To the Editor:
The vast majority of treatments for cystic fibrosis (CF) target the downstream consequences of the disease and are incompletely effective. The success of the CF transmembrane conductance regulator (CFTR) potentiator ivacaftor has illustrated the clinical benefits arising from restoration of CFTR protein function (1). This agent is applicable as a monotherapy for a minority of patients with specific, rare mutations. CFTR gene therapy, a mutation-independent alternative, has demonstrated proof of principle for gene transfer in animal models and human trials, but only one study (using a viral vector) has unsuccessfully assessed whether clinical outcomes can be improved (2). In preparation for a phase IIb clinical trial of repeatedly administered, nonviral, liposome-mediated CFTR gene transfer assessing clinically meaningful outcomes (3), the UK CF Gene Therapy Consortium (www.cfgenetherapy.org.uk) undertook a single-application safety and dose-ranging study (NCT00789867).
Some of the results of these studies have been previously reported in the form of abstracts (4, 5).
The chosen plasmid DNA expresses CFTR under the control of the human cytomegalovirus enhancer/elongation factor 1α sequence (6), a modified EF1a promoter aiming for extended duration of expression (7), and was rendered CpG-free to minimize a host inflammatory response (6). The cationic lipid, GL67A, was chosen on the basis of extensive preclinical testing (8). After informed consent, adult patients with CF received a single nebulized +/− nasal dose of pGM169/GL67A. Reconstitution and preparation of pGM169/GL67A was undertaken on the day, and doses were delivered in sealed negative-pressure cubicles after pretreatment with inhaled salbutamol (albuterol). Preplanned adjunctive therapies including ibuprofen, prednisolone, or paracetamol were administered to some patients.
The primary outcome of the clinical study was safety; assessment included examination, standard hematology/biochemistry, adverse events, spirometry, lung clearance index, chest computed tomography scan, gas transfer, bronchial biopsy histology, and immune markers. pGM169-specific DNA and mRNA were measured on nasal and lower airway brushings, with potential difference also measured bronchoscopically and nasally. For the latter, “responders” were defined as demonstrating chloride secretion 5 mV or more greater than their mean predose value, and greater than any of their predose responses.
A total of 35 subjects (Tables 1 and 2) received a nebulized dose (5 ml, n = 8; 10 ml, n = 10; 20 ml, n = 17) via an AeroEclipse II (Trudell Medical International, London, ON, Canada) breath-actuated nebulizer (9). Three subjects undertook slow delivery (∼75 vs. 25 min for each 5 ml). Standard spray devices were used for nasal delivery (2 ml, n = 21). According to pre-/post-device weighing, a mean (SD) of 88.7% (2.9%) of expected nebulized dose and 94.5% (15.0%) of expected nasal dose was delivered. There were two serious adverse events: one occurred after the predosing bronchoscopy (swelling of the uvula related to intubation) and led to observation overnight in hospital, and the other was an episode of pancreatitis occurring around Day 10 after dosing (10 ml nebulized cohort). The subject was exocrine pancreatic sufficient and had likely experienced previous similar, but undiagnosed, episodes.
Table 1.
Baseline Demographics
| All Nebulized Subjects | 20 ml | 10 ml | 5 ml | |
|---|---|---|---|---|
| Number of patients | 35 | 17 | 10 | 8 |
| Percentage male | 65.7 | 70.6 | 60 | 62.5 |
| Age, yr, median (range) | 27.4 (16.4–61.6) | 26.7 (17.3–50.1) | 33.2 (16.4–61.6) | 32.6 (24.3–46.4) |
| FEV1% predicted at recruitment, mean (SD) | 79 (14) | 78 (11) | 77 (13) | 84 (18) |
| Percentage homozygous p.Phe508del | 57.1 | 58.8 | 40 | 75 |
| Pancreatic insufficient, % | 82.9 | 88.2 | 80 | 75 |
| Nebulized antipseudomonal antibiotics, % | 80 | 82.4 | 70 | 87.5 |
| rhDNase, % | 40 | 29.4 | 60 | 37.5 |
| Hypertonic saline, % | 22.9 | 23.5 | 20 | 25 |
| Daily (long-term) azithromycin, % | 65.7 | 70.6 | 70 | 50 |
| Insulin, % | 17.1 | 23.5 | 10 | 12.5 |
| Inhaled steroids, % | 45.7 | 35.3 | 70 | 37.5 |
Definition of abbreviation: rhDNase = recombinant human DNase.
