Abstract
Sixty-one endophytic fungus strains with different colony morphologies were isolated from the leaves, stems and roots of Tephrosia purpurea with colonization rates of 66.95%, 37.50%, and 26.92%, respectively. Based on internal transcribed spacer sequence analysis, 61 isolates were classified into 16 genera belonging to 3 classes under the phylum Ascomycota. Of the 61 isolates, 6 (9.84%) exhibited antifungal activity against one or more indicator plant pathogenic fungi according to the dual culture test. Isolate TPL25 had the broadest antifungal spectrum of activity, and isolate TPL35 was active against 5 plant pathogenic fungi. Furthermore, culture filtrates of TPL25 and TPL35 exhibited greater than 80% growth inhibition against Sclerotinia sclerotiorum. We conclude that the endophytic fungal strains TPL25 and TPL35 are promising sources of bioactive compounds.
Keywords: Antifungal activity, Endophytic fungi, ITS sequence, Tephrosia purpurea
Currently, endophytic fungi are of biotechnological interest due to their potential use as biological control agents and as sources of novel, biologically active secondary metabolites. Many of the bioactive agents that are produced by plants (e.g., taxol) can also be produced by endophytic fungi [1]. Endophytic fungi yield a broad variety of substances, including antioxidants, novel antibiotics, and antimicrobial, immunosuppressant and antiparasitic compounds, and thus are rich sources of biologically active metabolites that have been widely exploited in medicine, agriculture, and industry [2,3]. Here, we isolated endophytic fungi from the plant Tephrosia purpurea.
T. purpurea is widely distributed in tropical, sub-tropical and arid regions. This perennial herb is an ingredient in traditional herbal formulations for hepatitis [4] and exhibits several hepatoprotective activities, including antimicrobial [5], wound-healing [6], antiulcer [7], immunomodulatory [8], and anticancer [9] activities. The plant is used commonly as an anti-inflammatory agent in the traditional Indian system of medicine [4]. Several phytochemicals have been isolated from T. purpurea, and their medicinal uses have been examined. Previous studies have mainly focused on the phytochemical and pharmacological activities of the plant. However, the role of the endophytic fungi that are associated with T. purpurea remains unclear.
To search for bioactive metabolites from endophytic fungi, we collected the medicinal plant T. purpurea which was not reported to be attacked by many plant pathogens or pests and then isolated the endophytic fungi. Here, we investigated the phylogenetic diversity of endophytic fungi from T. purpurea and evaluated their potential as biocontrol agents against a variety of pathogenic fungi. To our knowledge, this work is the first report describing endophytic fungi from T. purpurea.
MATERIALS AND METHODS
Sample collection
Healthy and asymptomatic leaves, stems and roots were collected randomly from 8 T. purpurea plants in the campus of Hunan Agricultural University, Changsha, China, from June to October 2013. Each tissue sample was used within 48 hr of collection. Finally, the plant parts were washed with running tap water to remove attached soil and then rinsed twice with distilled water and processed to isolate the endophytic fungi.
Surface sterilization and isolation of endophytic fungi
To kill epiphytic microorganisms, the samples were initially surface sterilized according to Petrini et al. [10], with some modifications. Samples from each tissue were immersed in 75% ethanol for 3~5 min soaked in a 0.1% mercuric chloride solution for 30~45 sec, depending on the tissue, and finally rinsed five times with distilled water. The samples were then dried on sterile tissue paper and cut into small pieces using a sterile pinch cutter. The leaves were cut into 0.5 × 0.5-cm squares, and the stems and roots were cut into 0.5-cm segments. Then, three to five segments were placed onto potato dextrose agar (PDA) containing 0.5 g/L streptomycin. All plates were incubated in the dark at 26℃, and observations were recorded daily for 4 wk. The hyphal tips of the developing fungal colonies were transferred to fresh PDA plates to obtain pure cultures. All strains were stored in 30% glycerol in a deep freezer at -80℃.
The colonization rate was calculated as the total number of segments colonized by endophytic fungi divided by the total number of incubated segments [11].
Phylogenetic analysis of culturable endophytic fungi
Actively growing mycelium of the endophytic fungi was scraped directly from the PDA plates. Genomic DNA was extracted using a fungal genomic DNA extraction kit (Sangon, Shanghai, China). The sequences for the consensus fungal primer ITS4 and ITS5 regions were 5'-TCCTCCGCTTATTGATATGC-3' and 5'-GGAAGTAAAAGTCGTAACAAGG-3', respectively [12]. DNA was amplified in a final volume of 50 µL containing 1 µL of template DNA, 2 µL of 10 pmol of each primer, and 25 µL of Dream Taq Green PCR Master Mix (2×) (Thermo Scientific, Waltham, MA, USA). The cycling program used was as follows: 95℃ for 5 min, followed by 30 cycles of 95℃ for 30 sec, 56℃ for 30 sec, and 72℃ for 45 sec; finally, the reaction was incubated at 72℃ for 10 min. The resulting PCR products were resolved using electrophoresis on 1% agarose gel in 0.5× TBE (45 mM Tris base, 45 mM boric acid, 1 mM ethylenediaminetetraacetic acid pH 8.0) running buffer and then stained with ethidium bromide and photographed under UV light. Sequencing was performed by a commercial company (Sangon).
