Abstract
Lung cancer stem cells (CSCs) have recently been isolated from lung cancer patient samples and have been reported to be responsible for tumor initiation, treatment resistance and tumor recurrence. We have previously shown that oncolytic Newcastle disease virus (NDV), strain FMW (NDV/FMW) induces apoptosis in drug-resistant lung cancer cells. However, how NDV exerts its oncolytic effect on lung CSCs remains to be investigated. Here we show that NDV/FMW replicates in, and lyses CSC-enriched lung cancer spheroids and inhibits the 3D growth potential of lung cancer spheroid and agar colonies. We demonstrate that NDV/FMW triggers caspase-dependent apoptosis in lung cancer spheroids as shown by increased caspase-3 processing and Poly (ADP-ribose) polymerase (PARP) cleavage. Notably, NDV/FMW infection results in the degradation of microtubule-associated protein 1 light chain 3 (LC3) II and P62, two hallmarks of autophagy maturation, indicating that NDV/FMW promotes autophagy flux in lung cancer cell spheroids. This was further confirmed by the appearance of an increased number of double-membrane vesicles as detected by transmission electron microscopy. We also show that NDV/FMW promotes autophagy degradation in lung cancer spheroids via inhibition of the AKT/mTOR pathway. In addition, treatment of spheroids with the autophagy inhibitor, chloroquine increases NDV/FMW-induced cytotoxicity. Collectively, our data show that oncolytic NDV/FMW may be a potential strategy in targeting lung CSCs.
Keywords: Newcastle disease virus, cancer stem cell, spheroid, lung cancer, apoptosis, autophagy
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide with a 5-year survival rate of less than 20% [1]. Although somewhat controversial, it is generally accepted that a subpopulation of lung cancer cells, referred to as lung cancer stem cells (CSCs) or tumor-initiating cells (TICs), contributes to lung tumor initiation and growth [2]. Lung CSCs or TICs have been isolated from human cell lines and patient samples [3-10], and are known to confer resistance to both radiotherapy and chemotherapy [11]. Therefore, novel treatment strategies such as oncolytic viruses (OVs) targeting lung CSCs or TICs are urgently required as OVs kill cancer cells through mechanisms distinct from conventional therapeutics [12]. A recent study has indicated that gene-armed oncolytic adenovirus may be a potential treatment approach for lung cancer through targeting of this CSC subpopulation within a tumor [13].
CSC eradication is critical from a clinical perspective. However, it has been recently recognized that tumors can harbor multiple phenotypically and/or genetically distinct CSCs and that the CSC phenotype may vary greatly between patients [14]. Such complexities hinder the direct study of CSC lysis. Accumulating evidence indicates that CSCs form multicellular three-dimensional (3D) spheres in vitro when grown in non-adherent serum-free conditions [15,16]. As such, 3D sphere cultures have been used to enrich for lung CSC populations [3,4,6,8,10].
The oncolytic Newcastle Disease Virus (NDV), an avian paramyxovirus, can replicate in multiple tumor types and exert strong cytotoxic effects [17-23]. In particular, NDV may be effective in the treatment of lung cancers, as its natural tropism is the respiratory tract of avian species. In support of this, several naturally occurring strains of NDV, such as 73-T, NDV-HUJ, Italien and Ulster, have displayed strong oncolytic effects on lung cancers in pre-clinical and clinical trials [24-27]. In addition, oncolytic NDV induces oncolysis in human lung adenocarcinoma A549 cells over-expressing the anti-apoptotic protein, Bcl-xL [28]. We have previously shown that the oncolytic NDV strain, FMW (NDV/FMW) induces apoptosis in both A549 wild-type and cisplatin-resistant (A549/DDP) cells in vitro and in vivo [29,30]. We have also shown that NDV/FMW-mediated oncolysis in cisplatin- or paclitaxel-resistant lung cancer cells is enhanced by pharmacological modulation of autophagy [30].
In this study, we report that NDV/FMW replicates in, and lyses lung CSC-enriched spheroids. Moreover, we have shown that NDV/FMW induces apoptosis and subsequent autophagy in 3D spheroids. Taken together, our study suggests a potential role of oncolytic NDV in the lysis of lung cancer cells with stem cell-like properties and may be used as a novel strategy to target lung CSCs.
Materials and methods
Cell lines
The human large cell lung cancer cell line NCI-H460, the human adenocarcinoma MOR cell line MOR and chicken embryo fibroblast cell line DF1 were obtained from the American Type Culture Collection (ATCC). H460 and MOR cells were cultured in Roswell Park Memorial Institute (RPMI-1640) medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml) at 37°C and 5% CO2. DF1 cells were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS. H460 and MOR cells were seeded (1 × 103/well) in ultra-low attachment 96-well plates and maintained in serum-free DMEM/F12 medium supplemented with 10 ng/ml basic fibroblast growth factor (bFGF), 20 ng/ml epidermal growth factor (EGF) and B27 (B27 and medium at a 1:50 volume ratio). Seven days after seeding, the propagated spheroid bodies were collected and digested by StemPro Accutase to single cell suspensions to generate a second generation of spheroids.