Table 2.
Postdosing Responses
| Time Point of Maximal Effect | 20 ml | 10 ml | 5 ml | P Value | |
|---|---|---|---|---|---|
| Systemic | |||||
| Fever, % of group >38°C | 6-8 h | 93.3 | 50 | 0 | 0.0003 |
| Maximum temperature, mean (SD) | 6-8 h | 38.6 (0.5) | 38.0 (0.7) | 37.4 (0.3) | 0.0002 |
| Blood leukocytes, mean (SD) | 8 h | 15.8 (3.2) | 14.1 (4.5) | 12.8 (3.8) | 0.22 |
| Blood neutrophils, mean (SD) | 8 h | 13.9 (3.4) | 11.3 (4.1) | 9.8 (3.5) | 0.06 |
| Serum C-reactive protein, median (IQR) | Day 2 | 59 (36-73) | 51 (25-88) | 38 (5-78) | 0.4 |
| Pulmonary | |||||
| FEV1, % of group with >20% relative drop | 66.6 | 33.3 | 0 | 0.02 | |
| FEV1 relative % drop, mean (SD) | ∼6 h | 24.6 (9.3) | 17.5 (7.8) | 16.8 (4.0) | 0.08 |
| FVC relative % drop, mean (SD) | ∼6 h | 20.7 (2.9) | 13.7 (2.2) | 14.7 (2.2) | 0.22 |
| FEV1/FVC ratio, absolute % | ∼6 h | −3.1 | −2.7 | −1.7 | 0.85 |
Definition of abbreviation: IQR = interquartile range.
Postdosing responses reported only for patients not receiving adjunctive treatments (20 ml, n = 15; 10 ml, n = 6; 5 ml, n = 6).
Results for two 20-ml patients receiving prednisolone and ibuprofen, respectively, lay within group spread. Data from six patients receiving paracetamol (10 ml, n = 4; 5 ml, n = 2) showed a trend to a reduction in systemic responses, but as numbers are small they have not been subjected to formal statistical analysis. Paracetamol was included in the future protocol to mitigate against the possibility that a patient might experience an influenza-like response and become unblinded. Three-group data were compared with analysis of variance, Kruskall-Wallis, or Fisher’s exact tests, as appropriate.
Overall, in the trial, 94.3% of subjects experienced at least one adverse event, the majority of which were mild to moderate in severity and resolved spontaneously or with standard antipyretics. The most common occurred on the day of dosing and largely resolved within 24–48 hours (Tables 1 and 2): Typically, within the first few hours after dosing, a mild, self-limiting influenza-like systemic response was seen, most frequently in the 20-ml patients. This was not affected by slow delivery or coadministration of ibuprofen or prednisolone but was clearly dose-related and reduced by paracetamol. Symptoms of headache and/or tiredness were reported by 82, 70, and 13%, and raised serum inflammatory markers were recorded in 100, 60, and 63% of the 20-, 10-, and 5-ml groups respectively, with dose-related trends in maximal values. No patient dosed with 5 ml had a temperature higher than 38°C (Table 2). A relatively asymptomatic, dose-related, restrictive drop in spirometry was also observed, with no change in respiratory rate or oxygen saturation. No patient dosed with 5 ml showed a more than 20% relative fall in FEV1 (Table 2). The 20-ml group showed a small, significant (P < 0.05) mean (SD) drop in gas transfer (transfer factor for carbon monoxide corrected for alveolar volume and hemoglobin concentration) on Day 2 of 4.5% (6.0%), which returned to baseline values by Day 14. No changes were seen in the other cohorts. Two of the 20-ml patients had small areas of ground glass opacity reported on their Day 2 chest computed tomography scans, which resolved by Day 14. No significant changes were seen in endobronchial histology (20 ml; n = 10).
Consistent with the proposed excretion route for lipids, small but significant serum creatinine rises within the normal range could be detected 8 hours after dosing in the 20- and 10-ml groups, but not the 5-ml cohort; there were no other biochemical changes. Bilirubin rose on Day 1 in all dosing groups, as with creatinine, remaining within the normal range, and normalized by Day 2. There was no evidence of immune responses based on double-stranded DNA antibodies or human CFTR-specific T cells.
