Abstract
Rationale: Anaerobic bacteria are present in large numbers in the airways of people with cystic fibrosis (PWCF). In the gut, anaerobes produce short-chain fatty acids (SCFAs) that modulate immune and inflammatory processes.
Objectives: To investigate the capacity of anaerobes to contribute to cystic fibrosis (CF) airway pathogenesis via SCFAs.
Methods: Samples of 109 PWCF were processed using anaerobic microbiological culture with bacteria present identified by 16S RNA sequencing. SCFA levels in anaerobic supernatants and bronchoalveolar lavage (BAL) were determined by gas chromatography. The mRNA and/or protein expression of two SCFA receptors, GPR41 and GPR43, in CF and non-CF bronchial brushings and 16HBE14o− and CFBE41o− cells were evaluated using reverse transcription polymerase chain reaction, Western blot analysis, laser scanning cytometry, and confocal microscopy. SCFA-induced IL-8 secretion was monitored by ELISA.
Measurements and Main Results: Fifty-seven (52.3%) of 109 PWCF were anaerobe positive. Prevalence increased with age, from 33.3% to 57.7% in PWCF younger (n = 24) and older (n = 85) than 6 years of age. All evaluated anaerobes produced millimolar concentrations of SCFAs, including acetic, propionic, and butyric acids. SCFA levels were higher in BAL samples of adults than in those of children. GPR41 levels were elevated in CFBE41o− versus 16HBE14o− cells; CF versus non-CF bronchial brushings; and 16HBE14o− cells after treatment with cystic fibrosis transmembrane conductance regulator inhibitor CFTR(inh)-172, CF BAL, or inducers of endoplasmic reticulum stress. SCFAs induced a dose-dependent and pertussis toxin–sensitive IL-8 response in bronchial epithelial cells, with a higher production of IL-8 in CFBE41o− than in 16HBE14o− cells.
Conclusions: This study illustrates that SCFAs contribute to excessive production of IL-8 in CF airways colonized with anaerobes via up-regulated GPR41.
Keywords: anaerobic bacteria, cystic fibrosis, inflammation, short-chain fatty acids
At a Glance Commentary
Scientific Knowledge on the Subject
A large number of culture-dependent and culture-independent studies have revealed a significant anaerobic bioburden in cystic fibrosis (CF) airways; however, the role of anaerobic bacteria in the pathogenesis of infection and inflammation in the CF airways remains largely underexplored.
What This Study Adds to the Field
In the present study, we demonstrate a high prevalence of anaerobes in the airways of people with CF and show that this prevalence increases with age. Short-chain fatty acids (SCFAs) were secreted by anaerobic bacteria in vitro and were also detected in vivo in CF bronchoalveolar lavage. SCFAs induced a significant IL-8 response in bronchial epithelial cells that was more pronounced in CF than in normal bronchial epithelium. Two receptors for SCFAs, GPR41 and GPR43, were expressed in bronchial epithelial cells. However, only GPR41 was upregulated in CF compared with normal airway epithelium in vitro and in vivo. GPR41 upregulation was intrinsically driven by the lack of cystic fibrosis transmembrane conductance regulator activity and endoplasmic reticulum stress and was further corroborated by inflammatory stimuli. This study provides novel insight into the pathophysiological role of anaerobic bacteria in the CF airways.
The main cause of morbidity and mortality in cystic fibrosis (CF) is respiratory failure resulting from persistent microbial infection and neutrophil-dominated inflammation (1, 2). Bacteria such as Pseudomonas aeruginosa, Staphylococcus aureus, Haemophilus influenzae, and Burkholderia cepacia complex have been recognized as key pathogens in CF airway infection (3). The detection of bacterial pathogens in CF depends mostly on routine aerobic culture techniques. Consequently, the prevalence of anaerobic and other fastidious bacteria has remained largely understudied. With the implementation of enhanced culture-dependent and culture-independent molecular diagnostics–based techniques, interest in the prevalence and potential pathogenic role of anaerobes has been sparked (4–6).
Anaerobic bacteria are present in the airways of 66–91% people with CF (PWCF) (7, 8) in numbers equal to those of P. aeruginosa, a well-established pathogen in the CF airways (3, 7). Studies exploring the role of anaerobes have failed to show a direct impact on airway function in CF (7, 8). However, more recent studies have associated decreasing CF airway microbial diversity, to which anaerobic bacteria contribute, with poorer lung function (9–11). Contrary to this, Prevotella intermedia was shown to induce a humoral immune response in PWCF and to mediate an influx of neutrophils and macrophages, thereby increasing CF airway inflammation (12). Furthermore, anaerobes belonging to the oropharyngeal flora have been shown to enhance the virulence of P. aeruginosa in a number of infection models (13–17).
Anaerobic bacteria comprise an integral part of the normal gut microflora, where they produce copious amounts of short-chain fatty acids (SCFAs), including acetic, propionic, and butyric acids (18). In addition to their role as fuel for intestinal epithelial cells, SCFAs modulate various processes, including cell proliferation and differentiation, hormone secretion, metabolic homeostasis, and modulation of immune and inflammatory responses (18–23), by binding to the recently deorphanized G protein–coupled receptors, GPR41 (also called free fatty acid receptor 3 or FFAR3) and GPR43 (FFAR2) and by the inhibition of histone deacetylase (HDAC) (24–26).
In the present study, we investigated the capacity of anaerobic bacteria, found in the CF airways, to induce an inflammatory response by the production of SCFAs. The results revealed that these anaerobes produce copious amounts of SCFAs that were also present in the bronchoalveolar lavage (BAL) of PWCF. SCFAs induced a dose-dependent and pertussis toxin (PTX)–sensitive IL-8 response in bronchial epithelium that was higher in CF than in normal bronchial epithelial cells. This effect coincided with increased GPR41 expression in CF compared with normal bronchial epithelial cells that was intrinsically driven by lack of cystic fibrosis transmembrane conductance regulator (CFTR) activity and endoplasmic reticulum (ER) stress, in addition to the proinflammatory milieu of the CF airways.
Some of the results of this study were previously reported in the form of abstracts (27–29).
Methods
Comprehensive details on all methods, including quantification of SCFAs, quantification of SCFA receptors, CF and non-CF bronchial epithelial cell culture and treatments, and measurement of IL-8, are provided in the online supplement.
