Abstract
Deficiency or excess of certain trace elements has been considered as risk factor for prostate cancer. This study was aimed to detect differential changes and mutual correlations of selected trace elements in prostate cancer tissue versus benign prostatic hyperplasia tissue. Zinc, copper, iron, calcium and selenium were analysed in histologically proven 15 prostate cancer tissues and 15 benign prostatic hyperplasia tissues using atomic absorption spectrophotometer. Unpaired two tailed t test/Mann–Whitney U test and Pearson correlation coefficient were used to compare the level of trace elements, elemental ratios and their interrelations. As compared to benign prostatic tissue, malignant prostatic tissue had significantly lower selenium (p = 0.038) and zinc (p = 0.043) concentrations, a lower zinc/iron ratio (p = 0.04) and positive correlation of selenium with zinc (r = 0.71, p = 0.02) and iron (r = 0.76, p = 0.009). Considerably divergent interrelationship of elements and elemental ratios in prostate cancer versus benign prostatic hyperplasia was noted. Understanding of differential elemental changes and their interdependence may be useful in defining the complex metabolic alterations in prostate carcinogenesis with potential for development of element based newer diagnostic, preventive and therapeutic strategies. Further studies may be needed to elucidate this complex relationship between trace elements and prostate carcinogenesis.
Keywords: Prostate cancer; Trace elements; Benign prostatic hyperplasia; Prostate carcinogenesis, metal carcinogenesis; Prostate cancer prevention
Introduction
It is well known that some of the heavy metals are essential while some have toxic and carcinogenic effects in humans [1]. Studies on concentration of these trace metals in human play an important role in better understanding of biochemical and metabolic processes occurring in cells. Instability (overabundance or deficiency) in the composition of these trace metals may play a critical role in cancer biology; however, their precise role in initiation, promotion and inhibition of carcinogenic process is still undefined.
For understanding the relationship between prostate cancer and metals, it may be important to identify differences in homeostasis of trace elements between two common diseases of aging prostate i.e. benign prostatic hyperplasia (BPH) and prostate cancer (PCa). As both of these conditions have different histopathology and clinical behaviour, different metabolic alterations should account for these pathological processes. Alterations in levels of certain trace elements especially selenium and zinc have been identified in prostate cancer [2–5]. However, administration of Se to subjects in clinical trials [6] and Zn in case control studies [7] failed to prevent the prostate cancer. Thus, in addition to absolute tissue levels of various metals, their ratios and mutual correlations may help in understanding the complex metabolic dynamics of trace elements in prostate cancer. Only isolated studies have examined this complex interrelationship [8, 9] or ratios [10] of the trace elements in prostate cancer tissue. The aim of present study was to assess differences in levels, ratios and inter-relationship of five elements i.e. Copper (Cu), Calcium (Ca), Iron (Fe), Zinc (Zn) & Selenium (Se) between benign versus malignant prostate tissue. The understanding of this disturbed homeostasis of trace elements in cancerous tissue may have significant implications for development of element related preventive or therapeutic interventions for prostate cancer.
Materials and Methods
This study was approved by the institutional review board and ethics committee of our institution. Informed consent was obtained from all individual participants included in the study.
Patients and Protocol
The study was conducted on prostatic tissues obtained from 30 patients who attended our tertiary care teaching institution for surgical management of BPH (group 1 = 15 patients) and prostate cancer (group 2 = 15 patients). Exclusion criteria included diabetes, any other malignancy, any hormone therapy or steroid therapy. In BPH group, transurethral resection (TUR) chips were used where as in prostate cancer group either channel TUR chips or tissue obtained from radical prostatectomy specimens after sectioning the prostate were used. Parts of the selected tissues were separately sent to confirm the representative histopathology. Concentration of Zn, Cu, Fe and Ca was analysed in all 30 patients (15 BPH and 15 PCa samples) and that of Se was analysed in 20 patients (10 BPH and 10 PCa samples).
