Abstract
Rationale: Cytomegalovirus (CMV), which is one of the most common infections after lung transplantation, is associated with chronic lung allograft dysfunction and worse post-transplantation survival. Current approaches for at-risk patients include a fixed duration of antiviral prophylaxis despite the associated cost and side effects.
Objectives: We sought to identify a specific immunologic signature that predicted protection from subsequent CMV.
Methods: CMV-seropositive lung transplantation recipients were included in the discovery (n = 43) and validation (n = 28) cohorts. Polyfunctional CMV-specific immunity was assessed by stimulating peripheral blood mononuclear cells with CMV pp65 or IE-1 peptide pools and then by measuring T-cell expression of CD107a, IFN-γ, tumor necrosis factor-α (TNF-α), and IL-2. Recipients were prospectively monitored for subsequent viremia. A Cox proportional hazards regression model that considered cytokine responses individually and in combination was used to create a predictive model for protection from CMV reactivation. This model was then applied to the validation cohort.
Measurements and Main Results: Using the discovery cohort, we identified a specific combination of polyfunctional T-cell subsets to pp65 that predicted protection from subsequent CMV viremia (concordance index 0.88 [SE, 0.087]). The model included both protective (CD107a−/IFN-γ+/IL-2+/TNF-α+ CD4+ T cells, CD107a−/IFN-γ+/IL-2+/TNF-α+ CD8+ T cells) and detrimental (CD107a+/IFN-γ+/IL-2−/TNF-α− CD8+ T cells) subsets. The model was robust in the validation cohort (concordance index 0.81 [SE, 0.103]).
Conclusions: We identified and validated a specific T-cell polyfunctional response to CMV antigen stimulation that provides a clinically useful prediction of subsequent cytomegalovirus risk. This novel diagnostic approach could inform the optimal duration of individual prophylaxis.
Keywords: cytomegalovirus, lung transplantation, immunologic monitoring
At a Glance Commentary
Scientific Knowledge on the Subject
Cytomegalovirus (CMV) is one of the most common infections after lung transplantation and is associated with chronic allograft dysfunction and worse survival. There is an unmet clinical need to more precisely determine post-transplantation risk for CMV and tailor prophylaxis.
What This Study Adds to the Field
A CMV-specific polyfunctional T-cell response can distinguish recipients at low risk for subsequent CMV viremia. This novel approach can individualize the duration of antiviral prophylaxis.
Cytomegalovirus (CMV) infection and disease are some of the most common complications after solid organ transplantation. In addition to the direct tissue injury of invasive disease, CMV has indirect effects, including increased risk of other infections and organ rejection (1, 2). Lung transplantation recipients are at the highest risk for CMV, with a significant number of at-risk patients developing infection or disease, particularly after prophylaxis ends. In a lung transplantation recipient, a single episode of CMV infection, even with current treatments, significantly increases the risk for chronic allograft dysfunction and death (3–5).
Accordingly, international consensus guidelines recommend antiviral prophylaxis in lung transplantation recipients who are pretransplantation CMV seropositive or pretransplantation CMV seronegative, but who receive an organ from a seropositive donor (1). Pretransplantation seropositive recipients represent the majority of transplantation recipients at risk for CMV, and current guidelines recommend a minimum of 6 months of antiviral prophylaxis for this cohort (1). However, due to the high cost of antiviral treatments (e.g., valganciclovir), risk of bone marrow suppression, and potential development of subsequent viral resistance, the optimal duration of prophylaxis is controversial, with practices varying across centers (6). Thus, there is an unmet clinical need to more precisely determine post-transplantation risk for CMV beyond pretransplantation recipient and donor serostatus, with the ultimate goal of tailoring CMV prophylaxis duration to an individual’s specific risk.
Because T-cell responses are critical to CMV immune control, previous experimental assays in solid organ transplantation recipients have attempted to measure T-cell responses to CMV lysate or antigens to predict future infections. The most widely used commercial assay (QuantiFERON-CMV, Qiagen, Venlo, the Netherlands) is an ELISA that measures CD8+ T-cell response to HLA class I restricted CMV peptides by IFN-γ production. The assay has multiple limitations, including HLA restriction, low sensitivity, and a high indeterminate rate (7, 8). In the absence of a reliable assay, the current standard of care in seropositive lung transplantation recipients is to apply a fixed duration of prophylaxis, which subjects some patients to overtreatment with the risks of medication toxicity, while undertreating others who remain at higher risk for CMV.
We sought to determine if immunologic profiling of the CMV-specific immune T-cell responses could define an individual risk for CMV infection in CMV-seropositive lung transplantation recipients. T-cell polyfunctionality is operationally defined by the simultaneous measurement of three or more antigen-driven effector functions within a single cell, including production of IL-2, tumor necrosis factor-α (TNF-α), IFN-γ, and expression of the degranulation marker CD107a. Our group has previously profiled polyfunctional T cells by flow cytometry after stimulation by CMV pp65 and by IE-1 peptide pools in immunosuppressed lung transplantation recipients (9). Because of the importance of T-cell polyfunctionality in high-quality vaccine immunity and control of HIV infection (10–12), we hypothesized that a polyfunctional CMV-specific immune response would discriminate CMV risk and provide a novel and clinically relevant diagnostic test.
Methods
Discovery and Validation Study Cohorts
Lung transplantation recipients who were serologically positive for CMV before transplantation, who had not yet developed CMV post-transplantation (on or off prophylaxis), and who were at least 4 weeks post-transplantation, were eligible for the study. Exclusion criteria included age <18 years, multiorgan transplantation, and retransplantation. Discovery and validation subjects were independent. The study samples were collected between April 2011 and March 2013, and the study was approved by the Duke University Institutional Review Board (protocol Pro00007005).