Study Population
A total of 109 PWCF (age range, 0–61 yr; mean age, 20.7 ± 12.1 yr; 65 males, 44 females) were recruited into this study. Seventy-three PWCF were attending Beaumont Hospital, Dublin, Ireland (age range, 18–61 yr; mean age, 27.4 ± 8.2 yr; 44 males, 29 females); 24 were attending Our Lady’s Children’s Hospital, Crumlin, Ireland (age range, 0–6 yr; mean age, 3.5 ± 1.6 yr; 11 males, 13 females); and 12 were attending Temple Street Children’s University Hospital, Dublin, Ireland (age range, 7–17 yr; mean age, 14.6 ± 2.4 yr; 10 males, 2 females). CF was confirmed by sweat testing and/or genotyping. Only clinically stable PWCF were included in this study. Clinical stability was defined as no change in symptoms, FEV1 within 10% of best value in the previous 6 months, and no new antibiotics started. Full informed consent was obtained from all participants or their parents before the collection of samples, and ethical approval was obtained from Beaumont Hospital, Our Lady’s Children’s Hospital, and Temple Street Children’s University Hospital institutional review boards.
Collection and Processing of Sputum and BAL
Spontaneously expectorated sputum was collected in a sterile container that was placed into an Anaerogen Compact anaerobic pouch (Thermo Fisher Scientific, Waltham, MA) and immediately transported to an anaerobic cabinet for further processing. BAL samples were obtained from individuals undergoing diagnostic or therapeutic fiberoptic bronchoscopy as part of routine care. In adults, 60 ml of sterile 0.9% NaCl was instilled into the right or left subsegmental bronchi. In children, flexible fiberoptic bronchoscopy was performed via a laryngeal airway mask. To prevent upper airway contamination, suction was not performed until the tip of the bronchoscope was past the carina. During the procedure, 1 ml/kg of sterile 0.9% NaCl was instilled twice in the right middle lobe and twice in the lingula, and all four samples were pooled. BAL intended for anaerobic bacterial isolation and identification was handled as described above for sputum, and BAL samples intended for SCFA determination were filtered through gauze and centrifuged at 500 × g for 10 minutes at 4°C. The microbiological culture and identification of bacteria in sputum and BAL were performed as described previously (7).
Bronchial Brushing Sample Collection
All patients (control subjects and PWCF) were undergoing diagnostic or therapeutic fiberoptic bronchoscopy as part of routine care. Bronchial brushings were recovered as previously described (30).
Statistical Analysis
Data were analyzed with GraphPad Prism 4.0 software (GraphPad Software, San Diego, CA). Unless stated otherwise, all data are presented as mean ± SEM and are representative of at least three independent experiments. Normal data were compared using Student’s t test; nonnormal data were compared by Mann-Whitney U test; and, where appropriate, analysis of variance was performed. Differences were considered significant at P < 0.05.
Results
Anaerobic Bacteria Are Present in a High Percentage of PWCF
To determine the prevalence of anaerobic bacteria in the CF airways, 80 sputum and 29 BAL samples of 109 PWCF were collected and analyzed (one sample per patient). The presence of at least one obligate anaerobe was found in 57 (52.3%) of 109 analyzed samples. The most frequently detected obligate anaerobes belonged to the genera Prevotella (35.8%), Actinomyces (16.5%), Veillonella (8.3%), and Fusobacterium (5.5%) (see Table E1 in the online supplement). The most commonly occurring aerobes (facultative anaerobes) belonged to the genera Pseudomonas (44.0%), Staphylococcus (41.3%), and Streptococcus (40.4%), the latter including S. anginosus, S. constellatus, S. intermedius, S. parasanguinis, and S. sanguinis. The prevalence of obligate anaerobes was greater than that of P. aeruginosa (43.2%). Higher prevalence of anaerobes was observed in PWCF older than 6 years of age (57.7%; n = 85; mean age, 25.6 ± 8.9 yr; 54 males and 31 females) than in PWCF younger than 6 years of age (33.3%; n = 24; mean age, 3.5 ± 1.6 yr; 11 males and 13 females) (Figure 1A), demonstrating that the anaerobic prevalence increases with age. On the basis of these data, four representative obligate anaerobes and one facultative anaerobe were selected for further work: Prevotella melaninogenica, Actinomyces odontolyticus, Veillonella parvula, Fusobacterium nucleatum, and Streptococcus sanguinis.
Figure 1.
Anaerobic species are found in high numbers in the cystic fibrosis (CF) airways and secrete copious amounts of short-chain fatty acids (SCFAs) in vitro and in vivo. (A) The percentage of anaerobe-positive cultures in children with CF up to 6 years of age (n = 24; mean age, 3.5 ± 1.6 yr; 11 males and 13 females) and people with cystic fibrosis (PWCF) older than 7 years of age (n = 85; mean age, 25.6 ± 8.9 yr; 54 males and 31 females). Sputum or bronchoalveolar lavage (BAL) samples of 109 PWCF were collected and screened for the presence of anaerobic bacteria as stated in the Methods section. For the sake of clarity, only four genera of the most frequent obligate anaerobes (Prevotella, Actinomyces, Veillonella, and Fusobacterium) and the most commonly occurring aerobes (facultative anaerobes) belonging to the genera Pseudomonas, Staphylococcus, and Streptococcus are shown. (B) SCFA levels in bacterial supernatants from American Type Culture Collection (ATCC) and patient isolate strains of Prevotella melaninogenica, Actinomyces odontolyticus, Veillonella parvula, Fusobacterium nucleatum, and Streptococcus sanguinis as determined by gas chromatography. Data are presented as mean ± SEM (n ≥ 3). (C) SCFA levels in BAL samples of children with CF (n = 7; mean age, 4.0 ± 2.2 yr; 2 males and 5 females) and adults with CF (n = 11; mean age, 29.2 ± 9.3 yr; 3 males and 8 females). (D) IL-8 levels in BAL samples of children with CF (n = 6; mean age, 4.5 ± 1.9 yr; 2 males and 4 females) and adults with CF (n = 10; mean age, 30.1 ± 9.7 yr; 3 males and 7 females) as evaluated with an IL-8 ELISA. (E) Correlation between IL-8 and acetic acid in BAL samples of PWCF (n = 16; r2 = 0.6409; P = 0.002). **P ≤ 0.01; ***P ≤ 0.001.
