Abstract
Background
Neuropathic pain, including paresthesia/dysesthesia in the lower extremities, always develops and remains for at least one month, to variable degrees, after percutaneous endoscopic lumbar discectomy (PELD). The recently discovered dual analgesic mechanisms of action, similar to those of antidepressants and anticonvulsants, enable nefopam (NFP) to treat neuropathic pain. This study was performed to determine whether NFP might reduce the neuropathic pain component of postoperative pain.
Methods
Eighty patients, who underwent PELD due to herniated nucleus pulposus (HNP) at L4-L5, were randomly divided into two equal groups, one receiving NFP (with a mixture of morphine and ketorolac) and the other normal saline (NS) with the same mixture. The number of bolus infusions and the infused volume for 3 days were compared in both groups. The adverse reactions (ADRs) in both groups were recorded and compared. The neuropathic pain symptom inventory (NPSI) score was compared in both groups on postoperative days 1, 3, 7, 30, 60, and 90.
Results
The mean attempted number of bolus infusions, and effective infused bolus volume for 3 days was lower in the NFP group for 3 days. The most commonly reported ADRs were nausea, dizziness, and somnolence, in order of frequency in the NFP group. The median NPSI score, and all 5 median sub-scores in the NFP group, were significantly lower than that of the NS group until postoperative day 30.
Conclusions
NFP significantly reduced the neuropathic pain component, including paresthesia/dysesthesia until 1 month after PELD. The common ADRs were nausea, dizziness, somnolence, and ataxia.
Keywords: Adverse drug reactions, Dysesthesia, Herniated discs, Intravenous infusion, Nefopam, Neuropathic pain, Percutaneous discectomy, Postoperative pain, Symptom assessment
INTRODUCTION
Percutaneous endoscopic lumbar discectomy (PELD) has become a mainstay of minimally invasive spine surgery (MISS) because of a small incision, low-degree damage to the normal tissue, the use of local anesthesia, an early ambulation immediately after surgery, and a short hospital stay [1].
However, one of the more common complications of PELD through the posterolateral approach is postoperative dysesthesia on the dermatomal distribution to variable degrees. Postoperative dysesthesia due to the existing dorsal root ganglion (DRG) injury is both a unique and inevitable complication of PELD. When postoperative dysesthesia occurs, even if the traversing root has been successfully decompressed, it hinders swift recovery and delays the patient's return to daily routines and normal functions [2].
The classic dermatomal presentation of tenderness over the spinous and transverse process of the vertebrae and interspinous area, pain in the gluteal region and thigh, and numbness on the leg and foot usually develops along the lower limb from the lumbosacral herniated nucleus pulposus (HNP) [3]. The tenderness and pain along the dermatome above the knee disappears immediately after PELD, however, the numbness below the knee changes its character into intractable paresthesia/dysesthesia. The paresthesia/dysesthesia tends to persist for at least 1 month postoperatively.
The analgesic nefopam (NFP) is one of the drugs for which the mechanism-of-action target is unknown but can be predicted. It is a non-opioid, non-steroidal, centrally-acting analgesic drug that is a derivative of the non-sedative benzoxazocine, developed and known in the 1960's as fenazocine. Although the mechanisms of analgesic action of NFP are not well understood, they are similar to those of triple neurotransmitter (serotonin, norepinephrine, and dopamine) reuptake inhibitors and anticonvulsants [4]. It has been used mainly as an analgesic drug for nociceptive pain, as well as a treatment for the prevention of postoperative shivering and hiccups [5,6,7,8]. Based on NFP's mechanisms of analgesic action, it is more suitable for the treatment of neuropathic pain.
This study was performed to determine whether perioperative intravenous NFP might reduce the neuropathic pain component, especially the paresthesia/dysesthesia of postoperative pain.
