Abstract
Sarcoidosis is a multi-system inflammatory disorder of unknown etiology resulting in formation of non-caseating granulomas. Cardiac involvement—which is associated with worse prognosis—has been detected in approximately 25% of individuals based on autopsy or cardiac imaging studies. Nevertheless, the diagnosis of cardiac sarcoidosis is challenging due to the low yield of endomyocardial biopsy, and the limited accuracy of various clinical criteria. Thus, no gold standard diagnostic criterion exists. This review will summarize the pathophysiology, diagnosis, and treatment of cardiac sarcoidosis with a focus on advanced cardiovascular imaging, We review the evidence to support a role for cardiac magnetic resonance (CMR) imaging in the initial evaluation of selected patients with suspected cardiac sarcoidosis, with cardiac positron emission tomography (PET) as an alternative or complementary initial diagnostic test in a subgroup of patients in whom CMR may be contra-indicated or when CMR is negative with continued clinical concern for myocardial inflammation. In addition to the diagnostic value of these tests, CMR and PET are also useful in identifying patients who have higher risk of adverse events such as ventricular tachycardia or death, in whom preventive therapies such as defibrillators should be more strongly considered. Although no randomized controlled trials for treatment of cardiac sarcoidosis exist, immunosuppressive therapy is often used. We review emerging evidence regarding the use of cardiac PET to identify and quantity the amount of myocardial inflammation as well as to guide the use of immunotherapy. Future studies are needed to determine the benefit of imaging guided therapies aimed at improving patient outcomes.
Keywords: Cardiac sarcoidosis, cardiac MRI, cardiac positron emission tomography (cardiac PET), review
Introduction
The first modern description of sarcoidosis is attributed to Dr. James Hutchinson in 1869, who depicted a patient with “color on his extremities” and “an attack of gout” (1). Although the cause of this rare disease is unknown, the onset is hypothesized to be precipitated by exposure to an unknown antigen with subsequent exaggerated immune response leading to granuloma formation in multiple organs (2). While the skin, lymph nodes, lungs, and eyes are commonly involved, any organ may be affected. The presence of cardiac disease is associated with significant morbidity and mortality; thus, there is an important need to diagnose and treat cardiac involvement. However, there are limitations to both clinical criteria and imaging tests that are used to detect cardiac involvement and a paucity of data on how to most effectively treat this condition.
While a positive endomyocardial biopsy can definitively establish the diagnosis of cardiac sarcoidosis, the sensitivity of biopsy is low due to the patchy involvement of disease (3). In addition, due to its invasive nature, performing an endomyocardial biopsy exposes patients to risks such as damage to the tricuspid valve, the right ventricular myocardium, or veins through which access is obtained. Within the last 20 years, advances in noninvasive cardiovascular imaging have improved our understanding of the epidemiology and pathophysiology of cardiac sarcoidosis. As a result, imaging tests have an important role in evaluating and managing patients with known or suspected cardiac sarcoidosis. This review will summarize the emerging role of non-invasive cardiovascular imaging in the diagnosis and management of cardiac sarcoidosis.
Epidemiology and clinical impact of cardiac sarcoidosis
The prevalence of sarcoidosis is 10–40/100,000 persons in the United States and Europe with a 3.8-fold greater risk in African-Americans compared to Caucasians (4). Organs commonly involved with sarcoidosis include the lungs, lymph nodes, skin, eye, and central nervous system. Cardiac involvement is diagnosed clinically in as few as 5% of patients with sarcoidosis, although autopsy studies have shown that cardiac involvement is present in up to 25% of autopsy specimens (5). Cardiac sarcoidosis is present up to 58% of Japanese patients based on autopsy findings (3), and is the leading cause of death in Japanese patients (up to 85%) with sarcoidosis (6). The prevalence of cardiac involvement among patients with systemic sarcoidosis screened by advanced imaging has varied widely from 3.7–54.9%, depending upon the techniques used and the population studied (7).
The underlying pathophysiology of cardiac sarcoidosis involves formation of non-caseating granulomas, which may involve any part of the heart, although the left ventricle is the most commonly affected chamber. The cardiac pathologic features include three successive histological stages: edema, granulomatous inflammation, and fibrosis leading to post-inflammatory scarring (8).
The primary clinical manifestations of cardiac sarcoidosis, in order of frequency, include conduction abnormalities and arrhythmias, congestive heart failure, and sudden death (9). The most important clinical predictor of mortality among patients with cardiac sarcoidosis has been reported to be left ventricular ejection fraction. Other clinical findings associated with mortality among a cohort of steroid-treated Japanese patients include New York Heart Association functional class, left ventricular end diastolic diameter, and sustained ventricular tachycardia (10). More recently, advanced cardiac imaging findings including late gadolinium enhancement (LGE) by cardiac magnetic resonance (CMR) imaging and myocardial inflammation by 18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) have been reported as predictors of adverse clinical outcomes even among individuals who have normal ejection fraction. Nevertheless, the incremental value of such tests to clinical parameters such as left ventricular ejection fraction (LVEF) requires further validation among larger cohorts (11-14).
Because of the poor prognosis of cardiac sarcoidosis, intense interest exists to investigate potential therapies that could reduce morbidity and mortality. Despite several decades of corticosteroid treatment for patients with cardiac sarcoidosis, no randomized trial exists to establish a definitive role and experts still debate the benefits versus harm as well as the optimal dosing and duration for therapy. Although many off-label indications exist, there is currently no FDA approved therapy for sarcoidosis. Traditionally, high dose steroid therapy is initiated, such as 40–60 mg prednisone daily, and then tapered and continued at a lower dose for at least 6 to 12 months. No specific regimen has been proven superior to others. More recently, centers have recognized the adjunctive role for steroid-sparing agents in an effort to minimize steroid induced weight gain, diabetes, and osteoporosis. Although data are lacking, clinicians have reported successful use of many agents, including methotrexate, infliximab, azathioprine, cyclosporine, antimalarials, pentoxifylline, azathioprine, and thalidomide, among others (15). Other immune modulating agents, such as the tumor necrosis-factor alpha inhibitors infliximab and adalimumab as well as anti-CD20 antibody rituximab, have been reported to have efficacy in case series and small cohorts but are not considered standard therapy currently (16). Heart or lung transplantation may be considered in select patients, even though reports of recurrent sarcoidosis after transplantation have rarely been reported (17). Pacemaker therapy in general follows standard guidelines and is indicated for symptomatic Mobitz type II second degree or third degree heart block (7). Even though heart block may improve with anti-inflammatory therapy, the disease course is not predictable; thus, pacemaker implantation is recommended regardless of the possibility of transient AV nodal recovery. The Heart Rhythm Society (HRS) expert consensus statement suggests it may be appropriate to also consider implantable cardiac defibrillator (ICD) implantation simultaneously for sarcoidosis patients with an indication for pacemaker implantation, due to the high risk of sudden death (level of evidence C). Otherwise, ICD implantation also relies upon general guidelines for primary and secondary prevention, with the additional consideration that cardiac sarcoidosis patients with fibrosis or inflammation detected by advanced imaging might be considered for ICD implantation on an individualized basis without otherwise meeting traditional criteria (7). These recommendations are based on the premise that such imaging findings have been shown to be associated with a higher rate of death or ventricular tachycardia (12). However, prospective studies have not been performed to definitively prove benefit versus harm of ICD implantation among such patients. Finally, as discussed below, 18F-FDG PET imaging has been evaluated retrospectively for its potential role to guide the need, intensity, and duration of anti-inflammatory therapies (18). In order to more completely encompass the complex and multi-organ system involvement of sarcoidosis, and recognizing the important and complementary roles of various disciplines (including pulmonology, ophthalmology, cardiology, endocrinology, neurology, rheumatology and radiology), some centers have created multi-disciplinary sarcoidosis clinics. We summarize in Tables 1 and 2 highlights of surgical, device, and medical interventions for cardiac sarcoidosis.