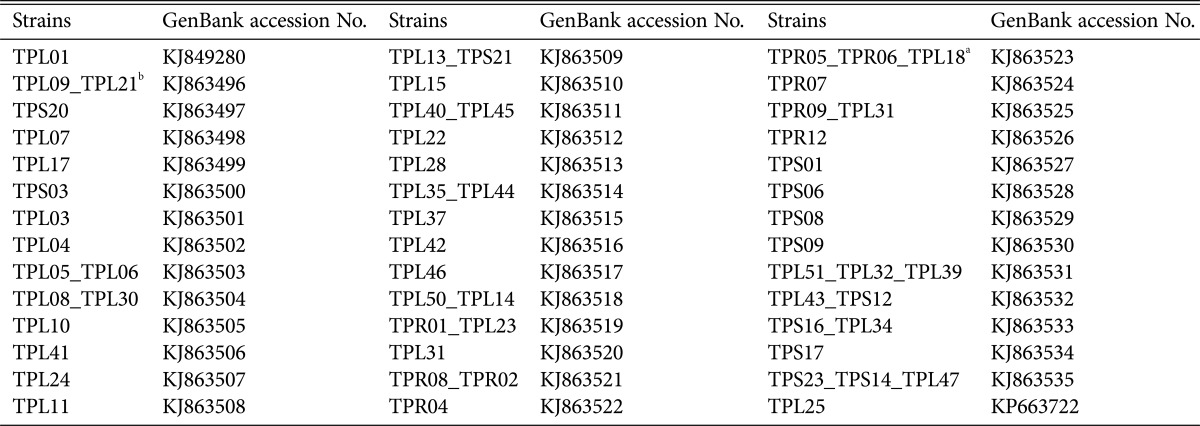
The sequences of the internal transcribed spacer fragments (ITS4~ITS5) were analyzed using the nucleotide BLAST program; the National Center for Biotechnology Information (NCBI) database was used to test for similarity. Phylogenetic trees were constructed using the neighbor-joining method of MEGA ver. 5 [13] including a bootstrap analysis of 1,000 replications. The confirmed rDNA sequences for each species were then deposited in GenBank under the accession numbers listed in Table 1.
Table 1. The codes and GenBank accession numbers of the isolated strains.
aThree strains.
bTwo strains: Although these strains exhibited different culture characteristics on potato dextrose agar, they had the same base site; thus, only one sequence was submitted to GenBank.
Bioassay of endophytic fungi against plant pathogenic fungi
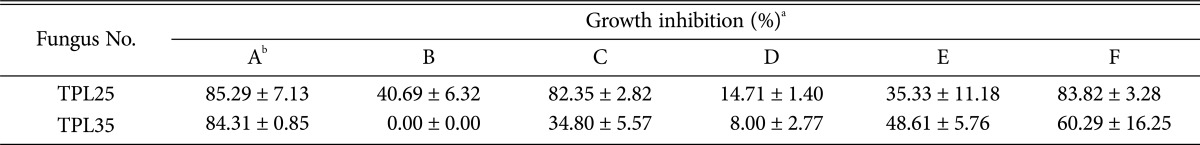
Cultures of the isolates were screened for their ability to interact with the Sclerotinia sclerotiorum, Phytophthora parasitica var. nicotianae, Phytophthora melonis, Botrytis cinerea, Colletotrichum gloeosporioides, and Rhizoctonia solani, plant pathogens of global importance that cause significant yield loss across many crops. Postharvest phytopathogenic fungi were obtained from the culture collection of the Plant Pathology Laboratory, Plant Protection Department, Hunan Agricultural University, Changsha, China. For dual culture testing, 6-mm disks containing endophytic fungi and plant pathogenic fungi were placed 4 cm apart on PDA. The plates were incubated at 26℃ for 3~7 days in the dark. The width of the inhibition zones between the pathogen and the endophytic fungi was scored as antifungal activity and was measured in millimeters. The result was recorded as no inhibition (-), weak inhibition (+), moderate inhibition (++), and strong inhibition (+++).
The culture filtrates of strains exhibiting strong activity were tested using the poisoned food technique of Grover and Moore [14] against six plant pathogenic fungi. Two plugs of mycelial agar, 6 mm in diameter, were obtained from the growing edges of 7-day-old cultures of endophytic fungus strains and cultured in a potato dextrose broth (PDB) medium (100 mL per conical flask) for 5~7 days at 26℃ and 200 rpm. Four flasks containing PDB medium were inoculated with each endophytic fungus, and one flask of medium was maintained as a control. The cultures were then centrifuged to remove hyphal mass at 4,000 rpm for 15 min, and the supernatant containing the excretory metabolites of the endophytic fungi was then filter-sterilized through a Millex-GP 0.22-µm syringe filter (Millipore, Billerica, MA, USA) before assaying the antifungal activity. PDA (15 mL) was poured into sterilized 75-mm Petri dishes, and sterilized culture filtrate (1 mL) of endophytic fungus was then added. The medium was supplemented with the same amount of PDB medium instead of culture filtrate for the control sets. Upon solidification of the medium, plant pathogen was inoculated at the center of the plate, and growth inhibition of the treatment against the control was measured by mycelial growth inhibition and calculated according to the formula of Pandey et al. [15]:
| Percentage of mycelial growth inhibition = (dc - dt)/dc × 100, |
where dc = the average diameter (in mm) of fungal colonies in the control, and dt = the average diameter (in mm) of fungal colonies in the treatment groups.
