Abstract
A nerve block is an effective tool for diagnostic and therapeutic methods. If a diagnostic nerve block is successful for pain relief and the subsequent therapeutic nerve block is effective for only a limited duration, the next step that should be considered is a nerve ablation or modulation. The nerve ablation causes iatrogenic neural degeneration aiming only for sensory or sympathetic denervation without motor deficits. Nerve ablation produces the interruption of axonal continuity, degeneration of nerve fibers distal to the lesion (Wallerian degeneration), and the eventual death of axotomized neurons. The nerve ablation methods currently available for resection/removal of innervation are performed by either chemical or thermal ablation. Meanwhile, the nerve modulation method for interruption of innervation is performed using an electromagnetic field of pulsed radiofrequency. According to Sunderland's classification, it is first and foremost suggested that current neural ablations produce third degree peripheral nerve injury (PNI) to the myelin, axon, and endoneurium without any disruption of the fascicular arrangement, perineurium, and epineurium. The merit of Sunderland's third degree PNI is to produce a reversible injury. However, its shortcoming is the recurrence of pain and the necessity of repeated ablative procedures. The molecular mechanisms related to axonal regeneration after injury include cross-talk between axons and glial cells, neurotrophic factors, extracellular matrix molecules, and their receptors. It is essential to establish a safe, long-standing denervation method without any complications in future practices based on the mechanisms of nerve degeneration as well as following regeneration.
Keywords: Axon, Chemical Neurolysis, Denervation, Electromagnetic field, Myelin, Nerve degeneration, Nerve regeneration, Peripheral nerve injury, Pulsed radiofrequency treatment, Wallerian degeneration
INTRODUCTION
Spinal joint pain is a representative painful disorder which is determined by a diagnostic block rather than imaging studies. A dual infiltration of local anesthetics (for example, lidocaine and bupivacaine) into the medial branch of the posterior ramus is considered as a confirmatory diagnostic method after a history of referred pain and tenderness on the facetal area. If the test shows a positive result of pain relief for different durations, a therapeutic nerve block is applied. However, if the effect of the therapeutic block lasts only for a short period and the pain recurs, a destructive procedure of nerve ablation or modulation may be an inevitable step for long-term pain relief. Unwanted nerve regeneration after nerve degeneration due to ablation or modulation seems to occur quite quickly in a clinical pain practice setting.
Currently available and frequently used nerve ablation and modulation methods include conventional radiofrequency ablation (RFA) using heat, chemical ablation using alcohol, and pulsed RFA using an electromagnetic field. The most important consideration for nerve ablation is to safely produce a limited precise lesioning, without unwanted damage to the other structures. However, it is not uncommon that the lesioning caused by conventional RFA is too small, or is limited to the nearest side of the nerve, and thus fails to denervate the targeted nerve completely. Therefore, a chemical ablation using alcohol is used instead of conventional RFA, due to the incomplete denervation and recurrence, despite the risk of adjacent structural damage [1]. The other decision for neural ablation or modulation depends on its contained neural components. If the targeted nerve lacks a motor component, neural ablation, rather than neural modulation, is chosen without hesitation.
This review aims to summarize the current pain practice of neural ablation (iatrogenic degeneration) or neural modulation and its action mechanisms and subsequent neural regeneration (recurrence of pain) from the ablation.
