Abstract
Objective:
To investigate whether the location and extent of the CT hyperdense artery sign (HAS) at presentation affects response to IV alteplase in the randomized controlled Third International Stroke Trial (IST-3).
Methods:
All prerandomization and follow-up (24–48 hours) CT brain scans in IST-3 were assessed for HAS presence, location, and extent by masked raters. We assessed whether HAS grew, persisted, shrank, or disappeared at follow-up, the association with 6-month functional outcome, and effect of alteplase. IST-3 is registered (ISRCTN25765518).
Results:
HAS presence (vs absence) independently predicted poor 6-month outcome (increased Oxford Handicap Scale [OHS]) on adjusted ordinal regression analysis (odds ratio [OR] 0.66, p < 0.001). Outcome was worse in patients with more (vs less) extensive HAS (OR 0.61, p = 0.027) but not in proximal (vs distal) HAS (p = 0.420). Increasing age was associated with more HAS growth at follow-up (OR 1.01, p = 0.013). Treatment with alteplase increased HAS shrinkage/disappearance at follow-up (OR 0.77, p = 0.006). There was no significant difference in HAS shrinkage with alteplase in proximal (vs distal) or more (vs less) extensive HAS (p = 0.516 and p = 0.580, respectively). There was no interaction between presence vs absence of HAS and benefit of alteplase on 6-month OHS (p = 0.167).
Conclusions:
IV alteplase promotes measurable reduction in HAS regardless of HAS location or extent. Alteplase increased independence at 6 months in patients with and without HAS.
Classification of evidence:
This study provides Class I evidence that for patients within 6 hours of ischemic stroke with a CT hyperdense artery sign, IV alteplase reduced intra-arterial hyperdense thrombus.
Arterial hyperattenuation on noncontrast CT, the hyperdense artery sign (HAS), is a consistently recognized CT sign of acute ischemic stroke.1 HAS is highly specific and moderately sensitive for intracranial arterial obstruction by thrombus.2 HAS is associated with increased stroke severity at presentation and worse long-term outcomes.3–5 There are, however, limited data on how the location, extent, or persistence of HAS relates to functional outcome following stroke, and importantly whether patients with (vs without) HAS benefit differently from IV thrombolysis with alteplase.6–9
The Third International Stroke Trial (IST-3) was a large (n = 3,035), multicenter, randomized controlled trial testing IV alteplase given within 6 hours of ischemic stroke.10 A central masked panel assessed prerandomization and follow-up CT for the presence of HAS.
We analyzed IST-3 imaging data to investigate whether, in this large, prospectively studied group of patients, the presence, location, and extent of HAS was associated with response to IV alteplase assessed as both change in HAS on short-term follow-up and also its effect on 6-month functional outcome.
METHODS
IST-3.
IST-3 was an international, multicenter, prospective, randomized, open, blinded endpoint (PROBE) trial of IV alteplase in acute ischemic stroke. Enrollment, data collection, image analysis, and CONSORT compliance have been described elsewhere.10,11 Briefly, adult patients with acute stroke of any severity, with no upper age limit, were eligible if IV alteplase could be started within 6 hours of stroke onset and CT or MRI had excluded intracranial hemorrhage and any structural stroke mimic. IST-3 used the uncertainty principle for enrollment, i.e., if the randomizing clinician believed that alteplase was clearly indicated or contraindicated, such a patient could not be enrolled; patients were only enrolled when there was genuine uncertainty over the benefit of alteplase for that individual.10,11 Stroke severity prior to randomization was assessed with the NIH Stroke Scale (NIHSS). After entry of baseline data, patients were randomized to receive IV alteplase (0.9 mg/kg) or control. Results were analyzed on an intention-to-treat basis. Patients were followed by postal questionnaire or masked telephone interview at 6 months and functional status assessed with the Oxford Handicap Scale (OHS).
Standard protocol approvals, registrations, and patient consents.
Ethical approval for IST-3 was granted by the Scotland A research ethics committee and by local ethics committees. Informed consent was obtained for all patients. IST-3 is registered with Current Controlled Trials, ISRCTN25765518.
Imaging protocol.
The IST-3 imaging protocol has been described previously.10,12 Briefly, CT scans were required to cover the brain from foramen magnum to vertex, with maximum slice thickness 4–5 mm through the posterior fossa and 8–10 mm for the cerebral hemispheres, with no slice gap. Thinner slices were also accepted. Scans were windowed on 80 Hounsfield units (HU) width and center level of 35–40 HU. Follow-up brain imaging (CT or MRI) was also required, to the same protocol, for all patients, between 24 and 48 hours after stroke onset. All imaging was reviewed centrally for quality control and validation.
Image analysis.
A centralized panel of neuroradiologists and neurologists experienced in reviewing stroke imaging analyzed all imaging with an online assessment tool, the Systematic Image Review System (SIRS), recording assessments on a validated structured pro forma1,13 available at www.sbirc.ed.ac.uk/research/imageanalysis.html, accessed November 23, 2015. Assessors were masked to all other imaging and clinical data. HAS presence was determined visually (i.e., the reader decided if a vessel appeared hyperdense; no objective HU measurements were made). Where present, the location and extent of HAS was recorded by selecting the 3 largest vessels affected from the following options: internal carotid artery (ICA), middle cerebral artery (MCA) mainstem, sylvian branches of MCA, anterior cerebral artery (ACA), posterior cerebral artery (PCA), vertebral artery, basilar artery. A subgroup of scans (n = 272) underwent a second independent read as part of the IST-3 angiography and perfusion imaging substudy.14 Secondary reads were performed by a different panel of assessors (see coinvestigator list on the Neurology® Web site at Neurology.org) using SIRS as described above. Scans that were read twice were used to test interrater reliability of HAS assessment.
Data analysis.
These analyses are restricted to patients with a noncontrast CT obtained prerandomization. The location of HAS was classified as proximal (ICA, MCA mainstem, vertebral or basilar arteries) or distal (ACA, PCA, or sylvian branches of the MCA). HAS extent was classified by the number of contiguous named vessels involved (0, 1, 2, or 3 as per the predefined options). We compared prerandomization and follow-up scans in all patients who had noncontrast CT performed at both times and calculated the change in HAS segment number from a minimum of −3 to a maximum of +3 (negative numbers indicate shrinkage and positive numbers indicate growth of HAS).
Statistics.
We used univariate and multivariate tests to examine differences between all IST-3 patients and the subset who were HAS-positive prerandomization, between the treatment and control groups with HAS, and for associations among the presence, extent, location, and persistence of HAS, baseline clinical features, and effect of alteplase.
For univariate analysis of parametric data, we used t tests to compare ratios and means; for nonparametric data, we used Mann-Whitney U tests. We calculated χ2 statistics for dichotomous data. We used Krippendorff α (K-α) to test interrater reliability. K-α results range from −1.0 to +1.0 where +1.0 equates to perfect agreement, 0.0 means no agreement, and −1.0 implies perfect disagreement.15
We used multivariate ordinal regression to calculate common odds ratios (ORs) with 6-month OHS and change in HAS segment number as dependent variables.16 We tested the effect of HAS presence, extent, and location on outcome in separate models. Consistent with the main IST-3 analyses,10,17 multivariate models were adjusted for the effect of patient age, NIHSS, and time from stroke onset to prerandomization scan since these variables predicted outcome in the main trial.10 To reduce confounding between HAS extent and location on outcome, the effect of location was assessed only among those with HAS in a single arterial segment. To stabilize ordinal regression estimates, we used the same approach as the original IST-3 analysis of functional outcome, where the more severe grades of OHS (4–6) were grouped as one, leaving 5 ordinal analysis groups.17 Similarly, the variable time from stroke onset to prerandomization scan was grouped into 6 1-hour windows (0–6 hours), the variable time from prerandomization scan to follow-up scan was grouped into 5 12-hour windows (≤12, 13–24, 25–36, 37–48, and >48 hours) and the variable change in HAS segment number was grouped into 3 outcomes (fewer segments = shrinkage, no change, more segments = growth).
