Abstract
Rationale: Frailty is associated with morbidity and mortality in abdominal organ transplantation but has not been examined in lung transplantation.
Objectives: To examine the construct and predictive validity of frailty phenotypes in lung transplant candidates.
Methods: In a multicenter prospective cohort, we measured frailty with the Fried Frailty Phenotype (FFP) and Short Physical Performance Battery (SPPB). We evaluated construct validity through comparisons with conceptually related factors. In a nested case–control study of frail and nonfrail subjects, we measured serum IL-6, tumor necrosis factor receptor 1, insulin-like growth factor I, and leptin. We estimated the association between frailty and disability using the Lung Transplant Valued Life Activities disability scale. We estimated the association between frailty and risk of delisting or death before transplant using multivariate logistic and Cox models, respectively.
Measurements and Main Results: Of 395 subjects, 354 completed FFP assessments and 262 completed SPPB assessments; 28% were frail by FFP (95% confidence interval [CI], 24–33%) and 10% based on the SPPB (95% CI, 7–14%). By either measure, frailty correlated more strongly with exercise capacity and grip strength than with lung function. Frail subjects tended to have higher plasma IL-6 and tumor necrosis factor receptor 1 and lower insulin-like growth factor I and leptin. Frailty by either measure was associated with greater disability. After adjusting for age, sex, diagnosis, and transplant center, both FFP and SPPB were associated with increased risk of delisting or death before lung transplant. For every 1-point worsening in score, hazard ratios were 1.30 (95% CI, 1.01–1.67) for FFP and 1.53 (95% CI, 1.19–1.59) for SPPB.
Conclusions: Frailty is prevalent among lung transplant candidates and is independently associated with greater disability and an increased risk of delisting or death.
Keywords: biomarker, body composition, disability, frailty, lung transplantation
At a Glance Commentary
Scientific Knowledge on the Subject
Frailty, originally a geriatric syndrome, reflects increased vulnerability to physiologic stressors. Emerging evidence has demonstrated frailty to be associated with morbidity and mortality in abdominal organ transplant and other surgical populations, but it has not been evaluated in lung transplantation.
What This Study Adds to the Field
We found that frailty, as ascertained using either of two established measures, is prevalent among adult lung transplant candidates at four U.S. transplant centers. In addition to establishing the construct validity of frailty in lung transplant candidates, this study shows that frailty is associated with greater disability and increased rates of death or delisting before transplant.
The aim of lung transplants is to extend survival, reduce disability, and improve health-related quality of life for persons with advanced lung diseases. Despite rigorous candidacy screening practices, improvements in surgical and medical management, and iterative advancements in organ allocation policies, nearly 20% of adults awaiting lung transplants die or are removed from the waiting list owing to disease progression before receiving a suitable donor offer (1). After lung transplants, nearly the same proportion of patients die within the first postoperative year (2). Notably, serious morbidity after transplant is increasing, with resultant disability and associated decrements in health-related quality of life (3, 4). Although known risk factors for death are already incorporated into lung allocation in the United States (Lung Allocation Score [LAS]), persistently high mortality and increasing morbidity underscore the need to identify novel risk factors for poor outcomes to maximize the individual and societal benefit of lung transplants (5).
Frailty is an independent risk factor for disability, perioperative complications, and mortality in older medical (6–9) and surgical patient populations (10–13). Conceptualized first in the field of geriatrics, frailty is defined as a generalized vulnerability to stressors resulting from an accumulation of physiologic deficits across multiple interrelated systems (14). These deficits, in turn, deplete the body’s physiologic reserves, resulting in a “state of risk” for disproportionate declines in health status following exposure to an additional stressor such as major surgery. Drawing from the geriatrics experience, frailty has become recognized more recently as a risk factor for poor outcomes in solid organ transplantation. Specifically, frailty has been found to be associated with delayed graft function and mortality in kidney transplant recipients and waitlist mortality in liver transplant candidates (15–17).
In this multicenter study, we aimed to establish the prevalence and validity of frailty in lung transplant candidates. We hypothesized that frailty would be common and independently associated with disability and an increased risk of death or delisting in lung transplant candidates. Some of the results of this study have been reported previously in the form of abstracts (18–24).
Methods
Study Design, Participants, and Settings
We analyzed participants in the Lung Transplant Body Composition Study, which is an ongoing observational prospective cohort study of the impact of preoperative body composition on outcomes after lung transplants. For this analysis, candidates for lung transplant age ≥18 years were recruited at the University of California, San Francisco (UCSF); Columbia University Medical Center (CUMC); the University of Pennsylvania; and the University of Pittsburgh. The study period was from March 17, 2011, to October 10, 2014; however, each center began enrolling subjects at different times within that period. Participants provided written informed consent for participation. Institutional review boards at each participating center approved this study.
Frailty Assessment
Several validated measures of frailty have been developed (25). Informed by differing conceptual underpinnings (26), some measures are composed of multidimensional assessments of physical functioning, whereas others include assessment of factors such as cognition, social isolation, or counts of major comorbidities (27). Because listing for lung transplants is contingent on adequate social support, cognitive functioning, and freedom from severe comorbidity, we hypothesized frailty measures that emphasized physical functioning would perform better in lung transplant candidates.
We applied two well-validated frailty measures that emphasize physical functioning. The Fried Frailty Phenotype (FFP) is an aggregate score of five constructs: shrinking, exhaustion, low physical activity, slowness, and weakness (14). Each construct is assigned 1 point if present or 0 if absent. Thus, the FFP score ranges from 0 to 5, with higher scores reflecting increased frailty. The FFP can be evaluated as a binary (frail, FFP ≥ 3), categorical (frail, FFP ≥ 3; prefrail, FFP = 1–2; not frail, FFP = 0), or ordinal scale (0–5) (14). The Short Physical Performance Battery (SPPB) is a three-component battery of lower extremity performance measures that includes gait speed, chair stands, and balance (6, 7). Each measure is scored from 0 to 4, with an aggregate score ranging from 0 to 12. In contrast to the FFP, lower SPPB scores reflect increased frailty. The SPPB can also be evaluated using a binary (frail, SPPB ≤ 7), categorical (frail, SPPB ≤ 7; prefrail, SPPB = 8–9; not frail, SPPB ≥ 10), or ordinal scale (0–12) (7). Details on the frailty measures and scoring are provided in Table E1 in the online supplement. Because of the timing of study initiation and related instrument introduction at each center, not all subjects underwent assessments with both the FFP and SPPB frailty measures.
