Abstract
Background
Children’s health programs in Sub-Saharan Africa have always been oriented primarily to infectious diseases and malnutrition. We are witnessing in the early 21st century an epidemiological transition marked by the decline of old diseases and the identification of new diseases including heart disease. Therefore, it is necessary to describe the spectrum of these diseases in order to better prepare health workers to these new challenges.
Methods
We conducted a cross-sectional study focused on heart disease diagnosed by echocardiography in children seen from January 2006 to December 2014 in a pediatric hospital of Yaounde. We collected socio-demographic data and the types of heart disease from registers, patients files as well as the electronic database of echocardiographic records.
Results
A total of 2,235 patients underwent echocardiographic examination during the study period including 1,666 subjects with heart disease. Congenital cardiopathies were found in 1,230 (73.8%) patients and acquired abnormalities in 429 (25.8%). Seven children (0.4%) had a combination of both types. Congenital heart defects (CHD) were dominated by ventricular septal defect (VSD). Acquired heart disease was mostly rheumatic valvulopathies. Dyspnea on exertion was the most frequent presenting complaint (87.6%). Discovery of a heart murmur was the principal clinical finding on physical examination (81.4%). The median age was 9 months for congenital heart disease and 132 months for acquired heart disease.
Conclusions
As infectious diseases recede and the diagnostic facilities are improving, pediatric heart diseases occupy a more important position in the spectrum of pediatric diseases in our context. However, the ability to evoke the diagnosis remains unsatisfactory by the majority of health personnel and therefore needs to be improved. Apart from congenital heart diseases, the impact of acquired heart diseases, rheumatic valvulopathy being the highest ranking, is remarkable in pediatrics. Awareness of health personnel for better management of child tonsillitis is more than ever a necessity. This preventive attitude of rheumatic heart disease is the main attitude available in our disadvantaged economic environment.
Keywords: Heart disease, congenital, acquired, echocardiography, diagnosis, child, Yaounde
Introduction
In order to attain the millennium development goals (MDGs), several health programs have been established aiming at reducing infectious diseases and malnutrition: the principal causes of death in children of developing countries (1-3). However Africa is witnessing an epidemiological transition marked by the emergence of non-communicable diseases among children (4-6). The highest ranking among such conditions include sickle cell anemia, diabetes and metabolic diseases, as well as heart disease in children (7).
Worldwide, congenital heart defects (CHD) are the main heart diseases found in children. Heart defects have a prevalence of eight cases per 1,000 live births across the globe, representing approximately 1.35 million newborns each year with CHD (8). The impact appears to vary only slightly by region of the world, but the biggest contrast lies in the differences of priorities that are granted according to whether it is in the Western or in the southern countries. Apart from these, the African child also faces the additional burden of acquired heart disease, the highest ranking being rheumatic valvulopathie (9,10). Rheumatic fever (RF), stimulated and promoted by precarious socio-economic conditions, has become an exceptional occurrence in high income countries. However, it remains a public health problem in low income countries (9-11).
Northern countries have reached an excellent level of management for these disorders through prevention strategies, prenatal diagnosis and even in utero treatment of some CHD. Sub-Saharan countries have not yet developed real screening strategies for these diseases that are thus mostly discovered late or when complications have occurred. Moreover, in our countries, heart disease in children is not yet considered as one of the priorities of governments. However, in recent years, thanks to improved technical facilities in hospitals, heart disease is emerging and is now one of the main points of interest. The increasingly widespread use of echocardiography has a significant impact on the diagnosis of heart diseases in children (12,13).
We therefore report a 9-year experience summarizing the circumstances of diagnosis and the different types of heart disease described in echocardiography performed in a group of children attending a pediatric hospital in Yaounde, Cameroon.
Methods
Study type
The study was cross sectional and covered the period from January 2006 to December 2014. Data were collected from records of echocardiography, patient’s files and the database stored in the echocardiography device.
Study site
The study was conducted at the Mother and Child Centre of the Chantal Biya Foundation (MCC/CBF). The MCC/CBF is a Pediatric Hospital in Yaounde, city of nearly two and a half million inhabitants. This hospital which serves as a university teaching hospital, with a capacity of 260 beds, is the largest pediatric hospital of Cameroon. It receives about 30,000 children a year and 9,000 are hospitalized. The medical staff has 15 pediatricians, 15 general practitioners and between 5-10 pediatric residents. A pediatric cardiology unit exists in this center since January 2006 for management of pediatric cardiopathies and where echocardiograms are performed. Patients are spontaneously taken to this unit or are referred by doctors from other hospitals of the city, or other regions and even from neighboring countries.