We performed tests of interaction (using Comprehensive Meta-Analysis software; Biostat, Englewood, NJ) to compare ORs for the effect of IV alteplase on change in HAS extent in distal vs proximal HAS and in 1 segment vs >1 segment HAS and to compare the effect of alteplase on outcome in those with vs those without HAS.
All other analyses were performed with IBM SPSS statistics software, version 20.0 (IBM Corporation, Armonk, NY). We considered p < 0.05 significant.
Primary research question.
To assess the response of HAS to IV alteplase and to determine if the presence, location, extent, or persistence of HAS modified the effect of alteplase on outcome.
Classification of evidence.
This study provides Class I evidence that IV alteplase (0.9 mg/kg) given within 6 hours of ischemic stroke onset accelerates shrinkage of HAS at follow-up 24–48 hours later.
RESULTS
Demographics and clinical and outcome data.
Most IST-3 patients had noncontrast CT performed both prerandomization (2,961/3,035, 97.6%) and at follow-up (2,779/3,035, 91.6%) 24–48 hours later. MRI was the imaging method in 56 and 151 patients, respectively. Prerandomization or follow-up CT scans were not available for central review in 18 (0.6%) and 105 cases (3.5%), respectively. Thus, the total number of patients with centrally reviewed CT both prerandomization and at follow-up was 2,731 (90.0% of 3,035). HAS data were missing for one follow-up CT (poor quality). There were no significant differences in the demographic and clinical data for the 2,961 patients with centrally reviewed prerandomization CT, nor for the 2,731 patients who also had follow-up CT when both groups were compared with 3,035 patients in the full IST-3 trial (data not shown).
The results of interrater reliability are as follows: for HAS identification, K-α = 0.40; for assessment of the largest artery involved, K-α = 0.46; and for assessment of HAS extent, K-α = 0.39.
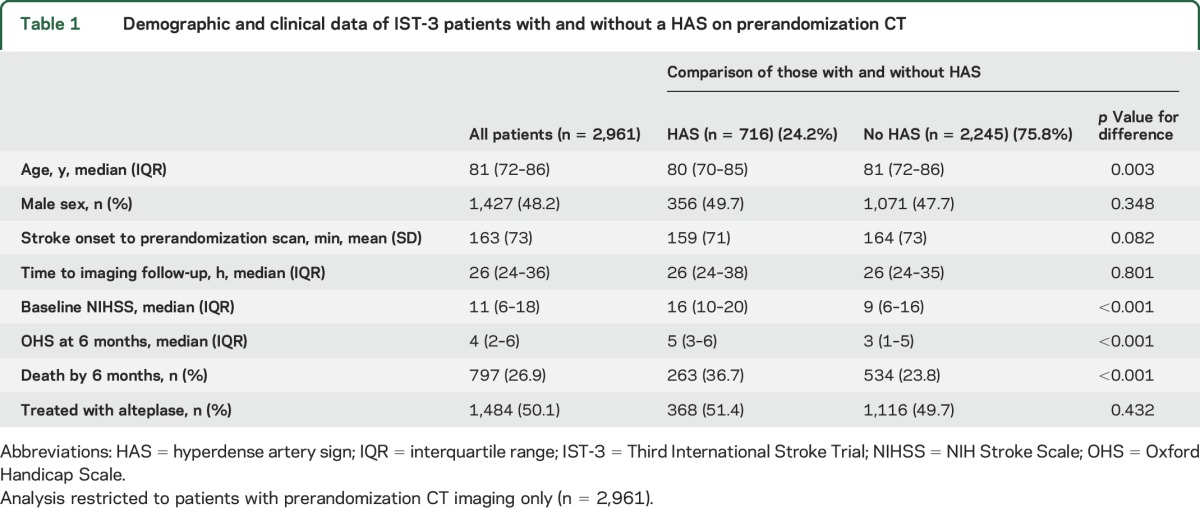
Of 2,961 patients with prerandomization CT, 716 (24.2%) demonstrated HAS. On univariate analysis, patients with baseline HAS were younger and had a more severe stroke and worse 6-month outcomes than those without HAS (table 1). Among patients with HAS, those allocated control had an increased median time between prerandomization and follow-up scans compared with patients allocated alteplase. None of the other demographic or clinical measures in patients with HAS were significantly different between alteplase and control groups (table e-1).
Table 1.
Demographic and clinical data of IST-3 patients with and without a HAS on prerandomization CT
For the multivariable ordinal analysis testing prerandomization variables associated with 6-month outcome in the whole group (OHS as dependent variable, n = 2,961), alteplase treatment allocation independently predicted a better outcome (lower OHS); OR 1.29, 95% confidence interval (CI) 1.12–1.50, p = 0.001. In the same analysis, the presence vs absence of HAS independently predicted a worse outcome (higher OHS, figure e-1); OR 0.66, 95% CI 0.55–0.80, p < 0.001. These results are adjusted for age, NIHSS, and time from stroke onset to scan (table 2 and figure 1).
Table 2.
Multivariable analyses testing the importance of HAS presence, extent, and location on 6-month outcome
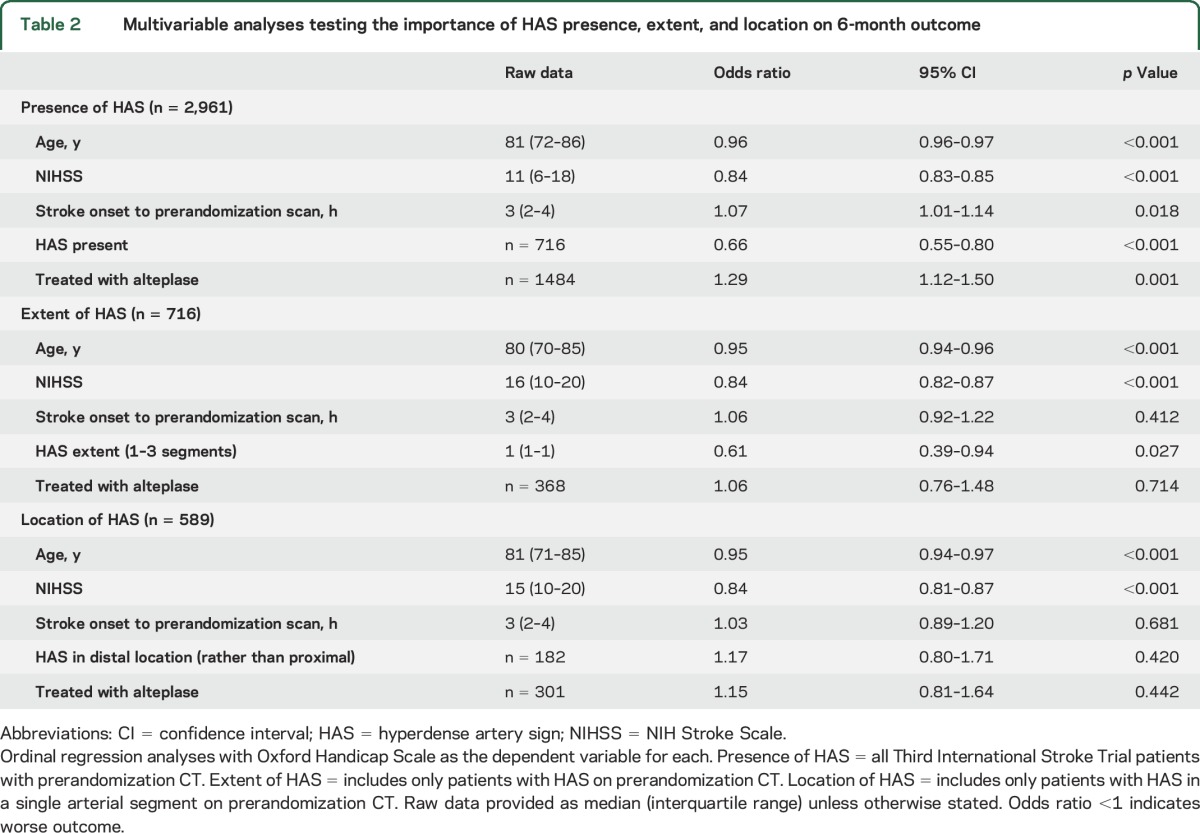
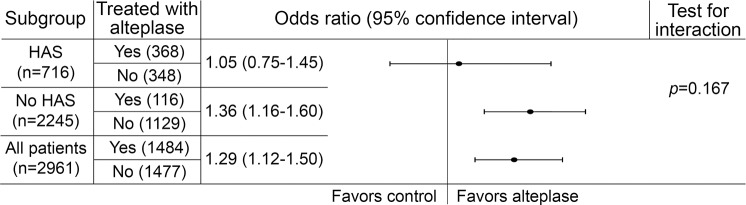
Figure 1. Separate ordinal regression analyses show odds ratios for the effect of alteplase treatment on 6-month functional outcome in the full group (n = 2,961) and in the subgroups with and without a hyperdense artery sign (HAS) on prerandomization CT.
