Abstract
Asthma is a common chronic disease without cure. Our understanding of asthma onset, pathobiology, classification, and management has evolved substantially over the past decade; however, significant asthma-related morbidity and excess healthcare use and costs persist. To address this important clinical condition, the NHLBI convened a group of extramural investigators for an Asthma Research Strategic Planning workshop on September 18–19, 2014, to accelerate discoveries and their translation to patients. The workshop focused on (1) in utero and early-life origins of asthma, (2) the use of phenotypes and endotypes to classify disease, (3) defining disease modification, (4) disease management, and (5) implementation research. This report summarizes the workshop and produces recommendations to guide future research in asthma.
Keywords: asthma, prevention, phenotype, disease modification, implementation
At a Glance
Scientific Knowledge on the Subject
This report summarizes the Workshop participants’ discussions, recommendations, and priorities for future research in asthma.
What This Study Adds to the Field
The report represents a collective body of scientific expert opinion conveyed to the NHLBI for use in strategic planning. The recommendations will be of interest to the scientific, professional, and patient communities because they constitute a summary of the directions asthma research may take in the near future.
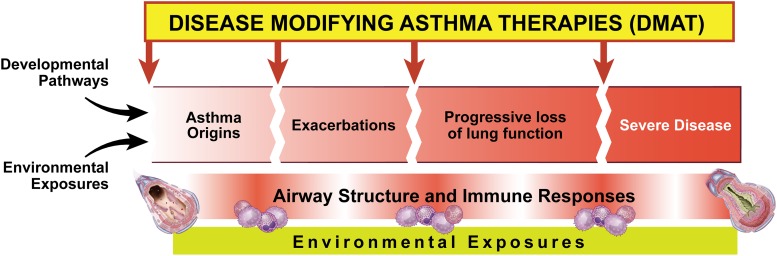
Since the NHLBI last convened an asthma research planning workshop in 2003 (1), the NHLBI and other organizations have made a number of efforts to summarize available evidence that reflects the advances and gaps in our understanding of the onset of asthma, and its pathobiology, classification, management, and research (2, 3). For example, research has identified prenatal and early childhood environmental, genetic, and immune risk factors for the development of asthma that act in concert, and perhaps sequentially, at critical developmental time points to determine an individual’s risk for the development of disease. Nevertheless, we remain uncertain how to prevent the onset of asthma, while recognizing that primary prevention of asthma is one ultimate goal (4). Ideally, preventing or perhaps encouraging specific environmental exposures at critical time points in developmental pathways would allow us to prevent the onset of asthma (Figure 1). Once a diagnosis is established, patients were traditionally classified as having mild, moderate, or severe asthma. We now refine the classification to reflect disease heterogeneity and the biological basis of such heterogeneity using clinical and/or molecular phenotypes. The current approach may allow us to make more informed treatment decisions that will modify or reverse the disease (i.e., disease-modifying asthma therapies) (Figure 1), rather than simply relieving symptoms temporarily. Until we can prevent asthma or modify existing disease, patient care will remain challenging because of our inability to predict or prevent exacerbations and the persistence of substantial barriers to the translation of scientific evidence into the everyday lives of patients. On September 18–19, 2014, the Division of Lung Diseases of NHLBI convened a group of extramural investigators to discuss their recommendations for the direction(s) of future asthma research and to identify opportunities for scientific advancement.
Figure 1.
Preventive strategies and disease-modifying therapies for asthma. The immune system and lung structure pathobiology in asthma arise from developmental pathways and exposures. Asthma exacerbations and progressive decline of lung function are morbid outcomes for asthma. Standard treatment is focused on reversing bronchoconstriction with β-adrenergic agonists (relievers) and treating inflammation with corticosteroids (long-term controllers) to alleviate the downstream signs and symptoms of asthma. Disease-modifying asthma therapies target upstream effectors and mechanisms underlying asthma pathobiology. Preventive strategies require understanding of the critical windows of environmental exposures over the life-course and the personal biological predispositions that put individuals at risk for development of asthma or its progression.
