To the Editor:
Smoking is a key contributor to airway disease in addition to nonpulmonary disorders (1–4). One proposed mechanism involves acquired dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR) protein, as cigarette smoke has been demonstrated to reduce CFTR activity both in vitro and in vivo (5–7) and is associated with clinical symptoms such as chronic bronchitis (7–10). Although direct smoke exposure to airway epithelial cells has been shown to cause respiratory CFTR dysfunction, it has only recently been observed that cigarette smoke is also associated with extrapulmonary (i.e., systemic) CFTR dysfunction, as detected by sweat chloride (9). This finding was confirmed in a distinct cross-sectional study using β-adrenergic sweat secretion rate as an alternative method to measure mild abnormalities in CFTR function (8). We hypothesized that smoking cessation will lead to improved systemic CFTR function, indicating a causal link in humans.
Methods
Smoking cessation program
Human protocols were approved by the University of Alabama at Birmingham’s Institutional Review Board, and all subjects provided written informed consent. Eligible participants were otherwise healthy smokers, 19–70 years old, who were willing to quit smoking. Required smoking intensity before enrollment was at least 20 cigarettes per day for 6 months or longer. Spirometry was performed according to American Thoracic Society criteria, and FEV1 and FEV1/FVC were required to be above the lower limit of normal. Subjects were evaluated at screening and then after smoking cessation (Figure 1A). Serum cotinine and exhaled carbon monoxide (CO) measurements were used to confirm abstinence. If a patient resumed smoking after cessation, further assessments were discontinued. Healthy smokers and healthy nonsmokers were used as controls with otherwise similar inclusion criteria. Controls had three repeated measures of sweat evaporimetry, with each test separated by at least 1 day (median, 10.5 d).
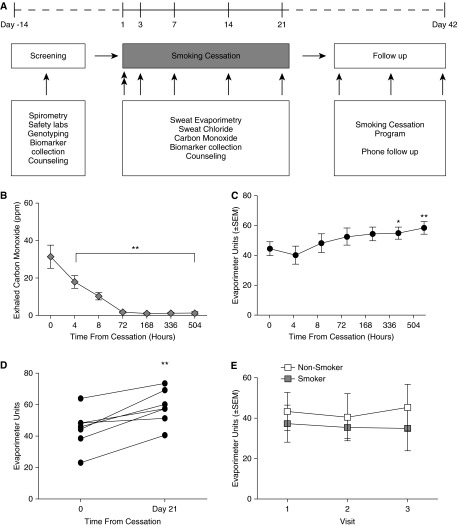
Figure 1.
The effect of smoking cessation on cystic fibrosis transmembrane conductance regulator (CFTR) function. (A) Study design. (B) Exhaled carbon monoxide measured at each point after smoking cessation. (C) CFTR-dependent β-adrenergic sweat secretion rate measured at each point after smoking cessation. (D) β-Adrenergic sweat secretion rate for each study subject that completed the study, before and 21 days after smoking cessation. (E) CFTR-dependent β-adrenergic sweat secretion rate measured at three points 1–7 days apart in healthy smokers and normal healthy nonsmokers who did not alter smoking habits during the study. White, nonsmokers; gray, smokers. *P < 0.05; **P < 0.01.
Sweat testing
Sweat evaporimetry was used to measure exocrine function of the sweat gland, and sweat chloride was used to determine ductular function of CFTR. Sweat evaporimetry was considered the primary endpoint because of its proposed sensitivity for minimal abnormalities in CFTR function (8, 11). Sweat rates were calculated as the maximal stable rate observed after β-adrenergic injection minus the lowest observed rate after the preceding atropine injection, as previously described (8, 11). The rate of evaporative water loss (kg water loss/m2/h) is expressed as evaporimeter units. Sweat chloride was measured by quantitative pilocarpine iontophoresis, using the Macroduct system (Westcor Inc., Logan, UT) (12–14).
Genetic testing
Genetic testing (50 mutation analysis) for CFTR mutations was performed by using a commercially accredited facility (Baylor Medical Genetics, Houston, TX). Patients with a CFTR mutation were excluded from the analysis. This test accounts for approximately 85% of the most common alleles found in the US population.
Statistics
Sweat tests were compared using repeated-measures analysis of variance or paired t test. Post hoc tests for multiple comparisons were calculated using Fisher’s least significant difference. All statistical tests were two-sided and performed at a 5% significance level, using GraphPad Prism (La Jolla, CA). For points containing missing data because of subject discontinuation (n = 1), available data were analyzed; last observed values were carried forward.