CLASSIFICATION OF PERIPHERAL NERVE INJURY INDUCED BY NEURAL ABLATION
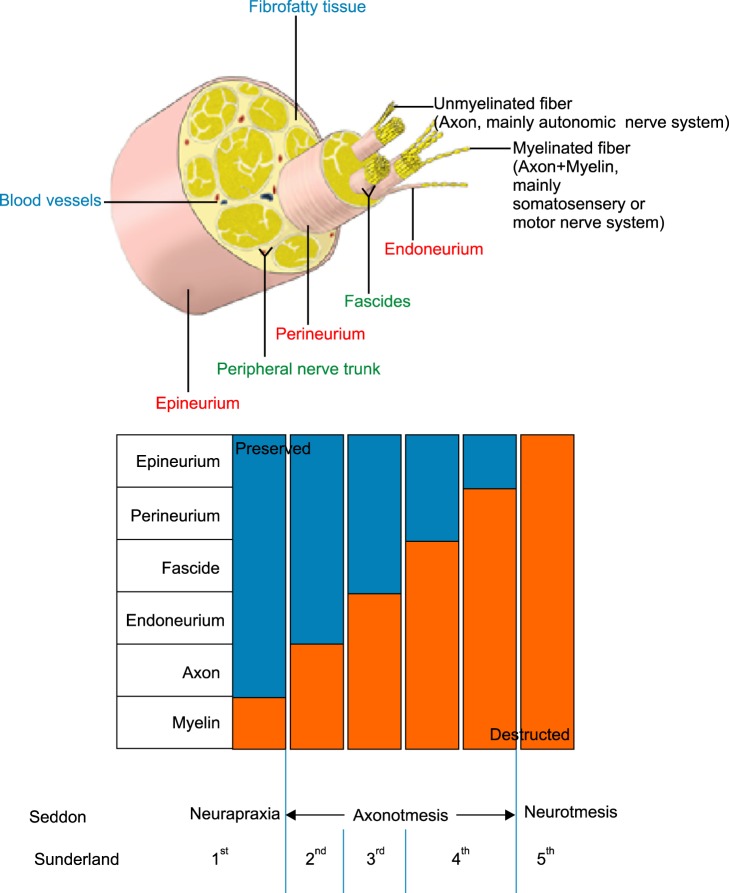
Neural ablation may be applied to an iatrogenic peripheral nerve injury (PNI). A peripheral nerve is composed of the myelin, axon, endoneurium, fascicle, perineurium, and epineurium. The endoneurium surrounds individual myelinated axons and groups of unmyelinated axons. Fascicles are collections of axons which are enclosed by the perineurium. The epifascicular (internal) epineurium lies between fascicles. The peripheral nerve trunk is a collection of fascicles. The epineurial (external) epineurium surrounds the nerve trunk proper. According to the extent of the nerve injury, the prognosis of nerve recovery is quite different (Fig. 1) [2,3].
Fig. 1. Comparison between Seddon's and Sunderland's classification of peripheral nerve injury. Sunderland subdivided axonotmesis into 3 types with different degrees of nerve disruption and different capabilities for spontaneous regeneration [7].
There are two well-known grading systems used to describe PNI. First, Seddon [4] described a scheme for describing 3 kinds of PNI in 1942, according to the extent of damage to axons and surrounding tissue layers. "Neurapraxia (non-action)", the first degree PNI, is used to describe a short-lived paralysis followed by recovery without any evidence of true regeneration. Common clinical examples are "Saturday night radial nerve palsy" and "leg-crossing peroneal nerve palsy" due to focal ischemia without any structural changes in the myelin. Axons are anatomically intact, but nonfunctional. Since the nerve cannot transmit impulses, the body part is paralyzed. Motor and sensory loss due to segmental demyelination can be observed. However, there is no axon disruption or Wallerian degeneration. The disruption of nerve conduction due to neurapraxia usually affects motor fibers rather than sensory fibers. However, muscle atrophy is not usually found. Loss of function persists until re-myelination occurs. If there is no ongoing compression, full recovery from neurapraxia without any intervention can be expected within 3-6 months [2,4].
"Axonotmesis", the second degree PNI, is actual damage to the nerve fibers, which produces complete peripheral degeneration. However, the sheath and its supporting connective tissues still remain. It means that the nerve as a mass of tissue is still in continuity. "Wallerian degeneration (degeneration of distal aspects of a nerve after injury to the cell body or proximal portion of the axon, anterograde or orthograde degeneration)" occurs when there is a disruption of the axon. Fragmentation of the axon and its myelin sheath is a characteristic feature. The myelin is a phospholipid membrane that wraps around axons to provide them with insulation. After axonal degeneration, myelin clearance is produced by Schwann cells in the peripheral nervous system or by oligodendrocytes in the central nervous system. The quick response of Schwann cells to axonal injury is performed by neuregulins [2,4,5,6].
