Abstract
Bone regeneration often requires continuous stimulation to promote local bone formation. In the present study, calcium phosphate (CaPi) was used to promote transfection of human bone morphogenetic protein 2 (BMP-2) cDNA plasmid, and poly (lactic-co-glycolic acid) (PLGA) was used to prepare microspheres of pBMP-2/CaPi (i.e., PLGA@pBMP-2/CaPi) using W/O/W double emulsion solvent evaporation method. We showed that PLGA@pBMP-2/CaPi microspheres were spherical with smooth surface, and the particle size ranged from 0.5 to 35 μm. Encapsulation efficiency was up to 30~50%. The release of BMP-2 cDNA from microspheres continued more than 30 days and constituted, less than 7.5% of total plasmid amount within the first 24 h. Real-time PCR results showed that co-culturing of PLGA@pBMP-2/CaPi with bone marrow-derived mesenchymal stem cells (BMSCs) increased calcium deposition and gene expressions of alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), SP7, and collagen type I (COLL I) in a time-dependent manner. Finally, X-ray analysis demonstrated that in vivo delivery of PLGA@pBMP-2/CaPi microspheres into the tibialis anterior muscles of rats promoted the generation of osteoblasts, bone tissue, and bone structure. The findings suggested that PLGA@pBMP-2/CaPi microspheres can promote ectopic osteogenesis in non-bone tissues, with strong prospects in promoting bone regeneration.
Keywords: BMP-2 plasmid, PLGA, calcium phosphate, microsphere, ectopic osteogenesis
Introduction
Bone loss of various etiologies is one of the most common clinical problems in stomatology. It not only affects the patient’s facial appe-arance, chewing, pronunciation, feeding capabilities and the subsequent denture, but also his or her psychological health. Current treatments for alveolar bone loss and atrophy include bone or bone substitute grafts, application of bone morphogenetic protein, chemical drugs, and stem cell tissue engineering [1-4]. However, all these treatments suffer from disadvantages such as the limited source of autologous bone, the risk of immune reactions and other diseases caused by allograft rejection, the damage to gastrointestinal tract, renal and alveolar bone caused by frequent and prolonged drug therapy, and ethical issues associated with the use of stem cells. Hence, there is an urgent need to find a safe, effective and convenient way to achieve the increased alveolar volume.
Bone morphogenetic protein-2 (BMP-2) is a member of the transforming growth factor-β super family. BMP-2 induces undifferentiated mesenchymal stem cells into chondrocytes and osteoblasts. The protein is involved in the growth and development of bone and the cartilage reconstruction process by promoting osteoblast differentiation and maturation, thus accelerating the repair of bone defects. BMP-2 can help the ectopic bone formation and has been approved for the clinical applications. However, the use of the bone morphogenetic protein is associated with short duration of action and high cost. In the present studies, we intend to use gene therapy techniques to delivery BMP gene into cells. The expression of bone morphogenetic protein in the cells is expected to overcome the disadvantages of exogenous BMP such as short half-life, the need to use large amounts of protein, and low potency.
Commonly used gene vectors include viral and non-viral vectors. Viral vectors have high transfection efficiency, but immunogenicity limits its application. Among various non-viral vectors, calcium phosphate is the main component of bone tissue. Therefore, calcium phosphate was selected as a gene delivery carrier in the present studies. In order to achieve the desirable therapeutic effect, the therapeutic stimulus at the defect site should be continuous and prolonged. Biodegradable polymers based on poly (lactic-co-glycolic acid) (PLGA) have been widely used in the controlled release systems and were approved by FDA. The present study use PLGA for the BMP gene encapsulation. The degradation of PLGA slowly releases BMP gene, which is continuously expressed at the bone defect site. The expression of BMP promoted bone regeneration.
Materials and methods
Chemical agents
Polylactic acid-glycolic acid (50:50 PLGA, MW = 30-60 kDa), polyvinyl alcohol (PVA), and calcium phosphate transfection kit were all purchased from Sigma (USA). Analytical grade dichloromethane was purchased from Beijing Chemical Plant (China).
Animals
All animals were provided by the Institutional Animal Care and Use Committee of Jilin University. Male Wistar rats (n=20) weighing 200-250 g were housed in a temperature- and humidity-controlled vivarium under a 12 h light/dark cycle. All animals had free access to food daily with water ad libitum. Animal experiments were approved by the local Institutional Animal Care and Use Committee. The housing and treatment of the animals followed the Guidance Suggestions for the Care and Use of Laboratory Animals formulated by the Ministry of Science and Technology of China.
Preparation and culturing of rat bone mesenchymal stem cells
To isolate BMSCs, tibias and femurs were dissected from the adult Wistar rats and washed with L-DMEM (DMEM media supplemented with L-glutamine). Both epiphyseal ends were cut to expose the marrow cavity. L-DMEM medium was used to flush the marrow cavity, and total fluid was collected. The fluid was subjected to the density gradient centrifugation followed by adherence separation. Bone marrow suspension was centrifuged (1000 rpm) in 15 ml centrifuge tube at room temperature for 3-5 min. After centrifugation, the pellet was gently suspended in 5 mL cell culture medium containing 10% fetal bovine serum. The resulting single cell suspension (2×104 cells/flask) was inoculated into 25 ml flasks, and cultured at 37°C in 5% CO2 humidified incubator. The cell culture medium was changed every 3 days. When the cells reached 80-90% confluency, they were harvested, replated on 25 ml plastic flask, and cultured to the next confluence. Cell morphology and growth were observed under an inverted microscope.