MATERIALS AND METHODS
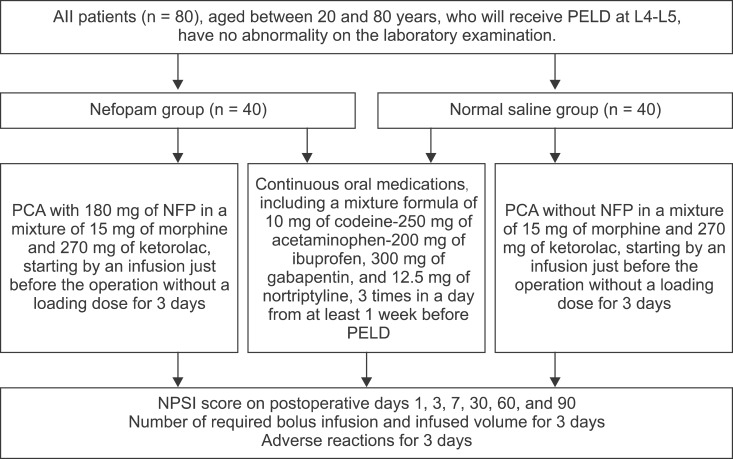
After Institutional Review Board approval was obtained, 80 patients, who underwent PELD due to HNP at L4-L5, were enrolled into this prospective, randomized, double blind study (Fig. 1).
Fig. 1. A flow chart. All 80 participants, who were to receive percutaneous endoscopic lumbar discectomy (PELD) at L4-L5, had already completed basic laboratory examination, including a complete blood count, liver and renal function tests, and electrolyte level tests before hospitalization. They randomly received a mixture of 15 mg of morphine and 270 mg of ketorolac with or without 180 mg of nefopam (NFP) (the NFP group or NS group) using a postoperative intravenous patient-controlled analgesia (PCA) device. The PCA use began with an infusion just before the operation, without a loading dose. The PCA was programmed for a total volume of 100 ml with a basal infusion rate of 1 ml/h, a bolus infusion volume of 1 ml, with a lockout interval of 1 hour without a 4 hour volume limit, for 3 days. The mean attempted number of bolus infusions, and effective infused bolus volume for 3 days were compared in both groups. The adverse reactions in both groups were also recorded and compared.
The enrollment took place in the pain clinic of a university hospital. Inclusion criteria for the inpatients were an age range of 20 to 80 years and an estimated glomerular filtration rate over 60 mg/dl.
Exclusion criteria included patients with contraindications of NFP administration, such as a history of epilepsy, myocardial infarction, convulsion, risk of urinary retention due to urethra or prostate problems, closed angle glaucoma, administrators of monoamine oxidase inhibitors, and those who were pregnant or breast feeding. Other excluded patients were those who could not understand or fill out the neuropathic pain symptom inventory (NPSI) score [9].
They had already taken oral medications, including a mixture of 10 mg of codein-250 mg of acetaminophen-200 mg of ibuprofen, 300 mg of gabapentin, and 12.5 mg of nortriptyline, 3 times in a day at least 1 week before PELD.
Using a computer-generated random allocations sequence, 80 patients who underwent PELD at L4-L5 were randomized and assigned into 2 equal groups: an NFP group and a normal saline (NS) group.
The mixture of analgesics with or without NFP was made by the same researcher. The operator and another researcher who started the patient-controlled analgesia (PCA) infusion pump, and checked the NPSI score during the study, did not know whether NFP was included or not. Both the participants and the care providers did not know whether the intravenous drugs contained NFP or not.
All 80 participants had already completed a basic laboratory examination, including a complete blood count, liver and renal function tests, and electrolyte level tests before hospitalization. They randomly received a mixture of 15 mg of morphine and 270 mg of ketorolac, with or without 180 mg of NFP (NFP group or NS group), using a postoperative intravenous PCA infusion device (Automed 3200, Ace Medical Corp., Ltd, Seoul, Korea). The PCA use began by an infusion just before the operation without a loading dose. The PCA was programmed as a total volume of 100 ml with a basal infusion rate of 1 ml/h, a bolus infusion volume of 1 ml, and a lockout interval of 1 hour without a 4 hour volume limit, for 3 days.