Antibodies, reagents and virus
The polyclonal rabbit anti-microtubule-associated protein 1A/1B-light chain 3 (LC3), monoclonal β-actin antibody, and rabbit polyclonal anti-P62 (SQSTM1) antibodies were obtained from Sigma-Aldrich. Anti-Nanog, anti-SOX2 and anti-Oct4 primary antibodies were purchased from Abcam. The following antibodies from Cell Signaling Technology were used: cleaved caspase-3, cleaved PARP, in addition to the phospho-specific antibodies, mTOR (Ser2448), Akt (Ser473) and p70 ribosomal protein S6 kinase (S6K) (Thr389). Rapamycin and chloroquine (CQ) were purchased from Sigma-Aldrich. The pan-caspase peptide inhibitor Z-VAD-FMK was purchased from Promega and prepared with dimethyl sulfoxide (DMSO). The propagation and titration of the oncolytic NDV strain, NDV/FMW, was performed as previously described [31]. Virus titer was expressed as log10 of 50% the infective dose (TCID50) in culture.
Virus infection
Spheroid cultures were infected as intact 3D cultures with NDV/FMW at a multiplicity of infection (MOI) of 10, or sham-infected with phosphate-buffered saline (PBS) in serum-free DMEM/F12 at 37°C for 1 h, after which time the virus was removed by centrifugation.
Cell death and colony formation assays
H460 and MOR spheroids were infected with NDV/FMW for the indicated times and stained with propidium iodide (PI) at 10 μg/ml. Subsequently, cells were visualized by a fluorescence microscopy where red fluorescencing cells were indicative of cell death. For colony formation assays, H460 and MOR spheroids were digested by accutase to single cell suspensions and seeded at a density of 500 cells per well in 6-well plates for 10 days. Colonies were photographed and expressed as the number of colonies ± SD.
Flow cytometry analysis
H460 and MOR sphere cells were treated with NDV/FMW for the indicated times, collected and dissociated into single cell suspensions. Apoptosis was assessed by flow cytometric analysis of membrane re-distribution of phosphatidylserine using Annexin V and propidium iodide (PI) double-staining [29].
Immunoblotting
Immunoblotting was carried out as previously described [32]. Briefly, spheroids were treated with NDV/FMW and harvested for the indicated times.
Animal experiments
BALB/c nude mice (female, 4-6 weeks old) were purchased from the Experimental Animal Center of Dalian Medical University (Dalian, China) and all procedures involving animals and their care complied with the China National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Ethical approval for the study was granted by the Ethics Committee of Dalian Medical University. For tumorigenicity assays, a total of 1 × 104 lung cancer cells or lung cancer spheroids/mouse were subcutaneously injected into the left rear back of mice. Cells (1 × 104) were injected in 100 μl of PBS per mouse (n = 3/group). Tumor growth was recorded weekly in two dimensions using a digital caliper. Tumor volume was calculated as [(length × width × width)/2] and compared as cubic millimeter.
Statistical analysis
The results are expressed as average ± standard deviation (SD). When data distribution approximated normality and two groups were compared, a Student’s t-test was used. Multiple comparisons between treatment groups and controls were evaluated using a one-way ANOVA. All statistical tests were performed using Prism GraphPad 5 software. P ≤ 0.05 was considered statistically significant.
Results
Oncolytic NDV/FMW replicates in lung CSC-enriched spheroid cultures
To establish multicellular spheroid cultures, lung cancer H460 and MOR cells were seeded in ultra-low attachment plates. Both H460 and MOR spheroids (≥ 100 μm in diameter) formed within 7 days of anchorage-independent culture (Figure 1A). To avoid the formation of aggregates, sub-cultured spheroids were used throughout the study. To examine whether H460 and MOR spheroids exhibit CSC-like properties, the expression of several transcription factors markers associated with stemness were examined. As shown in Figure 1B, the expression levels of Nanog, Sox2 and Oct4 were markedly increased in both H460 and MOR spheroid cultures compared to 2D cultured cells, suggesting that spheroids derived from these cells have the potential to self-renew, an important characteristic of CSCs. Furthermore, larger tumors were observed in nude mice inoculated with H460 or MOR spheroids relative to those formed in mice inoculated with H460 or MOR 2D cultures, respectively (Figure 1C), demonstrating that H460 and MOR spheroids display stronger tumor-initiating ability than their 2D counterparts. These data indicate that H460 and MOR spheroids display CSC-like properties.
Figure 1.
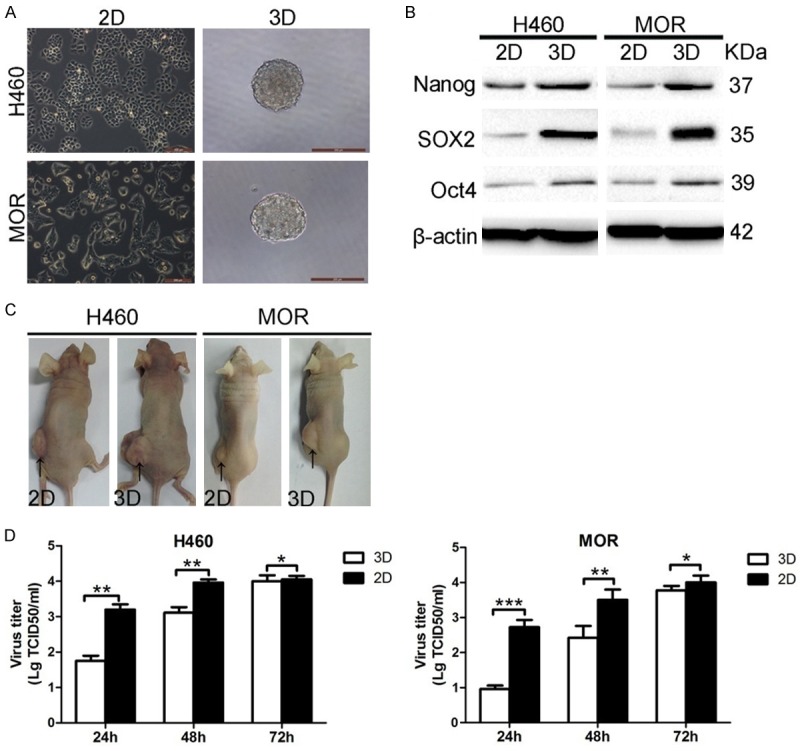
Oncolytic NDV/FMW replicates in lung CSC-enriched spheroid cultures. A. H460 and MOR cells cultured as monolayers and spheroids. Cells formed spheroid bodies following 7 days culture in serum-free medium (scale bar = 200 μm). B. Human embryonic stem cell makers, Nanog, SOX2 and Oct4, were up-regulated in spheroid cells (3D) relative to cells grown in culture (2D). All IB experiments were performed twice. C. Tumorigenicity of monolayer and spheroid cells in female BALB/c mice (1 × 104 cells per mouse). Spheroid cells initiated tumors earlier and formed larger xenografts than that observed with monolayer cells. The number of mice in each group was three. D. Monolayer cells and spheroid cells were infected with NDV/FMW (MOI = 0.01) for 48 h and viral titers were determined in DF-1 cells by TCID50 quantification assay. Data presented are mean ± SD calculated from three independent experiments (*P < 0.05; **P < 0.01; ***P < 0.001).
