart rate deceleration recognized as the serious potential factors to asphyxia. Patients suffered from asfixia were excluded from the study according to the laboratory findings including severe acidosis in arterial blood gases, and elevated levels of liver transaminases (AST, ALT), lactic dehydrogenase (LDH), creatine kinase (CK) in serum. The study group were consisted of 37 infants with RDS, and the control group was consisted of 42 infants born less than 34 weeks and having no problems other than premature birth. 200 mg/kg doses of surfactant (Curourf®, Chiesi Ltd. Parma, Italy) was used via endotracheal way for all infants with RDS. Supportive care such as mechanical ventilation, fluid electrolyte and nutritional support, close monitoring of vital signs were given to all patients.
Blood Sample Collection and Ischemia Modified Albumin Assay Method
Blood samples were collected from the patients in both study and control group before instituting mechanical ventilation and surfactant therapy for evaluation IMA serum levels. Prenatal, natal and post-natal records, demographic informations, clinical and radiological data of all patients were obtained from the medical records. Patients with, RDS were followed and cared according to blood gasses and oxygen index (OI). After obtaining blood samples in simple tubes which is containing no preservatives or separation gels, the sample was allowed to clot for 30–45 min and centrifuged before separating the serum. Serum samples were refrigerated at −20 °C and analyzed in the same laboratory within 1 month. Frozen samples were mixed thoroughly after thawing and centrifuged before analysis. Samples with more than a trace of hemolysis were discarded. Colorimetric assay was developed to screen human serum samples for decreased cobalt binding to albumin. The assay method involved adding 15 μL of 0.1 % cobalt chloride (Sigma, CoCl2·6H2O) in H2O to 50 μL of serum, gently mixing, and waiting 10 min for adequate cobalt-albumin binding. Fifteen microliters of dithiothreitol (DTT) (Sigma, 1.5 mg/mL H2O) was added as a colorizing agent and the reaction was quenched 2 min later by adding 1.0 mL of 0.9 % NaCl. Using a elisa reader at 450 nm (Bio-Tek ELX 800 Absorbance microplate reader, Bio-Rad, USA and Bio-Tek ELx50 microplate auto strip washer, Bio-Rad, USA) color development with DTT was compared to a serum-cobalt blank without DTT and reported in absorbance units (ABSU).
Statistical Analysis
For statistical analysis NCSS (Number Cruncher Statistical System) 2007 PASS (Power Analysis and Sample Size) 2008 Statistical Software (Utah, USA) was used. Student t test was used for comparing quantitative. Descriptive statistics for continuous variables were demonstrated as mean ± standard deviation and medians (minimum–maximum). Specificity, sensitivity, positive predictive value, negative predictive value, receiver operating characteristic curve (ROC) analysis were performed for cut-off values. Significance were taken at P < 0.05 levels.
Results
Thiry seven patients were diagnosed as RDS, and selected as the study group. 42 patients without respiratory symptoms were enrolled as the control group. There were no significant differences between the study and control groups in terms of gestational age, modes of delivery, birth weight (P > 0.05), except APGAR scores (P < 0.01) (Table 1). In the study group, resuscitation was applied to 30 (81.1 %) infants immediately after birth due to severe respiratory failure. However, infants in the control group did not need resuscitation (P < 0.001). OI of the patients in the study group was 27.7 ± 2.2 (25–32). The duration of mechanical ventilation therapy for patients in the study group was 3.8 ± 7.3 (1–38) days. Infants in the control group did not receive either mechanical ventilation therapy or nasal continuous positive airway pressure (NCPAP) therapy. The duration of NCPAP therapy of the patients in the study group was 3.7 ± 2.6 (3–15) days. The duration of hospitalization of the study group was 38.5 ± 16.3 (7–56) days; whereas, the duration of hospitalization of the control group was 26.0 ± 11.7 (7–52) days (P < 0.001). Surfactant was applied to all the 37 (100 %) infants in the study group (Table 1). Their oxygenation indexes was 29.7 ± 2.2 (25–37), and the duration of mechanical ventilation (day) was 3.8 ± 7.3 (1–38). IMA levels were found to be significantly higher in RDS group than those in the control groups (IMA levels were 0.91 ± 0.15 (0.15–1.25) ABSU and 0.63 ± 0.12 (0.38–0.97) ABSU, respectively, (P < 0.001) (Table 1).