A lidocaine patch (Lidotop patch, Teikoku Pharma-, Sanbonmatsu, Japan) was applied on the skin of the back, covering the anticipated path of the working channel 1 hour before surgery [1]. After a continuous infusion of 0.2 to 0.4 mg of dexmedetomidine (Precedex, Hospira Inc., Lake Forest, IL) and a local infiltration of 10 ml of 1% lidocaine into the skin, a discography was done and a skin incision was made. After a mechanical discectomy with forceps, the infusion of dexmedetomidine was stopped.
The mean attempted number of bolus infusions, and effective infused bolus volume for 3 days were compared in both groups. The neuropathic pain symptom inventory (NPSI) scores (Appendix 1) were evaluated preoperatively, and on postoperative days 1, 3, 7, 30, 60 and 90. The NPSI was self-evaluated by the patient every day, using both a visual analogue scale (VAS) score (from 0/"no pain" to 10/"worst pain imaginable") of neuropathic pain, including burning, pressure, squeezing, electric shocks, stabbing pain, as well as pain evoked by brushing, evoked by pressure, evoked by cold stimuli, pins and needles, and tingling, along with the duration of spontaneous pain and frequency of proved pain [9]. The median of the 5 sub-scores, including burning (superficial) spontaneous pain, pressing (deep) spontaneous pain, evoked pain, paresthesia/dysesthesia, and pins and needles/tingling, and total scores were compared in both groups during the study days. The adverse reactions (ADRs) in both groups were also recorded and compared [9]. Nausea was treated by 0.3 mg of ramosetron hydrochloride intravenously.
On the basis of a pilot study, we determined that a sample size of 80 participants per group was sufficient for this study using a desired power of 0.8 and an alpha level of 0.05. The primary outcome for power analysis was the pain score. The calculations were made for NPSI based on the VAS, using a clinically significant difference in the 2 groups, with a rating of a 25% reduction in the VAS and assuming a standard error rate of 15%.
The data were presented as a mean ± standard deviation or the median ± standard error. Demographic characteristics, including age and sex, were analyzed using the Student t test and the Chi-square test in the intergroup comparison. The mean number of infused bolus infusions and the infused bolus volume for 3 days were compared using the Student t-test. Median total and subtotal NPSI scores with spontaneous pain and pain attacks, and adverse reactions were compared using the Chi-squared test in the intragroup comparison. In all comparisons, a P value less than 0.05 was considered statistically significant. The statistical analyses were performed using SPSS 12.0 (IBM Corporation, Somers, New York).
RESULTS
There were no drop-out patients who stopped taking medicine or receiving intravenous NFP due to ADRs. The patients' demographic characteristics, including sex, age, duration of radicular pain, presence of foot drop, and previous back operations, were similar and had no obvious effect on the outcome (Table 1).
Table 1. Baseline Characteristics of the Study Participants.
Data are mean ± SD or numbers.
The mean attempted number of bolus infusions (4.4 ± 0.7 times), and effective infused bolus volume (3.2 ± 0.6 ml) for 3 days, were significantly reduced in the NFP group compared with those in the NS group (18.8 ± 3.8 times and 14.5 ± 3.2 ml, respectively).
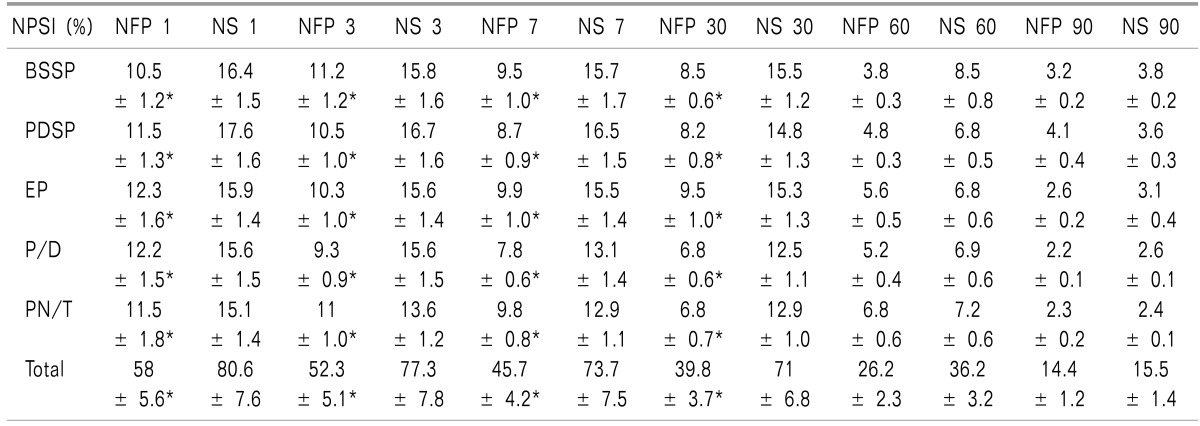
The median NPSI score in the NFP group was significantly lower than that of the NS group until postoperative day 30. All 5 median sub-scores in the NFP group, including paresthesia/dysesthesia, were significantly lower than those in the NS group until postoperative day 30 (Table 2).
