Abstract
Rationale: Cross-sectional studies of T-cell responses to self-antigens correlate with baseline emphysema severity.
Objectives: We investigated whether clinical and/or immunological factors could predict disease progression, such as emphysema, FEV1, and 6-minute-walk distance (6MWD), in former and active smokers in a 5-year prospective study.
Methods: We recruited 224 ever smokers over 40 years of age and with greater than a 15 pack-year smoking history.
Measurements and Main Results: Repeated spirometry, 6MWD, and peripheral blood T-cell cytokine responses to lung elastin fragments were measured. Baseline and repeat chest computed tomography (CT) scans (34 to 65 mo apart) were used to quantify emphysema progression. Of the 141 ever-smokers with baseline and repeat CT scans, the mean (SD) annual rate of change in percent emphysema was +0.46 (0.92), ranging from −1.8 to +4.1. In multivariable analyses, the rate of emphysema progression was greater in subjects who had lower body mass index (BMI) (+0.15 per 5-unit decrease in BMI; 95% confidence interval, +0.03 to +0.29). In active smokers, increased IFN-γ and IL-6 T-cell responses had a positive association with the annual rate of emphysema progression. Male sex and IL-6 T-cell responses to elastin fragments were significantly associated with annual 6MWD decline, whereas IL-13 was associated with an increase in annual 6MWD.
Conclusions: The rate of emphysema progression quantified by CT scans among ever-smokers was highly variable; clinical factors and biomarkers explained only some of the variability. Aggressive clinical care that targets active smokers with autoreactive T cells and low BMI may temporize progression of emphysema.
Keywords: emphysema progression, T cells, IFN-γ, IL-6, IL-17
At a Glance Commentary
Scientific Knowledge on the Subject
Cross-sectional studies have identified biomarkers and activated immune cells in a subset of ever-smokers with advanced emphysema. Whether biological or clinical factors could predict disease progression in former or active smokers is unknown.
What This Study Adds to the Field
Here we show that the rate of emphysema progression was greater in subjects who had lower body mass index. Furthermore, when compared with former smokers, active smokers with increased cytokine responses to self-antigens showed a higher rate of emphysema progression. Together, these findings indicate that prolonged exposure to smoke could lower the threshold for autoimmune inflammation and provide a new tool for identifying smokers who are predisposed to emphysema progression and could benefit from early intervention.
Chronic obstructive pulmonary disease (COPD) encompasses a spectrum of clinical conditions characterized by reduced maximum expiratory airflow with or without lung parenchymal destruction, or emphysema. The long-term adverse effects of cigarette smoke exposure ensure that despite a decline in the prevalence of smoking, there will still be a high prevalence of COPD, along with rising health-care costs for active and former (ever) smokers (1–4).
The spectrum of COPD varies greatly from mild to very severe disease, indicating that detailed phenotypic classification of COPD is critical for providing individualized treatment (5, 6). The “Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints” (ECLIPSE) was among the first unbiased observational studies of smokers that used multiple clinical parameters, pulmonary function tests (PFTs), serum biomarkers, and exercise tolerance to unequivocally document disease heterogeneity and variability in disease progression in ever-smokers (7, 8). This study found a poor correlation between FEV1, biomarkers, and functional outcomes, whereas active smoking and emphysema were identified as strong predictors for physiological decline (9). Currently, there are no specific prognostic tools to determine which smokers will develop emphysema, a known independent risk factor for lung cancer in smokers (3, 10). We and others have shown that spirometry, a well-established screening tool to evaluate airway obstruction, fails to identify emphysema in more than 20% of smokers (11, 12). There is thus a strong need to better understand the basic pathophysiology of emphysema and develop novel biomarkers that could aid in predicting disease progression.
In a cohort of 156 former and active smokers, we comprehensively examined lung disease using PFTs, quantitative chest computed tomography (CT) scans, annual 6-minute-walk distance (6MWD) measurements, and T-cell cytokine responses to human lung elastin fragments (EFs) to determine relevant factors that correlate with the emphysema phenotype (13). A cross-sectional analysis of this cohort showed that EF-induced secretion of IFN-γ and IL-6 from peripheral blood T cells significantly correlated with emphysema, indicating that autoreactive T cells are present in smokers with emphysema (13). In support of these findings, our interim analysis showed that after adjusting for multiple confounders, the same two biomarkers, IFN-γ and IL-6, showed a significant negative correlation with change in 6MWD over a 2-year period (13).
Here we report the longitudinal follow-up of the clinical and immunological variables that are associated with: (1) the annual progression of emphysema as determined by quantitative CT imaging, (2) the progression of obstructive lung disease as determined by FEV1 decline, (3) the change in physiological capacity as measured by annual 6MWD, (4) the incidence of upper and lower respiratory tract infections as recorded through monthly telephone calls, and (5) the 10-year mortality data. Our findings strongly suggest that personalized phenotypic characterization of smokers combined with detection of T-cell–based autoimmune responses could aid the identification of active smokers who are at risk for progression of emphysema, thereby providing a foundation for individualized therapy in COPD. Some of the results have been previously reported in the form of an abstract (14).
Methods
Study Design and Population
We recruited 224 former and active smokers as part of the Longitudinal Exacerbation Study of Chronic Obstructive Pulmonary Disease (LES-COPD) at Baylor College of Medicine. The demographic, schema of enrollment, and clinical characteristics of the volunteers have been described previously (11, 13) and are shown in Figure 1A and Table 1. Briefly, enrollment criteria included age greater than 40 years, greater than 15 pack-year smoking history, and no history of lung cancer, chest surgery, or chronic lung diseases other than COPD (e.g., sarcoidosis, fibrosis, etc.). Participants had no history of allergies or asthma and had not received oral or systemic corticosteroids during the 6 weeks before initial recruitment. Volunteers were enrolled from three clinics within the Texas Medical Center in Houston, Texas: the Ben Taub General Hospital, the Baylor Clinic, and the Michael E. DeBakey Veterans Affairs (VA) Hospital. All studies were approved by the Institutional Review Board at Baylor College of Medicine, and written informed consent was obtained from all study participants.
