Ullman and Spencer-Smith et al. use neonatal structural MRI to predict cognitive and academic functioning at 5 and 7 years in very preterm children. Univariable and multivariable models reveal associations between the neonatal volume of regions in the vicinity of the insula and putamen, white matter microstructure and childhood working memory and mathematical skills.
Keywords: prematurity, deformation based morphometry, diffusion tensor imaging, working memory, mathematics
Ullman and Spencer-Smith et al. use neonatal structural MRI to predict cognitive and academic functioning at 5 and 7 years in very preterm children. Univariable and multivariable models reveal associations between the neonatal volume of regions in the vicinity of the insula and putamen, white matter microstructure and childhood working memory and mathematical skills.
Abstract
School-age children born preterm are particularly at risk for low mathematical achievement, associated with reduced working memory and number skills. Early identification of preterm children at risk for future impairments using brain markers might assist in referral for early intervention. This study aimed to examine the use of neonatal magnetic resonance imaging measures derived from automated methods (Jacobian maps from deformation-based morphometry; fractional anisotropy maps from diffusion tensor images) to predict skills important for mathematical achievement (working memory, early mathematical skills) at 5 and 7 years in a cohort of preterm children using both univariable (general linear model) and multivariable models (support vector regression). Participants were preterm children born <30 weeks’ gestational age and healthy control children born ≥37 weeks’ gestational age at the Royal Women’s Hospital in Melbourne, Australia between July 2001 and December 2003 and recruited into a prospective longitudinal cohort study. At term-equivalent age ( ±2 weeks) 224 preterm and 46 control infants were recruited for magnetic resonance imaging. Working memory and early mathematics skills were assessed at 5 years (n = 195 preterm; n = 40 controls) and 7 years (n = 197 preterm; n = 43 controls). In the preterm group, results identified localized regions around the insula and putamen in the neonatal Jacobian map that were positively associated with early mathematics at 5 and 7 years (both P < 0.05), even after covarying for important perinatal clinical factors using general linear model but not support vector regression. The neonatal Jacobian map showed the same trend for association with working memory at 7 years (models ranging from P = 0.07 to P = 0.05). Neonatal fractional anisotropy was positively associated with working memory and early mathematics at 5 years (both P < 0.001) even after covarying for clinical factors using support vector regression but not general linear model. These significant relationships were not observed in the control group. In summary, we identified, in the preterm brain, regions around the insula and putamen using neonatal deformation-based morphometry, and brain microstructural organization using neonatal diffusion tensor imaging, associated with skills important for childhood mathematical achievement. Results contribute to the growing evidence for the clinical utility of neonatal magnetic resonance imaging for early identification of preterm infants at risk for childhood cognitive and academic impairment.
Introduction
Preterm birth is of major importance, with the proportion of infants born preterm continuing to grow and an increasing survival rate of those born very preterm (<32 weeks’ gestational age) and extremely preterm (<28 weeks’ gestational age) (Doyle et al., 2010; Costeloe et al., 2012; Howson et al., 2012). It is well documented that these children experience reduced cognitive and academic abilities compared with their term-born peers (Taylor et al., 2002; Anderson and Doyle, 2003; Aarnoudse-Moens et al., 2009; Johnson et al., 2009; Hutchinson et al., 2013; Simms et al., 2013b), and this disadvantage persists across the school years (Aarnoudse-Moens et al., 2009). Teacher ratings of academic attainment suggest over one-half of extremely preterm children attending mainstream school have a special education need or provision (Johnson et al., 2009, 2011). At school age preterm children are at increased risk for low achievement in literacy, reading and in particular mathematics (Taylor et al., 2002; Anderson and Doyle, 2003; Litt et al., 2005; Johnson et al., 2011).
A concerning proportion of preterm children suffer significant mathematical impairment, with large cohort studies reporting 10–18% of extremely preterm children perform more than 2 standard deviations (SD) below age expectations on standardized math tests (Anderson and Doyle, 2003; Johnson et al., 2011; Hutchinson et al., 2013). Many more preterm children experience mild mathematical impairment, with reports that up to 66% of extremely preterm children perform 1 to 2 SD below age expectations (Anderson and Doyle, 2003; Hutchinson et al., 2013), and these children might not raise sufficient concern to receive referral and intervention. High rates of school-age impairments in mathematics are concerning because of the long-term implications, including grade repetition, reduced school completion, and increased behavioural and emotional problems in childhood (Heath and Ross, 2000; Hudson et al., 2009), as well as reduced post-secondary school education, employment opportunities, and increased mental health and well-being problems in adulthood (Levine and Nourse, 1998; Hudson et al., 2009).
Mathematical achievement involves several skills. In preterm children low mathematical achievement has been consistently associated with reduced executive functions, and in particular working memory (Taylor et al., 2002; Mulder et al., 2010; Litt et al., 2012; Simms et al., 2015), which is the ability to hold a limited amount of information in mind and work with it over a short period of time. There is some suggestion that low mathematical achievement might also reflect difficulties in numerical skills such as number sequencing, identification and place value (Pritchard et al., 2009; Simms et al., 2013a, b), and estimation (Simms et al., 2013a, b). Recent work suggests different underlying associations between numerical skills and mathematical achievement in preterm and term-born children, with the association persisting after controlling for cognitive ability in 11-year-old preterm but not term-born children (Simms et al., 2013b).
Identifying early markers and methods for classifying preterm infants at risk for low mathematical achievement at school age would assist in advising families regarding referral to early intervention and on-going surveillance for educational difficulties. Establishing early markers for long-term outcome is particularly important given the prolonged developmental trajectory of skills important for mathematical achievement. Neonatal MRI, which describes a common pattern of diffuse white matter damage in preterm infants (Inder et al., 2005), is a promising marker of long-term cognitive outcomes. Various qualitative, manual and automated MRI methods using neonatal MRI have been associated with a range of cognitive skills at school-age, even after accounting for perinatal factors known to influence neurobehavioural development. For example, qualitative abnormalities on neonatal MRI have been associated with childhood general cognitive ability (Woodward et al., 2012), language abilities (Reidy et al., 2013) and working memory (Omizzolo et al., 2013), while reduced hippocampal volume has been associated with poorer memory function (Thompson et al., 2013).