Tissue Preparation and Trace Elements Analysis
Fresh prostate tissues were washed in deionised water and at least 1 gram of the tissue (range 1.050–1.428 g) was carefully weighed and stored at −20 °C. These samples were digested in a mixture of nitric: perchloric acid (6:1) in Kjeldahl flasks on slow heating. The heating was continued till white fumes evolved and the volume was reduced to 0.5–1.0 ml. The contents were made up to 10 ml with 0.1 N nitric acid, filtered and used for the analysis of zinc, copper, iron and calcium on a flame Atomic Absorption Spectrometer (AAS) (Perkin Elmer, AAnalyst 300). Selenium was analysed on an AAS equipped with a Graphite tube atomiser (Analytik Jena, Zeenit 700). The method was validated using the matrix spiking, recovery experiments, repeatability, reproducibility, limit of detection (LOD) and limit of quantitation (LOQ). Reagent blanks were also prepared and processed in the same manner for analysis. Recovery experiments indicated that the percentage recovery ranges from 80-120 % and the instrument calibration was done using the NIST traceable certified reference material (ICP multi-element standard solution IV from Merck, Darmstadt, Germany).Concentration of Zn, Cu, Fe and Ca are reported in µg/g while Se is reported in ng/g of wet tissue. Trace element analysis was done at Analytic Chemistry Laboratory, CSIR - Indian Institute of Toxicology Research, Lucknow, India. The following sequence was followed for the analysis on the guidelines of MET-CHEM-AA-01 and MET-CHEM-AA-02 as described by Neugebauer et al. [11]
Analysis sequence performed:
| Solution type | Acceptance criteria and comments |
|---|---|
| Calibration blank | To check the background contamination. There should not be any contamination |
| Sensitivity check standard | For determination of LOD and LOQ of the instrument |
| Standards (CRMs) | To check the linearity range of the instrument |
| Calibration blank | No contamination should be there in blank |
| Calibration verification standard | It should read between 80 and 120 % |
| Reagent blank | Should be lower than 10 times the acceptable limit of error for that particular analyte |
| Standard reference material | By matrix spiking. Recovery between 80 and 120 % |
| Establishment of LOD/LOQ for the method used | By replicate analysis of spiked samples at ppb level and after getting the LOQ value, practically demonstrated to get confidence in the method used |
| Samples | Absorbance should be <highest standard % RSD of two readings should be ±20 % Recovery of spiked sample should be 80–120 % Values less than LOQ evaluated |
| SRM and calibration blank | Used for checking the instrumental performance in between the sample analysis |
Statistical Analysis
The results are presented as mean ± SD. The unpaired t-test/Mann–Whitney U test was used to compare the various elements, ratios of elements, age and PSA between BPH and Cap groups. The Pearson correlation coefficient was calculated among the parameters to find the trend of correlation i.e. any correlation of trace elements with either each other in benign and cancerous tissues or other patient parameters i.e. age, ultrasonographic prostate size (in case of BPH) and PSA (in case of prostate cancer). The p value <0.05 was considered to be significant. All the statistical analysis was carried out using SPSS 16.0 version (Chicago, In., USA).
Results
Mean age was similar in both PCa and BPH groups (Table 1). Selenium and Zinc levels were significantly (p = 0.038 and p = 0.043 respectively) lower in PCa tissue as compared to BPH tissue. Levels of Cu, Ca and Fe were not statistically different (p = 0.23, 0.47 and 0.93) between PCa and BPH. On comparing the ratios of trace elements, Zn/Fe ratio was significantly (p = 0.04) lower whereas Fe/Se and Ca/Se ratios were slightly higher (p values 0.068 and 0.08 respectively) in PCa as compared to BPH.
Table 1.