RESULTS
Diversity of culturable endophytic fungi associated with T. purpurea
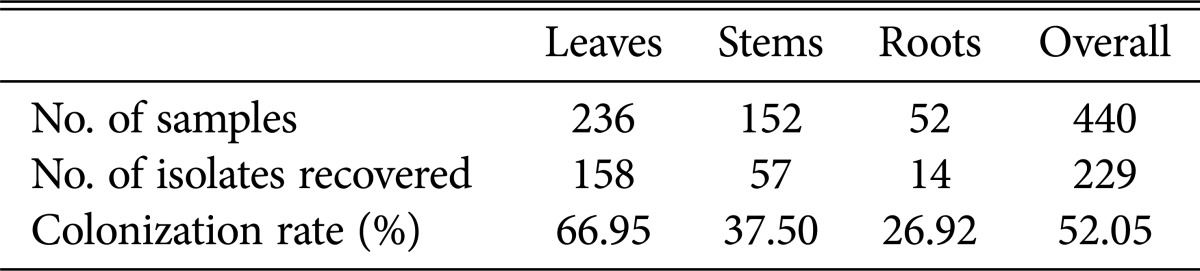
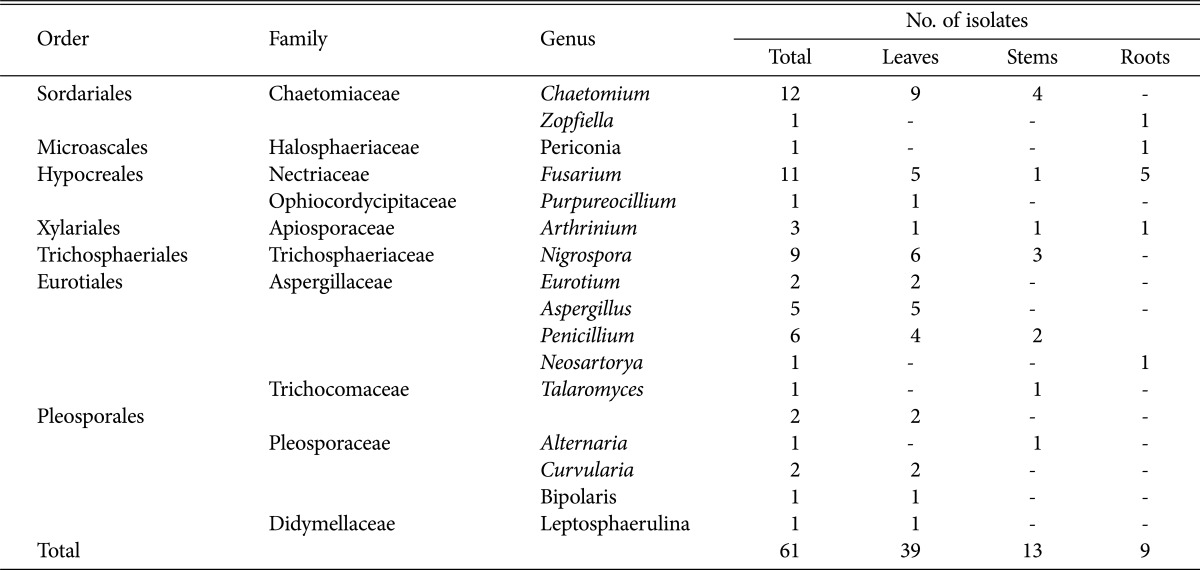
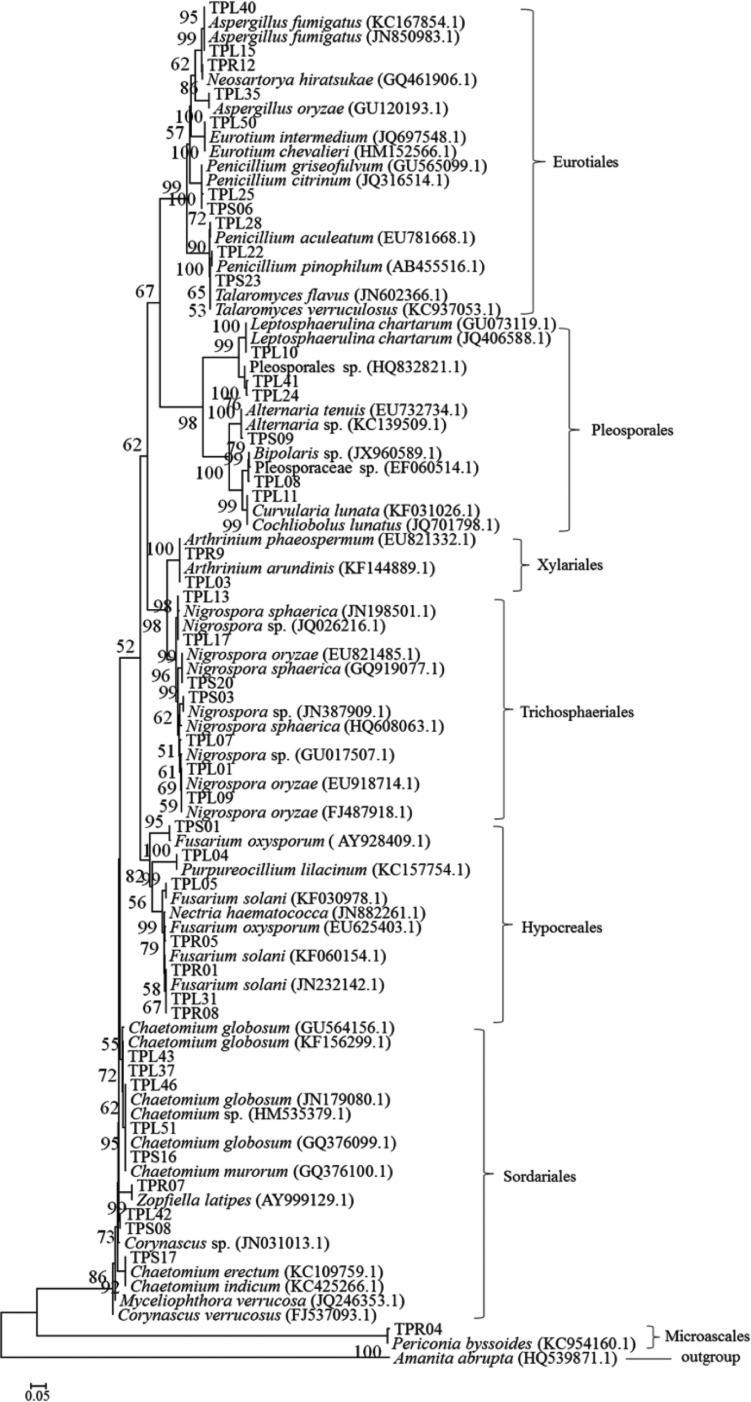
Sixty-one endophytic fungi with different colony morphologies among 239 isolated were isolated from 440 tissue segments (236 leaves, 152 stems, and 52 roots) of T. purpurea. Little difference was found in the colonization rates among the leaf (66.95%), stem (37.50%) and root (26.92%) samples (Table 2). Thirty-nine isolates originated from leaf samples, 13 were isolated from stems, and 9 were isolated from roots (Table 3). These endophytic fungi were identified by rDNA sequencing of the internal transcribed spacer region and by phylogenetic analysis. All isolated strains belonged to one of 3 classes—Sordariomycetes, Dothideomycetes and Eurotiomycetes—including the following 7 orders: Sordariales, Microascales, Hypocreales, Xylariales, Trichosphaeriales, Eurotiales, and Pleosporales. Of the 61 strains, 59 were identified at the genus level, including Chaetomium, Zopfiella, Fusarium, Purpureocillium, Arthrinium, Nigrospora, Eurotium, Aspergillus, Penicillium, Neosartorya, Talaromyces, Alternaria, Curvularia, Leptosphaerulina, Bipolaris, and Periconia, and 2 strains were identified at the order level, indicating that a great diversity of taxa are associated with T. purpurea. The phylogenetic relationships between these isolates and their related fungi are shown in Fig. 1. All of the isolated endophytic fungi were members of phylum Ascomycota.