Other Measurements
Sarcopenia, now defined by abnormally low lean body mass and decreased function, is a cardinal physiologic feature of frailty (28–30). Using exclusively low muscle mass thresholds, others have shown low lean muscle mass sarcopenia to be an independent risk factor for infections and mortality in liver transplant recipients (31–33). At UCSF and CUMC, lean body mass was assessed in Clinical and Translational Science Award–funded research centers using whole-body dual-energy X-ray absorptiometry (Lunar Prodigy DXA, GE Healthcare, Madison, WI, at UCSF; Discovery W, Hologic, Bedford, MA, at CUMC). The appendicular skeletal muscle mass index (ASMI) was calculated as previously described (34). Lean mass precision error (±1 SD) is 0.8 kg (35). We defined lean muscle mass sarcopenia as an ASMI of ≤5.45 kg/m2 for women and ≤7.26 kg/m2 for men (28).
Demographic and clinical variables at study entry were abstracted from medical records. Variables included body mass index (BMI; in kilograms per meter squared), race and/or ethnicity, diagnostic indication for transplant, hemoglobin (in grams per deciliter) (27, 36), FVC (in liters), 6-minute-walk distance (6MWD; in meters), and LAS.
Construct Validity Assessment
The convergent and discriminative validity of frailty measures was tested through comparisons with theoretically related clinical factors and measures of functioning. Factors tested included age, FVC, BMI, lean body mass (ASMI), grip strength, 6MWD, hemoglobin, and LAS. We hypothesized that frailty would be more strongly correlated with grip strength and exercise capacity than with age, BMI, or lung function. We anticipated that grip strength would correlate more strongly with the FFP than with the SPPB because it is the measure by which the FFP weakness construct is determined. On similar grounds, we also hypothesized that frailty measures would be positively associated with lean body mass and negatively associated with hemoglobin.
To further evaluate construct validity, we conducted an exploratory nested case–control study of selected biomarkers that have been shown to be associated with frailty in other populations (37). We measured serum IL-6 (38–40), tumor necrosis factor receptor 1 (40), insulin-like growth factor I (41), and leptin (42) in singletons at UCSF (Wolters Laboratory) using commercially available ELISA kits (R&D Systems, Minneapolis, MN). Blood was drawn in citrated tubes at the time of frailty assessments at UCSF and CUMC, then centrifuged within 45 minutes. The serum fraction was isolated and stored at −80°C for subsequent analysis. For this analysis, we identified all frail subjects by SPPB with a diagnosis of either pulmonary fibrosis or chronic obstructive pulmonary disease (COPD) for whom we had serum (n = 12) and 26 frail subjects by FFP and randomly selected one age-, sex-, and diagnosis-matched control for each individual from the nonfrail group.
Outcome Measures for Tests of Association
Disability
Disability was assessed with the Lung Transplant Valued Life Activities scale (LT-VLA). The LT-VLA is a validated measure of disability in lung transplantation (43). It considers disability across the full spectrum of functioning beyond activities of daily living and is associated with measures of functioning and health-related quality of life. It has a range from 0 to 3. Higher scores reflect increased disability; a difference of 0.3 is considered clinically meaningful.
Delisting or death before transplant
We treated delisting or death as a composite outcome. Dates of delisting and death as well as reason for delisting were obtained through medical record review. For delisting, only delisting due to becoming too ill for transplant was considered for the outcome. Time was calculated as the number of days from frailty assessment until date of death or delisting. Subjects were censored if they underwent lung transplant.
Analytical Approach
Baseline characteristics were compared by using Student’s t test, the Wilcoxon rank-sum test, or the χ2 test, as appropriate. We chose to perform statistical hypothesis tests of baseline characteristics between frail and nonfrail participants because these differences inform the construct validity of the frail phenotypes of interest. We used Spearman correlations to test convergent and discriminative validity. Lower scores on SPPB and higher scores on FFP indicate worse frailty. Therefore, for the correlation analyses, we reverse coded the SPPB so that the direction of the correlations would be consistent between measures. To account for multiple testing, we used the Benjamini-Hochberg procedure to control the false discovery rate at 0.05 (44). For all other analyses, we used a P value less than 0.05 as the threshold for statistical significance. To compare log-transformed biomarker levels in frail versus nonfrail subjects, we used the Wilcoxon rank-sum test. We used multivariate linear regression to estimate the association between both SPPB and FFP frailty and LT-VLA disability. Covariates were selected based on plausible associations with frailty and/or disability. In sequential models, all conditioned on center, we assessed unadjusted (model 1), demographic (model 2), acuity (model 3), and functioning factors (model 4). Although the covariates included in models 3 and 4 are conceptually related to frailty (e.g., BMI or 6MWD), we were interested in determining whether measures of frailty provided information above and beyond routinely collected clinical information. Our sample size required us to consider a parsimonious list of covariates.
We visually inspected the unadjusted association of frailty with delisting or death before lung transplant using Kaplan-Meier methods. We evaluated this association using established binary and categorical cutoffs for both the SPPB and FFP (7, 14). As a sensitivity analysis, we evaluated the association between SPPB and time to delisting or death across a range of binary cutoffs (45–47). To adjust for potential confounders, we used stratified Cox proportional hazards models, employing a similar approach to sequential modeling as used in examining the association between frailty and LT-VLA disability. We used individual and global tests for nonzero slope of covariates versus log survival time to evaluate the proportionality of hazards. The scales for SPPB and FFP are not directly comparable. Therefore, for both the regression and Cox model analyses, we standardized the scales by dividing each by its standard deviation.
One subject in the SPPB analyses and two subjects in the FFP analyses were missing one covariate value each relevant for model 4 (i.e., FVC or BMI). We used 10-fold multiple imputation by chained equations to account for these missing data (48). In examining the association between frailty and delisting or death, we performed an additional sensitivity analysis using competing risks analysis to estimate the association between frailty and delisting or death with lung transplant as the competing risk. Analyses were performed using Stata (version 13.1; StataCorp, College Station, TX) and SAS (version 9.3; SAS Institute, Cary, NC) software.
Results
Among the 395 subjects enrolled during the study period, 219 (55%) were male. The median age was 59 years (interquartile rage [IQR], 50–64), and the median LAS was 37.4 (IQR, 33.2–45.6). The most common indication for lung transplant was interstitial lung disease (56%), followed by COPD (30%) (Table E2). Of the cohort, 354 subjects completed FFP assessments, 262 completed SPPB assessments, and 200 underwent whole-body dual-energy X-ray absorptiometry (Table E2 and Figure E1).
We found that frailty was common: 10% were frail based on the SPPB (95% confidence interval [CI]: 7–14%) and 28% by the FFP (95% CI, 24–33%) (Table 1). There was no difference in the proportion of frail subjects by disease category. The median SPPB score was 11 (IQR, 9–11; SD, 2.1) and was skewed toward the not frail state (Figure E2A). The median FFP score was 2 (IQR, 1–3; SD, 1.2) and more normally distributed (Figure E2B). Table E3 shows the SPPB domains and the subject scores across each domain. Table E4 shows the proportion of subjects considered frail in each of the FFP domains. Although 35% of subjects had low muscle mass sarcopenia (95% CI, 28–42%), sarcopenia was not associated with frailty. Among frail subjects by SPPB, 22% had sarcopenia, whereas 34% of nonfrail subjects had sarcopenia (P = 0.47 by χ2 test). Among frail subjects by FFP, 41% had sarcopenia, whereas 33% of nonfrail subjects had sarcopenia (P = 0.33).