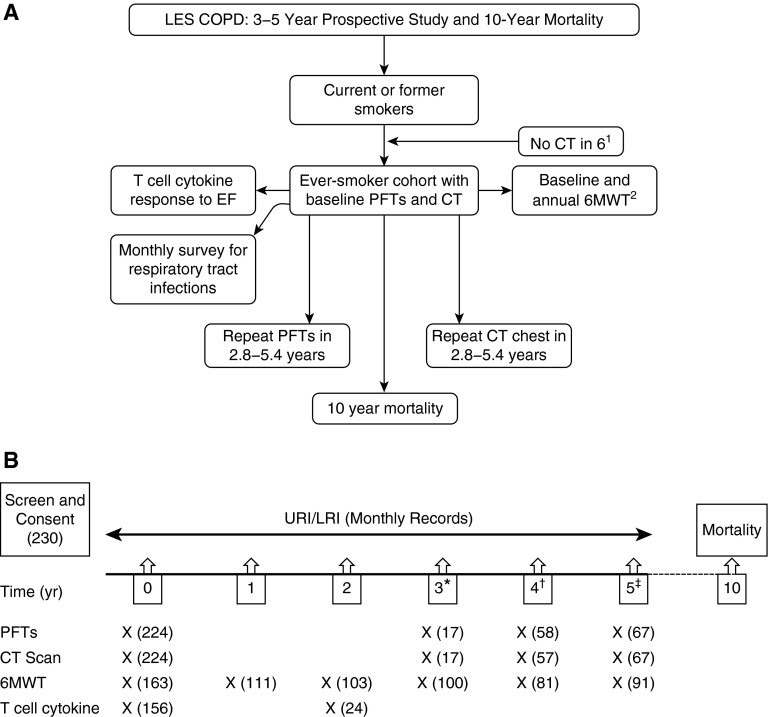
Figure 1.
(A) Schematic representation of the Longitudinal Exacerbation Study of Chronic Obstructive Pulmonary Disease (LES-COPD) study design. Participants underwent pulmonary function testing, chest computed tomography (CT) scan, T-cell cytokine measurement, and 6-minute-walk test (6MWT) as indicated in the boxes. The number of participants who did not complete the study is provided below: 1Six ever-smokers did not have CT scan performed (two died, three lost to follow-up, one withdrew consent). 2Subjects (n = 22) did not perform any 6MWT (3 died, 4 physically unable to perform the test, 3 withdrew consent, 12 lost to follow up). Forty-three subjects who performed 6MWT had incomplete data (1 died, 1 exacerbation, 1 inadequate test performed, 10 new nonpulmonary medical complaints that precluded performing the test, 18 lost to follow up). (B) Timeline of the LES-COPD data collection. Number of subjects in each data collection is shown in parentheses. EF = elastin fragment; LRI = lower respiratory tract infection; PFTs = pulmonary function tests; URI = upper respiratory tract infection. *Exact time range, 34–37 months. †Exact time range, 48–59 months. ‡Exact time range, 60–65 months.
Table 1.
Demographic and Clinical Characteristics of Subjects in the Longitudinal Exacerbation Study of Chronic Obstructive Pulmonary Disease Cohort
| Characteristic | Value (N = 224) |
|---|---|
| Age, mean ± SD, yr | 58 ± 10 |
| BMI, mean ± SD, kg/m2 | 29 ± 6 |
| Sex, no. (%) | |
| Female | 79 (35) |
| Male | 145 (65) |
| Smoking status, no. (%) | |
| Former | 91 (41) |
| Active | 133 (59) |
| Pack-year history, mean ± SD | 49 ± 37 |
| FEV1 % predicted, mean ± SD | 74 ± 25 |
| % Emphysema, mean ± SD | 12 ± 12 |
| Comorbidities, no. (%) | |
| CAD | 31 (14) |
| HTN | 108 (48) |
| Emphysema CT quantification, no. (%) | 122 (54) |
| Race/ethnicity, no. (%) | |
| White | 116 (52) |
| African American | 94 (42) |
| Hispanic | 8 (4) |
| Other | 6 (3) |
Definition of abbreviations: BMI = body mass index; CAD = coronary artery disease; CT = computed tomography; HTN = hypertension.
% Emphysema by computed tomography quantification indicating greater than 7% emphysema.
Longitudinal Follow-up and Respiratory Tract Infections
Subjects enrolled in the LES-COPD study were closely followed over the approximately 3- to 5-year study period (exact range, 2.8–5.4 yr) using monthly phone calls to record upper and lower respiratory tract infections (URI/LRIs) and yearly visits to conduct 6-minute walk tests (6MWT). A URI/LRI was defined by the presence of symptoms of rhinitis or pharyngitis, along with increased cough, shortness of breath, or sputum production and/or change in sputum color in the presence or absence of fever (temperature > 37.7°C) (15, 16). Because ever-smokers included those with COPD/emphysema, documented URI/LRIs were considered “COPD exacerbations” in the diseased group.
6MWT
Volunteers who met the criteria for COPD by the Global initiative for Obstructive Lung Disease or American Thoracic Society/European Respiratory Society guidelines, and/or showed evidence of emphysema on imaging underwent a baseline and yearly standard 6MWT throughout the length of the study (2.8–5.4 yr) (17). Briefly, volunteers were asked to assess their breathing status on a scale of 0 to 10 (10 being the most severe) at baseline and immediately after completion of their walk. Pulse oximetry readings (heart rate and O2 saturation) were recorded at the start and every 10 seconds throughout the walk. Total distance walked in 6 minutes and time to desaturation, as defined by a drop to less than 90% O2 saturation, were determined in each case (17). 6MWD was recorded annually.