Table 2. Colonization rates of endophytic fungi on leaves, stems and roots of Tephrosia purpurea.
Table 3. Distribution of endophytic fungi in Tephrosia purpurea.
Fig. 1. Phylogenetic tree of identified isolates that are associated with Tephrosia purpurea. The numbers at the nodes are percentages that indicate the levels of bootstrap support from 1,000 pseudoreplicates based on a neighbor-joining analysis. Bootstrap support values greater than 50% are shown above each branch. The scale bar represents 0.05 substitutions per nucleotide position.
Antifungal activity of the endophytic fungi
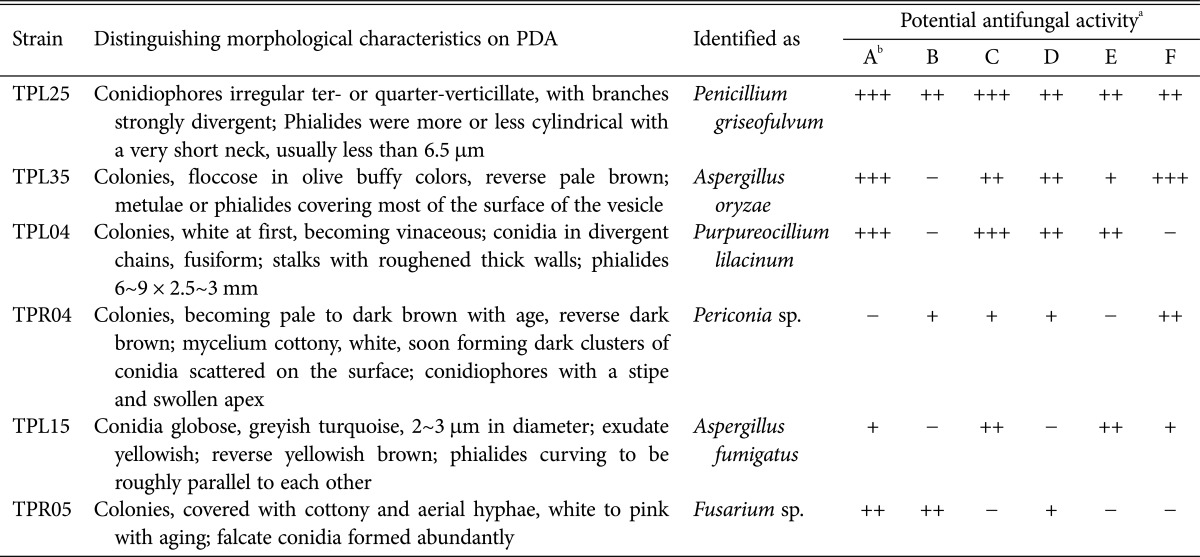
The antifungal activity of endophytic fungi isolated from T. purpurea measured by dual culture testing is shown in Table 4. Most of the fungi did not exhibit antifungal activity against six pathogenic fungi; however, 6 isolates did exhibit activity. In this experiment, the isolate TPL25 exhibited the broadest antifungal activity spectrum (Table 4); this isolate strongly inhibited the growth of S. sclerotiorum (Fig. 2) and P. melonis. TPL35 and TPL04 exhibited strong growth inhibition of S. sclerotiorum (Fig. 2) and exhibited high activity against P. parasitica var. nicotianae and P. melonis. Three isolates (TPR04, TPL15, and TPR05) exhibited moderate to weak antifungal activity against 3 or 4 species of pathogenic fungi of plants. Based on comparative efficacy, among the 6 endophytic fungi isolated from T. purpurea, TPL25 and TPL35 exhibited the strongest antifungal potential.
