Abstract
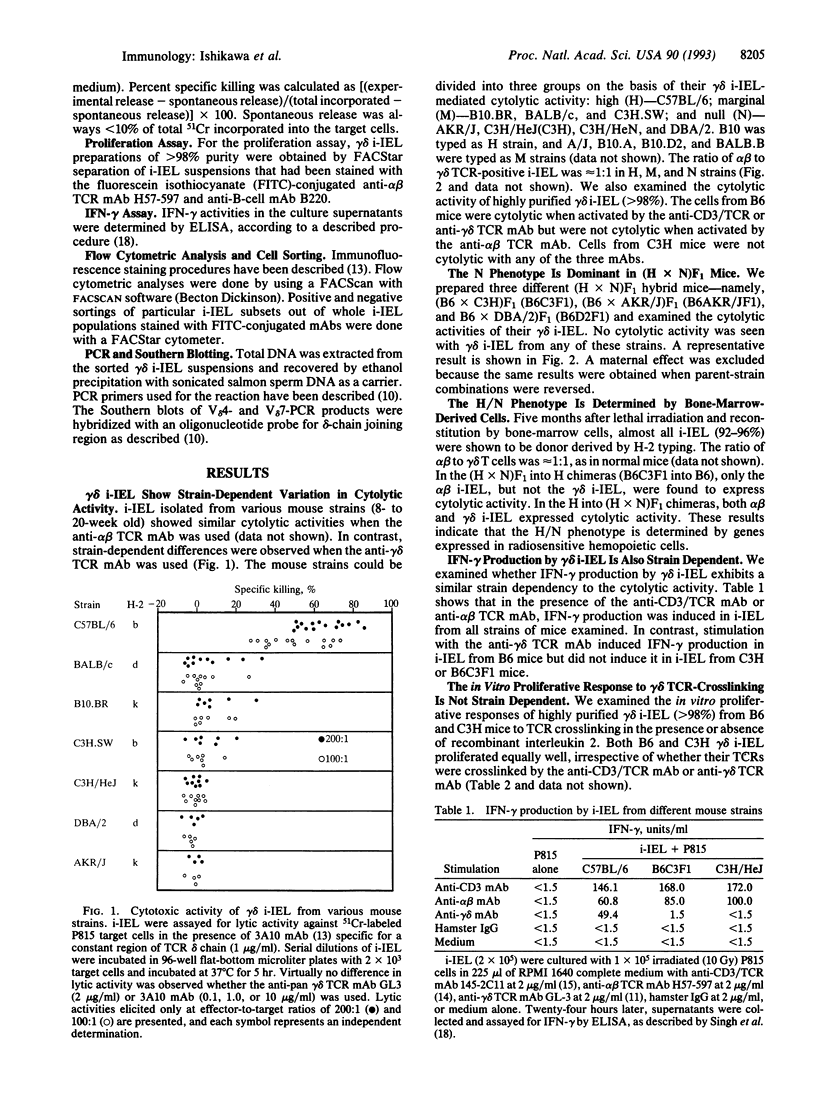
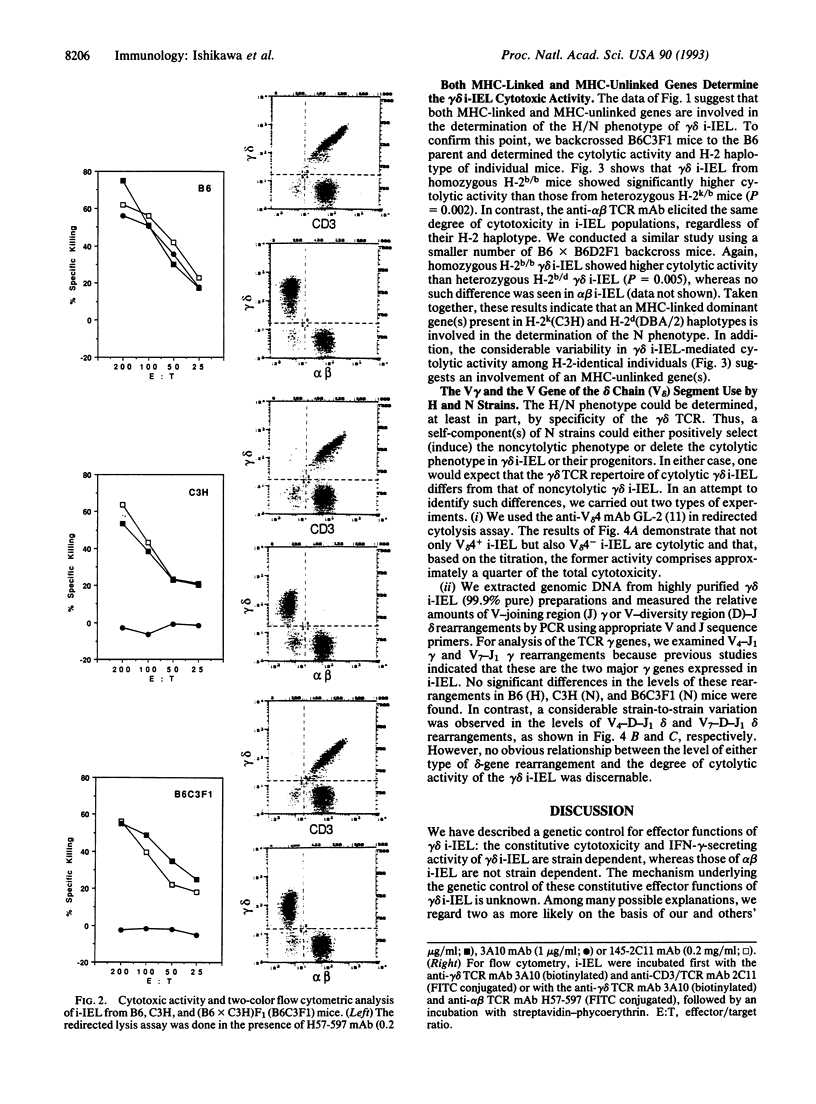
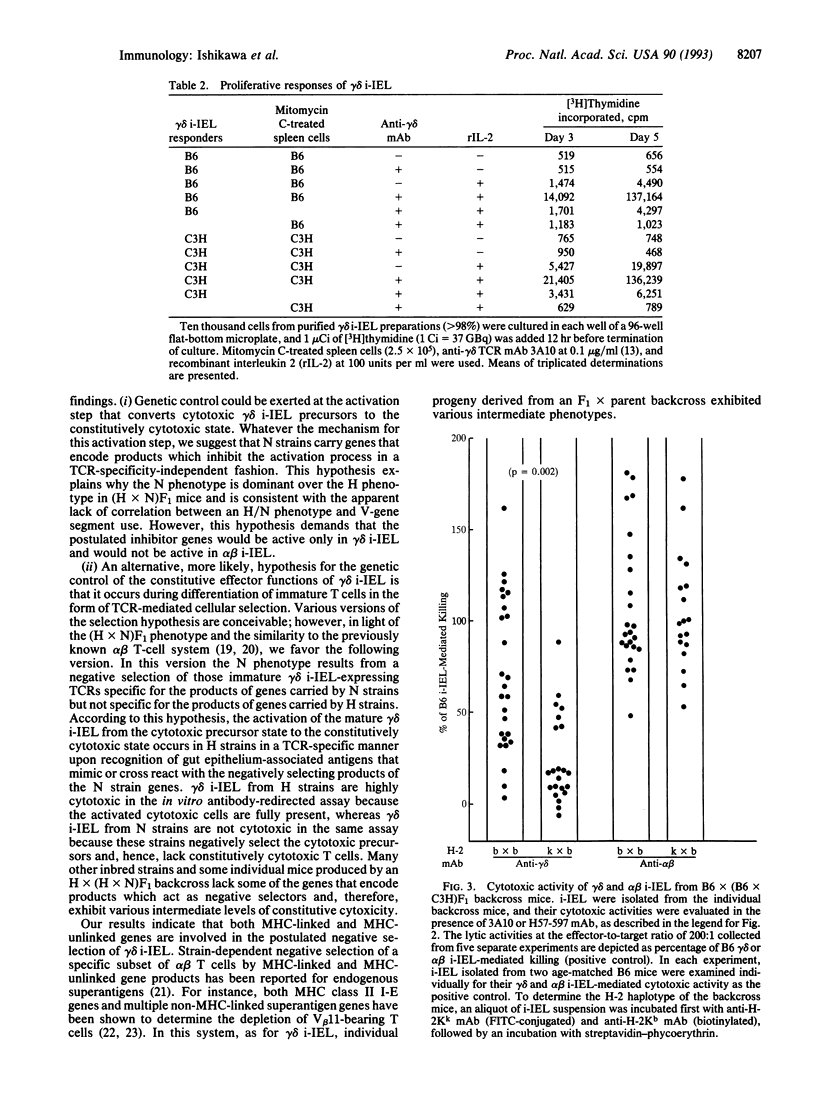
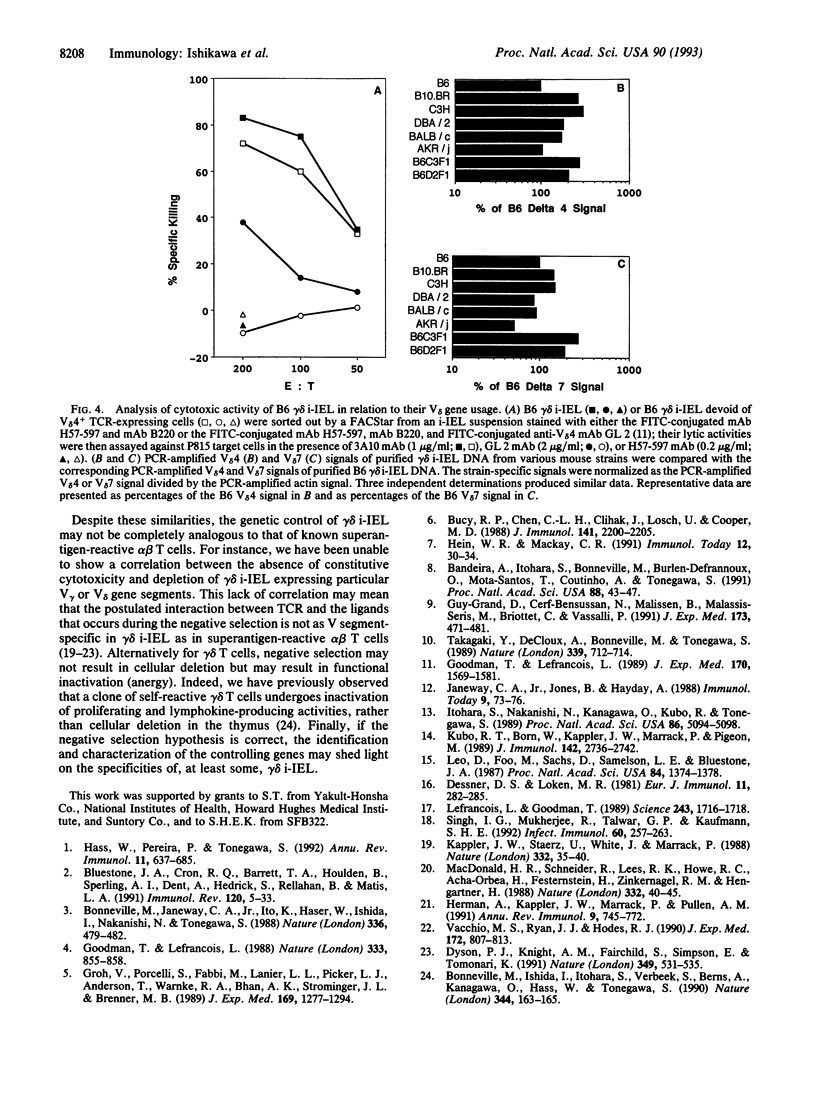
We have analyzed the cytolytic activity of freshly isolated intraepithelial T cells (i-IEL) from the intestines of several different mouse strains in an anti-T-cell receptor monoclonal antibody-mediated redirected lysis assay. The cytolytic activity of gamma delta i-IEL but not that of alpha beta i-IEL was strain dependent. Mouse strains could be divided into high (H), marginal (M), and null (N) strains. The anti-gamma delta T-cell receptor monoclonal antibody-induced interferon gamma production showed the same strain-dependent variability, but the proliferative responses to gamma delta T-cell receptor crosslinking did not show this variability. The N phenotype of gamma delta i-IEL was found to be dominant in (H x N)F1 mice. In radiation bone-marrow chimeras the H/N phenotype was determined by the genotype of the reconstituting bone-marrow-derived cells but was not determined by the genotype of the radioresistant host cells. Analysis of (H x N)F1 backcross animals indicated that at least two genes are involved in determination of the H/N phenotype. One of these genes is major-histocompatibility-complex linked. No difference in the use of the variable region segment of the gamma-chain or delta-chain was seen between the gamma delta i-IEL from H and N strains. Various models that might explain the strain-dependent gamma delta i-IEL phenotypes are discussed.