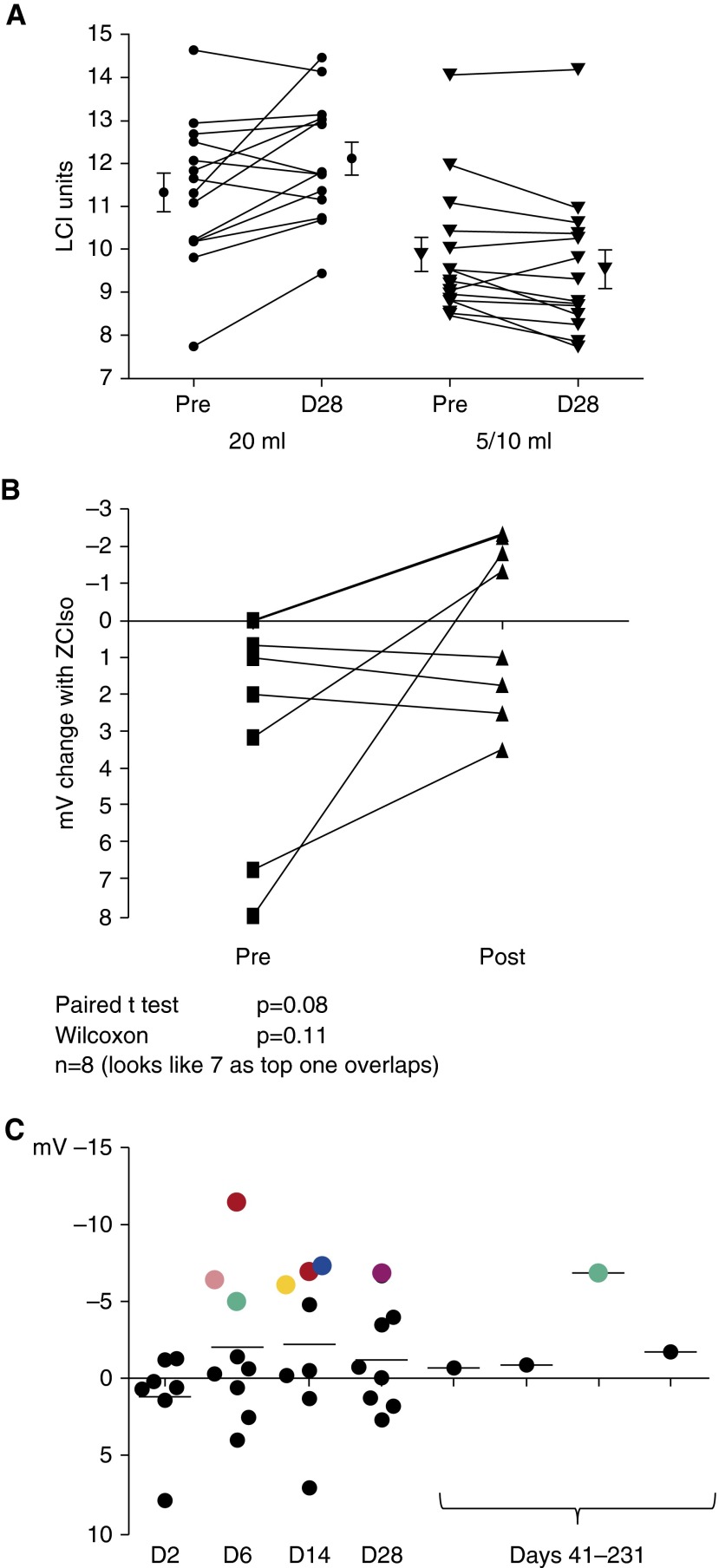
Lung clearance index, a sensitive marker of pulmonary dysfunction (10), was included as a safety assay. Fourteen 20-ml patients with paired predosing and 28-day postdosing values showed a small but significant increase (i.e., a deterioration; Figure 1A). In contrast, and unexpectedly, on post hoc analysis, 11 of 14 patients in the lower-dosing groups (5 and 10 ml) showed a small but significant improvement (Figure 1A).
Figure 1.