PFTs
Participants underwent baseline PFTs that were performed in the diagnostic laboratories of the enrollment sites (Michael E. DeBakey VA and Baylor College of Medicine clinics) using standardized equipment and following the American Thoracic Society/European Respiratory Society guidelines (18). If the FEV1 was reduced below 80% predicted or FEV1/FVC was below 70% predicted, the participants received two doses of bronchodilator (albuterol, 180 μg), and spirometry was repeated. Normative values for spirometry were based on National Health and Nutrition Examination Survey III data, and absolute lung volumes were measured using plethysmography (19). Lung volume normative values were based on the equations endorsed by the ERS (20), with the upper limits of normal calculated based on the 95% confidence interval (CI) as previously described (11).
Primary Outcome Measure: Quantitative Chest CT Scan
Participants underwent baseline PFTs and quantitative chest CT scans as well as repeat testing upon study completion (2.8–5.4 yr from enrollment). Lung CT scans were acquired with the subject in supine position during end-inspiration using a Siemens Cardiac Sensation cardiac scanner (Siemens Medical Solutions, Malvern, PA). The subjects were coached to take a full breath, with imaging parameters of 120 kVp, 100 to 130 mA ⋅ s, and a pitch of 1.75. The images were reconstructed at 1 and 5 mm thickness using both an intermediate (b35f) and a high (b65f) spatial frequency reconstruction algorithm. The deidentified CT images were transferred to CDs, sent to the University of British Columbia, and analyzed using the previously validated EmphylxJ custom software. The percentage of lung CT voxels with attenuation values less than −950 Hounsfield units (percentage low attenuation area), with correction for total lung volume, was used to quantify the percentage of emphysema (21, 22). The progression of emphysema was measured as the annual change in the percentage of emphysema from the baseline CT scan to the scan at study completion. Eighty-three subjects did not have a repeat CT scan at completion of the study; therefore, annual progression of emphysema was calculated based from the 141 subjects with 282 available CT scans.
T Cell–based Cytokine Response Studies
In vitro cytokine assays were performed on study participants using peripheral blood mononuclear cells as previously described (23). Briefly, CD4+ T cells and CD14+/CD1a+ antigen-presenting cells (APCs) were enriched (>90% purity) from peripheral blood mononuclear cells with magnetic cell sorting (Miltenyi Biotec, San Diego, CA). CD4+ T cells were cultured in the presence of irradiated autologous APCs using a ratio of 1:10 APCs to T cells. Duplicate wells were stimulated with lung-derived EFs or left untreated. After 3 to 5 days of coculture, supernatants were assayed for the presence of IFN-γ, IL-6, IL-10, IL-13, and IL-17 by Luminex assay (Milliplex Human Cytokine kit; Millipore, Billerica, MA). These values were expressed as fold-change in the cytokine production in EF-treated wells over untreated wells. For simplicity, this fold-change value will also be referred to as cytokine production or CD4+ T cell response.
Statistical Analysis
Associations between clinical and immunological variables and clinical outcomes (annual change in percentage emphysema, FEV1 [L], 6MWD, and URI/LRIs) were evaluated by multivariable linear and negative binomial regression modeling. Model variables were selected a priori based on medical literature including age, sex, smoking status (current and former), presence of coronary artery disease, body mass index (BMI), and baseline FEV1. Comparisons of two sets of unpaired data were made using Student's t test or chi-square test to determine clinical and immunological differences between the deceased and surviving subjects. All analyses were performed using Stata v11.1 software (StataCorp, College Station, TX) or Prism v5.0.2 (GraphPad Software, San Diego, CA). All P values were two-sided, with P < 0.05 considered statistically significant.
Results
Lower BMI Is Associated with Emphysema Progression
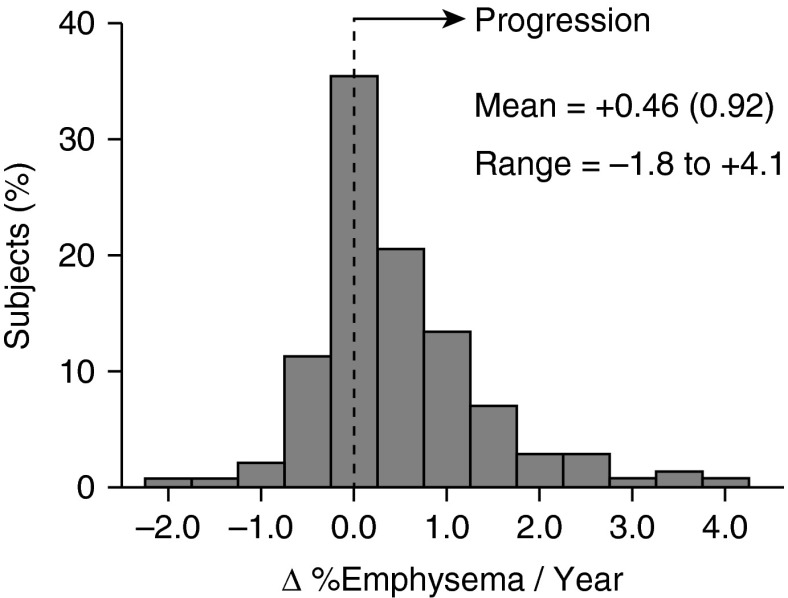
We examined the variation in annual changes in emphysema progression using 282 chest CT images in 141 subjects who underwent an initial scan and a repeat scan at the end of the study (Table 1). Percent emphysema was calculated from the CT scans as described in the Methods. Annual change in percentage emphysema was calculated as the difference between final and baseline percentage emphysema divided by the time between the two tests (range, 2.8–5.4 yr). The mean annual change in percentage emphysema (Δ percentage emphysema/yr) was +0.46 (SD, 0.92%), indicating that most subjects experienced progression of emphysema (Figure 2). The Δ percentage emphysema/yr varied considerably between subjects, ranging from −1.8 to +4.1.
Figure 2.