Clinical Protocol Management
Details of induction and immunosuppression are provided in the online supplement. All subjects received intravenous ganciclovir initially after transplantation and changed to valganciclovir once they were able to take oral medications. Valganciclovir was continued for 1 year post-transplantation, per center protocol. CMV prophylaxis was discontinued before 1 year for leukopenia or patient cost.
Sample Collection and Storage of Peripheral Blood Mononuclear Cells
Samples were collected at varying time points after transplantation in a cross-sectional manner. Details of sample collection and storage of peripheral blood mononuclear cells have been previously published and are also provided in the online supplement (9).
Cell Stimulation and Flow Cytometry in Assessing CMV-Specific Immunity
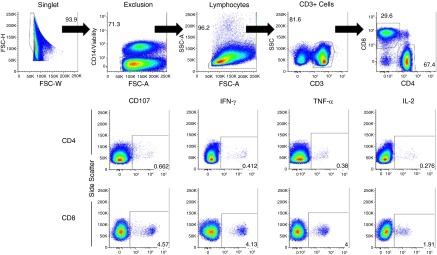
For each subject, 2 × 106 peripheral blood mononuclear cells were separately stimulated for 6 hours with a CMV pp65 peptide pool + costimulatory molecules (anti-CD28 and anti-CD49d) or with an IE-1 peptide pool + costimulatory molecules. CMV peptide pools consisted of overlapping 15-mer peptides (with 11 amino acid overlaps) that spanned the entire target region (pp65 or IE-1). A negative control (costimulatory molecules alone) and a positive control with staphylococcal enterotoxin B + costimulatory molecules were included for each subject. Brefeldin-A, Monensin, and anti-CD107a were added to the stimulation mixes before incubation. After incubation, EDTA was added to the samples, and the cells were washed and incubated with a cell surface antibody mix of anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD27, anti-CD57, anti-CD45RO, and live/dead fixable violet dye. After this incubation, the cells were washed, lysed, and permeabilized. The cells were then incubated with an intracellular antibody mix of anti–IL-2, anti–TNF-α, and anti–IFN-γ. After incubation, the cells were washed, and the cell pellets fixed in 1% formalin. Compensation beads were used, and data were acquired immediately with a LSRII flow cytometer (Becton-Dickinson, San Jose, CA). Additional details of the methods to assess CMV-specific immunity and gating strategy are provided in the online supplement, are displayed in Figure 1, and have been previously published (9).
Figure 1.
Gating strategy. A singlet gate identifies single cells that are then subset into viable (or live) cells. The live cells are then subset to lymphocytes. A CD3+ gate then identifies the T cells. T cells are then separated into CD4+and CD8+subsets. Individual cytokine expression was identified within these T-cell subsets. Boolean gating was used to identify different combinations of these markers as per Figure 2. FSC-A = forward scatter pulse area; FSC-H = forward scatter pulse height; FSC-W = forward scatter pulse width; SSC = side scatter; SSC-A = side scatter pulse area; TNF-α = tumor necrosis factor-α.
Assessment of CMV Infection and Disease
Serial blood draws with CMV detection by real-time PCR on plasma are done as part of routine clinical care at 1, 3, 6, 9, and 12 months post-transplantation and every 3 months thereafter. The CMV detection assay used was consistent through the study period. Additional testing is performed if a recipient stops prophylaxis before 1 year post-transplantation. CMV DNAemia was defined as having ≥250 copies/ml of viral DNA detected in the peripheral blood. Transbronchial biopsies are done at 1, 3, 6, 9, and 12 months post-transplantation, and then annually thereafter or for any clinical indication. IE-1 immunohistochemistry on biopsies was performed as clinically indicated.
Statistical Approach
Cell subset counts were gated as outlined in Figure 1 with subsequent Boolean gating. Frequencies relative to the total CD3 count were used for the analysis. Normalization to correct for possible variations in background expression of functional markers was done by taking log (base 2) ratios of the relative frequencies in the stimulation condition to those in the negative control.
To develop a CMV risk prediction model without dichotomization, the primary outcome variable was chosen to be the number of prophylaxis-free follow-up days, which was defined as the total number of (possibly noncontiguous) days off prophylaxis from date of transplantation until CMV event date, study censor date, or death date. For deaths during the study period, subjects who died, but who did not have evidence of CMV infection, were treated as right-censored data, whereas subjects who died and had evidence of CMV infection before death were treated as a CMV endpoint. We then fitted a Cox regression model using the adaptive least absolute shrinkage and selection operator (LASSO) optimizer (a covariate selection strategy) for these time-to-event outcomes against the log ratios in the discovery cohort. In the initial step, the best-standard LASSO fit was found by leave-one-out cross-validation (LOOCV), and the absolute values of the corresponding coefficient estimates were used as adaptive weights in the adaptive step. In the adaptive step, the best adaptive LASSO parameter was then found by another LOOCV, and the resulting best adaptive LASSO fit was chosen as the final model to derive the log risk score. Further details of the log risk score model are provided in the online supplement.
To identify cell subsets that could predict outcome and provide possible insight into pathogenesis, but which were not included in the final model (perhaps because of collinearity), a bootstrap analysis was done with 500 model fits, with the best adaptive LASSO found by LOOCV as in the main analysis. We then counted the number of times each pair of variables was selected together. Using this count as a dissimilarity measure, we performed hierarchical clustering with complete linkage and created a dendrogram to visualize the most informative cell subsets.
Once the final polyfunctional model was established, we compared its discriminatory ability with a model using the CD8+ IFN-γ data. The concordance index (13), which is a measure of the agreement between log risk score and time to CMV infection, was used to compare performance of the polyfunctional and single cytokine models. The cross-validation concordance index was calculated as the average of the concordance index for the 50 repetitions in 10 times repeated stratified 5-fold cross validation. Within each training set of the cross validation, (internal) LOOCV was used for tuning adaptive LASSO parameters.