Selected Anaerobic Strains Secrete Copious Amounts of SCFAs In Vitro
To investigate whether anaerobic bacteria found in the CF airway secrete SCFAs, bacterial supernatants from five representative species grown under anaerobic conditions were collected and analyzed using gas chromatography. American Type Culture Collection and patient isolated strains of all selected species secreted a variety of SCFAs in millimolar concentrations (Figure 1B). Each individual species had its own secretion profile, which was identical for the American Type Culture Collection and patient isolate strains. For example, whereas A. odontolyticus and S. sanguinis secreted acetic acid, V. parvula and F. nucleatum displayed more complex SCFA secretomes consisting of acetic and propionic acid and acetic, propionic, and butyric acids, respectively. P. melaninogenica, produced the highest number of SCFAs, including acetic, i-butyric, 2-methylbutyric, and i-valeric acids, albeit in lower concentrations. Of note, P. aeruginosa grown under anaerobic or aerobic conditions did not secrete any SCFAs (data not shown). These data suggest that anaerobic bacteria found in the CF airway produce copious amounts of SCFAs.
SCFAs Are Present in the Airways of PWCF and Elevated in Adults Compared with Children
To investigate whether anaerobes produce SCFAs in the CF airway in vivo, BAL samples of adults (n = 11; mean age, 29.2 ± 9.3 yr; 3 males and 8 females) and children with CF (n = 7; mean age, 4.0 ± 2.2 yr; 2 males and 5 females) were collected and analyzed using gas chromatography. Six SCFAs detected in bacterial supernatants were also observed in the micromolar concentration range in BAL samples of adult PWCF (Figure 1C). A significant increase in acetic acid (P < 0.001) was observed in BAL samples of adult PWCF compared with children with CF. Similarly, propionic and i-butyric acids were also elevated, but the difference was not statistically significant. These results illustrate that SCFAs are present in the airways of PWCF and are elevated in adult PWCF compared with children with CF.
IL-8 Is Elevated in BAL of Adults Compared with Children with CF and Correlates with Acetic Acid
IL-8 is an inflammatory marker in the airways of PWCF that mediates neutrophil trafficking to the bronchial lumen (31). As evaluated with an IL-8 ELISA, IL-8 was markedly increased in BAL samples of adult PWCF (n = 10; mean age, 30.1 ± 9.7 yr; 3 males and 7 females) compared with children with CF (n = 6; mean age, 4.5 ± 1.9 yr; 2 males and 4 females) (Figure 1D). A correlation analysis was performed to investigate whether this phenomenon was associated with SCFA levels in CF BAL. IL-8 levels displayed a strong correlation with acetic acid in BAL samples of children and adults with CF (n = 16; r2 = 0.6409; P = 0.002) (Figure 1E). Similarly, a significant correlation was also observed between IL-8 and acetic acid in BAL samples of adult PWCF (n = 10; r2 = 0.4200; P = 0.0427). No significant correlation between IL-8 and other SCFAs in CF BAL was observed (data not shown). Collectively, these results showcase that elevated IL-8 levels in CF BAL are associated with high levels of acetic acid in BAL.
Anaerobic Supernatants Induce an Inflammatory Response in CF Epithelial Cells
CFBE41o− cells were treated with supernatants from representative species and monitored for IL-8 secretion using ELISA. All five strains induced a dose- and PTX-dependent IL-8 response in CFBE41o− cells (Figure 2 and Figures E1 and E2). The highest IL-8 response was observed with V. parvula and F. nucleatum supernatants, which were shown to produce high amounts of acetic, propionic, and butyric acids. Interestingly, the IL-8 response induced by V. parvula and F. nucleatum was equal to or greater than that induced by the major CF pathogens S. aureus and P. aeruginosa. When CFBE41o− cells were treated with S. sanguinis or A. odontolyticus, which secreted acetic acid only, a less pronounced IL-8 response was observed and higher concentrations of bacterial supernatant (5% and 10%) were needed to achieve significant cytokine production (Figure E1). P. melaninogenica displayed an intermediate but significant impact on IL-8 production. Upon stimulation of 16HBE14o− cells with anaerobic supernatants, only F. nucleatum induced a significant IL-8 response compared with baseline levels (Figure 2). Furthermore, anaerobe-induced IL-8 responses in 16HBE14o− cells were strikingly lower than in CFBE41o− cells, suggesting that the CF epithelium is more susceptible to stimulation by anaerobic supernatants.
Figure 2.
Supernatants from anaerobic bacteria promote a proinflammatory response in cystic fibrosis airway epithelial cells. 16HBE14o− and CFBE41o− cells were treated with 5% bacterial supernatants or growth medium as a control for 24 hours, and IL-8 secretion was assessed with an ELISA. All data are presented as mean ± SEM of at least three independent experiments. **P ≤ 0.01; ***P ≤ 0.001. The dotted line represents the normalized IL-8 production in the presence of control medium.