Distribution of the rates of emphysema progression in the cohort of 141 subjects with baseline and final computed tomography (CT) scans. Percentage of emphysema (%emphysema) was quantified by the percentage of lung CT voxels with attenuation values less than −950 Hounsfield units (percentage low-attenuation area) in 141 subjects who completed both a baseline quantitative computed chest CT and repeat CT scan at 2.8 to 5.4 years after enrollment. The rate of emphysema progression was calculated as the change in %emphysema/yr based on the CT scans taken at baseline and on study completion. The mean rate of emphysema progression was +0.46%/yr ± 0.92 (SD) and a range of −1.8 to +4.1.
Using multivariable analysis and controlling for model variables (age, sex, BMI, smoking status, pack-year smoking history, presence of coronary artery disease, presence of hypertension, FEV1 % predicted, and percentage emphysema at baseline), we found that only lower BMI (+0.15% per 5-unit decrease in BMI; 95% CI, +0.02% to +0.29%; P = 0.02) was significantly associated with increased Δ emphysema/yr. There was also a trend for an association with hypertension, but it did not reach significance (+0.33%; 95% CI, −0.002% to +0.65%; P = 0.051).
T-Cell Cytokine Responses to EFs and Annual Change in Emphysema
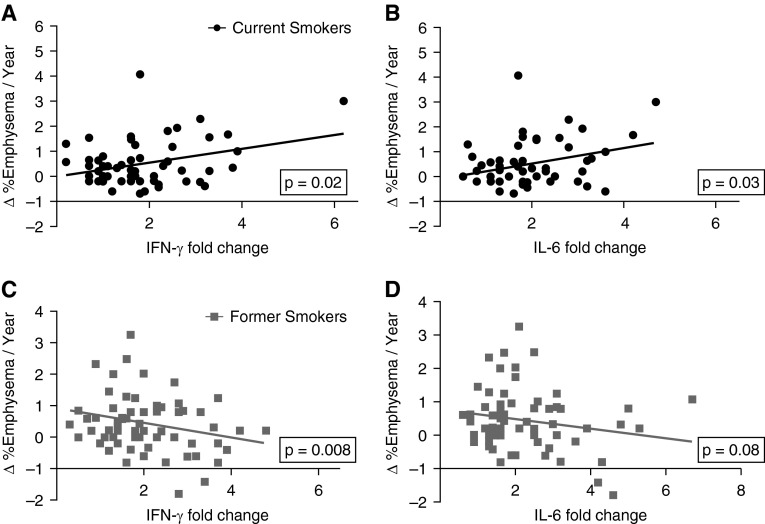
We have reported previously that T-cell expression of IFN-γ and IL-6 in response to human lung EFs has statistically significant positive associations with emphysema severity at baseline (13). We further examined the association between T-cell responses involving the same cytokines with emphysema severity as measured by CT scans performed at completion of the study. As expected, we found a significant association between T-cell expression of IFN-γ and IL-6 in response to human lung EFs and emphysema severity (see Figure E1 in the online supplement). Interestingly, although these two separate cross-sectional analyses showed significant associations between T-cell cytokine responses and emphysema, we did not find a significant association between T-cell responses to EFs and emphysema progression (Δ emphysema/yr) in the same cohort. However, subanalysis of the cohort after controlling for all model variables showed a significant positive correlation between T-cell responses (IFN-γ and IL-6 responses) and Δ emphysema/yr in active smokers, whereas there was a significant negative correlation between IFN-γ (but not IL-6) and emphysema progression in former smokers (Figures 3A–3D).
Figure 3.
Autoreactive T-cell responses to elastin fragments (EFs) in current smokers correlates with progression of emphysema. The annual change in percentage of emphysema was calculated using quantitative measurement of baseline and final computed tomography scans in 122 subjects with T-cell responses to lung EFs. (A) IFN-γ and (B) IL-6 fold changes were plotted against emphysema progression (Δ %emphysema/yr) in current smokers (n = 57; solid circles). Multivariate analysis (controlling for model variables: age, sex, smoking status, presence of coronary artery disease, presence of hypertension, body mass index, pack-year history, baseline percentage emphysema, and baseline FEV1 % predicted) showed significant positive association with the yearly progression of emphysema (IFN-γ, P = 0.02; IL-6, P = 0.03). (C) IFN-γ and (D) IL-6 were plotted against emphysema progression in former smokers (n = 65; shaded squares) (IFN-γ, P = 0.008; IL-6, P = 0.08).
CD4 T-Cell Responses to Lung EFs Correlate with Annual Decline in FEV1 (L)
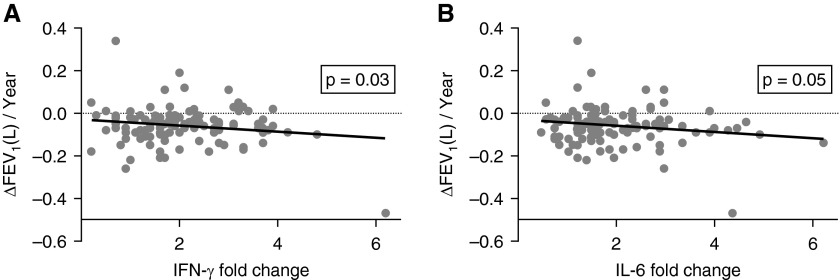
We next examined whether immune responses to EFs correlate with loss of lung function over time. Of the 224 subjects who enrolled, 142 subjects had completed PFTs at baseline and at the end of the study (2.8–5.4 yr after enrollment). Of these 142 subjects, 123 had peripheral blood drawn for measurement of T-cell cytokine (IL-6, IFN-γ, IL-13, IL-10, and IL-17) responses to lung EF stimulation. We have previously reported an inverse association between fold increase in IFN-γ, IL-17, and IL-6, and baseline FEV1 % predicted, and diffusing capacity of the lung for carbon monoxide (DlCO) % predicted, whereas no significant association was found for IL-13 or IL-10 (13). We found that there was also an inverse association between IFN-γ and IL-6 fold change and FEV1 % predicted and DlCO % predicted from PFTs performed at the completion of the study (Figure E2). Annual change in FEV1 (L) (ΔFEV1/yr) was calculated based on the initial and final PFT values. On multivariable analysis, there was a trend for association of active smoking with a greater decline in ΔFEV1/yr (−34 ml/yr, P = 0.053). Unexpectedly, male sex was strongly associated with a greater decline in ΔFEV1/yr than female sex (−48 ml/yr, P = 0.009). Furthermore, T-cell cytokine responses to EFs were also significantly associated with ΔFEV1/yr: increased IFN-γ and IL-6 expression in T cells in response to EFs were associated with greater decline in ΔFEV1/yr (−20 ml/yr per 1-unit fold change IFN-γ, P = 0.03; and −16 ml/yr per 1-unit fold change IL-6, P = 0.046) (Figure 4).