Odds ratio >1 (right of line) indicates better outcome (lower 6-month Oxford Handicap Scale). Results for the HAS and no HAS groups are adjusted for the effect of age, time from stroke onset to scan (hours), and NIH Stroke Scale (NIHSS) score. Results for the full group are adjusted for the effect of age, time from stroke onset to scan (hours), NIHSS, and presence/absence of HAS.
Location, extent, and persistence of HAS.
For 2,731 patients with CT performed prerandomization and at follow-up, HAS was identified on 674 (24.7% of 2,731) prerandomization and 520 (19.0%) follow-up scans. In 870 cases (31.9%), HAS was evident on at least one scan. On univariate analyses, 6-month outcome was worse if HAS was found only in proximal rather than only in distal vessels, if HAS was more vs less extensive, if HAS showed growth rather than shrinkage between scans, if HAS persisted at follow-up rather than disappeared, and if a new HAS developed between scans (table e-2).
For multivariate analyses testing the effect of HAS characteristics on outcome (OHS as dependent variable) among patients with HAS in the whole group (n = 716), HAS extent (1, 2, or 3 segments) independently predicted a worse outcome (higher OHS); OR 0.61, 95% CI 0.39–0.94, p = 0.027. In patients with HAS in only 1 arterial segment (n = 589), HAS location (proximal vs distal) was not independently associated with outcome, OR 1.17, 95% CI 0.80–1.71, p = 0.420. These analyses are adjusted for age, NIHSS, time from stroke onset to scan, and treatment allocation (table 2).
Effect of alteplase on HAS.
There were univariate associations between alteplase treatment and an increased likelihood of both HAS shrinkage and HAS disappearance (222/440 = 50.5% vs 166/429 = 38.7% shrank, χ2 = 12.6, p = 0.002, and 198/350 = 56.6% vs 151/323 = 46.7% disappeared, χ2 = 6.5, p = 0.011, respectively). In patients treated with alteplase, distal vs proximal HAS (65/86 = 75.6% vs 105/198 = 53.0%, respectively, χ2 = 12.7, p < 0.001) and single segment vs multisegment HAS (170/284 = 59.9% vs 28/66 = 42.4%, respectively, χ2 = 6.6, p = 0.010) were more likely to have disappeared at follow-up. Fewer patients who received alteplase had developed a new HAS in the interim between pre-randomization and follow-up CT, although the difference was not significant (90/957 = 9.4% vs 106/904 = 11.8%, χ2 = 2.2, p = 0.143).
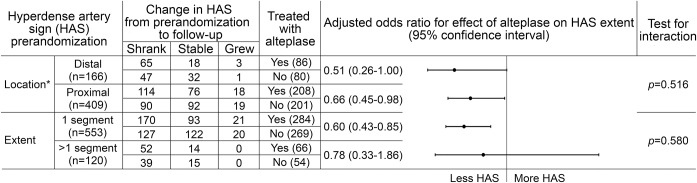
For multivariate analyses, testing factors associated with change in HAS segment number (dependent variable), alteplase treatment was an independent predictor of HAS shrinkage at follow-up (OR 0.77, 95% CI 0.65–0.93, p = 0.006), while HAS growth was more likely in older patients (OR 1.01, 1.00–1.02, p = 0.013) (table 3). Both proximal and distal HAS were equally likely to shrink following alteplase treatment (OR 0.66, 95% CI 0.45–0.98, and OR 0.51, 95% CI 0.26–1.00, respectively), with no evidence of an interaction between HAS location and alteplase effect (p = 0.516) (figure 2). HAS affecting a single arterial segment prerandomization was more likely to shrink following alteplase treatment (OR 0.60, 0.43–0.85), but alteplase was not an independent predictor of HAS shrinkage if more than 1 segment was affected prerandomization (OR 0.78, 95% CI 0.33–1.86). Nevertheless, there was no evidence of an interaction between HAS extent and alteplase effect (p = 0.580) (figure 2). These results are adjusted for patient age, time (between prerandomization and follow-up scans), and stroke severity.
Table 3.
Factors associated with change in extent of HAS from prerandomization to follow-up scan (n = 2,730a)
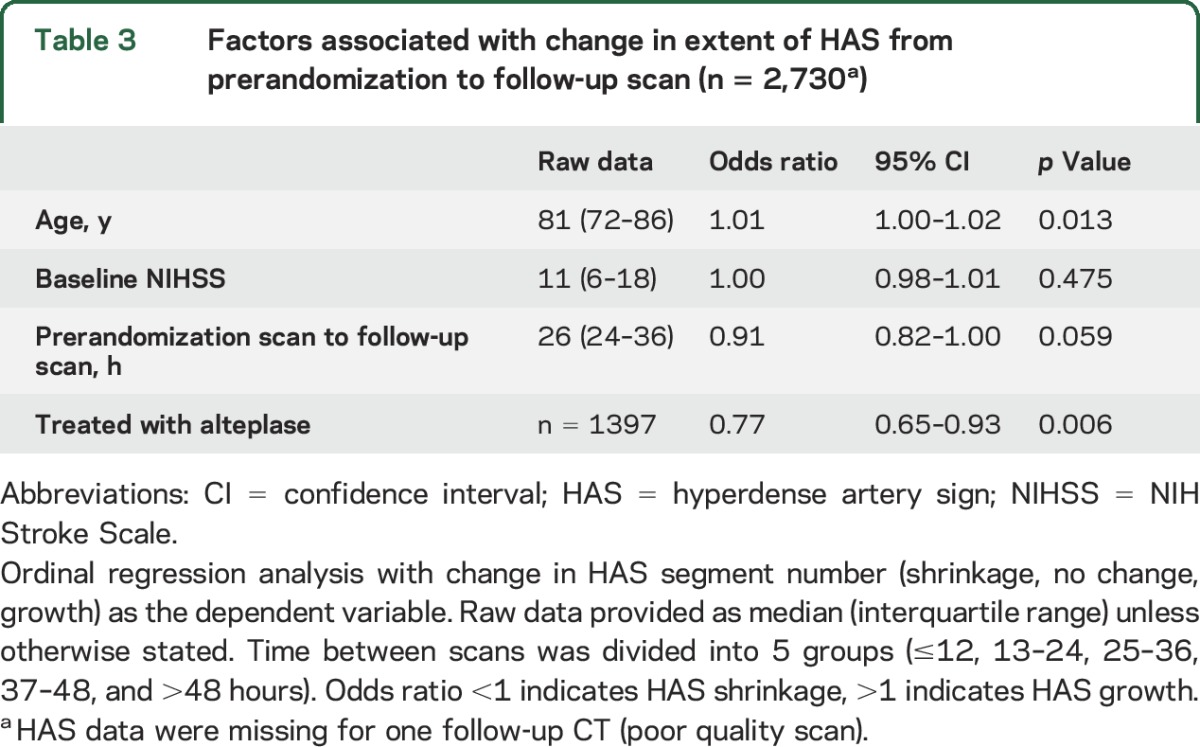
Figure 2. Ordinal regression analyses for the effect of treatment on change in HAS extent from prerandomization to follow-up scan.