Comparison of age, levels of various elements, elemental ratios and PSA between benign prostatic hyperplasia (BPH) and prostate cancer (PCa)
| Parameter/element/elemental ratio | BPH | PCa | p value |
|---|---|---|---|
| Age(years) | 67.9 ± 8.09 | 63.9 ± 12.1 | 0.2 |
| Ca (µg/g wet tissue) | 161.8 ± 78.5 | 140.7 ± 81.6 | 0.47 |
| Se(ng/g wet tissue) | 245.7 ± 195.6 | 91.5 ± 95.3 | 0.038 |
| Cu(µg/g wet tissue) | 5.02 ± 1.87 | 4.05 ± 2.41 | 0.23 |
| Zn(µg/g wet tissue) | 77.9 ± 43.4 | 49.2 ± 29.3 | 0.043 |
| Fe(µg/g wet tissue) | 55.04 ± 31.8 | 53.9 ± 38.4 | 0.93 |
| Zn/Se | 535 ± 347 | 797.5 ± 715.5 | 0.31 |
| Zn/Cu | 16.2 ± 8.05 | 13.2 ± 4.77 | 0.21 |
| Zn/Fe | 1.58 ± 0.7 | 1.07 ± 0.4 | 0.04 |
| Ca/Zn | 2.73 ± 1.89 | 3.29 ± 1.91 | 0.42 |
| Cu/Se | 28.01 ± 18.9 | 79.6 ± 98.8 | 0.12 |
| Ca/Cu | 35.4 ± 18.9 | 45.4 ± 36.9 | 0.36 |
| Fe/Cu | 11.16 ± 5.62 | 14.07 ± 7.38 | 0.23 |
| Ca/Se | 1179 ± 939.8 | 2404.5 ± 1868.6 | 0.08 |
| Ca/Fe | 3.54 ± 2.13 | 3.86 ± 4.17 | 0.79 |
| Fe/Se | 328.3 ± 215.6 | 673 ± 518 | 0.068 |
| PSA (ng/ml) | 2.3 ± 0.94 | 40.7 ± 44.7 | 0.001 |
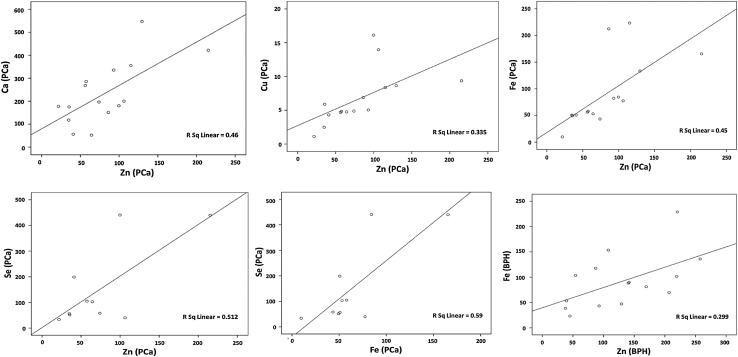
Table 2 and Fig. 1 show the results of correlation statistics. On examining the independent correlations of elements, the Zn level was positively correlated with Ca, Fe, Cu and Se (p values = 0.005, 0.006, 0.019 & 0.02 respectively) in PCa, whereas in BPH it was positively correlated with Fe (p value = 0.035) only. In malignant tissues, Se level was also positively correlated (p value = 0.009) with Fe levels. On examining the independent correlations between various trace elements and their ratios, Zn/Fe ratio was found to be positively correlated (p = 0.028) with Ca/Cu ratio in PCa.
Table 2.
Significant correlations between tissu level of element and their ratios—in benign prostatic hyperplasia (BPH) versus prostate cancer (PCa)
| Element/elemental ratio | Significant correlations (p < 0.05) | |||||
|---|---|---|---|---|---|---|
| In BPH | In PCa | |||||
| Correlation with | r value | p value | Correlation with | r value | p value | |
| Se | Cu/Se | −0.7 | 0.024 | Zn | 0.71 | 0.02 |
| Zn/Se | −0.7 | 0.023 | Fe | 0.76 | 0.009 | |
| Ca/Se | −0.69 | 0.026 | Ca/Se | −0.67 | 0.03 | |
| Fe/Se | −0.66 | 0.037 | ||||
| Zn | Fe | 0.54 | 0.035 | Fe | 0.67 | 0.006 |
| Zn/Cu | 0.77 | 0.001 | Ca | 0.67 | 0.005 | |
| Ca/Zn | −0.67 | 0.006 | Cu | 0.59 | 0.019 | |
| Zn/Fe | Zn/Cu | 0.55 | 0.033 | Ca/Cu | 0.56 | 0.028 |
| Ca/Zn | −0.57 | 0.026 | Ca/Fe | 0.73 | 0.002 | |
| Fe/Cu | −0.63 | 0.011 | ||||
| Fe | −0.55 | 0.033 | ||||
| Fe/Se | Fe/Cu | 0.66 | 0.035 | Cu/Se | 0.93 | 0.000 |
| Zn/Se | 0.71 | 0.020 | Zn/Se | 0.91 | 0.000 | |
| Ca/Se | 0.66 | 0.036 | ||||
| Fe | 0.64 | 0.044 | ||||
| Ca/Se | Ca/Cu | 0.83 | 0.003 | Ca/Fe | 0.65 | 0.039 |
| Ca | 0.74 | 0.013 | Ca/Zn | 0.67 | 0.031 | |
| Zn/Se | 0.65 | 0.040 | ||||
| Fe | Fe/Cu | 0.77 | 0.001 | Fe/Cu | 0.78 | 0.001 |
| Ca/Fe | −0.52 | 0.044 | ||||
| Zn/Se | Cu/Se | 0.90 | 0.0003 | Cu/Se | 0.94 | 0.00003 |
| Zn/Cu | Ca/Zn | −0.63 | 0.011 | Ca/Cu | 0.58 | 0.022 |
| Ca/Cu | Ca/Fe | 0.63 | 0.012 | Ca/Fe | 0.93 | 0.00001 |
| Ca | 0.75 | 0.001 | Ca/Zn | 0.88 | 0.00002 | |
| Cu | −0.518 | 0.048 | ||||
| Ca/Zn | – | – | – | Ca/Fe | 0.85 | 0.00004 |
Fig. 1.