MRI measures based on automated methods are appealing for large data sets and clinical grading because they provide a standardized and efficient method of analysis without time consuming measurements or visual assessments. Two structural MRI measures that are sensitive to brain development and pathology are structural Jacobian maps derived from deformation-based morphometry (DBM) (Ashburner et al., 1998) and fractional anisotropy maps derived using diffusion tensor imaging (DTI) (Basser and Pierpaoli, 1996). DBM is a method for identifying subtle macroscopic differences in brain shape and volume between different populations, and findings are described and interpreted as reflecting regional volumetric differences (Boardman et al., 2003, 2006). DTI characterizes water diffusion properties in the brain that are highly sensitive to differences in tissue microstructure between populations, and findings have been interpreted as reflecting white matter integrity (Basser and Pierpaoli, 1996). Thus, DBM and DTI are complimentary methods. Their application to neonatal magnetic resonance images using a whole brain analysis approach for predicting subsequent cognitive skills has not yet been examined in preterm populations.
Univariable statistical models, such as a general linear model (GLM), can be used to perform mass univariable tests at each voxel in order to examine associations between neuroimaging and cognitive measures (Woodward et al., 2006, 2012; Omizzolo et al., 2013; Reidy et al., 2013; Thompson et al., 2013). Multivariable models, which take all voxels of an image into account simultaneously, might be better suited to examine multivariable effects in large data sets, and might optimize the predictive ability of neonatal neuroimaging data. Machine learning algorithms, such as support vector regression (SVR), aim to model complex relationships in multivariable and often noisy data sets, which is generally the case for higher order cognitive functions that are underpinned by spatially different brain regions. For example, working memory relies on an integrated network of frontal and parietal regions in both healthy populations (Olesen et al., 2003; Short et al., 2013; Spencer-Smith et al., 2013) and in preterm children (Mürner-Lavanchy et al., 2014). In a recent study of working memory development using SVR models, fractional anisotropy, blood oxygen level-dependent (BOLD) and grey matter volume data were associated with working memory 2 years later in typically developing children, with fractional anisotropy maps from DTI showing the strongest prediction, r = 0.59, P < 0.001 (Ullman et al., 2014).
The current study aims to examine whether neonatal structural MRI measures derived from automated methods (Jacobian maps from DBM, fractional anisotropy maps from DTI) are associated with later childhood skills important for mathematical functioning, including working memory and early mathematical skills, in a cohort of preterm children using both univariable and multivariable models. We examine outcome at preschool age (5 years) and early school age (7 years).
Materials and methods
Participants
This study included participants in a prospective longitudinal cohort of children born <30 weeks’ gestational age and/or <1250 g at the Royal Women’s Hospital in Melbourne, Australia between July 2001 and December 2003. A total of 224 preterm infants (65% of those eligible, n = 348) were recruited (for details see Thompson et al., 2014). Infants with congenital anomalies were excluded (3%). Clinical data were collected during the perinatal period. Neurodevelopmental assessments were conducted at 2 years (n = 220), 5 years (n = 195) and 7 years (n = 198) of age, corrected for prematurity (Wilson-Ching et al., 2014). A control group of 46 healthy term-born infants (born ≥37 weeks’ gestational age) was also recruited for whom the same clinical data were collected and neurodevelopmental assessments conducted. Informed parental consent was obtained from all participants. All phases of the longitudinal study were approved by the Research and Ethics Committees at the Royal Children’s and Women’s Hospital, Melbourne. Cognitive profiles (Omizzolo et al., 2013; Murray et al., 2014) and neuroimaging findings (Thompson et al., 2014) have been previously reported, showing significantly reduced general intellectual functioning in very preterm compared with term-born controls.
MRI acquisition
Brain MRI was acquired at term equivalent age (40 weeks’ gestational age ± 2 weeks) in a 1.5 T General Electric MRI scanner using a birdcage quadrature 4-channel head coil in all 224 preterm infants and 46 controls. Prior to scanning the infants were fed, swaddled and immobilized in a vacuum fixation bean bag without sedation and then scanned during natural sleep. Data acquired included whole brain structural 3D T1 spoiled gradient recalled images (0.8–1.6 mm coronal slices; flip angle 45°; repetition time 35 ms; echo time 9 ms; field of view 210 × 157 mm; matrix 256 × 192; in plane voxel dimensions 0.82 mm2), T2 dual echo fast spin echo images with interleaved acquisition (1.7–3 mm coronal slices; repetition time 4000 ms; echo time 60/160 ms; field of view 220 × 165 mm; matrix 256 × 192, interpolated 512 × 512; in plane voxel dimensions 0.43 mm2), and line-scan diffusion images were acquired in 105 of the 224 infants (4–6 mm axial slices; two baselines, b = 5 s/mm2; six non-collinear gradient directions, b = 700 s/mm2; in plane voxel dimensions 0.86 mm2). T2 images were visually inspected and images with motion artefacts to the extent that the border between white and grey matter could not be visually distinguished were excluded (n = 5).
Cognitive outcomes at 5 and 7 years
Neurodevelopmental assessments at 5 and 7 years’ corrected age were conducted by clinicians blinded to perinatal data, parent interviews, and medical record review. Following is a description of the measures relevant to this study drawn from a broad assessment protocol.
Working memory
At 5 and 7 years’ corrected age the Backward Digit Span Test from the Working Memory Test Battery for Children (WMTB-C, designed for children 5–15 years; Pickering and Gathercole, 2001) was administered. The child listens to a string of digits and then recalls the digits in the reverse order. The number of digits increases across the test trials. The total number of correct trials is calculated and in this study we used raw scores in analyses.
Early mathematics
Developmentally appropriate measures were administered to estimate early mathematical abilities. At 5 years’ corrected age the Numbers Skills Scale from the Kaufman Survey of Early Academic and Language Skills (K-SEALS; Kaufman and Kaufman, 1993) was administered. This scale includes 20 items asking the child to select or name numbers, count, indicate knowledge of number concepts (e.g. smallest, half) and solve simple oral number problems. At 7 years’ corrected age the Math Computation task from the Wide Range Achievement Test (WRAT-4; Wilkinson and Robertson, 2006) was administered. The child is asked to count, identify numbers, solve simple oral number problems (add, subtract) and written math problems, and a total score is calculated. In analyses we used scaled scores of early mathematics task performances.
We used a conservative approach to address missing outcome data and only included performance scores for children who completed the task, without imputing values for children who failed to attempt or complete the task. Thus, the number of cases differs for each cognitive outcome.
Image preprocessing
Deformation-based morphometry
A total of 175 preterm and 38 control participants were available for inclusion in the DBM analyses. T2 images were used as they demonstrate better tissue contrast within the largely unmyelinated brain of neonates than T1 images. After bias field correction was applied (Tustison et al., 2010), each T2 image was aligned to a template corresponding to 40 weeks’ gestational age (www.brain-development.org) (Serag et al., 2012) using affine transformation. Non-linear registration of the magnetic resonance image to the template was performed to compute the deformation fields, a measure of regional volumetric difference. The Jacobian determinant matrices for the deformation fields were used as input data in the subsequent GLM and SVR analyses. The resulting P-values were displayed as maps to visualize patterns of significant associations with cognitive outcomes throughout the brain. Four infants were removed prior to statistical analysis due to unsatisfactory alignment to the reference template.
