Abstract
Angina, heart failure with preserved ejection fraction (HFpEF) and coronary microvascular dysfunction (CMD) in the absence of obstructive coronary artery disease (CAD) are more common in women and are associated with adverse cardiovascular prognosis. Cardiac magnetic resonance imaging (CMRI) is established for assessment of left ventricular (LV) morphology and systolic function and is increasingly used to assess myocardial perfusion and diastolic function. Indeed, stress CMRI allows measurement of myocardial perfusion reserve index (MPRI) using semi-quantitative techniques, and quantification of LV volumetric filling patterns provides valuable insight into LV diastolic function. The utility of these two techniques remains limited, because reference control values for MPRI and LV diastolic function in asymptomatic middle-aged, women have not previously been established. To address this limitation, we recruited twenty women, without clinical cardiovascular disease or cardiovascular risk factors, with normal maximal Bruce protocol exercise treadmill testing. Subjects underwent CMRI (1.5 tesla) using a standardized protocol of adenosine stress and rest perfusion and LV cinematic imaging. Commercially available with automated CMRI segmentation was used for calculation of MPRI, LV filling profiles, and ejection fraction. Mean age was 54±9 years and mean body mass index was 25±4 kg/m3. The exercise treadmill testing results demonstrated a normotensive group with normal functional capacity and hemodynamic response. We report reference control values for semi-quantitative MPRI as well as measures of LV systolic and diastolic function including ejection fraction, stroke volume, peak filling rate (PFR), PFR adjusted for end-diastolic volume (EDV) and stroke volume, time to PFR, and EDV index. The data herein provide reference values for MPRI and diastolic function in a cohort of healthy, middle-aged of women. These reference values may be used for comparison with a variety of patient populations, including women with CMD and HFpEF.
Keywords: Women, magnetic resonance imaging (MRI), myocardial perfusion, diastolic function
Introduction
Ischemic heart disease (IHD) is the leading cause of mortality among women. While gender-specific differences in the presentation and pathophysiology of IHD are increasingly recognized, the diagnosis of IHD has yet to utilize these differences (1,2). Approximately 50% of women with signs and symptoms of ischemia are found to have no obstructive coronary artery disease (CAD) (2). Rather than systolic dysfunction and obstructive CAD, women are more likely to present with heart failure with preserved ejection fraction (HFpEF) (3) and coronary microvascular dysfunction (CMD) (4). The gold-standard for the diagnosis of CMD is coronary reactivity testing, which is invasive and may be associated with adverse events (5). Noninvasive methods for evaluating CMD are needed. Cardiac magnetic resonance imaging (CMRI) is the gold standard tool for assessing cardiac morphology and global systolic function [i.e., left ventricular (LV) ejection fraction] (6). In addition to these traditional measures, CMRI also has the ability to assess myocardial perfusion and diastolic function.
Stress first-pass perfusion CMRI has a high diagnostic accuracy for detecting obstructive CAD and has demonstrated prognostic value for IHD (7), but data is limited for the diagnosis of CMD, which can produce more diffuse rather than segmental perfusion defects. Semi-quantitative perfusion analysis of adenosine stress CMRI has demonstrated subendocardial hypoperfusion in patients with cardiac syndrome X (8) and predicts prognosis in women with suspected myocardial ischemia and no obstructive CAD in the NHLBI-sponsored Women’s Ischemic Syndrome Evaluation (WISE) (9). Myocardial perfusion reserve index (MPRI) is an emerging semi-quantitative tool for the assessment of myocardial ischemia using pharmacologic stress CMRI has good reproducibility (10), and has demonstrated lower values in the subendocardium compared to the subepicardium (11). MPRI may therefore be a useful tool for evaluating CMD in women with suspected myocardial ischemia and non-obstructive CAD (12), but the range of normal values for MPRI has yet been established.
Diastolic dysfunction is common in the community (13) and is associated with increased morbidity (14) and mortality (15), and current guidelines emphasize the need for measuring diastolic function (16). While echocardiography has replaced invasive LV catheterization for measuring diastolic function and is widely available (17), CMRI has emerged as a reliable modality for measuring diastolic function and is not limited by the acoustic window, providing pristine visualization of the entire heart throughout the cardiac cycle, with excellent spatial and temporal resolution. Preliminary work using CMRI tissue tagging assessment of diastolic dysfunction has demonstrated that diastolic function is impaired in women with suspected CMD (18). However tissue tagging is not a method widely available for the assessment of diastolic dysfunction. The most common approach utilizes the volumetric filling profile of the LV, providing valuable insight into the rate and timing of LV relaxation. Indeed, this approach has previously been used several clinical trials (6) and at least one large community based study (19), and it is the approach being used in the WISE study. Despite the general acceptance of this technique in the literature, however, a need for normative values still remains to allow comparison to middle-aged women with suspected CMD.