Table 1.
Comparison of Demographics and Baseline Clinical Characteristics by Frailty Status
| Short Physical Performance Battery |
Fried Frailty Phenotype |
|||||
|---|---|---|---|---|---|---|
| Frail | Not Frail | P Value | Frail | Not Frail | P Value | |
| No. of subjects | 26 | 236 | 99 | 255 | ||
| Age, yr | 59 (50–65) | 59 (50–65) | 0.86 | 58 (46–63) | 59 (50–65) | 0.11 |
| Male | 13 (50%) | 135 (57%) | 0.48 | 50 (51%) | 142 (56%) | 0.38 |
| Female | 13 (50%) | 101 (43%) | 49 (49%) | 113 (44%) | ||
| Race/ethnicity | ||||||
| White, non-Hispanic | 15 (58%) | 180 (76%) | 0.07 | 65 (66%) | 210 (82%) | 0.003 |
| Black | 5 (19%) | 13 (6%) | 5 (5%) | 8 (3%) | ||
| Asian | 1 (4%) | 15 (6%) | 7 (7%) | 8 (3%) | ||
| Hispanic | 2 (8%) | 19 (8%) | 7 (7%) | 13 (5%) | ||
| Other | 1 (4%) | 4 (2%) | 14 (14%) | 11 (4%) | ||
| Diagnostic category* | ||||||
| Group A (COPD) | 6 (23%) | 78 (33%) | 0.38 | 25 (25%) | 70 (27%) | 0.85 |
| Group B (PAH) | 2 (8%) | 14 (6%) | 6 (6%) | 14 (5%) | ||
| Group C (CF) | 0 (0%) | 13 (6%) | 7 (7%) | 24 (9%) | ||
| Group D (ILD) | 18 (69%) | 131 (56%) | 61 (62%) | 147 (6%) | ||
| Body mass index, kg/m2 | 26.2 ± 7.5 | 26.1 ± 4.5 | 0.93 | 24.1 ± 4.6 | 26.0 ± 4.8 | 0.001 |
| Hemoglobin, g/dl | 12.0 ± 1.9 | 13.4 ± 2.0 | 0.004 | 12.5 ± 1.8 | 13.3 ± 2.0 | 0.001 |
| Creatinine, mg/dl | 0.9 ± 0.5 | 0.8 ± 0.2 | 0.83 | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.62 |
| FEV1, L | 1.4 (1.1–1.7) | 1.3 (0.8–1.9) | 0.49 | 1.2 (0.8–1.7) | 1.3 (0.8–1.8) | 0.58 |
| FVC, L | 1.7 (1.4–2.5) | 2.2 (1.6–2.7) | 0.12 | 1.9 (1.4–2.4) | 2.0 (1.5–2.6) | 0.16 |
| 6-minute-walk distance, m | 141 (36–213) | 355 (237–485) | <0.001 | 208 (134–323) | 333 (244–423) | <0.001 |
| LAS | 78 (62–94) | 45 (36–50) | <0.001 | 44 (35–61) | 37 (33–42) | <0.001 |
Definition of abbreviations: CF = cystic fibrosis; COPD = chronic obstructive pulmonary disease; ILD = interstitial lung disease; LAS = Lung Allocation Score; PAH = pulmonary arterial hypertension.
Data are presented as n (%), mean ± SD, or median (interquartile range).
Diagnostic categories used for calculation of the Lung Allocation Score (9).
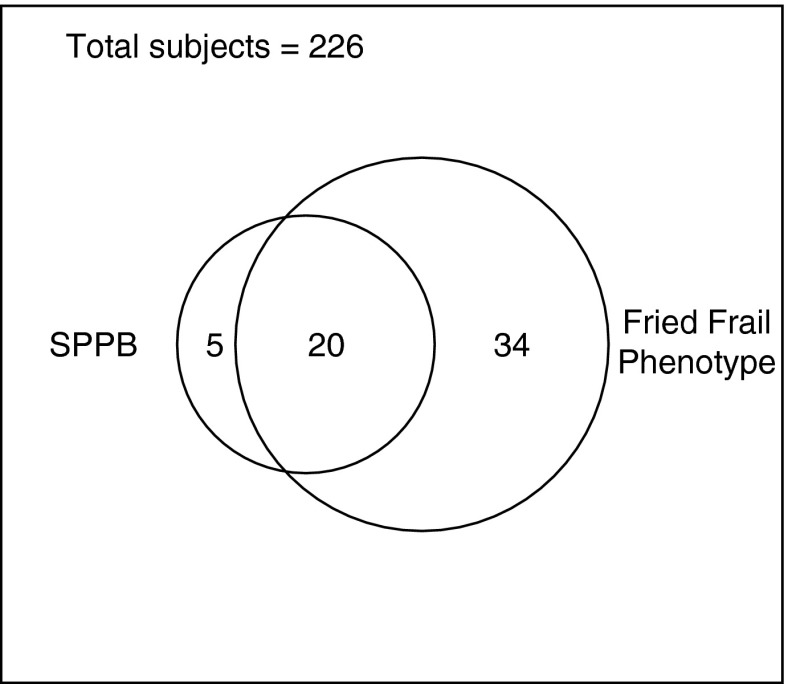
Although the prevalence varied by measure, both the SPPB and FFP appeared to measure similar conceptual constructs. Figure 1 shows that among those for whom both measures were available (n = 226), 80% of the subjects who were frail by SPPB were also frail by FFP. The strength and direction of correlations between SPPB and FFP and other measures were as hypothesized (Table 2). For example, both the SPPB and FFP negatively correlated with 6MWD, lean muscle mass, and grip strength, and both positively correlated with LAS. The strength and direction of the correlations were generally unchanged when stratified by age older than 60 years, pulmonary fibrosis, COPD, and in complete case analyses (stratifications are given in Tables E5–E9). We observed differences in biomarker levels consistent with findings in other populations, although not all comparisons achieved statistical significance. Frail subjects tended to have higher levels of IL-6 (SPPB, P = 0.10; FFP, P = 0.003) and tumor necrosis factor receptor 1 (SPPB, P = 0.001; FFP, P = 0.001)) and lower levels of insulin-like growth factor I (SPPB, P = 0.03; FFP, P = 0.16) and leptin (SPPB, P = 0.33; FFP, P = 0.08) (Figure 2A–2D).
Figure 1.
Comparison of the overlap of frailty diagnosis assessed by the Fried Frailty Phenotype (FFP) and Short Physical Performance Battery (SPPB). The rectangle represents the number of subjects with both SPPB and FFP assessments performed. Of the 25 frail subjects ascertained by SPPB, 20 were also frail by FFP (80%; 95% confidence interval, 64–96%). Of the 395 subjects in this study overall, 226 underwent both FFP and SPPB frailty assessments.