For working memory, 146 participants were included in the DBM analysis of Backward Digit Span at 5 years and 147 in the analysis of Backward Digit Span at 7 years. For early mathematics, 157 participants were included in the DBM analysis of Number Skills at 5 years score and 153 in the analysis of Math Computation at 7 years.
Diffusion tensor imaging
The quality of the DTI data was assessed both visually and by examining the distribution of the DTI based SVR predictions. A total of 105 preterm and 21 control participants were available for inclusion in the DTI analyses. After eddy current correction the data were fitted to a six-parameter tensor model (Jenkins et al., 2012). Individual fractional anisotropy maps were calculated. Fractional anisotropy maps were used for normalizing the images rather than T2 sequences or b0 images, as the fractional anisotropy maps demonstrated better contrast between major white matter tracts and surrounding tissue. A group-specific fractional anisotropy template was created in FSL by applying an affine transformation of the individual images to a representative subject with few artefacts. The subsequent images were averaged and used as the fractional anisotropy template. Linear affine transformation was applied to the individual fractional anisotropy maps to match the template. The subsequent spatially normalized fractional anisotropy maps were used in the GLM and SVR analyses.
For working memory, 88 participants were included in the DTI analysis of Backward Digit Span at 5 years and 84 in the analysis of Backward Digit Span at 7 years. For early mathematics, 93 participants were included in the DTI analysis of Number Skills at 5 years score and 87 in the analysis of Math Computation at 7 years.
Statistical analysis
General linear model
A standard mass univariable analysis was performed in FSL version 5.0 (www.fmrib.ox.ac.uk/fsl) using the preprocessed DBM and fractional anisotropy data. Each voxel was set as the dependent variable in a multiple regression model. The independent variables were the working memory and early mathematics variables at 5 and 7 years. The model was then estimated performing permutation testing (n = 10 000) using Randomise as implemented in the FMRIBs Software Library (Smith et al., 2004). After model estimation, contrast images for the outcome variable were calculated and contrast values were compared with the estimated empirical null distribution from the permutation testing. Significance was determined as clusters significant with FWE correction at a level of P < 0.05 using FSL based cluster enhancement for thresholding. Given correction for multiple comparisons is only applied to the P-values and not the correlation coefficients, we report only P-values (Kriegeskorte et al., 2009).
Support vector regression
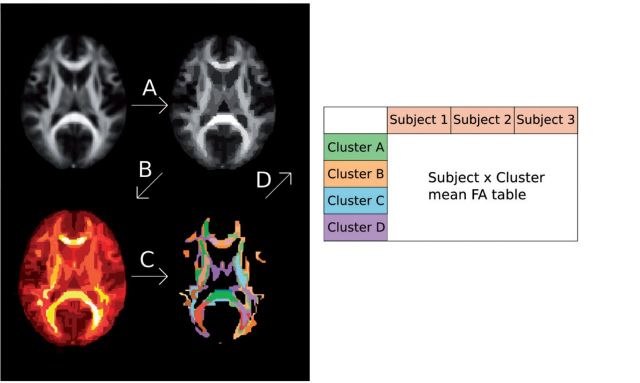
A multivariable pipeline was implemented separately for the preprocessed DBM and fractional anisotropy data. To obtain predictions with SVR that are not biased by overfitting, the SVR model needs to be trained and validated on separate data sets. In this study only one data set was available. We therefore used an internal validation in the form of a cross-validation. All calculations were performed using a leave-one-out cross-validation approach to provide an unbiased estimate of generalization. Parameters were adjusted in the algorithm and then estimated with a nested cross-validation in order to obtain unbiased approximations of the best parameters. This pipeline consisted of three steps (Fig. 1):
Features were agglomerated using a hierarchical clustering with spatial constraints (Ward, 1963). This algorithm calculates a hierarchical tree of the input data based on the Euclidean distance between the features. The tree is thereby cut at an arbitrary level resulting in a set number of clusters. The spatial constraints allow only neighbouring clusters to merge thereby resulting in spatially continuous clusters. The optimal number of clusters was set for each subject using only data from the other subjects, i.e. a nested cross-validation.
The resulting clusters were further pruned using a univariable correlation threshold. Pearson correlation between each cluster and the outcome was calculated. In a nested cross-validation loop the optimal correlation cut-off level was calculated. Only clusters with a higher correlation were subsequently used to train the multivariable model. This ensured that the clusters containing much noise and little relevant information were discarded to increase the model performance.
The clusters that were remaining after the univariable correlation selection were used to train a SVR model. To model the data we used a υ-SVR model, a regression adaptation of the Support Vector Machines. It transforms the initial input features to a higher dimensional kernel space and fits the regression function in this space (Cortes and Vapnik, 1995). Two parameters are set when training the model: υ-parameter and C-parameter. The υ-parameter determines the proportion of support vectors to use, which determines how sparse the model is. A low υ-parameter results in the use of only participants far away from the regression line in the kernel space to derive the coefficients. This can be beneficial when data are noisy and smaller variations close to the regression line are less meaningful. The υ-parameter was adjusted for the data set in a grid search. The second parameter is the C-parameter, which adjusts the regularization of the model. This is useful for reducing the risk of over fitting the model. The higher the C parameter is the more regularization is imposed on the coefficients. For more detailed information about Support Vector Machines see James et al. (2013). Here the C-parameter was set based on previous empirical research (Chalimourda, et al., 2004) to reduce computational load. The level used was the number of samples × the maximum of the dependent variable.
Figure 1.
Schematic overview of data processing prior to SVR modelling. (A) The first step involved hierarchical clustering of the data. (B) Second, correlation coefficients between each cluster and the cognitive outcome variable were calculated, here illustrated as a heat map. (C) Based on this heat map, the highest correlating features were selected. (D) Cluster values for each subject were calculated and transformed into a Subject × Feature matrix. Cross-validation was performed to determine the optimal parameters for each step. FA = fractional anisotropy.
When a significant relationship was found between neonatal MRI and a childhood cognitive outcome in the preterm group, we repeated the analysis in the control group to explore if the results were specific to the preterm population.