Table 2. The Total and Sub-total NPSI Scores during the Study Period.
*The median NPSI score in the NFP group was significantly lower than that of the NS group until postoperative day 30 (P < 0.05).
*All 5 median sub-scores in the NFP group, including paresthesia/dysesthesia, were significantly lower than those in NS group until postoperative day 30 (P < 0.05). NFP: nefopam, NS: normal saline, BSSP: Burning (superficial) spontaneous pain, PDSP: Pressing (deep) spontaneous pain, EP: Evoked pain, PDSP: Paresthesia/dysesthesia, PN/T: Pins and needles/Tingling.
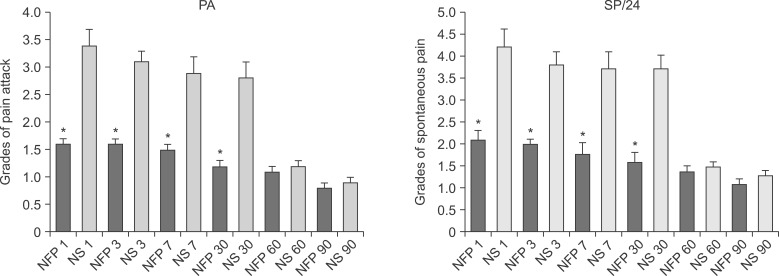
The median scores of the grades by the duration of spontaneous pain during the past 24 h, and the grades of the number of pain attack s during the past 24 h, was lower in NFP group from postoperative day 1 to 30 (P < 0.05) (Fig. 2).
Fig. 2. The median scores of the grades by the duration of spontaneous pain (SP) and the number of pain attacks (PA) during the study days. The grades by the duration of SP during the past 24 h was lower, and the grades by the number of PA during the past 24 h was lower in the nefopam (NFP) group from the postoperative day 1 to 30 (P < 0.05). NFP: nefopam, NS: normal saline, Grades by duration of SP: grade 1 (less than 1 h), grade 2 (between 1 and 3 h), grade 3 (between 4 and 7 h), grade 4 (8 and 12 h), and grade 5 (permanently), Grades by frequency of PA: grade 0 (no pain attack), grade 1 (between 1 and 5), grade 2 (between 6 and 10), grade 3 (between 11 and 20), and grade 4 (more than 20).
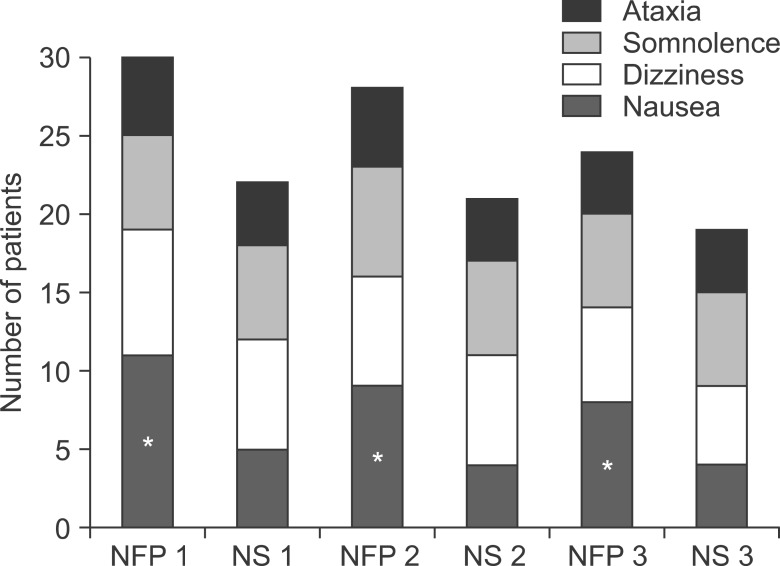
The most commonly reported ADRs during the administration of PCA for the postoperative 3 days were nausea, dizziness, somnolence, and ataxia, in order of frequency in both groups. Nausea in the NFP group was more frequent than that of the NS group (Fig. 3).