The final predictive model developed from the discovery cohort was then applied to the validation cohort. Additional details of the statistical approach are included in the online supplement.
Results
Study Cohort
The discovery cohort included 43 CMV serologically positive lung transplantation recipients (Table 1). The demographic features and indications for transplantation reflect the lung transplantation population at Duke University Medical Center. Cross-sectional blood draws were obtained a median of 363 days after transplantation (interquartile range [IQR]: 205–628). The median prophylaxis-free follow-up time, as calculated by the reverse Kaplan-Meier method (14), was 539 days (IQR: 502–777). The cohort was dichotomized for descriptive purposes (Table 1) into “Developed CMV” and “No CMV” based on the subsequent detection of CMV. In the Developed CMV group, CMV was detected at a median of 362 days (IQR: 219–439) after their blood draw. Of note, this group had a median of 254 prophylaxis days (IQR: 124–365) and a median of 82 off prophylaxis days (IQR: 54–168) before the development of CMV. The No CMV group, which was defined as having at least 100 days off prophylaxis, had a median of 369 prophylaxis days (IQR: 159–543) and a median of 539 days off prophylaxis (IQR: 358–795) without development of CMV. Three recipients died during the follow-up period.
Table 1.
Clinical and Demographic Characteristics of the 43 Serologically Cytomegalovirus-Positive Lung Transplantation Recipients in the Discovery Cohort
| Characteristics | Developed CMV* (N = 12) | No CMV (N = 31) |
|---|---|---|
| Male | 7 (58) | 14 (45) |
| Age at transplantation, yr | 68 (62–71) | 66 (59–69) |
| Race | ||
| White | 11 (92) | 26 (84) |
| African American | 1 (8) | 2 (6) |
| Other | 3 (10) | |
| Indication for transplantation | ||
| Obstructive disease | 6 (50) | 9 (29) |
| Restrictive disease | 6 (50) | 19 (61) |
| Cystic disease | 0 (0) | 2 (7) |
| Vascular disease | 0 (0) | 1 (3) |
| Lung transplantation operation | ||
| Bilateral | 7 (58) | 17 (55) |
| Single | 5 (42) | 14 (45) |
| Patients on prophylaxis atblood draw | 5 (42) | 11 (35) |
| Total prophylaxis days | 254 (124–365) | 369 (159–543) |
| Days from lung transplantation to blood draw | 342 (133–429) | 385 (212–660) |
| Off prophylaxis CMV-free days | 82 (54–168) | 539 (358–795) |
Definition of abbreviation: CMV = cytomegalovirus.
CMV is defined as DNAemia or pneumonitis. Subjects were classified as “No CMV” if they did not develop evidence of CMV in the study follow-up period. All subjects in this group had at least 100 days of prophylaxis-free time. Data are shown as n (%) or median (25th–75th percentile).
All had DNAemia; only one subject had concurrent pneumonitis.
The validation cohort included 28 seropositive recipients, as detailed in Table E1 in the online supplement. The validation cohort received post-transplantation prophylaxis, for a median of 446 days (IQR: 303–617), and had a reverse Kaplan-Meier estimate of the median prophylaxis-free follow-up of 417 days (95% confidence interval: 367–809).
Development of CMV Infection and/or Disease
In the discovery cohort of 43 lung transplantation recipients, 12 developed CMV DNAemia, with one developing concurrent CMV pneumonitis. Eleven of the 12 recipients developed CMV DNAemia during the first 180 prophylaxis-free days. In the validation cohort, 9 recipients developed CMV DNAemia during follow-up, and all of these CMV events were within the first 180 prophylaxis-free days.
Polyfunctional T-Cell Assessment and Subsequent CMV
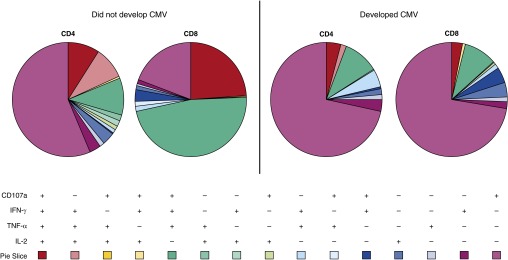
To determine whether T-cell polyfunctionality is predictive of protection from CMV in transplantation patients, we examined CD107a expression in addition to performing intracellular cytokine staining for IFN-γ, TNF-α, and IL-2 after stimulation of peripheral blood mononuclear cells with pools of CMV pp65 or IE-1 peptides according to the primary gating strategy depicted in Figure 1. Using Boolean gating, the different combinations of functional immune responses to pp65 and IE-1 were determined. These results are highlighted in Figure 2. Figure 2 depicts CD4 and CD8 T-cell responses from two representative subjects (one who developed CMV and one who did not) following pp65 stimulation.
Figure 2.
Polyfunctional response of one representative subject who subsequently developed cytomegalovirus (CMV) (right) and one subject who did not develop CMV (left) depicted by pie graphs. The different combinations of CD107a expression and intracellular IFN-γ, tumor necrosis factor-α (TNF-α), and IL-2 detection are noted by the different colors of the pie graph. Red, pink, and yellow pie slices are polyfunctional subsets, whereas the blue and purple pie slices are single-expression subsets. The protective subsets used in the final model include CD107a−/IFN-γ+/IL-2+/TNF-α+ CD4+T cells and CD107a−/IFN-γ+/IL-2+/TNF-α+ CD8+ T cells (pink pie slices), as well as the detrimental CD107a+/IFN-γ+/IL-2−/TNF-α− CD8+ T cell subset (dark blue pie slice).