Table 1. Summary of medical therapies for cardiac sarcoidosis based upon recommendations of the American College of Cardiology, American Heart Association, and the Heart Rhythm Society.
| Therapy | Medication | Mechanism | Potential benefit | Potential harm |
|---|---|---|---|---|
| Medical immunosupressive therapy | Prednisone (level of evidence C) | Anti-inflammatory, start 40–60 mg per day | No RCT data. An observational study of 23 cardiac sarcoid subjects suggests that 18F-FDG PET may guide steroid therapy (LVEF of 3.8% per reduction in SUV volume of 100 cm3 above a threshold value, P=0.022) (18) | Diabetes, weight gain, hypertension, insomnia, depression and irritability, fractures, infection |
| Methotrexate (level of evidence C) | Anti-metabolite and immune-modulator | Steroid-sparing. No RCT data. In a three year open-label study comparing 7 vs. 10 CS subjects treated with steroid or steroid + MTX, respectively, steroid + MTX had improved LVEF (44.5%±13.8% vs. 60.7%±14.3%, P=0.04) (19) | Thrombocytopenia, anemia, immunosuppression, pulmonary and liver toxicity, neurologic toxicity, infection | |
| Other immune-modulators (level of evidence C) | Varied | Steroid-sparing. Case reports only have included Infliximab, Azathioprine, Cyclosporine, Anti-malarials, Pentoxifylline, Azathioprine, Thalidomide | Anemia, immunosuppression, other specific toxicities | |
| Medical therapy for heart failure | ACE/ARB (level of evidence A) | Improves adverse cardiac remodeling | Class I to reduce mortality and morbidity of HFrEF. Class IIa for structural heart disease without impaired LVEF or symptoms (20) | Renal impairment, electrolyte abnormality, allergy, angioedema, cough |
| Beta-blockers (level of evidence C) | Negative inotrope, delays AV conduction | Class I to reduce mortality and morbidity for HFrEF (20) | Fatigue, cardiac conduction block, mood effects, erectile dysfunction | |
| Diuretics and restricted dietary sodium (level of evidence C) | Fluid and sodium excretion | Class I for HFrEF and symptoms (20) | Renal impairment, electrolyte abnormality, orthostasis |
18F-FDG PET, 18F-fluorodeoxyglucose positron emission tomography; LVEF, left ventricular ejection fraction; SUV, standardized uptake values; CS, cardiac sarcoidosis.
Table 2. Summary of device and surgical therapies for cardiac sarcoidosis based upon recommendations of the American College of Cardiology, American Heart Association, and the Heart Rhythm Society.
| Intervention | Mechanism | Potential benefit | Potential harm | |
|---|---|---|---|---|
| Device therapy | ICD, secondary prevention (level of evidence C) (7) | Defibrillation of potential recurrent VT/VF | Class I recommendation to reduced mortality in patients with structural heart disease and syncope, VT/VF, or sustained VT/VF inducible by EP study. Class III if life-expectancy <1 year (7) | Pain, infection, cost, lead fracture, need for re-implantation, inappropriate shock |
| ICD, primary prevention (level of evidence C) (7) | Defibrillation of potential VT/VF | Class I recommendation to reduce mortality in patients with structural heart disease and EF <30–35% despite medical therapy. Class IIa for those needing pacemaker, unexplained syncope, or sustained VT/VF inducible by EP study. LGE on CMR may be used to consider EP study. Class IIb for LVEF 36–49% or RVEF <40% despite medical therapy. Class III if life-expectancy <1 year (7) | Pain, infection, cost, lead fracture, need for re-implantation, inappropriate shock | |
| Pacemaker (level of evidence C) (7) | Prevention of immediately fatal arrhythmia | Class I recommendation to reduce mortality and symptoms from complete heart block and bradyarrhythmia (7,21) | Pain, infection, cost, lead fracture, re-implantation, device removal complex if heart block resolves | |
| Surgical | Heart and lung transplantation (level of evidence C) | Surgical transplant | Surgically replace organs affected by sarcoidosis with donor organs when end-stage organ dysfunction that may include refractory cardiogenic shock, IV inotrope dependence, peak VO2 <10 mL/kg per min with achievement of anaerobic metabolism, refractory VT/VF (20) | Infection, need for chronic immunosuppression, risk of surgery, acute and chronic rejection, chance of recurrence (17) |
ICD, implantable cardiac defibrillator; VT, ventricular tachycardia; VF, ventricular fibrillation; EP, electrophysiologic; EF, ejection fraction; LGE, late gadolinium enhancement; CMR, cardiac magnetic resonance.
Clinical criteria for diagnosis of cardiac sarcoidosis
The Japanese Ministry of Health and Welfare (JMH) Diagnostic guidelines for cardiac sarcoidosis were originally published in 1993 (6), and were updated in 2006 (22,23). These guidelines require histologic confirmation of cardiac involvement via myocardial biopsy or clinical confirmation via a combination of major and minor criteria (Table 3). There are several limitations to these criteria. Due to the patchy nature of non-caseating granulomatous infiltration of the myocardium and limitations of sampling via traditional approaches, endomyocardial biopsy has a low sensitivity (~30%) for the diagnosis of cardiac sarcoidosis (3). The clinical diagnostic criteria include cardiac imaging studies but still classify Gallium-67 uptake as a major criteria even though this test is no longer performed at most centers due to its limited diagnostic accuracy (25-27). The modified diagnostic criteria include CMR abnormalities, but only as a minor criteria, and do not include 18F-FDG PET, which has been shown to be an effective advanced imaging method for diagnosis and monitoring of treatment in patients with cardiac sarcoidosis (28). Additionally, the modified JMH criteria for clinical diagnosis perform poorly when compared to diagnostic accuracy by advanced imaging (11,12,29), reflecting the fact that CMR and 18F-FDG PET have a higher sensitivity than these criteria.