Table 4. Identification of endophytic fungi and activity against pathogenic fungi of plants according to the dual culture technique.
aWidth of growth inhibition zone (T): -, T=0mm; +, 0 < T ≤ 2 mm; ++, 2 < T ≤ 5 mm; +++, T > 5 mm.
bA, Sclerotinia sclerotiorum; B, Rhizoctonia solani; C, Phytophthora melonis; D, Colletotrichum gloeosporioides; E, Botrytis cinerea; F, Phytophthora parasitica var. nicotianae.
Fig. 2. Endophytic fungi from Tephrosia purpurea showing activity in a dual culture antagonistic study against the fungal pathogen Sclerotinia sclerotiorum (S).
Bioactivity of endophytic fungi culture filtrates against pathogenic fungi
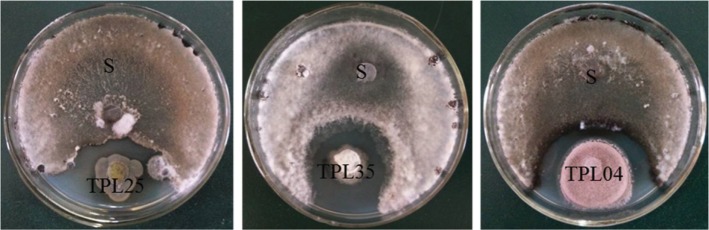
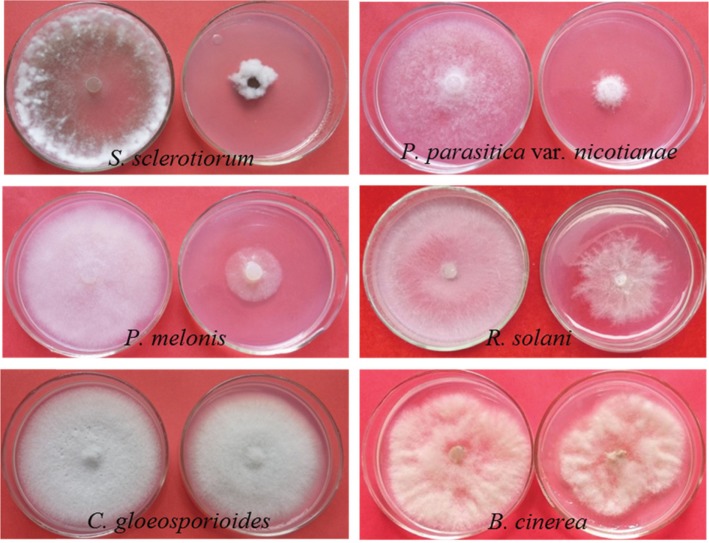
Culture filtrates of the endophytic fungi TPL25 and TPL35, which exhibited the strongest antifungal activity in dual culture testing, were tested using the poisoned food technique against six species of pathogenic fungi. As shown in Table 5 and Fig. 3, the culture filtrate of TPL25 exhibited a broad range of antifungal activity against all tested plant pathogens, including S. sclerotiorum (85.29%), P. parasitica var. nicotianae (83.82%), P. melonis (82.35%), R. solani (40.69%), B. cinerea (35.33%), and C. gloeosporioides (14.71%). Among the tested plant pathogens, S. sclerotiorum (84.31%) and P. parasitica var. nicotianae (60.29%) were inhibited most by the culture filtrate of TPL35; however, the culture filtrate of TPL35 exhibited moderate antifungal effects against B. cinerea (48.61%), P. melonis (34.80%) and C. gloeosporioides (8.00%), and did not exhibit antifungal activity against R. solani.
Table 5. Activity of culture filtrates of endophytic fungi against pathogenic fungi of plants.
aEach value represents the mean of three replicates ± standard deviation.
bA, Sclerotinia sclerotiorum; B, Rhizoctonia solani; C, Phytophthora melonis; D, Colletotrichum gloeosporioides; E, Botrytis cinerea; F, Phytophthora parasitica var. nicotianae.
Fig. 3. Growth inhibition of pathogenic fungus mycelia when treated with the TPL25 culture filtrate. The left plate in each picture was the control, and the right plate was treated with the TPL25 culture filtrate. S. sclerotiorum, Sclerotinia sclerotiorum; R. solani, Rhizoctonia solani; P. melonis, Phytophthora melonis; C. gloeosporioides, Colletotrichum gloeosporioides; B. cinerea, Botrytis cinerea; P. parasitica var. nicotianae, Phytophthora parasitica var. nicotianae.
DISCUSSION
Previous studies have noted that medicinal plants represent a potent, economically important source of microbial diversity [16,17,18]. Therefore, T. purpurea was used as a source to screen for antimicrobial endophytic fungi. In this study, endophytic fungi were successfully found in the leaves, stems and roots of this plant. Endophytic fungi were more prevalent on leaf tissues (66.95%) than on the stem (37.50%) or in root tissues (26.92%), similar to the results found in previous studies, in which endophytic fungi were isolated most often from leaves and stems, followed by the roots, with average colonization rates of 74.2, 55.6% and 9.4%, respectively [19]. This result was only slightly different from that obtained with Stipa grandis (which presented significantly higher endophytic fungi colonization rates in roots than in leaves) [20]. Interestingly, the endophytic fungi distribution patterns differed between the leaves, stems and roots. This phenomenon might be affected by tissue texture and differences in the tissue physiology and chemistry [21]. Compared with the above-ground parts, the root tissues of T. purpurea yielded an almost completely different endophytic mycoflora, which was characterized by low isolation rates and a different species composition.