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandeira A., Itohara S., Bonneville M., Burlen-Defranoux O., Mota-Santos T., Coutinho A., Tonegawa S. Extrathymic origin of intestinal intraepithelial lymphocytes bearing T-cell antigen receptor gamma delta. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):43–47. doi: 10.1073/pnas.88.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluestone J. A., Cron R. Q., Barrett T. A., Houlden B., Sperling A. I., Dent A., Hedrick S., Rellahan B., Matis L. A. Repertoire development and ligand specificity of murine TCR gamma delta cells. Immunol Rev. 1991 Apr;120:5–33. doi: 10.1111/j.1600-065x.1991.tb00585.x. [DOI] [PubMed] [Google Scholar]
- Bonneville M., Ishida I., Itohara S., Verbeek S., Berns A., Kanagawa O., Haas W., Tonegawa S. Self-tolerance to transgenic gamma delta T cells by intrathymic inactivation. Nature. 1990 Mar 8;344(6262):163–165. doi: 10.1038/344163a0. [DOI] [PubMed] [Google Scholar]
- Bonneville M., Janeway C. A., Jr, Ito K., Haser W., Ishida I., Nakanishi N., Tonegawa S. Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature. 1988 Dec 1;336(6198):479–481. doi: 10.1038/336479a0. [DOI] [PubMed] [Google Scholar]
- Bucy R. P., Chen C. L., Cihak J., Lösch U., Cooper M. D. Avian T cells expressing gamma delta receptors localize in the splenic sinusoids and the intestinal epithelium. J Immunol. 1988 Oct 1;141(7):2200–2205. [PubMed] [Google Scholar]