Table 3. Japanese Ministry of Health and Welfare Criteria for Diagnosis of Cardiac Sarcoidosis (Revised 2006).
| Histological diagnosis group |
| Cardiac sarcoidosis is confirmed when endomyocardial biopsy specimens demonstrate non-caseating epithelioid granulomas with histological or clinical diagnosis of extra-cardiac sarcoidosis |
| Clinical diagnosis group |
| Although endomyocardial biopsy specimens do not demonstrate non-caseating epithelioid granulomas, extra-cardiac sarcoidosis is diagnosed histologically or clinically and satisfies the following condition and more than one in six basic diagnostic criteria: |
| (I) Two or more of the four major criteria are satisfied |
| (II) One in four of the major criteria and two or more of the five minor criteria are satisfied |
| Major criteria |
| Advanced AV block |
| Basal thinning of the interventricular septum |
| Positive Gallium-67 uptake in the heart |
| Depressed left ventricular ejection fraction <50% |
| Minor criteria |
| Abnormal ECG findings: ventricular arrhythmias (VT or multifocal or frequent PVCs), complete RBBB, axis deviation, or abnormal Q waves |
| Abnormal ECHO: wall motion abnormality or morphological abnormality (aneurysm or wall thickening or ventricular dilation) |
| Perfusion defects on nuclear imaging: thallium-201, technetium 99m SPECT |
| Delayed gadolinium enhancement on CMR |
| Interstitial fibrosis or monocyte infiltration on cardiac biopsy |
Separately the United States National Institutes of Health developed a set of diagnostic criteria in 1999 (30), that was later revised and published by the World Association for Sarcoidosis and Other Granulomatous Disorders (WASOG) in 2014 (31). The WASOG criteria were in part referenced and expanded upon by a recent consensus statement from the HRS, which has suggested a more contemporary set of clinical criteria for the diagnosis of cardiac sarcoidosis. The HRS criteria acknowledge the inherent uncertainty related to diagnosing cardiac sarcoidosis and state that “it is probable that cardiac sarcoidosis is present” (defined as >50% likelihood) if there is a histological diagnosis of extra-cardiac sarcoidosis and the patient meets one or more of several criteria (Table 4). In the absence of a diagnostic endomyocardial biopsy, these criteria require the histological diagnosis of extra-cardiac sarcoidosis and, thus, cannot be used to diagnose the presence of isolated cardiac sarcoidosis. Given the aforementioned limitation of various criteria, there is significant heterogeneity in diagnosis and treatment algorithms for cardiac sarcoidosis (32).
Table 4. Heart Rhythm Society (HRS) consensus statement for diagnosis of cardiac sarcoidosis.
| Histological diagnosis of cardiac sarcoidosis |
| Endomyocardial biopsy specimens with non-caseating epithelioid granulomas and no alternative cause identified |
| Clinical diagnosis of probable cardiac sarcoidosis |
| Histologic diagnosis of extracardiac sarcoidosis and one or more of the following is present while reasonable alternative cardiac causes other than CS have been excluded: |
| Corticosteroid or immunosuppressive therapy responsive cardiomyopathy or heart block |
| Unexplained reduced LVEF (<40%) |
| Mobitz type two second degree heart block or third degree heart block |
| Depressed left ventricular ejection fraction <50% |
| Patchy uptake on cardiac FDG-PET in a pattern consistent with CS |
| Late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging in a pattern consistent with CS |
| Positive gallium uptake in a pattern consistent with CS |
Adapted from (7). “Probable Cardiac Sarcoidosis” defined as >50% likelihood. LVEF, left ventricular ejection fraction; FDG PET, fluorodeoxyglucose positron emission tomography; CS, cardiac sarcoidosis.
Echocardiographic findings in cardiac sarcoidosis
Although echocardiography has no pathognomonic finding for cardiac sarcoidosis, this modality warrants discussion since it will often be the first imaging test ordered for patients clinically suspected to have possible cardiac sarcoidosis. Such patients most commonly have known extra-cardiac sarcoidosis and have a sign (e.g., ECG abnormality) or symptom (e.g., palpitations, syncope), which raises suspicion for cardiac involvement. Echocardiographic findings may include regional wall motion abnormalities, aneurysms, thinning of the basal septum, dilated left ventricle, and impaired right or left ventricular systolic or diastolic function (33). However, echocardiography is insensitive for detecting early stages of disease and many patients with cardiac involvement can have normal findings. Further studies are needed in order to determine whether more sensitive methods for identifying abnormal myocardial contractility, such as strain imaging (34), could improve clinical outcomes through earlier detection of cardiac involvement. Due to comorbid lung disease, patients with sarcoidosis may have elevated right heart pressures. Notably, the presence of diastolic dysfunction is not specific for cardiac involvement (35). Thus, patients with a clinical suspicion of cardiac sarcoidosis generally warrant referral to advanced non-invasive cardiovascular imaging methods such as CMR or nuclear cardiology (36).
CMR imaging for diagnosis and prognosis
CMR imaging offers a multi-dimensional assessment of cardiac involvement of systemic sarcoidosis, allowing for a non-invasive detection of scar, biventricular function, edema, and myocardial perfusion defects. One of the attractive features of CMR imaging is the lack of ionizing radiation when compared with nuclear imaging. Additionally, although all medical imaging contrast agents have reported risks and benefits, gadolinium has a very low chance of serious adverse events. Among 37,788 patients in the EuroCMR registry who received gadolinium, two (0.005%) patients required inpatient admission for allergic reaction (37). Gadolinium based contrast agents have been associated with rare cases of nephrogenic systemic fibrosis (NSF) among patients with acute or chronic very severe renal impairment (GFR <30 mL/min), in whom gadolinium is now considered contra-indicated (38). A recent autopsy study of 13 patients who underwent at least 4 gadolinium administrations versus 10 controls noted microscopic brain deposits of gadolinium even in the setting of normal GFR, but without apparent detrimental effects and thus the consequences of potential microscopic gadolinium deposition are not known (39). In summary, gadolinium contrast CMR is currently considered to be a minimal risk imaging procedure for those with GFR >30 mL/min.
The chief diagnostic finding on CMR is the presence of LGE, which signifies the presence of fibrosis (Figure 1). While there are many different patterns of LGE that can be seen in patients with cardiac sarcoidosis, the most typical patterns include sub-epicardial and mid-wall LGE along the basal septum and/or inferolateral wall. When the septum is involved, contiguous involvement which includes the right ventricular insertion points is often present. It is also noteworthy, that marked edema can also increase the interstitial space, thereby resulting in LGE. Therefore, the intensity and size of LGE may decrease following immunosuppressive therapy (40).
