Abstract
Rationale: Carbon monoxide (CO) exposure is a leading cause of poison-related mortality. CO binds to Hb, forming carboxyhemoglobin (COHb), and produces tissue damage. Treatment of CO poisoning requires rapid removal of CO and restoration of oxygen delivery. Visible light is known to effectively dissociate CO from Hb, with a single photon dissociating one CO molecule.
Objectives: To determine whether illumination of the lungs of CO-poisoned mice causes dissociation of COHb from blood transiting the lungs, releasing CO into alveoli and thereby enhancing the rate of CO elimination.
Methods: We developed a model of CO poisoning in anesthetized and mechanically ventilated mice to assess the effects of direct lung illumination (phototherapy) on the CO elimination rate. Light at wavelengths between 532 and 690 nm was tested. The effect of lung phototherapy administered during CO poisoning was also studied. To avoid a thoracotomy, we assessed the effect of lung phototherapy delivered to murine lungs via an optical fiber placed in the esophagus.
Measurements and Main Results: In CO-poisoned mice, phototherapy of exposed lungs at 532, 570, 592, and 628 nm dissociated CO from Hb and doubled the CO elimination rate. Phototherapy administered during severe CO poisoning limited the blood COHb increase and improved the survival rate. Noninvasive transesophageal phototherapy delivered to murine lungs via an optical fiber increased the rate of CO elimination while avoiding a thoracotomy.
Conclusions: Future development and scaling up of lung phototherapy for patients with CO exposure may provide a significant advance for treating and preventing CO poisoning.
Keywords: carbon monoxide, poisoning, carboxyhemoglobin, phototherapy
At a Glance Commentary
Scientific Knowledge on the Subject
Carbon monoxide (CO) binds to Hb with an affinity 200 times greater than oxygen, reducing the blood oxygen-carrying capacity and impairing oxygen delivery. Treatment of CO poisoning involves breathing 100% oxygen to rapidly remove CO. Hyperbaric oxygen therapy further increases the rate of CO elimination but is often unavailable. Visible light is known to dissociate CO from Hb in vitro, with a single photon dissociating one CO molecule.
What This Study Adds to the Field
We demonstrated in vivo that direct lung illumination and noninvasive transesophageal lung illumination photodissociated CO from Hb in blood transiting the lungs of CO-poisoned mice. Phototherapy increased the amount of exhaled CO and enhanced the CO elimination when administered after CO exposure. When phototherapy was administered during CO breathing, illumination decreased the degree of CO intoxication. Here we describe the basic principles of in vivo phototherapy as an innovative therapeutic strategy for CO poisoning.
Acute carbon monoxide (CO) poisoning is one of the most common causes of poison-related death worldwide. In the United States, CO inhalation causes approximately 50,000 emergency room visits and more than 400 deaths each year (1). Even when it is not lethal, CO intoxication is associated with significant morbidity, including memory, attention, and affect disorders (2–5).
CO impairs tissue oxygenation by avidly binding Hb to form carboxyhemoglobin (COHb), which cannot transport oxygen (6). CO also causes direct tissue damage by binding to other heme-containing proteins. Binding of CO to cytochrome-c oxidase in the brain subverts oxidative metabolism and leads to the generation of reactive oxygen species (7–10). In the heart, binding of CO to myoglobin reduces oxygen availability, leading to arrhythmias and cardiac dysfunction (11–13).
According to current guidelines (14), the successful treatment of CO poisoning involves rapid removal of CO and restoration of oxygen delivery. No specific therapy to prevent or treat the tissue injury caused by CO is available. The COHb half-life (COHb-t1/2; the time to eliminate 50% of the blood concentration of COHb) reflects the efficacy of a treatment in removing CO. Breathing air, the COHb-t1/2 in humans is approximately 4–5 hours (15). Breathing normobaric oxygen (100% O2) reduces COHb-t1/2 to 1.5–2 hours (16). Hyperbaric oxygen (HBO) therapy can further reduce COHb-t1/2 to approximately 30 minutes (17). Inducing hyperventilation is as effective as HBO in reducing COHb-t1/2 (18–20). HBO and isocapnic hyperventilation, although relatively efficient in removing CO, have had limited therapeutic application. HBO is often not readily available and transporting patients to specialized hyperbaric units may result in long delays before commencing HBO therapy. HBO also has potential side effects, including barotrauma, oxygen toxicity, seizures, and pulmonary edema (21, 22). Isocapnic hyperventilation requires a large increase in minute ventilation, which may induce discomfort in awake patients.
In 1896, John Haldane observed that visible light irradiation efficiently dissociates CO from COHb (quantum yield ≈ 0.5–1) (23). In contrast, photodissociation of oxygen from oxygenated Hb is far less efficient (quantum yield ≈ 0.05–0.1) (24–26).
Smyczynski (27) suggested that light irradiation can be used to treat CO-poisoned patients by photodissociating COHb in blood passing through an extracorporeal oxygenator. We hypothesized that direct illumination of the lungs of CO-poisoned mice would cause COHb dissociation with rapid release of CO directly into the alveolus, thereby increasing the quantity of exhaled CO and the rate of CO elimination. We report the development of a murine model of CO poisoning and demonstrate the efficacy of lung phototherapy in both treating and preventing CO poisoning. Some of the results of these studies have been previously reported in the form of an abstract (28).