Development of a CMV Risk Prediction Score
A Cox proportional hazards regression model for prophylaxis-free follow-up time to CMV infection using the log ratios of stimulated to unstimulated cell subset relative frequencies as covariates was used. All recipients who developed CMV at any time point (n = 12) and all recipients without CMV (n = 31) were included with all available follow-up. Cell subset frequencies from the CMV pp65 stimulation (cross-validation concordance index = 0.82, with SE 0.020) were found to better predict outcomes compared with the IE-1 stimulation data (cross-validation concordance index = 0.79, with SE 0.019).
The analysis also considered if T-cell maturational markers might distinguish those at risk for CMV in addition to the intracellular cytokine markers given prior reports of effector memory cells (CD27−) predominating the CMV-specific T-cell population (15). Incorporating naive (CD45RO− CD27+), central memory (CD45RO+ CD27+), effector memory (CD27− CD45RO+), terminal effector (CD45RO− CD57+), and effector (CD45RO+ CD57+) cells in both CD4+ and CD8+ subsets did not improve the prediction of subsequent CMV over the CMV pp65 basic cell subset model alone. The clinical variables of sex, number of prophylaxis days post-transplantation, age at transplantation, indication for transplantation, and type of transplantation (single vs. bilateral) were also included in the initial model formation, but these did not improve the prediction of subsequent CMV in the model.
After adaptive LASSO shrinkage, only three cell subsets in the CMV pp65 stimulation condition were retained as useful for prediction, namely the polyfunctional CD107a−/IFN-γ+/IL-2+/TNF-α+ CD4+ and CD107a−/IFN-γ+/IL-2+/TNF-α+ CD8+ T cells, and the bifunctional CD107a+/IFN-γ+/IL-2−/TNF-α− CD8+ T cells. The two polyfunctional cell subsets were associated with decreased risk for CMV infection, whereas the bifunctional CD8+ T-cell subset was associated with an increased risk. Harrell’s concordance index, which is a measure of model prediction performance, was 0.88 (SE, 0.087). As described in the Methods section in the online supplement, these findings were robust under cross validation and bootstrap analysis (results provided in the online supplement).
Concordance between Prophylaxis-Free Follow-up Time to CMV Infection and Risk Scores Based on IFN-γ versus Polyfunctional Cytokine Production
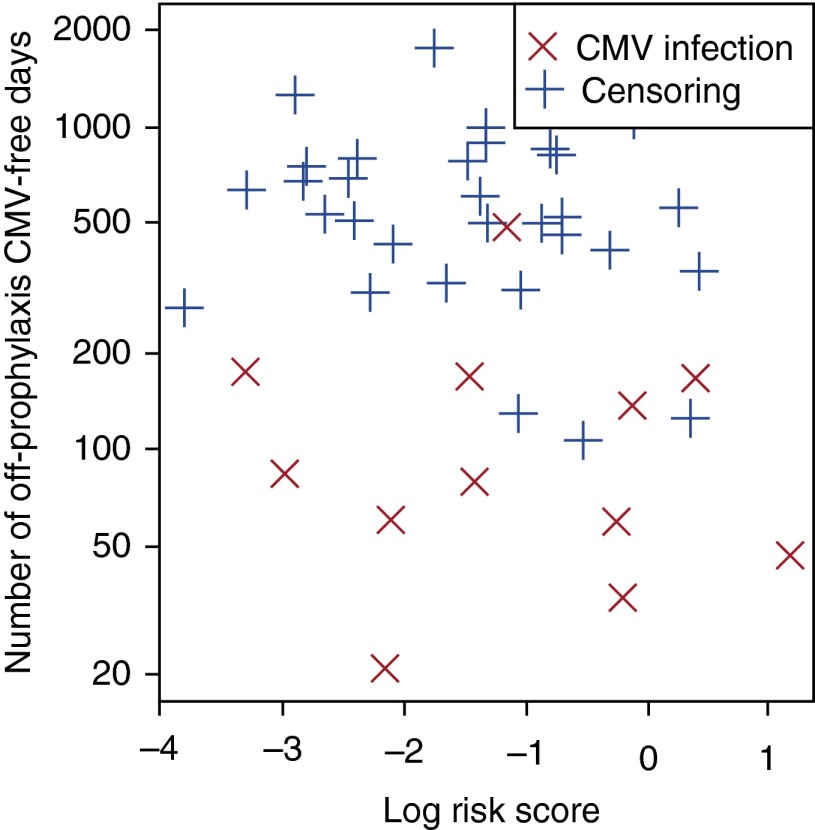
To evaluate the predictive ability of models based only on CD8+ IFN-γ+ cell subset frequencies stimulation (proxy for the QuantiFERON-CMV assay), we fit a standard Cox regression model using only CD8+IFN-γ+ after pp65 and IE-1 stimulation as covariates. In contrast to our polyfunctional cell subset model, the concordance index was only 0.58 (SE, 0.087), which was indicative of minimal ability to discriminate patients at low or high risk for CMV infection (Figure 3). This indicates that polyfunctional T-cell subset information, which is not available with single parameter assays such as the QuantiFERON-CMV, provides critical prognostic information and is essential for discrimination.
Figure 3.
Effectiveness of log risk score (x-axis) single-cytokine model derived from our discovery cohort to identify patients who develop cytomegalovirus (CMV) (red X) versus those free of CMV (blue +), adjusted for time after completion of prophylaxis (y-axis). Single-cytokine analysis was unable to discriminate a suitable risk score threshold.