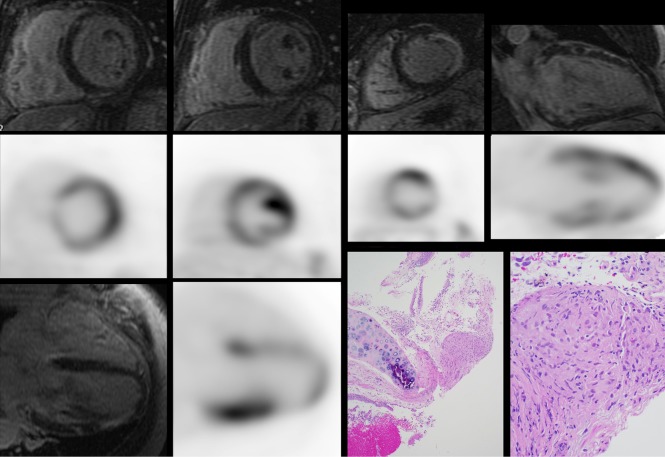
Figure 1.
A 67-year-old man without known sarcoidosis presented with abnormal chest X-ray after evaluation for a cough. A prior transbronchial biopsy was inconclusive. Evaluation demonstrated normal ECG, Holter monitor with brief episodes of non-sustained ventricular tachycardia, and globally hypokinetic left ventricular systolic function (LVEF 44%). CMR (black background) images with LGE demonstrated diffuse patchy hyperenhancement in the basal and mid ventricle in a non-ischemic pattern in addition to transmural enhancement in the thinned apical lateral wall. Corresponding cardiac PET (white background) demonstrated focal (basal lateral and apical anterior and lateral) on diffuse uptake. Transbronchial biopsy was repeated showing non-caseating granulomas (lower right, at low power 40× and high power 200×), consistent with pulmonary sarcoidosis. CT images of the chest had no coronary calcium that would have indicated significant calcified coronary artery disease. Histology images courtesy of Dr. Patrick Malafronte, Department of Pathology, Walter Reed National Military Medical Center. CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; PET, positron emission tomography.
While LGE is not included as a diagnostic criterion by the modified JMH guidelines, the presence of LGE has shown to be more sensitive than the JHM criteria for identifying cardiac involvement (29). One single-center study demonstrated that the presence of scar on LGE imaging was twice as sensitive as the JHM modified criteria to detect cardiac involvement, and LGE was associated with a nine-fold increased hazard of combined adverse events (death, defibrillator discharge, or pacemaker requirement) and 11.5-fold increased hazard for cardiac death (11). More recent studies investigating the role of LGE to forecast events have replicated these findings, showing myocardial scar to be a potent independent risk factor for death, appropriate ICD therapy, and ventricular arrhythmia (13). Additional CMR findings suggestive of cardiac involvement include thinning of the ventricular wall, the presence of myocardial edema by T2-weighted sequences, and the identification of global or regional ventricular dysfunction (41,42). CMR can also detect right-sided ventricular dysfunction, which can be due to elevated right heart pressures from pulmonary sarcoidosis or right ventricular granulomatous infiltration due to cardiac sarcoidosis. Right ventricular granulomatous infiltration has been reported at autopsy in 28/67 (42%) of patients with biopsy proven cardiac sarcoidosis who, prior to death, had clinical manifestations including arrhythmia or conduction block, heart failure, and recurrent pericardial effusion (43). Future research will include CMR T1- or T2-mapping techniques to further characterize myocardial disease in cardiac sarcoidosis and, potentially, as therapeutic endpoints to evaluate response to anti-inflammatory therapies.
The location of scar on LGE imaging has been used as a guide for endomyocardial biopsy (40,44). While the reduction in size and intensity of LGE may provide a method to assess for response to anti-inflammatory therapy in patients who are able to undergo serial CMR exams (40), nuclear imaging with 18F-FDG PET currently offers the most accurate and clinically meaningful imaging tool to monitor for response to anti-inflammatory therapy. While requiring further validation, emerging T2 mapping techniques, may ultimately provide a CMR based quantitative technique for following response to therapy (42).
Nuclear techniques for diagnosis and prognosis
Nuclear imaging provides an effective means of assessing myocardial perfusion and inflammation in patients with known or suspected cardiac sarcoidosis. Perfusion imaging is obtained from a resting SPECT study using 99mTechnetium (99mTc) or 201Thallium (201Th) while PET perfusion imaging can be performed with 13N-ammonia or 82Rubidium (82Rb) tracers (45,46). The most useful method to evaluate for myocardial inflammation is with 18F-FDG PET (47). While Gallium citrate (67Ga) imaging has been described as a JMH criterion for diagnosis of cardiac sarcoidosis, it is no longer used by most centers. Rather, 18F-FDG-PET has emerged as a superior technique, as it provides improved sensitivity and spatial resolution over 67Ga imaging (48). 18F-FDG, a glucose analog, is retained within cells with a high metabolic activity thus taking advantage of the high glycolytic activity of immune cells within granulomas (49). For accurate diagnosis, imaging with both a resting perfusion scan either by SPECT or PET as well as 18F-FDG PET imaging is needed in order to assess for the presence of both active inflammation and scar (50). Importantly although resting perfusion defects are often due to scar, when present, significant inflammation can cause resting perfusion defects due to compression of the microvasculature. Thus, in some instances, resting perfusion defects can improve following anti-inflammatory therapies.
Depending on metabolic conditions the normal myocardium can consume both glucose and free fatty acids. Several strategies for patient preparation have been developed to suppress normal myocardial uptake of 18F-FDG in order to distinguish active inflammation within granulomas from normal 18F-FDG uptake. Based on our experiences and those of others, we recommend a high fat/very low carbohydrate diet for at least 2 meals followed by a fast of at least 4 hours. The concomitant administration of intravenous unfractionated heparin has also been evaluated in some centers, but when used alone, is inferior to long-term fasting (51).
Following appropriate preparation, patients are administered 10–12 milliCuries of intravenous 18F-FDG and imaging is undertaken following approximately 90 minutes. A low-dose CT is acquired first for the purposes of attenuation correction. A dedicated cardiac acquisition as well as a separate whole body exam (from the skull base to the mid-thigh) is subsequently performed. For patients who have cardiac devices, image reconstruction is performed both with and without attenuation correction.
Interpretation of imaging requires direct comparison of perfusion and 18F-FDG PET imaging using conventional software, including both a visual and quantitative assessment. Several patterns of 18F-FDG PET abnormalities have been described including no uptake, diffuse uptake, focal uptake and focal on diffuse uptake (14). When combined with the resting perfusion data, these findings may show normal perfusion and 18F-FDG uptake, abnormal perfusion or 18F-FDG uptake, and abnormal perfusion and 18F-FDG uptake (12). These classifications can be extended to assess disease status with the following categorization: normal (normal perfusion and 18F-FDG uptake), early stage (no or mild perfusion defect and increased 18F-FDG uptake), progressive disease (moderate perfusion defect and increased 18F-FDG uptake), and fibrous disease (severe perfusion defect and minimal or no 18F-FDG uptake) (52). In addition the left ventricular involvement, focal 18F-FDG uptake can be useful for diagnosing inflammation involving the right ventricle.