Table 2.
Tests of Convergent (Positive Correlation) and Divergent (Negative Correlation) Validity of Frailty Phenotypes and Conceptually Related Demographic, Physiologic, and Functional Factors
| Correlation | SPPB (n = 262)* | FFP (n = 354)* |
|---|---|---|
| ASMI | −0.15 | −0.21† |
| BMI | −0.03 | −0.23† |
| Grip strength | −0.24† | −0.34† |
| 6MWD | −0.55† | −0.34† |
| FVC | −0.18† | −0.15† |
| LAS | 0.34† | 0.29† |
| Hemoglobin | −0.28† | −0.24† |
| Age | 0.18† | −0.11† |
Definition of abbreviations: 6MWD = 6-minute-walk distance; ASMI = appendicular skeletal muscle index; BMI = body mass index; FFP = Fried Frailty Phenotype by ordinal score; LAS = Lung Allocation Score; SPPB = Short Physical Performance Battery by ordinal score.
Because lower scores on the SPPB denote increased frailty and higher scores for FFP denote increased frailty, SPPB scores were reverse coded for this analysis.
ASMI: n = 141 for SPPB and n = 200 for FFP; hemoglobin: n = 192 for SPPB and n = 318 for FFP.
Significant at false discovery rate of 0.05.
Figure 2.
Box plots of (A) IL-6, (B) tumor necrosis factor (TNF) receptor 1, (C) insulin like growth factor (IGF)-1, and (D) leptin levels by frailty status. Horizontal lines within the box plots represent the medians, and borders of box plots represent the interquartile ranges (IQRs). Whiskers represent the highest value within 1.5 IQR of the upper quartile and lowest value within 1.5 IQR of the lower quantile. Dots represent outlier values. FFP = Fried Frailty Phenotype; SPPB = Short Physical Performance Battery. For FFP, comparisons are made between 26 cases and 26 age-, sex-, and diagnosis-matched controls. For SPPB, comparisons are made between 12 cases and 12 age-, sex-, and diagnosis-matched controls.
We found that frailty was strongly associated with increased disability (Table 3). After adjusting for age, sex, diagnosis, and center, each SD unit of worsening (i.e., decrease) in SPPB score was associated with a 0.25-point (95% CI, 0.17–0.34) increase in LT-VLA disability. After further adjusting for LAS, lung function, and BMI, each 1 SD unit worsening in SPPB score was associated with a 0.12-point higher LT-VLA disability (95% CI, 0.04–0.21). We observed a similar association between FFP frailty and disability. Each 1 SD unit worsening in FFP score (i.e., increase) was associated with a 0.26-point higher LT-VLA disability (95% CI, 0.19–0.34), adjusting for age, sex, diagnosis, and center, and a 0.17-point higher disability (95% CI, 0.09–0.25) after further adjusting for LAS, lung function, and BMI.
Table 3.
Association between Frail Phenotypes and Mean LT-VLA Disability
| SPPB (n = 253) | FFP (n = 254) | |
|---|---|---|
| Model 1 | 0.27 (0.19–0.35) | 0.28 (0.20–0.36) |
| Model 2 | 0.25 (0.17–0.34) | 0.26 (0.19–0.34) |
| Model 3 | 0.12 (0.04–0.21) | 0.18 (0.10–0.25) |
| Model 4 | 0.12 (0.04–0.21) | 0.17 (0.09–0.25) |
Definition of abbreviations: FFP = Fried Frailty Phenotype; LT-VLA = Lung Transplant Valued Life Activities; SPPB = Short Physical Performance Battery.
Effect estimates are increases in mean LT-VLA (95% confidence intervals) per 1 SD worsening in frailty score. Worsening is defined as a decrease in SPPB and an increase in FFP. Values greater than 0 indicate greater disability. An LT-VLA increase ≥0.3 is clinically meaningful.
Model 1: adjusted for center.
Model 2: model 1 + age, sex, diagnosis.
Model 3: model 2 + Lung Allocation Score.
Model 4: model 3 + FVC, body mass index.
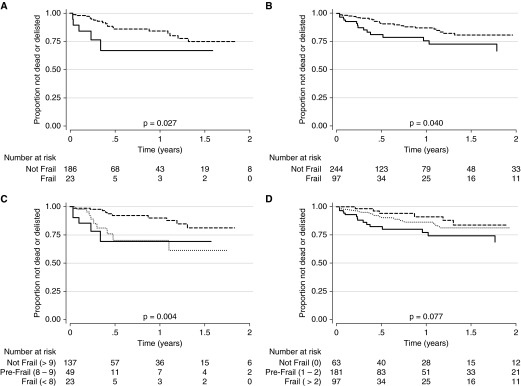
Frailty was also associated with an increased rate of delisting or death before lung transplant. In unadjusted analyses, frailty by either SPPB (score ≤ 7) or FFP (score ≥ 3) was associated with an increased estimated cumulative incidence of delisting or death (P < 0.05). The cumulative incidence of delisting or death by 1 year was 36% (95% CI, 16–68%) for subjects assessed to be frail by SPPB, compared with 16% for subjects who were not frail (95% CI, 10–25%) (Figure 3A). The cumulative incidence of delisting or death by 1 year was 27% (95% CI, 17–43%) for subjects assessed as frail based on FFP, compared with 13% for subjects who were not frail (95% CI, 8–20%) (Figure 3B). A monotonic stepup in risk was evident when we evaluated frailty as an ordinal categorical variable. For both the SPPB and FFP, estimated cumulative incidence of delisting or death was highest for frail subjects, intermediate among prefrail subjects, and lowest for the nonfrail subjects (Figures 3C, 3D). The association between frailty determined by SPPB and delisting or death was not substantively impacted by the binary cutoff used (data not shown).
Figure 3.
Time to delisting or death before lung transplant by (A) Short Physical Performance Battery (SPPB) binary score, (B) Fried Frailty Phenotype (FFP) binary score, (C) Short Physical Performance Battery ordinal score, and (D) Fried Frailty Phenotype ordinal score. Solid lines represent frail, dotted lines represent prefrail, and dashed lines represent not frail. For SPPB, lower scores denote increased frailty; for FFP, higher scores denote increased frailty.