Preparation PLGA@pBMP-2/CaPi microspheres
Calcium phosphate-BMP-2 cDNA plasmid complexes (pBMP-2/CaPi) were encapsulated intoPLGA microspheres using double emulsionsolvent evaporation method (W/O/W) [5], according to the instructions of manufacturer of calcium phosphate transfection kit. Specifically, 0.6 ml pBMP-2/CaPi (containing 36 µg of BMP-2 cDNA plasmid) were homogenized with 2 ml of 2% PLGA/DCM solution under ice cooling at 7500 rpm for 5 sec, followed by additional homogenization with 10 ml of 3% PVA solution on the ice bath in at 7500 rpm for 5 sec. Finally, the solution was added into 20 ml PVA (0.5%, w/v) solution with magnetic stirring at room temperature for 2 h, the residual DCM was evaporated, and microspheres were solidified. After centrifuging at 8000 rpm, microspheres were washed three times with deionized water, lyophilized, and stored at -20°C.
Determination of entrapment efficiency and burst release
PLGA entrapment efficiency of pBMP-2/CaPi was evaluated by measuring unbound pBMP-2 in the solution. Supernatant in the PLGA@pBMP-2/CaPi solution was recovered by centrifugation at 5,000 rpm for 15 min. Free pBMP-2 concentration in the supernatant was determined using a spectro photofluorometer (RF-1501, Shimadzu, Kyoto, Japan) with a Quant-It™ Pico Green dsDNA quantitation assay reagent (Life Technologies, Grand Island, NY, USA) [6]. Encapsulation efficiency was defined as percent of pBMP-2 encapsulated in pBMP-2/CaPi by PLGA with respect to initial amount of pBMP-2. Drug loading rate (wt%) was the percent of pBMP-2 entrapped in the microspheres in the total amount of PLGA@pBMP-2/CaPi [7]. The amount of pBMP-2 in the microspheres was determined by subtracting the amount of pBMP-2 recovered in the wash solutions from the initial amount of pBMP-2 added.
In vitro degradation
The lyophilized PLGA@pBMP-2/CaPi microspheres were added into PBS buffer (pH=7.4) with water bath shaking at 37°C. Immediately after dissolution (0 h), or after 24 h, one week (1 w), or one month (1 m), the morphology of microsphere samples was observed under scanning electron microscope (SEM).
Scanning electron microscopy
The product morphology and particle size was characterized by SEM (JSM-6700F, JEOL, USA). The sample was directly placed onto the sample holder with conductive coating. Excess sample was removed. The sample was treated by desiccation and spray-gold, and was observed after 80 sec. The accelerating voltage was set at 50 kV.
Transmission electron microscopy
The product morphology and particle size was also characterized by TEM. The PLGA@pBMP-2/CaPi were embedded in Epon resin (Polysciences, Inc., Warrington, Pennsylvania), and sectioned using a LKB-Huxley ltramicrotome (LKB Bromma, Sollentuna, Sweden) to a final thickness of 60-90 μm. Sections were stained with uranyl acetate-lead acetate and visualized by transmission electron microscopy (TEM) (Zeiss 9000; CarlZeiss, Oberkochen, Germany) [8]. Sample was treated with ultrasonic dispersion in ethanol first, followed by observation on a copper mesh. The accelerating voltage was set at 200 kV.
Alizarin red (AZR) staining
AZR staining was used to identify mineralization nodule formation after 21 days in the osteogenic induction culture. Briefly, to obtain 1% AZR staining buffer solution, 1 g AZR was added to Tris-HCl solution, which was made by adding 1 g Tris-HCl to 100 mL of distilled water with the pH adjusted to 7.8 using 0.1 M HCl. Experimental group (PLGA@pBMP-2/CaPi microspheres + BMSCs) and control group (BMSCs only or PLGA@CaPi microspheres + BMSCs) of cells were incubated for 21 days. After washing twice with PBS buffer, the cells were fixed with 95% ethanol for 10 min. After washing with PBS buffer again, the cells were incubated with 1% AZR staining buffer solution for 10 min at 37°C. In order to eliminate the non-specific bindings, the cells were incubated in 3 ml PBS solution for 30 min at room temperature and washed gently with PBS solution. The mineralization nodule formation was observed and photographed under an inverted microscope. To semi-quantify the mineralization nodule formation, 10% cetylpyridinium chloride in 10 mM Na2HPO4 was added to the well, followed by shaking for 10 min at room temperature. The absorbance was then measured at a wavelength of 562 nm using a microplate reader.