Cutoff Selection
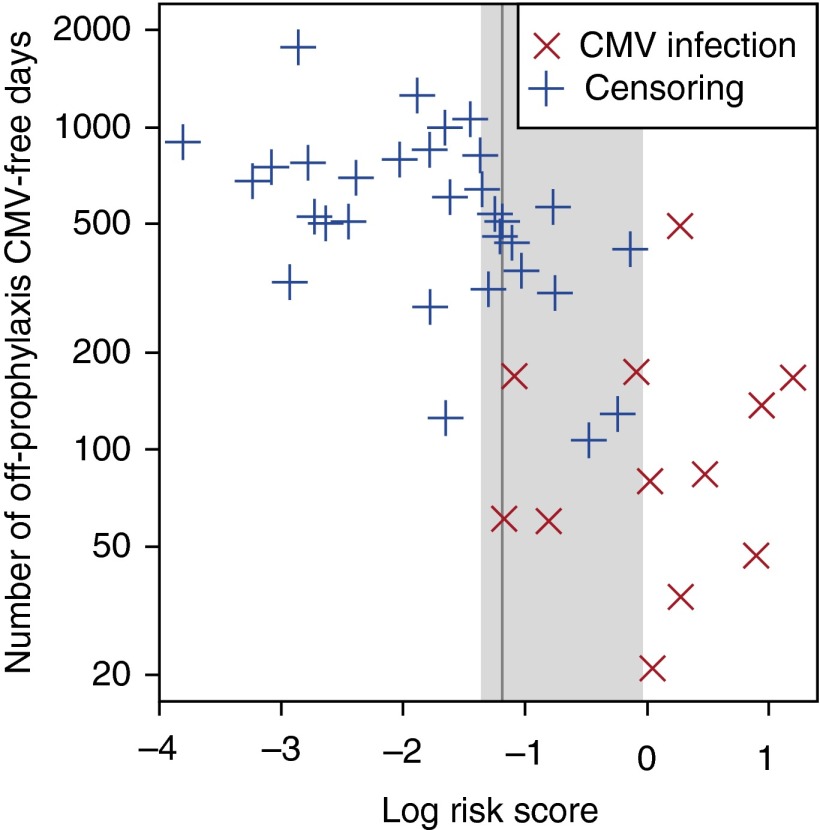
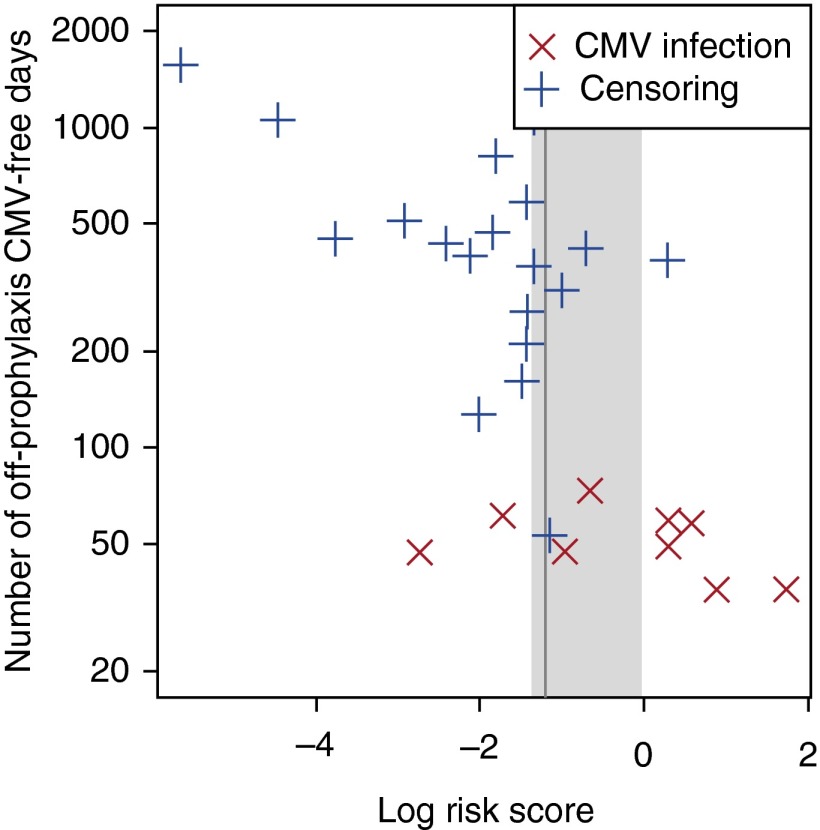
To establish a cutoff for the log risk score to discriminate between subjects at low and high risk for future CMV, we calculated the concordance between the prophylaxis-free, follow-up time to event and risk score dichotomized by a given cutoff. The cutoff that resulted in the highest concordance index was a log risk score of −1.2. Log risk scores in the range of −1.4 to 0.0 give a concordance index that falls within 1 SE of the optimal concordance index (Figure 4).
Figure 4.
Selection of the best cutoff for log risk score in the final polyfunctional subset model derived from the discovery data. For each candidate cutoff value, log risk scores of the 43 patients were dichotomized by the given threshold and concordance index between the dichotomized log risk scores, and off-prophylaxis times to cytomegalovirus (CMV) infection was computed. The vertical gray line is drawn at –1.2, the best cutoff that gives the highest concordance index. This cutoff value discriminated subsequently infected CMV patients and patients who did not develop CMV. The gray shaded area is drawn over the interval (−1.4, 0.0) that included all cutoff values whose concordance indexes were within 1 SE of the largest concordance index. For more conservative withdrawal of prophylaxis, a cutoff of −1.4 could be used.
External Validation
After the final model was fitted, we conducted a second independent study in a cohort of 28 independent serologically positive patients with no previous CMV, on prophylaxis at the time of blood draw and subsequently stopped prophylaxis at a time point after the blood draw. Demographic characteristics of these recipients are described in Table E1 in the online supplement. Nine of these recipients developed CMV infection over the follow-up period. Polyfunctional CD4+and CD8+ CMV-specific response to pp65 was measured as described previously. The model predictions were robust in the external validation. Using a cutoff of log score −1.2, the concordance index for this independent cohort using the final model was 0.81 (SE, 0.103). The distribution of the log risk score is shown in Figure 5. Kaplan-Meier plots for CMV-free survival in patients above and below the log risk thresholds for the discovery and validation cohorts are shown in Figure E2.