Study population
It consisted of patients aged 0 to 16 years with a heart defect, for whom diagnostic echocardiography was done for the first time at MCC/CBF during the study period. We excluded patients who were referred from other structures with an ultrasound diagnosis of heart disease already available.
At MCC/CBF, we use an Accuson Cypress Siemens echocardiograph with two multifrequency heart probes: 3V2c (3.5/3.0/2.5/2.0 MHz) and 7V3c (7.0/6.0/5.0/3.5 MHz). The same operator performs all echocardiograms, all transthoracic in TM-mode, two-dimensional, pulsed Doppler, continuous and color. Each patient is systematically explored in the following incidences in this chronological order: subcostal, parasternal long axis, parasternal short axis, apical 4-chamber view, apical 5-chamber and finally suprasternal. According to the appropriate incidence, the exploration can be done with the patient supine, lateral left or rarely right side (in cases of dextrocardia). All echocardiographic measurements were made following the recommendations of the “American Society of Echocardiography”. All diagnoses are based on standard criteria (14).
Rheumatic valvulopathy was diagnosed based on predefined criteria for different types of pathologies by the “World Heart Federation criteria for the echocardiographic diagnosis of rheumatic heart disease in individuals aged ≤20 years” (15);
Congenital heart disease was defined by the presence in the 2D color Doppler echocardiography or conventional images of the various anomalies (16);
Dilated cardiomyopathy (CMP) was declared with images of expanded heart chambers and reduced systolic function of the left ventricle (LV) in the absence of chronic increased afterload or volume overload (17,18);
Hypertrophic CMP was declared with discovery of an asymmetric hypertrophy predominantly affecting the interventricular septum (19,20);
The endomyocardial fibrosis (EMF) was diagnosed after findings of apical obliteration of one or both ventricles, thickening of the endocardium, atrioventricular valve leakage and dilatation of the atrium on the affected side (21);
Pericardial effusion was diagnosed with discovery of an anechoic space between the visceral and parietal pericardium.
The register of echocardiography had records of the exam numbers with: exam date, patient’s name, age, sex, weight, height, oxygen saturation (SaO2) on the finger, permanent residence, person who requested the exam, indication(s) of echocardiography, ultrasound diagnosis, pulmonary artery pressure (PAP), presence of symptoms of rheumatic valvulopathy and two telephone numbers of family members. The iconography was stored in the hard drive of the ultrasound machine and copied to an external hard drive once a month.
The data of patients diagnosed with heart disease were transcribed to a register of heart disease which also included parents’ professions, the presence or absence of dysmorphic features in the patient, or the reason(s) for the first consultation (from the patient’s medical book/file) and the main clinical signs of heart disease.
For the purposes of this study, we have classified the heart diseases as: congenital, acquired and mixed; which involve congenital and acquired lesions.
Ethical considerations
This study was approved by the ethics committee of the Chantal Biya Foundation.
Statistical analysis
The data were entered into Excel and then transcribed in SPSS version 11.0 (SPSS, Inc. Chicago, Illinois, USA). Continuous variables were expressed as median with interquartile range (IQR) and categorical variables as percentages. Differences between the proportions were analyzed using chi2 test. P values <0.05 were considered significant.
Results
Between January 2006 and December 2014, the MCC/CBF recorded 273,117 consultations for patients under the age of 16. A total number of 2,235 new patients had benefited from an echocardiography test, among which 1,666 had heart diseases. In addition, 117 already had an echocardiographic diagnosis of heart disease and were excluded from our study.
Origin of patients
The patients came from all regions of the country with more than half (59.1%) from the central region where our hospital is located. There were 15 patients of foreign origin: 2 from Nigeria, 3 from Equatorial Guinea, 4 from Chad and 6 from the Central African Republic. Figure 1 shows the geographic origin of patients diagnosed with heart disease.
Figure 1.
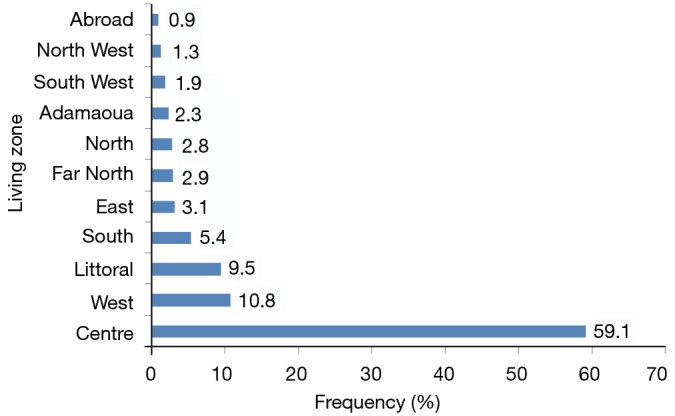
Place of residence of patients diagnosed for heart disease.