The objective of this study was therefore to determine reference values for MPRI and LV diastolic function in asymptomatic middle-aged women, using commercially available automated-CMRI segmentation software.
Methods
Twenty women were recruited at Cedars-Sinai Medical Center based on their age and hormone-use status to match CMD subjects in the WISE trial (NCT00000554) (20-22), to serve as a reference control group Baseline demographic and medical history was collected. Inclusion criteria included the absence of signs or symptoms of myocardial ischemia, absence of cardiac risk factors by Framingham/NCEP criteria, competence to give informed consent and performance of a normal standardized Bruce protocol exercise treadmill test (ETT). Exclusion criteria included contraindications to CMRI (AICD, pacemaker, untreatable claustrophobia or known angio-edema), contraindications to adenosine (including asthma, heart block and sinus node disease), significant COPD, any renal disease, pregnant and lactating women, and the inability to perform treadmill exercise.
Bruce protocol ETT
All subjects underwent maximum symptom-limited ETT according to the Bruce protocol. An abnormal test was defined as an inability to attain at least 85% of the maximum predicted heart rate for age, presence of ST-segment depression ≥1.0 mm horizontal or down-sloping at 80 msec after the J-point during exercise or recovery.
CMRI protocol
All study subjects underwent stress-rest CMRI (1.5T Magnetom Avanto, Siemens Healthcare, Erlangen, Germany). All participants were caffeine free for at least 24 hours prior to vasodilator stress. First-pass perfusion imaging was performed using gadolinium contrast of 0.05 mmol/kg (Gadodiamide, Omniscan, Amersham, Piscataway, NJ, USA), which was infused at 4 mL/sec via an intravenous access in the contralateral arm to that used for vasodilator stress. Contrast was followed by a 20 mL saline flush at 4 mL/sec. Adenosine was administered at a rate of 140 mcg/kg/min using an MRI-compatible infusion pump (Medex) for two minutes prior to the first-pass perfusion scan, and was continued until completion of the perfusion data acquisition (total duration of infusion 3 to 4 minutes).
Subjects received adenosine and underwent stress first-pass perfusion imaging followed 10 minutes later by rest first-pass perfusion imaging. Perfusion images were obtained in three LV short-axis slices (basal, mid and distal LV slice positions) with the following pulse sequence parameters: gradient echo-EPI hybrid perfusion imaging sequence perfusion imaging sequence, TE 1.1 msec, TR 148 msec (for each slice) with zero trigger delay, single preparation pulse, receiver bandwidth 1,420 Hz/pixel, echo train length 4, flip angle 20 degrees, slice thickness 8 mm, image matrix size 160×100 mm2, voxel size 2.2×2.2×8 mm3, parallel imaging factor 2, 3 slices imaged every heartbeat. In the event of a peak stress heart rate >120 beats per minute, the spatial coverage was reduced to two slices for the stress first-pass perfusion scan.
For long-axis and short-axis cine images a retrospectively gated steady-state free precession (true FISP) cine-imaging pulse sequence was used to provide excellent contrast between the blood pool and the myocardium. Cine imaging was performed in LV four chamber and two chamber long axis views and a stack of short axis images were acquired from above the atrio-ventricular valve plane to the apex with breath holding for each image using the following scan parameters: 25 cardiac phases/cycle, slice thickness 8 mm, 2 mm slice gap, TE 1.3 msec, TR 2.5 msec, flip angle 80 degrees, receiver BW 800 Hz/pixel, field of view optimized dependent on patient size to avoid wrap.