Workshop participants were divided into working groups that focused on five areas of asthma research: asthma origins and primary prevention; phenotypes and endotypes; disease modification; personalized asthma control strategies and management; and implementation research. Each working group identified barriers to progress and made recommendations for future research. During the workshop, several cross-cutting themes and associated research questions emerged from the working groups. This report provides a summary of the workshop participants’ discussion and cross-cutting themes, as well as their recommendations and priorities for future research in asthma
Asthma Origins and Primary Prevention
As a multifactorial disease, asthma onset, severity, and natural history vary, which reflects the combined effects of development-specific exposures (e.g., in utero vs. early-life vs. childhood exposures) and host responses to those exposures (4–6). Despite this heterogeneity, most asthma manifests in the preschool years; therefore, asthma origins and primary prevention research have focused on childhood onset of the disease. Although many epidemiological, genetic, environmental, and immune risk factors for asthma are known, distinguishing which are causal, and how these factors interact and lead to overt disease remains poorly understood. In clinical investigations, specific questions to be addressed include identifying which risk factors are causal, the mechanisms through which these factors initiate asthma, and how combinations of exposures interact to ultimately initiate disease. Studies that address both the relevance of specific developmental windows and the importance of coincident exposures are also necessary to understand the etiology of asthma. To do so, metrics for the exposome, originally defined as “encompasses(ing) life-course environmental exposures (including lifestyle factors), from the prenatal period onwards,” (7) will be needed. Incorporating comprehensive longitudinal exposure data (with relevant exposure specificity, time, dose, and frequency) collected from the prenatal period through the onset of asthma into rigorous analyses in a meaningful way remains a challenge. Furthermore, investigation into the role of extrapulmonary organ systems on the development of asthma through unique or shared mechanisms could provide insights into important developmental pathways.
Current barriers to our understanding of the origins of asthma include the limitations on clinical research that are permissible during gestation and the perinatal period, inadequacies of animal models in recapitulating the onset of human disease, and differences between human and experimental animal developmental stages. For example, although mouse allergen challenge models recapitulate many features of sensitization and allergic lung responses, acute exposures in mice differ substantially from the chronic/intermittent and diverse exposures antecedent to the onset of asthma in humans. Therefore, the utility of animal models to elucidate the origins of asthma is limited, particularly when assessing potential interventions for the primary prevention of asthma.
To address these issues, the following recommendations were made:
-
1.
Develop methods to assess the developing human infant immune system and lung.
-
2.
Define the critical windows in human development when environmental exposures are most likely to increase the risk of asthma or its onset, specifying the relevance of interactions between specific risk factors and the timing (critical window) of the exposure.
-
3.
Elucidate the critical windows in human development when non-environmental risk factors cause or influence the onset of asthma, specifying the relevance of interactions between specific risk factors and the timing (critical window) of the exposure.
-
4.
Test in utero or early-life strategies targeted to mitigate potential determinants of asthma onset (primary prevention).
-
5.
Generate and evaluate methods to measure and analyze the asthma-relevant “exposome” at different stages of human development (e.g., determine how peripubertal changes in the exposome differ by sex and the associated differences in immune response, lung development, and the onset of asthma).
Asthma Heterogeneity: Phenotypes and Endotypes
Several approaches have been taken to classify the disease heterogeneity observed in patients with asthma (8–12). For example, cluster analyses, which define sets of patient characteristics based on mathematical modeling to create clusters, have been used within specific cohorts. However, in clinical practice, prospective application of cluster analyses to individual patients has limited utility because of the granularity of phenotyping and analytical approaches used to aggregate candidate variables into a cluster. In addition, the natural history of phenotypes over the life span and the longitudinal course of phenotypes with treatment and among racially or ethnically diverse populations remain unclear. The potential integration of technologically advanced data (e.g., molecular or “omics” data or other biomarkers) to refine phenotypes into endotypic clusters further complicates the classification of disease. For example, patients may be characterized as having “type 2–high” asthma on the basis of elevated gene expression of markers of type 2 inflammation in airway epithelial cells or on the basis of type 2 cytokine expression in bronchial biopsies (13). Nonetheless, type 2–high patients may be similar to type 2–low patients (as defined by gene or cytokine expression) if they are compared on the basis of lung function or hyperresponsiveness. Moreover, the analytical approach to classification may be modified based on the purpose (e.g., predicting patient response to therapy) or disease status (e.g., patient on immunomodulatory therapy or having an exacerbation) (13–15).