Age at diagnosis and sex
Patient age at diagnosis ranged from one day to 15 years 10 months. Those with congenital heart disease were significantly younger (median age 9.0 months; IQR, 4–30 months) compared to those with acquired heart diseases (median age 132 months; IQR, 96-152 months). The males were 800 against 866 females, a sex ratio M/F 0.92.
Consultation methods
We received 1,354 (81.3%) patients on consultation (before admission into the echocardiography room) and 312 (18.7%) for the first time in the exam room for echocardiography. From the first forementioned group, consultation in our service was spontaneous (personal initiative of parents) in 703 (51.9%) patients. Health workers had referred 651 patients (48.1%) to our consultation among which 28.4%, 15.2%, 2.5% and 2.0% where referred by pediatricians, general practitioners, other specialists and nurses respectively.
Clinical presentation at diagnosis
We collected complaints from parents and from their health book at the first consultation of 1,172 patients (70.3%). The most frequent complaint was dyspnea on exertion. A single patient could have several complaints. Table 1 summarizes the different complaints presented.
Table 1. Presenting complaints of patients at the first consultation.
| Presenting complaint | Numbers (N=1,172) | Percentage (%) |
|---|---|---|
| Dyspnea on exertion | 1,027 | 87.6 |
| Failure to thrive | 720 | 61.4 |
| Cough | 592 | 50.5 |
| Sweating | 480 | 40.9 |
| Anorexia | 452 | 38.6 |
| Abdominal distension | 309 | 26.3 |
| Lower limb edema | 85 | 7.3 |
| systematic consultation | 72 | 6.1 |
| Thoracic deformity | 59 | 5.0 |
| Chest pain | 46 | 3.9 |
| Cyanosis | 45 | 3.8 |
During physical examination, the presence of a heart murmur was by far the most frequent sign found (81.3%). It was mostly a systolic murmur whose intensity was at least grade 3/6 in 902 patients (75.4%). Table 2 summarizes those signs. A patient could have several clinical signs. The heart disease diagnosis was made in 116 patients who were followed up for pathology or who had been brought in for well-child check.
Table 2. Clinical signs at physical examination.
| Clinical signs | Numbers (N=1,197) | Percentage (%) |
|---|---|---|
| Heart murmur | 974 | 81.4 |
| Dyspnea at rest | 791 | 66.1 |
| Hepatomegaly | 633 | 52.9 |
| Turgescent jugular veins | 345 | 28.8 |
| Cyanosis | 195 | 16.3 |
| Muffled heart sounds | 183 | 15.3 |
| Split second heart sound | 142 | 11.9 |
| Dysrhythmia | 104 | 8.7 |
| Lower limb edema | 96 | 8.0 |
| Thoracic deformity | 69 | 5.8 |
| Ascites | 62 | 5.2 |
| Facial dysmorphy | 58 | 4.8 |
| Pericardial rub | 27 | 2.3 |
| Others | 38 | 3.2 |
Ultrasound diagnosis
Echocardiographic examination enabled the discovery of 1,230 patients with congenital heart disease (73.8%), 429 with acquired heart disease (25.8%) and 7 mixed, an association of a congenital malformation and an acquired lesion (0.4%). Table 3 summarizes the different types of pathologies diagnosed.
Table 3. Different types of diagnosed heart diseases.
| Type of cardiopathy | Number (N=1,666) | Percentage (%) |
|---|---|---|
| Congenital heart defects (CHD) (N=1,230) | ||
| VSD | 460 | 37.2 |
| PS | 186 | 15.0 |
| PDA | 170 | 13.7 |
| ASD | 136 | 11.1 |
| ToF | 102 | 8.2 |
| AVCD | 66 | 5.3 |
| Other associations of congenital heart diseases | 58 | 4.7 |
| TA | 18 | 1.5 |
| TGV + ASD | 8 | 0.6 |
| PAVSD | 6 | 0.4 |
| TrA | 5 | 0.4 |
| APVR | 5 | 0.4 |
| Hypoplastic left heart | 4 | 0.3 |
| Coarctation of the aorta | 2 | 0.2 |
| Ebstein’s anomaly | 2 | 0.2 |
| Cardiac ectopy | 2 | 0.2 |
| Acquired cardiopathies (N=429) | ||
| Rheumatic valvulopathy | 206 | 48.0 |
| Cardiomyopathies | ||
| Dilated | 108 | 25.2 |
| Restrictive (EMF) | 53 | 12.4 |
| Hypertrophic | 10 | 2.3 |
| Isolated pericarditis | 50 | 11.7 |
| Right atrium tumor | 2 | 0.5 |
| Mixed cardiopathies (N=7) | ||
| VSD + MR | 3 | 42.9 |
| PDA + MR | 2 | 28.6 |
| ASD + MR | 2 | 28.6 |
PAVSD, pulmonary atresia with ventricular septal defect; AVCD, atrioventricular canal defect; ASD, atrial septal defect; VSD, ventricular septal defect; EMF, endomyocardial fibrosis; MR, mitral regurgitation; PDA, patent ductus arteriosus; APVR, anomalous pulmonary venous return; PS, pulmonary stenosis; TA, truncus arteriosus; TrA, tricuspid atresia; TGV, transposition of great vessels; ToF, Tetralogy of Fallot.