SCFAs Generate a Notable IL-8 Response in CF Epithelial Cells
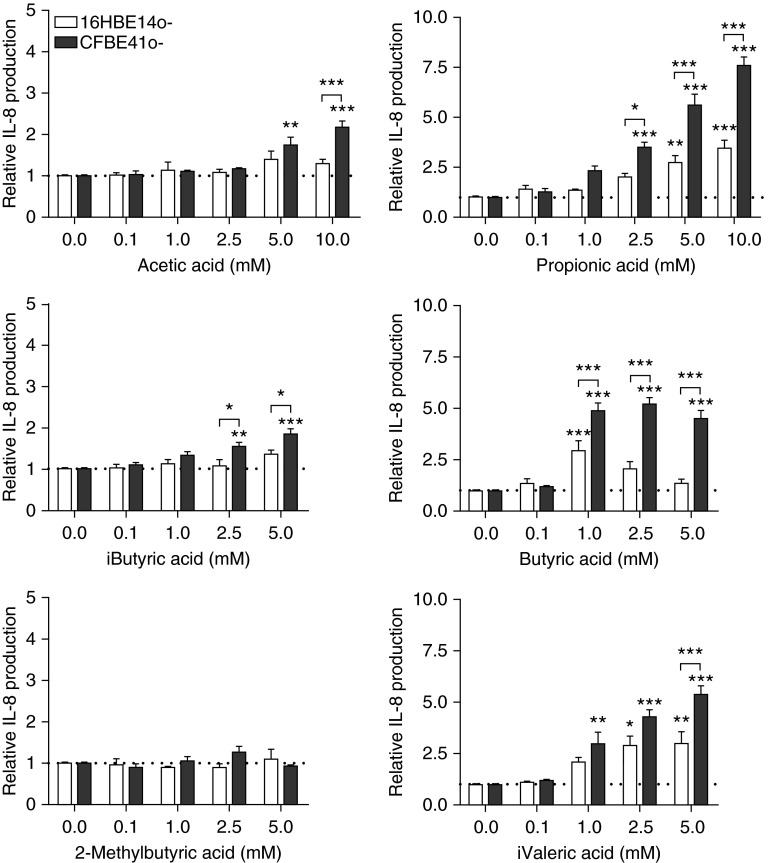
To elucidate whether SCFAs in anaerobic supernatants contribute to IL-8 secretion, bronchial epithelial cells were treated with SCFAs and analyzed for IL-8 secretion. The influence of SCFAs on cell viability was evaluated to exclude any SCFA-induced cytotoxicity (Figure E3), and in the following experiments the cells were treated with nontoxic concentrations of SCFAs. All SCFAs, apart from 2-methylbutyric acid, induced a significant and a dose-dependent IL-8 response from CFBE41o− cells (Figure 3) with the following potency in descending order: butyric acid (half-maximal effective concentration [EC50] = 0.170 mM; R2 = 0.8816), i-valeric acid (EC50 = 1.0 mM; R2 = 0.7711), propionic acid (EC50 = 4.6 mM; R2 = 0.9107), acetic acid (EC50 = 15.5 mM; R2 = 0.8443), i-butyric acid (EC50 = 64 mM; R2 = 0.8304). At the same time, no basal or SCFA-stimulated IL-1β levels were detected in CFBE41o− supernatants (data not shown). Additionally, a small but insignificant increase in SCFA-induced IL-6 over baseline was observed in CFBE41o− supernatants (Figure E4). However, this response was markedly lower than with IL-8, confirming that IL-8 is the predominant proinflammatory cytokine induced by SCFAs in CF bronchial epithelial cells. When CFBE41o− cells were treated with a combination of SCFAs mimicking the complex SCFA secretomes of P. melaninogenica, F. nucleatum, and V. parvula, no synergistic effect on IL-8 secretion was observed (Figure E5). SCFAs also induced a dose-dependent IL-8 response in 16HBE14o− cells (Figure 3); however, IL-8 secretion was significantly less pronounced in 16HBE14o− cells than in CFBE41o− cells, corroborating our earlier observations with anaerobic supernatants. These results confirm that SCFAs in anaerobic supernatants are at least in part responsible for the inflammatory response in bronchial epithelial cells in vitro.
Figure 3.
Short-chain fatty acids (SCFAs) induce a dose-dependent IL-8 response in bronchial epithelial cells. 16HBE14o− and CFBE41o− cells were treated with increasing concentrations of SCFAs for 24 hours and monitored for IL-8 production using an ELISA. All data are presented as means ± SEM of at least three independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. The dotted line represents the normalized IL-8 production in the presence of control medium.
Of note, upon incubation of representative SCFAs (acetic, propionic, and butyric acids) with human serum albumin or α1-antitrypsin, no changes in intrinsic fluorescence of either proteins were observed (Figure E6), suggesting that SCFAs do not bind human serum albumin or α1-antitrypsin. Moreover, the latter also failed to attenuate the SCFA-induced IL-8 response in CFBE41o− cells (Figure E7).
SCFA-mediated Release of IL-8 by CFBE41o− Cells Is GPR41 Dependent
Previous studies have shown that GPR41 couples exclusively through the PTX-sensitive Gαi/o family, whereas GPR43 displays dual coupling through Gαi/o and PTX-insensitive Gαq protein families (24, 25). To explore whether SCFA-mediated IL-8 release was indeed mediated by SCFA receptors, CFBE41o− cells were treated with PTX before SCFAs. PTX (250 ng/ml) significantly attenuated IL-8 secretion in CFBE41o− cells induced by all evaluated SCFAs (Figure 4A). Higher PTX concentrations (500 and 1,000 ng/ml) did not fully restore the IL-8 baseline levels (Figure E8). These data suggest the involvement of PTX-sensitive receptors GPR41 and GPR43 in SCFA-mediated cytokine response in CFBE41o− cells.
Figure 4.
Pertussis toxin and GPR41 small interfering RNA (siRNA) attenuate short-chain fatty acid (SCFA)-mediated IL-8 secretion in cystic fibrosis bronchial epithelial cells. (A) CFBE41o− cells were treated with pertussis toxin (PTX; 250 ng/ml) and increasing concentrations of SCFAs for 24 hours. Afterward, cell supernatants were monitored for IL-8 secretion using an ELISA. CFBE41o− cells were transfected with GPR41 or control siRNA and were evaluated for GPR41 mRNA and protein levels 48 hours after transfection using quantitative reverse transcription polymerase chain reaction and Western blot analysis, respectively. (B) mRNA expression of GPR41 relative to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was determined using the 2−ΔΔCt cycle threshold method, and the results are presented as fold differences. (C) Whole cell lysates were separated by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane, and detected with anti-GPR41 antibody. Signal intensities of all bands were quantified by densitometry and normalized against GAPDH, which served as a loading control. Bar graph depicts densitometric analysis of GPR41 expression. All data are presented as means ± SEM (n ≥ 3). (D) CFBE41o− cells were transfected with GPR41 or control siRNA (80 nM) 24 hours before treatment with acetic acid (10 mM), propionic acid (1 mM), and butyric acid (1 mM) for an additional 24 hours. Cell supernatants were monitored for IL-8 secretion using an ELISA. The results shown are representative of three independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
To further confirm the involvement of GPR41 in SCFA-mediated IL-8 response, a small interfering RNA (siRNA)–knockdown approach was employed. siRNA (80 nM)-mediated knockdown of GPR41 was confirmed using reverse transcription polymerase chain reaction and Western blot analysis 48 hours after transfection (Figure 4B and 4C). The reduction of GPR41 protein expression (∼50%), although not complete, was sufficient to produce a significant decrease in the IL-8 production induced by acetic acid (10 mM), propionic acid (1 mM), and butyric acid (1 mM) (Figure 4D). Collectively, these data corroborate the involvement of GPR41 in SCFA-mediated IL-8 release by CF bronchial epithelial cells.