Methods
Animals
All animal studies were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital, Boston, Massachusetts. We studied anesthetized and mechanically ventilated mice. Volume-controlled ventilation was provided at a respiratory rate of 90 breaths ⋅ min−1, a tidal volume of 10 ml ⋅ kg−1, and a positive end-expiratory pressure of 1 cm H2O. To directly illuminate the lungs, mice underwent a median thoracotomy (direct lung phototherapy). Mice were poisoned by breathing CO at 400 or 2,000 ppm in air for 1 hour, and subsequently treated by breathing either 100% oxygen or air with or without lung phototherapy.
In some experiments, mice were serially poisoned with 400 ppm CO for 5 minutes followed by 25 minutes of treatment with 100% O2, either alone or together with phototherapy at various wavelengths (λ), energy levels, and pulse durations.
Measurements and Calculations
During the poisoning and the treatment periods, inhaled and exhaled CO concentrations were measured and the areas under the curves (AUC) were calculated. Because the minute ventilation per kilogram body weight was known and fixed, the quantity of CO inhaled and exhaled over a period of time (ml/kg) was calculated as AUC (ppm ⋅ min) ⋅ 10−6 ⋅ 900 (ml/kg/min). The quantity of CO absorbed by the mouse during the poisoning was calculated as the difference between the quantity of CO inhaled and exhaled. The percentage of COHb in arterial blood was measured during and after CO poisoning and COHb half-life was calculated. In the experiments involving serial CO poisoning episodes in the same mouse, the CO elimination rate was determined by calculating the time necessary to eliminate 90% of the CO absorbed during the poisoning period (T90%CO).
Phototherapy
Laser devices were used to generate light at 532, 570, 592, 628, and 690 nm. The power of the light reaching the lung surface was measured with a power meter. The light irradiance and the radiant exposure were calculated according to standard formulae.
Transesophageal Lung Phototherapy
A 1-mm diameter optical fiber with a 1.2-cm-long diffusing tip, which emits light at 360 degrees, was placed via the oropharynx into the esophagus of the mouse. Intermittent phototherapy with light at 532 nm was tested. The power was set to 1.5 W and light was pulsed at 1 Hz with a 200-ms pulse width.
Statistical Analysis
Statistical analysis was performed using Sigma Plot 12.5 (SPPS, Inc., Chicago, IL). Data were analyzed using Student’s t test and a one-way analysis of variance with post hoc Bonferroni test (two-tailed). Two-way analysis of variance for repeated measurements was used to compare variables over time among groups. For survival analyses, Kaplan-Meier estimates were generated and compared using the log-rank test. Statistical significance was defined as a P value of less than 0.05. All data are expressed as mean ± SD unless specified otherwise. Additional details can be found in the online supplement Methods.
Results
Development of a Murine Model of CO Poisoning
To investigate whether visible light might be used to dissociate CO from Hb in vivo, we developed a murine model of CO poisoning. Mice were poisoned by inhaling 400 ppm CO for 1 hour and subsequently treated by breathing either air or 100% O2. The exhaled CO concentration and the AUC of exhaled CO concentration during the first 15 minutes of breathing 100% O2 were significantly greater than during the first 15 minutes of breathing air, indicating that a larger amount of CO was eliminated during this period (3.21 ± 0.22 vs. 1.57 ± 0.09 ml/kg; P < 0.001 values differ) (Figure 1A). In contrast, after approximately 20 minutes of treatment, the exhaled CO concentration was significantly higher in air-breathing mice, demonstrating the presence of a larger amount of CO remaining to be eliminated. At the end of the poisoning period, 28.0 ± 0.6% of circulating Hb was saturated with CO. Treatment by ventilation with 100% O2 decreased COHb concentration faster than treatment with air (COHb-t1/2: 8.2 ± 1.2 vs. 31.5 ± 3.1 min; P < 0.001) (Figure 1B). In this murine model, both the exhaled CO concentration and arterial COHb levels reflect the advantage of breathing 100% O2 over breathing air after CO poisoning.
Figure 1.
Murine model of carbon monoxide (CO) poisoning. (A) Inhaled and exhaled CO concentrations during poisoning (0–60 min) with 400 ppm CO and treatment (60–160 min) by breathing either air or 100% O2 (n = 5 per group). The red shaded area between the inhaled and exhaled CO curves (differential areas under the curves) during the poisoning period reflects the quantity of absorbed CO. The green and blue shaded regions beneath the exhaled CO concentration curves (areas under the curves) during the treatment period reflect the amount of CO eliminated while breathing either air or 100% O2, respectively. (A, inset) In the first 15 minutes of the treatment period, the quantity (ml/kg) of exhaled CO of mice breathing 100% O2 was significantly greater as compared with mice breathing air (*P < 0.001; Student’s t test). (B) Arterial blood carboxyhemoglobin (COHb) levels during poisoning with 400 ppm CO (0–60 min) and during treatment by breathing either air or 100% O2. Treatment with 100% O2 induced a more rapid decrease in arterial COHb levels as compared with air breathing and (B, inset) COHb half-life (COHb-t1/2) was significantly shorter (*P < 0.001; Student’s t test). All data represent mean ± SD.