In this study, Chaetomium and Fusarium species were the dominant endophytic fungal species in T. purpurea. Chaetomium species have often been reported to be endophytic fungi in host plants including Huperzia serrata [22], Nyctanthes arbor-tristis [23], Cinnamomum camphora [24] and Lycopersicon esculentum [25]. It has also been reported that the metabolites of Chaetomium sp. have bioactivity, including antifungal [26], antioxidant [27] and anticancer [28] activities. Fusarium is a cosmopolitan and common plant pathogen in nature, particularly Fusarium solani, which causes root rot resulting in considerable losses in many important crops [29]. However, Fusarium solani has also been reported as an endophytic fungi on the root tissues of tomato plants [30] and is present as an endophyte in Apodytes dimidiate, where it produces camptothecin and 10-hydroxycamptothecin, important precursors for the synthesis of the clinically useful anticancer drugs topotecan and irinotecan [31].
In contrast, only one strain was isolated from the roots of T. purpurea, and this strain belonged to the genus Periconia under the order Microascales, which might be of practical value. Li et al. [32] used Periconia sp., endophytic fungus isolated from Torreya grandifolia, to produce readily detectable quantities of the anticancer drug taxol. In another study, Periconia sp. (obtained from Piper longum L.) were used to produce piperine (5-(3,4-methylenedioxyphenyl)-1-piperidinopent-2,4-dien-1-one) under liquid culture [33]. Several interesting biologically active metabolites obtained from the endophytic fungi of medicinal plants have been studied [34]; for example, the polyketide compound 5-hydroxyramulosin, obtained from an endophytic Phoma sp. of Cinnamomum mollissimum, inhibited the fungal pathogen Aspergillus niger [35]. In our study, dual culture assay and culture filtrate testing were employed to evaluate the antimicrobial activity of sixty-one T. purpurea strains against pathogenic fungi of plants. Of the isolated strains, only 9.84% were active against one or more indicator pathogenic fungi used in the primary screening, supporting the viewpoints of Gong and Guo [36] and Zhao et al. [37]. Thus, the isolates obtained appear to exhibit narrow antimicrobial spectra, with some exceptions [38]. Ding et al. [38] found that fermentation broths of most of the endophytic fungi isolates they isolated from Camptotheca acuminata exhibited antifungal activity. It was suggested that the percentage of antibiotic-producing strains depends on the assay method used and on the species and the number of indicator microbes used in the screening [39]. The preliminary bioassay presented here might provide further guidance for the screening and identification of novel antimicrobial agents from this potential host plant.
In the dual culture assay, isolate TPL25 (identified as Penicillium griseofulvum) exhibited the broadest antifungal activity spectrum, and isolate TPL35 (identified as Aspergillus oryzae) was active against 5 fungal plant pathogens. Penicillium griseofulvum is well known for its production of griseofulvin, a widely useful antifungal antibiotic metabolic product that was isolated by Oxford et al. [40]. Kwon et al. [41] isolated two new potent compounds, phenylpyropene A and B, from a fermentation broth of P. griseofulvum F1959, which inhibit Acyl-CoA : cholesterol acyltransferase. This represents the first report of the isolation of P. griseofulvum as an endophytic fungus from a perennial herb. Aspergillus oryzae is important for the production of traditional fermented foods and beverages in Japan [42]. Ali et al. [43] reported that A. oryzae yields high amounts of L-dopa, which is a useful drug for Parkinson's disease. Furthermore, A. oryzae was also reported to exhibit antitumor activity in the study by Zhou et al. [44]. The activity of A. oryzae against pathogenic fungi, such as S. sclerotiorum and P. parasitica var. nicotianae, is reported here for the first time.
Mycelial growth inhibition assay of the strains TPL25 and TPL35 corroborated the activity exhibited by their fermentation broths. In addition, the results of the bioassays conducted here (in which greater than 80% growth inhibition was observed against some pathogenic fungi of plants) can be considered promising because they were obtained with culture filtrates that were not subjected to purification or concentration; the fermentation of endophytic fungi offers several advantages for producing bioactive compounds, including reproducibility and dependable production. Further research is warranted to identify the active metabolites in the culture filtrates and to evaluate these compounds as possible antimicrobials.
To the best of our knowledge, this study is the first ever collection of, and phylogenetic diversity analysis of, endophytic fungi from T. purpurea. The antifungal activities found provide a strong foundation for the isolation and purification of natural antimicrobial agents from the endophytic fungi of T. purpurea; these agents might prove useful in designing novel drugs that would provide sustainable solutions to various problems faced by modern society.
ACKNOWLEDGEMENTS
This work was supported by the National Nature Science Foundation of China (No. 31071715).