Endotyping and phenotyping research requires well-characterized cohorts with appropriate representation of the spectrum of disease and modifying factors, as well as bioinformatics and computational biology expertise. The complexity of managing large data sets, including access to electronic medical record data and lack of data harmonization among large cohort studies create significant barriers to progress.
To address these issues, the following recommendations were made:
-
1.
Sustain basic and translational research into the mechanisms underlying endotypes and phenotypes of asthma, including, but not limited to, the molecular phenotypes that underlie type 2–low asthma.
-
2.
Develop new models for molecular phenotyping, including organotypic cultures of cells from patients.
-
3.
Foster public–private scientific collaborations between academia and industry to uncover endotypes in human asthma as revealed by the increasing use of newly developed type 2–specific antagonists.
Disease Modification
Although asthma therapeutics have been traditionally identified as either “controllers” or “relievers,” this dichotomous classification does not address the therapeutic potential to modify the underlying disease. Ideally, disease-modifying asthma therapies (DMATs) should target fundamental pathobiological mechanisms involved in asthma and/or pathologic alterations in lung structure. Evidence suggests that the mucosal immune system (16), and specifically airway epithelial-derived signals, play a pivotal role in asthma (17). In addition, dysregulation of innate antiviral defenses (17) and/or the role of type 2 inflammation are important features of disease pathobiology in many individuals with asthma.
To achieve disease modification, disruption of the critical mechanisms of disease is likely to be necessary, and the timing of such disruption may also be important. Mouse models suggest that innate lymphoid cells (ILCs), which respond to epithelial-derived signals to mediate allergic airway inflammation (18, 19), may be useful to target the mucosal immune system. Although ILCs have been identified in the healthy human lung (20) and among patients with asthma (21), more research is needed to understand the role of ILCs in asthma, to quantify the degree of dysregulation in mucosal immunity, and to assess epithelial integrity (22) in human subjects. Moreover, the relationship between critical mechanisms of disease and clinically meaningful disease manifestations or outcomes requires further exploration. For example, airway remodeling can be an early event in the natural history of asthma, but it is often dissociated from inflammation or airflow limitations (23, 24). Finally, as described in the Asthma Heterogeneity: Phenotypes and Endotypes section, although the type 2–high phenotype (characterized by elevated gene or cytokine expression of markers of type 2 inflammation in airway epithelial cells or bronchial biopsies, respectively) has been targeted to modify disease, our understanding of the type 2–low phenotype and mechanisms of disease that are independent of type 2 inflammation continues to be a challenge.
To address these issues, the following recommendations were made:
-
1.
Promote investigation of asthma by multidisciplinary groups of scientists and clinicians with diverse expertise, and the ability to use new tools and animal models to explore the heterogeneity of disease mechanisms, identify the critical pathobiological mechanisms of disease, and understand the dynamic interactions between such processes.
-
2.
Evaluate the relationship between pathobiological pathways and clinical manifestations of disease to enable the identification of appropriate targets for DMATs.
-
3.
Assess critical windows for disease modification and clarify when end-effector mechanisms (e.g., smooth muscle structure or function, mucous production, or airway fibrosis) are reversible or modifiable.