"Neurotmesis (cutting)", the third degree PNI, shows a complete neural separation state. Most of the connective tissue framework is lost and distorted including the epineurium [4].
In 1951, Sunderland [5] further pursued Seddon's PNI classification by subdividing the axonotmesis into 3 categories - second, third, and fourth degrees - according to the severity of nerve injury. He subdivided it, considering the spontaneous regeneration after different degrees of axonotmesis. The "second degree PNI" is defined as a condition that shows axonal discontinuity, but preserved endoneurium, fascicular arrangement, and perineurium. The "third degree PNI" shows that the myelin, axon, and endoneurium are disrupted, but the fascicular arrangement and perineurium are still maintained. The "fourth degree of PNI" means that only the epineurium is still intact (Fig. 1) [5,7].
Currently available neural ablation methods focus on precise, target-oriented denervation, but recurrence of pain after a short period is always a problem in pain practice. Recurrence of pain means nerve regeneration after PNI. According to Sunderland's classification, it is first and foremost suggested that current neural ablations produce third degree PNI to the myelin, axon, and endoneurium without disruption of the fascicular arrangement, perineurium, and epineurium. The merit of Sunderland's third degree PNI is to produce a reversible injury. However, its shortcoming is recurrence of pain and the necessity of repeated ablative procedures [2].
NEURAL ABLATION METHODS CURRENTLY AVAILABLE
Neural ablation in current pain practice can be divided into chemical and physical (thermal and electromagnetic field) neurolysis. Both methods produce nerve injury and result in degeneration of the nerve fiber from the distal to the lesion, along with its myelin sheath, known as "Wallerian degeneration". The Wallerian degeneration causes a temporary interference in nerve cell transmission, resulting in a nociceptive block. This process does not completely disrupt the nerve cell, but preserves the basal lamina of the Schwann cells (non-neuronal cells that coat the axons in myelin). The Schwann cells in the peripheral nerve potentially allows for axonal regeneration with reconnection to the proximal end of the nerve fiber [8].
1. Alcohol Neurolysis
The representative chemical ablative agents are alcohol, phenol, and glycerol. They disrupt the transmission of pain signals for 3-6 months by causing Wallerian degeneration from the distal to the lesion [8]. Alcohol is the most commonly used agent for intractable visceral cancer pain to produce damage to the unmyelinated sympathetic chains and ganglia (Fig. 2) [9]. However, potential complications arising from chemical neurolysis of the peripheral nerve include necrosis of the skin and other non-target tissue, neuritis, anesthesia dolorosa, and prolonged motor paralysis [1]. Both the American Society of Anesthesiologists' task force on chronic pain management and the American Society of Regional Anesthesia and Pain Medicine recommended in 2010 that chemical denervation should not be used in the routine care of non-cancer patients with chronic pain [10].
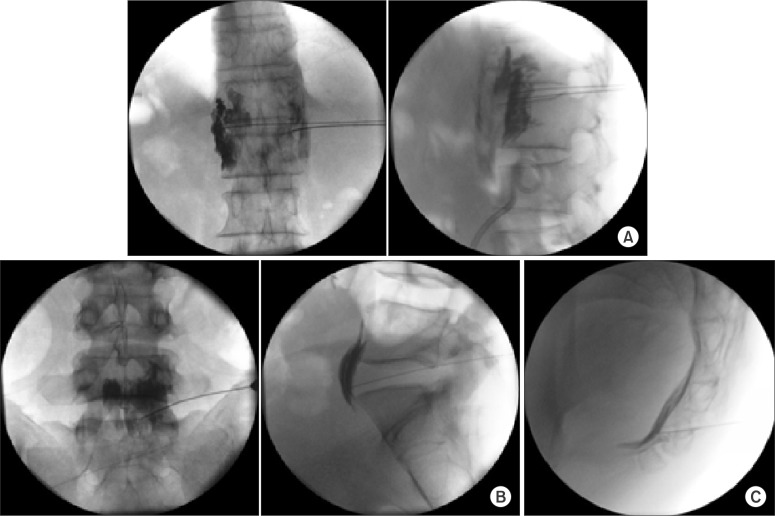
Fig. 2. Sympathetic neurolysis for visceral cancer pain. (A) Celiac plexus neurolysis, (B) Superior hypogastric neurolysis, and (C) Ganglion impar neurolysis.