Scatter plot diagrams showing significant (p < 0.05) correlations between trace elements in prostate cancer (PCa) and benign prostatic hyperplasia (BPH) (concentration of Zn, Ca, Cu and Fe is shown in µg/g wet tissue and that of Se is shown in ng/g wet tissue)
On comparing the correlation coefficients (Table 3) of elements between PCa and BPH, the correlation of Se with Fe was significantly different (r = -0.13 vs. 0.76, Z-score = 2.15, p = 0.03) in these two conditions. Among elemental ratios, Ca/Zn had significantly different correlations with Zn/Cu and Zn/Fe in PCa (no correlation) as compared to BPH (negative correlation). Further, Ca/Zn to Ca/Cu, Ca/Fe to Ca/Cu and Fe/Se to Cu/Se correlations were significantly different between PCa (strongly positive) and BPH (mild positive or no correlation).
Table 3.
Significant differences on comparisons of correlation coefficients of elements and their ratios between benign prostatic hyperplasia (BPH) and prostate cancer (PCa)
| Correlated elements or elemental ratios |
Correlation coefficients in |
Z-score | p value | |
|---|---|---|---|---|
| BPH | PCa | |||
| Se & Fe | −0.13 | 0.76 | 2.15 | 0.03 |
| Ca/Zn & Zn/Cu | −0.63 | 0.22 | 2.39 | 0.017 |
| Ca/Zn & Zn/Fe | −0.57 | 0.37 | 2.55 | 0.01 |
| Ca/Zn & Ca/Cu | 0.44 | 0.88 | 2.25 | 0.024 |
| Ca/Fe & Ca/Cu | 0.63 | 0.93 | 2.24 | 0.025 |
| Fe/Se & Cu/Se | 0.56 | 0.93 | 2 | 0.045 |
In the PCa group, comparison of parameters between high grade (Gleason > 7, n = 5) versus non-high grade (Gleason ≤ 7, n = 10) tumors, showed no differences in age, tissue element levels and ratios of elements (p values >0.05). PSA was significantly (p < 0.0001) higher in high grade tumors as compared to low grade tumors. There was no correlation of element levels or their ratios with age, PSA (in PCa group) or size of prostate gland (in BPH group).
Discussion
Obtained concentration ranges for various elements in our study were similar to that reported in literature for wet benign and malignant prostatic tissues [10, 12, 13]. Of the five measured elements, a significant difference was noticed in the concentration of Se and Zn only between PCa and BPH groups. While higher levels of Fe and Cu have been reported in diet [14], studies noted an insignificant difference in the levels of these elements in prostatic tissue [10, 12], blood [15] and hair [16] of PCa patients as compared to BPH patients. In malignant prostatic tissue, some [10] reported a significantly lower Ca level, whereas others [12] reported a significantly higher Ca concentration as compared to BPH. Hence, our findings revealing insignificant differences in Fe, Cu and Ca concentration between PCa and BPH tissue conform to these reports.
Reports regarding the prostatic tissue selenium changes in PCa versus BPH have been somewhat conflicting. Guntupalli et al. [17] showed significantly lower Se levels in PCa and BPH both (vs. normal prostatic tissue) with more significant decrease in case of cancer. In contrast, Zachara et al. [18] found significantly (p < 0.01) higher Se level in PCa as compared to both healthy controls and benign prostate hyperplasia. Zaichick and Zaichick [8] found Se to be significantly higher in BPH tissue as compared to normal and malignant tissues. In our study, Selenium level was found to be significantly lower (p = 0.01) in PCa versus BPH. Although, observational epidemiological studies suggest an inverse association between selenium and risk of prostate cancer [19, 20], randomized controlled trials of selenium supplementation have reported conflicting results [21, 22]. In the Selenium and Vitamin E Cancer Prevention Trial (SELECT) clinical trial, selenium supplementation had no effect on prostate cancer risk [23]. Rather it might increase the risk of high-grade PCa among men with high selenium status [24]. Among men with non-metastatic prostate cancer, there is suggestive evidence that genetic variation in selenoproteins and related antioxidant enzymes may be associated with risk of high-grade disease at diagnosis and prostate cancer recurrence [25].