Phototherapy Increases the Rate of CO Elimination
To evaluate the effect of direct illumination of the lungs on the CO elimination rate, mice were subjected to a median sternotomy to expose both lungs. After breathing 400 ppm CO for 1 hour, the mice were treated by ventilation with either air or 100% O2, with or without phototherapy (see Figure E1B in the online supplement). Pulmonary phototherapy added to 100% O2 breathing induced an increase of exhaled CO concentration during the first 5 minutes of treatment, indicating a greater CO elimination (2.49 ± 0.19 vs. 1.71 ± 0.10 ml/kg; P < 0.001) (Figure 2A). Commencing at 8 minutes of treatment, exhaled CO levels were significantly higher in mice treated with 100% O2 alone as compared with light and 100% O2, demonstrating the presence of a larger amount of CO remaining to be eliminated. The reduction of blood COHb concentration during treatment with 100% O2 and phototherapy was greater than treatment with 100% O2 alone, and the COHb-t1/2 was significantly shorter (3.8 ± 0.5 vs. 8.2 ± 1.2 min; P < 0.001) (Figure 2B).
Figure 2.
Phototherapy increases the rate of carbon monoxide (CO) elimination in mice treated by breathing either 100% O2 or air. Effect of treatment by breathing 100% O2 and phototherapy at 628 nm and 54 mW ⋅ cm−2 irradiance on (A) inhaled and exhaled CO concentrations and (B) arterial carboxyhemoglobin (COHb) levels (n = 5 per group). (A, inset) The quantity (ml/kg) of CO exhaled during the first 5 minutes of treatment was higher in mice treated with 100% O2 and phototherapy as compared with mice breathing 100% O2 alone (*P < 0.001; Student’s t test). (B, inset) COHb half-life (COHb-t1/2) was significantly shorter in mice treated by breathing 100% O2 and phototherapy as compared with mice breathing 100% O2 alone (*P < 0.001; Student’s t test). Effect of treatment by breathing air and phototherapy on (C) inhaled and exhaled CO concentrations and (D) arterial COHb levels (n = 5 per group). (C, inset) The quantity (ml/kg) of CO exhaled during the first 25 minutes of treatment was higher in mice treated with air and phototherapy as compared with mice breathing air alone (*P < 0.001; Student’s t test). (D, inset) COHb-t1/2 was significantly shorter in mice treated with phototherapy as compared with mice breathing air alone. (*P < 0.001; Student’s t test). All data represent mean ± SD.
To evaluate the efficacy of phototherapy with a lower concentration of inspired O2, we tested whether lung phototherapy could increase the CO elimination rate while mice were breathing air. Phototherapy added to air breathing was associated with an increased quantity of CO eliminated during the first 25 minutes of treatment as compared with air breathing without phototherapy (3.11 ± 0.16 vs. 2.12 ± 0.12 ml/kg; P < 0.001) (Figure 2C) and COHb-t1/2 was significantly shorter (19.2 ± 1.3 vs. 31.5 ± 3.1 min; P < 0.001) (Figure 2D). There was no difference in systemic arterial pressure, heart rate, or rectal or lung surface temperature between mice treated with or without phototherapy (see Figures E2 and E3). These results show that after CO poisoning, direct lung phototherapy increased the rate of CO elimination in mice breathing either 100% O2 or air.
Effect of Light at Various Wavelengths on the In Vivo CO Elimination Rate
Light with a wide range of wavelengths (280–620 nm) photodissociates COHb in vitro (24). To test whether visible light at different wavelengths could dissociate COHb in vivo, mice were poisoned with CO and treated with direct lung phototherapy at 532, 570 (green), 592 (yellow), or 628 (red) nm. To exclude nonspecific effects of lung illumination on the CO elimination rate, light at 690 nm (infrared), which does not dissociate COHb in vitro, was also tested. Phototherapy at 532, 570, 592, or 628 nm, when added to 100% O2 treatment, significantly decreased the T90%CO when compared with 100% O2 alone (respectively, 8.7 ± 1.2, 7.2 ± 0.2, 8.8 ± 2.2, 10.2 ± 2.7 vs. 21.8 ± 2.4 min; each comparison P < 0.005) (Figure 3A). In contrast, phototherapy at 690 nm did not decrease the T90%CO when compared with 100% O2 breathing alone (19.4 ± 1.5 vs. 21.8 ± 2.4 min; P = 0.892) (Figure 3A). These results show that phototherapy at wavelengths between 532 and 628 nm increases the rate of CO elimination in CO-poisoned mice.
Figure 3.
Effect of the wavelength and irradiance of light on the carbon monoxide (CO) elimination rate. (A) Effect of phototherapy at 532 (n = 5), 570 (n = 3), 592 (n = 5), 628 (n = 7), and 690 nm (n = 6) wavelength and 54 mW ⋅ cm−2 irradiance (I) on the time necessary to eliminate 90% of the CO absorbed during the poisoning period (T90%CO). *P < 0.005 versus 100% O2 alone, one-way analysis of variance (ANOVA). (B) Effect of phototherapy at 628 nm using either low (n = 4), medium (n = 5), or high (n = 5) power (I = 24, 54, and 80 mW ⋅ cm−2) on COHb half-life (COHb-t1/2). *P < 0.001 versus 100% O2 alone, †P < 0.05 versus low power; §P = 0.07 versus medium power, one-way ANOVA. (C) Effect of intermittent phototherapy at 532 nm, 80, 160, or 250 mW ⋅ cm−2 I and pulse widths of 100, 500, 1,000 ms at a constant frequency of 1 Hz on the T90%CO (n = 2–4 per treatment group). *P < 0.001 versus 100% O2 alone, one-way ANOVA. †P < 0.001 versus pulse width 500 and 1,000 ms within the same irradiance, §P < 0.01 versus I 160 mW ⋅ cm−2 within the same pulse duration, two-way ANOVA. (D) Relationship between the radiant exposure of light at 532 nm and the T90%CO, R2 = 0.93 (see text for details). All data represent mean ± SD. COHb = carboxyhemoglobin.