Groups of heart diseases and their ages at diagnosis
Patients with congenital heart disease were significantly younger than those with acquired heart disease. Table 4 summarizes the distribution of types of heart diseases by age groups.
Table 4. Distribution of heart diseases by age groups.
| Type of cardiopathy | Age group (years) |
Total, N (%) | ||
|---|---|---|---|---|
| ≤ 5, N (%) | 6-10, N (%) | 11-15, N (%) | ||
| Congenital heart diseases | 1,132 (67.9) | 63 (3.8) | 35 (2.1) | 1,230 (73.8) |
| Acquired cardiopathy | 99 (6.0) | 172 (10.3) | 158 (9.5) | 429 (25.8) |
| Mixed cardiopathy | 0 (0) | 2 (0.1) | 5 (0.3) | 7 (0.4) |
| Total | 1,238 (73.9) | 232 (14.2) | 196 (11.9) | 1,666 [100] |
Congenital heart diseases
Among the 1,230 with congenital heart disease, 154 (12.5%) were cyanotic while 1,076 (87.5%) were acyanotic. Ventricular septal defect (VSD) was by far the most common congenital heart disease while Tetralogy of Fallot (ToF) was the most common cyanotic congenital heart disease found. Atrioventricular canal was predominant in patients with clinical features of down syndrome (90.9%). We found two cases of cardiac ectopy; one of which was probably a component of a class 2 pentalogy of Cantrell.
Acquired heart disease
Rheumatic valvulopathies
We found rheumatic valvular lesions in 206 patients. Mitral valve lesion was constant (100%). All patients with rheumatic heart disease had mitral regurgitation (MR). It was isolated in 125 (60.7%) patients. In the other patients, it was associated with other valvulopathies. The least affected valve was the pulmonary valve, seen in only 4 patients (2%). Table 5 summarizes the different types of rheumatic valvular lesions found. We noticed authentic rheumatic valvular lesions in three children under 2 years.
Table 5. Distribution of the different rheumatic valvular lesions depending on the age of patients.
| Valvular lesion | Age of patient (months) |
Total N (%) | |||
|---|---|---|---|---|---|
| <24, N (%) | 60-119, N (%) | ≥120, N (%) | |||
| Isolated MR | 2 (1.6) | 125 (60.7) | 70 (56.0) | 45 (36.4) | 125 (60.7) |
| MR + AR | 1 (2.9) | 34 (16.5) | 12 (35.3) | 17 (50.0) | 34 (16.5) |
| MR + TR | 0 (0.0) | 08 (3.9) | 2 (25.0) | 5 (72.5) | 08 (3.9) |
| MR + AR + TR | 0 (0.0) | 17 (8.3) | 5 (29.4) | 12 (70.6) | 17 (8.3) |
| MR + AR + TR + PR | 0 (0.0) | 4 (2.0) | 2 (50.0) | 1 (25.0) | 4 (2.0) |
| MR + MS + AR + TR | 0 (0.0) | 9 (4.3) | 1 (11.1) | 8 (88.9) | 9 (4.3) |
| MR + MS + TR | 0 (0.0) | 2 (1.0) | 0 (0.0) | 2 (100) | 2 (1.0) |
| MR + MS + AR | 0 (0.0) | 7 (3.3) | 0 (0.0) | 7 (100) | 7 (3.3) |
| Total | 3 (1.5) | 206 [100] | 92 (44.7) | 97 (47.0) | 206 [100] |
AR, aortic regurgitation; MR, mitral regurgitation; PR, pulmonary regurgitation; TR, tricuspid regurgitation; MS, mitral stenosis.