Table 1.
Demographic and clinical characteristics of the patients, and IMA levels
| Variables | RDS (n = 37) % mean ± SD (min–max) | Control (n = 42) % mean ± SD (min–max) | P |
|---|---|---|---|
| Gender | |||
| Female | 21 (56.8 %) | 20 (47.6 %) | 0.33 |
| Male | 16 (43.2 %) | 22 (52.4 %) | 0.58 |
| Gestasyon (week) | 30.0 ± 2.9 (27–34) | 30.3 ± 1.7 (28–34) | 0.85 |
| Birth weight (g) | 1574.4 ± 298.5 (860–2090) | 1581 ± 301.1 (900–2120) | 0.75 |
| Mode of delivery | |||
| NVYD | 16 (43.2 %) | 23 (54.8 %) | 0.31 |
| C/S | 21 (56.8 %) | 19 (45.23 %) | 0.23 |
| APGAR 1st min | 6.0 ± 0.7 (1–7) | 8.0 ± 0.5 (7–8) | <0.001* |
| APGAR 5th min | 7.9 ± 0.7 (7–9) | 9.3 ± 0.6 (9–10) | <0.001* |
| Gravida | 2.8 ± 1.7 (1–5) | 2.7 ± 1.6 (1–5) | 0.75 |
| Parity | 2.6 ± 1.5 (1–5) | 2.6 ± 1.3 (1–5) | 0.78 |
| IMA (ABSU) | 0.91 ± 0.15 (0.15–1.25) | 0.63 ± 0.12 (0.38–0.97) | <0.001* |
NVD normal vaginal delivery, C/S cesarean section, ABSU absorbance units
* Significant differences between control and RDS. Values of P < 0.05 were considered
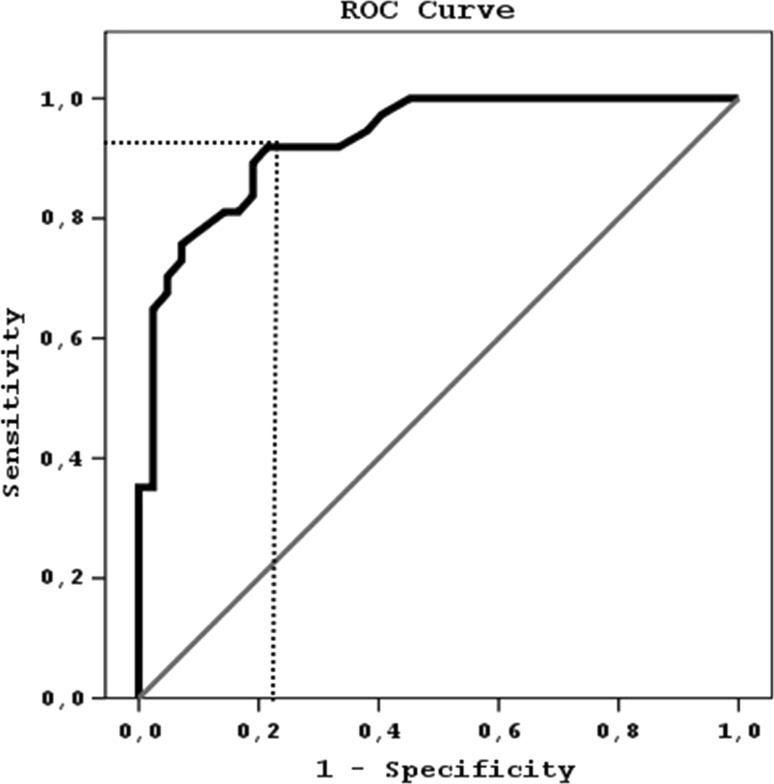
There was no correlation between birth weight (ρ = −0.132, P = 0.44), gestational age (ρ = 0.009, P = 0.94) and modes of delivery (ρ = −0.02, P = 0.96) and IMA levels in the study group. The ROC curve of IMA for prediction of RDS was shown in Fig. 1. Cut-off value of IMA was determined as 0.72 ABSU (sensitivity of IMA was 91.8 %, specificity was 78.6 %, positive predictive value was 79.1 %, and negative predictive value was 91.7 %. Area under curve (AUC) was 0.93 (P < 0.001; 95 % CI, 0.88–0.98).