Effect of Irradiance and Radiant Exposure on the CO Elimination Rate
To investigate the relationship between the light energy and the COHb photodissociation efficiency, mice were poisoned with CO and treated with 100% O2 combined with continuous direct lung phototherapy at 628 nm using either a low, medium, or high power. Each of these three energy levels decreased blood COHb-t1/2 as compared with mice treated by breathing 100% O2 alone (4.8 ± 0.5, 3.8 ± 0.5, 3.1 ± 0.2 vs. 8.2 ± 1.2 min; each comparison P < 0.001) (Figure 3B). The results show that continuous phototherapy with an irradiance as low as 24 mW ⋅ cm−2 effectively increased the rate of CO elimination. Moreover, the reduction of COHb-t1/2 obtained with phototherapy was enhanced with an increase of the energy of incident light.
Heat produced by continuous irradiation might damage the lung. One approach to reducing tissue heating is to administer the light intermittently. To investigate the effect of intermittent phototherapy on the CO elimination rate, we tested light at three energy levels and three different pulse widths at a constant frequency of 1 Hz. When using light at low energy and with a short pulse width, T90%CO was not decreased when compared with breathing 100% O2 alone (Figure 3C). At each energy level, the longer the pulse the greater was the reduction in T90%CO. At each pulse width tested, T90%CO was reduced when the irradiance was increased from 80 to 160 mW ⋅ cm−2. There was no further reduction when the irradiance was increased to 250 mW ⋅ cm−2. These results show that intermittent phototherapy can also increase the rate of CO elimination, although the effect is less than continuous phototherapy.
To evaluate the combined effects of pulse width and light irradiance on the CO elimination rate, we calculated the radiant exposure for each treatment. The relationship between the CO elimination rate and the radiant exposure is described by an exponential decay curve (Y = (Y0−Plateau) ⋅ exp(−K ⋅ X) + Plateau, R2 = 0.93) (Figure 3D): when the radiant exposure is greater than 0.08 J ⋅ cm−2, a plateau is reached (Plateau = 7.0 ± 0.6 min), and a further increase of the radiant exposure (the product of irradiance and pulse duration) does not increase the CO elimination rate.
Phototherapy Decreases CO Uptake during Poisoning
To test whether phototherapy might prevent CO poisoning during CO exposure, mice breathed 400 ppm CO with or without simultaneous direct lung phototherapy. The exhaled CO concentration was significantly greater in mice treated with phototherapy during poisoning (Figure 4A) and the amount of CO absorbed by these mice was significantly less than control mice (3.00 ± 0.11 vs. 4.23 ± 0.26 ml/kg; P < 0.001) (Figure 4B). In mice receiving phototherapy during CO exposure, arterial blood COHb levels at 20, 40, and 60 minutes were significantly lower than in control animals (13.4 ± 1.1 vs. 16.5 ± 1.9, 16.8 ± 1.1 vs. 24.2 ± 1.2, 18.4 ± 0.6 vs. 28.0 ± 0.6% respectively; P < 0.001) (Figure 4C). These results demonstrate that direct lung illumination during ongoing CO exposure is associated with reduced CO absorption and lower arterial COHb levels.
Figure 4.
Phototherapy decreases carbon monoxide (CO) uptake during poisoning. Mice breathed 400 ppm CO for 1 hour with (n = 5) or without (n = 6) simultaneous phototherapy at 628 nm. (A) Inhaled and exhaled CO concentrations during the poisoning. Starting 4 minutes after initiation of CO exposure, exhaled CO concentration was higher in phototherapy-treated mice as compared with control animals (*P < 0.05 vs. exhaled CO control animals; P interaction <0.001; two-way analysis of variance for repeated measurements). The red shaded area between the inhaled and exhaled CO concentration curves reflects the amount of CO absorbed by mice concomitantly treated with phototherapy. The yellow shaded area reflects the additional amount of CO absorbed by control mice. (B) Cumulative CO uptake during poisoning calculated from the red and yellow areas in A. *P < 0.001, Student’s t test. (C) Arterial blood carboxyhemoglobin (COHb) levels at 20, 40, and 60 minutes were significantly lower in phototherapy-treated mice than in control animals (*P < 0.001 vs. control animals; P interaction <0.001; two-way analysis of variance for repeated measurements). All data represent mean ± SD.
Phototherapy Improves Survival in Ongoing CO Poisoning
Because phototherapy during CO poisoning decreased the amount of absorbed CO, we explored whether phototherapy might improve the survival rate of mice breathing high levels of CO. Mice breathed 2,000 ppm CO with or without simultaneous direct lung phototherapy. Without phototherapy, all of the mice died within 30 minutes of CO exposure. In contrast, five of six mice breathing CO while receiving phototherapy survived for 60 minutes (P < 0.001) (Figure 5A). During CO poisoning, the blood COHb levels in phototherapy-treated mice were significantly less than in control mice (34.1 ± 3.5% vs. 49.9 ± 1.9% at 10 min and 38.5 ± 4.0% vs. 62.0 ± 0.9% at 20 min, respectively; P < 0.001) (Figure 5B). In the first 15 minutes of CO exposure, the systolic arterial pressure decreased and the heart rate transiently increased in both groups (Figures 5C and 5D). Thereafter blood pressure and heart rate dramatically decreased in untreated mice, whereas they remained constant in phototherapy-treated mice. During the study period, blood lactate levels were significantly lower in phototherapy-treated mice as compared with untreated mice (3.4 ± 1.0 vs. 4.6 ± 1.0 mmol ⋅ L−1 at 10 min, P = 0.05 and 3.7 ± 1.0 vs. 11.0 ± 1.5 mmol ⋅ L−1 at 20 min; P < 0.001) (Figure 5E). These results show that direct lung phototherapy improves the survival rate of mice undergoing CO poisoning. The reduced degree of CO intoxication was associated with lower blood COHb and lactate levels.