GPR41 Expression Is Increased in CF Compared with Normal Bronchial Epithelial Cells
To confirm the expression of SCFA receptors in human bronchial epithelium, mRNA and protein levels of GPR41 and GPR43 were evaluated. Western blot analysis revealed that the GPR41 receptor was expressed by both 16HBE14o− and CFBE41o− cells, with significantly higher protein expression in CFBE41o− cells than in 16HBE14o− cells (P < 0.001) (Figure 5A). GPR43 was expressed in 16HBE14o− and CFBE41o− cells at equivalent levels (Figure 5A). The cell surface expression of SCFA receptors was further examined with laser scanning cytometry and confocal microscopy. Staining for the GPR41 receptor was significantly increased on the surface of CFBE41o− cells compared with 16HBE14o− cells as assessed with laser scanning cytometry (55.5 ± 19.5%; P < 0.001) (Figure 5B). Although both cell lines displayed intracellular staining for GPR41 receptor as visualized with confocal microscopy (Figure 5C), a significant increase in colocalization of the GPR41 receptor with the plasma membrane marker Na+/K+-ATPase was observed in CFBE41o− cells (11.9 ± 2.0%) compared with 16HBE14o− cells (6.9 ± 1.1%, P = 0.0434). No differences in GPR43 cell surface expression were detected in 16HBE14o− and CFBE41o− cells when analyzed with laser scanning cytometry or confocal microscopy (Figure E9). These results suggest that although GPR41 and GPR43 are both present in bronchial epithelial cells, only GPR41 is up-regulated at the surface of CF epithelial cells compared with normal bronchial epithelial cells.
Figure 5.
Short-chain fatty acid receptor GPR41 is upregulated at the surface of cystic fibrosis (CF) bronchial epithelial cells. (A) A representative Western blot depicting GPR41 and GPR43 protein expression in 16HBE14o− and CFBE41o− cells. Whole cell lysates were separated by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane, and detected with anti-GPR41 or anti-GPR43 antibody. Signal intensities of all bands were quantified by densitometry and normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which served as a loading control. Bar graph depicts densitometric analysis of GPR41 expression in 16HBE14o− and CFBE41o− cells. Data are shown as means ± SEM (n ≥ 3). (B) GPR41 cell surface staining was analyzed in CFBE41o− and 16HBE14o− cells using laser scanning cytometry as stated in the Methods section. Histograms representative of three independent experiments are shown. Inset values represent fold increases in mean fluorescence intensity between cells stained with primary anti-GPR41 and secondary antibody (black line) and control cells stained only with the secondary antibody (gray line). (C) 16HBE14o− or CFBE41o− cells were grown on coverslips and probed with anti-GPR41 and anti-Na+/K+-ATPase antibody, respectively, followed by fluorescein isothiocyanate (FITC)-labeled and Alexa Fluor 568 secondary antibody, respectively. Controls were labeled with secondary antibodies only and are shown in Figure E11. Cell nuclei were visualized using 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; blue), GPR41 and Na+/K+-ATPase are represented as green and red fluorescence, and colocalization is shown in white. Images shown are representative of three independent experiments. Scale bar, 5 μm. Quantitative reverse transcription polymerase chain reaction was performed (D) in vitro using CFBE41o− and 16HBE14o− epithelial cells and (E) in vivo using bronchial brushings from people with cystic fibrosis (n = 3; mean age, 23.0 ± 4.0 yr; 2 males and 1 female) and control subjects without CF (n = 3; mean age, 56.3 ± 9.5 yr; 3 females). Expression of GPR41 relative to GAPDH was determined using the 2−ΔΔCt cycle threshold method, and the results are presented as fold differences. Data are presented as means ± SEM (n ≥ 3). **P ≤ 0.01; ***P ≤ 0.001.
To investigate whether GPR41 mRNA was also increased in CFBE41o− cells, quantitative reverse transcription polymerase chain reaction was performed. GPR41 mRNA levels were significantly increased in CFBE41o− compared with 16HBE14o− cells (P < 0.001) (Figure 5D). The in vitro trend was also observed in vivo as an increase in GPR41 mRNA levels in bronchial brushings from PWCF compared with non-CF controls (P = 0.0064) (Figure 5E).
Endoplasmic Reticulum Stress Up-regulates GPR41 Expression in CF Bronchial Epithelial Cells In Vitro
Previous studies have associated dysfunctional CFTR with ER stress and activation of the unfolded protein response (UPR) (32). To elucidate whether the latter could have an impact on GPR41 expression, 16HBE14o− cells were treated with tunicamycin (2 μg/ml) or thapsigargin (2 μM), which are well-established inducers of ER stress and UPR (33). ER stress and UPR induction by these compounds was confirmed by monitoring the expression of glucose-regulated proteins 78 and 94 (Figure E10). Tunicamycin and thapsigargin significantly increased GPR41 protein levels in 16HBE14o− cells (P = 0.0297 and P = 0.0198, respectively) (Figure 6A). These data suggest that ER stress and UPR may have a role in GPR41 up-regulation in CF bronchial epithelial cells.
Figure 6.
The mechanism of GPR41 upregulation in cystic fibrosis (CF) bronchial epithelial cells. (A) 16HBE14o− cells were treated with thapsigargin (2 μM) or tunicamycin (2 μg/ml) for 24 hours, and GPR41 protein levels were evaluated by Western blot analysis. Whole cell lysates were separated by 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane, and detected with anti-GPR41 antibody. Signal intensities of all bands were quantified by densitometry and normalized against glyceraldehyde 3-phosphate dehydrogenase (GAPDH), which served as a loading control. Bar graph depicts densitometric analysis of the GPR41 expression. (B) 16HBE14o− cells were treated with a cystic fibrosis transmembrane conductance regulator inhibitor (CFTR[inh]-172; CFTRi) (10 μM) or 10% CF bronchoalveolar lavage (BAL) for 24 hours and 6 hours, respectively, and the GPR41 mRNA levels were evaluated using quantitative reverse transcription polymerase chain reaction as described in the Methods section. Expression of GPR41 relative to GAPDH was determined using the 2−ΔΔCt cycle threshold method, and the results are presented as fold differences. (C) 16HBE14o− cells were treated with CFTR(inh)-172 (20 μM) for 48 hours or 10% CF BAL for 24 hours, and GPR41 protein levels were evaluated with Western blot analysis as described above. Data are presented as means ± SEM of at least three independent experiments. *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. DMSO = dimethyl sulfoxide; Thaps. = thapsigargin; Tun. = tunicamycin.