In vitro delivery
To evaluate the in vitro delivery efficiency of PLGA@pBMP-2/CaPi microspheres, we first prepared PLGA@pEGFP/CaPi microspheres, in which EGFP gene served as a fluorescent marker, using the same method as described above. After three passages of MC3T3-E1 cells, the cells were rinsed twice with PBS buffer, digested with 0.25% trypsin, and re-suspended in α-DMEM medium after centrifugation. Cells were then loaded on the 6-well cell culture plate (2×105 cells/well). On the next day, the cell culture medium was replaced by serum-free medium, followed by addition of 0.3 mg of PLGA@pEGFP/CaPi microspheres which contained about 5 μg of cDNA plasmid. Cells were co-cultured with PLGA@pEGFP/CaPi microspheres overnight. On the next day, the cell culture medium was replaced with medium containing serum, and the cells were examined by fluorescent microscopy.
Using the same method, BMSCs cells were incubated with PLGA@pBMP-2/CaPi microspheres. Cell culture medium was changed every three days, with simultaneous replacement of PLGA@pBMP-2/CaPi microspheres. After 1, 3, 7 or 14 days of incubation, the total RNAs were extracted, and mRNA expression levels of alkaline phosphatase (ALP), runt-related transcription factor 2 (RUNX2), SP7, and collagen type I (COLL I) genes were measured using RT-qPCR (Table 1). After incubation for 21 days, AZR staining was used to examine the mineralization nodule formation in the BMSCs.
Table 1.
Primer sequences for PCR
| Gene | Gene Bank Accession | Sequences of probes | Length (bp) | Product (bp) |
|---|---|---|---|---|
| Actb | NM_007393.3 | F: 5’-CATCCGTAAAGACCTCTATGCCAAC-3’ | 25 | 171 |
| R: 5’-ATGGAGCCACCGATCCACA-3’ | 19 | |||
| Runx2 | NM_001145920.1 | F: 5’-GCACAAACATGGCCAGATTCA-3’ | 21 | 126 |
| R: 5’-AAGCCATGGTGCCCGTTAG-3’ | 19 | |||
| Sp7 | NM_130458.3 | F: 5’-AAGTTATGATGACGGGTCAGGTACA-3’ | 25 | 129 |
| 5’-AGAAATCTACGAGCAAGGTCTCCAC-3’ | 25 | |||
| COLL I | NM_007742.3 | F: 5’-GACATGTTCAGCTTTGTGGACCTC-3’ | 24 | 119 |
| R: 5’-GGGACCCTTAGGCCATTGTGTA-3’ | 22 | |||
| Alp | NM_007431.2 | F: 5’-CTCAACACCAATGTAGCCAAGAATG-3’ | 25 | 75 |
| R: 5’-GGCAGCGGTTACTGTGGAGA-3’ | 20 |
In vivo delivery
Surgery was done under anesthetic (60 mg/kg of ketamine and 8 mg/kg of xylazine). PLGA@pBMP-2/CaPi microspheres or PLGA@CaPi control microspheres were injected into the tibialis anterior muscles of Wistar rats (n=20). After 4 or 8 weeks following the injections, rats were transcardially perfused, tissues around the injection sites were collected, fixed in 10% formalin solution, and X-ray photographs were taken. One month after decalcification with 10% EDTA, the tissues were embedded in paraffin, and 3 μm slices were prepared, followed by hematoxylin-eosin (HE) staining.
Results
pBMP-2/CaPi particle size
The results of the particle size measurements are shown on Figure 1. Dynamic Light Scattering (DLS) showed that the calcium phosphate particles size was about 350 nm. The particle size of pBMP-2/CaPi was around 600 nm, suggesting that BMP-2 cDNA plasmid was precipitated with calcium phosphate. A narrow distribution of particle size indicates that no obvious aggregation phenomena take place (Figure 1).
Figure 1.

Particle size of (A) calcium phosphate and (B) BMP-2/CaPi complex.
Surface morphology of PLGA@pBMP-2/CaPi microspheres
SEM results showed that PLGA@pBMP-2/CaPi microspheres were in good and uniform shape with small holes caused by solvent evaporation distributed evenly on the surface (Figure 2). The diameter of microspheres was between 0.5-30 μm. The different sizes may contribute to the rate of degradation, thus helping in achieving the sustained release. The small holes distributed on the surface of the microspheres can help the external liquid to enter, there by accelerating the degradation process.
Figure 2.

SEM photographs of (A) PLGA@pBMP-2/CaPi microspheres and (B) microspheres surface.
TEM analysis
To verify whether the pBMP-2/CaPi was successfully entrapped into PLGA microspheres, PLGA@pBMP-2/CaPi microspheres were embedded with an epoxy resin, sectioned, and observed under TEM. The results showed thatsubstance with uneven density inside microspheres had a size ranging from 100 nm to 1 μm. X-ray diffraction analysis confirmed the presence of crystals (Figure 3). Because the existing crystals were calcium phosphate only, which were co-precipitated with BMP-2 cDNA plasmid, these results suggested that pBMP-2/CaPi was successfully entrapped into PLGA microspheres.