Figure 5.
Phototherapy improves survival in ongoing carbon monoxide (CO) poisoning. (A) Survival rate of mice breathing 2,000 ppm CO in air for 1 hour with or without simultaneous phototherapy at 532 nm (n = 6 per group). All control mice died within 30 minutes of commencing CO exposure. In contrast, five of six phototherapy-treated mice breathing 2,000 ppm CO survived for 60 minutes. *P < 0.001, phototherapy versus control animals, log-rank test. (B) Arterial carboxyhemoglobin (COHb) levels during poisoning were significantly lower in phototherapy-treated mice compared with control animals (*P < 0.001; P interaction <0.001; two-way analysis of variance [ANOVA] for repeated measurements). (C) Systolic arterial pressure (SAP; mean ± SEM), commencing at minute 21 of exposure, decreased in control mice, whereas SAP remained constant in phototherapy-treated mice (*P < 0.001; P interaction <0.001; two-way ANOVA for repeated measurements). (D) Heart rate (HR, mean ± SEM), commencing from minute 17, decreased in control animals but not in phototherapy-treated mice (*P < 0.001; P interaction <0.001; two-way ANOVA for repeated measurements). (E) Blood lactate levels during poisoning were significantly lower in mice treated with lung phototherapy compared with untreated mice (*P < 0.001; †P = 0.05, P interaction <0.001; two-way ANOVA for repeated measurements at time 0, 10, and 20 min). All data represent mean ± SD except for SAP and HR.
Esophageal Phototherapy Increases the Rate of CO Elimination
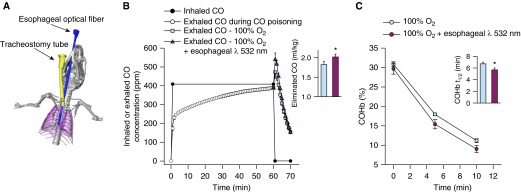
To evaluate the possibility of delivering phototherapy to murine lungs without a thoracotomy, we examined the effect of phototherapy delivered to the lungs via an optical fiber placed in the mouse esophagus. To optimize the position of the esophageal optical fiber in relation to the lungs, we obtained a computed tomography scan of a living, anesthetized, and mechanically ventilated 25 g mouse (Figure 6A). The optimal location of the fiber to irradiate the lower portion of the lungs was reached when the end of the fiber was 3.9 cm from the mandibular incisors.
Figure 6.
Esophageal phototherapy increases the rate of carbon monoxide (CO) elimination. (A) Computed tomography scan of a living, anesthetized, and mechanically ventilated 25-g mouse with an optical fiber placed in the esophagus as the three-dimensional reconstruction form. The esophageal optical fiber is depicted in blue, the lungs in purple, and the tracheostomy tube in yellow. (B) Inhaled and exhaled CO concentrations of mice during poisoning with 400 ppm CO (0–60 min) and treatment (60–70 min) by breathing 100% O2 alone or combined with intermittent transesophageal phototherapy at 532 nm (n = 6 per group). (B, inset) The areas under the curves of exhaled CO concentration during the first 5 minutes of treatment were greater in mice treated with 100% O2 and esophageal phototherapy as compared with mice breathing 100% O2 alone (*P < 0.001; Student’s t test). (C) Arterial blood carboxyhemoglobin (COHb) levels decreased more rapidly in the first 10 minutes of treatment with esophageal phototherapy and 100% O2 breathing as compared with breathing 100% O2 alone and (C, inset) COHb half-life (COHb-t1/2) was significantly shorter (*P < 0.001; Student’s t test). All data represent mean ± SD.
After poisoning, mice were treated with 100% O2 alone or combined with transesophageal lung phototherapy. The quantity of CO eliminated during the first 5 minutes of treatment was significantly greater in mice treated with esophageal phototherapy (2.02 ± 0.07 vs1.83 ± 0.08 ml/kg; P = 0.001) (Figure 6B) and COHb-t1/2 was significantly shorter with phototherapy than in mice breathing 100% O2 alone (5.7 ± 0.3 vs. 6.8 ± 0.3 min; P < 0.001) (Figure 6C). These results demonstrate that esophageal phototherapy increased the rate of CO elimination when compared with 100% O2 breathing alone.
Discussion
The development of a model of CO poisoning in anesthetized and mechanically ventilated mice allowed us to continuously monitor the rate of CO uptake and elimination and assess the efficacy of therapeutic interventions. Direct irradiation of murine lungs with visible light at various wavelengths between 532 and 628 nm increased the rate of CO elimination after CO poisoning, even when the light was administered by intermittent pulses and at low energy levels. Direct lung phototherapy administered during CO poisoning reduced the degree of CO intoxication and improved the survival rate. Without performing a thoracotomy, we demonstrated that noninvasive transesophageal administration of light to the lungs of CO-exposed mice using an orally placed optical fiber significantly increased the CO elimination rate.