Dysfunctional CFTR and the Inflammatory CF Environment Promote GPR41 Up-regulation in CF Bronchial Epithelial Cells In Vitro
To explore the possibility that GPR41 up-regulation in the CF airway epithelium could also be associated with reduced CFTR activity, 16HBE14o− cells were treated with a CFTR inhibitor, CFTR(inh)-172 (10 μM), and subsequently evaluated for GPR41 mRNA expression. CFTR inhibition significantly increased GPR41 mRNA levels in 16HBE14o− cells (P = 0.0166) (Figure 6B), matching them to levels found in CFBE41o− cells (Figure 5). Similarly, 10% CF BAL caused a significant up-regulation of GPR41 mRNA in 16HBE14o− cells (P < 0.001) (Figure 6B). These findings were translated to the protein level, as both CFTR(inh)-172 and 10% CF BAL significantly elevated GPR41 protein levels in 16HBE14o− cells (P = 0.0233 and P = 0.0098, respectively) (Figure 6C). Similarly, GlyH-101, another inhibitor of CFTR, also increased GPR41 protein levels (data not shown). These results imply that reduced CFTR activity may be associated with GPR41 up-regulation in CF bronchial epithelial cells in vitro, an effect that is also driven by the inflammatory CF airway milieu.
Discussion
Recent advances in culture-independent molecular techniques for bacterial identification have sparked a renewed interest in the role of anaerobic bacteria in the CF airways. Therefore, we aimed to explore the contribution of anaerobes via SCFAs to the pathogenesis of CF airway disease. In the present study, we show that anaerobic bacteria commonly found in the CF airways secrete SCFAs in vitro and in vivo and, by acting through SCFA receptors, mediate IL-8 release, promoting an inflammatory response in the CF airway.
Similarly to previous studies (7, 8, 16), our cohort of PWCF displayed a high prevalence of anaerobic bacteria (52.3%). This number was greater than that of P. aeruginosa (43.2%), an established CF airway pathogen, confirming that anaerobes are prevalent in the CF airways and may play a role during the course of the disease. Indeed, the percentage of PWCF positive for anaerobes increased with their age. Five representative species that were selected on the basis of their prevalence, secreted high quantities of SCFAs, with each displaying a distinct SCFA secretome (Figure 1B). SCFAs were also detected in CF BAL and were elevated in BAL samples of adult PWCF compared with children with CF (Figure 1C). This suggests that, alongside anaerobes, SCFAs also increase with age. Although SCFAs were previously studied in the context of allergic airway inflammation (34), the present study is the first, to our knowledge, to prove the presence of SCFAs in the lower airways.
CF airway disease is characterized by an exaggerated neutrophil influx that plays a key role in tissue damage and disease progression (35). We have previously shown that IL-8, a potent neutrophil chemotactic agent, is readily secreted from CF airway epithelium by various stimuli (36–38) and is found in high concentrations in CF BAL (31). Anaerobic supernatants induced a potent and dose-dependent IL-8 response that was more pronounced in CF than in normal bronchial epithelium (Figure 2 and Figure E1). Similarly, SCFAs generated a dose-dependent IL-8 response in bronchial epithelial cells that was higher in CF than in normal bronchial epithelial cells (Figure 3), suggesting that SCFAs in anaerobic supernatants contribute to the IL-8 response and may have a detrimental role in the CF airways by promoting neutrophil mobilization.
In addition to the activation of airway epithelial cells to produce cytokines, SCFAs are known to display a plethora of other effects. SCFAs were recently shown to act as chemotactic agents for neutrophils via GPR43 (39). Butyric acid was also reported to impair neutrophil reactive oxygen species production, phagocytosis, and microbial killing (40). This suggests that, in addition to IL-8-mediated recruitment of neutrophils, SCFAs may display a direct effect on neutrophil migration to the CF airways and on their function.
Both SCFA receptors, GPR41 and GPR43, couple to the PTX-sensitive Gαi/o family of proteins, but only GPR43 also couples to the PTX-insensitive Gαq family of proteins (24, 25). PTX blocks the signaling mediated by Gαi/o proteins, and PTX treatment significantly reduced the SCFA-mediated IL-8 response (Figure 4), confirming that SCFAs mediate their effects via SCFA receptors GPR41 and GPR43. However, increasing the concentration of PTX did not return the levels of IL-8 production to baseline after stimulation by propionic and butyric acids (Figure E8). Propionic and butyric acids are well-established inhibitors of HDAC (41), and previous studies have shown that butyric acid induces an IL-8 response in human intestinal epithelial cells by inhibiting HDAC activity and promoting histone acetylation (26). Therefore, SCFA-induced IL-8 response in bronchial epithelial cells may also be mediated by HDAC inhibition in addition to SCFA receptors. Nevertheless, even a partial reduction (∼50%) of GPR41 protein expression induced by siRNA silencing was sufficient for significant inhibition of SCFA-mediated IL-8 production (Figure 4D), corroborating the role of GPR41 in SCFA-mediated IL-8 release by CF bronchial epithelial cells.
GPR41 expression is increased in CF bronchial epithelium in vitro and in vivo (Figure 5), and the mechanism of up-regulation was subsequently investigated. F508del-CFTR is the most common cause of CF. It causes misfolding of CFTR and its subsequent degradation after synthesis, leading to a lack of the functional CFTR on the cell surface (42). Treatment with CFTR(inh)-172, a CFTR inhibitor, increased mRNA and protein levels of GPR41 in 16HBE14o− cells (Figure 6B and 6C), suggesting that the lack of CFTR activity could be associated with GPR41 up-regulation in CF bronchial epithelial cells and that the mechanism could be related to defective chloride ion and/or bicarbonate conductance. Previously, we demonstrated altered expression of microRNAs (miRNAs) in CF (30). miR-182, miR-23b, and miR-544 were shown to be decreased in CF bronchial brushings (30), and we identified them as targeting GPR41 mRNA by using various miRNA target prediction databases (43, 44). However, overexpression of these miRNAs failed to reduce GPR41 mRNA and protein expression in CFBE41o− cells (data not shown), excluding the possibility of regulation of GPR41 expression by these miRNAs. Accumulation of misfolded F508del-CFTR in the ER causes ER stress that activates UPR, a signal transduction pathway to alleviate this stress and restore ER homeostasis (32, 45, 46). Exposure of 16HBE14o− cells to tunicamycin or thapsigargin, well-known inducers of ER stress and UPR, increased protein expression of GPR41 (Figure 6A), implying that the ER stress and UPR may also, independent of CFTR activity, contribute to GPR41 up-regulation. The CF airway also harbors high concentrations of proinflammatory markers, including proteases and chemokines (31). Treatment of 16HBE14o− cells with CF BAL increased GPR41 mRNA and protein levels (Figure 6B and 6C), suggesting that, in addition to intrinsic up-regulation of GPR41 in CF, the latter could further be corroborated by the inflammatory milieu of the CF airways.