Each SD unit of worsening in SPPB score was associated with a twofold relative increase in the risk of delisting or death after stratifying by center (hazard ratio [HR], 2.04; 95% CI, 1.49–2.79) (Table 4). After additionally stratifying by diagnosis and adjusting for age and sex, the risk estimate increased to threefold per 1 SD unit worsening in SPPB score (HR, 2.99; 95% CI, 1.77–5.06). Further adjusting for LAS, BMI, and 6MWD decreased the risk estimate to 2.40 (HR, 2.40; 95% CI, 1.03–5.59). We found a similar but attenuated relationship between frailty measured by the FFP and delisting or death before lung transplant. Each SD unit of worsening in the FFP score was associated with a 37% relative increase in the risk of delisting or death after stratifying by center (HR, 1.37; 95% CI, 0.98–1.91). Additional stratification by diagnosis and adjustment for age and sex did not appreciably change the risk estimate, but it did narrow the confidence interval (HR, 1.38; 95% CI, 1.01–1.89). Unlike SPPB, after further adjustment for LAS, BMI, and 6MWD, FFP-defined frailty was no longer a risk factor for delisting or death before lung transplant (HR, 0.95; 95% CI, 0.66–1.35). Results from the sensitivity analysis using competing risks demonstrated similar patterns of risk across frailty measures and models (Table E10).
Table 4.
Associations between Frail Phenotypes and the Risk of Delisting or Death before Lung Transplant
| SPPB (n = 209) | FFP (n = 341) | |
|---|---|---|
| Model 1 | 2.04 (1.49–2.79) | 1.37 (0.98–1.91) |
| Model 2 | 2.99 (1.77–5.06) | 1.38 (1.01–1.89) |
| Model 3 | 2.20 (1.14–4.25) | 1.14 (0.85–1.54) |
| Model 4 | 2.40 (1.03–5.59) | 0.95 (0.66–1.35) |
Definition of abbreviations: FFP = Fried Frailty Phenotype; SPPB = Short Physical Performance Battery.
Effect estimates are hazard ratios (95% confidence intervals) per 1 SD worsening in frailty score. Worsening is defined as a decrease in SPPB and an increase in FFP.
Model 1: unadjusted, stratified by center.
Model 2: adjusted for age and sex, stratified by center and diagnosis.
Model 3: adjusted for model 2 + Lung Allocation Score.
Model 4: adjusted for model 3 + body mass index, 6-minute-walk distance.
Discussion
In this multicenter cohort study of adult lung transplant candidates, we found that frailty, as assessed using two distinct measures, was common and independently associated with patient-reported disability and with subsequent delisting or death before transplant. We also found that the FFP and SPPB measures have reasonable construct validity; that is, they correlate with measures of impairment, functioning, and illness (including the LAS) and show differences in biomarker levels consistent with findings in other populations. Taken together, our findings suggest that frailty assessment might provide important morbidity and mortality risk information above and beyond typically captured clinical measures and the LAS. Our findings may be particularly relevant today, given that older and sicker patients are prioritized for lung transplants and morbidity and mortality remain persistently and unacceptably high. Frailty assessment provides a novel, objective measure that can be used to identify lung transplant candidates at increased risk before transplant for post-transplant disability and poor outcomes. Our findings also raise important questions about whether preoperative frailty might be associated with heightened risk for complications after transplant.
Our findings of the association of frailty with disability and mortality in lung transplant candidates are consistent with observations in other populations. Frailty measures operationalize what clinicians intuitively recognize as a sense that a patient may poorly tolerate a new physiologic stressor such as surgery. Fried, who was pivotal in initiating the construct, proposed that frailty could be conceptualized as an accumulation of physiologic deficits resulting in attenuated physiologic reserves (14). These reserves are required for the dynamic maintenance of homeostasis in the face of new physiologic stressors. Such attenuated reserves, or “homeostenosis” (26), result in a state of risk in which new stressors such as major surgery may exceed these reserves, resulting in catastrophic system failure. Concurrent with this conceptual definition, Fried and others developed operational measures of frailty (7, 14, 27), showing that it is associated with important outcomes in geriatric populations, including hip fracture, disability, institutionalization, poorer health-related quality of life, and mortality (6, 14, 49, 50). Following on this work, others found that preoperative frailty assessment predicted perioperative complications and mortality following cardiac and abdominal surgery above and beyond existing risk stratification tools (12, 13, 51–53). Most recently, these measures have been shown to predict death in liver transplant candidates as well as delayed graft function and death in kidney transplant recipients (15–17).
Frailty conceptually ties together a number of the previously observed risk factors for poor outcomes in patients with advanced lung disease and lung transplants. For example, low BMI and hypoalbuminemia are associated with both frailty and sarcopenia (presumably because abnormally low lean muscle mass and function is a putative driver of the frail phenotype) (29, 40, 42, 54, 55). We previously demonstrated that preoperative underweight status and hypoalbuminemia are strongly associated with mortality after lung transplant (34, 56). Also, low lean muscle mass sarcopenia is common in patients with COPD and in lung transplant candidates (34, 47). Consistent with these reports, lean muscle mass correlated with both of the frailty measures used in this study.
Protein biomarkers provide some insights into potential mechanisms driving the frailty phenotype. For example, leptin levels tended to be lower in our frail subjects. Leptin, a measure of adipose tissue mass, is lower in patients with cachexia as well as frailty (42, 57, 58). Chronic inflammation is considered another potential cause of the frail phenotype (40, 59). Increased IL-6 and chronic cytomegalovirus infection are associated with frailty as well as with primary graft dysfunction and acute and chronic allograft rejection following lung transplant (38, 39, 60–65). Our future efforts will be focused on understanding the mechanisms underpinnings suggested by our biomarker findings. Importantly, recent work in older populations suggests that frailty may be reversible through targeted exercise- and nutrient-based interventions (66–68). Targeting at-risk, frail patients before lung transplant may reduce perioperative complications, reduce the risk of death, and mitigate disability and risk of poorer health-related quality of life after transplant.
Although our findings support the validity and relevance of frailty assessment in lung transplant candidates, the strength of the associations between frailty and disability and delisting or death appeared to differ by instrument. Although conceptually frailty has face validity and the instruments used in this study have reasonable construct validity, neither was developed specifically for adults with advanced lung disease. Thus, some frailty components, such as low activity or exhaustion in the FFP, may be confounded by lung disease. For example, the activity scale used in the FFP instrument includes activities that few lung transplant candidates are likely to perform, such as jogging and tennis. Supporting this possibility is the high prevalence of frailty by FFP observed in our study compared with other populations and the stronger observed association between the SPPB and disability and delisting or death compared with the FFP after controlling for relevant covariates. Alternatively, whereas the prevalence of frailty by FFP is high relative to other populations, lung disease may cause frailty, which could also explain, in part, the prevalence of frailty that we observed. Many advanced lung diseases are associated with systemic inflammation, hypoxia, chronic infections, increased resting energy expenditure, corticosteroid and other immunomodulator use, and immobility, which are all theoretical causes of frailty (69–74). Future work should focus on refining the operational measure of frailty in lung disease to improve risk prediction.