18F-FDG PET imaging is a useful tool for the early diagnosis and assessment of cardiac sarcoidosis. Several studies have shown increased sensitivity of 18F-FDG imaging over 67Ga, 201Tl and 99mTc imaging for the diagnosis of cardiac sarcoidosis (26,53,54). However, comparison of the diagnostic accuracy of 18F-FDG PET versus cardiac CMR has been limited due to an imperfect reference standard and few studies that incorporate both modalities in large numbers of patients. For example, investigators often cannot conclusively determine whether small areas of LGE on CMR not detected by 18F-FDG PET represent false positive LGE or true disease. Similarly, small amounts of inflammation on 18F-FDG PET not detected by LGE-CMR could represent false positive 18F-FDG PET or early cardiac sarcoidosis without fibrosis. To date, only one small study compared the diagnostic accuracy of 18F-FDG PET versus CMR. It concluded that PET may have a higher sensitivity (88% vs. 75%), although these estimates were based on only 8 patients who were positive by the JMHW criteria and was therefore not statistically significant (8,49).
It should be recognized that the findings of CMR (fibrosis by LGE) and 18F-FDG PET (increased glucose uptake due to increased macrophage infiltration/inflammation) are complementary as they identify different pathologies. Our experience has generally been that CMR may be more sensitive for initial diagnosis whereas 18F-FDG PET likely has greater utility for serial imaging of inflammation and response to anti-inflammatory therapy (see below).
Cardiac sarcoidosis has the potential to lead to heart failure and ventricular arrhythmias (49) with sudden cardiac death as the major cause of mortality among patients with cardiac sarcoidosis (43). A retrospective analysis of 44 patients with cardiac sarcoidosis and implantable cardioverter-defibrillators (ICDs) showed a significant relationship between the presence of active myocardial inflammation on PET imaging as a risk for ventricular arrhythmias in those with preserved or depressed LVEF (55). On the other hand, a prospective study that excluded those with known heart disease, an independent predictor of survival in this population (10), did not show an increased risk of ventricular arrhythmia over a follow-up period of 1.8 years in those with preserved LVEF (49). In a larger retrospective study of 125 patients with suspected cardiac sarcoidosis who underwent 82Rb and 18F-FDG PET imaging and were followed for a mean interval of 1.5 years, it was found that the presence of a perfusion defect and increased 18F-FDG uptake was associated with an increased risk of death or ventricular arrhythmia (hazard ratio of 3.9, P<0.01), even after adjusting for LVEF, the presence of extra-cardiac sarcoidosis, and the Japanese Ministry of Health and Welfare clinical criteria. The same study showed increased risk for cardiac events among CS patients who had focal right ventricular uptake of 18F-FDG (12). A separate study by Ahmadian, et al. developed a quantitative measure of FDG-volume intensity called Cardiac Metabolic Activity (CMA). The authors evaluated CMA and qualitative PET in thirty-one patients referred for suspected cardiac sarcoidosis. Eight patients experienced twelve adverse clinical events and CMA was independently associated with future events with an odds ratio of 2.6 (P=0.03) (56). Further prospective data is needed to fully understand the role of 18F-FDG PET imaging in the diagnosis and prognosis of patients with cardiac sarcoidosis.
Cardiac PET for serial assessment of LVEF and response to steroids
Cardiac PET has a growing role in the management of patients with cardiac sarcoidosis. The assessment of response to therapy has traditionally relied upon the use of standardized uptake values (SUV) of 18F-FDG (52). SUV is quantified by the decay corrected uptake of a tracer within tissue divided by the dose of tracer adjusted to body weight (57). Changes in the ratio of myocardial to cerebellar 18F-FDG and the coefficient of variation of SUV values have also been used to assess disease activity over time (50). Prior small studies demonstrated improvement in 18F-FDG uptake on serial PET exams with concurrent improvement in conduction abnormalities and ventricular arrhythmias in patients with cardiac sarcoidosis (26) and a significant reduction in the coefficient of variation of SUV in patients with cardiac sarcoidosis who were treated with steroids (47). Other observational data has indicated that left ventricular ejection fraction may improve with corticosteroid therapy (58,59)
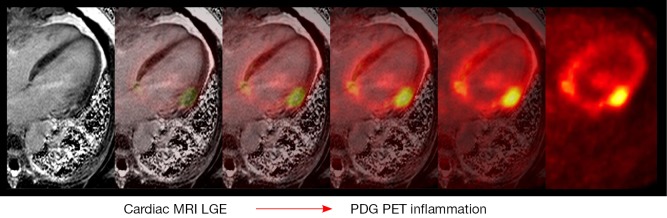
A study by Osborne et al. (18) examined the relationship between 18F-FDG uptake, as quantified by SUV, and LVEF on serial PET exams in 23 patients with cardiac sarcoidosis with baseline reduced LVEF (43%±13%) who underwent a total of 90 exams over a median follow up period of 2 years (Figure 2). Of these patients, a majority was treated with beta-blocker, ACE-I or ARB therapy, and corticosteroids and had an ICD during the study. The authors demonstrated a significant inverse linear relationship between maximum SUV and LVEF (expected increase in LVEF of 7.9% per reduction in SUV maximum of 10 g/mL, P=0.008) and a significant relationship between SUV volume above a threshold value and LVEF (expected increase in LVEF of 3.8% per reduction in SUV volume of 100 cm3 above a threshold value, P=0.022). Additional analysis demonstrated that patients could be separated into treatment responders and non-responders based upon their clinical course with a significant difference in expected change in LVEF between the groups (8.6%±5.2% vs. −5.5%±3.4%, P=0.03). On the basis of these results, the authors concluded that steroid therapy is associated with improvement in LVEF and potentially lower adverse event incidence in patients who respond to medical therapy with reduced inflammation on serial 18F-FDG PET studies., Use of serial 18F-FDG PET imaging may help to guide immunosuppressive therapy among patients with cardiac sarcoidosis either by intensification of therapy for patients with ongoing inflammation or reduction of therapy for those without active inflammation.
Figure 2.
Serial FDG PET exams showing change in inflammation. The results of three serial studies over 25 months from a 46-year-old man with cardiac sarcoidosis treated with corticosteroids are shown. The color maps demonstrate the intensity of FDG uptake in a sagittal view. The grayscale images demonstrate serial perfusion images using 82-rubidium (top) and metabolism images using FDG (bottom) in three distinct axes at approximately the same location. For each scan, the measurements of LV ejection fraction, SUV maximum and SUV volumes are displayed. Adapted from (18). FDG PET, fluorodeoxyglucose positron emission tomography; SUV, standardized uptake values.