Covariates
In this study we acknowledged factors previously shown to influence cognitive development (Woodward et al., 2006), including gestational age at birth, small for gestational age, patent ductus arteriosus, bronchopulmonary dysplasia (requiring oxygen at 36 weeks), administration of postnatal corticosteroids, and confirmed sepsis determined by medical record review. In addition, white matter injury (normal, mild, moderate, severe) was determined based on neonatal MRI review by a qualified neurologist using a structured scale assessing: cystic lesions, focal signal abnormality, myelination in the posterior limb on the internal capsule and corona radiate, callosal thinning, lateral ventricular diameter, and white matter volume measured via biparietal diameter (Kidokoro et al., 2013). Significant injury associated with intraventricular haemorrhage and periventricular leukomalacia are noted in the white matter injury score and were therefore not included as separate covariates. Analyses were performed initially with gestational age at scan included as a covariate, and then with both gestational age at scan and birth (a major mediator of altered brain development that links the risk of adverse neurobehavioural outcome) as covariates, and finally analyses were performed also including all of the clinical perinatal factors as covariates. This approach enabled us to determine if neonatal brain structure measures (DBM and DTI) using univariable and multivariable analysis approaches (GLM and SVR) are unique and therefore useful markers of later cognitive and academic outcome in children born very preterm.
Results
Characteristics of the very preterm participants
Characteristics of the preterm participants who completed neonatal MRI are presented in Table 1. Cognitive assessments were completed at 5 years [mean (M) = 5.04, SD = 0.17, n = 195) and 7 years (M = 7.49, SD = 0.25, n = 197]. Consistent with the literature (Simms et al., 2015), we observed a positive association between working memory and early mathematics scores at 5 and 7 years in this cohort of preterm children: Backward Digit Span at 5 and 7 years (r = 0.45, P < 0.001, n = 156), Number Skills at 5 years and Math Computation at 7 years (r = 0.69, P < 0.001, n = 172), Backward Digit Span and Number Skills at 5 years (r = 0.52, P < 0.001, n = 180), and Backward Digit Span and Math Computation at 7 years (r = 0.54, P < 0.001, n = 179).
Table 1.
Perinatal characteristics and cognitive scores of the preterm group that participated in neonatal MRI
| Descriptive characteristics of the MRI sample | |
|---|---|
| n | 224 |
| Males, n (%) | 114 (50.9) |
| Clinical perinatal characteristics | |
| Gestational age at birth, M (SD) weeks | 27.5 (1.9) |
| Singleton | 58% |
| Birth weight, M (SD) g | 961 (225) |
| Small for gestational age | 8.9% |
| Patent ductus arteriosus | 49.1% |
| Oxygen administered at 36 weeks | 33.5% |
| Postnatal corticosteroid use | 8.8% |
| Sepsis episodes | 43.8% |
| Intraventricular haemorrhage: Grade I / II / III / IV | 3.2% / 5.9% / 1.8% / 1.8% |
| White matter injury: mild / moderate / severe | 36.2% / 14.5% / 5.4% |
| Gestational age at MRI, M (SD) weeks | 40.3 (1.7) |
| General intelligence | |
| Full-scale IQ at 7 years, M (SD), % impaired e | 97.1 (13.8), 18.4% |
| Working memory | |
| Backward Digit Span at 5 years, M (SD), % impaired a | 76.7 (13.1), 68.5% |
| Backward Digit Span at 7 years, M (SD), % impaired b | 87.4 (16.7), 52.5% |
| Early mathematics | |
| Number Skills at 5 years, M (SD), % impaired c | 97.4 (12.6), 15.5% |
| Math Computation at 7 years, M (SD), % impaired d | 89.7 (17.8), 38.8% |
a n = 181; b n = 177; c n = 193; d n = 188; e n = 190.
Standard scores are reported for working memory and early mathematics. Impairment defined as <−1 SD relative to the test mean of 100.
Deformation-based morphometry
General linear model
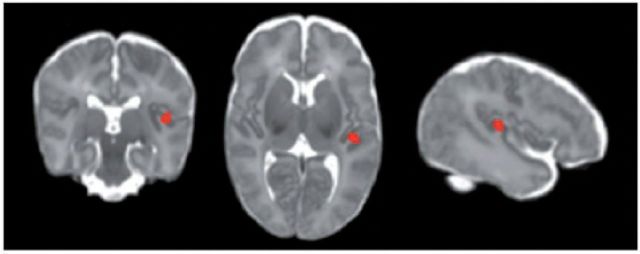
For working memory, the preterm neonatal Jacobian map was not significantly associated with Backward Digit Span at 5 years after correcting for gestational age at scan (n = 146, all P > 0.10), nor when both gestational age at scan and birth were covaried, or after additionally accounting for clinical perinatal factors. The association between the neonatal Jacobian map and Backward Digit Span at 7 years approached significance after correcting for gestational age at scan (n = 147, P = 0.07). After correcting for both gestational age at scan and birth, increasing tissue volume in the left insula region of the neonatal Jacobian map was associated with higher Backward Digit Span scores at 7 years (Fig. 2; FDR-corrected for multiple comparisons at P < 0.05, cluster size 603 voxels), and this association approached significance after correcting for clinical perinatal factors (P = 0.07).
Figure 2.
Localized region in the neonatal Jacobian map associated with working memory using GLM. FDR-corrected P < 0.05. Increasing tissue volume in the left insula region was associated with higher Backward Digit Span score at 7 years corrected age after adjusting for gestational age at scan and birth.
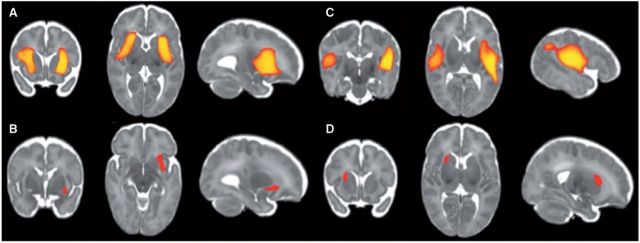
For early mathematics, the preterm neonatal Jacobian map was significantly associated with number skills at 5 years after correcting for gestational age at scan (FDR-corrected for multiple comparisons at P < 0.05, n = 146, size of first cluster 17 750, size of second cluster 17 726 voxels) (Fig. 3A), and this pattern of association remained significant after correcting for both gestational age at scan and birth (FDR-corrected for multiple comparisons at P < 0.05, size of first cluster 13 086 voxels, size of second cluster 12 553 voxels). After accounting for clinical perinatal factors, increasing tissue volume in the left insula region of the neonatal Jacobian map was positively associated with higher number skills scores at 5 years (FDR-corrected for multiple comparisons at P < 0.05, cluster size 421 voxels). Similarly, the neonatal Jacobian map was associated with Math Computation at 7 years after correcting for gestational age at scan (FDR-corrected for multiple comparisons at P < 0.05, size of first cluster 21 591, size of second cluster 8514 voxels) (Fig. 3B), and this pattern of association remained significant after correcting for both gestational age at scan and birth. After accounting for clinical perinatal factors, increasing tissue volume in the right putamen region was associated with higher scores, FDR-corrected for multiple comparisons at P < 0.05, cluster size 731 voxels.