We also noted a lower (p = 0.043) Zn level in malignant tissue similar to many studies showing a significantly lower tissue zinc levels in prostate cancer as compared to normal prostatic tissue [9, 10, 13, 17] or BPH tissue [9, 10, 13]. By contrast, isolated studies have reported a significantly higher tissue level of zinc in PCa as compared to BPH [12] and normal prostatic tissue [27] or an insignificantly lower level of zinc level in PCa as compared to normal prostatic tissue [28]. There are conflicting reports about tissue zinc levels in BPH as well; some found a decrease [17] while others an increase [13] or no change [18, 26] as compared to normal prostatic tissue. The potential reasons for a variable difference in tissue Zn level between PCa and BPH in studies may be—unwanted possible representative tissue sampling errors or ethnic related. Despite the evidence [29] for decrease in serum and tissue Zn as well as significance of zinc in PCa in experimental studies, the epidemiological data have shown mixed results between zinc status and PCa, varying from direct [30–32] to inverse [33–35] correlation and no significant correlation [7, 36]. Higher levels of zinc supplementation have been shown to increase prostatic tissue zinc levels and induce intraepithelial carcinoma prostate in murine model [37].
Inconsistency of Zn and inefficacy of Se in significantly altering the natural history of prostate cancer suggest that permissive, balancing and differential roles of various other elements may be equally important in the complex process of carcinogenesis. In order to probe this complexity of interdependence of various elements, we compared the ratios and interrelationship among various elements and elemental ratios in PCa versus BPH.
To the best of our knowledge, the only study available on the changes in ratios of metal ions in BPH or PCa is by Sapota et al. [10] for Zn, se, Cu, Fe and Ca. Of these, they reported 2.4 times lower Zn/Cu ratio (53 vs. 130), 2.9 times lower Zn/Se ratio (440 vs. 1290), 2 times lower Ca/Zn (1/7 vs. 1/3.5) and 1.2 times (8.2 vs. 10) lower Cu/Se ratio in PCa as compared to BPH. No significant differences (p values >0.05) for these ratios between PCa and BPH was observed in the present study. Among ratios, the most remarkable difference in the present study was a lower Zn/Fe ratio for PCa (vs. BPH) (p = 0.04). In the present study, somewhat higher Fe/Se and Ca/Se (p = 0.068 and 0.08 respectively) ratio in PCa (vs. BPH) could be noticed. So, there seems to be a relative deficiency of Zn and/or Se with regard to Fe in PCa tissue. Significance of this imbalance of these elements for prostate carcinogenesis needs further confirmation and exploration by larger studies.
There is scarcity of published data referring to inter-elemental correlations of various elements in normal, benign and malignant prostates [8, 9, 38]. Among Se, Zn and Fe, Zaichick and Zaichick [8] reported a positive trilateral correlation with each other in normal prostatic tissue, no correlation at all in BPH and a positive correlation of Zn with Se in PCa. However, in another study on prostatic tissues of healthy males, Zaichick [38] reported a significant positive correlation of the prostatic Zn with Ca, Cu, and Fe content and no correlation with Se. In our study, Zn was found to have positive correlations with Ca, Cu, Fe and Se in case of PCa and with Fe only in case of BPH. Se had a direct positive correlation with Zn (r = 0.71, p = 0.02) and Fe (r = 0.77, p = 0.009) in cancerous tissue. The positive correlation between Zn and Se in malignant tissue seems a consistent phenomenon as it was observed by Zaichick [8] as well. This interdependence between Se and Zn may partially explain the similar inconsistency of Se or Zn alone in preventing PCa. Fe had positive correlation with both Se and Zn in PCa as compared to BPH where it had positive correlation with Zn only. The significance of the noted positive correlation of Fe with Se in cancerous tissue needs further confirmation and exploration as this correlation (r = 0.76, p = 0.009) differed significantly (Z score = 2.15, p = 0.03) from an insignificant (r = −0.13, p = 0.7) correlation between them in BPH.