Fig. 1.
ROC curve showing the predictive value of serum ischemia-modified albumin (IMA) levels in the diagnosis of respiratory dystress syndrome (RDS) in preterm newborn. Area under curve (AUC) for IMA was 0.93 (P < 0.001; 95 % CI, 0.88–0.98)
Discussion
In the present study, we have evaluated the serum levels and the efficacy of IMA in infants with RDS. To the best of our knowledge, the present study is the first report evaluating neonatal IMA levels in clinical evidence of RDS. We also described the relationship between IMA levels and RDS that may be associated with hypoxia and oxidative stress in newborns. These results showed that IMA levels were significantly higher in infants with diagnosed RDS than those in infants in the control group. In this study, IMA sensitivity, negative predictive value and AUC values in RDS were significantly higher than the control group. Therefore, IMA may be a useful marker for distinguishing severity of preterm infants that may possibly have serious hypoxia and clinically severe RDS early in the course of the disease at admission to NICU.
The mechanism of IMA generation remains unexplained. It has been suggested that IMA is a modification of serum albumin, and it results from oxidative stress and concurrently produced superoxide-free oxygen radicals that occur during ischemic events, regardless of tissue specificity [7–9, 12]. Recently, some studies have reported that IMA has a relationship with various ischemia-related conditions, such as acute coronary syndrome, ischemia of liver, brain, kidney, and bowel in adults [7, 15, 16]. And also, it has been accepted as a highly specific marker for myocardial ischemia and it has been indicated that the level of IMA remained high during the presence of ischemia [8, 17, 18].
In patients with RDS, hypoxemia is usually the result of V/Q mismatch or right-to-left shunting, diffusion abnormalities and hypoventilation which may decrease oxygenation. V/Q mismatch is a major cause of hypoxemia in infants with RDS. V/Q mismatch usually is caused by poor ventilation of alveoli relative to their perfusion. Shunting can be seen in the intrapulmonary different regions due to RDS. Therefore, infants with RDS expose to severe hypoxia [4]. Current studies have determined that cord blood levels of IMA are higher in perinatal asfixia and complicated deliveries associated with fetal distress, hypoxia, and oxidative stress in newborns [11, 12, 19, 20], and IMA has been declared to be a good marker for diagnosis of asphyxia. RDS is known a severe disease that leads to severe hypoxia and further deleterious neurodevelopmental outcomes of preterm infants and mortality without appropriate respiratory support and surfactant therapy. In the present study, IMA levels were determined significantly higher infants with RDS. Current results show that IMA can be used to display hypoxia in infants with RDS.
In the newborn period, few studies have reported the affectivity of IMA for that particular population. Yakut et al. [13] have reported that IMA levels are sensitive and effective marker for diagnosing NEC in close association with intestinal hypoxia, monitoring and decision-making for surgery in preterm infants. Kumral et al. [12] have stated that asphyxia in low birth weight infants exists more than term infants, and this situation increases reactive oxygen species [21]. Therefore, oxidative stress in preterm infants with insufficient oxygenation may contribute to increase in the IMA levels. This hypoxia may contribute to adverse neurodevelopmental outcomes and multiorgan failure other than respiratory system. Furthermore, reducing motor and cognitive skills of preterm infants could be seen during long-term follow up.