Personalized Asthma Control Strategies and Management
As indicated by the asthma phenotypes and endotypes working group, there is substantial heterogeneity in “asthma,” and this heterogeneity is reflected by a broad range of therapeutic responses in clinical trials (8, 25–27). Such heterogeneity makes it challenging to manage asthma, particularly in the absence of prognostic indicators. Moreover, diagnostic biomarkers that could be of great value for young children and for determining the earliest origins of disease (28) are also not yet available. Individualized, evidence-based approaches to managing asthma on the basis of disease endotypes are lacking (29–33), as are approaches to management in diverse healthcare settings. In addition to cross-sectional approaches to defining an individual’s disease characteristics, there is a paucity of longitudinal research linking molecular mechanisms and environmental exposures with clinical manifestations of disease (such as lung function changes or exacerbations) that might improve our understanding of the heterogeneity of responses to treatment.
For precision in asthma management, more mechanistic approaches to care are needed. These new approaches would be based on an integrated understanding of the individual patient’s biological mechanisms, including the interplay among the exposome, genetics, epigenetics, immune response, psychosocial support and lung physiology. To address these issues, the following recommendations were made:
-
1.
Design nontraditional clinical trials to identify the causes of disease heterogeneity and to facilitate the discovery of diagnostic and prognostic biomarkers.
-
2.
Evaluate alternative methods to assess risk factors, mechanisms, and predictors of heterogeneity in the response to treatment, including medication-related adverse effects and morbidity.
-
3.
Identify effective longitudinal asthma management models that integrate essential components of care (e.g., diagnosis, monitoring, education, exposures, medications, adherence) to prevent asthma, improve asthma control, or mitigate disease progression while improving efficiency and adaptation over time.
-
4.
Asthma control strategies and management models should address the needs and behaviors of patients of all ages (soliciting patient feedback to do so), with different social, economic, and cultural backgrounds in a variety of healthcare settings.
Implementation Research
Implementation research investigates the processes by which evidence results in the modification of patient care (34). In asthma, implementation research integrates the relevant context in which care occurs, the needs and concerns of patients, the most appropriate provider and location for care to occur, and the organizations and communities in which patients live and care providers work. Current examples of advances made through implementation research include interventions to achieve evidence-based practices in some communities (35) and use of school programs (36, 37) for children with asthma. Other examples have defined modifiable barriers that may be addressed to improve asthma care (38).
Despite significant advances in our understanding of the management of asthma over the past decade, there are numerous barriers to translating the efficacy measured in randomized clinical trials into the everyday lives of patients. One key issue is that advances in therapy have not been equally distributed among different racial and ethnic groups. In particular, recent improvements in asthma outcomes are larger among non-Hispanic white Americans than among African Americans (39). Systematic reviews have shown that many healthcare providers do not adhere to clinical practice guidelines, and efforts to improve providers’ compliance have had limited success (40). In addition, many interventions are now available for broad-scale dissemination, but these interventions lack an efficient mechanism for dissemination. The establishment of standard definitions of asthma outcomes based on collaboration among investigators, NHLBI, and National Institute of Allergy and Infectious Disease (41) may facilitate comparative effectiveness research particularly when interventions are disseminated across different communities. Despite previously weak incentives that have impeded implementation, current secular influences, most notably the Affordable Care Act (42) and Patient-Centered Outcomes Research Institute (PCORI) have created opportunities to which the asthma research community should respond.
To address these issues, the following recommendations were made:
-
1.Create a continuous learning/training collaborative of implementation researchers with the following goals:
-
a.Develop new study designs and methodologies that enable the integration of evidence into clinical practice efficiently while assessing implementation outcomes to meet the dynamic needs of patients with asthma. This will require patient engagement in the research process.
-
b.Investigate best practices for the dissemination of scientific discoveries and evidence-based practices in a variety of clinical care settings.
-
c.Study how novel models of health care and its delivery promote implementation and dissemination in vulnerable and underserved populations that have a disproportionate burden of asthma, as well as in novel contexts, including nonmedical facilities (e.g., school, community center) and alternative healthcare providers (e.g., patient navigator, community health worker).
-
a.
-
2.
Develop a standard training curriculum within the implementation research collaborative for junior investigators to learn how to overcome challenges and attain success in implementation research.
-
3.