Thus, inability to retain high levels of Se and Zn in comparison to Fe may lead to imbalance between oxygen free radicals and antioxidative defense and thus possibly initiate and promote prostate carcinogenesis by oxidative DNA damage. Zn and Se deficiency may also lead to impaired immune function of cells. As Se and Zn supplementation alone have not resulted in consistent anticancer effects, targeting the intracellular Fe could be a potentially useful additional strategy. Recently, desferrioxamine by causing Fe depletion has been shown to suppress the critical oncogenic STAT3 (signal transducer and activator3) activity in vivo and vitro, on various cancer cell lines including those of prostate cancer [39].
Limitations of our study are small sample size and lack of normal prostate controls. Reasons for small sample size were; funding restrictions, the lesser prevalence of PCa in our geographical area, inclusion of the only samples where an adequate amount (at least 1 g) of representative tissue could be collected and inclusion of patients who didn’t have any hormonal manipulation (like 5 alpha reductase inhibitors for BPH and hormonal ablation for PCa) prior to tissue sampling. Obtaining a normal prostate tissue from healthy age matched controls would have been an ethical issue for the study.
Despite these limitations, our study confirms the considerably divergent interrelationship of elements and their ratios in PCa versus BPH as noted by others as well [40]. Further understanding of such complex interrelationship of elements in carcinoma prostate may raise implications for anticancer strategies by either targeting the appropriate trace elements directly to produce an unfavourable environment of elements for carcinogenesis or exploiting these changes for new targeted therapies.
Conclusions
The malignant prostatic tissue has significantly lower zinc and selenium level, a lower Zn/Fe ratio and a positive direct correlation of Se with Zn and Fe as compared to benign prostatic hyperplasia tissue. Although importance of these findings either as a cause or effect of pathological process remains to be further investigated, understanding of such relative and differential elemental changes and their interdependence may be useful in defining the complex metabolic alterations in prostate carcinogenesis with potential for development of element based diagnostic, preventive or therapeutic strategies.
Acknowledgments
Authors are thankful to King George’s Medical University, Lucknow, India for providing funding for this Intramural Research project.
Conflict of interest
Bhupendra Pal Singh, Shailendra Dwivedi, Urmila Dhakad, Ramesh Chandra Murthy, Vimal Kumar Choubey, Apul Goel and Satya Narayan Sankhwar declare that they have no conflict of interest.
Funding
This study was funded by Intramural Research Grant Fund from King George’s Medical University, Lucknow, India. (KGMU/Res.cell/Uro/10/Ref.Code: XLI ECM/B-P9).
Ethical standard
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Vinceti M, Venturelli M, Sighinolfi C, TrerotoliP Bonvicini F, Ferrari A, et al. Case-control study of toenail cadmium and prostate cancer risk in Italy. Sci Total Environ. 2007;373:77–78. doi: 10.1016/j.scitotenv.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Platz EA, Helzlsouer KJ. Selenium, zinc, and prostate cancer. Epidemiol Rev. 2001;23:93–101. doi: 10.1093/oxfordjournals.epirev.a000801. [DOI] [PubMed] [Google Scholar]
- 3.Arnold WN. Selenium and the prostate. J Appl Biomed. 2005;3:59–66. [Google Scholar]
- 4.Karimi G, Shahar S, Homayouni N, Rajikan R, Abu Bakar NF, Othman MS. Association between trace element and heavy metal levels in hair and nail with prostate cancer. Asian Pac J Cancer Prev. 2012;13:4249–4253. doi: 10.7314/APJCP.2012.13.9.4249. [DOI] [PubMed] [Google Scholar]
- 5.Kaba M, Pirincci N, Yuksel MB, Gecit I, Gunes M, Ozveren H, et al. Serum levels of trace elements in patients with prostate cancer. Asian Pac J Cancer Prev. 2014;15:2625–2629. doi: 10.7314/APJCP.2014.15.6.2625. [DOI] [PubMed] [Google Scholar]
- 6.Klein EA, Thompson IM. Chemoprevention of prostate cancer: an updated view. World J Urol. 2012;30:189–194. doi: 10.1007/s00345-011-0822-9. [DOI] [PubMed] [Google Scholar]
- 7.Park SY, Wilkens LR, Morris JS, Henderson BE, Kolonel LN. Serum zinc and prostate cancer risk in a nested case-control study: the multiethnic cohort. Prostate. 2013;73:261–266. doi: 10.1002/pros.22565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaichick S, Zaichick V. Trace elements of normal, benign hypertrophic and cancerous tissues of the human prostate gland investigated by neutron activation analysis. Appl Radiat Isot. 2012;70:81–87. doi: 10.1016/j.apradiso.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 9.Picurelli L, Olcina PV, Roig MD, Ferrer J. Determination of Fe, Mg, Cu, and Zn in normal and pathological prostatic tissue. Actas Urol Esp. 1991;15:344–350. [PubMed] [Google Scholar]
- 10.Sapota A, Darago A, Taczalski J, Kilanowicz A. Disturbed homeostasis of zinc and other essential elements in the prostate gland dependent on the character of pathological lesions. Biometals. 2009;22:1041–1049. doi: 10.1007/s10534-009-9255-y. [DOI] [PubMed] [Google Scholar]
- 11.Neugebauer EA, Sans Cartier GL, Wakeford BJ. Methods for the determination of metals in wildlife tissues using various atomic absorption spectrophotometry techniques. Cat.2000: CW69-5/337E.