Ordinal regression analyses with change in HAS segment number (shrinkage, no change, growth) as the dependent variable assessing the importance of prerandomization HAS location (proximal = internal carotid artery, middle cerebral artery mainstem, vertebral or basilar arteries; distal = anterior or posterior cerebral arteries or sylvian branches of the middle cerebral artery) and HAS extent on the effect of alteplase. *Location analysis does not include patients with HAS in both proximal and distal arteries. Odds ratio <1 (left of line) indicates HAS shrinkage, >1 (right of line) indicates HAS growth. Results are adjusted for the effect of age, time from stroke onset to scan (hours), and NIH Stroke Scale score.
Effect of alteplase on patient outcome in those with HAS.
Results of multivariate analysis of alteplase effect on outcome (OHS as dependent variable) for the subgroups with (OR 1.05, 0.75–1.45) and without (OR 1.36, 1.16–1.60) HAS on prerandomization CT are shown in figure 1. There was no evidence of an interaction with the presence vs absence of HAS (p = 0.167).
DISCUSSION
In this analysis of IST-3, we show that IV alteplase both reduces the persistence (more likely to shrink, or disappear) and limits the formation (less likely to grow) of HAS compared to patients allocated control. We found that alteplase increases HAS shrinkage/disappearance independent of age, baseline stroke severity, and time between prerandomization and follow-up scans. While HAS disappearance following treatment with alteplase was more common for small or distal vs large or proximal HAS on univariate analysis, the alteplase effect on HAS reduction/shrinkage was similar across all subgroups on multivariate regression analysis. We found no difference in the likelihood of HAS shrinkage for proximal vs distal HAS or for single segment vs multisegment HAS, i.e., alteplase reduces all HAS but is less able to remove the larger volume completely, usually more proximally sited HAS. We should note that alteplase treatment did not independently predict reduction of multisegment HAS but there was a trend toward such an association and this analysis is underpowered. Our findings are particularly relevant given recent evidence supporting the use of endovascular clot retrieval in ischemic stroke18–20 since not all patients will have the opportunity to receive, or be eligible for, such treatment. In addition, we and others7,8 have shown improved patient outcome where HAS shrinks or disappears with IV alteplase. Conversely, increasing patient age was an independent predictor of HAS growth, which may help to explain poorer stroke outcomes in the elderly.
Our work demonstrates a novel association between IV thrombolysis and measurable growth/shrinkage of HAS using randomized controlled trial data. We found no evidence that presence or absence of HAS materially altered the benefit of alteplase (the favorable shift in 6-month OHS). While the ordinal regression results for the HAS group alone are nonsignificant, this subgroup is underpowered and should be interpreted with caution. The appropriate interpretation is that there is no evidence of a difference in treatment effect between those with and without HAS at baseline, not that alteplase has no effect in those with HAS. To understand the apparent benefit of alteplase in patients without HAS, the presence of undetected thrombosis may be an answer; a recent report demonstrated benefit from thrombolysis for patients without apparent arterial obstruction on angiography.21
In IST-3, HAS was present in approximately 25% of patients within 6 hours of acute stroke. This is consistent with other published large series using similar scanning protocols22,23 although HAS prevalence rates of 47%–59% were reported in smaller studies.24,25 We confirmed that patients with HAS have more severe strokes and worse 6-month outcomes than patients without HAS. The effect of HAS presence on outcome is independent of age, time from stroke onset, stroke severity, and treatment with IV alteplase, i.e., patients with and without HAS both benefit from alteplase. Other authors have demonstrated that HAS independently predicts outcome,8,26,27 but our work also demonstrates that more extensive HAS independently predicts worse outcome among patients with the sign. We did not demonstrate an independent association between HAS location and outcome despite highly significant univariate results as demonstrated elsewhere9; however, our multivariate analysis of outcome by HAS location is limited by the exclusion of those HAS affecting more than one arterial segment and may be underpowered.
Our study has limitations. First, some CT scans were not thin-section but prevalence rates for HAS are greater with thin-slice protocols.2 Our prerandomization HAS prevalence may therefore be an underestimate. Second, our estimates of interrater reliability ranged from K-α 0.39 to 0.46. Prior studies have reported kappa (numerically equivalent to K-α) for the identification of HAS in the 0.36–0.91 range, but these studies rarely involved more than 2 readers, whereas our panel consisted of 10 individuals masked to correlative clinical and imaging data.2,28 Third, we did not define HAS based on HU measurements. Our central image analysis was performed qualitatively to reflect acute stroke care. A recent cohort study demonstrated that following IV alteplase, ischemic stroke patients with persistent arterial occlusion had lower mean density of thrombus compared to those who achieved recanalization.29 We are assessing the effect of thrombus density on outcome in IST-3 in separate analyses. Fourth, we estimated hyperdensity extent based on the number of arterial segments affected. A volumetric measurement may have provided a more accurate assessment of changes in HAS volume. However, it remains unclear if HAS volume can be measured reliably. Our method of assessing thrombus extent is relevant to daily practice. Fifth, we used HAS disappearance as a surrogate indicator of arterial recanalization in the absence of angiographic data for all patients. We are analyzing available angiography data from IST-3 in a separate analysis. Sixth, multivariate models were adjusted only for variables that were associated with outcome in the main IST-3 analysis10 and for the variable time from stroke onset to scan since this latter variable would likely affect the appearance of HAS. Seventh, the limitations of the full IST-3 trial have been previously discussed10 including use of the uncertainty principle for enrolment and the potential introduction of bias through the adoption of an open design. Finally, even with 3,000 patients, IST-3 has insufficient power to reliably explore interactions between HAS and the effect of alteplase, but we found little evidence that patients with HAS responded differently to alteplase than those without HAS.
We confirmed, using data from a large randomized controlled trial, associations between the presence and extent of HAS and poor 6-month outcomes. We show clearly that alteplase accelerates shrinkage of HAS, regardless of HAS location and extent. Furthermore, we found no evidence that the improvement in functional outcome following alteplase was materially different in the presence or absence of HAS. Therefore, while HAS is useful to support a diagnosis of acute ischemic stroke and helps predict outcome, our data suggest that the presence or absence of the sign should not preclude the use of IV alteplase where clinically appropriate.
Supplementary Material
ACKNOWLEDGMENT
The IST-3 collaborative group thanks the patients who participated in the study.