Figure 3.
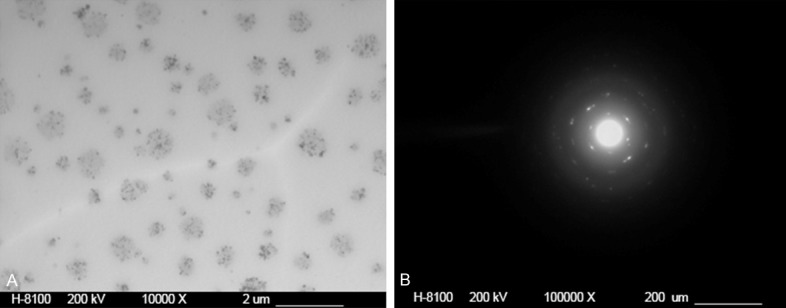
(A) TEM and (B) X-ray photographs of PLGA@pBMP-2/CaPi microspheres.
Entrapment efficiency and burst release rate
To date, there is a variety of methods used to prepare drug-carrying microspheres [9,10]. However, since cDNA plasmid is water soluble, the W/O/W method is the most suitable for preparation of PLGA@pBMP-2/CaPi microspheres. The entrapment efficiency of PLGA@pBMP-2/CaPi microspheres prepared in this study was about 30-50%, and 24-hour burst release rate was less than 7.5%. PLGA@pBMP-2/CaPi microspheres can release BMP-2 cDNA plasmid for up to 30 days (Figure 4).
Figure 4.
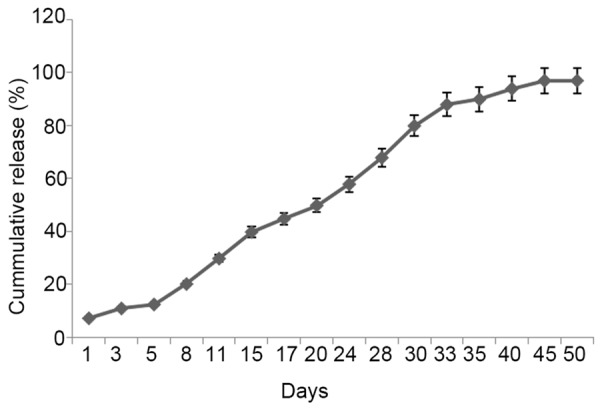
In vitro release of PLGA@pBMP-2/CaPi microspheres.
In vitro degradation
The results of in vitro degradation of PLGA@pBMP-2/CaPi microspheres are shown in Figure 5. One day after dissolution in PBS buffer, the surface of PLGA@pBMP-2/CaPi microspheres began to swell, erode, and deform. The surface was no longer smooth, showing adhesion. One week later, some of microspheres were completely disintegrated, showing polymer frag mentation. One month later, almost all of the microspheres eroded and degraded to short-chain polymers.
Figure 5.

SEM photographs of the degradation of PLGA@pBMP-2/CaPi microspheres in PBS buffer after: (A) 0 h, (B) 24 h, (C) 1 week, or (D) 1 month.
In vitro delivery
PLGA@pEGFP/CaPi microspheres were incuba-ted with MC3T3-E1. After 24 h of incubation, a small percentage of cells showed expression of green fluorescence. The expression of green fluorescence started to increase after 48 h and peaked after 72 h (Figure 6).
Figure 6.
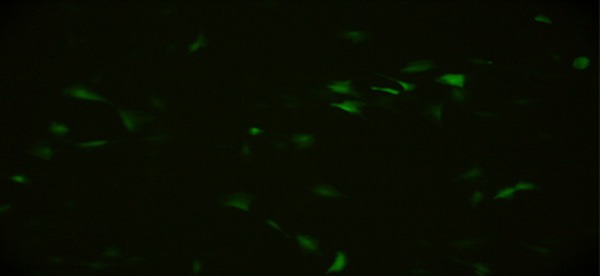
Photograph of fluorescent microscopy for PLGA@pEGFP/CaPi and BMSCs co-cultured for three days.
After 21 days of incubation of PLGA@pBMP-2/CaPi microspheres with BMSCs, Alizarin Red staining showed that PLGA@pBMP-2/CaPi microspheres increased calcium nodule formation relative to the control groups (Figure 7). Furthermore, RT-qPCR showed that PLGA@pBMP-2/CaPi microspheres started to increase mRNA expression levels of RUNX2 and COLL I after 3 days of incubation with BMSCs. Additionally, the mRNA expression levels of ALP, RUNX2, SP7, and COLL I were all increased after 7 or 14 days of incubation (Figure 8).
Figure 7.

Effects of PLGA@pBMP-2/CaPi microspheres on the mineralization nodule formation. Representative images showed red calcium nodule that were visible to naked eyes. Semi-quantitative analysis showed the calcium nodule staining of experimental and control groups. Asterisks represent the significant effects, p<0.05.
Figure 8.