Choosing the right imaging test for diagnosis of suspected cardiac sarcoidosis
We have provided a general algorithm for the use of advanced imaging in the diagnosis and management of cardiac sarcoidosis (Figure 3), which is also consistent with HRS guidelines (7), although all guidelines recognize that evaluation must be individualized to each patient rather than adhere strictly to any proposed algorithm. Because CMR involves no ionizing radiation, is generally well tolerated, and in our experience may have a higher negative predictive value to exclude disease, our recommendation is to first use CMR, recognizing that some patients with cardiac devices or other contra-indications may need to undergo 18F-FDG PET and that among selected patients with normal CMR, it may still be reasonable to perform 18F-FDG PET if a high clinical suspicion persists.
Figure 3.
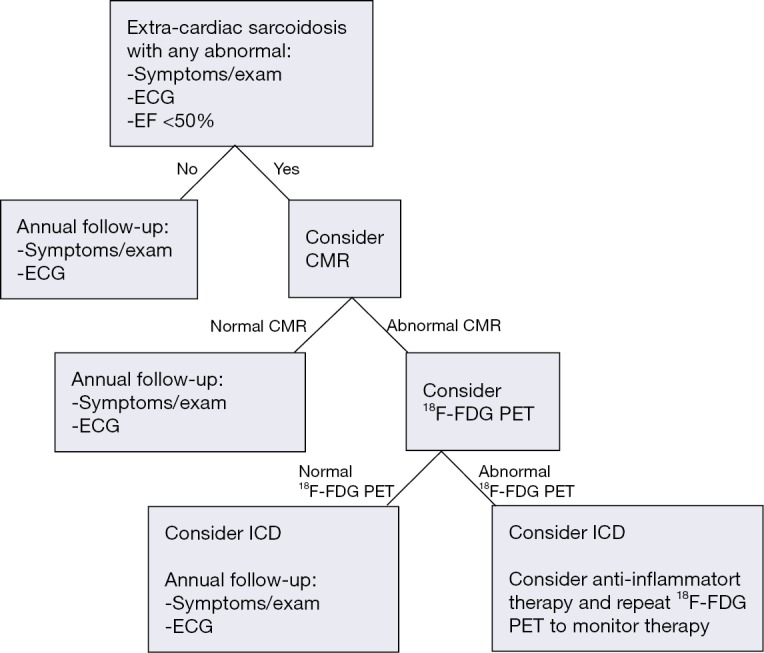
Use of CMR and 18F-FDG PET for the diagnosis and monitoring of cardiac sarcoidosis. Patients with normal CMR are unlikely to have significant cardiac involvement and may be monitored clinically. Select patients with high clinical suspicion of cardiac sarcoidosis and normal CMR might be considered for 18F-FDG PET. CMR may be preferable to 18F-FDG PET as a first line test to minimize ionizing radiation, although local institutional expertise may influence test choice. Patients with inflammation by 18F-FDG PET should be considered for anti-inflammatory therapy and repeat 18F-FDG PET imaging in 3–6 months to evaluate response to therapy. EF, ejection fraction; CMR, cardiac magnetic resonance; 18F-FDG PET, 18F-fluorodeoxyglucose positron emission tomography; ICD, implantable cardiac defibrillator.
For patients with no known history of extracardiac sarcoidosis and a nonspecific finding, such as right bundle branch block or syncope, one could begin with echocardiography due to its widespread availability and ease of interpretation. On the other hand, when a higher suspicion of cardiac involvement is present (i.e., the presence of heart block and/or ventricular tachycardia with no known explanation, particularly if extra-cardiac sarcoidosis is also present), proceeding directly to advanced imaging will be most useful. Often, the value of CMR in such scenarios lies in the fact that it can also identify other alternative cardiomyopathies or infiltrative diseases that may account for the patient’s clinical presentation. It is noteworthy that isolated cardiac sarcoidosis, whereby the disease is only confined to the heart, is under-recognized. Accordingly, the absence of any known extra-cardiac sarcoidosis should not be used to exclude potential cardiac involvement.
Interestingly, a survey of physicians treating sarcoidosis determined that most would screen patients who have known extra-cardiac sarcoidosis for possible cardiac involvement with history, physical, and ECG. Some respondents also reported screening by cardiac CMR, due to CMR’s high sensitivity to identify a large proportion of sarcoidosis patients who have cardiac involvement that would otherwise go undetected (32).
In addition to uncertainties about the best initial screening process, it is similarly unclear when to consider repeat testing in patients who initially screen negative. While a negative CMR has a high sensitivity to exclude cardiac involvement, the “warranty period” of a normal CMR is unknown. Furthermore, it remains unproven whether advanced imaging can meaningfully improve prognosis, either by initiation or modulation of immunotherapy or as a decision point for ICD implantation. At this time, there is no evidence to guide when one might consider re-imaging a patient with known extracardiac sarcoidosis and a baseline CMR without LGE. For this reason, the recent HRS guideline recommends to continue to monitor symptoms, exam, ECG, and echocardiogram among patients with extra-cardiac sarcoidosis and previously normal advanced imaging and considering repeat imaging for clinical change (7).
18F-FDG PET offers a reasonable alternative to CMR, although it does expose patients to ionizing radiation and does require careful patient preparation. On the other hand, for patients with glomerular filtration rate below 30 mL/min, ferromagnetic devices such as defibrillators or pacemakers (with the exception of a few approved devices), or other contra-indication to CMR, imaging with 18F-FDG PET may be useful. 67Ga scanning was traditionally offered and is still included in the 2006 modified JMH criteria for cardiac sarcoidosis, but this test is not commonly performed in the United States and is not considered first line due to its inferior accuracy when compared to perfusion and 18F-FDG PET (48).
Hybrid imaging—PET and CMR
Recently, CMR has been combined with 18F-FDG PET (Figure 4) either separately through co-registration of distinctly acquired scans or in combination via a PET-CMR hybrid scanner (60). Combined PET-CMR offers the advantage of an accurate assessment of function by CMR, identification of fibrosis/scar by CMR using LGE, and assessment of inflammation via 18F-FDG PET. Although the field of PET-CMR remains in its infancy and does not have immediate widespread clinical applicability, the potential to combine both of these imaging techniques together has generated great optimism for future applications.
Figure 4.
Co-registration of separately acquired cardiac CMR, left panel with cardiac PET, right panel. The CMR was notable for a region of focal late gadolinium enhancement in the basal anterolateral wall. PET was significant for active inflammation in the same area in addition to an area of less intense inflammation in the basal anteroseptum that did not have significant LGE. CMR, cardiac magnetic resonance; PET, positron emission tomography; LGE, late gadolinium enhancement.