- Dessner D. S., Loken M. R. DNL 1.9: a monoclonal antibody which specifically detects all murine B lineage cells. Eur J Immunol. 1981 Apr;11(4):282–285. doi: 10.1002/eji.1830110403. [DOI] [PubMed] [Google Scholar]
- Dyson P. J., Knight A. M., Fairchild S., Simpson E., Tomonari K. Genes encoding ligands for deletion of V beta 11 T cells cosegregate with mammary tumour virus genomes. Nature. 1991 Feb 7;349(6309):531–532. doi: 10.1038/349531a0. [DOI] [PubMed] [Google Scholar]
- Goodman T., Lefrancois L. Intraepithelial lymphocytes. Anatomical site, not T cell receptor form, dictates phenotype and function. J Exp Med. 1989 Nov 1;170(5):1569–1581. doi: 10.1084/jem.170.5.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman T., Lefrançois L. Expression of the gamma-delta T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 1988 Jun 30;333(6176):855–858. doi: 10.1038/333855a0. [DOI] [PubMed] [Google Scholar]
- Groh V., Porcelli S., Fabbi M., Lanier L. L., Picker L. J., Anderson T., Warnke R. A., Bhan A. K., Strominger J. L., Brenner M. B. Human lymphocytes bearing T cell receptor gamma/delta are phenotypically diverse and evenly distributed throughout the lymphoid system. J Exp Med. 1989 Apr 1;169(4):1277–1294. doi: 10.1084/jem.169.