CMRI analysis
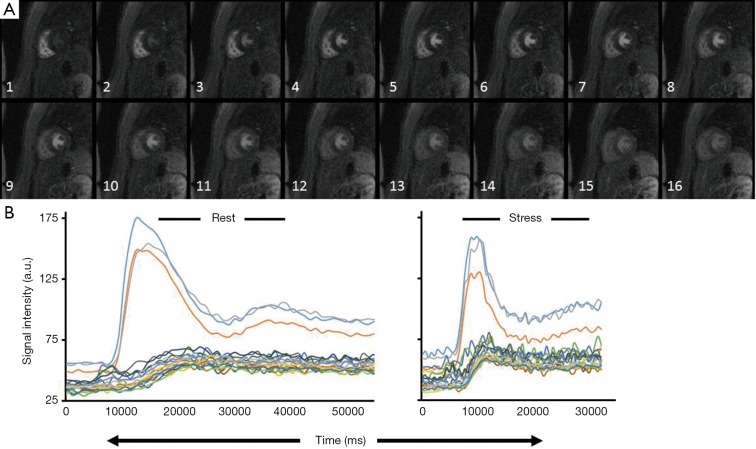
CAAS MRV software (Pie Medical Imaging, B.V., Netherlands) with semi-automated CMRI segmentation was used for calculation of MPRI, ventricular volumetric filling profiles, and ejection fraction. CMRI data were analyzed in a dedicated core laboratory with review of all data contours by an experienced reader. The myocardial region for first pass perfusion evaluation was defined by manually tracing endocardial and epicardial contours to avoid inclusion of the ventricular blood pools and to exclude any linear dark rim artifact at the LV cavity/endocardial border. If the LV outflow tract was imaged in the basal imaging slice, this anteroseptal segment was excluded from analysis. LV cavity region of interest was adjusted to include the region of maximal signal intensity. Relative upslope was defined as the ratio between the maximum upslope of the first-pass myocardial perfusion time-intensity curve divided by the maximum upslope of the first-pass LV cavity time-intensity curve. For analysis of the MPRI, the relative upslope at stress was divided by the relative upslope at rest, and the American Heart Association standard 16-segment model was used. A good quality first-pass signal intensity time curve was defined as having a clearly defined single bolus LV input function and smooth and uniform segmental signal intensity slopes during the first pass of contrast (Figure 1). The mean MPRI was the average of all 16 segments. Reproducibility analysis has previously shown good intra- and inter-observer reproducibility, with intra-observer coefficient of variation of 3.6% and inter-observer coefficient of variation of 7.5% (10).
Figure 1.
Cardiac magnetic resonance imaging (CMRI) endocardial and epicardial contours. (A) Representative image for a control subject demonstrating first pass gadolinium perfusion. Demonstrated is the initial uptake of gadolinium in the right ventricular cavity followed by gadolinium uptake in the left ventricular (LV) cavity and finally uptake into the myocardium; (B) signal intensity time curves at rest (left) and in response to 140 mcg/kg/min of adenosine (stress) (right). Intensity over time curves are used to calculate the relative upslope (RU), defined as the ratio between the maximum upslope of the selected curve divided by the maximum upslope of the LV cavity curve. Myocardial perfusion reserve index (MPRI), RU at stress/RU at rest.
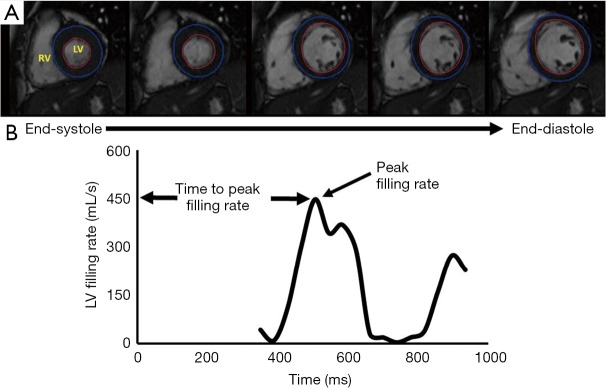
For analysis of LV diastolic function, volumetric diastolic filling was plotted over time, demonstrated in Figure 2, and used to calculate peak filling rate (PFR), PFR adjusted for end-diastolic volume (EDV) and time to PFR. Cine images had endocardial and epicardial contours placed for every frame of each slice from end systole to end diastole. The base of the LV was defined using the two long axis views. Papillary muscles were contoured separately and were included in the myocardial mass and excluded from LV volume.
Figure 2.
Volumetric filling rate. (A) Representative cinematic images beginning from end systole and advancing to end diastole, illustrating left ventricular (LV) filling over time. LV endocardial border is highlighted using the red tracing whereas the epicardial border is highlighted with the blue tracing; (B) representative volumetric filling profile from a control patient, illustrating a change in volume over time. Peak filing is taken as the highest rate of LV filing. Time to peak filing is the time from ventricular depolarization (QRS complex) to the peak ventricular filing rate.