GLOSSARY
- ACA
anterior cerebral artery
- CI
confidence interval
- HAS
hyperdense artery sign
- HU
Hounsfield units
- ICA
internal carotid artery
- IST-3
Third International Stroke Trial
- MCA
middle cerebral artery
- NIHSS
NIH Stroke Scale
- OHS
Oxford Handicap Scale
- OR
odds ratio
- PCA
posterior cerebral artery
- SIRS
Systematic Image Review System
Footnotes
Supplemental data at Neurology.org
Contributor Information
Collaborators: IST-3 Collaborative Group, Peter Sandercock, Richard I Lindley, Joanna M Wardlaw, Colin Baigent, David Chadwick, Pippa Tyrrell, Gordon Lowe, Martin Dennis, Geoff Cohen, Karen Innes, Heather Goodare, Richard I Lindley, Graeme J Hankey, Karl Matz, Michael Brainin, Gord Gubitz, Stephen J Phillips, Stefano Ricci, Antonio Arauz, Eivind Berge, Karsten Bruins Slot, Anna Czlonkowska, Adam Kobayashi, Manuel Correia, Phillippe Lyrer, Stefan Engelter, Veronica Murray, Andreas Terent, Bo Norrving, Per Wester, Graham Venables, Joanna M Wardlaw, Andrew Farrall, Zoe Morris, Rüdiger von Kummer, Lesley Cala, Anders von Heijne, Alessandro Adami, Andre Peeters, Gillian Potter, Nick Bradey, Joanna M Wardlaw, Rüdiger von Kummer, Andrew Farrall, Robin Sellar, Alessandro Adami, Philip White, Andrew Demchuk, Matthew Adams, Grant Mair, Bernard Yan, G Venables, C Blank, H Bowler, C Doyle, K Endean, K Harkness, E Parker, M Randall, C Roffe, N Ahmad, A Arora, S Brammer, J Chembala, B Davies, S Ellis, E Epstein, K Finney, C Jackson, C Jadun, R Kinston, H Maguire, I Memon, I Natarajan, M Poulson, R Sanyal, S Sills, A Vreeburg, E Ward, P Sandercock, R Al-Shahi Salman, R Davenport, M Dennis, P Hand, S Hart, I Kane, S Keir, M MacLeod, L McKinlay, H Milligan, E Sandeman, J Stone, C Sudlow, P Taylor, J Wardlaw, C Warlow, W Whiteley, A Williams, M Brown, B Athwal, V Bassan, N Bhupathiraju, J Bowler, C Davie, D Doig, R Erande, S Gilbert, L Ginsberg, R Greenwood, S Gregoire, N Harding, N Losseff, R Luder, N Passeron, R Perry, P Rayson, R Simister, S Stone, D Werring, J Barrett, H Aitken, S Cherian, R Davis, S Downham, L Godd, V Gott, D Jose, V Little, D Lowe, L Luxford, M McGrory, P Owings, N Price, J Richards, G Sangster, J Sherlock, S Vargese, I Wakefield, P Weir, P Guyler, T Attygale, S Chandler, L Coward, S Feasey, C Khuoge, T Loganathan, S Martin, A O'Brien, D Sinha, V Thompson, S Tysoe, R Walsh, K Metcalf, J Cochius, R Fulcher, N Gange, C Green, J Jagger, M Lee, P Myint, J Potter, G Ravenhill, S Shields, N Shinh, T Staunton, E Thomas, W Woodward, P Worth, N Wyatt, W Sunman, P Bath, P Berman, J Clarke, C Gaynor, F Hammonds, R Harwood, K Mitchell, S Munshi, S Pacey, A Shetty, N Sprigg, H Stear, G Subramanian, A Wills, A Rudd, H Audebert, A Bhalla, J Birns, R Chowdhury, G Cluckie, I Davies, C Gibbs, P Holmes, N Mitchell, F Schiavone, E White, M Yeung, A Mehrzad, V Baliga, E Brown, L Burnside, B Esisi, J Kent, P Orr, D Stead, E Wayman, R Durairaj, C Cullen, R Kumar, H Martin, D McDowell, A Sharma, V Sutton, R White, T Hughes, K Ali, J Anderson, K Baker, K Bethune, K Bethune, M Booth, M Cossburn, S Halpin, M Hourihan, E Marsh, K Peall, R Powell, H Shetty, M Wardle, M Williams, K Muhiddin, J Beavan, M Clarke, R Donneley, S Elliott, P Fox, P Gorman, M Harper, M Mangoyana, I Memon, L Mills, L Wright, L Warburton, J Baron, P Barry, D Day, T Harold, P Martin, J Mitchell, E O'Brien, J Rycarte, M Turnham, G Cloud, L Choy, B Clarke, C Griffin, O Halse, I Jones, F Kennedy, U Khan, R Lewis, A Loosemore, C Lovelock, H Markus, B Moynihan, J O'Reilly, O Paul, A Pereira, M Punter, P Rich, D Rolfe, F Schiavone, M James, J Bell, A Bowring, L Boxall, J Cageao, H Eastwood, S Elyas, F Hall, S Harries, A Hemsley, S Jackson, S Keenan, P Mudd, A Sekhar, D Strain, J Sword, N Wedge, M MacLeod, M Bruce, A Joyson, M Kemp, K McMullan, J Reid, O Robb, J Webster, S Wilkinson, P Sharma, P Bentley, H Jenkins, A Kar, T Sachs, D Cohen, R Bathula, J Devine, M Mpelembue, D Hargroves, I Balogun, L Cowie, A Maidment, D Rand, J Rowe, H Rudenko, D Smithard, L Wray, J Paterson, J Brown, J Hampton, S Jamieson, R Rose, A Volans, K Chatterjee, G Abbott, R Brookes, C Castle, C Kelly, S Leason, A Nallasivan, A Sen, D Collas, M Cottle, N Damani, P Jacob, D Oza, D Werring, A Kenton, N Adab, L Aldridge, H Allroggen, Y Brown, R Cross, L Galvin, K Ghosh, A Grubneac, A Lindahl, H Mehta, M Pritchard, C Randall, P Ray, A Shehu, S Thelwell, D Jenkinson, J Bell, T Black, O David, J Kwan, A Orpen, C Ovington, D Tiwari, Z ud Din Babar, A Hassan, A Bailey, J Bamford, C Bedford, R Bellfield, J Cooper, L Dunsmure, J Greig, M Keeling, L Mandizvidza, J Rankine, E Roberts, P Wanklyn, T Webb, S Williamson, J Coyle, S Crane, C Croser, P Duffey, R Evans, E Iveson, M Keeling, G Kitching, M Porte, C Rhymes, D Barer, M Armstrong, M Bokhari, T Cassidy, B McClelland, G Gunathilagan, P Dolke, S Jain, S Jones, A Maidment, L Rosser, G Thomas, C White, P Sanmuganathan, C Scholtz, E Stratford, M O'Donnell, H Goddard, G Hoadley, J Howard, S Leach, J McIlmoyle, A Stewart, A Strain, F Huwez, P Croot, N Gadi, N Mguni, U Umasankar, G Mead, B Chapman, A Coull, S Hart, A Kinnear, B Morrow, F Morrow, D Ames, J Ball, S Bannerjee, J Chataway, K Rashed, C Buckley, D Donaldson, D Hayward, C Lawson, L Sekaran, K Bharaj, F Justin, G Jutlla, D Phiri, S Sethuraman, M Tate, D Sandler, P Carr, G Jones, J Lyons, K Warren, L Kalra, A Davis, J Jarosz, D Manawadu, L Sztriha, D Chadha, A Holford, P Willcoxson, L Shaw, D Button, A Cunningham, L Dow, J Dutson, T Hall, C Hardy, N Jakeman, P Kaye, B Madigan, K O'Brien, D Pressdee, M Price, L Robinson, C Taylor, D Williamson, D Sandler, P Carr, J Lyons, J McCormack, C Stretton, P Earnshaw, E Brown, S Bruce, C Church, S Desai, B Esisi, M Myint, N Watt, C Price, S Elliott, H Graham, R Lakey, K Mitchelson, P Murphy, L Ball, S Caine, J Dovey, J Hughes, A Steele, K Dizayee, A Brown, T Chattopadhyay, J Cheetham, H Cochrane, A Datta, M Datta-chaudhuri, C Fox, D Kilroy, S Krishnamoorthy, F Levy, S Metha, P Ngoma, B Venkatesh, K Ali, R Gautam, N Henderson, M Jones, S Murphy, G Spurling, I Wiggam, C Boyd, K Fullerton, P Gray, M Kinnaird, S MacNair, C Morgan, M Reid, S Tauro, S Loharuka, D Balmforth, P Cox, G Fletcher, A Ledger, A