- 12.Yaman M, Atici D, Bakirdere S, Akdeniz I. Comparison of trace metal concentrations in malign and benign human prostate. J Med Chem. 2005;48:630–634. doi: 10.1021/jm0494568. [DOI] [PubMed] [Google Scholar]
- 13.Christudoss P, Selvakumar R, Fleming JJ, Gopalakrishnan G. Zinc status of patients with benign prostatic hyperplasia and prostate carcinoma. Indian J Urol. 2011;27:14–18. doi: 10.4103/0970-1591.78405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goluch-Koniuszy Z, Rygielska M, Nowacka I. Nutritional status and nutritional habits of men with benign prostatic hyperplasia or prostate cancer—preliminary investigation. Acta Sci Pol Technol Aliment. 2013;12:319–330. [PubMed] [Google Scholar]
- 15.Adedapo KS, Arinola OG, Shittu OB, Kareem OI, Okolo CA, Nwobi LN. Diagnostic value of lipids, total antioxidants, and trace metals in benign prostate hyperplasia and prostate cancer. Niger J Clin Pract. 2012;15:293–297. doi: 10.4103/1119-3077.100623. [DOI] [PubMed] [Google Scholar]
- 16.Quyang SY, Li SL. Investigations of trace elements in hair of patients with prostate carcinoma, benign prostatic hypertrophy, and normal controls. Hunan Yi Ke Da Xue Xue Bao. 2000;25:279–280. [PubMed] [Google Scholar]
- 17.Guntupalli JN, Padala S, Gummuluri AV, Muktineni RK, Byreddy SR, Sreerama L, et al. Trace elemental analysis of normal, benign hypertrophic and cancerous tissues of the prostate gland using the particle-induced X-ray emission technique. Eur J Cancer Prev. 2007;16:108–115. doi: 10.1097/01.cej.0000228409.75976.b6. [DOI] [PubMed] [Google Scholar]
- 18.Zachara BA, Szewczyk-Golec K, Wolski Z, Tyloch J, Skok Z, Bloch-Boguslawska E, et al. Selenium level in benign and cancerous prostate. Biol Trace Elem Res. 2005;103:199–206. doi: 10.1385/BTER:103:3:199. [DOI] [PubMed] [Google Scholar]
- 19.Hurst R, Hooper L, Norat T, Lau R, Aune D, Greenwood DC, et al. Selenium and prostate cancer: systematic review and meta-analysis. Am J Clin Nutr. 2012;96:111–122. doi: 10.3945/ajcn.111.033373. [DOI] [PubMed] [Google Scholar]
- 20.Geybels MS, Verhage BA, van Schooten FJ, Goldbohm RA, van den Brandt PA. Advanced prostate cancer risk in relation to toenail selenium levels. J Natl Cancer Inst. 2013;105:1394–1401. doi: 10.1093/jnci/djt186. [DOI] [PubMed] [Google Scholar]
- 21.Vinceti M, Dennert G, Crespi CM, Zwahlen M, Brinkman M, Zeegers MP, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2014;3:CD005195. doi: 10.1002/14651858.CD005195.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandair D, Rossi RE, Pericleous M, Whyand T, Caplin ME. Prostate cancer and the influence of dietary factors and supplements: a systematic review. Nutr Metab(Lond) 2014 doi: 10.1186/1743-7075-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) JAMA. 2009;301:39–51. doi: 10.1001/jama.2008.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristal AR, Darke AK, Morris JS, Tangen CM, Goodman PJ, Thompson IM, et al. Baseline selenium status and effects of selenium and vitamin e selenium supplementation on prostate cancer risk. J Natl Cancer Inst. 2014 doi: 10.1093/jnci/djt456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerstenberger JP, Bauer SR, Van Blarigan EL, Sosa E, Song X, Witte JS, et al. Selenoprotein and antioxidant genes and the risk of high-grade prostate cancer and prostate cancer recurrence. Prostate. 2015;75:60–69. doi: 10.1002/pros.22892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaichick VYe, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29:565–574. doi: 10.1007/BF02552202. [DOI] [PubMed] [Google Scholar]