Figure 4.
Autoreactive T-cell responses to elastin fragments (EFs) correlate with decreased FEV1 (L). (A) IFN-γ fold change and (B) IL-6 fold change T-cell responses to lung EF, controlled for model variables (age, sex, smoking status, presence of coronary artery disease, presence of hypertension, body mass index, pack-year history, baseline percentage emphysema, and baseline FEV1 % predicted), were plotted against the change in FEV1 (L) in 123 subjects who had pulmonary function tests at baseline and at final visit (2.8–5.4 yr), as well as cytokine data.
Sex, Pack-Year Smoking History, and Autoreactive T Cells Correlate with Physiological Decline
Study participants received baseline and annual measurements of walking distance (6MWD). Baseline 6MWT was performed in 163 subjects, of whom 140 had at least one repeat study: 7 at 1 year, 14 at 2 years, 20 at 3 years, 14 at 4 years, and 85 subjects at 5 years completed 6MWTs. We measured annual change in 6MWD using the difference between the final recorded and the baseline 6MWD divided by the exact number of years between the two measurements (Δ6MWD/yr) that allowed adjusting for the variability in time between testing in each subject. Using multivariable analysis, we found that male, but not female, sex was associated with a further decrease in annual 6MWD (−16 m, P = 0.02). We found a trend for association of pack-year smoking history with a further decrease in annual 6MWD (−1.4 m for each 10-yr increase in pack-year history, P = 0.051). Immunologically, IL-6 fold change was strongly associated with a decrease in annual 6MWD (−7.1 m per 1-unit increase in IL-6 fold change, P = 0.008). IFN-γ and IL-17 fold change both showed a nonsignificant trend for association with greater decline in 6MWD. Interestingly, IL-13 fold change was associated with a statistically significant increase in annual 6MWD (+3.7 m per 1-unit increase in IL-13 fold change, P = 0.03) (Table 2).
Table 2.
Multivariable Analysis of Clinical and Immunological Characteristics with Annual Change in 6-Minute-Walk Distance
| Annual Change in 6MWD (95% CI) | P Value | |
|---|---|---|
| Age, per 1-yr increase | −0.5 m (−1.1 to +0.17) | 0.15 |
| Male sex | −16 m (−29 to −2.6) | 0.02 |
| Smoking status, active vs. former | −9.5 m (−22 to +2.6) | 0.12 |
| Pack-year history, per 10 pack-year increase | −1.4 m (−2.7 to +0.006) | 0.051 |
| BMI, per 1-unit increase | +0.8 m (−0.03 to +1.7) | 0.06 |
| CAD | −10 m (−25 to +4.5) | 0.17 |
| HTN | −6.8 m (−17 to +3.7) | 0.20 |
| FEV1 % predicted, per 1% increase | +0.2 m (−0.12 to +0.43) | 0.26 |
| % Emphysema, per 1% increase | −0.2 m (−0.73 to +0.33) | 0.46 |
| IFN-γ fold change, per 1-unit increase | −3.7 m (−9.5 to +2.0) | 0.20 |
| IL-17 fold change, per 1-unit increase | −3.9 m (−8.6 to +0.84) | 0.11 |
| IL-6 fold change, per 1-unit increase | −7.1 m (−12 to −1.9) | 0.008 |
| IL-13 fold change, per 1-unit increase | +3.7 m (+0.32 to +7.0) | 0.03 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; BMI = body mass index; CAD = coronary artery disease; CI = confidence interval; HTN = hypertension.
Airway Obstruction and Pack-Year Smoking History Associated with Respiratory Tract Infections
We recorded respiratory tract infections occurring in the 224 participants by monthly telephone interview. URI/LRIs were defined using validated criteria (15, 16). When controlling for model variables (age, sex, BMI, smoking status, presence of coronary artery disease, presence of hypertension, percentage FEV1, percentage emphysema, and pack-year smoking history), the only variables predictive of respiratory tract infections (e.g., COPD exacerbations) was FEV1 % predicted (P < 0.0001). URI/LRI severity was further classified as those that required hospitalization or were managed with outpatient care; when stratifying for severity of the illness, both FEV1 % predicted and pack-year smoking history were significantly associated with respiratory tract infections that required hospitalization (P < 0.0001 and P = 0.002, respectively). Only FEV1 % predicted was significantly associated with respiratory tract infections managed as an outpatient (P < 0.0001).
Autoreactive T-Cell Responses Are Associated with Increased Mortality
Over a 10-year follow-up period, there were 48 deaths in the LES-COPD cohort of 224 ever-smokers. Major causes of death included respiratory failure, malignancy, and cardiac disease (Table 3). The deceased subjects (n = 48) were predominantly men, older, and had a greater pack-year smoking history (Table 3). As expected, we also found more evidence of cardiovascular disease, severe emphysema, and obstructive lung disease among the deceased (Table 3). Interestingly, in a subpopulation of 39 deceased and 117 living subjects for whom we had autoreactive T-cell data, we found significant differences in their cytokine signatures: IFN-γ, IL-17, and IL-6 T-cell responses to EFs were greater in the deceased subjects than in the surviving subjects (P = 0.02, P = 0.02, and P = 0.04, respectively). Consistent with a positive association with preservation of 6MWD, IL-13 expression in T cells was significantly higher in the surviving subjects than in the deceased subjects (P = 0.04) (Table 4).