References
- 1.Stierle A, Strobel G, Stierle D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of pacific yew. Science. 1993;260:214–216. doi: 10.1126/science.8097061. [DOI] [PubMed] [Google Scholar]
- 2.Strobel G, Daisy B. Bioprospecting for microbial endophytes and their natural products. Microbiol Mol Biol Rev. 2003;67:491–502. doi: 10.1128/MMBR.67.4.491-502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aly AH, Debbab A, Proksch P. Fungal endophytes: unique plant inhabitants with great promises. Appl Microbiol Biotechnol. 2011;90:1829–1845. doi: 10.1007/s00253-011-3270-y. [DOI] [PubMed] [Google Scholar]
- 4.Dalwadi PP, Patel JL, Patani PV. Tephrosia purpurea Linn (Sharpunkha, Wild Indigo): a review on phytochemistry and pharmacological studies. Indian J Pharm Biol Res. 2014;2:108–121. [Google Scholar]
- 5.Kumar GS, Jayaveera KN, Kumar CK, Sanjay UP, Swamy BM, Kumar DV. Antimicrobial effects of Indian medicinal plants against acne-inducing bacteria. Trop J Pharm Res. 2007;6:717–723. [Google Scholar]
- 6.Lodhi S, Pawar RS, Jain AP, Singhai AK. Wound healing potential of Tephrosia purpurea (Linn.) Pers. in rats. J Ethnopharmacol. 2006;108:204–210. doi: 10.1016/j.jep.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande SS, Shah GB, Parmar NS. Antiulcer activity of Tephrosia purpurea in rats. Indian J Pharmacol. 2003;35:168–172. [Google Scholar]
- 8.Damre AS, Gokhale AB, Phadke AS, Kulkarni KR, Saraf MN. Studies on the immunomodulatory activity of flavonoidal fraction of Tephrosia purpurea. Fitoterapia. 2003;74:257–261. doi: 10.1016/s0367-326x(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 9.Kishore K, Roy D. Tephrosia purpurea Pers. (Fabaceae): a common winter weed of Chhattisgargh, India: as a source of anticancer drug. Indian J Appl Pure Biol. 2011;26:53–55. [Google Scholar]
- 10.Petrini O, Sieber TN, Toti L, Viret O. Ecology, metabolite production, and substrate utilization in endophytic fungi. Nat Toxins. 1992;1:185–196. doi: 10.1002/nt.2620010306. [DOI] [PubMed] [Google Scholar]
- 11.Li HY, Shen M, Zhou ZP, Li T, Wei YL, Lin LB. Diversity and cold adaptation of endophytic fungi from five dominant plant species collected from the Baima Snow Mountain, Southwest China. Fungal Divers. 2012;54:79–86. [Google Scholar]
- 12.White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninshy JJ, White TJ, editors. PCR protocols: a guide to methods and applications. London: Academic Press; 1990. pp. 315–322. [Google Scholar]
- 13.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grover RK, Moore JD. Toxicometric studies of fungicides against brown rot organisms Sclerotinia fructicola and S. laxa. Phytopathology. 1962;52:876–880. [Google Scholar]
- 15.Pandey DK, Tripathi NN, Tripathi RD, Dixit SN. Fungitoxic and phytotoxic properties of the essential oil of Hyptis suaveolens. J Plant Dis Prot. 1982;89:344–349. [Google Scholar]
- 16.Li H, Qing C, Zhang Y, Zhao Z. Screening for endophytic fungi with antitumour and antifungal activities from Chinese medicinal plants. World J Microbiol Biotechnol. 2005;21:1515–1519. [Google Scholar]
- 17.Wiyakrutta S, Sriubolmas N, Panphut W, Thongon N, Danwisetkanjana K, Ruangrungsi N, Meevootisom V. Endophytic fungi with anti-microbial, anti-cancer and anti-malarial activities isolated from Thai medicinal plants. World J Microbiol Biotechnol. 2004;20:265–272. [Google Scholar]
- 18.Huang WY, Cai YZ, Hyde KD, Corke H, Sun M. Biodiversity of endophytic fungi associated with 29 traditional Chinese medicinal plants. Fungal Divers. 2008;33:61–75. [Google Scholar]
- 19.Sun BD, Chen AJ, Cao WW, Zhou YG, Liu HY. Endophytic fungi associated with the medicinal plant, Achyranthes bidentata Blume (Amaranthaceae) Afr J Microbiol Res. 2013;7:1357–1365. [Google Scholar]
- 20.Su YY, Guo LD, Hyde KD. Response of endophytic fungi of Stipa grandis to experimental plant function group removal in Inner Mongolia steppe, China. Fungal Divers. 2010;43:93–101. [Google Scholar]
- 21.Wang Y, Guo LD. A comparative study of endophytic fungi in needles, bark, and xylem of Pinus tabulaeformis. Can J Bot. 2007;85:911–917. [Google Scholar]
- 22.Chen XY, Qi YD, Wei JH, Zhang Z, Wang DL, Feng JD, Gan BC. Molecular identification of endophytic fungi from medicinal plant Huperzia serrata based on rDNA ITS analysis. World J Microbiol Biotechnol. 2011;27:495–503. [Google Scholar]
- 23.Gond SK, Mishra A, Sharma VK, Verma SK, Kumar J, Kharwar RN, Kumar A. Diversity and antimicrobial activity of endophytic fungi isolated from Nyctanthes arbor-tristis, a well-known medicinal plant of India. Mycoscience. 2012;53:113–121. [Google Scholar]
- 24.Kharwar RN, Maurya AL, Verma VC, Kumar A, Gond SK, Mishra A. Diversity and antimicrobial activity of endophytic fungal community isolated from medicinal plant Cinnamomum camphora. Proc Natl Aacd Sci India Sect B Biol Sci. 2012;82:557–565. [Google Scholar]