Manoj, M Wilkinson, D Nicholl, S Clegg, S Hurdowar, S Kausar, K Law, A Singal, S Sturman, P Gompertz, J Evanson, A Farrell, A Petrou, K Saastamoinen, T Sachs, A Salek-Haddadi, R Yadava, J O'Connell, H Brew, S Butler, S Crawford, C Gray, D Gulliver, N Majmudar, R O'Brien, M Wani, L Dacey, L Davies, R Evans, D Harris, T Jones, S Storton, S Punekar, A Ashton, S Duberley, H Emsley, C Gilmour, B Gregary, L Hough, S Philip, S Wuppalapati, K Fotherby, P Bourke, D D'Costa, K Kauldhar, D Leung, R Lodwick, S McBride, D Morgan, M Qaiyum, G Sahota, M Srinvasan, I Kane, N Chuter, L Garrad, M Hookway, S Ivatts, G Kennedy, K Darawil, L Al Dhahirl, S Andole, M Baig, P Dugh, K Dunne, H Kariuki, M Khan, S Rathnayaka, M Power, K Dynan, J Finnerty, A Heaney, C Leonard, K McKnight, J Turkington, B Wroath, B Dewan, S Cotton, M Gardiner, T Saunders, B Vincent, D Sims, P Guest, E Jones, J McCormack, D Nicholl, J Savanhu, R Tongue, M Willmot, D Eveson, S Dawson, M Dickens, M Fotherby, R Hunt, S Khan, T Kumar, R Marsh, A Mistri, T Robinson, J Thompson, P Aghoram, T Daniel, M Gatehouse, S Hussein, A Jackson, T Shanganya, E Strachan, G Tan, B Richard, S Elaine, S Hanson, S Mosely, H Reed, M Williams, R Saksena, S Cook, D Demuran, M Keating, R Needle, V Paramsothy, A Sebastian, R Sivakumar, A Wright, R Grue, E Barberan, C Dickson, C Douglas, J Jellicoe, T Marsden, J Priestley, E Quick, C Sherrington, A Singh, C Smith, J Stevens, P Tyrell, J Wainwright, M Ardron, J Birchall, R Shekhar, C Barsted, S Coleman, S Fletcher, J Graham, A Buchan, J Hinkle, J Kennedy, A Manoj, M Westwood, A Mohd Nor, S Allder, B Hyams, A Pace, E Orugun, C Brewer, L Huntley, R Jolly, C Summers, K Sharobeem, J Khaira, J Leahy, E Linehan, G Moore, J Rizkalla, J Wilkinson, D Kelly, C Hilaire, O Otaiku, L Connell, G Delaney-Sagar, G James, L Lomax, D Matthew, J Simpson, H Whittle, S Sanmuganathan, S Burrows, A Mahmood, G Durward, S Barker, J Cantle, P Crawford, S Evans, V Pressly, N Weir, V Cvoro, K McCormick, A Czlonkowska, J Bembenek, M Bilik, G Chabik, W Czepiel, J Dzierka, M Gluszkiewicz, K Grabska, B Janus-Laszuk, J Jedrzejewska, A Kobayashi, T Litwin, A Oskedra, A Piorkowska, M Skowronska, A Sliwinska, U Stepien, P Sobolewski, A Gajewska, M Grzesik, R Hatalska-Zerebiec, I Labudzka, B Loch, A Medrykowska, M Sledzinska, A Sobota, W Szczuchniak, G Wolak, I Zdyb, W Nyka, D Gasecki, K Chwojnicki, A Gojska, B Karaszewski, G Kozera, M Kwarciany, M Nowak, M Swierkocka-Miastkowska, S Szczyrba, M Wisniewska, E Wnorowska, P Richter, A Bochynska, M Chahwan, A Graban, R Rola, A Stepien, B Brodacki, M Grotowska, J Kotowicz, J Staszewski, J Swistak, S Zaloga, J Stoiñski, K Czajkowaka-Fornal, P Czubak, A Kaczor, J Kraska, E Nowakowska-Sledz, J Ozdoba-Rot, E Zawadzka, M Fudala, D Adamczyk, W Brola, I Guldzinska, K Kaluzny, M Kucharska-Lipowska, M Mosiolek, M Polewczyk, M Ziomek, G Opala, M Arkuszewski, M Kudlacik, P Malgorzata, M Swiat, W. Orlowskiego, U Fiszer, M Lenska-Mieciek, S Cenciarelli, A Barilaro, R Condurso, F Coppola, S Dioguardi, E Gallinella, A Mattioni, C Menichetti, S Ricci, F Casoni, M Bacchelli, M Cavazzuti, M Malagoli, A Zini, G Benemio, M Celani, R Allegrucci, V Bondo, S Cupella, L Guerra, S Guerrieri, C Ottaviani, E Righetti, C Rossi, N Sacchi, M Scucchi, V Stefanini, T Mazzoli, A Bigaroni, L Greco, R Paris, P Parise, S Ricci, A Ciccone, L Basso, R Causarano, P Doneda, E Ferrante, A Gatti, A Guccione, A Gullo, F Imbesi, S Jann, R Marazzi, E Moro, C Motto, D Parodi, A Protti, M Riva, A Rosiello, I Santilli, R Sterzi, P Tiraboschi, G Venturelli, F Iemolo, R Campagna, G Campagnolo, A Carnemolla, N D'Apico, G D'Asta, S Giannarita, A Giordano, E Sanzar, E Bottacchi, S Cordera, G Corso, M Di Giovanni, G Giardini, C Lia, T Meloni, M Pesenti Campagnoni, P Tosi, A Adami, G Rossato, T Zuppini, G Procaccianti, T Sacquegna, C Gandolfo, M Balestrino, C Bruno, L Castellan, M Del Sette, A Ferrari, C Finocchi, N Reale, D Rizzi, M Del Sette, L Benedetti, C Capellini, E Carabelli, E Cibei, M Godani, G Guariglia, E Landini, A Mannironi, B Nucciarone, S Parodi, S Tonelli, E Traverso, D Zito, P Brustenghi, F Corea, O Flamini, S Lolli, G Pelliccia, R Ricci, S Stefanucci, M Zampolini, D Consoli, F Galati, P Postorino, G Rinaldi, E Carapelle, G Grilli, M Guido, L Specchio, N Checcarelli, G Borin, L Chiveri, R Clerici, E Corengia, L Gandola, P Garavaglia, M Guidotti, A Martegani, M Mauri, F Muscia, F Raudino, F Chiodo Grandi, A Bratina, N Carraro, M Gaio, A Granato, N Koscica, M Naccarato, V Sarra, P Schincariol, C Vilotti, Z Zugna, D Idone, C Bonato, E De Angelis, A Forgione, M Gambera, F Recchia, S Tamburin, P Tinazzi Martini, G Zanette, Spoleto S Grasselli, G Agnelli, A Andrea, A Billecia, V Caso, V Casso, R Fabiola, P Fanelli, M Paciaroni, B Sergio, M Vemti, M Silvestri, L Altarini, A Bonfante, M Bonornetti, B Costa, N D'Attoma, N Deluca, F Frattini, R Niego, D Rafaele, V Ravenna, M Turazzini, E Lundström, L Jonsson, U Söderström, A Terént, V Murray, A Alvelius, M Arbin von, I Dalenbring, Å Doverhall, Å Franzén-Dahlin, N Greilert, M Hallberg, A Heijne von, E Isaksson, H Kumpulainen, A Laska, A Lundström, C Martin, J Muhrbeck, E Näslund, N Ringart, E Rooth, R Undén, P Waldenström, M Esbjornsson, M Petranek, B Cederin, E Bertholds, A Elgåsen, T Johansson, B Witteborn, M Kwiatkowska, E Gustafsson, T Noren, J Saaf, J Teichert, M Bertilsson, S Nilsson, S Oestberg, L Welin, K Fredricson, L Pehn, J Hambraeus, I Lonn, B Hojeberg, A Adolfsson, M Anzen, T Wallen, R Schloenzig, P Söderström, A Wennerberg, F Buchwald, K Abul-Kasim, A Berkeskold, J Petersson, E Poromaa, P Wester, R Backlund, A Sjöström, B Hedström, E Campbell, K Johnsson, B Karlsson, N Lekokotla, C Lundahl, A Risedal, P Sandgren, A Svensson, S Bysell, E Smedberg, A Vestberg Bysell, V Sjögren, B Högvall, G Andsberg, T Cronberg, A Lindgren, H Wannberg, F Ax, L Nyren, J Sanner, H Andersson, F Andler, S Holmgård, R Johansson, I Magnussan, K Nilsson, J Rådberg, B Indredavik, H Ellekjær, A Østvik, G Rohweder, D Steckhan, J Storvold, E Berge, Y Rønning, R Aakvik, K Bruins Slot, G Knutsen, M Moxness, R Pettersen, T Wyller, C Wahl, O Iversen, S Johnsen, B Norderhus, L Steffensen, E Stensland, T