The oncolytic NDV strain FMW (NDV/FMW), which has been shown to be cytotoxic in lung cancer cells [29-31], was used throughout this study. To examine whether NDV/FMW replicates in these CSC-enriched spheroid cultures, H460 and MOR spheroids were infected with NDV/FMW at the multiplicity of infection (MOI) of 0.01 and multi-step viral growth curves were determined. A time-dependent increase in virus yield in NDV/FMW-infected spheroids was observed, indicating that NDV/FMW replicates in CSC-enriched spheroid cultures. Interestingly, virus yield in 3D spheroid cultures was generally lower than that in 2D cultures at all time-points examined (Figure 1D).
NDV/FMW lyses lung CSC-enriched spheroid cultures
We first examined the ability of NDV/FMW to abrogate the 3D, CSC-like growth potential of lung cancer cells. Cultures (3D) of H460 and MOR cells were infected with NDV/FMW (MOI = 1) or mock-infected and examined for spheroid formation at 7 days post infection (p. i.), respectively. NDV/FMW-infected H460 and MOR cells did not grow under these conditions compared to mock-infected cells (Figure 2A). Similarly, NDV/FMW infection completely significantly abrogated the clonogenic survival ability of both cell lines when cultured in soft agar (Figure 2B). These findings indicate that NDV/FMW has the ability to prevent lung cancer growth under 3D conditions, including the formation of spheroids and agar colonies.
Figure 2.
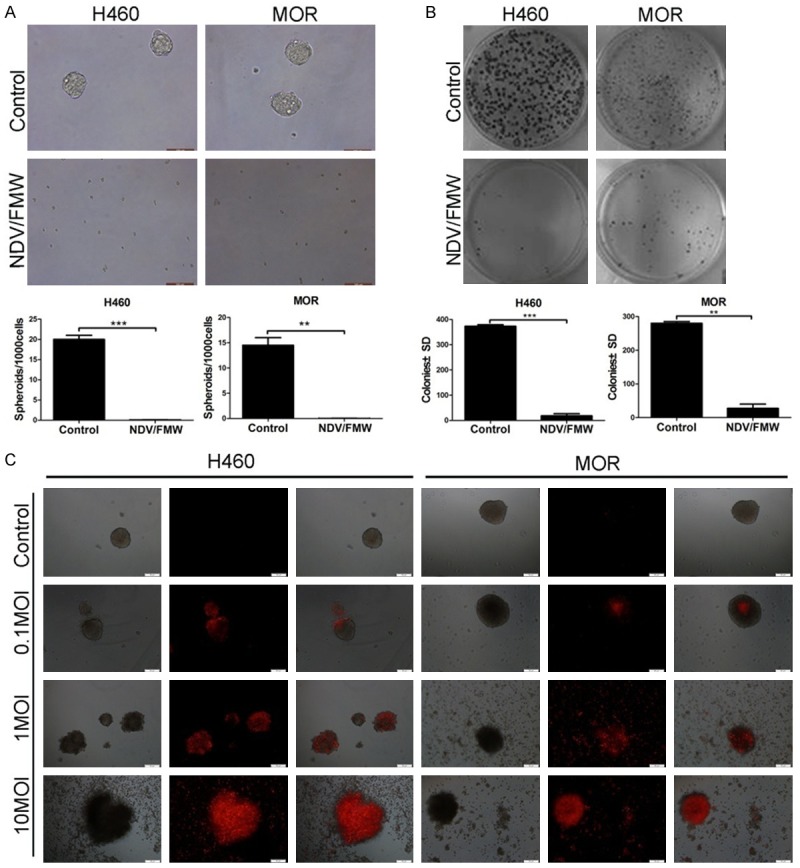
NDV/FMW exhibits oncolytic activity in lung cancer spheroid cultures. 3D cultures of H460 and MOR cells were mock-infected or infected with NDV/FMW (MOI = 1; 48 h) and examined for spheroid (A) and colony formation (B). Results are expressed as number of spheroids/1000 cells and colonies ± SD from three independent experiments (scale bar = 100 μm). H460 and MOR spheroids were mock-infected or infected with NDV/FMW (MOI = 10, 1, or 0.1) and stained with PI (C). Spheroids mock-infected or infected with NDV/FMW at MOIs (0.1, 1 and 10) were stained with PI at 48 h and imaged under phase contrast and red fluorescence microscopy (scale bar = 50 μm).