Table 3.
Univariate Analysis of Factors Associated with Mortality at 10 Years
| Deceased (n = 48) | Alive (n = 176) | P Value | |
|---|---|---|---|
| — | |||
| Age, mean ± SD, yr | 66 ± 8 | 56 ± 9 | <0.001 |
| Male, no. (%) | 47 (98) | 98 (56) | <0.001 |
| Current smokers, no. (%) | 22 (46) | 111 (63) | 0.047 |
| Pack-years, mean ± SD | 79 ± 42 | 41 ± 31 | <0.0001 |
| BMI, mean ± SD, kg/m2 | 27 ± 6 | 30 ± 7 | 0.01 |
| CAD, no. (%) | 16 (33) | 15 (8.5) | <0.0001 |
| HTN, no. (%) | 27 (56) | 81 (46) | 0.3 |
| FEV1 % predicted, mean ± SD | 55 ± 21 | 79 ± 24 | <0.0001 |
| DlCO % predicted, mean ± SD | 48 ± 17 | 69 ± 19 | <0.0001 |
| % Emphysema, mean ± SD | 20 ± 13 | 9.9 ± 10 | <0.0001 |
| Cause of death* | |||
| Malignancy | 9 | — | — |
| Natural causes | 6 | — | — |
| Respiratory failure | 16 | — | — |
| Cardiac | 9 | — | — |
| Unknown | 8 | — | — |
Definition of abbreviations: BMI = body mass index; CAD = coronary artery disease; DlCO = diffusing capacity of the lung for carbon monoxide; HTN = hypertension.
% Emphysema by computed tomography scan quantification. P value was determined by Student's t test or chi-square test.
Respiratory failure: pneumonia, failure to wean; malignancy: lung, head and neck, prostate, leukemia; cardiac: myocardial infarction, congestive heart failure; natural causes: age associated; unknown: no definitive cause identified.
Table 4.
Univariate Analysis of Cytokine Production Patterns and Mortality at 10 Years
| Deceased | Alive | P Value | |
|---|---|---|---|
| Number | 39 | 117 | — |
| IFN-γ | 2.5 ± 1.2 | 1.9 ± 1.0 | 0.02 |
| IL-17 | 2.3 ± 1.4 | 1.8 ± 1.0 | 0.02 |
| IL-6 | 2.5 ± 1.0 | 2.1 ± 1.1 | 0.04 |
| IL-13 | 1.9 ± 1.0 | 2.3 ± 1.7 | 0.04 |
Data presented as mean ± SD unless otherwise noted. P value was determined by comparisons of two sets of unpaired data using Student's t test to determine clinical and immunological differences between the deceased and surviving subjects.
Discussion
In this longitudinal study of 224 active and former smokers, we investigated multiple clinical and immunological parameters to determine which were predictive of emphysema progression. Our assessment included baseline and repeat PFTs, quantitative lung CT scans, monthly documentation of URI/LRIs, annual 6MWT, and an assessment of mortality over 10 years of follow-up. Comparing two chest CT scans and PFTs performed approximately 3 to 5 years apart and combined with annual 6MWTs, we calculated the annual rate of change of emphysema, FEV1, and 6MWD. We found significant variability in the annual rate of emphysema progression over the study period as expected given the highly heterogeneous nature of our study population. Specific associations with emphysema progression included decreased BMI and active smoking. Despite the fact that our study was much smaller and used different methods for quantifying emphysema, these findings are entirely consistent with those reported in other studies (e.g., ECLIPSE) (9, 21, 24).
However, our study extends well beyond prior reports by adding insight into the biological and clinical parameters that are associated with emphysema progression. Specifically, our findings suggest that distinct emphysema endotypes may exist that could be distinguished by unique comorbidities. We found no correlation between emphysema progression and URI/LRI (COPD exacerbations) as seen in the entire cohort or within the active and former smoker subgroups. In addition to examining whether clinical factors could predict emphysema progression, we also examined whether immune responses to autoantigens (e.g., lung EFs) could predict disease progression. We have previously shown that the mean fold change in T-cell cytokine responses to EFs in active and former smokers are not significantly different, but those with emphysema have nearly double the response when compared with the nonemphysema group, a highly significant difference (13). In this study, we validated our prior findings and show further that emphysema correlates significantly with autoreactive T-cell responses (e.g., IFN-γ and IL-6 secretion). These cytokine responses were associated with emphysema severity based on cross-sectional analysis of baseline and repeat CT scans but were not significantly associated with longitudinal emphysema progression (the annual change in percent emphysema). Subgroup analysis of active smokers, however, revealed a significant positive association between immune responses—specifically, increased T-cell production of IFN-γ and IL-6 in response to EFs—and longitudinal emphysema progression. These findings are consistent with the pathological role played by type 1 and 17 cytokines in emphysema as we previously demonstrated, specifically, their role in up-regulating elastolytic matrix metalloproteinase activity (23, 25)
Conversely, in former smokers, we found a negative association between IFN-γ production and emphysema progression, whereas IL-6 did not reach statistical significance. Because these subgroups had divergent associations, when combined there was no observable association between cytokine production and emphysema progression. These results suggest that active exposure to cigarette smoke may mediate the extent to which immune responses are associated with lung destruction, highlighting the role of as yet poorly explored epigenetic factors in lung inflammation and emphysema progression (26).