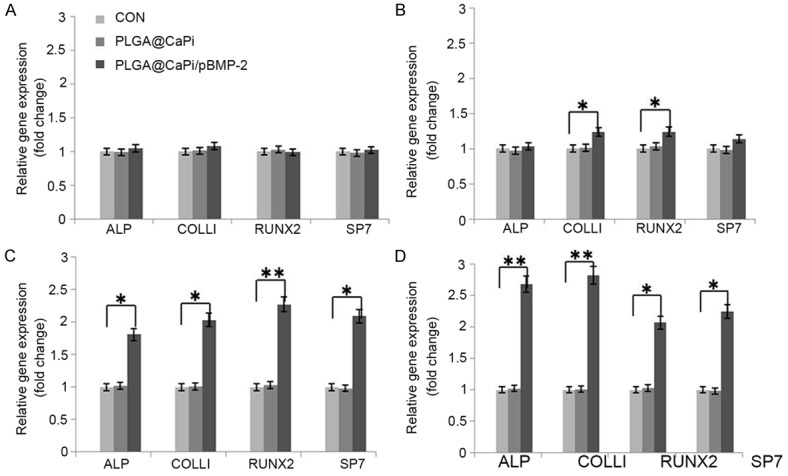
Effects of PLGA@pBMP-2/CaPi microspheres on mRNA expressions of ALP, COLL-1, SP7, and Runx2 genes after (A) 1, (B) 3, (C) 7, or (D) 14 days of incubation with BMSCs. mRNA levels for each gene of interest were calculated by normalizing the quantified mRNA amount to β-actin. *ANOVA simple main effect; p<0.05; **ANOVA simple main effect; p<0.01.
In vivo delivery
Eight weeks afte injections of PLGA@pBMP-2/CaPi microspheres into the tibialis anterior muscles in rats, X-ray analysis indicated that there was a high density area within the injection site (Figure 9A). HE staining results suggested the increased activity of osteoblasts and a new bone formation eight weeks after injections of PLGA@pBMP-2/CaPi microspheres (Figure 9B and 9C).
Figure 9.
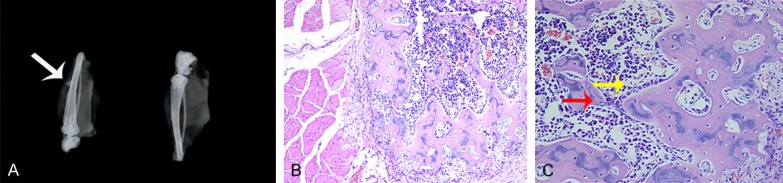
Effects of PLGA@pBMP-2/CaPi microspheres on bone formation in vivo. A. Eight weeks after injections of PLGA@pBMP-2/CaPi microspheres into thetibialis anterior muscles in rats, X-ray analysis indicated there was a high density area within the injection site (arrow). B, C. HE staining results suggested that there were increased activity of osteoblasts and newbone formation after eight weeks following injections of PLGA@pBMP-2/CaPi microspheres.
Discussion
This study reports successful preparation of PLGA@pBMP-2/CaPi microspheres using double emulsion solvent evaporation method (w/o/w). We showed that PLGA@pBMP-2/CaPi microspheres were spherical with smooth surface, and the particle size ranged from 0.5 to 35 μm. Additionally, encapsulation efficiency was up to 30~50%. The release of BMP-2 cDNA continued for more than 30 days, with less than 7.5% of total DNA released in the first 24 h. Real-time PCR results showed that co-culturing of PLGA@pBMP-2/CaPi with bone marrow-derived mesenchymal stem cells increased calcium deposition and mRNA expressions of ALP, RUNX2, SP7, and COLL I in a time-dependent manner. Finally, X-ray analysis demonstrated that in vivo delivery of PLGA@pBMP-2/CaPi microspheres into the tibialis anterior muscles of rats promoted the generation of osteoblasts, bone tissue, and bone structure.
BMP proteins are one of the most important growth factors in bone formation and regeneration [11,12]. Forty-three subtypes of BMPs have been identified to date. All the BMP subtypes belong to the TGF family except BMP-1. BMPs are able to induce ectopic bone formation and stimulate mesenchymal stem cells to differentiate into mature osteoblasts. A large number of studies have confirmed that BMP can promote healing of bone defects and fractures [13-15]. The application of BMP-2 for the treatment of tibial fractures is approved by FDA. However, BMP-2 treatment for fractures or bone defects often requires relatively high doses of proteins and long-term and frequent application due to the short half-life of BMP-2. These limitations might be overcome by using gene therapy approaches, which can be used to produce endogenous BMPs by in vivo transfection of the target cells.