Basic mechanisms of alcohol neurolysis are clearly unknown, but it damages nerves by denaturing proteins and fatty substance extraction. In other words, the mechanisms involve extraction of cholesterol/phospholipid and cerebroside from the neural membranes, and precipitation of mucoproteins and lipoprotein [11].
Currently, a recommended application of alcohol neurolysis is sympathetic neurolysis for the treatment of visceral cancer pain. However, the risks of chemical neurolysis to the peripheral nerve are considered to outweigh its benefits. In cases of recurrence following RFA for spinal joint pain, including facet and sacroiliac joint pain, alcohol ablation in the medial branch and sacroiliac joint may be the next step [1].
2. Radiofrequency Ablation (RFA)
1) Thermal (conventional, continuous) RFA
Thermal RF, using a constant high frequency (500 kHz), creates a thermal neurodestructive lesion at the active tip of an insulated cannula. The generator produces an alternating current to induce coagulative necrosis at the target tissue. The electrical field causes charged molecules to oscillate and finally generate heat in the surrounding tissues. The lesion that is created is spheroid in shape, with its long axis along the active tip of the cannula. Therefore, the cannula must be placed parallel to the target nerve in clinical practice [12].
However, it is difficult to place the cannula parallel to the targeted nerve. First, prior to applying the RFA, stimulation tests for the sensory (50 Hz) and motor (2 Hz) nerves are performed after placing the cannula parallel to the running path of the nerve. Anatomic variants resulting from degenerative changes or congenital variations of the peripheral nerve result in a time and effort-consuming task to place the cannula in the correct place [1].
Second, the shortcoming of RFA using a cannula is lesioning only on the affected side of the targeted nerve. Ideally, the given thermal RF reaches the whole nerve, from the epineurium to myelinated or unmyelinated nerve fibers. However, the recurrence from RF denervation is faster than that of alcohol denervation [1]. Alcohol denervation can theoretically make the whole nerve wet while causing unwanted adjacent tissue damage.
Third, sympathetic ganglions rather than its chains are the target structures for the sympathectomy. Several anatomic and clinical studies have tried to find a correct position for the needle insertion to approach the sympathetic ganglia [13,14,15]. However, there is no stimulation method for the sympathetic ganglia yet.
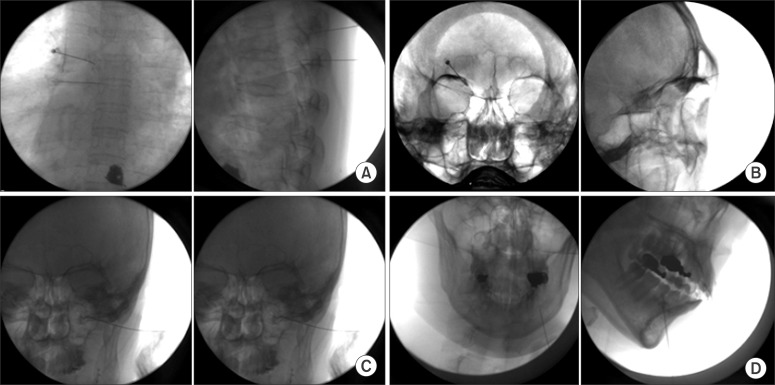
Most conventional RF lesions are applied at probe temperatures of between 60℃ and 90℃ for 90 to 120 seconds in clinical procedures. RF thermal lesions produce irreversible protein denaturation when the tissue temperature rises above 60℃ [16]. An indiscriminate destruction of myelinated fibers, coagulative necrosis, hemorrhages, and massive edema can be found in the RF lesion [17]. Even though sensory and motor tests are performed before the ablation, unwanted motor deficit rather than sensory loss may remain for a certain period of time. Thermal conventional RFA is currently applied to the medial branch of the posterior ramus for the treatment of facet joint pain, and the 3 branches of the trigeminal nerve for the treatment of trigeminal neuralgia and postherpetic neuralgia (Fig. 3).