As we have shown in our study that acute transient hypoxia occurred in RDS infants. We thought that this hypoxia mainly occurred because of RDS. Because of the fact that as we obviously can see that preterm infant in the control group did not have severe respiratory compromises after birth. And also, infants in the control group did not receive serious respiratory support. Higher IMA levels in preterm infants with RDS suggested that preterm infants with RDS may suffer from clinical hypoxia/asphyxia. And asphyxia may further lead to increase severity of respiratory distress and multiorgan failure. In this regard, increased IMA levels could warn the attending physicians in NICU.
Conclusion
RDS is a serious respiratory condition in preterm infants that may have severe different kind of adverse outcomes due to hypoxia. Therefore, IMA levels in infants with RDS might be a good marker at showing asphyxia/hypoxia in the early course of RDS and early days of preterm birth. Elevated blood IMA levels may be accepted as a useful marker for hypoxia in newborn with RDS. However, the results of the present study are still preliminary and future prospective and controlled studies including larger series are warranted.
Footnotes
Hasan Kahveci and Cuneyt Tayman are the first author.
References
- 1.Rodrigez RJ, Martin RJ, Fanroff AA. Respiratory dystress syndrome and its manangement. In: Martin RJ, Fanaroff AA, Walsh MC, editors. Fanaroff and Matin’s neonatal perinatal disease of the fetus and infant. 8. Philadelphia: Elsevier-Mosby; 2006. pp. 1097–1122. [Google Scholar]
- 2.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants–2013 update. Neonatology. 2013;103:353–368. doi: 10.1159/000349928. [DOI] [PubMed] [Google Scholar]
- 3.Dani C, Reali MF, Bertini G, Wiechmann L, Spagnolo A, Tangucci M, et al. Risk factors for the development of respiratory distress syndrome and transient tachypnoea in newborn infants. Italian group of Neonatal Pneumology. Eur Respir J. 1999;14:155–159. doi: 10.1034/j.1399-3003.1999.14a26.x. [DOI] [PubMed] [Google Scholar]
- 4.Gitto E, Reiter RJ, Karbownik M, Xian-Tan D, Barberi I. Respiratory distress syndrome in the newborn: role of oxidative stress. Intensive Care Med. 2001;27:1116–1123. doi: 10.1007/s001340100977. [DOI] [PubMed] [Google Scholar]
- 5.Thornberg E, Thiringer K, Odeback A, Milsom I. Birth asphyxia: incidence, clinical course and outcome in a Swedish population. Acta Paediatr. 1995;84:927–932. doi: 10.1111/j.1651-2227.1995.tb13794.x. [DOI] [PubMed] [Google Scholar]
- 6.Nordstrom L, Arulkumaran S. Intrapartum fetal hypoxia and biochemical markers: a review. Obstet Gynecol Surv. 1998;53:645–657. doi: 10.1097/00006254-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Gaze DC. Ischemia modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab Pharmacokinet. 2009;24:333–341. doi: 10.2133/dmpk.24.333. [DOI] [PubMed] [Google Scholar]
- 8.Shen XL, Lin CJ, Han LL, Lin L, Pan L, Pu XD. Assessment of ischemia-modified albumin levels for emergency room diagnosis of acute coronary syndrome. Int J Cardiol. 2011;149:296–298. doi: 10.1016/j.ijcard.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Sadler PJ, Tucker A, Viles JH. Involvement of a lysine residue in the N-terminal Ni2+ and Cu2+ binding site of serum albumins. Comparison with Co2+, Cd2 + and Al3+ Eur J Biochem. 1994;220:193–200. doi: 10.1111/j.1432-1033.1994.tb18614.x. [DOI] [PubMed] [Google Scholar]