Conclusions
Cardiac sarcoidosis, which occurs in approximately one quarter of patients with extra-cardiac disease but can also be isolated only to the heart, is associated with conduction abnormalities, arrhythmias, heart failure, and sudden cardiac death. Since no gold standard for diagnosis of cardiac sarcoidosis exists, clinicians often must combine clinical data with advanced imaging. While all patients with extra cardiac sarcoidosis require yearly screening with EKG, history, and physical exam, imaging should only be used when there is clinical suspicion or an abnormal sign (e.g., EKG changes such as new conduction block or ventricular tachycardia) or symptoms (e.g., palpitations or pre-syncope). In patients with a high clinical suspicion of cardiac sarcoidosis, CMR offers an excellent screening test, as the absence of LGE is associated with a high negative predictive value for excluding disease as well as with an excellent prognosis. In patients who have contra-indications to CMR, 18F-FDG PET with resting myocardial perfusion imaging can also be used for the diagnosis of cardiac (and extra-cardiac) disease. Furthermore, the serial assessment of inflammation via 18F-FDG PET may be used to follow response to therapy, thereby guiding the choice and duration of therapy. Nevertheless, while there is increased recognition that imaging with CMR and PET can identify patients with a higher risk of adverse events, no randomized trials exist regarding the benefit of immunosuppressive therapies. Future studies are needed to determine the benefit of imaging guided therapies aimed at improving patient outcomes.
Acknowledgements
None.
Disclaimer: The opinions and assertions contained herein are the authors’ alone and do not represent the views of the Walter Reed National Military Medical Center, the US Army, or the Department of Defense.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.James DG, Sharma OP. From Hutchinson to now: a historical glimpse. Curr Opin Pulm Med 2002;8:416-23. [DOI] [PubMed] [Google Scholar]
- 2.Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet 2014;383:1155-67. [DOI] [PubMed] [Google Scholar]
- 3.Ardehali H, Howard DL, Hariri A, et al. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J 2005;150:459-63. [DOI] [PubMed] [Google Scholar]
- 4.Rybicki BA, Major M, Popovich J, Jr, et al. Racial differences in sarcoidosis incidence: a 5-year study in a health maintenance organization. Am J Epidemiol 1997;145:234-41. [DOI] [PubMed] [Google Scholar]
- 5.Iwai K, Takemura T, Kitaichi M, et al. Pathological studies on sarcoidosis autopsy. II. Early change, mode of progression and death pattern. Acta Pathol Jpn 1993;43:377-85. [DOI] [PubMed] [Google Scholar]
- 6.Guideline for Diagnosis of Cardiac Sarcoidosis: Study Report on Diffuse Pulmonary Diseases. Tokyo Japan: Ministry of Health, Labour and Welfare, 1993:23-4. [Google Scholar]
- 7.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm 2014;11:1305-23. [DOI] [PubMed] [Google Scholar]
- 8.Ohira H, Tsujino I, Ishimaru S, et al. Myocardial imaging with 18F-fluoro-2-deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging 2008;35:933-41. [DOI] [PubMed] [Google Scholar]
- 9.Nery PB, Beanlands RS, Nair GM, et al. Atrioventricular block as the initial manifestation of cardiac sarcoidosis in middle-aged adults. J Cardiovasc Electrophysiol 2014;25:875-81. [DOI] [PubMed] [Google Scholar]
- 10.Yazaki Y, Isobe M, Hiroe M, et al. Prognostic determinants of long-term survival in Japanese patients with cardiac sarcoidosis treated with prednisone. Am J Cardiol 2001;88:1006-10. [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation 2009;120:1969-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol 2014;63:329-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC Cardiovasc Imaging 2013;6:501-11. [DOI] [PubMed] [Google Scholar]
- 14.Tahara N, Tahara A, Nitta Y, et al. Heterogeneous myocardial FDG uptake and the disease activity in cardiac sarcoidosis. JACC Cardiovasc Imaging 2010;3:1219-28. [DOI] [PubMed] [Google Scholar]
- 15.Kim JS, Judson MA, Donnino R, et al. Cardiac sarcoidosis. Am Heart J 2009;157:9-21. [DOI] [PubMed] [Google Scholar]
- 16.Vorselaars AD, Cremers JP, Grutters JC, et al. Cytotoxic agents in sarcoidosis: which one should we choose? Curr Opin Pulm Med 2014;20:479-87. [DOI] [PubMed] [Google Scholar]
- 17.Osborne M, Kolli S, Padera R, et al. Use of multimodality imaging to diagnose cardiac sarcoidosis as well as identify recurrence following heart transplantation. J Nucl Cardiol 2013;20:310-2. [DOI] [PubMed] [Google Scholar]
- 18.Osborne MT, Hulten EA, Singh A, et al. Reduction in 18F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. J Nucl Cardiol 2014;21:166-74. [DOI] [PubMed] [Google Scholar]
- 19.Nagai S, Yokomatsu T, Tanizawa K, et al. Treatment with methotrexate and low-dose corticosteroids in sarcoidosis patients with cardiac lesions. Intern Med 2014;53:427-33. [DOI] [PubMed] [Google Scholar]
- 20.Hunt SA, American College of Cardiology ; American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol 2005;46:e1-82. [DOI] [PubMed] [Google Scholar]
- 21.Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2013;61:e6-75. [DOI] [PubMed] [Google Scholar]
- 22.Soejima K, Yada H. The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol 2009;20:578-83. [DOI] [PubMed] [Google Scholar]
- 23.Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord 2007;27:89-102. [Google Scholar]
- 24.Mc Ardle BA, Leung E, Ohira H, et al. The role of F(18)-fluorodeoxyglucose positron emission tomography in guiding diagnosis and management in patients with known or suspected cardiac sarcoidosis. J Nucl Cardiol 2013;20:297-306. [DOI] [PubMed] [Google Scholar]
- 25.Okayama K, Kurata C, Tawarahara K, et al. Diagnostic and prognostic value of myocardial scintigraphy with thallium-201 and gallium-67 in cardiac sarcoidosis. Chest 1995;107:330-4. [DOI] [PubMed] [Google Scholar]
- 26.Yamagishi H, Shirai N, Takagi M, et al. Identification of cardiac sarcoidosis with (13)N-NH(3)/(18)F-FDG PET. J Nucl Med 2003;44:1030-6. [PubMed] [Google Scholar]
- 27.Ohira H, Tsujino I, Yoshinaga K. 18F-Fluoro-2-deoxyglucose positron emission tomography in cardiac sarcoidosis. Eur J Nucl Med Mol Imaging 2011;38:1773-83. [DOI] [PubMed] [Google Scholar]