Figure 5.
Application of a polyfunctional T-cell subset model to the validation cohort. The vertical gray line is drawn at −1.2 log risk score. The gray shaded area is drawn over the interval (−1.4, 0.0) that included all cutoff values whose concordance indexes were within 1 SE of the largest concordance index. The concordance index between the dichotomized log risk scores and off-prophylaxis times to cytomegalovirus (CMV) infection was 0.81 (SE, 0.103).
Discussion
Establishing a diagnostic test that reliably predicts future CMV risk in at-risk solid organ transplantation recipients is a critical and unmet need of the transplant community. Previous attempts to measure CMV-specific immunity have explored the enzyme-linked immunospot assay (ELISPOT), HLA-restricted tetramer technology, ATP release assays, and intracellular cytokine response to CMV peptides, lysate, and infected cells (16–18). These assays have been limited by a focus on a single cytokine output (IFN-γ), poor specificity, HLA restriction, and unacceptably high rates of uninterpretable results.
Because of these limitations, a reliable and clinically useful method to risk stratify recipients does not exist. Thus, seropositive lung transplant recipients typically receive a fixed duration of antiviral prophylaxis. Although this can result in lower rates of CMV infection, it also subjects a large number of patients to a costly, potentially toxic therapy. At the same time, it results in discontinuation of therapy in some patients who could benefit from continued prophylaxis. In contrast, our novel diagnostic approach is the first to consider multiple polyfunctional cell subsets in a prediction model to determine future CMV risk. This approach represents a paradigm shift in analyzing CMV-specific immunity after organ transplantation, moving from single cytokine functional assays to a polyfunctional approach.
Our results demonstrate that a polyfunctional approach is considerably more reliable than assays that measure a single cytokine response. If we considered just IFN-γ production from CD8+ T cells after pp65 and IE-1 stimulation in our discovery cohort, there was no reliable prediction of subsequent CMV infection. Similarly, two other studies in lung transplantation recipients that used QuantiFERON-CMV did not find the assay reliable in predicting CMV risk (19, 20).
An advantage of flow cytometry–based assays, as opposed to QuantiFERON-CMV or ELISPOT IFN-γ assays, is the ability to distinguish responses by CD4+ and CD8+ cells. Importantly, our analysis indicates that both CD4+ and CD8+ polyfunctional T cells are associated with protection against CMV infection. This may be surprising because of the predominant role of CD8+ in viral infections (21), especially for prevention and control of primary CMV disease after transplantation (7, 22, 23). However, other transplantation studies have also indicated an important role for CD4+ T cells in CMV viral control after transplantation (24–28). Interestingly, in a study of renal, heart, and lung transplantation recipients, lung transplantation recipients had fewer CMV reactive CD4+ cells compared with other solid organ transplantation recipients and healthy control subjects (30). This relative paucity of CMV reactive CD4+ cells may account for the higher level of CMV infection seen in lung transplantation recipients compared with other transplant recipients. Thus, our finding of CD4+ cells as being critical to CMV reactivation post-transplantation is consistent with previous limited studies in this area.
Our analysis indicates that the model was more predictive of CMV infection risk when using data from pp65 stimulated samples than from IE-1 stimulated samples, although both stimulations elicited polyfunctional T-cell responses. In contrast to our findings, a previous study of thoracic organ transplantation recipients that used IE-1 or pp65 stimulation with an IFN-γ readout by flow cytometry noted that the early postoperative detection of IE-1–specific CD8+cells, but not pp65-specific CD4+ cells, was associated with protection from CMV infection (17). However, these differences may reflect the limited number of lung transplantation patients included (n = 4), early assessment time points (Day 0 and Day 14 postoperative), or the reliance on single IFN-γ cytokine measurements (17).
Finally, an important interpretation of our results is that T cells are capable of mounting a polyfunctional cytokine response that correlates to infection risk. A polyfunctional T-cell response is associated with control of both HIV-1 and HIV-2, mycobacteria tuberculosis (latent disease), hepatitis C, as well as other infections (10, 11, 29). In addition, a polyfunctional cytokine response after vaccination is considered protective (30, 31). Because of this body of work, it is not surprising that the single cytokine approach (commercially available as QuantiFERON-CMV or T Track assay) is inadequate to predict subsequent CMV in a lung transplantation recipient. A polyfunctional signature was required to accurately and reproducibly predict those at high risk for subsequent CMV. In parallel to our findings, CMV-specific CD4+ cells in vitro that concurrently produced IFN-γ+, IL-2+, and TNF-α+ expressed the costimulatory molecule CD40L and degranulation markers at higher levels than cells that expressed only single cytokines (32). This provides some insights into the potential mechanism by which polyfunctional cells might regulate host CMV-specific immunity, because triple cytokine producing cells appear to be functionally superior both in vitro and in vivo systems. However, additional studies are needed to more precisely define the mechanisms by which specific degrees of polyfunctionality confer protective CMV-specific immunity after lung transplantation.
Our study has several limitations. First, although we discovered and validated a novel polyfunctional response that predicts CMV infection after lung transplantation, our clinical cohort sizes were small. This assay will need to be validated in a larger, independent cohort. Second, our study used samples obtained at variable post-transplantation time points. It is encouraging that, despite the variation in sampling time frame, we still identified a robust protective signature. It will be useful to determine how changes in immunosuppression or time after lung transplantation affect polyfunctional responses. In addition, it will be important to validate this signature over time in individual patients. Further understanding the changes in this specific immune signature is the focus of future work.