- 27.Banas A, Kwiatek WM, Banas K, Gajda M, Pawlicki B, Cichocki T. Correlation of concentrations of selected trace elements with Gleason grade of prostate tissues. J Biol Inorg Chem. 2010;15:1147–1155. doi: 10.1007/s00775-010-0675-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryś M, Nawrocka AD, Miekoś E, Zydek C, Foksiński M, Barecki A, et al. Zinc and cadmium analysis in human prostate neoplasms. Biol Trace Elem Res. 1997;59:145–152. doi: 10.1007/BF02783239. [DOI] [PubMed] [Google Scholar]
- 29.Gumulec J, Masarik M, Adam V, Eckschlager T, Provaznik I, Kizek R. Serum and tissue zinc in epithelial malignancies: a meta-analysis. PLoS ONE. 2014;18(9):e99790. doi: 10.1371/journal.pone.0099790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leitzmann MF, Stampfer MJ, Wu K, Colditz GA, Willett WC, Giovannucci EL. Zinc supplement use and risk of prostate cancer. J Natl Cancer Inst. 2003;95:1004–1007. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Coogan P, Palmer JR, Strom BL, Rosenberg L. Vitamin and mineral use and risk of prostate cancer: the case-control surveillance study. Cancer Causes Control. 2009;20:691–698. doi: 10.1007/s10552-008-9282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallus S, Foschi R, Negri E, Talamini R, Franceschi S, Montella M, et al. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol. 2007;52:1052–1056. doi: 10.1016/j.eururo.2007.01.094. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez A, Peters U, Lampe JW, White E. Zinc intake from supplements and diet and prostate cancer. Nutr Cancer. 2009;61:206–215. doi: 10.1080/01635580802419749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Vitamin and mineral supplement use is associated with reduced risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:887–892. [PubMed] [Google Scholar]
- 35.Wagner SE, Burch JB, Hussey J, Temples T, Bolick-Aldrich S, Mosley-Broughton C, et al. Soil zinc content, groundwater usage, and prostate cancer incidence in South Carolina. Cancer Causes Control. 2009;20:345–353. doi: 10.1007/s10552-008-9248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platz EA, Helzlsouer KJ, Hoffman SC, et al. Prediagnostic toenail cadmium and zinc and subsequent prostate cancer risk. Prostate. 2002;52:288–296. doi: 10.1002/pros.10115. [DOI] [PubMed] [Google Scholar]
- 37.Ko YH, Woo YJ, Kim JW, Choi H, Kang SH, Lee JG, et al. High-dose dietary zinc promotes prostate intraepithelial neoplasia in a murine tumor induction model. Asian J Androl. 2010;12:164–170. doi: 10.1038/aja.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zaichick V, Nosenko S, Moskvina I. The effect of age on 12 chemical element contents in the intact prostate of adult men investigated by inductively coupled plasma atomic emission spectrometry. Biol Trace Elem Res. 2012;147:49–58. doi: 10.1007/s12011-011-9294-4. [DOI] [PubMed] [Google Scholar]
- 39.Lui GY, Kovacevic Z, Menezes S, Kalinowski DS, Merlot AM, Sahni S, et al. Novel Thiosemicarbazones Regulate the Signal Transducer and Activator of Transcription 3 (STAT3) Pathway: inhibition of Constitutive and Interleukin 6 (IL6)—nduced Activation by Iron Depletion. Mol Pharmacol. 2015;87:543–560. doi: 10.1124/mol.114.096529. [DOI] [PubMed] [Google Scholar]
- 40.Qayyum MA, Shah MH. Comparative study of trace elements in blood, scalp hair and nails of prostate cancer patients in relation to healthy donors. Biol Trace Elem Res. 2014;162:46–57. doi: 10.1007/s12011-014-0123-4. [DOI] [PubMed] [Google Scholar]