To determine whether oncolytic NDV/FMW lyses lung cancer sphere cells, H460 and MOR spheroids were infected with different MOIs (0.1, 1, 10) or mock-infected with phosphate-buffered saline (PBS). Cell death in response to infection was then examined by staining with propidium iodide (PI) and imaged by light microscopy (Figure 2C). The majority of cells within the spheroids infected with NDV/FMW at an MOI of 1 or 10 showed significant staining with PI at 48 h p. i., thereby indicating cell death.
NDV/FMW induces apoptosis of 3D spheroid cultures
We have previously shown that NDV/FMW induces apoptosis of lung cancer cells grown as 2D cultures [33]. To address the mechanism by which NDV/FMW exerts its oncolytic effect on lung cancer spheroids, H460 and MOR spheroid cultures were infected with NDV/FMW (MOI = 10) for 48 h or mock-infected. Spheroids were then dissociated and analyzed by flow cytometry with FITC-conjugated Annexin V and PI double staining. NDV/FMW infection significantly increased the percentages of both early and late apoptotic cells in H460 and MOR spheroids, indicating apoptotic induction by NDV/FMW (Figure 3A). Consistently, two classical markers of apoptosis, caspase-3 and Poly (ADP-ribose) polymerase (PARP) cleavage, were detected in NDV/FMW-treated cells at 48 h p. i. as indicated by immunoblot (Figure 3B). To confirm that NDV/FMW-induced apoptosis in these cells is caspase-dependent, H460 and MOR spheroids were pre-treated with the broad-specificity caspase inhibitor, Z-VAD-FMK, and subsequently infected with NDV/FMW (MOI = 1) for 72 h. Pre-treatment with Z-VAD-FMK decreased both caspase-3 processing and PARP cleavage in virus-infected cells compared to virus-infected cells only (Figure 3C). Inhibition with Z-VAD-FMK significantly decreased the number of apoptotic cells in NDV/FMW-infected spheroids (Figure 3D). These data indicate a role for caspase activity in NDV/FMW-induced apoptosis of H460 and MOR lung spheroids.
Figure 3.
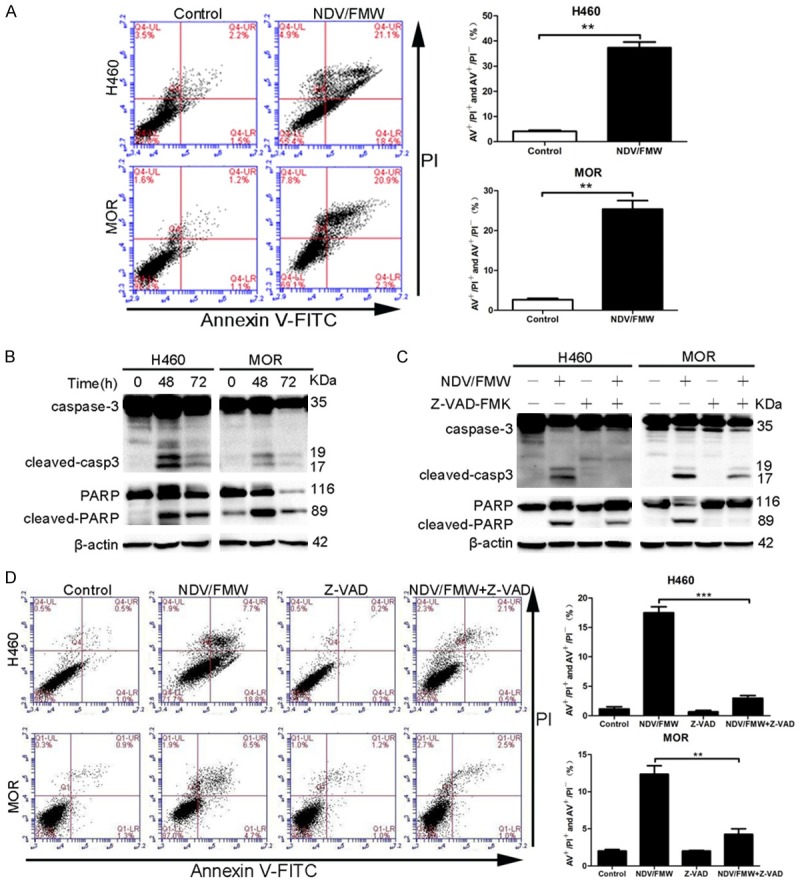
NDV induces apoptosis of 3D spheroid cultures. A and B. H460 and MOR spheroid cells were mock-treated or infected with NDV/FMW (MOI = 10; 48 h) and double-stained with Annexin V and propidium iodide (PI) and analyzed by flow cytometry. A. The cell population in the right lower quadrant (PI-negative, Annexin V-positive) and the right upper quadrant (Annexin V/PI positive) are represented. Data shown are representative of three independent experiments (**P < 0.01). B. Spheroid cells were mock-infected or infected with NDV/FMW (MOI = 10; 48 and 72 h). Activation of caspase-3 and cleaved poly (ADP-ribose) polymerase (PARP) was examined by immunoblot analysis (n = 2). To control for loading, β-actin was used. C. Spheroid cells were mock-infected or infected with NDV/FMW (MOI = 1; 72 h) in the absence or presence of the pan-caspase inhibitor Z-VAD-FMK (100 μm). Total protein lysates were separated by SDS-PAGE and probed with antibodies against cleaved and total caspase-3 and PARP. All IB experiments were performed twice. D. Apoptosis was also assessed by flow cytometry. The percentage of apoptotic cells are represented as mean ± SD from three independent experiments (**P < 0.01, ***P < 0.001).