Statistical analysis
All statistical analysis was performed using SAS (ver. 9.2; The SAS Institute, Cary, NC, USA). Summary data are expressed as means ± standard deviation (SD) for continuous variables and frequencies (%) for categorical ones.
Results
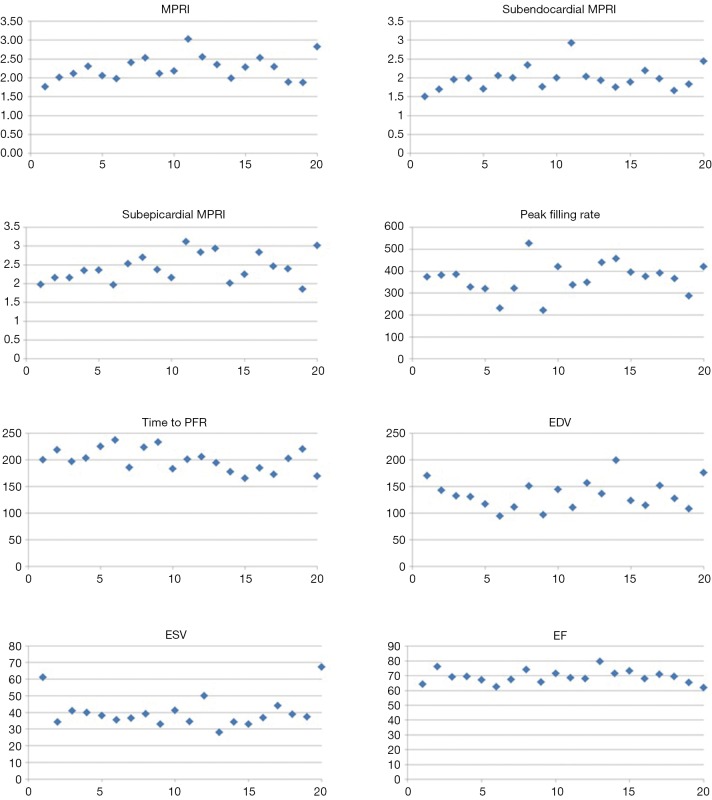
The group demographic variables are shown in Table 1. The population was middle-aged women. The Bruce Protocol results are shown in Table 1 and demonstrate a normotensive group with normal functional capacity and hemodynamic response. CMRI variables of MPRI and LV diastolic filling are shown in the Table 2 and Figure 3 for this reference control population.
Table 1. Demographic data (n=20).
| Variable | Mean ± SD or N (%) |
|---|---|
| Age (years) | 54±9 |
| Body mass index | 25±4 |
| Risk factors | |
| Hypertension | 0 |
| Dyslipidemia | 0 |
| Diabetes | 0 |
| Current smoker | 1 (5) |
| Former smoker | 7 (33) |
| Bruce protocol parameters: | |
| Heart rate (bpm) | 73±8 |
| Resting systolic blood pressure (mmHg) | 121±24 |
| Resting diastolic blood pressure (mmHg) | 73±11 |
| Peak heart rate (bpm) | 167±15 |
| Exercise peak systolic blood pressure (mmHg) | 162±13 |
| Exercise peak diastolic blood pressure (mmHg) | 81±12 |
| Total metabolic equivalents (METS) | 12±3 |
| Total exercise duration (Minutes) | 10:13±2:02 |
SD, standard deviation.
Table 2. Reference control values for pharmacological MPRI and LV systolic and diastolic function (n=20).
| CMRI parameters | Mean ± SD |
|---|---|
| MPRI | 2.19±0.38 |
| MPRI subendocardial | 1.95±0.37 |
| MPRI subepicardial | 2.32±0.42 |
| Peak filling rate (mL/sec) | 366.5±75.2 |
| Peak filling rate (mL/sec) (adjusted for end diastolic volume) | 2.9±0.4 |
| Peak filling rate (mL/sec) (adjusted for stroke volume) | 4.2±0.6 |
| Time to peak filling rate (msec) | 200.0±19.7 |
| End diastolic volume/end diastolic volume index (mL) | 128.6/73.0±27.7/14.5 |
| End systolic volume/end systolic volume index (mL) | 36.8/20.9±7.9/4.0 |
| Resting LV ejection fraction (%) | 70.4±3.9 |
CMRI, cardiac magnetic resonance imaging; MPRI, myocardial perfusion reserve index; LV, left ventricular; SD, standard deviation.