Cardiomyopathy (CMP)
We found mostly dilated (63.2%) CMP and restrictive CMP (31.0%). Table 6 shows different types of CMP found.
Table 6. Different types of cardiomyopathy (CMP).
| Type of CMP | Number | % |
|---|---|---|
| Dilated cardiomyopathies (N=108) | ||
| Dilated CMP of sickle cell disease | 66 | 38.6 |
| Dilated CMP in patients on anticancer treatment | 22 | 12.9 |
| Idiopathic dilated CMP | 20 | 11.7 |
| Other cardiomyopathies (N=63) | ||
| Hypertrophic CMP | 10 | 5.8 |
| Restrictive CMP | 53 | 31.0 |
| Total | 171 | 100.0 |
CMP, cardiomyopathy.
Dilated CMP found in sickle cell disease patients was hyperkinetic in 61 patients (92.4%) and hypokinetic in 5 patients (7.6%). Restrictive CMP was exclusively in patients with EMF. We found a dilated CMP in 20 children without any particular chronic condition such as sickle cell disease, HIV infection or chronic anemia. These were classified in the category of idiopathic dilated CMP.
Other acquired heart diseases
These were 48 cases of isolated pericarditis, and two cases of left atrial tumors.
Discussion
Geographical origin of patients
The patients seen at our unit were from all the ten regions of the country and some from abroad. However, the vast majority (58.7%) lived in the central region where our hospital is located. The proximity of the structure probably contributes to this predominance. Moreover, this site is located in a town where there are several university hospitals with competent staff including cardiologists and pediatric specialists, more qualified to establish a diagnosis and refer to our unit.
Consultation method
Patients were most often brought for consultations by their parents (51.9%). However, 651 patients (39.1%) were referred mostly by pediatricians although these specialists are fewer compared to nurses who referred very few patients, probably due to lack of skills to make the diagnosis.
Clinical presentation at diagnosis
Dyspnea on exertion which was the most frequently presented complain, was a sign of heart failure. Patients arrived late, at the stage of complications. Ignorance of signs of heart disease or underestimation of disease severity by parents contributed to the delay. In addition, most health workers ignore these signs, contributing to the diagnostic error. Apart from reference health centers, nurses are the first to see children brought by their parents. Only 2% of children were referred by a nurse. It is thus necessary to train them on certain aspects of pediatric semiology.
Presence of a heart murmur was the first clinical sign found on physical examination. This is a common sign of heart disease in children (22). The murmurs were generally of high intensity indicating their organic character (22,23). The fact that such obvious murmurs were not previously detected either shows that children were not at all auscultated, at least for the cardiovascular examination, or health workers who auscultated had ignored its semiological value. Other signs were essentially signs of heart failure revealing complication of the disease. There would be a real need for training of health personnel in the clinical evaluation of the cardiovascular system, including/especially cardiac auscultation.
Types of heart diseases
Congenital heart diseases
Congenital heart diseases accounted for three quarters of our sample while the remaining quarter was acquired heart diseases. For congenital heart diseases, VSD were most common (37.2%) as described in the literature (24,25). This was followed by pulmonary stenosis and PDA. These two anomalies are very common in congenital rubella (26). In our environment, the Rubella vaccination coverage among women of childbearing age is very low (27). It is estimated that many children with these two malformations suffer from congenital rubella. We still have not had the opportunity to systematically investigate the existence of rubella antibodies to enable us to directly incriminate this infection. ToF occupied 5th place (8.2%) among all congenital heart diseases in our series and the first cause of cyanotic congenital heart disease as described in the literature (25,28). This heart disease does not cause early heart failure, although it can be quite symptomatic depending on the anatomical type. The discovery, sometimes, in older patients who however manifest typical clinical signs demonstrates the ignorance of parents and some of the health personnel on the condition. ASD, described in the literature as the second ranking condition was found in the fourth position in our series with a frequency of 12.6%. These are usually asymptomatic anomalies or anomalies tolerated for long periods. In our series we found primarily symptomatic abnormalities since the patients were seen after appearance of complications. ASD that are not large rarely get complicated. Auscultation reveals a murmur of mild intensity that can easily go unnoticed. In addition, recurrent bronchitis that is most often found in the clinical characteristics is not always linked to the heart condition by unaccustomed staff, especially in an environment where respiratory infections are frequent. Coarctation of the aorta was rare in our series. Only two cases were reported. All these cases were referred by a pediatrician in the neonatology department of our hospital. This condition accounts for only 0.2% of heart disease in our series, unlike the 6% reported in literature (25,28). This is therefore an anomaly probably under-diagnosed in our environment. Screening should be done in the first hours and the first days of life by the systematic palpation of the femoral pulse by trained personnel. This is far from the case in our maternities where there is usually no doctor, let alone any pediatrician. The examination of the newborn by a trained staff before discharge from a maternity is not routine in most hospitals. Moreover, for economic reasons, the majority of women give birth in private clinics or health centers with ill-qualified paramedics. An unmanaged tight coarctation evolves very rapidly over a few days to clinical signs of cardiovascular collapse similar to a septic shock, a very common finding in our neonatal units. It is possible that many patients have died of coarctation while incorrectly diagnosed with septic shock.