- 10.Caglar GS, Tasci Y, Goktolga U, Oztas E, Pabuccu R, Ozdemir ED, et al. Maternal and umbilical cord ischemia-modified albumin levels in nonreassuring fetal heart rate tracings regarding the mode of delivery. J Matern Fetal Neonatal Med. 2013;26:528–531. doi: 10.3109/14767058.2012.743519. [DOI] [PubMed] [Google Scholar]
- 11.Dursun A, Okumus N, Zenciroglu A. Ischemia-modified albumin (IMA): could it be useful to predict perinatal asphyxia? J Matern Fetal Neonatal Med. 2012;25:2401–2405. doi: 10.3109/14767058.2012.697943. [DOI] [PubMed] [Google Scholar]
- 12.Kumral A, Okyay E, Guclu S, Gencpinar P, Islekel GH, Oguz SS, et al. Cord blood ischemia-modified albumin: is it associated with abnormal Doppler findings in complicated pregnancies and predictive of perinatal asphyxia? J Obstet Gynaecol Res. 2013;39:663–671. doi: 10.1111/j.1447-0756.2012.02055.x. [DOI] [PubMed] [Google Scholar]
- 13.Yakut I, Tayman C, Oztekin O, Namuslu M, Karaca F, Kosus A. Ischemia-modified albumin may be a novel marker for the diagnosis and follow-up of necrotizing enterocolitis. J Clin Lab Anal. 2014;28:170–177. doi: 10.1002/jcla.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal V, David RJ, Harris VJ. Classification of acute respiratory disorders of all newborns in a tertiary care center. J Natl Med Assoc. 2003;95:585–595. [PMC free article] [PubMed] [Google Scholar]
- 15.Sbarouni E, Georgiadou P, Kremastinos DT, Voudris V. Ischemia modified albumin: is this marker of ischemia ready for prime time use? Hellenic J Cardiol. 2008;49:260–266. [PubMed] [Google Scholar]
- 16.Gunduz A, Turkmen S, Turedi S, Mentese A, Yulug E, Ulusoy H, et al. Time-dependent variations in ischemia-modified albumin levels in mesenteric ischemia. Acad Emerg Med. 2009;16:539–543. doi: 10.1111/j.1553-2712.2009.00414.x. [DOI] [PubMed] [Google Scholar]
- 17.Ustün Y, Engin-Ustün Y, Oztürk O, Alanbay I, Yaman H. Ischemia-modified albumin as an oxidative stress marker in preeclampsia. J Matern Fetal Neonatal Med. 2011;24:418–421. doi: 10.3109/14767058.2010.497879. [DOI] [PubMed] [Google Scholar]
- 18.van Rijn BB, Franx A, Sikkema JM, van Rijn HJ, Bruinse HW, Voorbij HA, et al. Ischemia modified albumin in normal pregnancy and preeclampsia. Hypertens Pregnancy. 2008;27:159–167. doi: 10.1080/10641950701885147. [DOI] [PubMed] [Google Scholar]
- 19.Gugliucci A, Hermo R, Monroy C, Numaguchi M, Kimura S. Ischemia-modified albumin levels in cord blood: a case-control study in uncomplicated and complicated deliveries. Clin Chim Acta. 2005;362:155–160. doi: 10.1016/j.cccn.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 20.Ukinc K, Eminagaoglu S, Ersoz HO, Erem C, Karahan C, Hacihasanoglu AB, et al. A novel indicator of widespread endothelial damage and ischemia in diabetic patients: ischemia-modified albumin. Endocrine. 2009;36:425–432. doi: 10.1007/s12020-009-9236-5. [DOI] [PubMed] [Google Scholar]
- 21.Davis JM, Auten RL. Maturation of the antioxidant system and the effects on preterm birth. Semin Fetal Neonatal Med. 2010;15:191–195. doi: 10.1016/j.siny.2010.04.001. [DOI] [PubMed] [Google Scholar]