Asak, J Aaseth, T Rotnes, J Sparby, S Wetterhus, H Hallan, A Aardal, T Graven, H Hansbakk Skjetne, B Klykken, K Lindqvist, A Tommy, T Engstad, M Antonsen, R Bajic, W Fønnebø, S Hykkerud, I Lyngmo, A Nyrnes, S Rogne, S Sparr, O Kildahl-Andersen, K Pedersen, H Ulrichsen, O Skogen, I Alnes, R Hukari, Y Seljeseth, P Vadset, G Knutsen, B Fure, H Ihle-Hansen, N Johnsen, L Kornberg, S Schuler, M Heibert, M Lillebø, O Aasen, I Eskeland, T Hamre, S Hareide, H Helset, K Kolnes, B Lødemel, H Ose Velle, S Reite, E Velle, R Grimley, E Ahern, C Cocks, M Courtney, R Devin, J Endacott, C Fawcett, V Harrington, C Johnston, M Koltermann, S Murray, K Ng, G Styles, A Tampiyappa, C Levi, K Chung, L Dark, M Evans, Y Gawarikar, E Kerr, A Loiselle, F Miteff, A Moore, W O'Brien, M Parsons, D Quain, A Royan, M Russell, N Spratt, J Sturm, D Crimmins, D Griffiths, P Kavelieros, J Kinsella, A Malhotra, B O'Brien, A Schutz, M Webb, S Whyte, V Zenteno, R Lindley, A Bleasel, N Cordato, A Duggins, V Fung, L Gomes, N Ingham, J Ip, P Landau, J Morris, S Vucic, G Hankey, A Claxton, N Lillywhite, C Lueck, C Andrews, G Danta, C Das, I Harvey, A Hughes, C McColl, A Oon, R Tuck, S Read, M Badve, M Broad, G Cadigan, H Cavanagh, J Chalk, D Copsinis, K Etherington, R Henderson, R Hull, J O'Sullivan, J Pandian, L Ross-Lee, M Roxas, N Sheikh, G Skinner, A Wong, H Dewey, A Brodtmann, G Donnan, A Hughes, M Karonen, H Ma, T Mulcahy, S Petrolo, L Walker, D Young, J Zavala, M Thieben, C Harris, M Krause, S Lane, H Park, M Shaffi, J Wood, C Bladin, A Buckland, K Coughlan, B Coulton, A Gilligan, P Lee, S Mullen, Z Ross, P Sien Loh, C Szoeke, M Silva, F Afonso, J Gabriel, P Guimarães, A Velon, M Castelo-Branco, F Alvarez, V Branco, C Coxo, P Goulao, D Leal, S Morgado, R Oliveira, F Paiva, A Rodrigues, M Simoes, G Lopes, T Almeida, M Cardoso, J Chaves, C Correia, M Correia, J Damásio, R Felgueiras, J Pereira, A Tuna, C Ferreira, E Lourenco, A Machado, R Mare, J Rocha, A Peeters, K Matz, M Brainin, G Funk, V Reiner-Deitemyer, J Ferrari, A Flamm-Horak, G Gruber, R Rattinger, W Muellbacher, D Doppelbauer, R Kalchmayr, W Schima, T Wieser, M Zart, P Lyrer, L Bonati, S Engelter, F Fluri, S Muller, E Radue, A Tiemessen, L Walz, F Weisskopf, S Wetzel, A Luft, D Fetz, B Hertler, A Pangalu, G Gubitz, P Boulton, J Jarrett, J Moeller, S Phillips, A Arauz, L Bermudez, J Calleja, and R Garcia
AUTHOR CONTRIBUTIONS
Grant Mair: developed study concepts and design, analyzed data, prepared the manuscript, edited and agreed on the final manuscript. Rüdiger von Kummer: developed image analysis, analyzed imaging, prepared the manuscript, edited and agreed on the final manuscript. Zoe Morris: analyzed imaging, edited and agreed on the final manuscript. Anders von Heijne: analyzed imaging, edited and agreed on the final manuscript. Nick Bradey: analyzed imaging, edited and agreed on the final manuscript. Lesley Cala: analyzed imaging, edited and agreed on the final manuscript. André Peeters: analyzed imaging, edited and agreed on the final manuscript. Andrew J. Farrall: analyzed imaging, edited and agreed on the final manuscript. Alessandro Adami: analyzed imaging, edited and agreed on the final manuscript. Gillian Potter: analyzed imaging, edited and agreed on the final manuscript. Geoff Cohen: analyzed data, edited and agreed on the final manuscript. Peter A.G. Sandercock: developed study concepts and design, prepared the manuscript, edited and agreed on the final manuscript. Richard I. Lindley: developed study concepts and design, edited and agreed on the final manuscript. Joanna M. Wardlaw: obtained funding, developed study concepts and design, analyzed imaging, prepared the manuscript, edited and agreed on the final manuscript, guarantor of study.
STUDY FUNDING
The startup phase of IST-3 was supported by a grant from the Stroke Association, UK (TSA 04/99). The expansion phase was funded by the Health Foundation UK (2268/1282). The scan reading development was funded by Chest, Heart Stroke Scotland (R100/7). The main phase of the trial is funded by UK Medical Research Council (MRC) (grant numbers G0400069 and EME 09-800-15) and managed by NIHR on behalf of the MRC-NIHR partnership; the Research Council of Norway; Arbetsmarknadens Partners Forsakringsbolag (AFA) Insurances Sweden; the Swedish Heart Lung Fund; The Foundation of Marianne and Marcus Wallenberg, Stockholm County Council; Karolinska Institute Joint ALF-project grants Sweden; the Polish Ministry of Science and Education (grant number 2PO5B10928); the Australian Heart Foundation; Australian National Health and Medical Research Council (NHMRC); the Swiss National Research Foundation; the Swiss Heart Foundation; the Foundation for Health and Cardio-/Neurovascular Research, Basel, Switzerland; the Assessorato alla Sanita, Regione dell'Umbria, Italy; and, Danube University, Krems, Austria. Boehringer-Ingelheim GmbH donated drug and placebo for the 300 patients in the double-blind phase, but thereafter had no role in the trial. The UK Stroke Research Network (SRN study ID 2135) adopted the trial on 1/5/2006, supported the initiation of new UK sites, and in some centers, and, after that date, data collection was undertaken by staff funded by the network or working for associated NHS organizations. IST-3 acknowledges the support of the NIHR Stroke Research Network, NHS Research Scotland (NRS), through the Scottish Stroke Research Network, and the National Institute for Social Care and Health Research Clinical Research Centre (NISCHR CRC). The central imaging work was undertaken at the Brain Imaging Research Centre (www.sbirc.ed.ac.uk), a member of the Scottish Imaging Network: A Platform for Scientific Excellence (SINAPSE) collaboration (www.sinapse.ac.uk), at the Division of Clinical Neurosciences, University of Edinburgh. SINAPSE is funded by the Scottish Funding Council (SFC) and the Chief Scientist Office of the Scottish Executive (CSO). Additional support was received from Chest Heart and Stroke Scotland, DesAcc, University of Edinburgh, Danderyd Hospital R&D Department, Karolinska Institutet, Oslo University Hospital, and the Dalhousie University Internal Medicine Research Fund.