Acquired heart diseases
They were dominated by rheumatic valvulopathies representing 48% of acquired heart diseases and 12.4% of all heart diseases. The sequelae of rheumatic fever have become exceptional in Western countries (29-31).
They still represent a real health problem in developing countries in general and Africa in particular (32). Worldwide, about 470,000 cases of rheumatic fever are reported each year and nearly 233,000 deaths from rheumatic fever and rheumatic carditis. Nearly all cases occur in developing countries. Poverty, poor sanitation and inadequate health systems promote rheumatic fever which results in valvular complications (33,34). In our country, no strategy recommended by the World Federation of the heart is implemented (11). The cases we found represent only a small portion of the actual existing cases. They were more often already advanced cases. A study in the population would surely have shown significantly more subjects with rheumatic fever sequelae. Marijon et al. who did screening in schools of Cambodia and Mozambique found that 2–3% of students were carriers of rheumatic valvular lesions. Almost all of these valvulopathies had not been diagnosed previously (34). Mitral valve involvement was a constant finding in our series. Other valves involvements, when they were present, were associated to mitral valve injury. The mitral valve in our environment can be used in echocardiographic lab as a “sentinel” to detect the rheumatic valuvulopathy in patients at risk of having one.
Cardiomyopathies were the next most frequent after rheumatic valvulopathies. They were dominated by the CMP of sickle cell disease. Sickle cell disease is a common haemoglobinopathy in sub Saharan Africa. In Cameroon, the severe form represents 2% of the population (35). This condition is characterized by chronic anemia with consequences on some organs including the heart (36,37). Chronic anemia of sickle cell disease is the cause of a compensatory elevation in cardiac output, resulting in left ventricular overload characterized by dilation of the LV with an increased end-systolic volume. This is the clinical picture of dilated hyperkinetic CMP (38).
Age at diagnosis and different types of heart diseases
It was not surprising that congenital heart diseases were diagnosed earlier than the acquired diseases. If not for the deficiencies in our screening system, diagnosis of congenital heart diseases would have been done earlier as is the case in Western countries (39,40). In our study, early diagnosis depended very much on the severity of the heart disease. Thus all cases (100%) of tricuspid atresia (TrA), hypoplastic left heart were diagnosed in neonatal period. Similarly, anomalous pulmonary venous return, truncus arteriosus (TA) and Epstein’s disease were discovered in the neonatal period, representing 75%, 66.6% and 66.6% of cases respectively. All these heart abnormalities have particularly obvious signs that will worry parents and cause them to consult earlier than for other heart diseases. The age of diagnosis of rheumatic valvulopathy was higher. Rheumatic fever is a disease of school-age children with its complications appearing most often in adolescence (41). Note that, in our study however, three authentic cases of rheumatic valvulopathies in infants less than two years were found. Two of these children had a cleft lip and palate while the other suffered from adenoids hypertrophies. All these diseases are responsible for recurrent upper respiratory tract infections and have probably been the site of streptococcal infections that cause rheumatic sequelae. Cardiopathies of Sickle cell disease are late complications due to chronic anemia and vasculopathies (38). EMF is also a disease of adolescents and young adults (21,42).
Strengths and limitations
The strength of our study lies in its implementation in a pediatric center populate with all social classes from all regions of the country and even abroad. It is thus possible to have a fairly accurate idea of the spectrum of heart disease in these patients. Moreover, the presence of pediatricians and other cardiology specialists both in our hospital and in neighboring hospitals facilitated recruiting. However, our study also has some limitations; it is but a cross sectional and retrospective study and we did not have all of the clinical information of all patients.
Conclusions
Heart diseases represent an important burden in the child’s disease in Cameroon. These heart diseases are significantly dominated by congenital defects. However, rheumatic cardiopathies are strongly represented, unlike in the West, where they have almost disappeared. These conditions are unfortunately under-diagnosed, which is why they are usually discovered late; at the stage of complications. Much remains to be done in terms of training of medical personnel for the early detection of these diseases that are part of the new challenges of Africa at the time of the epidemiological transition. Awareness and education of people as far as primary prevention of rheumatic fever is concerned are particularly necessary to reduce the predominance of cardiac sequelae.