In conclusion, we demonstrate a unique immunological approach to improve CMV risk stratification that considers CMV-specific polyfunctional cytokine production. This novel assay could directly affect and improve clinical lung transplantation care by avoiding excessive prophylaxis in some individuals and undertreatment in others. By individualizing treatment, we can ultimately reduce the burden of both prophylaxis and of CMV after lung transplantation and improve long-term patient outcomes. Our results suggest that a similar approach to CMV prevention would be useful to explore across all the commonly transplanted solid organs and bone marrow transplantation, because CMV remains a common and serious problem among all transplantation recipients. Finally, it is conceivable that the polyfunctional CMV signature identified through these studies will provide a useful tool in which to monitor the host immune response after immunization, because several CMV vaccines are currently under development for use in disease prevention in solid organ transplantation and other at-risk immunocompromised populations.
Footnotes
Supported by Biomarker Factory, LLC (a Duke University/LabCorp joint company), and by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001117. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Contributions: Study conception, study design, interpretation of the data, drafting, and revision of the manuscript: L.D.S. Data analysis and interpretation, drafting, and revision of the manuscript: C.C. Data analysis and interpretation: D.K. Study conception, study design, data acquisition, analysis of data, and revision of the manuscript: J.S.Y. Study conception and data acquisition: J.A.M. Study conception, study design, analysis of data, and revision of manuscript: C.A.F.C. Data acquisition and analysis of data: R.J.O. Study design, data acquisition, and analysis of data: S.D.S. Study conception, study design, interpretation of the data, and revision of the manuscript: S.M.P. Study conception, study design, interpretation of the data, and drafting and revision of the manuscript: K.J.W.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201504-0733OC on September 16, 2015.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, Humar A Transplantation Society International CMV Consensus Group. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013;96:333–360. doi: 10.1097/TP.0b013e31829df29d. [DOI] [PubMed] [Google Scholar]
- 2.Roman A, Manito N, Campistol JM, Cuervas-Mons V, Almenar L, Arias M, Casafont F, del Castillo D, Crespo-Leiro MG, Delgado JF, et al. ATOS Working Group. The impact of the prevention strategies on the indirect effects of CMV infection in solid organ transplant recipients. Transplant Rev (Orlando) 2014;28:84–91. doi: 10.1016/j.trre.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 3.Snyder LD, Finlen-Copeland CA, Turbyfill WJ, Howell D, Willner DA, Palmer SM. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am J Respir Crit Care Med. 2010;181:1391–1396. doi: 10.1164/rccm.200911-1786OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westall GP, Michaelides A, Williams TJ, Snell GI, Kotsimbos TC. Bronchiolitis obliterans syndrome and early human cytomegalovirus DNAaemia dynamics after lung transplantation. Transplantation. 2003;75:2064–2068. doi: 10.1097/01.TP.0000069234.04901.A3. [DOI] [PubMed] [Google Scholar]
- 5.Paraskeva M, Bailey M, Levvey BJ, Griffiths AP, Kotsimbos TC, Williams TP, Snell G, Westall G. Cytomegalovirus replication within the lung allograft is associated with bronchiolitis obliterans syndrome. Am J Transplant. 2011;11:2190–2196. doi: 10.1111/j.1600-6143.2011.03663.x. [DOI] [PubMed] [Google Scholar]
- 6.Zuk DM, Humar A, Weinkauf JG, Lien DC, Nador RG, Kumar D. An international survey of cytomegalovirus management practices in lung transplantation. Transplantation. 2010;90:672–676. doi: 10.1097/TP.0b013e3181ea3955. [DOI] [PubMed] [Google Scholar]
- 7.Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, Kalpoe J, Lisboa L, Ely L, Kaul DR, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis. 2013;56:817–824. doi: 10.1093/cid/cis993. [DOI] [PubMed] [Google Scholar]
- 8.Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, Venkataraman S, Humar A. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009;9:1214–1222. doi: 10.1111/j.1600-6143.2009.02618.x. [DOI] [PubMed] [Google Scholar]
- 9.Snyder LD, Medinas R, Chan C, Sparks S, Davis WA, Palmer SM, Weinhold KJ. Polyfunctional cytomegalovirus-specific immunity in lung transplant recipients receiving valganciclovir prophylaxis. Am J Transplant. 2011;11:553–560. doi: 10.1111/j.1600-6143.2010.03405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Silva TI, Peng Y, Leligdowicz A, Zaidi I, Li L, Griffin H, Blais ME, Vincent T, Saraiva M, Yindom LM, et al. Correlates of T-cell-mediated viral control and phenotype of CD8(+) T cells in HIV-2, a naturally contained human retroviral infection. Blood. 2013;121:4330–4339. doi: 10.1182/blood-2012-12-472787. [DOI] [PubMed] [Google Scholar]
- 12.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 13.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Shuster JJ. Median follow-up in clinical trials. J Clin Oncol. 1991;9:191–192. doi: 10.1200/JCO.1991.9.1.191. [DOI] [PubMed] [Google Scholar]