Immune responses to lung EFs also predicted disease progression as measured by FEV1. We show that annual changes in FEV1 (ΔFEV1/yr) significantly correlated with IFN-γ and IL-6 T-cell responses. Using multivariable analysis and controlling for model variables, increasing elastin-specific T-cell secretion of IFN-γ and IL-6 were associated with greater decline in ΔFEV1/yr. In addition to these immunological parameters, active smoking status showed a trend, but only male sex was significantly associated with greater decline in ΔFEV1/yr. The former finding, although it did not reach significance in this cohort, has been validated in the Lung Health Study, where an aggressive smoking intervention program significantly reduced the decline in FEV1 in some smokers (27), and in the ECLIPSE, where increased rate of FEV1 decline was observed among current smokers (28). Furthermore, in support of a genetic risk linking IL-6 to disease progression, the IL6-174G/C single nucleotide polymorphism has been found to be associated with a more rapid decline in FEV1 and susceptibility to COPD in smokers (29).
The association of autoimmune responses with emphysema severity and progression is also supported by recent studies in mice showing that prolonged exposure to smoke could lower the threshold for autoimmune inflammation and lung destruction (30, 31). Our findings of the potential association of elastin-specific immune responses with emphysema progression are of particular interest. Specifically, this finding suggests that autoreactivity to elastin in susceptible smokers may be due to decreased antigen-specific immune tolerance that in turn leads to the induction of elastin-specific T cells that could produce disease in many elastin-rich organs beyond the lungs, especially including the skin and blood vessels (32–34).
Some of the limitations of this study include potential error from quantification of emphysema using CT scans that is related to the increased inflammation as seen in patients with severe COPD. Such inflammation could spuriously enhance lung tissue density and thus reduce CT-based detection of true emphysema, and this could have biased the results toward the null. Also, patient drop-out related to emphysema-dependent mortality may have influenced the results. Finally, in this single-center study, a relatively small cohort provided a limited power to detect statistically significant differences with multiple biomarkers. Our findings therefore call for larger cohort studies that could potentially reveal the importance of additional comorbidities and endotypes on long-term survival in emphysema.
In summary, our findings further provide strong evidence that immunological and clinical factors can predict disease progression in ever-smokers. Specifically, active smokers who display autoreactive T cells with increased IFN-γ and IL-6 responses to EFs show a higher rate of emphysema progression. These support recent studies in mice showing that prolonged exposure to smoke could lower the threshold for autoimmune inflammation and lung destruction (30, 31). Importantly, our findings suggest novel means for detecting smokers who are at high risk for development and progression of emphysema and who might be suitable for early targeted therapeutic intervention.
Footnotes
Supported by the National Institutes of Health grants HL082487 and HL110883 (F.K. and D.B.C.), a Veterans Affairs merit award, and Baylor College of Medicine Cardiovascular Research Institute Pilot Project Award (F.K.).
Author Contributions: F.K. and D.B.C. contributed to study design; S.P. and S.H. contributed to subject recruitment, institutional review board application, and data collection; H.C. quantified emphysema; S.B., C.-L.T., D.B.C., and F.K. contributed to statistical analysis, manuscript composition, and data analysis; and L.P. contributed to data analysis. F.K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201504-0736OC on August 4, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Carter BD, Abnet CC, Feskanich D, Freedman ND, Hartge P, Lewis CE, Ockene JK, Prentice RL, Speizer FE, Thun MJ, et al. Smoking and mortality: beyond established causes. N Engl J Med. 2015;372:631–640. doi: 10.1056/NEJMsa1407211. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. Plos Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson DO, Weissfeld JL, Balkan A, Schragin JG, Fuhrman CR, Fisher SN, Wilson J, Leader JK, Siegfried JM, Shapiro SD, et al. Association of radiographic emphysema and airflow obstruction with lung cancer. Am J Respir Crit Care Med. 2008;178:738–744. doi: 10.1164/rccm.200803-435OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Punturieri A, Szabo E, Croxton TL, Shapiro SD, Dubinett SM. Lung cancer and chronic obstructive pulmonary disease: needs and opportunities for integrated research. J Natl Cancer Inst. 2009;101:554–559. doi: 10.1093/jnci/djp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vestbo J. COPD: definition and phenotypes. Clin Chest Med. 2014;35:1–6. doi: 10.1016/j.ccm.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Vestbo J, Vogelmeier C, Small M, Higgins V. Understanding the GOLD 2011 Strategy as applied to a real-world COPD population. Respir Med. 2014;108:729–736. doi: 10.1016/j.rmed.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Agusti A, Calverley PMA, Celli B, Coxson HO, Edwards LD, Lomas DA, MacNee W, Miller BE, Rennard S, Silverman EK, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11:122. [Google Scholar]
- 8.Faner R, Tal-Singer R, Riley JH, Celli B, Vestbo J, MacNee W, Bakke P, Calverley PMA, Coxson H, Crim C, et al. ECLIPSE Study Investigators. Lessons from ECLIPSE: a review of COPD biomarkers. Thorax. 2014;69:666–672. doi: 10.1136/thoraxjnl-2013-204778. [DOI] [PubMed] [Google Scholar]
- 9.Vestbo J, Agusti A, Wouters EFM, Bakke P, Calverley PMA, Celli B, Coxson H, Crim C, Edwards LD, Locantore N, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints Study Investigators. Should we view chronic obstructive pulmonary disease differently after ECLIPSE? A clinical perspective from the study team. Am J Respir Crit Care Med. 2014;189:1022–1030. doi: 10.1164/rccm.201311-2006PP. [DOI] [PubMed] [Google Scholar]