In conclusion, we propose a novel mechanism by which anaerobic bacteria, through the production of SCFAs, contribute to the inflammatory environment in the CF airways, in this way potentially amplifying mobilization of neutrophils, tissue destruction, and disease progression. These data suggest that eradication of anaerobes in PWCF may be beneficial by resolving the excessive inflammation brought on by these microorganisms.
Acknowledgments
Acknowledgment
The authors thank David Cunningham for technical assistance with gas chromatography and the patients with cystic fibrosis for taking part in this study.
Footnotes
Supported by Science Foundation Ireland and the Health Research Board under grant SFI/08/US/B1676 and by the National Institutes of Health under grant 5R01 HL092964-04.
Author Contributions: Study conception: B.M., M.A.M., R.D., C.M.G., and N.G.M.; experiments and data analysis: B.M., M.A.M., G.M.L., J.H., M.W., M.M.T., C.M.G., and N.G.M.; patient recruitment: M.A.M., K.M., A.A.A., C.G., F.H., D.S., and P.M.; clinical data collection: B.M., M.A.M., G.M.L., and C.G.; interpretation of experimental results: B.M., C.M.G., and N.G.M.; drafting of the manuscript: B.M., M.S.M., C.M.G., and N.G.M.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201505-0943OC on August 12, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Davies JC, Alton EWFW, Bush A. Cystic fibrosis. BMJ. 2007;335:1255–1259. doi: 10.1136/bmj.39391.713229.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen TS, Prince A. Cystic fibrosis: a mucosal immunodeficiency syndrome. Nat Med. 2012;18:509–519. doi: 10.1038/nm.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folkesson A, Jelsbak L, Yang L, Johansen HK, Ciofu O, Høiby N, Molin S. Adaptation of Pseudomonas aeruginosa to the cystic fibrosis airway: an evolutionary perspective. Nat Rev Microbiol. 2012;10:841–851. doi: 10.1038/nrmicro2907. [DOI] [PubMed] [Google Scholar]
- 4.Bittar F, Richet H, Dubus JC, Reynaud-Gaubert M, Stremler N, Sarles J, Raoult D, Rolain JM. Molecular detection of multiple emerging pathogens in sputa from cystic fibrosis patients. PLoS One. 2008;3:e2908. doi: 10.1371/journal.pone.0002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sibley CD, Grinwis ME, Field TR, Eshaghurshan CS, Faria MM, Dowd SE, Parkins MD, Rabin HR, Surette MG. Culture enriched molecular profiling of the cystic fibrosis airway microbiome. PLoS One. 2011;6:e22702. doi: 10.1371/journal.pone.0022702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser PM, Bernard T, Greub G, Jaton K, Pagni M, Hafen GM. Microbiota present in cystic fibrosis lungs as revealed by whole genome sequencing. PLoS One. 2014;9:e90934. doi: 10.1371/journal.pone.0090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tunney MM, Field TR, Moriarty TF, Patrick S, Doering G, Muhlebach MS, Wolfgang MC, Boucher R, Gilpin DF, McDowell A, et al. Detection of anaerobic bacteria in high numbers in sputum from patients with cystic fibrosis. Am J Respir Crit Care Med. 2008;177:995–1001. doi: 10.1164/rccm.200708-1151OC. [DOI] [PubMed] [Google Scholar]
- 8.Worlitzsch D, Rintelen C, Böhm K, Wollschläger B, Merkel N, Borneff-Lipp M, Döring G. Antibiotic-resistant obligate anaerobes during exacerbations of cystic fibrosis patients. Clin Microbiol Infect. 2009;15:454–460. doi: 10.1111/j.1469-0691.2008.02659.x. [DOI] [PubMed] [Google Scholar]
- 9.Delhaes L, Monchy S, Fréalle E, Hubans C, Salleron J, Leroy S, Prevotat A, Wallet F, Wallaert B, Dei-Cas E, et al. The airway microbiota in cystic fibrosis: a complex fungal and bacterial community—implications for therapeutic management. PLoS One. 2012;7:e36313. doi: 10.1371/journal.pone.0036313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zemanick ET, Harris JK, Wagner BD, Robertson CE, Sagel SD, Stevens MJ, Accurso FJ, Laguna TA. Inflammation and airway microbiota during cystic fibrosis pulmonary exacerbations. PLoS One. 2013;8:e62917. doi: 10.1371/journal.pone.0062917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox MJ, Allgaier M, Taylor B, Baek MS, Huang YJ, Daly RA, Karaoz U, Andersen GL, Brown R, Fujimura KE, et al. Airway microbiota and pathogen abundance in age-stratified cystic fibrosis patients. PLoS One. 2010;5:e11044. doi: 10.1371/journal.pone.0011044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ulrich M, Beer I, Braitmaier P, Dierkes M, Kummer F, Krismer B, Schumacher U, Gräpler-Mainka U, Riethmüller J, Jensen PØ, et al. Relative contribution of Prevotella intermedia and Pseudomonas aeruginosa to lung pathology in airways of patients with cystic fibrosis. Thorax. 2010;65:978–984. doi: 10.1136/thx.2010.137745. [DOI] [PubMed] [Google Scholar]
- 13.Sibley CD, Duan K, Fischer C, Parkins MD, Storey DG, Rabin HR, Surette MG. Discerning the complexity of community interactions using a Drosophila model of polymicrobial infections. PLoS Pathog. 2008;4:e1000184. doi: 10.1371/journal.ppat.1000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan Y, Teng D, Burke AC, Haase EM, Scannapieco FA. Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb Pathog. 2009;46:73–79. doi: 10.1016/j.micpath.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Pustelny C, Komor U, Pawar V, Lorenz A, Bielecka A, Moter A, Gocht B, Eckweiler D, Müsken M, Grothe C, et al. Contribution of Veillonella parvula to Pseudomonas aeruginosa-mediated pathogenicity in a murine tumor model system. Infect Immun. 2015;83:417–429. doi: 10.1128/IAI.02234-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field TR, Sibley CD, Parkins MD, Rabin HR, Surette MG. The genus Prevotella in cystic fibrosis airways. Anaerobe. 2010;16:337–344. doi: 10.1016/j.anaerobe.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Duan K, Dammel C, Stein J, Rabin H, Surette MG. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol. 2003;50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x. [DOI] [PubMed] [Google Scholar]