Fig. 3. Conventional radiofrequency ablation in the (A) thoracic medial branch of the posterior ramus, (B) supraorbital branch, (C) infraorbital branch, and (D) mental branch of the trigeminal nerve.
2) Pulsed RFA
Compared with thermal conventional RFA, pulsed RFA at less than 42℃ can avoid possible heat complications, such as neuritis, motor dysfunction, and deafferentation pain (or anesthesia dolorosa) [18]. It uses a 500 KHz current, applying 2 bursts/s with each pulse lasting 20 ms over a 120 second interval [19]. Therefore, pulsed RFA should be given for a longer duration than continuous RFA, and in a repeated manner. It is known to produce an electromagnetic field at the front of the cannula, using a radiofrequency current in short, high–voltage bursts. After pulsed RFA, the internal ultrastructural components of the axons show microscopic damage, including abnormal mitochondrial membranes and morphologic changes of the mitochondria, and disruption/disorganization of the microfilaments and microtubules. The damage appears to be more pronounced for thin unmyelinated C-fibers than for thick myelinated A-delta and A-beta fibers [20].
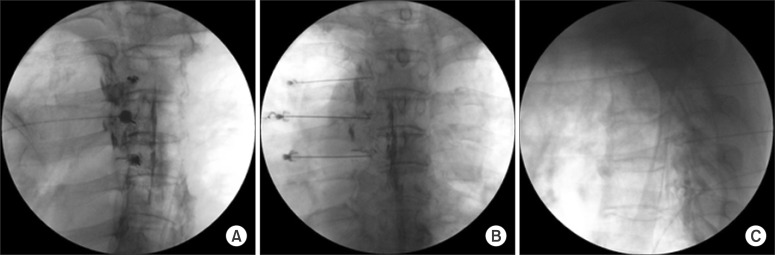
Even though pulsed RFA has several merits that improve the weak points of conventional continuous thermal RFA, it seems to be weaker for sensory denervation. Therefore, it is better to apply it if some damage to the nerve already existed, and the damage is difficult to repair or correct using current modalities. The current application of pulsed RFA in pain practices is to the dorsal root ganglia for the treatment of postherpetic neuralgia, which has scarred the dorsal horn of the spinal cord and dorsal root ganglion at 42℃ for 120 seconds, repeated 2 or 3 times (Fig. 4) [21]. It may be applied to the injured dorsal root ganglion in type 2 complex regional pain syndrome. Applying pulsed RFA is definitely not recommended for the treatment of radicular pain, due to the compressed dorsal root ganglion, in cases of surgically correctable spinal stenosis.
Fig. 4. Pulsed radiofrequency ablation in the thoracic dorsal root ganglia for the treatment of postherpetic neuralgia (PHN). (A) Oblique view: The target point is below the pedicle. (B) Anteroposterior view: The needle is advanced to the dorsal root ganglion below the pedicle. (C) Lateral view: The depth of the needle is adjusted under the lateral view. A contrast medium spreads to the left posterior epidural space, and the dorsal root ganglia become apparent.
NEURAL REGENERATION AFTER NEURAL ABLATION
Nerve regeneration after peripheral nerve injury is good for patients who want to recover from sensory, motor, or reflex deficits. However, nerve regeneration after nerve ablation in patients with intractable pain means recurrence of pain in a clinical pain practice setting.