Studies have shown that secretion of endogenous BMPs after transfection at the fracture site can lead to better results than direct application of exogenous recombinant BMP proteins [16-18]. Transfection of genes into target cells need vectors, either viral or non-viral, both of which have been used for transfection of osteogenic growth factors for the treatment of bone defects. Subcutaneous or intramuscular injections of adenovirus carrying BMP-7 gene can induce bone formation after four weeks [19]. Additionally, intramuscular injections of adenovirus carrying BMP-2 gene can induce bone formation in immune deficient mice after two weeks and in normal mice after three weeks [20]. Furthermore, injections of adenovirus carrying BMP-2 gene with tetracycline-sensitive promoter could induce bone formation in ectopic and orthotopic sites after applying doxycycline [21]. While adenoviral vectors have high transfection efficiency, they are associated with immunogenicity problems. Retroviral vectors infect only proliferating cells, have low transfection efficiency, and may increase the risk of genetic mutation. However, plasmid vectors are relatively simple, safe, and associated with much reduced antigenicity. For instance, repeated injections of BMP-2-containing plasmids into the muscles of rats could induce bone formation [22]. Degradable matrices containing BMP-4 expressing plasmid can promote the healing of segmental gaps created in the adult rat femur after implantation [23].
Currently, BMP-2 gene therapy is done either by direct injection or biological entrapment and implantation. Although the direct injection is simple, it is hard to control the gene carrier diffusion and fast elimination. Moreover, its transient effect at the defect site cannot serve a continuous function. Therefore, it is particularly important to choose an appropriate gene vector complex as sustained delivery system at the defect site. Due to its good biocompatibility and biodegradability, PLGA is often used as slow or controlled release vehicle of drugs, proteins, genes and other biologically active molecules.
PLGA is typically prepared in the form of scaffold or microspheres for transportation of drugs or growth factors. Huang et al. utilized PLGA scaffold to incorporate BMP-2 protein, on which the MSC cells were inoculated. It was found that the expression of type I collagen and VEGF mRNA was promoted [24]. Lee et al. prepared PLGA microspheres carrying BMP-2 protein and applied them in rat calvaria critical bone defect model. It was found that the BMP-2 protein microspheres promoted the healing of bone defects [25]. The advantage of scaffold is its bone growth induction effect in the application to large bone defects, in favor of cell climbing. The advantages of microspheres or microparticles include large surface area, high drug payload, and absence of selectivity for defect shape. The present study is focused on alveolar bone loss in periodontal and alveolar bone defects that do not have uniform sizes or shapes. Therefore, microspheres are more suitable carriers for the treatment of clinical alveolar bone loss in periodontitis.
Currently studies on non-viral vector-mediated BMP delivery have focused on liposomes [26], gene activated matrix [23] and ultrasound-mediated gene transfer [27]. Few studies have been conducted using calcium phosphate for BMP-2 transfection for the bone regeneration. Given that calcium phosphate is required for the mineralization of bone tissues and that calcium phosphate is the classic non-viral gene vector with high biocompatibility, the present study used calcium phosphate co-precipitation method to prepare microspheres.
In order to verify whether pBMP-2 and calcium phosphate complex was entrapped into PLGA microspheres, PLGA@pBMP-2/CaPi microspheres were embedded into epoxy resin, sectioned, and examined under TEM. The results showed that substance with uneven density inside microspheres had a size ranging from 100 nm to 1 μm. X-ray diffraction analysis confirmed the presence of crystals. Because the existing crystals were calcium phosphate only, which were co-precipitated with BMP-2 cDNA plasmid, these findings suggested that pBMP-2/CaPi was successfully entrapped into PLGA microspheres. Furthermore, PLGA@pEGFP/CaPi microspheres were incubated with MC3T3-E1 cells. After 24 h of incubation, a small percentage of cells showed expression of green fluorescence. The expression of green fluorescence started to increase after 48 hours and peaked after 72 hours. These results indicated pEGFP-carrying PLGA microspheres did not alter the biological activity of EGFP-containing plasmid. However, fewer cells expressed EGFP protein, likely due to transfection of the genes contained in microspheres into MC3T3-E1 cells only after the plasmid’s release from microspheres. Compared with the transfection efficiency in the direct in vitro transfection experiments during which the cell number and the amount of plasmid DNA can be well controlled, it is difficult to match the number of cells with the amount of released plasmid DNA.
A large number of studies have reported that in vitro or in vivo BMP-2 gene therapy can induce ectopic bone formation. For example, injections of bone progenitor cells into thigh muscles in nude mice after transfection of adenoviral vector-carried BMP-2 gene in vitro can promote new bone formation [28]. Additionally, Laurencin and colleagues used a retroviral vector to transfect BMP-2 gene into bone marrow cells with constructed PLA/HA as a scaffold for engineering bone tissue, and then implanted transfected cells in immune deficient mice. They found a large number of high-density bone shadow formed around the scaffold, suggesting that BMP-2 gene transfected bone marrow cells and induced osteogenic activity [29]. Furthermore, Schek and colleagues re-suspended Ad-BMP-7 in an aqueous gel and injected it into muscles in nude mice. Four weeks later, they found that 80% of the muscle turned into bone tissue, thus demonstrating that the hydrogel may provide a protective role in preventing the viral infectivity declining and is a good gene carrier [30]. Nonetheless, studies using calcium phosphate-mediated transfection method have not been reported in BMP-2 gene delivery. In the present study, eight weeks after injections of PLGA@pBMP-2/CaPi microspheres into the tibialis anterior muscles in rats, X-ray analysis indicated that there was a high density area within the injection site. HE staining results suggested that there were increased activity of osteoblasts and the newbone formation after eight weeks following the injections of PLGA@pBMP-2/CaPi microspheres. Due to the absence of osteogenic cells or any relevant cytokines within the muscle, it is highly likely that high density area formed in the muscle was the newly formed bone as the result of the transfections of BMP-2 genes from PLGA@pBMP-2/CaPi microspheres.