Direct lung phototherapy combined with breathing either air or 100% O2 markedly reduced the blood COHb-t1/2 by 40 and 55% respectively. The high efficiency of lung phototherapy suggests that the pulmonary circulation is an ideal location to induce COHb photodissociation. Photodissociation of COHb in nonpulmonary tissues is ineffective, because displaced CO either rebinds to Hb or diffuses into peripheral tissues, the latter potentially worsening CO toxicity. In contrast, when CO photodissociates from COHb in the pulmonary vasculature, where the alveolar partial pressure of O2 is much greater than the pressure of CO, the O2 pressure gradient drives O2 molecules to bind to deoxygenated Hb at a far greater rate than CO (the rate constant of O2 binding to Hb is 10 times faster than CO binding) (29), thus CO is photodissociated from COHb, diffuses into the alveolus, and is eliminated during exhalation.
Theoretical models have been proposed for treating CO-poisoned patients by photodissociating COHb in blood passing through an extracorporeal oxygenator or skin (27, 30). Because the rate of CO elimination depends on the amount of blood taking part in gas exchange and because the entire cardiac output passes through the lungs, the pulmonary vasculature is an ideal location in which the largest quantity of blood can be treated per unit time.
Previous in vitro studies reported that the photodissociation process is wavelength independent between 280 and 620 nm (31). Kuz’min and colleagues (32) showed that the in vitro photodissociation of COHb has a peak of efficiency at 570 nm. When treating CO poisoning with phototherapy in vivo, an additional factor comes into play: the ability of light to penetrate tissue. When comparing wavelengths with varying in vitro efficiency in photodissociating COHb, the longer wavelengths have greater tissue penetration (33) and thereby longer wavelengths are able to photodissociate CO from Hb in vivo more efficiently than shorter wavelengths. The great efficacy of light at 628 nm at increasing the rate of CO elimination reported in this study suggests that this wavelength can be more effective than shorter wavelengths in treating CO poisoning in vivo, where lung tissue penetration is required.
In our poisoning model, direct lung phototherapy administered during severe CO poisoning prevented death by reducing the rate of increase and the maximum level of blood COHb. Moreover, oxyhemoglobin levels were significantly higher in CO-poisoned mice receiving phototherapy as compared with control animals, whereas blood deoxyhemoglobin levels were similar in both groups (see Table E1), demonstrating that pulmonary phototherapy selectively dissociates CO from Hb.
Translation of pulmonary phototherapy to larger animals and humans will most likely require irradiation with higher light energy levels. The use of intermittent light pulses, which we demonstrated to be effective in improving CO elimination, and the development of tissue cooling systems will limit the risk of heat-induced injury in larger animals. Laser light delivered to the stomach via an optical fiber has been used to eradicate Helicobacter pylori (34, 35), showing that phototherapy can be delivered in humans without causing tissue damage. It is possible that light can be delivered to the lungs via one or more optical fibers placed in the respiratory tree through an endotracheal tube or in the pleural space through a chest tube. Other approaches of light delivery could also be developed, such as the inhalation or injection of light-emitting microparticles that localize in the lungs. This study describes and demonstrates the fundamental principles of in vivo phototherapy, and provides the basis for scaling up the development and testing of lung phototherapy in larger animals and humans.
In clinical practice, HBO once initiated provides efficient removal of CO from poisoned patients (36). Unfortunately, HBO administration is often not available or delayed, because it requires transporting patients to specialized centers. Delays of HBO initiation may explain why clinical trials have failed to confirm a benefit of HBO over breathing normobaric oxygen (37–40). Developing a strategy of light delivery that is noninvasive, portable, readily available in the field, and highly effective in treating CO-poisoned patients will provide a critical advance in the treatment of CO poisoning.
This study has some limitations. First, we were unable to show an outcome benefit related to the use of phototherapy after CO poisoning. In the mouse, severe CO poisoning was an acute lethal event. Lactate only increased during the terminal cardiovascular collapse and in this brief period we were unable to institute any effective treatment. Second, the effect of transesophageal phototherapy on COHb half-life was smaller than direct lung phototherapy. To deliver light via an optical fiber through the esophagus, we used a KTP laser generating light at 532 nm, which poorly penetrates tissues. To reduce the heating effect on internal tissue we used intermittent phototherapy. These factors may explain why transesophageal phototherapy, as we tested it, was less efficient than direct phototherapy.
In summary, continuous or intermittent pulmonary phototherapy at wavelengths between 532 and 628 nm photodissociates blood COHb passing through the pulmonary circulation, thereby increasing the CO elimination rate of CO-poisoned mice. Photodissociation of COHb was effective in preventing CO poisoning. Transesophageal irradiation of the lungs via an orally placed esophageal optical fiber significantly increased the rate of CO elimination, suggesting that noninvasive approaches may be applicable to larger species including humans. The successful development and application of lung phototherapy could provide a useful therapeutic strategy for field use in treating patients who have been exposed to CO and for preventing CO poisoning in subjects at risk of CO exposure.
Acknowledgments
Acknowledgment
The authors thank Reginald Greene, M.D., and Gregory R. Wojtkiewicz of the Massachusetts General Hospital Department of Radiology for assistance in both performing and three-dimensional reconstruction of the murine computed tomography scans. They also thank Antonio Ortega-Martinez, M.S., for technical support in setting up the laser optics.