Figure 3.
Localized regions in the neonatal Jacobian map associated with early mathematics using GLM. FDR-corrected P < 0.05. Increasing tissue volume in the insula and putamen regions was associated with higher scores for Number Skills at 5 years corrected age (A) and Math Computation at 7 years corrected age (B) after adjusting for gestational age at scan, and this pattern was also observed after adjusting for gestational age at birth. Increasing tissue volume in the left insula region was associated with better Number Skills at 5 years (C), and increasing tissue volume in the right putamen region was associated with better Math Computation at 7 years (D) after adjusting for gestational age at scan (FDR-corrected P < 0.05).
When analyses were repeated in the control group, no significant associations were found between the neonatal Jacobian map and Backward Digit Span at 7 years (P > 0.2, n = 34), Number Skills at 5 years (P > 0.6, n = 33) or Math Computation at 7 years (P > 0.5, n = 36).
Support vector regression
The preterm neonatal Jacobian map was not significantly associated with working memory using Backward Digit Span at 5 or 7 years, or early mathematics outcomes using Number Skills at 5 years or Math Computation at 7 years (all P > 0.1). This pattern of results was observed after correcting for gestational age at scan, both gestational age at scan and birth, and after correcting for clinical perinatal factors.
In summary, increasing tissue volume in localized regions in the neonatal Jacobian map were positively associated with early mathematics at 5 and 7 years in preterm children using GLM but not SVR. There was some suggestion that increasing tissue volume in localized regions were positively associated with working memory at 7 years using GLM but not SVR.
Diffusion tensor imaging
General linear model
The preterm neonatal fractional anisotropy map was not significantly associated with working memory using Backward Digit Span at 5 or 7 years (all P > 0.65), or early mathematics using Number Skills at 5 years or Math Computation at 7 years (all P > 0.45). This pattern of results was observed after correcting for gestational age at scan, both gestational age at scan and birth, and after correcting for clinical perinatal factors.
Support vector regression
For working memory, the preterm neonatal fractional anisotropy map was positively associated with Backward Digit Span at 5 years after correcting for gestational age at scan (r = 0.36, P < 0.001, n = 88), both gestational age at scan and birth (r = 0.36, P < 0.001, n = 88; Fig. 4), and after correcting for all of the clinical perinatal factors (P < 0.01). However, the neonatal fractional anisotropy map was not associated with Backward Digit Span at 7 years (P > 0.20, n = 84), a pattern of results that was observed after correcting for gestational age at scan, both gestational age at scan and birth, and after correcting for clinical perinatal factors.
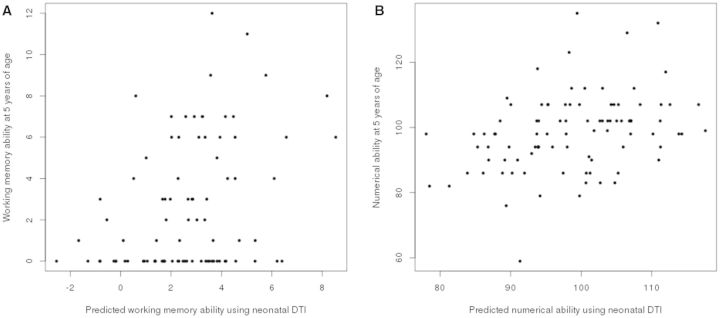
Figure 4.
Correlation between expected and measured working memory and early mathematics at 5 years corrected age based on neonatal DTI data. (A) Scatterplot of expected and measured Backward Digit Span score (r = 0.36, P < 0.001). (B) Scatterplot of expected and measured Number Skills score (r = 0.35, P < 0.001).
For early mathematics, the preterm neonatal fractional anisotropy map was positively associated with Number Skills at 5 years after correcting for gestational age at scan (r = 0.36, P < 0.001, n = 93), both gestational age at scan and birth (r = 0.35, P < 0.001, n = 93; Fig 4), and after correcting for clinical neonatal factors (P < 0.05). The neonatal fractional anisotropy map was not associated with Math Computation at 7 years (P > 0.70, n = 87), a pattern of results that was observed after correcting for gestational age at scan, both gestational age at scan and birth, and after correcting for clinical perinatal factors.
To examine if different associations might be observed for preterm infants with no evidence of white matter injury, we explored the relationship between degree of white matter injury and neonatal fractional anisotropy maps by correlating these measures, and no significant associations were found (P > 0.1). When including white matter injury as a predictor together with the SVR prediction of the DTI, white matter injury did not contribute significantly to the model (P > 0.3).
When analyses were repeated in the control group, no significant predictive ability of the neonatal DTI was found for Number Skills at 5 years (P > 0.3, n = 21) or Backward Digit Span at 5 years (P > 0.7, n = 21).
In summary, neonatal fractional anisotropy maps were positively associated with later working memory and early mathematics at 5 years but not 7 years in preterm children using SVR but not GLM.
Discussion
Using structural MRI measures acquired in the neonatal period, we were able to identify some brain regions that are associated with later childhood working memory and mathematical skills in our cohort of very preterm children. We identified localized regions in the neonatal Jacobian map that were associated with early mathematics using GLM but not SVR. Specifically, increasing tissue volume in the insula and putamen was associated with better early mathematics at 5 and 7 years, and this persisted even after adjusting for important clinical factors known to influence neurobehavioural development. There was some suggestion that increasing tissue volume in the left insula was associated with better working memory at 7 years (but not 5 years) using GLM. The neonatal fractional anisotropy maps were positively associated with working memory and early mathematics at age 5 (but not at 7 years) using SVR but not GLM, and these associations persisted after accounting for relevant clinical factors. Highlighting the robustness of these results, the inclusion of the prediction based on the neonatal fractional anisotropy in the regression model with all neonatal clinical factors increased the amount of variance that could be explained in working memory (R2 0.19 compared with 0.08) and early mathematics (R2 0.27 compared with 0.21) at age 5. The specificity of these results to preterm children was examined by replicating the analyses in the small healthy term-born control group, which did not identify significant associations between neonatal MRI and childhood mathematical skills. Together these results contribute to the growing evidence for the clinical utility of neonatal neuroimaging for identifying preterm infants at-risk for suffering cognitive and academic difficulties in childhood (Woodward et al., 2006, 2012; Omizzolo et al., 2013; Reidy et al., 2013; Thompson et al., 2013), although we acknowledge the techniques used in this study are not currently readily available to be used in a clinical setting. In the future, this knowledge could assist in identifying infants at risk of mild academic impairments who might not raise sufficient concern but would benefit from monitoring and referral to early intervention. Such an approach could assist in reducing the large number of preterm children performing below their peers in mathematics (Anderson and Doyle, 2003; Hutchinson, et al., 2013), and in turn reduce grade repetition, behavioural and emotional problems in childhood, as well as increase post-secondary school education and employment opportunities and reduce mental health and well-being problems in adulthood.