Figure 3.
Reference control myocardial perfusion and LV function values (n=20). A scatter plot of the CMRI values for the reference controls is provided for the MPRI, subendocardial MPRI, subepicardial MPRI, PFR (mL/sec), time to PFR (msec), EDV (mL), ESV (mL), and resting LV EF (%). CMRI, cardiac magnetic resonance imaging; MPRI, myocardial perfusion reserve index; PFR, peak filling rate; EDV, end-diastolic volume; ESV, end-systolic volume; LV, left ventricular; EF, ejection fraction.
Discussion
We report reference control values for semi-quantitative MPRI and LV diastolic function using CMRI images of middle-aged females with normal exercise capacity and normal ETT. MPRI, ejection fraction, stroke volume, PFR, PFR adjusted for EDV and stroke volume, time to PFR, and EDV index are identified in this asymptomatic population. Our results may be applied to future CMRI studies that investigate a variety of disease afflicting middle-aged women (e.g., non-obstructive coronary microvascular disease).
Myocardial perfusion reserve index (MPRI)
Traditionally, CMRI is a modality that is largely interpreted qualitatively. Semi-quantitative measures of CMRI can be used to improve diagnostic accuracy, and reduce inter-reader variability. Qualitative analysis of myocardial first-pass perfusion imaging has been validated for detection of obstructive CAD. Using semi-quantitative MPRI may help to identify diffuse hypoperfusion caused by microvascular and/or endothelial coronary dysfunction (6). We have recently found that an MPRI threshold of 1.84 best predicts the presence of CMD defined by invasive coronary reactivity testing (23).
Our results extend prior work on MPRI in healthy subjects. Larghat et al. (24) studied 17 young, healthy, overweight subjects (9 male and 8 female) with a mean age 34±8 years and BMI 26±5. Using the QMASS quantitative software and the mid-ventricular LV slice only, the investigators identified a lower MPRI in the subendocardium than in the subepicardium (1.54±0.3 vs. 1.81±0.35, P=0.03), which is consistent with our findings. While both studies found a similar regional difference between the subendocardium and the subepicardium, our MPRI results had higher absolute values. This contradicts the commonly held belief that older subjects have lower global MPRI. We rationalize that this difference may be explained by subject selection and differences between image acquisition and analysis.
Attention to detail is needed when evaluating myocardial first pass perfusion images by automated CMRI segmentation software. Inclusion of non-myocardial image data (blood pool or surrounding epicardial fat) or inadvertent inclusion of papillary muscle within the LV cavity input region of interest can impact the observed MPRI. Limitations of spatial resolution and respiratory or cardiac motion also impact accuracy of measurement of the data during software post processing and are probable contributing factors to the reported inter-study variation in MPRI. Improvements in CMRI quality that result from hardware and software advances at the point of image acquisition are likely to result in decreased variance in the measurements made at post processing. Standardization of the user interaction with the software is also important, and having a single user post process all data will further decrease the variation in measurements.
LV diastolic function
Our current study findings can be compared with limited prior studies of diastolic filling measured by cine CMRI and several reported normal ranges for LVEDV in reference control groups. For example, Maceira et al. (25) studied 120 subjects, with 10 men and 10 women in each of six age deciles from 20 to 80 years. When comparing the results of their study for females 50-59 years (n=10), LV end-diastolic volume (LVEDV) was demonstrated to be 126±21 mL, which is similar to our findings of 128.6±27.7 mL. Maceira et al. (25) were able to demonstrate a relationship between gender, age and body surface area, LV volumes and function (systolic and diastolic) with the use of CMRI. Alfakih et al. (26) reported a similar normal range for LVEDV in 17 females with mean age 48±5.5 years who had steady state free precession MRI at 1.5T, LVEDV 133.3±18.7 mL.
LV diastolic filling rate has also previously been reported in healthy controls. Rodriguez-Granillo et al. (27) measured LV diastolic function by CMRI in 25 subjects (15 women and 10 men) with a mean age 59±13 years; however, several important differences preclude the interpretation of this study. For example, these investigations were performed using a higher field strength, the post processing method was different, and the population included a cohort of individuals with a history of hypertension. We extend this prior work by: (I) focusing on middle aged women, a population often underrepresented in the literature; and (II) only including women who met our strict inclusion criteria, ensuring our reference values reflect a truly healthy population.