Footnotes
Supported by the Leducq Fondation and National Institutes of Health grant R01DK082971 (D.B.B.).
Author Contributions: L.Z. performed all the experiments, analyzed and interpreted the data, performed statistical analyses, and wrote, drafted, and edited the manuscript. C.L. and A.N. measured blood carboxyhemoglobin levels and drafted and edited the manuscript. W.F. and W.A.F. set up the laser optics, constructed the esophageal optical fiber, and drafted and edited the manuscript. D.B.B. provided valuable advice and suggestions and drafted and edited the manuscript. R.R.A. and W.M.Z. conceived the study, designed and supervised the experiments, reviewed the data, and drafted, edited, corrected, and reviewed the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201503-0609OC on July 27, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention (CDC) Carbon monoxide--related deaths—United States, 1999-2004. MMWR Morb Mortal Wkly Rep. 2007;56:1309–1312. [PubMed] [Google Scholar]
- 2.Weaver LK, Hopkins RO, Chan KJ, Churchill S, Elliott CG, Clemmer TP, Orme JF, Jr, Thomas FO, Morris AH. Hyperbaric oxygen for acute carbon monoxide poisoning. N Engl J Med. 2002;347:1057–1067. doi: 10.1056/NEJMoa013121. [DOI] [PubMed] [Google Scholar]
- 3.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40:433–435. doi: 10.1001/archneur.1983.04050070063016. [DOI] [PubMed] [Google Scholar]
- 4.Thom SR, Taber RL, Mendiguren II, Clark JM, Hardy KR, Fisher AB. Delayed neuropsychologic sequelae after carbon monoxide poisoning: prevention by treatment with hyperbaric oxygen. Ann Emerg Med. 1995;25:474–480. doi: 10.1016/s0196-0644(95)70261-x. [DOI] [PubMed] [Google Scholar]
- 5.Hampson NB. Hyperbaric oxygen: a plea for uniform nomenclature. Undersea Hyperb Med. 1999;26:267. [PubMed] [Google Scholar]
- 6.Haldane J. The action of carbonic oxide on man. J Physiol. 1895;18:430–462. doi: 10.1113/jphysiol.1895.sp000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown SD, Piantadosi CA. In vivo binding of carbon monoxide to cytochrome c oxidase in rat brain. J Appl Physiol (1985) 1990;68:604–610. doi: 10.1152/jappl.1990.68.2.604. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J, Piantadosi CA. Mitochondrial oxidative stress after carbon monoxide hypoxia in the rat brain. J Clin Invest. 1992;90:1193–1199. doi: 10.1172/JCI115980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brown SD, Piantadosi CA. Recovery of energy metabolism in rat brain after carbon monoxide hypoxia. J Clin Invest. 1992;89:666–672. doi: 10.1172/JCI115633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savolainen H, Kurppa K, Tenhunen R, Kivistö H. Biochemical effects of carbon monoxide poisoning in rat brain with special reference to blood carboxyhemoglobin and cerebral cytochrome oxidase activity. Neurosci Lett. 1980;19:319–323. doi: 10.1016/0304-3940(80)90281-5. [DOI] [PubMed] [Google Scholar]
- 11.Gandini C, Castoldi AF, Candura SM, Locatelli C, Butera R, Priori S, Manzo L. Carbon monoxide cardiotoxicity. J Toxicol Clin Toxicol. 2001;39:35–44. doi: 10.1081/clt-100102878. [DOI] [PubMed] [Google Scholar]
- 12.Coburn RF. The carbon monoxide body stores. Ann N Y Acad Sci. 1970;174:11–22. doi: 10.1111/j.1749-6632.1970.tb49768.x. [DOI] [PubMed] [Google Scholar]
- 13.Coburn RF, Ploegmakers F, Gondrie P, Abboud R. Myocardial myoglobin oxygen tension. Am J Physiol. 1973;224:870–876. doi: 10.1152/ajplegacy.1973.224.4.870. [DOI] [PubMed] [Google Scholar]
- 14.Hampson NB, Piantadosi CA, Thom SR, Weaver LK. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am J Respir Crit Care Med. 2012;186:1095–1101. doi: 10.1164/rccm.201207-1284CI. [DOI] [PubMed] [Google Scholar]
- 15.Peterson JE, Stewart RD. Absorption and elimination of carbon monoxide by inactive young men. Arch Environ Health. 1970;21:165–171. doi: 10.1080/00039896.1970.10667215. [DOI] [PubMed] [Google Scholar]
- 16.Weaver LK, Howe S, Hopkins R, Chan KJ. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;117:801–808. doi: 10.1378/chest.117.3.801. [DOI] [PubMed] [Google Scholar]
- 17.Pace N, Strajman E, Walker EL. Acceleration of carbon monoxide elimination in man by high pressure oxygen. Science. 1950;111:652–654. doi: 10.1126/science.111.2894.652. [DOI] [PubMed] [Google Scholar]
- 18.Killick EM, Marchant JV. Resuscitation of dogs from severe acute carbon monoxide poisoning. J Physiol. 1959;147:274–298. doi: 10.1113/jphysiol.1959.sp006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Douglas TA, Lawson DD, Ledingham IM, Norman JN, Sharp GR, Smith G. Carbogen in experimental carbonmonoxide poisoning. BMJ. 1961;2:1673–1675. doi: 10.1136/bmj.2.5268.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher JA, Rucker J, Sommer LZ, Vesely A, Lavine E, Greenwald Y, Volgyesi G, Fedorko L, Iscoe S. Isocapnic hyperpnea accelerates carbon monoxide elimination. Am J Respir Crit Care Med. 1999;159:1289–1292. doi: 10.1164/ajrccm.159.4.9804040. [DOI] [PubMed] [Google Scholar]