- 25.Larran S, Mónaco C, Alippi HE. Endophytic fungi in leaves of Lycopersicon esculentum Mill. World J Microbiol Biotechnol. 2001;17:181–184. [Google Scholar]
- 26.Kumar S, Kaushik N, Edrada-Ebel R, Ebe R, Proksch P. Isolation, characterization, and bioactivity of endophytic fungi of Tylophora indica. World J Microbiol Biotechnol. 2011;27:571–577. [Google Scholar]
- 27.Debbab A, Aly AH, Edrada-Ebel RA, Müller WE, Mosaddak M, Hakiki A, Ebel R, Proksch P. Bioactive secondary metabolites from the endophytic fungus Chaetomium sp. isolated from Salvia officinalis growing in Morocco. Biotechnol Agron Soc Environ. 2009;13:229–234. [Google Scholar]
- 28.Krohn K, Kouam SF, Kuigoua GM, Hussain H, Cludius-Brandt S, Flörke U, Kurtán T, Pescitelli G, Bari LD, Draeger S, et al. Xanthones and oxepino[2, 3-b] chromones from three endophytic fungi. Chemistry. 2009;15:12121–12132. doi: 10.1002/chem.200900749. [DOI] [PubMed] [Google Scholar]
- 29.Lim HS, Kim YS, Kim SD. Pseudomonas stutzeri YPL-1 genetic transformation and antifungal mechanism against Fusarium solani, an agent of plant root rot. Appl Environ Microbiol. 1991;57:510–516. doi: 10.1128/aem.57.2.510-516.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kavroulakis N, Ntougias S, Zervakis GI, Ehaliotis C, Haralampidis K, Papadopoulou KK. Role of ethylene in the protection of tomato plants against soil-borne fungal pathogens conferred by an endophytic Fusarium solani strain. J Exp Bot. 2007;58:3853–3864. doi: 10.1093/jxb/erm230. [DOI] [PubMed] [Google Scholar]
- 31.Shweta S, Zuehlke S, Ramesha BT, Priti V, Mohana Kumar P, Ravikanth G, Spiteller M, Vasudeva R, Uma Shaanker R. Endophytic fungal strains of Fusarium solani, from Apodytes dimidiata E Mey. ex Arn (Icacinaceae) produce camptothecin, 10-hydroxycamptothecin and 9-methoxycamptothecin. Phytochemistry. 2010;71:117–122. doi: 10.1016/j.phytochem.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 32.Li JY, Sidhu RS, Ford EJ, Long DM, Hess WM, Strobel GA. The induction of taxol production in the endophytic fungus: Periconia sp from Torreya grandifolia. J Ind Microbiol Biotechnol. 1998;20:259–264. [Google Scholar]
- 33.Verma VC, Lobkovsky E, Gange AC, Singh SK, Prakash S. Piperine production by endophytic fungus Periconia sp. isolated from Piper longum L. J Antibiot (Tokyo) 2011;64:427–431. doi: 10.1038/ja.2011.27. [DOI] [PubMed] [Google Scholar]
- 34.Kaul S, Gupta S, Ahmed M, Dhar MK. Endophytic fungi from medicinal plants: a treasure hunt for bioactive metabolites. Phytochem Rev. 2012;11:487–505. [Google Scholar]
- 35.Santiago C, Fitchett C, Murno MH, Jalil J, Santhanam J. Cytotoxic and antifungal activities of 5-hydroxyramulosin, a compound produced by an endophytic fungus isolated from Cinnamomum mollisimum. Evid Based Complement Alternat Med. 2012;2012:689310. doi: 10.1155/2012/689310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gong LJ, Guo SX. Endophytic fungi from Dracaena cambodiana and Aquilaria sinensis and their antimicrobial activity. Afr J Biotechnol. 2009;8:731–736. [Google Scholar]
- 37.Zhao J, Cai X, Li J, Zhang Y, Peng Y, Zhou L. Endophytic fungi from rhizomes of Paris polyphylla var. yunnanensis and their antibacterial activity. J Biotechnol. 2008;136(Suppl):S609. [Google Scholar]
- 38.Ding T, Jiang T, Zhou J, Xu L, Gao ZM. Evaluation of antimicrobial activity of endophytic fungi from Camptotheca acuminata (Nyssaceae) Genet Mol Res. 2010;9:2104–2112. doi: 10.4238/vol9-4gmr809. [DOI] [PubMed] [Google Scholar]
- 39.Shnit-Orland M, Kushmaro A. Coral mucus-associated bacteria: a possible first line of defense. FEMS Microbiol Ecol. 2009;67:371–380. doi: 10.1111/j.1574-6941.2008.00644.x. [DOI] [PubMed] [Google Scholar]
- 40.Oxford AE, Raistrick H, Simonart P. Studies in the biochemistry of micro-organisms: Griseofulvin, C17H17O6Cl, a metabolic product of Penicillium griseo-fulvum Dierckx. Biochem J. 1939;33:240–248. doi: 10.1042/bj0330240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwon OE, Rho MC, Song HY, Lee SW, Chung MY, Lee JH, Kim YH, Lee HS, Kim YK. Phenylpyropene A and B, new inhibitors of acyl-CoA: cholesterol acyltransferase produced by Penicillium griseofulvum F1959. J Antibiot (Tokyo) 2002;55:1004–1008. doi: 10.7164/antibiotics.55.1004. [DOI] [PubMed] [Google Scholar]
- 42.Machida M, Asai K, Sano M, Tanaka T, Kumagai T, Terai G, Kusumoto K, Arima T, Akita O, Kashiwagi Y, et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 2005;438:1157–1161. doi: 10.1038/nature04300. [DOI] [PubMed] [Google Scholar]
- 43.Ali S, Ikram-ul-Haq, Qadeer MA. Novel technique for microbial production of 3,4-dihydroxy phenyl L-alanine by a mutant strain of Aspergillus oryzae. Electron J Biotechnol. 2002;5:118–124. [Google Scholar]
- 44.Zhou HR, Luan HB, Wang H, Dong KM, Miao L. Optimization of the fermentation medium of an antitumor endophytic fungus Aspergillus oryzae YX-5 isolated from Ginkgo biloba. Microbiol China. 2014;41:1358–1367. [Google Scholar]