Nerve repair may be initiated through three mechanisms within 30 minutes of an injury: 1) remyelination, 2) collateral sprouting from preserved axons, and 3) regeneration [7]. Collateral sprouting can contribute reinnervation in partial nerve injuries, especially when less than 20-30% of all the axons are affected. When more than 90% of the axons are injured, the primary nerve repair mechanism is regenerated from the injury site [7]. The success of regeneration from the proximal stump depends on its distance from the injury site.
Macrophages migrate into the distal stump, and may be involved in initiating Schwann cell proliferation. Schwann cells play a vital role in promoting regeneration. The Schwann cells increase synthesis of surface cell adhesion molecules (CAMs) and prepare the basement membrane containing extracellular matrix proteins, such as laminin and fibronectin. The nerve growth factor (NGF) receptors on the Schwann cells, lining the endothelial tubes in the distal stump, increase. The NGF stimulates regenerating axonal sprouting [22].
The stimulation effects of the NGF receptors on the Schwann cells, after nerve injury, radiate retrograde from the peripheral to the nerve cell body. Neuronal survival is facilitated by the activation of trophic factors, including neurotrophins, neurpoietic cytokines, insulin-like growth factors (IGFs), and glial cell line derived neurotrophic factors (GDNFs). Axotomized neurons must switch from a transmitting mode to a growth mode for regeneration. They express growth-associated proteins (GAPs), such as GAP-43, tubulin, actin, neuropeptides, and cytokines [23].
Peripheral nerve regeneration, common only for flesh wound trauma, may have problems due to the lack of specificity. Compared with normal, accurate reinnervation of motor and sensory targets, various abnormal reconnections and reinnervation may occur: 1) Abortive regeneration undergoes chronic target denervation or atrophy on the muscles or sensory receptors. 2) Misdirected reinnervation of axons reconnects to an inappropriate target, which results in the motor nerve connecting to the sensory receptor or the sensory nerve connecting to the muscle. 3) Misdirected motor reinnervation leads to functionally inappropriate muscle targets (flexor muscles versus extensor muscles). 4) Hyperinnervation of muscle targets with misdirected motor reinnervation may result in coactivation of flexor and extensor muscles [24].
In clinical practice, nerve regeneration after ablation may recover according to its thickness from tactile sense, motor, proprioception, temperature, pain, and sympathetic tone. However, an itching sensation without pain sometimes remains after denervation, and it recurs at first without recurrence of pain. It is called "neuropathic itching", which relates to the transient receptor potential (TRP) channels [25].
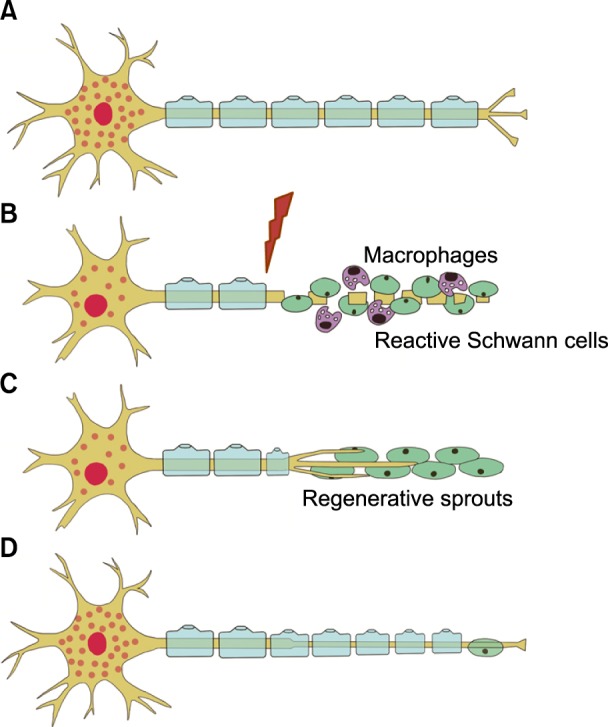
The regeneration of a sectioned axon involves the transformation of a stable axonal segment into a highly motile tip, called a "growth cone". The growth cone senses the surrounding environment and leads the elongation of the regenerating axon. It is important that the growth cones elongate following the endoneurial tubes of the distal nerve to eventually reinnervate their peripheral target organs. If they do not reach the distal stump, they may sprout within the proximal stump forming a "neuroma". In the distal stump, denervated Schwann cells proliferate on the basal membrane of Büngner, over which growth cones advance (Fig. 5) [23].