- 18.Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu J, Zhou Z, Hu Y, Dong S. Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J Genet Genomics. 2012;39:375–384. doi: 10.1016/j.jgg.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Xiong Y, Miyamoto N, Shibata K, Valasek MA, Motoike T, Kedzierski RM, Yanagisawa M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc Natl Acad Sci USA. 2004;101:1045–1050. doi: 10.1073/pnas.2637002100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, et al. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nat Commun. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miletta MC, Petkovic V, Eblé A, Ammann RA, Flück CE, Mullis PE. Butyrate increases intracellular calcium levels and enhances growth hormone release from rat anterior pituitary cells via the G-protein-coupled receptors GPR41 and 43. PLoS One. 2014;9:e107388. doi: 10.1371/journal.pone.0107388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- 25.Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- 26.Fusunyan RD, Quinn JJ, Fujimoto M, MacDermott RP, Sanderson IR. Butyrate switches the pattern of chemokine secretion by intestinal epithelial cells through histone acetylation. Mol Med. 1999;5:631–640. [PMC free article] [PubMed] [Google Scholar]
- 27.Mirković B, Murray M, Lavelle G, Bergin DA, Devery R, Greene CM, McElvaney NG. Role of short chain fatty acids, produced by anaerobic bacteria, in the cystic fibrosis airway [abstract] Am J Respir Crit Care Med. 2013;187:A5285. doi: 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mirković B, Murray M, Lavelle GM, Devery R, Molloy K, Greene CM, McElvaney NG. Short chain fatty acids, produced by anaerobic bacteria, elicit a pro-inflammatory response in the cystic fibrosis airway [abstract] Am J Respir Crit Care Med. 2014;189:A1718. doi: 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mirković B, Murray MA, Lavelle GM, Molloy K, Gunaratnam C, Devery R, Greene CM, McElvaney NG. Short chain fatty acids, produced by anaerobic bacteria, elicit a pro-inflammatory response in the cystic fibrosis airway [abstract] Am J Respir Crit Care Med. 2015;191:A3803. doi: 10.1164/rccm.201505-0943OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oglesby IK, Bray IM, Chotirmall SH, Stallings RL, O’Neill SJ, McElvaney NG, Greene CM. miR-126 is downregulated in cystic fibrosis airway epithelial cells and regulates TOM1 expression. J Immunol. 2010;184:1702–1709. doi: 10.4049/jimmunol.0902669. [DOI] [PubMed] [Google Scholar]
- 31.Bergin DA, Hurley K, Mehta A, Cox S, Ryan D, O’Neill SJ, Reeves EP, McElvaney NG. Airway inflammatory markers in individuals with cystic fibrosis and non-cystic fibrosis bronchiectasis. J Inflamm Res. 2013;6:1–11. doi: 10.2147/JIR.S40081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bartoszewski R, Rab A, Jurkuvenaite A, Mazur M, Wakefield J, Collawn JF, Bebok Z. Activation of the unfolded protein response by ΔF508 CFTR. Am J Respir Cell Mol Biol. 2008;39:448–457. doi: 10.1165/rcmb.2008-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. doi: 10.1016/B978-0-12-385114-7.00004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, et al. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- 35.Cohen-Cymberknoh M, Kerem E, Ferkol T, Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax. 2013;68:1157–1162. doi: 10.1136/thoraxjnl-2013-203204. [DOI] [PubMed] [Google Scholar]
- 36.Cosgrove S, Chotirmall SH, Greene CM, McElvaney NG. Pulmonary proteases in the cystic fibrosis lung induce interleukin 8 expression from bronchial epithelial cells via a heme/meprin/epidermal growth factor receptor/Toll-like receptor pathway. J Biol Chem. 2011;286:7692–7704. doi: 10.1074/jbc.M110.183863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greene CM, Carroll TP, Smith SGJ, Taggart CC, Devaney J, Griffin S, O’Neill SJ, McElvaney NG. TLR-induced inflammation in cystic fibrosis and non-cystic fibrosis airway epithelial cells. J Immunol. 2005;174:1638–1646. doi: 10.4049/jimmunol.174.3.1638. [DOI] [PubMed] [Google Scholar]
- 38.Walsh DE, Greene CM, Carroll TP, Taggart CC, Gallagher PM, O’Neill SJ, McElvaney NG. Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J Biol Chem. 2001;276:35494–35499. doi: 10.1074/jbc.M103543200. [DOI] [PubMed] [Google Scholar]
- 39.Vinolo MAR, Ferguson GJ, Kulkarni S, Damoulakis G, Anderson K, Bohlooly-Y M, Stephens L, Hawkins PT, Curi R. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS One. 2011;6:e21205. doi: 10.1371/journal.pone.0021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinolo MAR, Hatanaka E, Lambertucci RH, Newsholme P, Curi R. Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem Funct. 2009;27:48–55. doi: 10.1002/cbf.1533. [DOI] [PubMed] [Google Scholar]
- 41.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2008;19:587–593. doi: 10.1016/j.jnutbio.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16:45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betel D, Wilson M, Gabow A, Marks DS, Sander C. The microRNA.org resource: targets and expression. Nucleic Acids Res. 2008;36(Database issue):D149–D153. doi: 10.1093/nar/gkm995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Betel D, Koppal A, Agius P, Sander C, Leslie C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010;11:R90. doi: 10.1186/gb-2010-11-8-r90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blohmke CJ, Mayer ML, Tang AC, Hirschfeld AF, Fjell CD, Sze MA, Falsafi R, Wang S, Hsu K, Chilvers MA, et al. Atypical activation of the unfolded protein response in cystic fibrosis airway cells contributes to p38 MAPK-mediated innate immune responses. J Immunol. 2012;189:5467–5475. doi: 10.4049/jimmunol.1103661. [DOI] [PubMed] [Google Scholar]
- 46.Kerbiriou M, Le Drévo M-A, Férec C, Trouvé P. Coupling cystic fibrosis to endoplasmic reticulum stress: differential role of Grp78 and ATF6. Biochim Biophys Acta. 2007;1772:1236–1249. doi: 10.1016/j.bbadis.2007.10.004. [DOI] [PubMed] [Google Scholar]