Fig. 3. Adverse reactions. The most commonly reported adverse reactions during the administration of patient-controlled analgesia for the postoperative 3 days were nausea, dizziness, somnolence, and ataxia, in order of frequency in both groups. Nausea in the NFP group was more frequent than that of the normal saline (NS) group (P < 0.05).
DISCUSSION
This study was performed to evaluate whether NFP could relieve neuropathic pain, especially paresthesia/dysesthesia, after PELD, while non-steroidal anti-inflammatory drugs and opioids were considered to relieve somatic pain. Even though the infused volume of opioid and ketorolac was larger in the NS group for the postoperative 3 days, the mean total NPSI score including the paresthesia/dysesthesia score, spontaneous pain, and pain attacks were lower in the NFP group. It meant that the NFP relieved the neuropathic pain developed after mechanical decompression and adhesiolysis between the dorsal root ganglion and dorsal horn and herniated nucleus pulposus. In addition, the relatively lower pain scores were maintained in the NFP group until the postoperative follow-up days 7 and 30.
The mean trial number of required bolus infusions and effective infused bolus volume for the 3 days were significantly larger in the NS group. It meant that unsolved residual neuropathic pain was left after morphine and ketorolac covered somatic pain in patients receiving PELD. In clinical practice, the patients who had paresthesia/dysesthesia also complained about burning, electric shocks, pins and needles, and tingling sensations. These symptoms were representative neuropathic pain symptoms.
NFP has been known to have similar properties to triple receptor reuptake inhibitors (antidepressants). It had Ki values for 5-HT2A, 5-HT2B, and 5-HT2C of 1,685, 329.5, and 56 nM, respectively. Similarly, it had a Ki of 531 nM against a dopamine transporter. This, in turn, led us to test the molecule against the norepinephrine and serotonin transporters, where its Ki values, at 33 and 29 nM, were more substantial still [4]. It also has an anticonvulsant effect [10,11]. It prevents N-methyl-D-aspartate (NMDA)-mediated excitotoxicity following stimulation of L-type voltage sensitive calcium channels [12]. It also blocks voltage-sensitive sodium channels, and modulates glutamatergic transmission in another study [13]. These 2 analgesic mechanisms of NFP might reduce neuropathic pain, including paresthesia/dysesthesia from immediately after the operation to 30 days, and reduce chronification of pain [14].
Recently, the concept of preemptive analgesia has been changed into preventive analgesia. Preemptive analgesia can reduce acute postoperative pain; however, minimizing the development of chronic pain conditions can only be successful in combination with intraoperative and postoperative pain therapy as well as social and psychological support when indicated (preventive analgesia) [15]. It is rare to develop permanent paresthesia/dysesthesia on the lower legs below the knees. The dysesthesia tends to remain or to change into paresthesia on the extremities distally, especially below the ankle until 1-2 months. The NFP infused before the beginning of the PELD produced reduced postoperative paresthesia/dysesthesia for 30 days.
The infusion of dexmedetomidine during the operation guarantees self-respiratory activity even in a prone position, while the patient's response may prevent potential nerve damage. However, the drawback of dexmedetomidine is delayed emergence. The delayed emergence delays complaints about postoperative pain for at least 3 hours after the operation.