DISCLOSURE
G. Mair reports no disclosures relevant to the manuscript. R. von Kummer has received funding from Lundbeck, Covidien, Brainsgate, and Boehringer Ingelheim. Z. Morris, A. von Heijne, N. Bradey, and L. Cala report no disclosures relevant to the manuscript. A. Peeters has received funding from Boehringer Ingelheim. A. Farrall, A. Adami, G. Potter, and G. Cohen report no disclosures relevant to the manuscript. P. Sandercock has received funding from Boehringer Ingelheim. R. Lindley has received funding from Boehringer Ingelheim and Covidien. J. Wardlaw has received funding from Medical Research Council, Efficacy and Mechanisms Evaluation, Chest Heart Stroke Scotland. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Wardlaw JM, Farrall AJ, Perry D, et al. Factors influencing the detection of early computed tomography signs of cerebral ischemia: an Internet-based, international multiobserver study. Stroke 2007;38:1250–1256. [DOI] [PubMed] [Google Scholar]
- 2.Mair G, Boyd E, Chappell FM, et al. Sensitivity and specificity of the hyperdense artery sign for arterial occlusion in acute ischemic stroke. Stroke 2015;46:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kharitonova T, Ahmed N, Thorén M, et al. Hyperdense middle cerebral artery sign on admission CT scan: prognostic significance for ischaemic stroke patients treated with intravenous thrombolysis in the safe implementation of thrombolysis in Stroke International Stroke Thrombolysis Register. Cerebrovasc Dis 2009;27:51–59. [DOI] [PubMed] [Google Scholar]
- 4.Manelfe C, Larrue V, von Kummer R, et al. Association of hyperdense middle cerebral artery sign with clinical outcome in patients treated with tissue plasminogen activator. Stroke 1999;30:769–772. [DOI] [PubMed] [Google Scholar]
- 5.Novotna J, Kadlecova P, Czlonkowska A, et al. Hyperdense cerebral artery computed tomography sign is associated with stroke severity rather than stroke subtype. J Stroke Cerebrovasc Dis 2014;23:2533–2539. [DOI] [PubMed] [Google Scholar]
- 6.Nichols C, Khoury J, Brott T, Broderick J. Intravenous recombinant tissue plasminogen activator improves arterial recanalization rates and reduces infarct volumes in patients with hyperdense artery sign on baseline computed tomography. J Stroke Cerebrovasc Dis 2008;17:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kharitonova T, Thoren M, Ahmed N, et al. Disappearing hyperdense middle cerebral artery sign in ischemic stroke patients treated with intravenous thrombolysis: clinical course and prognostic significance. J Neurol Neurosurg Psychiatry 2009;80:273–278. [DOI] [PubMed] [Google Scholar]
- 8.Paliwal PR, Ahmad A, Shen L, et al. Persistence of hyperdense middle cerebral artery sign on follow-up CT scan after intravenous thrombolysis is associated with poor outcome. Cerebrovasc Dis 2012;33:446–452. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Davis S, Mitchell P, Dowling R, Yan B. Proximal hyperdense middle cerebral artery sign predicts poor response to thrombolysis. PLoS One 2014;9:e96123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The IST-3 Collaborative Group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 2012;379:2352–2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sandercock P, Lindley R, Wardlaw J, et al. Third International Stroke Trial (IST-3) of thrombolysis for acute ischaemic stroke. Trials 2008;9:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wardlaw JM, Sandercock PAG, Lindley RI, et al. Association between brain imaging signs, early and late outcomes, and response to intravenous alteplase after acute ischaemic stroke in the third International Stroke Trial (IST-3): secondary analysis of a randomised controlled trial. Lancet Neurol 2015;14:485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wardlaw JM, von Kummer R, Farrall AJ, Chappell FM, Hill M, Perry D. A large web-based observer reliability study of early ischaemic signs on computed tomography: the Acute Cerebral CT Evaluation of Stroke Study (ACCESS). PLoS One 2010;5:e15757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wardlaw JM, von Kummer R, Carpenter T, et al. Protocol for the perfusion and angiography imaging sub-study of the third International Stroke Trial (IST-3) of alteplase treatment within six hours of acute ischemic stroke. Int J Stroke. Epub 2013. [DOI] [PubMed]
- 15.Hayes AF, Krippendorff K. Answering the call for a standard reliability measure for coding data. Commun Methods Meas 2007;1:77–89. [Google Scholar]
- 16.Roozenbeek B, Lingsma HF, Perel P, et al. The added value of ordinal analysis in clinical trials: an example in traumatic brain injury. Crit Care 2011;15:R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sandercock P, Lindley R, Wardlaw J, Whiteley W, Murray G; IST-3 Collaborative Group. Statistical analysis plan for the third International Stroke Trial (IST-3): part of a “thread” of reports of the trial. Int J Stroke 2012;7:186–187. [DOI] [PubMed] [Google Scholar]
- 18.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 19.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 20.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 21.Lahoti S, Gokhale S, Caplan L, et al. Thrombolysis in ischemic stroke without arterial occlusion at presentation. Stroke 2014;45:2722–2727. [DOI] [PubMed] [Google Scholar]
- 22.Davalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke 1999;30:2631–2636. [DOI] [PubMed] [Google Scholar]
- 23.Qureshi AI, Ezzeddine MA, Nasar A, et al. Is IV tissue plasminogen activator beneficial in patients with hyperdense artery sign? Neurology 2006;66:1171–1174. [DOI] [PubMed] [Google Scholar]
- 24.Kim EY, Yoo E, Choi HY, Lee JW, Heo JH. Thrombus volume comparison between patients with and without hyperattenuated artery sign on CT. AJNR Am J Neuroradiol 2008;29:359–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Kummer R, Meyding-Lamade U, Forsting M, et al. Sensitivity and prognostic value of early CT in occlusion of the middle cerebral artery trunk. AJNR Am J Neuroradiol 1994;15:9–15. [PMC free article] [PubMed] [Google Scholar]
- 26.Aries MJ, Uyttenboogaart M, Koopman K, et al. Hyperdense middle cerebral artery sign and outcome after intravenous thrombolysis for acute ischemic stroke. J Neurol Sci 2009;285:114–117. [DOI] [PubMed] [Google Scholar]
- 27.Mustanoja S, Meretoja A, Putaala J, et al. Outcome by stroke etiology in patients receiving thrombolytic treatment: descriptive subtype analysis. Stroke 2011;42:102–106. [DOI] [PubMed] [Google Scholar]
- 28.Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment: systematic review. Radiology 2005;235:444–453. [DOI] [PubMed] [Google Scholar]
- 29.Niesten JM, van der Schaaf IC, van der Graaf Y, et al. Predictive value of thrombus attenuation on thin-slice non-contrast CT for persistent occlusion after intravenous thrombolysis. Cerebrovasc Dis 2014;37:116–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.