Establish a comparative cost-effectiveness core center within the collaborative that uses a systematic approach to compare various interventions for asthma.
Cross-Cutting Themes to Direct Future Research
Throughout the workshop and in the discussions of all of the working groups, several common recommendations emerged that merit further discussion because of the potential for synergistic advances in asthma research.
Informatics
The importance of advancing the use of informatics tools to enable asthma research was emphasized. One goal was to allow the creation, harmonization, and merging of large datasets, including prospective patient populations and existing administrative or electronic health record data. This would enable the identification of phenotypes on a population level using existing data. Enrollment of homogenous patient populations in clinical trials could be facilitated by such phentoypes. Ideally, these large datasets would also integrate existing clinical and “omics” data (e.g., genomic, proteomic, lipidomic, and microbiomic). Of critical importance, such datasets would require the use of a harmonized set of terms to define patient characteristics and outcomes, and provide links to available standardized protocols and repositories for biospecimen collection in current and future asthma cohorts. Such rigorously collected and integrated data would be useful to further clarify the heterogeneity of disease to allow development of predictive indexes to target populations at risk for disease development, DMATs, and new approaches to asthma management that account for the healthcare setting. Similarly, a registry of data from the electronic health records of patients with asthma would provide insight into the current state of asthma care/practices across the nation and promote planning of how to scale and efficiently implement discoveries to the population level.
Longitudinal Cohorts
The need for longitudinal cohorts of patients with asthma was also recommended for several purposes. First is to better understand the origins of the disease and the interdependence of both disease-promoting and disease-suppressing exposures and developmental changes over the lifespan. Such cohorts would also allow investigation of the durability and natural history of clinical phenotypes and molecular endotypes from infancy to adulthood, and help predict individuals at high risk for severe disease. Longitudinal collection of biospecimens would also allow for identification of predisease biomarkers and DMATs at different stages of life for patients with a variety of phenotypes.
Translational Research
The promotion of translational research incorporating multidisciplinary teams was also championed. For example, clinical phenotypes and molecular endotypes could be prospectively validated using evidence-based clinical management strategies to improve asthma care across the severity spectrum. Such teams would require the promotion of training to fill knowledge gaps, such as information technology skills to create user-friendly large datasets. Investigators with expertise in molecular phenotypes would need to engage in research planning with the informatics, genetics, clinical, and drug development experts. Such efforts may be useful to identify novel approaches to defining biomarkers for predicting the response to treatment or the disease course.
Mechanisms of Disease
Although much has been learned in the last decade regarding the underlying mechanisms of asthma, the principal focus has been on type 2–high asthma. With the remarkable development by industry of several new therapeutics that target molecular mechanisms for type 2 immune responses, their translation to clinical practice will provide a virtual learning laboratory for the importance of type 2 immunity in asthma. Renewed efforts for basic and translational research are needed for type 2–independent mechanisms in asthma, including immune, neural, and structural mechanisms, as well as developmental and regenerative pathways. These and other precision interventions and tools will allow for the clarification of type 2–dependent and –independent pathways in the pathobiology of asthma exacerbations, underlying disease, and progression.
Prevention, Disease Modification, and Cure
Asthma remains without primary prevention or cure. Multidisciplinary teams should be encouraged to investigate early-life events, including environmental exposures, to identify high-risk patients and populations to target for primary prevention. In the near term, new mechanistic-based therapies offer promise for a new era of disease modification with reductions in asthma morbidity, including improved quality of life, lung function, and reduced risk of exacerbations even for those with severe, persistent disease. Implementation research on the delivery of current asthma medications and new DMATs will also be needed to realize the full potential of existing and emerging therapies. Finally, long-term approaches to disease modification will enable the ultimate goal of asthma secondary prevention and cure.
Conclusions
The emerging themes from the workshop are intended to be a challenge for investigators to connect technological advances to the understanding of asthma pathobiology for disease management and its eventual dissemination to the general population. The ultimate goals are to develop effective strategies for the primary prevention of asthma and to discover or create precision interventions for asthma patients that optimize airway function and the health of individuals and their communities. These priority topics will be used to create a strategic approach for asthma research to achieve these goals, thereby improving patient outcomes and reducing the public health burden of asthma.