Our study has notable strengths. We performed a relatively large prospective multicenter study. We evaluated the construct validity of frailty by two different established instruments in comparison with relevant clinical measures and biomarkers that represent putative mechanistic pathways driving frailty. In estimating the association between frailty and key clinical outcomes, our study size enabled us to control for multiple relevant covariates. Despite these strengths, we did not have a sufficient number of subjects to confidently estimate the impact of frailty across clinically relevant stratified analyses, such as by age category or diagnostic subgroup. For example, our cohort included relatively few subjects with cystic fibrosis or pulmonary arterial hypertension. Also, it is possible that unmeasured covariates could explain the observed association between frailty and disability and delisting or death. Additionally, requirements for pulmonary rehabilitation before listing for lung transplant varied across the participating centers. Whereas one center requires completion of pulmonary rehabilitation at a program of the patient’s choosing, others less stringently recommend regular exercise. Although our models were conditioned or stratified by center, it is possible that this methodological approach did not fully account for the potential impact of pulmonary rehabilitation. Further, because these measures were implemented at different times at the four participating centers, not all subjects underwent all measurements. Finally, and importantly, this study did not investigate the impact of preoperative frailty on outcomes after lung transplant. Further work should clarify this impact and the relevance of frailty across clinically important strata of lung transplant candidates before frailty can be used to inform patient management, determine transplant candidacy, or be considered in lung allocation schema. Indeed, if preoperative frailty is not associated with poorer postoperative outcomes, frail patients may benefit from prioritization, whereas if it is, frailty assessments may help identify patients unlikely to benefit from lung transplantation.
In summary, despite stringent selection criteria, frailty is common and associated with disability, delisting, and death in candidates awaiting lung transplant. This study provides support for the importance of frailty in lung transplantation and is consistent with the emerging importance of refined assessments of functioning and body composition in the lung transplant population. Multipronged efforts aimed at refining clinical assessments, understanding the mechanisms, and developing interventions targeting perturbations in body composition may provide important insights into the high morbidity and mortality in patients with advanced lung disease and lung transplants.
Acknowledgments
Acknowledgment
The authors greatly appreciate the hundreds of patients who have participated in the Lung Transplant Body Composition Study. The authors also appreciate the efforts of Paul Vaughn, PTA, in guidance regarding the measurement of the Short Physical Performance Battery; Janet La, B.S., for her technical assistance in performing the biomarker measurements; and Michael Peters, M.D., for his advice and expertise in the measurement of IL-6.
Footnotes
Supported by NHLBI grant K23 HL111115 and a Nina Ireland Program in Lung Health Award (J.P.S.); NHLBI grant K23 HL121406 (J.M.D.); and NHLBI grants R01 HL081619, HL096845, HL115354, HL087115 (J.D.C.), and HL114626 (D.J.L.). This project was also supported in part by the Rocco Guinta Research Fund and an Irving Pilot Award from the Irving Institute for Clinical and Translational Research. A portion of this study was supported by the National Center for Advancing Translational Sciences (previously the National Center for Research Resources) at the National Institutes of Health (UCSF-CTSI UL1 RR024131; CUMC-CTSA UL1 TR000040).
Author Contributions: J.P.S., J.M.D., C.J.G., P.D.B., P.P.K., J.D.C., and D.J.L. made substantial contributions to the conception and design of the work; J.P.S. wrote the first draft of the manuscript; and J.P.S., J.M.D., C.J.G., P.D.B., R.S., J.R.G., J.A.G., S.H., P.P.K., L.E.L., J.K., J.D.C., and D.J.L. revised the manuscript for important intellectual content. All authors made substantial contributions to the acquisition, analysis, or interpretation of data for the work. All authors approved the manuscript. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201506-1150OC on August 11, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Valapour M, Skeans MA, Heubner BM, Smith JM, Schnitzler MA, Hertz MI, Edwards LB, Snyder JJ, Israni AK, Kasiske BL. OPTN/SRTR 2012 Annual Data Report: lung. Am J Transplant. 2014;14:139–165. doi: 10.1111/ajt.12584. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Lund LH, Meiser B, et al. International Society for Heart and Lung Transplantation. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report—2014; focus theme: retransplantation. J Heart Lung Transplant. 2014;33:1009–1024. doi: 10.1016/j.healun.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell BG, Mooney JJ, Lee PH, Levitt JE, Chhatwani L, Nicolls MR, Zamora MR, Valentine V, Weill D, Dhillon GS. Increased resource use in lung transplant admissions in the Lung Allocation Score era. Am J Respir Crit Care Med. 2015;191:302–308. doi: 10.1164/rccm.201408-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyu DM, Zamora MR. Medical complications of lung transplantation. Proc Am Thorac Soc. 2009;6:101–107. doi: 10.1513/pats.200808-077GO. [DOI] [PubMed] [Google Scholar]
- 5.Egan TM, Murray S, Bustami RT, Shearon TH, McCullough KP, Edwards LB, Coke MA, Garrity ER, Sweet SC, Heiney DA, et al. Development of the new lung allocation system in the United States. Am J Transplant. 2006;6:1212–1227. doi: 10.1111/j.1600-6143.2006.01276.x. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 8.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, Walston JD, Fried LP Cardiovascular Health Study Research Group. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 9.Sancarlo D, Pilotto A, Panza F, Copetti M, Longo MG, D’Ambrosio P, D’Onofrio G, Ferrucci L, Pilotto A. A Multidimensional Prognostic Index (MPI) based on a comprehensive geriatric assessment predicts short- and long-term all-cause mortality in older hospitalized patients with transient ischemic attack. J Neurol. 2012;259:670–678. doi: 10.1007/s00415-011-6241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristjansson SR, Nesbakken A, Jordhøy MS, Skovlund E, Audisio RA, Johannessen HO, Bakka A, Wyller TB. Comprehensive geriatric assessment can predict complications in elderly patients after elective surgery for colorectal cancer: a prospective observational cohort study. Crit Rev Oncol Hematol. 2010;76:208–217. doi: 10.1016/j.critrevonc.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Lee DH, Buth KJ, Martin BJ, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;121:973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 12.Lee JS, He K, Harbaugh CM, Schaubel DE, Sonnenday CJ, Wang SC, Englesbe MJ, Eliason JL Michigan Analytic Morphomics Group (MAMG) Frailty, core muscle size, and mortality in patients undergoing open abdominal aortic aneurysm repair. J Vasc Surg. 2011;53:912–917. doi: 10.1016/j.jvs.2010.10.111. [DOI] [PubMed] [Google Scholar]