NDV/FMW promotes autophagy flux in 3D spheroid cultures via inhibition of the AKT/mTOR pathway
Recent work in our laboratory has shown that NDV/FMW triggers autophagy of lung cancer cells [31]. To investigate whether NDV/FMW perturbs the autophagic machinery in 3D spheroid cultures, the conversion of LC3I (cytosolic form) to LC3II (autophagosome-bound lipidated form) together with the expression of P62/SQSTM1, two well-known autophagy markers, was examined in H460 and MOR spheroids. NDV/FMW reduced both LC3I and LC3II levels in H460 spheroid cultures at 48 and 72 h p. i., while P62 levels was strongly reduced at 72 h p. i. (Figure 4A). Interestingly, while expression of LC3II was not observed in NDV/FMW-infected MOR spheroid cultures over time, LC3I and P62 levels were reduced in virus-infected MOR spheroid cultures (Figure 4A). This was most pronounced at 72 h. These findings suggest that NDV/FMW can promote autophagy flux in lung cancer spheroids. This was further supported by TEM-based ultra-structural analysis of H460 and MOR spheroids in response to NDV/FMW, where several autophagosomes were detected in NDV/FMW spheroids, but to a lesser extend in mock-treated cells (Figure 4B).
Figure 4.
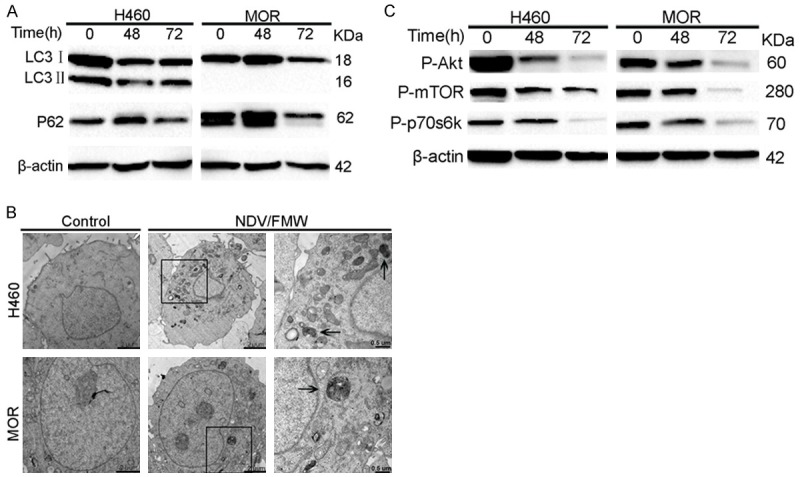
NDV/FMW promotes autophagy flux in 3D spheroid cultures via inhibition of the AKT/mTOR pathway. Spheroid cells were mock-infected or infected with NDV/FMW at an MOI of 10 at the indicated time points. Activation of P62, LC3I to LC3II conversion. (A)And phosphorylated Akt, mTOR and p70S6K were analyzed by immunoblot analysis using β-actin as a loading control. All IB experiments were performed twice (C). Transmission electron microscopy analysis of cells infected with NDV/FMW for 72 h (B).
To explore the mechanism underlying NDV/FMW-activated autophagy in H460 and MOR spheroids, the phosphorylation status of the class I PI3K/AKT/mTOR/p70S6K pathway was examined. This has been reported to negatively regulate autophagosome formation [34,35]. NDV/FMW infection reduced the phosphorylation levels of AKT, mTOR and p70S6K in H460 and MOR spheroids (Figure 4C), indicating an inhibition of this regulatory pathway in the autophagy of these cells. No changes in the levels of total AKT, mTOR and p70S6K were found (data not shown). Based on these observations, down-regulation of the class I PI3K/AKT/mTOR/p70S6K signaling pathway plays a critical role in NDV/FMW-induced autophagy in H460 and MOR spheroids.
Pharmacological inhibition of autophagy enhances NDV/FMW-induced cytotoxicity of 3D spheroid cultures
Pharmacological modulation of autophagy enhances NDV/FMW-mediated oncolysis in drug-resistant lung cancer cells [30]. To investigate whether autophagy plays a role in NDV/FMW-mediated oncolysis of lung cancer spheroids, rapamycin (Rapa), an autophagy inducer, and the autophagy inhibitor chloroquine (CQ) were used to elucidate this potential effect. We and others have shown that these compounds and their analogs, RAD001 and hydroxychloroquine, respectively, can be used to potentiate the anti-tumor effects of several oncolytic viruses in the pre-clinical setting [30,36,37]. In this study, both compounds had no cytotoxic effect on H460 and MOR spheroids at concentrations used in preliminary studies. However, pre-treatment with CQ increased further caspase-3 processing and PARP cleavage induced by NDV/FMW, whereas treatment with Rapa decreased NDV/FMW-induced caspase-3 processing and PARP cleavage in H460 and MOR 3D spheroid cultures (Figure 5A). Levels of apoptosis in NDV/FMW-infected spheroids were increased following treatment with CQ, while on the contrary, apoptosis was decreased in response to Rapa (Figure 5B).
Figure 5.