Footnotes
Supported by the NHLBI, National Institutes of Health.
Author Contributions: Conception and design: B.D.L., S.C.E., C.O., P.G.W., S.N.G., N.N.J., M.M.C., M.M.F., and P.J.N. Writing and contributing important intellectual content: all authors.
Originally Published in Press as DOI: 10.1164/rccm.201505-0963WS on August 25, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Busse W, Banks-Schlegel S, Noel P, Ortega H, Taggart V, Elias J NHLBI Working Group. Future research directions in asthma: an NHLBI Working Group report. Am J Respir Crit Care Med. 2004;170:683–690. doi: 10.1164/rccm.200311-1539WS. [DOI] [PubMed] [Google Scholar]
- 2.National Asthma Education and Prevention Program. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007 Aug [accessed 2015 Oct 29]. Report No.: 07-4051. Available from: http://www.nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf.
- 3.Busse WW, Morgan WJ, Taggart V, Togias A. Asthma outcomes workshop: overview. J Allergy Clin Immunol. 2012;129(3) Suppl:S1–S8. doi: 10.1016/j.jaci.2011.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jackson DJ, Hartert TV, Martinez FD, Weiss ST, Fahy JV. Asthma: NHLBI workshop on the primary prevention of chronic lung diseases. Ann Am Thorac Soc. 2014;11:S139–S145. doi: 10.1513/AnnalsATS.201312-448LD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker ML, Holt KE, Anderson GP, Teo SM, Sly PD, Holt PG, Inouye M. Elucidation of pathways driving asthma pathogenesis: development of a systems-level analytic strategy. Front Immunol. 2014;5:447. doi: 10.3389/fimmu.2014.00447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vrijheid M, Slama R, Robinson O, Chatzi L, Coen M, van den Hazel P, Thomsen C, Wright J, Athersuch TJ, Avellana N, et al. The human early-life exposome (HELIX): project rationale and design. Environ Health Perspect. 2014;122:535–544. doi: 10.1289/ehp.1307204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wild CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14:1847–1850. doi: 10.1158/1055-9965.EPI-05-0456. [DOI] [PubMed] [Google Scholar]
- 8.Wenzel SE. Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med. 2012;18:716–725. doi: 10.1038/nm.2678. [DOI] [PubMed] [Google Scholar]
- 9.Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH. Cluster analysis and clinical asthma phenotypes. Am J Respir Crit Care Med. 2008;178:218–224. doi: 10.1164/rccm.200711-1754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, D’Agostino R, Jr, Castro M, Curran-Everett D, Fitzpatrick AM, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med. 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzpatrick AM, Teague WG, Meyers DA, Peters SP, Li X, Li H, Wenzel SE, Aujla S, Castro M, Bacharier LB, et al. National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. Heterogeneity of severe asthma in childhood: confirmation by cluster analysis of children in the National Institutes of Health/National Heart, Lung, and Blood Institute Severe Asthma Research Program. J Allergy Clin Immunol. 2011;127:382–389.e1–e13. doi: 10.1016/j.jaci.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boudier A, Curjuric I, Basagaña X, Hazgui H, Anto JM, Bousquet J, Bridevaux PO, Dupuis-Lozeron E, Garcia-Aymerich J, Heinrich J, et al. Ten-year follow-up of cluster-based asthma phenotypes in adults: a pooled analysis of three cohorts. Am J Respir Crit Care Med. 2013;188:550–560. doi: 10.1164/rccm.201301-0156OC. [DOI] [PubMed] [Google Scholar]