Echocardiography appears to be an important diagnostic tool cost-effective and non-invasive. Practice should be encouraged in pediatric setting for the diagnosis of congenital heart diseases and rheumatic valve diseases screening.
Acknowledgements
We are grateful to all the staff of Mother and Child Center, Chantal Biya Foundation, especially those of the pediatric cardiology unit for their great support.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Nations Unies. Objectifs du Millénaire pour le développement rapport 2009. Available online: http://www.un.org/fr/millenniumgoals/pdf/PR%20Global%20MDG09%20FR.pdf
- 2.Lapeyre F. Objectifs du millénaire pour le développement: outils de développement ou cheval de troie des politiques néolibérales? Available online: https://www.uclouvain.be/cps/ucl/doc/dvlp/documents/2006-1edito-1(1).pdf
- 3.Unicef. The State of The World’s Children 2009: Maternal and Newborn Health. 2009. Available online: http://www.unicef.org/sowc09/
- 4.Touze JE. Cardiovascular diseases and the epidemiological transition in tropical regions. Med Trop (Mars) 2007;67:541-2. [PubMed] [Google Scholar]
- 5.World Health Organization. Health statistics and information systems. Available online: http://www.who.int/healthinfo/en/
- 6.World Health Organization. Noncommunicable diseases: a major health challenge of the 21st century. Available online: https://www.rcpe.ac.uk/sites/default/files/files/malik_moledina_globalhealthessay.pdf
- 7.van der Sande MA. Cardiovascular disease in sub-Saharan Africa: a disaster waiting to happen. Neth J Med 2003;61:32-6. [PubMed] [Google Scholar]
- 8.van der Linde D, Konings EE, Slager MA, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241-7. [DOI] [PubMed] [Google Scholar]
- 9.Zühlke L, Mirabel M, Marijon E. Congenital heart disease and rheumatic heart disease in Africa: recent advances and current priorities. Heart 2013;99:1554-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ansell B. Rheumatic Heart Disease. Ann Rheum Dis 1962;21:394. [Google Scholar]
- 11.WHO Study Group on Rheumatic Fever and Rheumatic Heart Disease (2001 : Geneva S, Organization WH. Rheumatic fever and rheumatic heart disease: report of a WHO expert consultation, Geneva, 20 October - 1 November 2001. 2004. Available online: http://apps.who.int/iris/handle/10665/42898
- 12.Sani MU, Mukhtar-Yola M, Karaye KM. Spectrum of congenital heart disease in a tropical environment: an echocardiography study. J Natl Med Assoc 2007;99:665-9. [PMC free article] [PubMed] [Google Scholar]
- 13.Ogah OS, Adegbite GD, Akinyemi RO, et al. Spectrum of heart diseases in a new cardiac service in Nigeria: an echocardiographic study of 1441 subjects in Abeokuta. BMC Res Notes 2008;1:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheitlin MD, Alpert JS, Armstrong WF, et al. ACC/AHA Guidelines for the Clinical Application of Echocardiography. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. Circulation 1997;95:1686-744. [DOI] [PubMed] [Google Scholar]
- 15.Reményi B, Wilson N, Steer A, et al. World Heart Federation criteria for echocardiographic diagnosis of rheumatic heart disease--an evidence-based guideline. Nat Rev Cardiol 2012;9:297-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chetboul V, Pouchelon JL. Diagnostic échodoppler des cardiopathies congénitales. EMC – Vétérinaire 2004;1:175-90.
- 17.Thomas DE, Wheeler R, Yousef ZR, et al. The role of echocardiography in guiding management in dilated cardiomyopathy. Eur J Echocardiogr 2009;10:iii15-21. [DOI] [PubMed] [Google Scholar]
- 18.Venugopalan P. Pediatric Dilated Cardiomyopathy: Practice Essentials, Background, Pathophysiology. 2014. Available online: http://emedicine.medscape.com/article/895187-overview#a1
- 19.Colan SD. Hypertrophic cardiomyopathy in childhood. Heart Fail Clin 2010;6:433-44, vii-iii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seggewiss H, Rigopoulos A. Management of hypertrophic cardiomyopathy in children. Paediatr Drugs 2003;5:663-72. [DOI] [PubMed] [Google Scholar]
- 21.Falase AO, Kolawole TM, Lagundoye SB. Endomyocardial fibrosis. Problems in differential diagnosis. Br Heart J 1976;38:369-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Segaier M, Lilja O, Lukkarinen S, et al. Computer-based detection and analysis of heart sound and murmur. Ann Biomed Eng 2005;33:937-42. [DOI] [PubMed] [Google Scholar]
- 23.Rein AJ, Omokhodion SI, Nir A. Significance of a cardiac murmur as the sole clinical sign in the newborn. Clin Pediatr (Phila) 2000;39:511-20. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002;39:1890-900. [DOI] [PubMed] [Google Scholar]
- 25.Robert-Gnansiaa E, Francannetb C, Bozioc A, et al. Épidémiologie, étiologie et génétique des cardiopathies congénitales. EMC - Cardiologie-Angéiologie 2004;1:140-60.