Results
Nine subjects provided consent for the study. Seven met inclusion criteria and were enrolled in the smoking cessation program. One subject resumed smoking 10 days into the study and was subsequently withdrawn, but was included in the analysis with available data. Baseline characteristics are provided in Table 1. The median age was 47 years (range, 27–59 yr), and 43% were female. The median FEV1 and FEV1/FVC ratios were 3.94 L (101% predicted) and 0.76, respectively, reflecting near-normal lung function. Smoking intensity was relatively heavy, with a median history of 19 pack-years (range, 12–43 pack-years) and a current use of 1.5 packs/day (range, 1–2 packs/d), and was greater than prior populations studied previously (8). CO levels dropped rapidly after smoking cessation (Figure 1B).
Table 1.
Subject Characteristics
| Subject | Age (yr) | Packs/d | Pack-Years | Sex | Race |
|---|---|---|---|---|---|
| 1 | 57 | 1.5 | 64.5 | Male | White |
| 2 | 47 | 1 | 17 | Female | African American |
| 3 | 36 | 1 | 17 | Female | White |
| 4 | 37 | 2 | 38 | Male | White |
| 5 | 59 | 1.5 | 61.5 | Male | African American |
| 6 | 27 | 1 | 12 | Female | White |
| 7 | 56 | 1.5 | 57 | Male | African American |
The mean (± standard deviation) CFTR-dependent sweat secretion rate for healthy smokers was 44.5 ± 12.3 at the start of cessation and increased to 58.5 ± 10.9 on Day 21 (P < 0.005; Figures 1C and 1D). A statistically significant difference from time 0 was observed by Day 14 (55.0 ± 10.8; P < 0.05) and persisted at Day 21. Findings remained significant when the subject who resumed smoking was omitted from the analysis. Sweat chloride was normal (18.4 ± 12.0 mEq/L) at baseline and did not significantly change after smoking cessation. Similarly, evaporative sweat loss induced by cholinergic (non–CFTR dependent) stimulus was not affected by smoking cessation (70.6 ± 12.7 Day 0 vs. 70.1 ± 6.3 Day 21), consistent with prior cross-sectional studies (8).
In comparison to changes in evaporimetry on smoking cessation among healthy smokers, healthy smokers (n = 4) who did not participate in the smoking cessation program and who maintained stable smoking habits (median age, 43 yr [range, 27–61 yr]; 50% female) exhibited no change in CFTR-dependent sweat secretion rate (Figure 1E). Similarly, normal nonsmokers (n = 5; median age, 41 yr [range, 34–49 yr]; 40% female) also had stable sweat evaporimetry (Figure 1E).
Discussion
Acquired CFTR dysfunction in smoking-related lung disease was only recently described to be present beyond the airway (8, 14). Although this has potentially significant ramifications as a result of the association of cigarette smoking with other disorders in which CFTR has an etiologic role (e.g., pancreatitis, male infertility, diabetes mellitus), these studies were limited by their cross-sectional design and could not determine causality in humans. This study represents the most viable alternative to asking patients to begin smoking, allowing causality to be inferred in the setting of smoking cessation. As such, these data represent the first to demonstrate that systemic CFTR dysfunction induced by cigarette smoke can also recover (8). This observation is significant, as it solidifies the mechanistic link between cigarette smoke exposure and systemic CFTR dysfunction. Moreover, these results indicate that β-adrenergic sweat rate can be a sensitive marker to monitor changes in CFTR dysfunction among individuals and is stable over time, suggesting its potential as a biomarker for measuring the recovery of CFTR function in the setting of therapeutic trials with an agent intended to augment CFTR activity in patients with chronic bronchitis (7, 15). Of interest, in this fashion it performed superior to sweat chloride, suggesting sweat rate may be a relatively dynamic measure; however, this relatively small population may not have been representative, as sweat chloride was relatively low compared with higher values observed in a two larger studies (8, 9); this points out the heterogeneity of sweat chloride abnormalities among smokers.
Acknowledgments
Acknowledgment
The authors thank Dr. E. J. Sorscher, Dr. R. Kimberly, and Dr. L. Guay-Woodford for infrastructural support and a number of helpful discussions. The authors also acknowledge Sherry Tidwell, Heather Hathorne, Scott House, and Ginger Reeves for performing sweat testing. The authors are also grateful to the subjects who volunteered for the study. The sponsors had no role in the design of the study, the collection and analysis of data, or the preparation of the manuscript.