In summary, the present study reports successful preparation of a novel PLGA@pBMP-2/CaPi microspheres using double emulsion solvent evaporation (w/o/w) method. The use of these microspheres helps to achieve sustained release of BMP-2 cDNA plasmid locally, and promoted ectopic osteogenesis in vivo. Our study suggested that PLGA@pBMP-2/CaPi microspheres can promote ectopic osteogenesis in non-bone tissues, with strong prospects in promoting bone regeneration.
Acknowledgements
This study was supported by the National Natural Science Foundation for the Youth of China (No. 81400488), the National Science Foundation of China (No. 30830108 and No. 81271111), the Jilin Provincial Science and Technology Department (No. 20150414002GH), College students’ innovative entrepreneurial training plan of Jilin University (No. 2014A78334 and No. 2015781128), China Postdoctoral Science Foundation (No. 2015M581406), the Jilin Provincial Education Department (No. 2015-529).
Disclosure of conflict of interest
None.
References
- 1.Fellah BH, Gauthier O, Weiss P, Chappard D, Layrolle P. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials. 2008;29:1177–1188. doi: 10.1016/j.biomaterials.2007.11.034. [DOI] [PubMed] [Google Scholar]
- 2.Biasibetti A, Aloj D, Di Gregorio G, Masse A, Salomone C. Mechanical and biological treatment of long bone non-unions. Injury. 2005;36(Suppl 4):S45–50. doi: 10.1016/j.injury.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Koulocheris P, Weyer N, Liebehenschel N, Otten JE, Gutwald R, Schmelzeisen R. Suppurative maxillary sinusitis in patients with bisphosphonate-associated osteonecrosis of the maxilla: report of 2 cases. J Oral Maxillofac Surg. 2008;66:539–542. doi: 10.1016/j.joms.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Cui W, Wang Q, Chen G, Zhou S, Chang Q, Zuo Q, Ren K, Fan W. Repair of articular cartilage defects with tissue-engineered osteochondral composites in pigs. J Biosci Bioeng. 2011;111:493–500. doi: 10.1016/j.jbiosc.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Qiao C, Zhang K, Jin H, Miao L, Shi C, Liu X, Yuan A, Liu J, Li D, Zheng C, Zhang G, Li X, Yang B, Sun H. Using poly (lactic-co-glycolic acid) microspheres to encapsulate plasmid of bone morphogenetic protein 2/polyethylenimine nanoparticles to promote bone formation in vitro and in vivo. Int J Nanomedicine. 2013;8:2985–2995. doi: 10.2147/IJN.S45184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ho J, Wang H, Forde GM. Process considerations related to the microencapsulation of plasmid DNA via ultrasonic atomization. Biotechnol Bioeng. 2008;101:172–181. doi: 10.1002/bit.21876. [DOI] [PubMed] [Google Scholar]
- 7.Andreas K, Zehbe R, Kazubek M, Grzeschik K, Sternberg N, Baumler H, Schubert H, Sittinger M, Ringe J. Biodegradable insulin-loaded PLGA microspheres fabricated by three different emulsification techniques: investigation for cartilage tissue engineering. Acta Biomater. 2011;7:1485–1495. doi: 10.1016/j.actbio.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 8.Miao L, Zhang K, Qiao C, Jin X, Zheng C, Yang B, Sun H. Antitumor effect of human TRAIL on adenoid cystic carcinoma using magnetic nanoparticle-mediated gene expression. Nanomedicine. 2013;9:141–150. doi: 10.1016/j.nano.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Walter E, Moelling K, Pavlovic J, Merkle HP. Microencapsulation of DNA using poly(DL-lactide-co-glycolide): stability issues and release characteristics. J Control Release. 1999;61:361–374. doi: 10.1016/s0168-3659(99)00151-0. [DOI] [PubMed] [Google Scholar]
- 10.Hirosue S, Muller BG, Mulligan RC, Langer R. Plasmid DNA encapsulation and release from solvent diffusion nanospheres. J Control Release. 2001;70:231–242. doi: 10.1016/s0168-3659(00)00353-9. [DOI] [PubMed] [Google Scholar]
- 11.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 12.Chen D, Zhao M, Harris SE, Mi Z. Signal transduction and biological functions of bone morphogenetic proteins. Front Biosci. 2004;9:349–358. doi: 10.2741/1090. [DOI] [PubMed] [Google Scholar]
- 13.Mathavan N, Bosemark P, Isaksson H, Tagil M. Investigating the synergistic efficacy of BMP-7 and zoledronate on bone allografts using an open rat osteotomy model. Bone. 2013;56:440–448. doi: 10.1016/j.bone.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 14.Draenert ME, Kunzelmann KH, Forriol F, Hickel R, Draenert K. Primary cancellous bone formation with BMP and micro-chambered beads: experimental study on sheep. Bone. 2013;52:465–473. doi: 10.1016/j.bone.2012.08.120. [DOI] [PubMed] [Google Scholar]