Our findings demonstrate that increased tissue volumes in regions located around the insula and putamen during the neonatal period are associated with better early mathematics in preterm children. A pattern of distributed bilateral regions in the Jacobian map was associated with childhood skills important for mathematical achievement, and only the core of the clusters survived statistical testing when neonatal clinical covariates were included. There is an intrinsic lower anatomical specificity in DBM due to the lack of segmentation (Ashburner et al., 1998). Although we cannot draw conclusions about the specific anatomical locale of the significant clusters from our results, findings are consistent with previous DBM study findings showing reduced volume in thalamus, globus pallidus and putamen in preterm compared with term-born infants at term corrected age (Boardman, et al., 2003, 2006) and observations of reductions in insula and putamen volumes in preterm compared with term-born adults (Nosarti et al., 2002). Of particular interest, insula and putamen are involved in the salience network, a neurocognitive resting state network focused in fronto-insula cortex, dorsal cingulate cortex and subcortical structures (Seeley et al., 2007). While the salience network is reportedly similar in preterm and term-born individuals (Damaraju et al., 2010; White et al., 2014), recent findings suggest a specific role of the insula in the reduced connectivity seen in very preterm compared with term individuals (White et al., 2014). The association we observed between neonatal alterations around the insula and putamen and childhood working memory and early mathematics might be explained by aberrant connectivity between the neurocognitive networks.
Analysis of the neonatal fractional anisotropy map did not identify regions that were associated with childhood working memory and early mathematics using a univariable analysis approach. This is in contrast to previous reports using a regions of interest approach. For example, Progribna and colleagues (2014) identified higher fractional anisotropy in the subventricular zone associated with better general cognitive and language abilities at 18 and 22 months’ corrected age in extremely low birth weight infants (n = 39). In the current study, significant associations were observed using a multivariable analysis approach (all voxels considered simultaneously), suggesting widely distributed regions relating to childhood working memory and early mathematics. Our results would support the further development of methods of neonatal DTI multivariable analysis approaches for the early identification of preterm infants destined for future cognitive and academic difficulties.
We used two different analytic approaches to examine the associations of neonatal MRI measures and childhood cognitive skills important for mathematical functioning: GLM (a univariable algorithm) and SVR (a multivariable algorithm), which have unique benefits for examining different types of data. The univariable method analyses each voxel separately and will therefore be independent of noise in other voxels as well as the number of voxels when fitting the regression function. However, univariable models are not able to take into account noisy signal distributed across many voxels, which independently is not sufficient for significance. Multivariable methods might be able to use the distributed signal over many voxels as well as examine the relationship between the voxels. However, multivariable models can suffer from contamination of noise from other non-informative voxels, which can lead to a lower performing model if the effect is univariable and spatially localized. The possible differences in results between the univariable and multivariable models are of interest; however, results cannot be compared statistically.
We examined skills important for mathematical achievement in children at 5 (preschool age) and 7 years of age (school age), which are rapid stages in neurological and cognitive development. For example, in prefrontal cortex synaptic elimination continues across these ages and myelination is increasing with a peak at ∼7–9 years, thought to reflect interregional connectivity important for cognitive development (for a review see Spencer-Smith and Anderson, 2009). Our pattern of results might in part reflect differences in developmental process occurring at 5 and 7 years of age. In addition, the pattern of results might reflect varying sensitivities of the measures we used to estimate working memory and early mathematics. Working memory was estimated by performance on the Backward Digit Span test. Although the same measure was administered at 5 and 7 years, it is possible the test elicits different information at different ages, e.g. the pattern of performances was negatively skewed at 5 years and normally distributed at 7 years. It could be considered a limitation that we used a working memory test involving digits, given mathematical impairment might reflect reduced number knowledge. Early mathematics was estimated by performance at age 5 by the Number Skills scale (K-SEALS) and at age 7 by the Math Computation subtest (WRAT-4). Both tests summarize the child’s ability to count, recognize numbers, and solve simple math problems. In addition the scores for children at 5 years incorporated knowledge of number concepts such as smallest and half, and scores for children at 7 years incorporated written math test performance, reflecting developmentally appropriate functioning. Different measures of early mathematics at 5 and 7 years might have influenced the pattern of results, however, as expected, we observed a high correlation between Number Skills and Math Computation scores (r = 0.69, P < 0.001). Using tests of early mathematics that provide a summary of several skills important for mathematical performance limited our potential to examine the nature of mathematical impairments in very preterm children. However, it is worth noting that performance on working memory and early mathematics tests were correlated, but were differentially associated with neonatal MRI measures, suggesting that performance on these tests provide some common as well as unique information about skills important for mathematical performance in very preterm children.
We repeated analyses with significant findings in the healthy term-born control group. Results for the DTI and the DBM analyses in the control group did not replicate the significant results observed in the preterm sample. However, due to the lower number of children in the control group it is difficult to draw conclusions regarding the presence or absence of a predictive effect in the neonatal magnetic resonance images of healthy term-born children. Based on previous studies (Short et al., 2013; Ullman et al., 2014) prediction of cognitive development is to some degree expected in healthy children. Further studies are needed to clarify whether there is a difference in the amount of variance that can be predicted in healthy term-born and preterm children. However, the usefulness of cognitive predictions may be more evident in preterm-born children due to the higher risk for clinically significant cognitive deficits.
Our study has some limitations that should be acknowledged. The preterm infants were scanned over a time period during which scanning protocols were upgraded. This resulted in a range of slice thicknesses within our cohort, which possibly led to inconsistencies, although neonatal DTI slice thickness was not associated with performance scores on tests of working memory or early mathematics (all P > 0.47). There are important technological limitations to the diffusion acquisition used in our study. DTI was acquired in only six non-collinear directions, and today diffusion acquisitions include more directions to provide more accurate estimations of diffusion metrics. We acknowledge that there is rapid maturation and a dramatic decrease in brain water content with increasing gestational age during the perinatal period. As a result, there may be changes in diffusivity measures over the 4-week gestational age range at which the preterm infants were scanned, which was a possible confounder. To address this, we statistically corrected for gestational age at MRI in all analyses. Although we adjusted analyses for some important clinical factors, we acknowledge that several factors associated with childhood cognitive functioning were not included as covariates, such as interventions received. It would have been interesting to examine associations between neonatal DTI and DBM measures and later childhood skills in a larger control group of healthy children born full-term to determine if the study findings are unique to the preterm population. Unfortunately the control group for this longitudinal cohort study had small numbers and subsequently insufficient statistical power, therefore direct comparison between the strength and significance of association in preterm and term-born groups is not appropriate. However, performing these analyses in a larger group of healthy children born full-term might provide different results given previously described differences between preterm and term-born groups in neonatal Jacobian maps (Boardman et al., 2006).