Footnotes
Author Contributions: M.T.D. and S.M.R. conceived of the experiments; C.A.C. and S.V.R. conducted research; C.A.C., S.V.R., B.L., F.J.A., M.T.D., and S.M.R. analyzed the data; C.A.C., M.T.D., and S.M.R. wrote the manuscript; M.T.D. and S.M.R. supervised the project; and S.M.R. is the guarantor of the manuscript and takes responsibility for the integrity of the data and accuracy of the data analysis.
This research was sponsored by the National Institutes of Health (1R01 HL105487 to S.M.R., P30 DK072482 to the University of Alabama at Birmingham CF Research Center, and 5 UL1 RR025777 to the University of Alabama at Birmingham Center for Clinical and Translational Science).
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Kawakami N, Takatsuka N, Shimizu H, Ishibashi H. Effects of smoking on the incidence of non-insulin-dependent diabetes mellitus: replication and extension in a Japanese cohort of male employees. Am J Epidemiol. 1997;145:103–109. doi: 10.1093/oxfordjournals.aje.a009080. [DOI] [PubMed] [Google Scholar]
- 2.Daniell HW. Osteoporosis and smoking. JAMA. 1972;221:509. [PubMed] [Google Scholar]
- 3.Godtfredsen NS, Lam TH, Hansel TT, Leon ME, Gray N, Dresler C, Burns DM, Prescott E, Vestbo J. COPD-related morbidity and mortality after smoking cessation: status of the evidence. Eur Respir J. 2008;32:844–853. doi: 10.1183/09031936.00160007. [DOI] [PubMed] [Google Scholar]
- 4.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 5.Cantin AM, Hanrahan JW, Bilodeau G, Ellis L, Dupuis A, Liao J, Zielenski J, Durie P. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 6.Clunes LA, Davies CM, Coakley RD, Aleksandrov AA, Henderson AG, Zeman KL, Worthington EN, Gentzsch M, Kreda SM, Cholon D, et al. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sloane PA, Shastry S, Wilhelm A, Courville C, Tang LP, Backer K, Levin E, Raju SV, Li Y, Mazur M, et al. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One. 2012;7:e39809. doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Courville CA, Tidwell S, Liu B, Accurso FJ, Dransfield MT, Rowe SM. Acquired defects in CFTR-dependent β-adrenergic sweat secretion in chronic obstructive pulmonary disease. Respir Res. 2014;15:25. doi: 10.1186/1465-9921-15-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188:1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dransfield MT, Wilhelm AM, Flanagan B, Courville C, Tidwell SL, Raju SV, Gaggar A, Steele C, Tang LP, Liu B, et al. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinton P, Molyneux L, Ip W, Dupuis A, Avolio J, Tullis E, Conrad D, Shamsuddin AK, Durie P, Gonska T. β-adrenergic sweat secretion as a diagnostic test for cystic fibrosis. Am J Respir Crit Care Med. 2012;186:732–739. doi: 10.1164/rccm.201205-0922OC. [DOI] [PubMed] [Google Scholar]
- 12.Hammond KB, Turcios NL, Gibson LE. Clinical evaluation of the macroduct sweat collection system and conductivity analyzer in the diagnosis of cystic fibrosis. J Pediatr. 1994;124:255–260. doi: 10.1016/s0022-3476(94)70314-0. [DOI] [PubMed] [Google Scholar]
- 13.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raju SV, Jackson PL, Courville CA, McNicholas CM, Sloane PA, Sabbatini G, Tidwell S, Tang LP, Liu B, Fortenberry JA, et al. Cigarette smoke induces systemic defects in cystic fibrosis transmembrane conductance regulator function. Am J Respir Crit Care Med. 2013;188:1321–1330. doi: 10.1164/rccm.201304-0733OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambert JA, Raju SV, Tang LP, McNicholas CM, Li Y, Courville CA, Farris RF, Coricor GE, Smoot LH, Mazur MM, et al. Cystic fibrosis transmembrane conductance regulator activation by roflumilast contributes to therapeutic benefitin chronic bronchitis. Am J Respir Cell Mol Biol. 2014;50:549–558. doi: 10.1165/rcmb.2013-0228OC. [DOI] [PMC free article] [PubMed] [Google Scholar]