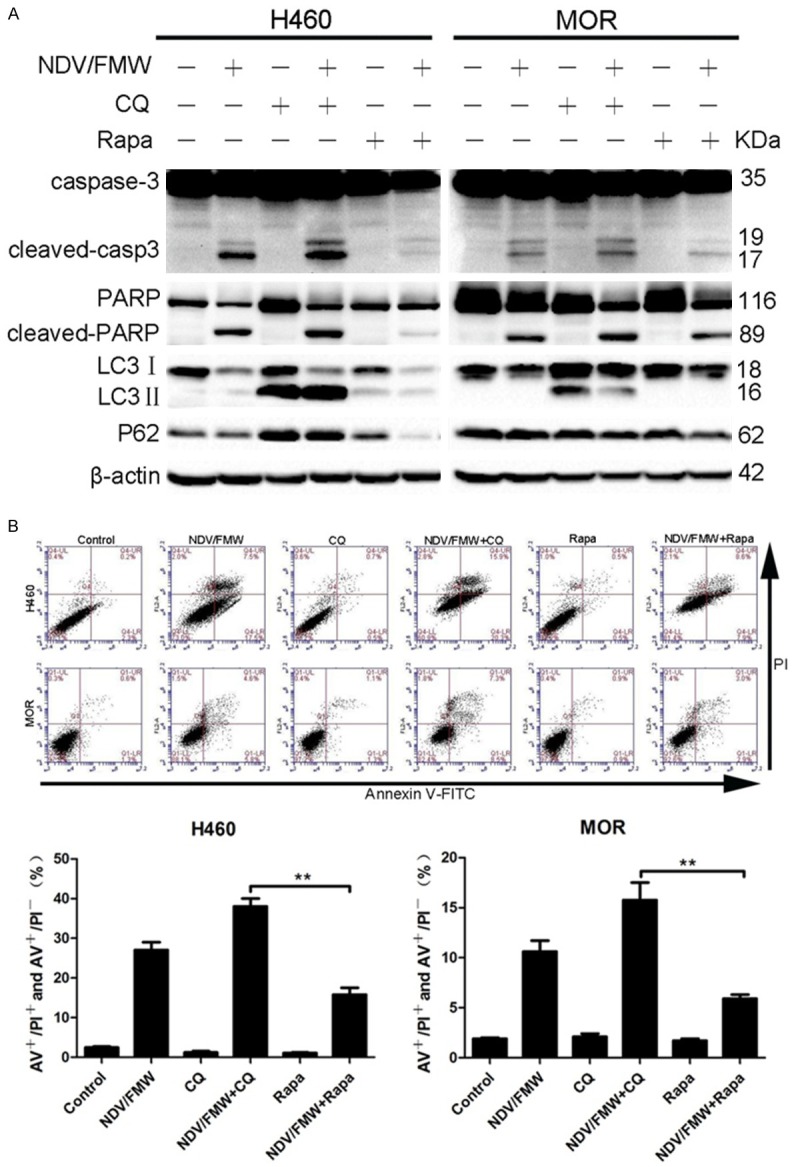
Inhibition of autophagy enhances NDV/FMW mediated oncolysis of lung cancer spheroid cultures. Spheroid cells were mock-infected or infected with NDV/FMW (MOI = 1; 72 h) in the absence or presence of the autophagy inducer, Rapamysin (Rapa, 200 nM) and the autophagy inhibitor, chloroquine (CQ, 10 μM). Protein lysates were electrophoresed on SDS-PAGE gels and probed with antibodies to caspase-3, PARP, LC3I, LC3II and p62. All IB experiments were performed twice (A). Apoptosis of control and infected cells in response to treatment with CQ and Rapa was analyzed by flow cytometry (B). Data are representative of mean ± SD from three independent experiments (**P < 0.01).
The effect of Rapa and CQ on NDV/FMW- induced autophagy flux was examined in 3D spheroid cultures. The pre-treatment of H460 spheroids with CQ resulted in enhanced LC3II accumulation and increased P62 levels upon NDV/FMW infection compared with virus-infected cells only (Figure 5A). Similarly, increased levels of LC3I were detected in MOR spheroids pre-treated with CQ and infected with NDV/FMW relative to with virus-infected cells only. These would suggest that autophagy may play a pro-survival role in NDV/FMW-induced oncolysis of 3D spheroid cultures.
Discussion
To our knowledge, this is the first report showing that oncolytic NDV exerts a robust cytotoxic effect in lung CSC-enriched spheroids. Recent studies have reported that lung cancer 3D sphere cultures are enriched with CSCs [10]. In this study, when cultured in 3D conditions, the lung cancer cell lines, H460 and MOR, formed spheres and were shown to exhibit more stem-cell like properties than when cultured in 2D conditions. Although some 3D cancer cultures are known to resist oncolytic viral therapy owing to reduced virus penetration and spread [38-40], in this study oncolytic NDV/FMW replicated efficiently in lung cancer spheroids. Furthermore, NDV/FMW showed substantial oncolytic activity in CSC-enriched lung cancer 3D cultures. In addition to lysing CSC-enriched cultures, NDV/FMW inhibited the 3D growth potential of H460 and MOR cells, highlighting the use of oncolytic NDV as a promising therapeutic strategy for lung cancer, via targeting of the CSC subpopulation.
Our previous work indicated that apoptosis is the main cell death pathway involved in NDV/FMW-mediated oncolytic effects in cisplatin-resistant lung cancer cells and parental cell [29]. In this study, NDV/FMW also induced apoptosis of lung cancer 3D spheroid cultures. Interestingly, we observed that autophagy is induced following the induction of apoptosis in response to NDV/FMW infection. These findings are in line with our previous observations that NDV/FMW triggers autophagy in U251 glioma cells and A549 lung cancer cells [30,31]. Further analysis of the signaling pathways involved in autophagy induction indicates that NDV/FMW promotes autophagy flux in lung cancer 3D spheroids via inhibition of the AKT/mTOR pathway, consistent with our previous observations in lung cancer 2D cultures [30].