Fig. 5. Degeneration and regeneration after peripheral nerve injury. (A) Normal neuron and nerve fiber. (B) Wallerian degeneration. The axotomy results in fragmentation of the distal axon and myelin sheaths. Schwann cells proliferate. Macrophages invade the distal nerve segment, and phagocytize degrading materials. (C) Schwann cells in the distal segment line up in bands of Büngner. Axonal sprouts advance embedded in the Schwann cells and are attracted by gradients of neurotrophic factors. (D) Axonal reconnection with end organs and maturation and remyelination of the nerve fiber [22].
CONCLUSIONS
According to Sunderland's classification, it is first and foremost suggested that current neural ablations produce third degree PNI to the myelin, axon, and endoneurium without any disruption of the fascicular arrangement, perineurium, and epineurium.
Neural ablation methods currently available include chemical and physical modalities. Following the motor and sensory differential diagnostic test, the ablation method considered as the most common and safest method is a thermal, conventional, continuous RFA. However, a size-limited or one-sided lesioning, compared with alcohol ablation, may result in a short duration of pain relief. It should be applied to the pure sensory nerve, or a motor-mixed nerve with the smallest motor component. It is used for the denervation of the peripheral branch of the trigeminal nerve or the medial branch of the posterior ramus.
Alcohol ablation is routinely applied to visceral cancer pain using a celiac plexus, superior hypogastric, or ganglion impar neurolysis. It may be used in cases of pain recurrence after thermal conventional continuous RFA in order to increase the duration of pain relief in somatic pain.
Pulsed RFA uses an electromagnetic field - instead of heat less than 42℃ - for the treatment of neuropathic pain. However, it may reduce the complications of thermal conventional continuous RFA; the effective duration is shorter than that of thermal conventional continuous RFA. It is commonly used for the treatment of postherpetic neuralgia to the dorsal root ganglion.
Pain recurrence after denervation in medical practice, and nerve regeneration from a third degree nerve injury from both ablation techniques, have a common pathway. This pathway includes macrophage migration, Schwann cell proliferation, CAMs for preparing the basement membrane, NGF on the Schwann cell for axonal sprouting, and increased trophic factors (Table 1).
Table 1. Comparison of Representative Nerve Ablation and Modulation Methods.
PNI: peripheral nerve injury, CAMs: surface cell adhesion molecules, NGF: nerve growth factor.
An ideal neural ablation technique has satisfactory ablation power, with adequate action duration without complications such as anesthesia dolorosa, neuritis, and motor or reflex deficits.
ACKNOWLEDGEMENTS
This work was supported by a 2-year research grant of Pusan National University.