(A) Lung clearance index (LCI) increased (worsened) by a mean (SEM) of 0.75 (0.3) units in the 20-ml group (P = 0.03), whereas it decreased (improved) in the 5-/10-ml patients by 0.32 (0.1) (P = 0.04). The difference in change between the groups was significant (P = 0.003). (B) Because of the invasive nature of the procedure, bronchoscopic responses were assessed only in a subset of the 20-ml cohort. Chloride secretion was assessed in the lower airway by change in response to a zero chloride solution containing isoprenaline (isoproterenol) (ZCIso). Of the eight subjects with available pre- and post-treatment measurements, there was a trend (P = 0.08) toward non–cystic fibrosis (more negative) responses. (C) Nasal potential difference was measured in the group receiving nasal dosing. Sixteen subjects had scorable pretreatment chloride responses (zero chloride solution) and at least one post-treatment value. The figure shows the post-treatment values as a delta from the mean pretreatment. A more negative value indicates change toward non–cystic fibrosis. Responders (a post-treatment value at least 5 mV more negative than the mean pretreatment and greater than any of the [maximum 3] pretreatment values), are shown in color, with measures from the same patient being coded in the same color.
With respect to bronchial samples, 10 patients (all 20 ml) had paired pre- and postdosing bronchoscopies. pGM169-specific DNA was detected in all bronchial brushing samples at levels ∼1000-fold higher than in the nasal samples. pGM169-specific mRNA was detected in 2 of 10 postdosing samples. Paired bronchoscopic potential difference measurements were interpretable for 8 of 10 patients. There was a trend toward an increase in chloride secretion (Figure 1B) but no changes in sodium-related parameters.
With respect to nasal samples, pGM169-specific DNA was detected in all 15 brushing samples taken between Day 2 and Day 14 after dosing and in two of six samples at Day 28. pGM169-specific mRNA was detected in 3 of 21 postdosing samples, with all positive samples being observed at either Day 14 (n = 2) or Day 28 (n = 1). In keeping with previous published data, there were no changes in sodium parameters on nasal potential difference. In contrast, 6 of 16 subjects (37.5%) demonstrated a “response” in terms of chloride secretory capacity. Responses were seen most commonly in the zero chloride perfusion phase and at the 14-day point; they were of sustained duration in one subject (Figure 1C).
These data were important in informing the design of the phase IIb trial. Thus, based on these findings, 5 ml was selected as the optimal dose, with paracetamol being used as an adjuvant to minimize the risk of unblinding. Although well-tolerated, the adverse effects of the 20-ml doses were considered prohibitive for use in a repeated administration trial. We consider that the efficient delivery of large volumes of viscous fluid into the airways led acutely to both the influenza-like and restrictive responses, analogous to those seen after bronchoalveolar lavage, and masking the effect of plasmid DNA CpG depletion. At lower volumes, the latter effect was “revealed,” allowing safe dosing of 5 ml. The unexpected improvement in lung clearance index after only one administration at the lower doses was intriguing; larger numbers and longer follow-up are needed to confirm or refute this finding. The variable responses both in molecular and CFTR functional terms underscore the technical challenges inherent in these assays and the limited sensitivity to low levels of gene expression (11). The clean safety profile and encouraging improvements in a sensitive measure of airway health lend support to progression to a phase IIb multidose trial designed to detect clinical improvements after prolonged administration.
Acknowledgments
Members of the UK Cystic Fibrosis Gene Therapy Consortium:
Eric W. F. W. Alton, Katie J. Bayfield, Nicholas Bell, A. Christopher Boyd, June Brand, Andrea Brum, Tabinda Burney, Roberto Calcedo, Paula Carvelli, Faye Chalmers, Mario Chan, Seng H. Cheng, D. David S. Collie, Steve Cunningham, Heather Davidson, Gwyneth Davies, Jane C. Davies, Lee A. Davies, Sarah Davis, Maria Dewar, Ann Doherty, Jackie Donovan, Natalie S. Dwyer, Mathew Embley, Duncan M. Geddes, James S. R. Gibson, Deborah R. Gill, Andrew P. Greening, Uta Griesenbach, David M. Hansell, Luci Hellings, Tracy E. Higgins, Stephen C. Hyde, Laura Hyndman, J. Alastair Innes, Joseph Jacob, Nancy Jones, Brian F. Keogh, Belinda Lees, Maria P. Limberis, Paul Lloyd-Evans, Michelle C. Manvell, Dominique McCormick, Gerry McLachlan, Fiona McLean, Cuixiang Meng, Gordon D. Murray, Nikki Newman, Andrew G. Nicholson, Javier Parra-Leiton, David J. Porteous, Ian A. Pringle, Phil Reid, Gina Rivellini, Clare J. Saunders, Ronald K. Scheule, Sarah Sheard, Helen Sheridan, Andrew Simpson, Keith Smith, Stephen N. Smith, Samia Soussi, Barbara Stevenson, Stephanie G. Sumner-Jones, Nia Voase, Marguerite Y. Wasowicz, Abigail Wilson, James M. Wilson, Paul Wolstenholme-Hogg, and Monica Yanez-Lopez.