We have previously demonstrated that the autophagy inhibitor, CQ, increases NDV-mediated oncolysis of cisplatin-resistant A549 cells both in vitro and in vivo [30]. In the current study, CQ enhanced NDV/FMW-mediated cytotoxicity in lung cancer 3D spheroids, suggesting that NDV/FMW in combination with autophagy inhibitors may be a strategy to enhance NDV/FMW oncolytic activity in lung cancer 3D spheroids. Autophagy has been shown to be involved in OV-mediated lysis of cancer 3D cultures [41]. Colunga et al. reported that the oncolytic virus ΔPK, induces LC3II accumulation and P62 clearance in melanoma 3D cultures with enriching CSCs [41]. The authors demonstrated that calpain-dependent clearance of p62 contributes to ΔPK oncolytic activity in breast and melanoma 3D sphere cells [41]. These observations and ours highlight that targeting autophagy could enhance OV-induced oncolysis.
In conclusion, our data show that oncolytic NDV has the ability to kill lung cancer 3D spheroids and may provide a compelling rationale for the clinical translation of oncolytic NDV in the treatment of lung cancer patients.
Acknowledgements
This work was supported by the National Science Foundation of China (81372471 to Songshu Meng, 81502674 to Ke Jiang). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 2.Giangreco A, Groot KR, Janes SM. Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med. 2007;175:547–553. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- 3.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS One. 2008;3:e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen YC, Hsu HS, Chen YW, Tsai TH, How CK, Wang CY, Hung SC, Chang YL, Tsai ML, Lee YY, Ku HH, Chiou SH. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS One. 2008;3:e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, Conticello C, Ruco L, Peschle C, De Maria R. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15:504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- 6.Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, Pratesi G, Fabbri A, Andriani F, Tinelli S, Roz E, Caserini R, Lo Vullo S, Camerini T, Mariani L, Delia D, Calabro E, Pastorino U, Sozzi G. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proc Natl Acad Sci U S A. 2009;106:16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7:330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD, Fink LM, Ma Y, Wong MP. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One. 2010;5:e14062. doi: 10.1371/journal.pone.0014062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mancini R, Giarnieri E, De Vitis C, Malanga D, Roscilli G, Noto A, Marra E, Laudanna C, Zoppoli P, De Luca P, Affuso A, Ruco L, Di Napoli A, Mesiti G, Aurisicchio L, Ricci A, Mariotta S, Pisani L, Andreetti C, Viglietto G, Rendina EA, Giovagnoli MR, Ciliberto G. Spheres derived from lung adenocarcinoma pleural effusions: molecular characterization and tumor engraftment. PLoS One. 2011;6:e21320. doi: 10.1371/journal.pone.0021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morrison BJ, Steel JC, Morris JC. Sphere culture of murine lung cancer cell lines are enriched with cancer initiating cells. PLoS One. 2012;7:e49752. doi: 10.1371/journal.pone.0049752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barr MP, Gray SG, Hoffmann AC, Hilger RA, Thomale J, O’Flaherty JD, Fennell DA, Richard D, O’Leary JJ, O’Byrne KJ. Generation and characterisation of cisplatin-resistant nonsmall cell lung cancer cell lines displaying a stem-like signature. PLoS One. 2013;8:e54193. doi: 10.1371/journal.pone.0054193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cripe TP, Wang PY, Marcato P, Mahller YY, Lee PW. Targeting cancer-initiating cells with oncolytic viruses. Mol Ther. 2009;17:1677–1682. doi: 10.1038/mt.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang Y, Xu H, Huang W, Ding M, Xiao J, Yang D, Li H, Liu XY, Chu L. Targeting lung cancer stem-like cells with TRAIL gene armed oncolytic adenovirus. J Cell Mol Med. 2015;19:915–923. doi: 10.1111/jcmm.12397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Visvader JE, Lindeman GJ. Cancer stem cells: current status and evolving complexities. Cell Stem Cell. 2012;10:717–728. doi: 10.1016/j.stem.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 16.Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, Pilotti S, Pierotti MA, Daidone MG. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65:5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 17.Zamarin D, Palese P. Oncolytic Newcastle disease virus for cancer therapy: old challenges and new directions. Future Microbiol. 2012;7:347–367. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schirrmacher V. Oncolytic Newcastle disease virus as a prospective anti-cancer therapy. A biologic agent with potential to break therapy resistance. Expert Opin Biol Ther. 2015;5:1757–71. doi: 10.1517/14712598.2015.1088000. [DOI] [PubMed] [Google Scholar]
- 19.Cuadrado-Castano S, Sanchez-Aparicio MT, Garcia-Sastre A, Villar E. The therapeutic effect of death: Newcastle disease virus and its antitumor potential. Virus Res. 2015;209:56–66. doi: 10.1016/j.virusres.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schirrmacher V, Fournier P. Multimodal cancer therapy involving oncolytic newcastle disease virus, autologous immune cells, and bi-specific antibodies. Front Oncol. 2014;4:224. doi: 10.3389/fonc.2014.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buijs PR, van Amerongen G, van Nieuwkoop S, Bestebroer TM, van Run PR, Kuiken T, Fouchier RA, van Eijck CH, van den Hoogen BG. Intravenously injected Newcastle disease virus in non-human primates is safe to use for oncolytic virotherapy. Cancer Gene Ther. 2014;21:463–471. doi: 10.1038/cgt.2014.51. [DOI] [PubMed] [Google Scholar]