References
- 1.Joo YC, Park JY, Kim KH. Comparison of alcohol ablation with repeated thermal radiofrequency ablation in medial branch neurotomy for the treatment of recurrent thoracolumbar facet joint pain. J Anesth. 2013;27:390–395. doi: 10.1007/s00540-012-1525-0. [DOI] [PubMed] [Google Scholar]
- 2.Vargas ME, Barres BA. Why is Wallerian degeneration in the CNS so slow? Annu Rev Neurosci. 2007;30:153–179. doi: 10.1146/annurev.neuro.30.051606.094354. [DOI] [PubMed] [Google Scholar]
- 3.Ross LM, Lamperti ED. Arteries & veins of the rhoracic wall. In: Gilroy AM, MacPherson BR, Ross LM, editors. Atlas of anatomy. New York (NY): Thieme Medical Publishers, Ins; 2005. p. 57. [Google Scholar]
- 4.Seddon HJ. A classification of nerve injuries. BMJ. 1942;2:237–239. doi: 10.1136/bmj.2.4260.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunderland S. A classification of peripheral nerve injuries producing loss of function. Brain. 1951;74:491–516. doi: 10.1093/brain/74.4.491. [DOI] [PubMed] [Google Scholar]
- 6.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurg Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Hsu M. Significance of clinical treatments on peripheral nerve and its effect on nerve regeneration. J Neurol Disord. 2014;2:168. [Google Scholar]
- 9.Koyyalagunta D, Burton AW. The role of chemical neurolysis in cancer pain. Curr Pain Headache Rep. 2010;14:261–267. doi: 10.1007/s11916-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 10.American Society of Anesthesiologists Task Force on Chronic Pain Management; American Society of Regional Anesthesia and Pain Medicine. Practice guidelines for chronic pain management: an updated report by the American Society of Anesthesiologists Task Force on Chronic Pain Management and the American Society of Regional Anesthesia and Pain Medicine. Anesthesiology. 2010;112:810–833. doi: 10.1097/ALN.0b013e3181c43103. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995;80:290–295. doi: 10.1097/00000539-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 12.Iannuccilli JD, Prince EA, Soares GM. Interventional spine procedures for management of chronic low back pain-a primer. Semin Intervent Radiol. 2013;30:307–317. doi: 10.1055/s-0033-1353484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong JH, Oh MJ. Comparison of multilevel with single level injection during lumbar sympathetic ganglion block: efficacy of sympatholysis and incidence of psoas muscle injection. Korean J Pain. 2010;23:131–136. doi: 10.3344/kjp.2010.23.2.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim WH, Kim SK, Lee CJ, Kim TH, Sim WS. Determination of adequate entry angle of lumbar sympathetic ganglion block in Korean. Korean J Pain. 2010;23:11–17. doi: 10.3344/kjp.2010.23.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rocco AG, Palombi D, Raeke D. Anatomy of the lumbar sympathetic chain. Reg Anesth. 1995;20:13–19. [PubMed] [Google Scholar]
- 16.Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14:199–208. doi: 10.1038/nrc3672. [DOI] [PubMed] [Google Scholar]
- 17.Erdine S, Bilir A, Cosman ER, Cosman ER., Jr Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009;9:407–417. doi: 10.1111/j.1533-2500.2009.00317.x. [DOI] [PubMed] [Google Scholar]
- 18.Chua NH, Vissers KC, Sluijter ME. Pulsed radiofrequency treatment in interventional pain management: mechanisms and potential indications-a review. Acta Neurochir (Wien) 2011;153:763–771. doi: 10.1007/s00701-010-0881-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rozen D, Parvez U. Pulsed radiofrequency of lumbar nerve roots for treatment of chronic inguinal herniorraphy pain. Pain Physician. 2006;9:153–156. [PubMed] [Google Scholar]
- 20.Liuzzi FJ, Tedeschi B. Peripheral nerve regeneration. Neurosurg Clin N Am. 1991;2:31–42. [PubMed] [Google Scholar]
- 21.Byrd D, Mackey S. Pulsed radiofrequency for chronic pain. Curr Pain Headache Rep. 2008;12:37–41. doi: 10.1007/s11916-008-0008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- 23.Allodi I, Udina E, Navarro X. Specificity of peripheral nerve regeneration: interactions at the axon level. Prog Neurobiol. 2012;98:16–37. doi: 10.1016/j.pneurobio.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 24.Grinsell D, Keating CP. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed Res Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bíró T, Tóth BI, Marincsák R, Dobrosi N, Géczy T, Paus R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim Biophys Acta. 2007;1772:1004–1021. doi: 10.1016/j.bbadis.2007.03.002. [DOI] [PubMed] [Google Scholar]