- 13.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, et al. Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 15.Garonzik-Wang JM, Govindan P, Grinnan JW, Liu M, Ali HM, Chakraborty A, Jain V, Ros RL, James NT, Kucirka LM, et al. Frailty and delayed graft function in kidney transplant recipients. Arch Surg. 2012;147:190–193. doi: 10.1001/archsurg.2011.1229. [DOI] [PubMed] [Google Scholar]
- 16.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant. 2014;14:1870–1879. doi: 10.1111/ajt.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McAdams-DeMarco MA, Law A, King E, Orandi B, Salter M, Gupta N, Chow E, Alachkar N, Desai N, Varadhan R, et al. Frailty and mortality in kidney transplant recipients. Am J Transplant. 2015;15:149–154. doi: 10.1111/ajt.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hook JL, Sonett J, Wilt J, Shah L, Robbins H, Sanyal S, Philip N, Peterson ER, Arcasoy S, Lederer DJ. The frailty phenotype in lung transplantation: preliminary results [abstract] Am J Respir Crit Care Med. 2012;187:A5328. [Google Scholar]
- 19.Lederer DJ, Sonett JR, Philip NA, Larkin M, Peterson ER, Desai A, Sanyal S, Shah L, Robbins HA, Raza K, et al. Frailty and early mortality after lung transplantation: preliminary results [abstract] J Heart Lung Transplant. 2013;32(4 Suppl):S119–S120. [Google Scholar]
- 20.Podolanczuk A, Peterson ER, Shah L, Robbins H, Philip N, Desai A, Larkin M, Ravichandran SN, Arcasoy SM, Lederer DJ. Gender, race, and clinical characteristics of frail lung transplant candidates [abstract] Am J Respir Crit Care Med. 2013;187:A2197. [Google Scholar]
- 21.Restivo M, Peterson ER, Shah L, Robbins H, Philip N, Desai A, Larkin M, Smith N, Arcasoy SM, Lederer DJ. Frailty is independently associated with reduced six-minute walk distance in lung transplant candidates [abstract] Am J Respir Crit Care Med. 2013;187:A2198. [Google Scholar]
- 22.Singer JP, Diamond JM, Gries CJ, McDonnough J, Blanc PD, Shah R, Dean MY, Hersch B, Dolan J, Arcasoy S, et al. Frailty is associated with pre-operative delisting and death in lung transplant candidates [abstract] J Heart Lung Transplant. 2015;34(4 Suppl):S15. [Google Scholar]
- 23.Singer JP, Katz PP, Dean MY, Chen J, Su B, Kern R, Leard LE, Hays SR, Kukreja J, Blanc PD. Frailty is common in lung transplant candidates and associated with poorer health-related quality of life [abstract] J Heart Lung Transplant. 2013;32(4 Suppl):S43. [Google Scholar]
- 24.Singer JP, Peterson ER, Golden J, Snyder ME, Lim B, Desai A, Sonett JR, Kukreja J, Arcasoy SM, Katz PP, et al. Lung transplant and body composition [abstract] J Heart Lung Transplant. 2014;33(4 Suppl):S79. [Google Scholar]
- 25.Rodríguez-Mañas L, Féart C, Mann G, Viña J, Chatterji S, Chodzko-Zajko W, Gonzalez-Colaço Harmand M, Bergman H, Carcaillon L, Nicholson C, et al. FOD-CC group (Appendix 1) Searching for an operational definition of frailty: a Delphi method based consensus statement: the frailty operative definition-consensus conference project. J Gerontol A Biol Sci Med Sci. 2013;68:62–67. doi: 10.1093/gerona/gls119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowdry EV, Allen E. Problems of ageing: biological and medical aspects. Baltimore: Williams & Wilkins; 1942. [Google Scholar]
- 27.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10:104–114. doi: 10.1016/j.arr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 29.Cesari M, Landi F, Vellas B, Bernabei R, Marzetti E. Sarcopenia and physical frailty: two sides of the same coin. Front Aging Neurosci. 2014;6:192. doi: 10.3389/fnagi.2014.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, Tomiyama K, Yagi S, Mori A, Uemoto S. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. 2013;13:1549–1556. doi: 10.1111/ajt.12221. [DOI] [PubMed] [Google Scholar]
- 33.Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, Malani PN. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396–1402. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer JP, Peterson ER, Snyder ME, Katz PP, Golden JA, D’Ovidio F, Bacchetta M, Sonett JR, Kukreja J, Shah L, et al. Body composition and mortality after adult lung transplantation in the United States. Am J Respir Crit Care Med. 2014;190:1012–1021. doi: 10.1164/rccm.201405-0973OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–1112. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 36.Shamliyan T, Talley KM, Ramakrishnan R, Kane RL. Association of frailty with survival: a systematic literature review. Ageing Res Rev. 2013;12:719–736. doi: 10.1016/j.arr.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Läärä E. Study designs for biobank-based epidemiologic research on chronic diseases. Methods Mol Biol. 2011;675:165–178. doi: 10.1007/978-1-59745-423-0_6. [DOI] [PubMed] [Google Scholar]
- 38.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002;50:1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 39.Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- 40.Hubbard RE, O’Mahony MS, Savva GM, Calver BL, Woodhouse KW. Inflammation and frailty measures in older people. J Cell Mol Med. 2009;13:3103–3109. doi: 10.1111/j.1582-4934.2009.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16:153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 42.Hubbard RE, O’Mahony MS, Calver BL, Woodhouse KW. Nutrition, inflammation, and leptin levels in aging and frailty. J Am Geriatr Soc. 2008;56:279–284. doi: 10.1111/j.1532-5415.2007.01548.x. [DOI] [PubMed] [Google Scholar]
- 43.Singer JP, Blanc PD, Dean YM, Hays S, Leard L, Kukreja J, Golden J, Katz PP. Development and validation of a lung transplant-specific disability questionnaire. Thorax. 2014;69:437–442. doi: 10.1136/thoraxjnl-2013-204557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. JR Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 45.Penninx BW, Ferrucci L, Leveille SG, Rantanen T, Pahor M, Guralnik JM. Lower extremity performance in nondisabled older persons as a predictor of subsequent hospitalization. J Gerontol A Biol Sci Med Sci. 2000;55:M691–M697. doi: 10.1093/gerona/55.11.m691. [DOI] [PubMed] [Google Scholar]
- 46.Volpato S, Cavalieri M, Sioulis F, Guerra G, Maraldi C, Zuliani G, Fellin R, Guralnik JM. Predictive value of the Short Physical Performance Battery following hospitalization in older patients. J Gerontol A Biol Sci Med Sci. 2011;66:89–96. doi: 10.1093/gerona/glq167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones SE, Maddocks M, Kon SS, Canavan JL, Nolan CM, Clark AL, Polkey MI, Man WD. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax. 2015;70:213–218. doi: 10.1136/thoraxjnl-2014-206440. [DOI] [PubMed] [Google Scholar]