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy-Grand D., Cerf-Bensussan N., Malissen B., Malassis-Seris M., Briottet C., Vassalli P. Two gut intraepithelial CD8+ lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J Exp Med. 1991 Feb 1;173(2):471–481. doi: 10.1084/jem.173.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Hein W. R., Mackay C. R. Prominence of gamma delta T cells in the ruminant immune system. Immunol Today. 1991 Jan;12(1):30–34. doi: 10.1016/0167-5699(91)90109-7. [DOI] [PubMed] [Google Scholar]
- Herman A., Kappler J. W., Marrack P., Pullen A. M. Superantigens: mechanism of T-cell stimulation and role in immune responses. Annu Rev Immunol. 1991;9:745–772. doi: 10.1146/annurev.iy.09.040191.003525. [DOI] [PubMed] [Google Scholar]
- Itohara S., Nakanishi N., Kanagawa O., Kubo R., Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor gamma delta: analysis of gamma delta T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Staerz U., White J., Marrack P. C. Self-tolerance eliminates T cells specific for Mls-modified products of the major histocompatibility complex. Nature. 1988 Mar 3;332(6159):35–40. doi: 10.1038/332035a0. [DOI] [PubMed] [Google Scholar]
- Kubo R. T., Born W., Kappler J. W., Marrack P., Pigeon M. Characterization of a monoclonal antibody which detects all murine alpha beta T cell receptors. J Immunol. 1989 Apr 15;142(8):2736–2742. [PubMed] [Google Scholar]
- Lefrancois L., Goodman T. In vivo modulation of cytolytic activity and Thy-1 expression in TCR-gamma delta+ intraepithelial lymphocytes. Science. 1989 Mar 31;243(4899):1716–1718. doi: 10.1126/science.2564701. [DOI] [PubMed] [Google Scholar]