- 13.Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, Arron JR, Fahy JV. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med. 2009;180:388–395. doi: 10.1164/rccm.200903-0392OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore WC, Hastie AT, Li X, Li H, Busse WW, Jarjour NN, Wenzel SE, Peters SP, Meyers DA, Bleecker ER National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Sputum neutrophil counts are associated with more severe asthma phenotypes using cluster analysis. J Allergy Clin Immunol. 2014;133:1557–1563.e5. doi: 10.1016/j.jaci.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ortega H, Li H, Suruki R, Albers F, Gordon D, Yancey S. Cluster analysis and characterization of response to mepolizumab. A step closer to personalized medicine for patients with severe asthma. Ann Am Thorac Soc. 2014;11:1011–1017. doi: 10.1513/AnnalsATS.201312-454OC. [DOI] [PubMed] [Google Scholar]
- 16.Belkaid Y, Artis D. Immunity at the barriers. Eur J Immunol. 2013;43:3096–3097. doi: 10.1002/eji.201344133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtzman MJ, Byers DE, Alexander-Brett J, Wang X. The role of airway epithelial cells and innate immune cells in chronic respiratory disease. Nat Rev Immunol. 2014;14:686–698. doi: 10.1038/nri3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker JA, Barlow JL, McKenzie AN. Innate lymphoid cells--how did we miss them? Nat Rev Immunol. 2013;13:75–87. doi: 10.1038/nri3349. [DOI] [PubMed] [Google Scholar]
- 19.Halim TY, Steer CA, Mathä L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnig C, Cernadas M, Dutile S, Liu X, Perrella MA, Kazani S, Wechsler ME, Israel E, Levy BD. Lipoxin A4 regulates natural killer cell and type 2 innate lymphoid cell activation in asthma. Sci Transl Med. 2013;5:174ra26. doi: 10.1126/scitranslmed.3004812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georas SN, Rezaee F. Epithelial barrier function: at the front line of asthma immunology and allergic airway inflammation. J Allergy Clin Immunol. 2014;134:509–520. doi: 10.1016/j.jaci.2014.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bossley CJ, Fleming L, Gupta A, Regamey N, Frith J, Oates T, Tsartsali L, Lloyd CM, Bush A, Saglani S. Pediatric severe asthma is characterized by eosinophilia and remodeling without T(H)2 cytokines. J Allergy Clin Immunol. 2012;129:974–982.e13. doi: 10.1016/j.jaci.2012.01.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James AL, Elliot JG, Jones RL, Carroll ML, Mauad T, Bai TR, Abramson MJ, McKay KO, Green FH. Airway smooth muscle hypertrophy and hyperplasia in asthma. Am J Respir Crit Care Med. 2012;185:1058–1064. doi: 10.1164/rccm.201110-1849OC. [DOI] [PubMed] [Google Scholar]
- 25.Szefler SJ, Chmiel JF, Fitzpatrick AM, Giacoia G, Green TP, Jackson DJ, Nielsen HC, Phipatanakul W, Raissy HH. Asthma across the ages: knowledge gaps in childhood asthma. J Allergy Clin Immunol. 2014;133:3–13, quiz 14. doi: 10.1016/j.jaci.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarjour NN, Erzurum SC, Bleecker ER, Calhoun WJ, Castro M, Comhair SA, Chung KF, Curran-Everett D, Dweik RA, Fain SB, et al. NHLBI Severe Asthma Research Program (SARP) Severe asthma: lessons learned from the National Heart, Lung, and Blood Institute Severe Asthma Research Program. Am J Respir Crit Care Med. 2012;185:356–362. doi: 10.1164/rccm.201107-1317PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holgate ST. Stratified approaches to the treatment of asthma. Br J Clin Pharmacol. 2013;76:277–291. doi: 10.1111/bcp.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sahiner UM, Yavuz ST, Buyuktiryaki B, Cavkaytar O, Arik Yilmaz E, Tuncer A, Sackesen C. Serum basal tryptase levels in healthy children: correlation between age and gender. Allergy Asthma Proc. 2014;35:404–408. doi: 10.2500/aap.2014.35.3769. [DOI] [PubMed] [Google Scholar]