- 15.Kern F, Khatamzas E, Surel I, Frömmel C, Reinke P, Waldrop SL, Picker LJ, Volk HD. Distribution of human CMV-specific memory T cells among the CD8pos. subsets defined by CD57, CD27, and CD45 isoforms. Eur J Immunol. 1999;29:2908–2915. doi: 10.1002/(SICI)1521-4141(199909)29:09<2908::AID-IMMU2908>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 16.Nickel P, Bold G, Presber F, Biti D, Babel N, Kreutzer S, Pratschke J, Schönemann C, Kern F, Volk HD, et al. High levels of CMV-IE-1-specific memory T cells are associated with less alloimmunity and improved renal allograft function. Transpl Immunol. 2009;20:238–242. doi: 10.1016/j.trim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 17.Bunde T, Kirchner A, Hoffmeister B, Habedank D, Hetzer R, Cherepnev G, Proesch S, Reinke P, Volk HD, Lehmkuhl H, et al. Protection from cytomegalovirus after transplantation is correlated with immediate early 1-specific CD8 T cells. J Exp Med. 2005;201:1031–1036. doi: 10.1084/jem.20042384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egli A, Humar A, Kumar D. State-of-the-art monitoring of cytomegalovirus-specific cell-mediated immunity after organ transplant: a primer for the clinician. Clin Infect Dis. 2012;55:1678–1689. doi: 10.1093/cid/cis818. [DOI] [PubMed] [Google Scholar]
- 19.Westall GP, Mifsud NA, Kotsimbos T. Linking CMV serostatus to episodes of CMV reactivation following lung transplantation by measuring CMV-specific CD8+ T-cell immunity. Am J Transplant. 2008;8:1749–1754. doi: 10.1111/j.1600-6143.2008.02294.x. [DOI] [PubMed] [Google Scholar]
- 20.Weseslindtner L, Kerschner H, Steinacher D, Nachbagauer R, Kundi M, Jaksch P, Simon B, Hatos-Agyi L, Scheed A, Klepetko W, et al. Prospective analysis of human cytomegalovirus DNAemia and specific CD8+ T cell responses in lung transplant recipients. Am J Transplant. 2012;12:2172–2180. doi: 10.1111/j.1600-6143.2012.04076.x. [DOI] [PubMed] [Google Scholar]
- 21.van Lier RA, ten Berge IJ, Gamadia LE. Human CD8(+) T-cell differentiation in response to viruses. Nat Rev Immunol. 2003;3:931–939. doi: 10.1038/nri1254. [DOI] [PubMed] [Google Scholar]
- 22.Sester M, Sester U, Gärtner BC, Girndt M, Meyerhans A, Köhler H. Dominance of virus-specific CD8 T cells in human primary cytomegalovirus infection. J Am Soc Nephrol. 2002;13:2577–2584. doi: 10.1097/01.asn.0000030141.41726.52. [DOI] [PubMed] [Google Scholar]
- 23.Pipeling MR, West EE, Osborne CM, Whitlock AB, Dropulic LK, Willett MH, Forman M, Valsamakis A, Orens JB, Moller DR, et al. Differential CMV-specific CD8+ effector T cell responses in the lung allograft predominate over the blood during human primary infection. J Immunol. 2008;181:546–556. doi: 10.4049/jimmunol.181.1.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tu W, Potena L, Stepick-Biek P, Liu L, Dionis KY, Luikart H, Fearon WF, Holmes TH, Chin C, Cooke JP, et al. T-cell immunity to subclinical cytomegalovirus infection reduces cardiac allograft disease. Circulation. 2006;114:1608–1615. doi: 10.1161/CIRCULATIONAHA.105.607549. [DOI] [PubMed] [Google Scholar]
- 25.Gerna G, Lilleri D, Chiesa A, Zelini P, Furione M, Comolli G, Pellegrini C, Sarchi E, Migotto C, Bonora MR, et al. Virologic and immunologic monitoring of cytomegalovirus to guide preemptive therapy in solid-organ transplantation. Am J Transplant. 2011;11:2463–2471. doi: 10.1111/j.1600-6143.2011.03636.x. [DOI] [PubMed] [Google Scholar]
- 26.Chiereghin A, Gabrielli L, Zanfi C, Petrisli E, Lauro A, Piccirilli G, Baccolini F, Dazzi A, Cescon M, Morelli MC, et al. Monitoring cytomegalovirus T-cell immunity in small bowel/multivisceral transplant recipients. Transplant Proc. 2010;42:69–73. doi: 10.1016/j.transproceed.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Sester M, Sester U, Gärtner B, Heine G, Girndt M, Mueller-Lantzsch N, Meyerhans A, Köhler H. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation. 2001;71:1287–1294. doi: 10.1097/00007890-200105150-00018. [DOI] [PubMed] [Google Scholar]
- 28.Sester U, Gärtner BC, Wilkens H, Schwaab B, Wössner R, Kindermann I, Girndt M, Meyerhans A, Mueller-Lantzsch N, Schäfers HJ, et al. Differences in CMV-specific T-cell levels and long-term susceptibility to CMV infection after kidney, heart and lung transplantation. Am J Transplant. 2005;5:1483–1489. doi: 10.1111/j.1600-6143.2005.00871.x. [DOI] [PubMed] [Google Scholar]
- 29.Ciuffreda D, Comte D, Cavassini M, Giostra E, Bühler L, Perruchoud M, Heim MH, Battegay M, Genné D, Mulhaupt B, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 30.Makedonas G, Betts MR. Polyfunctional analysis of human t cell responses: importance in vaccine immunogenicity and natural infection. Springer Semin Immunopathol. 2006;28:209–219. doi: 10.1007/s00281-006-0025-4. [DOI] [PubMed] [Google Scholar]
- 31.Precopio ML, Betts MR, Parrino J, Price DA, Gostick E, Ambrozak DR, Asher TE, Douek DC, Harari A, Pantaleo G, et al. Immunization with vaccinia virus induces polyfunctional and phenotypically distinctive CD8(+) T cell responses. J Exp Med. 2007;204:1405–1416. doi: 10.1084/jem.20062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kannanganat S, Ibegbu C, Chennareddi L, Robinson HL, Amara RR. Multiple-cytokine-producing antiviral CD4 T cells are functionally superior to single-cytokine-producing cells. J Virol. 2007;81:8468–8476. doi: 10.1128/JVI.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]