- Leo O., Foo M., Sachs D. H., Samelson L. E., Bluestone J. A. Identification of a monoclonal antibody specific for a murine T3 polypeptide. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1374–1378. doi: 10.1073/pnas.84.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Schneider R., Lees R. K., Howe R. C., Acha-Orbea H., Festenstein H., Zinkernagel R. M., Hengartner H. T-cell receptor V beta use predicts reactivity and tolerance to Mlsa-encoded antigens. Nature. 1988 Mar 3;332(6159):40–45. doi: 10.1038/332040a0. [DOI] [PubMed] [Google Scholar]
- Singh I. G., Mukherjee R., Talwar G. P., Kaufmann S. H. In vitro characterization of T cells from Mycobacterium w-vaccinated mice. Infect Immun. 1992 Jan;60(1):257–263. doi: 10.1128/iai.60.1.257-263.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagaki Y., DeCloux A., Bonneville M., Tonegawa S. Diversity of gamma delta T-cell receptors on murine intestinal intra-epithelial lymphocytes. Nature. 1989 Jun 29;339(6227):712–714. doi: 10.1038/339712a0. [DOI] [PubMed] [Google Scholar]
- Vacchio M. S., Ryan J. J., Hodes R. J. Characterization of the ligand(s) responsible for negative selection of V beta 11- and V beta 12-expressing T cells: effects of a new Mls determinant. J Exp Med. 1990 Sep 1;172(3):807–813. doi: 10.1084/jem.172.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]