Prior studies have compared CMRI and echocardiographic measures of diastolic function (28,29). Mendoza et al. (28) studied diastolic function parameters for 115 post-myocardial infarction men and women who underwent both CMRI and echocardiography within one day. They separated the subjects into four groups based on echocardiography parameters: normal diastolic filling, grade 1 diastolic dysfunction, grade 2 diastolic dysfunction, and grade 3 diastolic dysfunction. They analyzed 36 men and 4 women (mean age 50±10 years) with normal diastolic filling by echocardiography, which corresponded to PFR of 266±76 mL/sec (3.3±1.2 when adjusted for stroke volume). They also studied 18 men and 3 women (mean age 62±7 years) with grade 1 diastolic dysfunction, which corresponded to PFR of 207±58 mL/sec (2.7±0.6 when adjusted for stroke volume). In their study, PFR increased with diastolic dysfunction grade similar to E/e’, while time to PFR prolonged with grade 1 and shortened with grade 3 diastolic dysfunction similar to echo deceleration time. However, our healthy women (mean age 54±9 years) had PFR 366.5±75.2 mL/sec (4.2±0.6 when adjusted for stroke volume), which is significantly higher than their post-myocardial infarction subjects with normal or grade 1 diastolic dysfunction. In fact, our PFR values were more similar to their group of subjects with echo grade 3 diastolic dysfunction which corresponded to PFR 353±91 mL/sec (4.1±0.8 when adjusted for stroke volume). This discrepancy is likely due to sex-differences as their subjects were predominantly men as well as the presence of underlying CAD and cardiovascular risk factors. This discrepancy also highlights the importance of obtaining reference values for age- and sex-matched healthy controls without cardiovascular risk factors.
Limitations
The current study is limited to a pilot of 20 reference control subjects. While the subjects were carefully screening to by asymptomatic and had no evidence of ischemia by maximal exercise treadmill testing, they did not undergo coronary angiography to exclude obstructive CAD nor did they undergo invasive coronary reactivity testing to correlate with our non-invasive microvascular results. Careful attention to MR field strength, pulse sequence, and imaging software will also need to be taken into account when comparing the present results with those collected at other sites.
Conclusions
The data herein provide CMRI reference control values for MPRI and LV diastolic filling in a group of healthy, asymptomatic, middle-aged women. These data can now be used to compare the MPRI and diastolic parameters with other middle aged women afflicted with disease. Because the data were analyzed using commercially available, automated CMRI segmentation software, we hope that the results and techniques will allow for easy translation to other clinical research centers around the world.
Acknowledgements
Funding: This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, T32HL116273, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, and the Linda Joy Pollin Women’s Heart Health Program, Cedars-Sinai Medical Center, Los Angeles. B. Sharif: work is funded by this grant: NIH K99HL124323; PJ. Slomka: NIH grant support for SPECT analysis (and grants from Siemens medical systems for PET imaging not related to this grant); LE. J. Thomson: work in the core lab is supported via funding from the NHLBI WISE grant, and Gilead.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bugiardini R, Bairey Merz CN. Angina with "normal" coronary arteries: a changing philosophy. JAMA 2005;293:477-84. [DOI] [PubMed] [Google Scholar]
- 2.Merz CN. The Yentl syndrome is alive and well. Eur Heart J 2011;32:1313-5. [DOI] [PubMed] [Google Scholar]
- 3.Meyer S, Brouwers FP, Voors AA, et al. Sex differences in new-onset heart failure. Clin Res Cardiol 2015;104:342-50. [DOI] [PubMed] [Google Scholar]
- 4.Pepine CJ, Anderson RD, Sharaf BL, et al. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uren NG, Camici PG, Melin JA, et al. Effect of aging on myocardial perfusion reserve. J Nucl Med 1995;36:2032-6. [PubMed] [Google Scholar]
- 6.Duncan R. Non-invasive cardiac imaging for the quantification of ventricular function:potential and future applications. Newcastle: Newcastle University, 2013. [Google Scholar]
- 7.El Aidi H, Adams A, Moons KG, et al. Cardiac magnetic resonance imaging findings and the risk of cardiovascular events in patients with recent myocardial infarction or suspected or known coronary artery disease: a systematic review of prognostic studies. J Am Coll Cardiol 2014;63:1031-45. [DOI] [PubMed] [Google Scholar]