After developing paresthesia/dysesthesia on the legs, it is difficult to control even using anticonvulsants and antidepressants. The definite but transient method for alleviating dysesthesia after PELD may be a transforaminal epidural block targeting adhesion between the dorsal root ganglion/dorsal horn of the spinal cord and the HNP. Preoperative oral medications for neuropathic pain and the perioperative transforaminal epidural block may reduce the frequency and strength of paresthesia/dysesthesia. Modification of approach, such as a floating retraction technique, may reduce the possibility of developing postoperative dysesthesia [2]. If the distance from the exiting root to the facet, at the lower disc level, according to a preoperative magnetic resonance image scan is narrow, an alternative surgical method is recommended in one study [16].
Differentiation between dysesthesia and paresthesia after PELD was not performed while checking NPSI scores in this study. Clinically, intensive explanation of the difference between dysesthesia and paresthesia was given to the patients. However, they could not understand and differentiate their pain. In other words, patients never complain about paresthesia until the paresthesia had some unpleasant components, so we call it dysesthesia. It might safely be said that all complaints about paresthesia were dysesthesia in this study.
The most common ADR in the NFP group was nausea. Because of the larger infused volume of morphine and ketorolac (without NFP) in the NS group, the adverse reactions due to morphine and ketorolac was increased. However, the frequency of the adverse reactions, such as nausea, dizziness, somnolence, and ataxia, in the NS group, was similar. A previous long-term study revealed that the most frequent adverse reactions were sweating, nausea, tachycardia, malaise, and vomiting [17]. Cold sweating could be prevented if the NFP was injected slowly over 15 minutes with a single shot or by continuous infusion in this study.
Persistent postsurgical pain is a frequent and often disabling complication of many surgical procedures. Nerve injury–induced neuropathic pain has repeatedly been proposed as a major cause of persistent postsurgical pain. The prevalence of probable or definite nerve injury-induced neuropathic pain was high in patients with persistent pain after thoracic and breast surgeries—66% and 68% respectively. In patients with persistent postsurgical pain after groin hernia repair, the prevalence of nerve injury-induced neuropathic pain was 31%, and after total hip or knee arthroplasty, it was 6% [18]. Perioperative NFP may be helpful to reduce anticipated neuropathic pain in these kinds of operations.
There are few available intravenous anticonvulsants and antidepressants in current clinical practice. It means that it is difficult to administer intravenous drugs for neuropathic pain perioperatively. If perioperative intravenous NFP shows adequate analgesia for neuropathic pain, it could be exchanged for the oral formula after discharge [19].
In conclusion, NFP significantly reduced the neuropathic pain component, including paresthesia/dysesthesia of postoperative pain, after PELD, until 1 month postoperation.
Appendix 1
Neuropathic Pain Symptom Inventory (Korean Version)
References
- 1.Kim KH. Use of lidocaine patch for percutaneous endoscopic lumbar discectomy. Korean J Pain. 2011;24:74–80. doi: 10.3344/kjp.2011.24.2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho JY, Lee SH, Lee HY. Prevention of development of postoperative dysesthesia in transforaminal percutaneous endoscopic lumbar discectomy for intracanalicular lumbar disc herniation: floating retraction technique. Minim Invasive Neurosurg. 2011;54:214–218. doi: 10.1055/s-0031-1287774. [DOI] [PubMed] [Google Scholar]
- 3.Loeser JD. Pain of neurologic origin in the hips and lower extremities. In: Loeser JD, Bonica JJ, editors. Bonica's management of pain. 3rd ed. Philadelphia (PA): Lippincott Williams & Wilkins; 2001. pp. 1565–1578. [Google Scholar]