- 26.Bourquin JB. Les malformations du nouveau-né causées par des viroses de la grossesse et plus particulièrement par la rubéole: embryopathie rubéoleuse. Champagne-Ardenne: E. Le François, 1948. [Google Scholar]
- 27.World Health Organization. Lutte contre la rubéole et le syndrome de rubéole congénitale (SRC) dans les pays en développement. 2001. Available online: http://www.chu-rouen.fr/page/doc/DOC_8464
- 28.Iselin M. Cardiopathies congénitales. Encycl Méd-Chir 1999;32-015-A-12.
- 29.Bertrand E, Gérard R. Comparison of hospital prevalence of rheumatic heart diseases and acute rheumatic arthritis in France and Africa. Arch Mal Coeur Vaiss 1993;86:291-5. [PubMed] [Google Scholar]
- 30.Olivier C. Le rhumatisme articulaire aigu chez l'enfant aujourd'hui = Rheumatic fever in today's children. La Presse médicale 1998;27:1159-67. [PubMed] [Google Scholar]
- 31.Rotta J, Tikhomirov E. Les maladies à streptocoques dans le monde: situation actuelle et perspectives. Bull World Health Organ 1988;66:15-21. [Google Scholar]
- 32.Carapetis JR, Steer AC, Mulholland EK, et al. The global burden of group A streptococcal diseases. Lancet Infect Dis 2005;5:685-94. [DOI] [PubMed] [Google Scholar]
- 33.Feldman T. Rheumatic heart disease. Curr Opin Cardiol 1996;11:126-30. [DOI] [PubMed] [Google Scholar]
- 34.Marijon E, Ou P, Celermajer DS, et al. Prevalence of rheumatic heart disease detected by echocardiographic screening. N Engl J Med 2007;357:470-6. [DOI] [PubMed] [Google Scholar]
- 35.Comité régional de l’Afrique. La drépanocytose dans la Région africaine: situation actuelle et perspectives: rapport du Directeur régional. 2006. Available online: http://apps.who.int/iris/handle/10665/5600
- 36.Batra AS, Acherman RJ, Wong WY, et al. Cardiac abnormalities in children with sickle cell anemia. Am J Hematol 2002;70:306-12. [DOI] [PubMed] [Google Scholar]
- 37.Lamers L, Ensing G, Pignatelli R, et al. Evaluation of left ventricular systolic function in pediatric sickle cell anemia patients using the end-systolic wall stress-velocity of circumferential fiber shortening relationship. J Am Coll Cardiol 2006;47:2283-8. [DOI] [PubMed] [Google Scholar]
- 38.Retentissement cardiaque de la drépanocytose chez l’enfant. Médecine thérapeutique / Pédiatrie 2008;11:52-4.
- 39.Massin M, Malekzadeh-Milani S, Dessy H. Diagnostic des cardiopathies congénitales. Available online: https://www.google.com.hk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&cad=rja&uact=8&ved=0CB4QFjAAahUKEwiZ_Obi94fJAhUFw2MKHTynCC0&url=http%3A%2F%2Fwww.amub.be%2Frevue-medicale-bruxelles%2Fdownload%2F328&usg=AFQjCNHgZHtyAkDSzx9ZYlLRadccSVPimQ [PubMed]
- 40.Perthus I, Amar E, De Vigan C, et al. État des lieux des registres de malformations congénitales en France en 2008. Bull Epidemiol Hebd 2008;28:246-8. [Google Scholar]
- 41.Marijon E, Mirabel M, Celermajer DS, et al. Rheumatic heart disease. Lancet 2012;379:953-64. [DOI] [PubMed] [Google Scholar]
- 42.Williams AW, Ball JD, Davies JN. Endomyocardial fibrosis in Africa: its diagnosis, distribution and nature. Trans R Soc Trop Med Hyg 1954;48:290-305; discussion, 306-11. [DOI] [PubMed] [Google Scholar]