- 8.Panting JR, Gatehouse PD, Yang GZ, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med 2002;346:1948-53. [DOI] [PubMed] [Google Scholar]
- 9.Doyle M, Weinberg N, Pohost GM, et al. Prognostic value of global MR myocardial perfusion imaging in women with suspected myocardial ischemia and no obstructive coronary disease: results from the NHLBI-sponsored WISE (Women's Ischemia Syndrome Evaluation) study. JACC Cardiovasc Imaging 2010;3:1030-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goykhman P, Mehta PK, Agarwal M, et al. Reproducibility of myocardial perfusion reserve - variations in measurements from post processing using commercially available software. Cardiovasc Diagn Ther 2012;2:268-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shufelt CL, Thomson LE, Goykhman P, et al. Cardiac magnetic resonance imaging myocardial perfusion reserve index assessment in women with microvascular coronary dysfunction and reference controls. Cardiovasc Diagn Ther 2013;3:153-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation 2010;121:2317-25. [DOI] [PubMed] [Google Scholar]
- 13.Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194-202. [DOI] [PubMed] [Google Scholar]
- 14.Kane GC, Karon BL, Mahoney DW, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011;306:856-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mogelvang R, Sogaard P, Pedersen SA, et al. Cardiac dysfunction assessed by echocardiographic tissue Doppler imaging is an independent predictor of mortality in the general population. Circulation 2009;119:2679-85. [DOI] [PubMed] [Google Scholar]
- 16.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147-239. [DOI] [PubMed] [Google Scholar]
- 17.Paulus WJ, Tschöpe C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J 2007;28:2539-50. [DOI] [PubMed] [Google Scholar]
- 18.Nelson MD, Szczepaniak LS, Wei J, et al. Diastolic dysfunction in women with signs and symptoms of ischemia in the absence of obstructive coronary artery disease: a hypothesis-generating study. Circ Cardiovasc Imaging 2014;7:510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol 2002;156:871-81. [DOI] [PubMed] [Google Scholar]
- 20.Sharaf BL, Pepine CJ, Kerensky RA, et al. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women's Ischemia Syndrome Evaluation [WISE] Study Angiographic Core Laboratory). Am J Cardiol 2001;87:937-41; A3. [DOI] [PubMed]
- 21.Bairey Merz CN, Olson M, McGorray S, et al. Physical activity and functional capacity measurement in women: a report from the NHLBI-sponsored WISE study. J Womens Health Gend Based Med 2000;9:769-77. [DOI] [PubMed] [Google Scholar]
- 22.Bairey Merz CN, Johnson BD, Sharaf BL, et al. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol 2003;41:413-9. [DOI] [PubMed] [Google Scholar]
- 23.Thomson LE, Wei J, Agarwal M, et al. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging 2015;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larghat A, Biglands J, Maredia N, et al. Endocardial and epicardial myocardial perfusion determined by semi-quantitative and quantitative myocardial perfusion magnetic resonance. Int J Cardiovasc Imaging 2012;28:1499-511. [DOI] [PubMed] [Google Scholar]
- 25.Maceira AM, Prasad SK, Khan M, et al. Normalized left ventricular systolic and diastolic function by steady state free precession cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2006;8:417-26. [DOI] [PubMed] [Google Scholar]
- 26.Alfakih K, Plein S, Thiele H, et al. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady-state free precession imaging sequences. J Magn Reson Imaging 2003;17:323-9. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Granillo GA, Mejía-Campillo M, Rosales MA, et al. Left ventricular filling patterns in patients with previous myocardial infarction measured by conventional cine cardiac magnetic resonance. The International Journal of Cardiovascular Imaging 2012;28:795-801. [DOI] [PubMed] [Google Scholar]
- 28.Mendoza DD, Codella NC, Wang Y, et al. Impact of diastolic dysfunction severity on global left ventricular volumetric filling - assessment by automated segmentation of routine cine cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2010;12:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaji K, Codella NC, Prince MR, et al. Automated segmentation of routine clinical cardiac magnetic resonance imaging for assessment of left ventricular diastolic dysfunction. Circ Cardiovasc Imaging 2009;2:476-84. [DOI] [PubMed] [Google Scholar]