- 15.Hagi TT, Wu G, Liu Y, Hunziker EB. Cellmediated BMP-2 liberation promotes bone formation in a mechanically unstable implant environment. Bone. 2010;46:1322–1327. doi: 10.1016/j.bone.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Gazit D, Turgeman G, Kelley P, Wang E, Jalenak M, Zilberman Y, Moutsatsos I. Engineered pluripotent mesenchymal cells integrate and differentiate in regenerating bone: a novel cellmediated gene therapy. J Gene Med. 1999;1:121–133. doi: 10.1002/(SICI)1521-2254(199903/04)1:2<121::AID-JGM26>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 17.Kofron MD, Laurencin CT. Bone tissue engineering by gene delivery. Adv Drug Deliv Rev. 2006;58:555–576. doi: 10.1016/j.addr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Pelled G, Ben-Arav A, Hock C, Reynolds DG, Yazici C, Zilberman Y, Gazit Z, Awad H, Gazit D, Schwarz EM. Direct gene therapy for bone regeneration: gene delivery, animal models, and outcome measures. Tissue Eng Part B Rev. 2010;16:13–20. doi: 10.1089/ten.teb.2009.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franceschi RT, Wang D, Krebsbach PH, Rutherford RB. Gene therapy for bone formation: in vitro and in vivo osteogenic activity of an adenovirus expressing BMP7. J Cell Biochem. 2000;78:476–486. doi: 10.1002/1097-4644(20000901)78:3<476::aid-jcb12>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Musgrave DS, Bosch P, Ghivizzani S, Robbins PD, Evans CH, Huard J. Adenovirusmediated direct gene therapy with bone morphogenetic protein-2 produces bone. Bone. 1999;24:541–547. doi: 10.1016/s8756-3282(99)00086-1. [DOI] [PubMed] [Google Scholar]
- 21.Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, Galun E, Gazit Z, Jorgensen C, Gazit D. Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther. 2004;9:587–595. doi: 10.1016/j.ymthe.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Osawa K, Okubo Y, Nakao K, Koyama N, Bessho K. Osteoinduction by repeat plasmid injection of human bone morphogenetic protein-2. J Gene Med. 2010;12:937–944. doi: 10.1002/jgm.1515. [DOI] [PubMed] [Google Scholar]
- 23.Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, McCauley LK, Davidson BL, Roessler BJ. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996;93:5753–5758. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang W, Carlsen B, Wulur I, Rudkin G, Ishida K, Wu B, Yamaguchi DT, Miller TA. BMP-2 exerts differential effects on differentiation of rabbit bone marrow stromal cells grown in twodimensional and three-dimensional systems and is required for in vitro bone formation in a PLGA scaffold. Exp Cell Res. 2004;299:325–334. doi: 10.1016/j.yexcr.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 25.Lee JW, Kang KS, Lee SH, Kim JY, Lee BK, Cho DW. Bone regeneration using a microste-reolithography-produced customized poly (propylene fumarate)/diethyl fumarate photopolymer 3D scaffold incorporating BMP-2 loaded PLGA microspheres. Biomaterials. 2011;32:744–752. doi: 10.1016/j.biomaterials.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 26.Park J, Lutz R, Felszeghy E, Wiltfang J, Nkenke E, Neukam FW, Schlegel KA. The effect on bone regeneration of a liposomal vector to deliver BMP-2 gene to bone grafts in peri-implant bone defects. Biomaterials. 2007;28:2772–2782. doi: 10.1016/j.biomaterials.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Sheyn D, Kimelman-Bleich N, Pelled G, Zilberman Y, Gazit D, Gazit Z. Ultrasoundbased nonviral gene delivery induces bone formation in vivo. Gene Ther. 2008;15:257–266. doi: 10.1038/sj.gt.3303070. [DOI] [PubMed] [Google Scholar]
- 28.Lou J, Xu F, Merkel K, Manske P. Gene therapy: adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 1999;17:43–50. doi: 10.1002/jor.1100170108. [DOI] [PubMed] [Google Scholar]
- 29.Laurencin CT, Attawia MA, Lu LQ, Borden MD, Lu HH, Gorum WJ, Lieberman JR. Poly (lactide-co-glycolide)/hydroxyapatite delivery of BMP-2-producing cells: a regional gene therapy approach to bone regeneration. Biomaterials. 2001;22:1271–1277. doi: 10.1016/s0142-9612(00)00279-9. [DOI] [PubMed] [Google Scholar]
- 30.Schek RM, Hollister SJ, Krebsbach PH. Delivery and protection of adenoviruses using biocompatible hydrogels for localized gene therapy. Mol Ther. 2004;9:130–138. doi: 10.1016/j.ymthe.2003.10.002. [DOI] [PubMed] [Google Scholar]