- 4.Gregori-Puigjané E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, et al. Identifying mechanism-of-action targets for drugs and probes. Proc Natl Acad Sci U S A. 2012;109:11178–11183. doi: 10.1073/pnas.1204524109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore RA, Derry S, McQuay HJ, Wiffen PJ. Single dose oral analgesics for acute postoperative pain in adults. Cochrane Database Syst Rev. 2011:CD008659. doi: 10.1002/14651858.CD008659.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alfonsi P, Adam F, Passard A, Guignard B, Sessler DI, Chauvin M. Nefopam, a nonsedative benzoxazocine analgesic, selectively reduces the shivering threshold in unanesthetized subjects. Anesthesiology. 2004;100:37–43. doi: 10.1097/00000542-200401000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heel RC, Brogden RN, Pakes GE, Speight TM, Avery GS. Nefopam: a review of its pharmacological properties and therapeutic efficacy. Drugs. 1980;19:249–267. doi: 10.2165/00003495-198019040-00001. [DOI] [PubMed] [Google Scholar]
- 8.Podranski T, Bouillon TW, Riva T, Kurz AM, Oehmke MJ. Compartmental pharmacokinetics of nefopam during mild hypothermia. Br J Anaesth. 2012;108:784–791. doi: 10.1093/bja/aer517. [DOI] [PubMed] [Google Scholar]
- 9.Bouhassira D, Attal N, Fermanian J, Alchaar H, Gautron M, Masquelier E, et al. Development and validation of the Neuropathic Pain Symptom Inventory. Pain. 2004;108:248–257. doi: 10.1016/j.pain.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 10.Czuczwar M, Czuczwar K, Cięszczyk J, Kiś J, Saran T, Łuszczki JJ, et al. Nefopam enhances the protective activity of antiepileptics against maximal electroshock-induced convulsions in mice. Pharmacol Rep. 2011;63:690–696. doi: 10.1016/s1734-1140(11)70580-1. [DOI] [PubMed] [Google Scholar]
- 11.Novelli A, Groppetti A, Rossoni G, Manfredi B, Ferrero-Gutiérrez A, Pérez-Gómez A, et al. Nefopam is more potent than carbamazepine for neuroprotection against veratridine in vitro and has anticonvulsant properties against both electrical and chemical stimulation. Amino Acids. 2007;32:323–332. doi: 10.1007/s00726-006-0419-6. [DOI] [PubMed] [Google Scholar]
- 12.Novelli A, Díaz-Trelles R, Groppetti A, Fernández-Sánchez MT. Nefopam inhibits calcium influx, cGMP formation, and NMDA receptor-dependent neurotoxicity following activation of voltage sensitive calcium channels. Amino Acids. 2005;28:183–191. doi: 10.1007/s00726-005-0166-0. [DOI] [PubMed] [Google Scholar]
- 13.Verleye M, André N, Heulard I, Gillardin JM. Nefopam blocks voltage-sensitive sodium channels and modulates glutamatergic transmission in rodents. Brain Res. 2004;1013:249–255. doi: 10.1016/j.brainres.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 14.Kim KH, Abdi S. Rediscovery of nefopam for the treatment of neuropathic pain. Korean J Pain. 2014;27:103–111. doi: 10.3344/kjp.2014.27.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sittl R, Irnich D, Lang PM. Update on preemptive analgesia: options and limits of preoperative pain therapy. Anaesthesist. 2013;62:789–796. doi: 10.1007/s00101-013-2225-3. [DOI] [PubMed] [Google Scholar]
- 16.Choi I, Ahn JO, So WS, Lee SJ, Choi IJ, Kim H. Exiting root injury in transforaminal endoscopic discectomy: preoperative image considerations for safety. Eur Spine J. 2013;22:2481–2487. doi: 10.1007/s00586-013-2849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Durrieu G, Olivier P, Bagheri H, Montastruc JL; Overview of adverse reactions to nefopam: an analysis of the French Pharmacovigilance database. Fundam Clin Pharmacol. 2007;21:555–558. doi: 10.1111/j.1472-8206.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 18.Haroutiunian S, Nikolajsen L, Finnerup NB, Jensen TS. The neuropathic component in persistent postsurgical pain: a systematic literature review. Pain. 2013;154:95–102. doi: 10.1016/j.pain.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Joo YC, Ko ES, Cho JG, Ok YM, Jung GY, Kim KH. Intravenous nefopam reduces postherpetic neuralgia during the titration of oral medications. Korean J Pain. 2014;27:54–62. doi: 10.3344/kjp.2014.27.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]