Footnotes
Supported by the UK Cystic Fibrosis Trust and the National Institute for Health Research Respiratory Biomedical Research Unit at the Royal Brompton & Harefield NHS Foundation Trust and Imperial College London and the Biomedical Research Centre, based at Imperial College Healthcare NHS Trust and Imperial College London.
This letter has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griesenbach U, Alton EW UK Cystic Fibrosis Gene Therapy Consortium. Gene transfer to the lung: lessons learned from more than 2 decades of CF gene therapy. Adv Drug Deliv Rev. 2009;61:128–139. doi: 10.1016/j.addr.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 3.Alton EW, Armstrong DK, Ashby D, Bayfield KJ, Bilton D, Bloomfield EV, Boyd AC, Brand J, Buchan R, Calcedo R, et al. UK Cystic Fibrosis Gene Therapy Consortium. Repeated nebulisation of non-viral CFTR gene therapy in patients with cystic fibrosis: a randomised, double-blind, placebo-controlled, phase 2b trial. Lancet Respir Med. doi: 10.1016/S2213-2600(15)00245-3. [online ahead of print] 3 Jun 2015; DOI: 10.1016/S2213-2600(15)00245-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies JC, Gill D, Griesenbach U, Voase N, Davies G, Higgins T, Innes JA, Boyd C, Porteous D, Hyde S, et al. Evaluation of safety and gene expression with single dose of pGM169/GL67A administered to the nose and lung of individuals with CF: the UK CF Gene Therapy Consortium Pilot Study [abstract] Pediatr Pulmonol. 2009;(Suppl 32):305. [Google Scholar]
- 5.Davies JC, Gill D, Griesenbach U, Voase N, Davies G, Higgins T, Innes JA, Boyd C, Porteous D, Hyde S, et al. Evaluation of safety and gene expression with single dose of pGM169/GL67A administered to the nose and lung of individuals with CF: the UK CF Gene Therapy Consortium Pilot Study [abstract] Thorax. 2009;64:A70. [Google Scholar]
- 6.Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, Nunez-Alonso G, Green AM, Bazzani RP, Sumner-Jones SG, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26:549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 7.Gill DR, Smyth SE, Goddard CA, Pringle IA, Higgins CF, Colledge WH, Hyde SC. Increased persistence of lung gene expression using plasmids containing the ubiquitin C or elongation factor 1alpha promoter. Gene Ther. 2001;8:1539–1546. doi: 10.1038/sj.gt.3301561. [DOI] [PubMed] [Google Scholar]
- 8.McLachlan G, Davidson H, Holder E, Davies LA, Pringle IA, Sumner-Jones SG, Baker A, Tennant P, Gordon C, Vrettou C, et al. Pre-clinical evaluation of three non-viral gene transfer agents for cystic fibrosis after aerosol delivery to the ovine lung. Gene Ther. 2011;18:996–1005. doi: 10.1038/gt.2011.55. [DOI] [PubMed] [Google Scholar]
- 9.Davies LA, Nunez-Alonso GA, McLachlan G, Hyde SC, Gill DR. Aerosol delivery of DNA/liposomes to the lung for cystic fibrosis gene therapy. Hum Gene Ther Clin Dev. 2014;25:97–107. doi: 10.1089/humc.2014.019. [DOI] [PubMed] [Google Scholar]
- 10.Kent L, Reix P, Innes JA, Zielen S, Le Bourgeois M, Braggion C, Lever S, Arets HG, Brownlee K, Bradley JM, et al. European Cystic Fibrosis Society Clinical Trial Network (ECFS-CTN) Standardisation Committee. Lung clearance index: evidence for use in clinical trials in cystic fibrosis. J Cyst Fibros. 2014;13:123–138. doi: 10.1016/j.jcf.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Rose AC, Goddard CA, Colledge WH, Cheng SH, Gill DR, Hyde SC. Optimisation of real-time quantitative RT-PCR for the evaluation of non-viral mediated gene transfer to the airways. Gene Ther. 2002;9:1312–1320. doi: 10.1038/sj.gt.3301792. [DOI] [PubMed] [Google Scholar]