Conclusion
Structural MRI measures identified some regions of the brain during the neonatal period associated with childhood skills important for academic achievement in our cohort of very preterm children. Localized regions in the Jacobian map were associated with early mathematics at 5 and 7 years, even after accounting for important perinatal clinical factors, using GLM but not SVR. There was some suggestion that regions in the map were associated with working memory at 7 years. Neonatal fractional anisotropy maps were associated with working memory and early mathematics at 5 years, even after accounting for clinical factors, using SVR but not GLM. These results contribute to the growing evidence for the clinical utility of neonatal MRI for identifying preterm infants at risk for cognitive and academic impairment in childhood.
Acknowledgements
We gratefully thank Merilyn Bear for recruitment, Michael Kean and the radiographers at Melbourne Children’s MRI Centre, the Victorian Infant Brain Studies (VIBeS) and Developmental Imaging groups at the Murdoch Childrens Research Institute, as well as the families who participated in this study.
Glossary
Abbreviations
- DBM
deformation-based morphometry
- DTI
diffusion tensor imaging
- GLM
general linear model
- SVR
support vector regression
Funding
This study was supported by Australia’s National Health and Medical Research Council (Centre for Clinical Research Excellence 546519 to L.D. and P.A.; Project grants 237117 to L.D., 491209 to P.A., Senior Research Fellowship 628371 to P.A., Early Career Fellowship 1012236 and Career Development Fellowship 1085754 to D.T.), National Institutes of Health (HD058056), United Cerebral Palsy Foundation (USA), Leila Y. Mathers Charitable Foundation (USA), the Brown Foundation (USA), the Victorian Government’s Operational Infrastructure Support Program, and The Royal Children’s Hospital Foundation. H.U. is supported by the Clinical Scientist Training Program, Karolinska Institutet (Sweden).
References
- Aarnoudse-Moens C, Weisglas-Kuperus N, van Goudoever B, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009; 124: 717–28. [DOI] [PubMed] [Google Scholar]
- Anderson P, Doyle L. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. J Am Med Assoc 2003; 289: 3264–72. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Hutton C, Frackowiak R, Johnsrude I, Price C, Friston K. Identifying global anatomical differences: deformation based morphometry. Hum Brain Mapp 1998; 6: 348–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996; 113: 209–19. [DOI] [PubMed] [Google Scholar]
- Boardman JP, Bhatia K, Counsell S, Allsop J, Kapellou O, Rutherford MA, et al. An evaluation of deformation-based morphometry applied to the developing human brain and detection of volumetric changes associated with preterm birth. In 6th International Conference on Medical Imaging Computing and Computer-Assisted Intervention. Lect Notes Comput Sci 2003; 2878: 697–704. [Google Scholar]
- Boardman J, Counsell S, Rueckert D, Kapellou O, Bhatia K, Aljabar P, et al. Abnormal deep grey matter development following preterm birth detected using deformation-based morphometry. Neuroimage 2006; 32: 70–8. [DOI] [PubMed] [Google Scholar]
- Chalimourda A, Schölkopf B, Smola AJ. Experimentally optimal v in support vector regression for different noise models and parameter settings. Neural Netw 2004; 17: 127–41. [DOI] [PubMed] [Google Scholar]
- Cortes C, Vapnik V. Support-vector networks. Mach Learn 1995; 20: 273–97. [Google Scholar]
- Costeloe K, Hennessy E, Haider S, Stacey F, Marlow N, Draper E. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012; 345: e7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle LW, Roberts G, Anderson PJ. Outcomes at 2 years of infants <28 weeks’ gestational age born in Victoria in 2005. J Pediatri 2010; 156: 49–53. [DOI] [PubMed] [Google Scholar]
- Damaraju E, Phillips J, Lowe J, Ohls R, Calhourn V, Caprihan A. Resting-state functional connectivity differences in premature children. Front Syst Neurosci 2010; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson C, Price D, Gross J. The long term costs of numeracy difficulties. London: Every Child a Chance Trust (KPMG); 2009. [Google Scholar]
- Heath N, Ross S. Prevalence and expression of depressive symptomatology in students with and without learning disability. Learn Disabil Q 2000; 23: 24–36. [Google Scholar]
- Howson C, Kinney M, Lawn J. Born too soon: the global actin report on preterm birth. Geneva, Switzerland: March of Dimes, PMNC, Save the Children, WHO; 2012. [Google Scholar]
- Hutchinson E, De Luca C, Doyle L, Roberts G, Anderson P. School-age outcomes of extremely preterm or extremely low birth weight children. Pediatrics 2013; 131: e1053–61. [DOI] [PubMed] [Google Scholar]
- Inder T, Warfield S, Wang H, Huppi P, Volpe J. Abnormal cerebral structure is present at term in premature infants. Pediatrics 2005; 115: 286–94. [DOI] [PubMed] [Google Scholar]
- James G, Witten D, Hastie T, Tibshirani R. An introduction to statistical learning: with applications in R. Springer Science: Business Media New York; 2013. [Google Scholar]
- Jenkins M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage 2012; 62: 782–90. [DOI] [PubMed] [Google Scholar]
- Johnson S, Fawke J, Hennessy E, Rowell V, Thomas S, Wolke D, et al. Neurodevelopmental disability through 11 years of age in children born before 26 weeks of gestation. Pediatrics 2009; 124: e249–57. [DOI] [PubMed] [Google Scholar]
- Johnson S, Wolke D, Hennessy E, Marlow N. Educational outcomes in extremely preterm children: neuropsychological correlates and predictors of attainment. Dev Neuropsychol 2011; 36: 74–95. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman survey of early academic and language skills. Circle Pines, MD: American Guidance Service; 1993. [Google Scholar]
- Kidokoro H, Neil J, Inder T. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol 2013; 34: 2208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci 2009; 12: 535–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine P, Nourse S. What follow-up studies say about post school life for young men and women with learning disabilities: a critical look at the literature. J Learn Disabil 1998; 31: 212–33. [DOI] [PubMed] [Google Scholar]
- Litt J, Taylor H, Klein N, Hack M. Learning disabilities in children with very low birthweight: prevalence, neuropsychological correlates, and educational interventions. J Learn Disabil 2005; 38: 130–41. [DOI] [PubMed] [Google Scholar]