- 29.Agache I, Akdis C, Jutel M, Virchow JC. Untangling asthma phenotypes and endotypes. Allergy. 2012;67:835–846. doi: 10.1111/j.1398-9995.2012.02832.x. [DOI] [PubMed] [Google Scholar]
- 30.Wagener AH, Yick CY, Brinkman P, van der Schee MP, Fens N, Sterk PJ. Toward composite molecular signatures in the phenotyping of asthma. Ann Am Thorac Soc. 2013;10:S197–S205. doi: 10.1513/AnnalsATS.201302-035AW. [DOI] [PubMed] [Google Scholar]
- 31.Castro M, Fain SB, Hoffman EA, Gierada DS, Erzurum SC, Wenzel S National Heart, Lung, and Blood Institute’s Severe Asthma Research Program. Lung imaging in asthmatic patients: the picture is clearer. J Allergy Clin Immunol. 2011;128:467–478. doi: 10.1016/j.jaci.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Auffray C, Adcock IM, Chung KF, Djukanovic R, Pison C, Sterk PJ. An integrative systems biology approach to understanding pulmonary diseases. Chest. 2010;137:1410–1416. doi: 10.1378/chest.09-1850. [DOI] [PubMed] [Google Scholar]
- 33.Bunyavanich S, Schadt EE. Systems biology of asthma and allergic diseases: a multiscale approach. J Allergy Clin Immunol. 2015;135:31–42. doi: 10.1016/j.jaci.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peters DH, Adam T, Alonge O, Agyepong IA, Tran N. Implementation research: what it is and how to do it. BMJ. 2013;347:f6753. doi: 10.1136/bmj.f6753. [DOI] [PubMed] [Google Scholar]
- 35.Cloutier MM, Wakefield DB. Translation of a pediatric asthma-management program into a community in Connecticut. Pediatrics. 2011;127:11–18. doi: 10.1542/peds.2010-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerald LB, McClure LA, Mangan JM, Harrington KF, Gibson L, Erwin S, Atchison J, Grad R. Increasing adherence to inhaled steroid therapy among schoolchildren: randomized, controlled trial of school-based supervised asthma therapy. Pediatrics. 2009;123:466–474. doi: 10.1542/peds.2008-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cicutto L, Gleason M, Szefler SJ. Establishing school-centered asthma programs. J Allergy Clin Immunol. 2014;134:1223–1230, quiz 1231. doi: 10.1016/j.jaci.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Apter AJ, Wan F, Reisine S, Bender B, Rand C, Bogen DK, Bennett IM, Bryant-Stephens T, Roy J, Gonzalez R, et al. The association of health literacy with adherence and outcomes in moderate-severe asthma. J Allergy Clin Immunol. 2013;132:321–327. doi: 10.1016/j.jaci.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, Liu X. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief, no 94. Hyattsville, MD: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 40.Okelo SO, Butz AM, Sharma R, Diette GB, Pitts SI, King TM, Linn ST, Reuben M, Chelladurai Y, Robinson KA. Interventions to modify adherence to asthma guidelines. Comparative Effectiveness Review No. 95 (Prepared by Johns Hopkins University Evidence-based Practice Center under Contract No. 290-2007-10061-I). AHRQ Publication No. 13-EHC022-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2013 May [accessed 2015 Oct 29]. Available from: www.effectivehealthcare.ahrq.gov/reports/final.cfm.
- 41.Busse WW, Morgan WJ, Taggart V, Togias A, Akinbami LJ, Brown R, Cabana MD, Clark N, Camargo CA, Jr, Campbell JD, et al. Standardizing asthma outcomes in clinical research: report of the Asthma Outcomes Workshop. J Allergy Clin Immunol. 2012;129(3) Suppl:S1–S142. doi: 10.1016/j.jaci.2011.12.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fung V, Graetz I, Galbraith A, Hamity C, Huang J, Vollmer WM, Hsu J, Wu AC. Financial barriers to care among low-income children with asthma: health care reform implications. JAMA Pediatr. 2014;168:649–656. doi: 10.1001/jamapediatrics.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]