- 48.Rubin DB. Multiple imputation for non-response in surveys. New York: Wiley; 1997. [Google Scholar]
- 49.Cacciatore F, Abete P, Mazzella F, Viati L, Della Morte D, D’Ambrosio D, Gargiulo G, Testa G, Santis D, Galizia G, et al. Frailty predicts long-term mortality in elderly subjects with chronic heart failure. Eur J Clin Invest. 2005;35:723–730. doi: 10.1111/j.1365-2362.2005.01572.x. [DOI] [PubMed] [Google Scholar]
- 50.Masel MC, Ostir GV, Ottenbacher KJ. Frailty, mortality, and health-related quality of life in older Mexican Americans. J Am Geriatr Soc. 2010;58:2149–2153. doi: 10.1111/j.1532-5415.2010.03146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48:78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Rønning B, Wyller TB, Seljeflot I, Jordhøy MS, Skovlund E, Nesbakken A, Kristjansson SR. Frailty measures, inflammatory biomarkers and post-operative complications in older surgical patients. Age Ageing. 2010;39:758–761. doi: 10.1093/ageing/afq123. [DOI] [PubMed] [Google Scholar]
- 53.Sündermann S, Dademasch A, Praetorius J, Kempfert J, Dewey T, Falk V, Mohr FW, Walther T. Comprehensive assessment of frailty for elderly high-risk patients undergoing cardiac surgery. Eur J Cardiothorac Surg. 2011;39:33–37. doi: 10.1016/j.ejcts.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 54.Hubbard RE, Lang IA, Llewellyn DJ, Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol A Biol Sci Med Sci. 2010;65:377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 55.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272:1036–1042. [PubMed] [Google Scholar]
- 56.Baldwin MR, Arcasoy SM, Shah A, Schulze PC, Sze J, Sonett JR, Lederer DJ. Hypoalbuminemia and early mortality after lung transplantation: a cohort study. Am J Transplant. 2012;12:1256–1267. doi: 10.1111/j.1600-6143.2011.03965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mantovani G, Macciò A, Mura L, Massa E, Mudu MC, Mulas C, Lusso MR, Madeddu C, Dessì A. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J Mol Med (Berl) 2000;78:554–561. doi: 10.1007/s001090000137. [DOI] [PubMed] [Google Scholar]
- 58.Murdoch DR, Rooney E, Dargie HJ, Shapiro D, Morton JJ, McMurray JJ. Inappropriately low plasma leptin concentration in the cachexia associated with chronic heart failure. Heart. 1999;82:352–356. doi: 10.1136/hrt.82.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morley JE, Baumgartner RN. Cytokine-related aging process. J Gerontol A Biol Sci Med Sci. 2004;59:M924–M929. doi: 10.1093/gerona/59.9.m924. [DOI] [PubMed] [Google Scholar]
- 60.Allen JG, Lee MT, Weiss ES, Arnaoutakis GJ, Shah AS, Detrick B. Preoperative recipient cytokine levels are associated with early lung allograft dysfunction. Ann Thorac Surg. 2012;93:1843–1849. doi: 10.1016/j.athoracsur.2012.02.041. [DOI] [PubMed] [Google Scholar]
- 61.Iacono A, Dauber J, Keenan R, Spichty K, Cai J, Grgurich W, Burckart G, Smaldone G, Pham S, Ohori NP, et al. Interleukin 6 and interferon-γ gene expression in lung transplant recipients with refractory acute cellular rejection: implications for monitoring and inhibition by treatment with aerosolized cyclosporine. Transplantation. 1997;64:263–269. doi: 10.1097/00007890-199707270-00015. [DOI] [PubMed] [Google Scholar]
- 62.Lu KC, Jaramillo A, Lecha RL, Schuessler RB, Aloush A, Trulock EP, Mendeloff EN, Huddleston CB, Alexander Patterson G, Mohanakumar T. Interleukin-6 and interferon-γ gene polymorphisms in the development of bronchiolitis obliterans syndrome after lung transplantation. Transplantation. 2002;74:1297–1302. doi: 10.1097/00007890-200211150-00017. [DOI] [PubMed] [Google Scholar]
- 63.Mathur A, Baz M, Staples ED, Bonnell M, Speckman JM, Hess PJ, Jr, Klodell CT, Knauf DG, Moldawer LL, Beaver TM. Cytokine profile after lung transplantation: correlation with allograft injury. Ann Thorac Surg. 2006;81:1844–1850. doi: 10.1016/j.athoracsur.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 64.Saito T, Takahashi H, Kaneda H, Binnie M, Azad S, Sato M, Waddell TK, Cypel M, Liu M, Keshavjee S. Impact of cytokine expression in the pre-implanted donor lung on the development of chronic lung allograft dysfunction subtypes. Am J Transplant. 2013;13:3192–3201. doi: 10.1111/ajt.12492. [DOI] [PubMed] [Google Scholar]
- 65.Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, et al. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Am J Epidemiol. 2010;171:1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cameron ID, Fairhall N, Langron C, Lockwood K, Monaghan N, Aggar C, Sherrington C, Lord SR, Kurrle SE. A multifactorial interdisciplinary intervention reduces frailty in older people: randomized trial. BMC Med. 2013;11:65. doi: 10.1186/1741-7015-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fairhall N, Langron C, Sherrington C, Lord SR, Kurrle SE, Lockwood K, Monaghan N, Aggar C, Gill L, Cameron ID. Treating frailty - a practical guide. BMC Med. 2011;9:83. doi: 10.1186/1741-7015-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Latham NK, Harris BA, Bean JF, Heeren T, Goodyear C, Zawacki S, Heislein DM, Mustafa J, Pardasaney P, Giorgetti M, et al. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: a randomized clinical trial. JAMA. 2014;311:700–708. doi: 10.1001/jama.2014.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150:11–16. doi: 10.1164/ajrccm.150.1.8025735. [DOI] [PubMed] [Google Scholar]
- 70.Schols AM, Buurman WA, Staal van den Brekel AJ, Dentener MA, Wouters EF. Evidence for a relation between metabolic derangements and increased levels of inflammatory mediators in a subgroup of patients with chronic obstructive pulmonary disease. Thorax. 1996;51:819–824. doi: 10.1136/thx.51.8.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Congleton J. The pulmonary cachexia syndrome: aspects of energy balance. Proc Nutr Soc. 1999;58:321–328. doi: 10.1017/s0029665199000439. [DOI] [PubMed] [Google Scholar]
- 72.Takabatake N, Nakamura H, Abe S, Inoue S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. The relationship between chronic hypoxemia and activation of the tumor necrosis factor-α system in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;161:1179–1184. doi: 10.1164/ajrccm.161.4.9903022. [DOI] [PubMed] [Google Scholar]
- 73.Eisner MD, Blanc PD, Yelin EH, Sidney S, Katz PP, Ackerson L, Lathon P, Tolstykh I, Omachi T, Byl N, et al. COPD as a systemic disease: impact on physical functional limitations. Am J Med. 2008;121:789–796. doi: 10.1016/j.amjmed.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Breyer MK, Rutten EP, Locantore NW, Watkins ML, Miller BE, Wouters EF ECLIPSE Investigators (Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints) Dysregulated adipokine metabolism in chronic obstructive pulmonary disease. Eur J Clin Invest. 2012;42:983–991. doi: 10.1111/j.1365-2362.2012.02686.x. [DOI] [PubMed] [Google Scholar]