- 22.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, Merghoub T, Wolchok JD, Allison JP. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ganar K, Das M, Sinha S, Kumar S. Newcastle disease virus: current status and our understanding. Virus Res. 2014;184:71–81. doi: 10.1016/j.virusres.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phuangsab A, Lorence RM, Reichard KW, Peeples ME, Walter RJ. Newcastle disease virus therapy of human tumor xenografts: antitumor effects of local or systemic administration. Cancer Lett. 2001;172:27–36. doi: 10.1016/s0304-3835(01)00617-6. [DOI] [PubMed] [Google Scholar]
- 25.Yaacov B, Eliahoo E, Lazar I, Ben-Shlomo M, Greenbaum I, Panet A, Zakay-Rones Z. Selective oncolytic effect of an attenuated Newcastle disease virus (NDV-HUJ) in lung tumors. Cancer Gene Ther. 2008;15:795–807. doi: 10.1038/cgt.2008.31. [DOI] [PubMed] [Google Scholar]
- 26.Bian H, Wilden H, Fournier P, Peeters B, Schirrmacher V. In vivo efficacy of systemic tumor targeting of a viral RNA vector with oncolytic properties using a bispecific adapter protein. Int J Oncol. 2006;29:1359–1369. [PubMed] [Google Scholar]
- 27.Schirrmacher V, Bai L, Umansky V, Yu L, Xing Y, Qian Z. Newcastle disease virus activates macrophages for anti-tumor activity. Int J Oncol. 2000;16:363–373. [PubMed] [Google Scholar]
- 28.Mansour M, Palese P, Zamarin D. Oncolytic specificity of Newcastle disease virus is mediated by selectivity for apoptosis-resistant cells. J Virol. 2011;85:6015–6023. doi: 10.1128/JVI.01537-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng S, Zhou Z, Chen F, Kong X, Liu H, Jiang K, Liu W, Hu M, Zhang X, Ding C, Wu Y. Newcastle disease virus induces apoptosis in cisplatin-resistant human lung adenocarcinoma A549 cells in vitro and in vivo. Cancer Lett. 2012;317:56–64. doi: 10.1016/j.canlet.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Jiang K, Li Y, Zhu Q, Xu J, Wang Y, Deng W, Liu Q, Zhang G, Meng S. Pharmacological modulation of autophagy enhances Newcastle disease virus-mediated oncolysis in drug-resistant lung cancer cells. BMC Cancer. 2014;14:551. doi: 10.1186/1471-2407-14-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng C, Zhou Z, Jiang K, Yu S, Jia L, Wu Y, Liu Y, Meng S, Ding C. Newcastle disease virus triggers autophagy in U251 glioma cells to enhance virus replication. Arch Virol. 2012;157:1011–1018. doi: 10.1007/s00705-012-1270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meng S, Gui Q, Xu Q, Lu K, Jiao X, Fan J, Ge B, Ke Y, Zhang S, Wu J, Wang C. Association of Shp2 with phosphorylated IL-22R1 is required for interleukin-22-induced MAP kinase activation. J Mol Cell Biol. 2010;2:223–230. doi: 10.1093/jmcb/mjq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bian J, Wang K, Kong X, Liu H, Chen F, Hu M, Zhang X, Jiao X, Ge B, Wu Y, Meng S. Caspase- and p38-MAPK-dependent induction of apoptosis in A549 lung cancer cells by Newcastle disease virus. Arch Virol. 2011;156:1335–1344. doi: 10.1007/s00705-011-0987-y. [DOI] [PubMed] [Google Scholar]
- 34.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. J Pathol. 2010;221:117–124. doi: 10.1002/path.2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alonso MM, Jiang H, Yokoyama T, Xu J, Bekele NB, Lang FF, Kondo S, Gomez-Manzano C, Fueyo J. Delta-24-RGD in combination with RAD001 induces enhanced anti-glioma effect via autophagic cell death. Mol Ther. 2008;16:487–493. doi: 10.1038/sj.mt.6300400. [DOI] [PubMed] [Google Scholar]
- 37.Lun XQ, Jang JH, Tang N, Deng H, Head R, Bell JC, Stojdl DF, Nutt CL, Senger DL, Forsyth PA, McCart JA. Efficacy of systemically administered oncolytic vaccinia virotherapy for malignant gliomas is enhanced by combination therapy with rapamycin or cyclophosphamide. Clin Cancer Res. 2009;15:2777–2788. doi: 10.1158/1078-0432.CCR-08-2342. [DOI] [PubMed] [Google Scholar]
- 38.Hong CS, Fellows W, Niranjan A, Alber S, Watkins S, Cohen JB, Glorioso JC, Grandi P. Ectopic matrix metalloproteinase-9 expression in human brain tumor cells enhances oncolytic HSV vector infection. Gene Ther. 2010;17:1200–1205. doi: 10.1038/gt.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu G, Su W, Jin G, Xu F, Hao S, Guan F, Jia W, Liu F. Glioma stem cells targeted by oncolytic virus carrying endostatin-angiostatin fusion gene and the expression of its exogenous gene in vitro. Brain Res. 2011;1390:59–69. doi: 10.1016/j.brainres.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 40.Dmitrieva N, Yu L, Viapiano M, Cripe TP, Chiocca EA, Glorioso JC, Kaur B. Chondroitinase ABC I-mediated enhancement of oncolytic virus spread and antitumor efficacy. Clin Cancer Res. 2011;17:1362–1372. doi: 10.1158/1078-0432.CCR-10-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Colunga A, Bollino D, Schech A, Aurelian L. Calpain-dependent clearance of the autophagy protein p62/SQSTM1 is a contributor to DeltaPK oncolytic activity in melanoma. Gene Ther. 2014;21:371–378. doi: 10.1038/gt.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]