- 21.Piantadosi CA. A mini-forum on air breaks and O2 toxicity in clinical HBO2 therapy. Undersea Hyperb Med. 2004;31:185. [PubMed] [Google Scholar]
- 22.Weaver LK. Hyperbaric oxygen in the critically ill. Crit Care Med. 2011;39:1784–1791. doi: 10.1097/CCM.0b013e31821858d1. [DOI] [PubMed] [Google Scholar]
- 23.Haldane J, Smith JL. The oxygen tension of arterial blood. J Physiol. 1896;20:497–520. doi: 10.1113/jphysiol.1896.sp000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman BM, Gibson QH. On the photosensitivity of liganded hemoproteins and their metal-substituted analogues. Proc Natl Acad Sci USA. 1978;75:21–25. doi: 10.1073/pnas.75.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sawicki CA, Gibson QH. Dependence of the quantum efficiency for photolysis of carboxyhemoglobin on the degree of ligation. J Biol Chem. 1979;254:4058–4062. [PubMed] [Google Scholar]
- 26.Bersuker IB, Stavrov SS. Amsterdam: Elsevier Science Publishers; 1988. Structure and properties of metalloporphyrins and hemoproteins: the vibrionic approach. [Google Scholar]
- 27.Smyczynski MS.Extracorporeal photodynamic blood illumination (irradiation) for the treatment of carbon monoxide poisoningUS patent 20130101464 A1, 2013
- 28.Zazzeron L, Nakagawa A, Liu C, Franco W, Farinelli WA, Anderson RR, Zapol WM. Pulmonary phototherapy: preventing and treating carbon monoxide poisoning [abstract] Am J Respir Crit Care Med. 2015;191:A6449. doi: 10.1164/rccm.201503-0609OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper CE. Nitric oxide and iron proteins. Biochim Biophys Acta. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 30.Asimov MM.The phenomenon of laser-induced photodissociation of hemoglobin complexes in cutaneous blood vessels and its biomedical application. Presented at the International Conference on Advanced Optoelectronics & Lasers. September 10–14, 2010. Sevastopol, Ukraine [Google Scholar]
- 31.Bucher T, Kaspers I. Photochemische spaltung des kohlenoxydmyoglobins durch ultraviolette strahlung (wirksamkeit der durch die proteinkomponente des pigments absorbierten quanten) Biochim Biophys Acta. 1947;1:21–34. [Google Scholar]
- 32.Kuz’min VV, Salamin VV, Provorov AS. Study of photodissociation parameters of carboxyhemoglobin. Quantum Electron. 2008;38:695–701. [Google Scholar]
- 33.Welch AJ, Van Gemert MJ. New York: Springer Science and Business Media, LLC; 1995. Optical-thermal response of laser-irradiated tissue. [Google Scholar]
- 34.Ganz RA, Viveiros J, Ahmad A, Ahmadi A, Khalil A, Tolkoff MJ, Nishioka NS, Hamblin MR. Helicobacter pylori in patients can be killed by visible light. Lasers Surg Med. 2005;36:260–265. doi: 10.1002/lsm.20161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lembo AJ, Ganz RA, Sheth S, Cave D, Kelly C, Levin P, Kazlas PT, Baldwin PC, III, Lindmark WR, McGrath JR, et al. Treatment of Helicobacter pylori infection with intra-gastric violet light phototherapy: a pilot clinical trial. Lasers Surg Med. 2009;41:337–344. doi: 10.1002/lsm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf SJ, Lavonas EJ, Sloan EP, Jagoda AS American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Critical Issues in the Management of Adult Patients Presenting to the Emergency Department with Carbon Monoxide Poisoning. Clinical policy: critical issues in the management of adult patients presenting to the emergency department with acute carbon monoxide poisoning. J Emerg Nurs. 2008;34:e19–e32. doi: 10.1016/j.jen.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 37.Scheinkestel CD, Bailey M, Myles PS, Jones K, Cooper DJ, Millar IL, Tuxen DV. Hyperbaric or normobaric oxygen for acute carbon monoxide poisoning: a randomised controlled clinical trial. Med J Aust. 1999;170:203–210. doi: 10.5694/j.1326-5377.1999.tb140318.x. [DOI] [PubMed] [Google Scholar]
- 38.Annane D, Chadda K, Gajdos P, Jars-Guincestre M-CC, Chevret S, Raphael J-CC. Hyperbaric oxygen therapy for acute domestic carbon monoxide poisoning: two randomized controlled trials. Intensive Care Med. 2011;37:486–492. doi: 10.1007/s00134-010-2093-0. [DOI] [PubMed] [Google Scholar]
- 39.Juurlink DN, Stanbrook MB, McGuigan MA. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev. 2000;(2):CD002041. doi: 10.1002/14651858.CD002041. [DOI] [PubMed] [Google Scholar]
- 40.Raphael JC, Elkharrat D, Jars-Guincestre MC, Chastang C, Chasles V, Vercken JB, Gajdos P. Trial of normobaric and hyperbaric oxygen for acute carbon monoxide intoxication. Lancet. 1989;2:414–419. doi: 10.1016/s0140-6736(89)90592-8. [DOI] [PubMed] [Google Scholar]