- Litt J, Taylor H, Margevicius S, Schluchter M, Andreias L, Hack M. Academic achievement of adolescents born extremely low birth weight. Acta Paediatr 2012; 101: 1240–5. [DOI] [PubMed] [Google Scholar]
- Mulder H, Pitchford N, Marlow N. Processing speed and working memory underlie academic attainment in very preterm children. Arch Dis Child Fetal Neonatal Ed 2010; 95: F267–72. [DOI] [PubMed] [Google Scholar]
- Murray AL, Scratch SE, Thompson DK, Inder TE, Doyle LW, Anderson JFI, et al. Neonatal brain pathology predicts adverse attention and processing speed in very preterm and/or very low birth weight children. Neuropsychology 2014; 28: 552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mürner-Lavanchy I, Ritter B, Spencer-Smith M, Perrig W, Schroth G, Steinlin M, et al. Visuospatial working memory in preterms and controls—impact of age and performance. Deve Cogn Neurosci 2014; 9: 106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosarti C, Al-Asady M, Frangou S, Stewart A, Rifkin L, Murray R. Adolescents who were born very preterm have decreased brain volumes. Brain 2002; 125: 1616–23. [DOI] [PubMed] [Google Scholar]
- Olesen P, Nagy Z, Westerberg H, Klingberg T. Combined analysis of DTI and fMRI data reveals a joint maturation of white and grey matter in a fronto-parietal network. Cogn Brain Res 2003; 181: 48–57. [DOI] [PubMed] [Google Scholar]
- Omizzolo C, Scratch S, Stargatt R, Kidokoro H, Thompson D, Lee K, et al. Neonatal brain abnormalities and memory and learning outcomes at 7 years in children born very preterm. Memory 2013; 22: 605–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering S, Gathercole S. Working Memory Test Battery for Children (WMTB-C) manual. London: Psychological Corporation Ltd; 2001. [Google Scholar]
- Pritchard V, Clark C, Liberty K, Champion P, Wilson K, Woodward L. Early school-based learning difficulties in children born very preterm. Early Hum Dev 2009; 85: 215–24. [DOI] [PubMed] [Google Scholar]
- Progribna U, Burson K, Lasky R, Narayana P, Evans P, Parikh N. Role of diffusion tensor imaging as an independent predictor of cognitive and language development in extremely low-birth-weight infants. Am J Neuroradiol 2014; 35: 790–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidy N, Mogan A, Thompson D, Inder T, Doyle L, Anderson P. Impaired language abilities in children born very preterm and/or very low birth weight and the influence of neonatal white matter abnormality. J Pediatr 2013; 162: 719–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W, Menon V, Schatzberg A, Keller J, Glover G, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27: 2349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serag A, Aljabar P, Ball G, Counsell S, Boardman J, Rutherford M, et al. Construction of a consistent high-definition spatio-temporal atlas of the developing brain using adaptive kernel regression. Neuroimage 2012; 59: 2255–65. [DOI] [PubMed] [Google Scholar]
- Short S, Elison J, Goldman B, Styner M, Gu H, Connelly M, et al. Associations between white matter mictostructure and infants' working memory. NeuroImage 2013; 64: 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms V, Cragg L, Gilmore C, Marlow N, Johnson S. Mathematics difficulties in children born very preterm: current research and future directions. Arch Dis Child Fetal Neonatal Ed 2013a; 98: F457–63. [DOI] [PubMed] [Google Scholar]
- Simms V, Gilmore C, Cragg L, Clayton S, Marlow N, Johnson S. Nature and origins of mathematics difficulties in very preterm children: a difference in etiology than Developmental Dyscalculia. Pediatr Res 2015; 77: 389–95. doi:10.1038/pr.2014.184 [DOI] [PubMed] [Google Scholar]
- Simms V, Gilmore C, Cragg L, Marlow N, Wolke D, Johnson S. Mathematics difficulties in extremely preterm children: evidence of a specific deficit in basic mathematics processing. Pediatr Res 2013b; 73, 236–44. [DOI] [PubMed] [Google Scholar]
- Smith S, Jenkinson M, Woolrich M, Beckmann C, Behrens T, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23: 208–19. [DOI] [PubMed] [Google Scholar]
- Spencer-Smith M, Anderson V. Healthy and abnormal development of the prefrontal cortex. Dev Neurorehabil 2009; 12: 279–97. [DOI] [PubMed] [Google Scholar]
- Spencer-Smith M, Ritter B, El-Koussy M, Kiefer C, Steinlin M, Everts R. Age, sex and performance influence the visuospatial working memory network in childhood. Dev Neuropsychol 2013; 38: 236–55. [DOI] [PubMed] [Google Scholar]
- Taylor H, Burant C, Holding P, Klin N, Hack M. Sources of variability in sequelae of very low birth weight. Child Neuropsychol 2002; 8: 163–78. [DOI] [PubMed] [Google Scholar]
- Thompson D, Adamson C, Roberts G, Faggian N, Wood S, Warfield S, et al. Hippocampal shape variations at term equivalent age in very preterm infants compared with term controls: perinatal predictors and functional significance at age 7. Neuroimage 2013; 70: 278–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson D, Lee KJ, Egan GF, Warfield SK, Doyle LW, Anderson PJ, et al. Regional white matter microsctructure in very preterm infants: predictors and 7 year outcomes. Cortex 2014; 52: 60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imag, 2010; 29: 1310–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman H, Almeida R, Klingberg T. Structural maturation and brain activity predict future working memroy capacity during childhood development. J Neurosci 2014; 34: 1592–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JH. Hierarchical grouping to optimize an objective function. J Am Stat Assoc 1963; 58: 236–44. [Google Scholar]
- White T, Symington I, Castellanos N, Brittain P, Walsh S, Nam KW, et al. Dysconnectivity of neurocognitive networks at rest in very-preterm born adults. Neuroimage 2014; 4: 352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G, Robertson G. Wide range achievement test, 4th edn4th edn. Lutz, FL: Psychological Assessment Resources; 2006. [Google Scholar]
- Wilson-Ching M, Pascoe L, Doyle LW, Anderson PJ. Effects of correcting for prematurity on cognitive test scores in childhood. J Paediatr Child Health 2014; 50: 182–8. [DOI] [PubMed] [Google Scholar]
- Woodward L, Anderson P, Austin N, Howard K, Inder T. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med 2006; 355: 685–94. [DOI] [PubMed] [Google Scholar]
- Woodward L, Clark C, Bora S, Inder T. Neonatal white matter abnormalities an important predictor of neurocognitive outcome for very preterm children. PLoS One 2012; 7: e51879. [DOI] [PMC free article] [PubMed] [Google Scholar]