- 28.Youssef G, Leung E, Mylonas I, et al. The use of 18F-FDG PET in the diagnosis of cardiac sarcoidosis: a systematic review and metaanalysis including the Ontario experience. J Nucl Med 2012;53:241-8. [DOI] [PubMed] [Google Scholar]
- 29.Smedema JP, Snoep G, van Kroonenburgh MP, et al. Evaluation of the accuracy of gadolinium-enhanced cardiovascular magnetic resonance in the diagnosis of cardiac sarcoidosis. J Am Coll Cardiol 2005;45:1683-90. [DOI] [PubMed] [Google Scholar]
- 30.Judson MA, Baughman RP, Teirstein AS, et al. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999;16:75-86. [PubMed] [Google Scholar]
- 31.Judson MA, Costabel U, Drent M, et al. The WASOG Sarcoidosis Organ Assessment Instrument: An update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis 2014;31:19-27. [PubMed] [Google Scholar]
- 32.Hamzeh NY, Wamboldt FS, Weinberger HD. Management of cardiac sarcoidosis in the United States: a Delphi study. Chest 2012;141:154-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burstow DJ, Tajik AJ, Bailey KR, et al. Two-dimensional echocardiographic findings in systemic sarcoidosis. Am J Cardiol 1989;63:478-82. [DOI] [PubMed] [Google Scholar]
- 34.Smedema JP. Tissue Doppler imaging in cardiac sarcoidosis. Eur J Echocardiogr 2008;9:579-80. [DOI] [PubMed] [Google Scholar]
- 35.Patel AR, Klein MR, Chandra S, et al. Myocardial damage in patients with sarcoidosis and preserved left ventricular systolic function: an observational study. Eur J Heart Fail 2011;13:1231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubrey SW, Falk RH. Diagnosis and management of cardiac sarcoidosis. Prog Cardiovasc Dis 2010;52:336-46. [DOI] [PubMed] [Google Scholar]
- 37.Bruder O, Schneider S, Pilz G, et al. 2015 Update on Acute Adverse Reactions to Gadolinium based Contrast Agents in Cardiovascular MR. Large Multi-National and Multi-Ethnical Population Experience With 37788 Patients From the EuroCMR Registry. J Cardiovasc Magn Reson 2015;17:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beckett KR, Moriarity AK, Langer JM. Safe Use of Contrast Media: What the Radiologist Needs to Know. Radiographics 2015;35:1738-50. [DOI] [PubMed] [Google Scholar]
- 39.McDonald RJ, McDonald JS, Kallmes DF, et al. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015;275:772-82. [DOI] [PubMed] [Google Scholar]
- 40.Shimada T, Shimada K, Sakane T, et al. Diagnosis of cardiac sarcoidosis and evaluation of the effects of steroid therapy by gadolinium-DTPA-enhanced magnetic resonance imaging. Am J Med 2001;110:520-7. [DOI] [PubMed] [Google Scholar]
- 41.Schulz-Menger J, Wassmuth R, Abdel-Aty H, et al. Patterns of myocardial inflammation and scarring in sarcoidosis as assessed by cardiovascular magnetic resonance. Heart 2006;92:399-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crouser ED, Ono C, Tran T, et al. Improved Detection of Cardiac Sarcoidosis Using Magnetic Resonance with Myocardial T2 Mapping. Am J Respir Crit Care Med 2014;189:109-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roberts WC, McAllister HA, Jr, Ferrans VJ. Sarcoidosis of the heart: a clinicopathologic study of 35 necropsy patients (group I) and review of 78 previously described necropsy patients (group II). Am J Med 1977;63:86-108. [DOI] [PubMed] [Google Scholar]
- 44.Parsai C, O’Hanlon R, Prasad SK, et al. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson 2012;14:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dilsizian V, Bacharach SL, Beanlands RS, et al. ASNC imaging guidelines for nuclear cardiology procedures: PET myocardial perfusion and metabolism clinical imaging. J Nucl Cardiol 2009;16:651. [Google Scholar]
- 46.Holly TA, Abbott BG, Al-Mallah M, et al. Single photon-emission computed tomography. J Nucl Cardiol 2010;17:941-73. [DOI] [PubMed] [Google Scholar]
- 47.Gotthardt M, Bleeker-Rovers CP, Boerman OC, et al. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med 2010;51:1937-49. [DOI] [PubMed] [Google Scholar]
- 48.Nishiyama Y, Yamamoto Y, Fukunaga K, et al. Comparative evaluation of 18F-FDG PET and 67Ga scintigraphy in patients with sarcoidosis. J Nucl Med 2006;47:1571-6. [PubMed] [Google Scholar]
- 49.Mehta D, Lubitz SA, Frankel Z, et al. Cardiac involvement in patients with sarcoidosis: diagnostic and prognostic value of outpatient testing. Chest 2008;133:1426-35. [DOI] [PubMed] [Google Scholar]
- 50.Skali H, Schulman AR, Dorbala S. 18F-FDG PET/CT for the assessment of myocardial sarcoidosis. Curr Cardiol Rep 2013;15:352. [PMC free article] [PubMed] [Google Scholar]
- 51.Morooka M, Moroi M, Uno K, et al. Long fasting is effective in inhibiting physiological myocardial 18 F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res 2014;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okumura W, Iwasaki T, Toyama T, et al. Usefulness of fasting 18F-FDG PET in identification of cardiac sarcoidosis. J Nucl Med 2004;45:1989-98. [PubMed] [Google Scholar]
- 53.Ishimaru S, Tsujino I, Takei T, et al. Focal uptake on 18F-fluoro-2-deoxyglucose positron emission tomography images indicates cardiac involvement of sarcoidosis. Eur Heart J 2005;26:1538-43. [DOI] [PubMed] [Google Scholar]
- 54.Langah R, Spicer K, Gebregziabher M, et al. Effectiveness of prolonged fasting 18f-FDG PET-CT in the detection of cardiac sarcoidosis. J Nucl Cardiol 2009;16:801-10. [DOI] [PubMed] [Google Scholar]
- 55.Betensky BP, Tschabrunn CM, Zado ES, et al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart Rhythm 2012;9:884-91. [DOI] [PubMed] [Google Scholar]
- 56.Ahmadian A, Brogan A, Berman J, et al. Quantitative interpretation of FDG PET/CT with myocardial perfusion imaging increases diagnostic information in the evaluation of cardiac sarcoidosis. J Nucl Cardiol 2014;21:925-39. [DOI] [PubMed] [Google Scholar]
- 57.Dweck MR, Jones C, Joshi NV, et al. Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76-86. [DOI] [PubMed] [Google Scholar]
- 58.Chiu CZ, Nakatani S, Zhang G, et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol 2005;95:143-6. [DOI] [PubMed] [Google Scholar]
- 59.Grutters JC, van Den Bosch JM. Corticosteroid treatment in sarcoidosis. Eur Respir J 2006;28:627-36. [DOI] [PubMed] [Google Scholar]
- 60.Nekolla SG, Martinez-Moeller A, Saraste A. PET and MRI in cardiac imaging: from validation studies to integrated applications. Eur J Nucl Med Mol Imaging 2009;36 Suppl 1:S121-30. [DOI] [PubMed] [Google Scholar]