- 10.Spitz MR, Hong WK, Amos CI, Wu X, Schabath MB, Dong Q, Shete S, Etzel CJ. A risk model for prediction of lung cancer. J Natl Cancer Inst. 2007;99:715–726. doi: 10.1093/jnci/djk153. [DOI] [PubMed] [Google Scholar]
- 11.Hesselbacher SE, Ross R, Schabath MB, Smith EO, Perusich S, Barrow N, Smithwick P, Mammen MJ, Coxson H, Krowchuk N, et al. Cross-sectional analysis of the utility of pulmonary function tests in predicting emphysema in ever-smokers. Int J Environ Res Public Health. 2011;8:1324–1340. doi: 10.3390/ijerph8051324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaggiari E, Zompatori M, Verduri A, Chetta A, Bnà C, Ormitti F, Sverzellati N, Rabaiotti E. Early smoking-induced lung lesions in asymptomatic subjects: correlations between high resolution dynamic CT and pulmonary function testing. Radiol Med (Torino) 2005;109:27–39. [PubMed] [Google Scholar]
- 13.Xu C, Hesselbacher S, Tsai C-L, Shan M, Spitz M, Scheurer M, Roberts L, Perusich S, Zarinkamar N, Coxson H, et al. Autoreactive T cells in human smokers is predictive of clinical outcome. Front Immunol. 2012;3:267. doi: 10.3389/fimmu.2012.00267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsai CL, Garcia JM, Perusich S, Coxson H, Krowchuk N, Corry DB, Kheradmand F. Factors associated with progression of emphysema quantified by CT scan in ever smokers: the 5-year prospective LES-COPD study. Am J Respir Crit Care Med. 2014;189:A5105. [Google Scholar]
- 15.Greenberg SB, Allen M, Wilson J, Atmar RL. Respiratory viral infections in adults with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:167–173. doi: 10.1164/ajrccm.162.1.9911019. [DOI] [PubMed] [Google Scholar]
- 16.Singh M, Lee SH, Porter P, Xu C, Ohno A, Atmar RL, Greenberg SB, Bandi V, Gern J, Amineva S, et al. Human rhinovirus proteinase 2A induces TH1 and TH2 immunity in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2010;125:1369–1378.e2. doi: 10.1016/j.jaci.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crapo R, Enright P, Zeballos J ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CPM, Gustafsson P, Hankinson J, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 19.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 20.Stocks J, Quanjer PH Official Statement of the European Respiratory Society. Reference values for residual volume, functional residual capacity and total lung capacity: ATS Workshop on Lung Volume Measurements. Eur Respir J. 1995;8:492–506. doi: 10.1183/09031936.95.08030492. [DOI] [PubMed] [Google Scholar]
- 21.Coxson HO, Dirksen A, Edwards LD, Yates JC, Agusti A, Bakke P, Calverley PM, Celli B, Crim C, Duvoix A, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators. The presence and progression of emphysema in COPD as determined by CT scanning and biomarker expression: a prospective analysis from the ECLIPSE study. Lancet Respir Med. 2013;1:129–136. doi: 10.1016/S2213-2600(13)70006-7. [DOI] [PubMed] [Google Scholar]
- 22.Coxson HO, Lam S. Quantitative assessment of the airway wall using computed tomography and optical coherence tomography. Proc Am Thorac Soc. 2009;6:439–443. doi: 10.1513/pats.200904-015AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan M, Cheng HF, Song LZ, Roberts L, Green L, Hacken-Bitar J, Huh J, Bakaeen F, Coxson HO, Storness-Bliss C, et al. Lung myeloid dendritic cells coordinately induce TH1 and TH17 responses in human emphysema. Sci Transl Med. 2009;1:4ra10. doi: 10.1126/scitranlsmed.3000154. [DOI] [PubMed] [Google Scholar]
- 24.Regan EA, Hokanson JE, Murphy JR, Make B, Lynch DA, Beaty TH, Curran-Everett D, Silverman EK, Crapo JD. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7:32–43. doi: 10.3109/15412550903499522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, et al. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. Plos Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kheradmand F, Shan M, Xu C, Corry DB. Autoimmunity in chronic obstructive pulmonary disease: clinical and experimental evidence. Expert Rev Clin Immunol. 2012;8:285–292. doi: 10.1586/eci.12.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, Conway WA, Jr, Enright PL, Kanner RE, O’Hara P, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 28.Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, Bakke P, Calverley PMA, Celli B, Coxson HO, Crim C, et al. ECLIPSE Investigators. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365:1184–1192. doi: 10.1056/NEJMoa1105482. [DOI] [PubMed] [Google Scholar]
- 29.He JQ, Foreman MG, Shumansky K, Zhang X, Akhabir L, Sin DD, Man SFP, DeMeo DL, Litonjua AA, Silverman EK, et al. Associations of IL6 polymorphisms with lung function decline and COPD. Thorax. 2009;64:698–704. doi: 10.1136/thx.2008.111278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan X, Shan M, You R, Frazier MV, Hong MJ, Wetsel RA, Drouin S, Seryshev A, Song LZ, Cornwell L, et al. Activation of C3a receptor is required in cigarette smoke-mediated emphysema. Mucosal Immunol. 2015;8:874–885. doi: 10.1038/mi.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shan M, You R, Yuan X, Frazier MV, Porter P, Seryshev A, Hong J-S, Song LZ, Zhang Y, Hilsenbeck S, et al. Agonistic induction of PPARγ reverses cigarette smoke-induced emphysema. J Clin Invest. 2014;124:1371–1381. doi: 10.1172/JCI70587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams MC, Murchison JT, Edwards LD, Agustí A, Bakke P, Calverley PMA, Celli B, Coxson HO, Crim C, Lomas DA, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) investigators. Coronary artery calcification is increased in patients with COPD and associated with increased morbidity and mortality. Thorax. 2014;69:718–723. doi: 10.1136/thoraxjnl-2012-203151. [DOI] [PubMed] [Google Scholar]
- 33.Lahousse L, van den Bouwhuijsen QJA, Loth DW, Joos GF, Hofman A, Witteman JCM, van der Lugt A, Brusselle GG, Stricker BH. Chronic obstructive pulmonary disease and lipid core carotid artery plaques in the elderly: the Rotterdam Study. Am J Respir Crit Care Med. 2013;187:58–64. doi: 10.1164/rccm.201206-1046OC. [DOI] [PubMed] [Google Scholar]
- 34.Patel BD, Loo WJ, Tasker AD, Screaton NJ, Burrows NP, Silverman EK, Lomas DA. Smoking related COPD and facial wrinkling: is there a common susceptibility? Thorax. 2006;61:568–571. doi: 10.1136/thx.2005.053827. [DOI] [PMC free article] [PubMed] [Google Scholar]