Abstract
Rationale: Alveolar macrophages (AMs) play a key role in host defense to inhaled bacterial pathogens, in part by secreting inflammatory mediators. Cystic fibrosis (CF) airways exhibit a persistent, robust inflammatory response that may contribute to the pathophysiology of CF. Recent findings have linked endoplasmic reticulum stress responses mediated by inositol-requiring enzyme 1α–dependent messenger RNA splicing (activation) of X-box–binding protein-1 (XBP-1s) to inflammation in peripheral macrophages. However, the role of XBP-1s in CF AM function is not known.
Objectives: To evaluate inflammatory responses of AMs from chronically infected/inflamed human CF lungs and test whether XBP-1s is required for AM-mediated inflammation.
Methods: Basal and LPS-induced inflammatory responses were evaluated in primary cultures of non-CF versus CF AMs. XBP-1s was measured and its function was evaluated in AMs using 8-formyl-7-hydroxy-4-methylcoumarin (4μ8C), an inhibitor of inositol-requiring enzyme 1α–dependent XBP-1s, and in THP-1 cells stably expressing XBP-1 shRNA, XBP-1s, or a dominant-negative XBP-1.
Measurements and Main Results: CF AMs exhibited exaggerated basal and LPS-induced production of tumor necrosis factor-α and IL-6, and these responses were coupled to increased levels of XBP-1s. In non-CF and CF AMs, LPS-induced cytokine production was blunted by 4µ8C. A role for XBP-1s in AM inflammatory responses was further established by data from dTHP-1 cells indicating that expression of XBP-1 shRNA reduced XBP-1s levels and LPS-induced inflammatory responses; and LPS-induced inflammation was up-regulated by expression of XBP-1s and inhibited by dominant-negative XBP-1.
Conclusions: These findings suggest that AMs contribute to the robust inflammation of CF airways via an up-regulation of XBP-1s-mediated cytokine production.
Keywords: cystic fibrosis, airway inflammation, alveolar macrophage, UPR, IRE1α/XBP-1
At a Glance Commentary
Scientific Knowledge on the Subject
Alveolar macrophages (AMs) play a crucial role in pulmonary innate defense in part by secreting inflammatory mediators. The specific contribution of AMs to inflammatory responses observed in cystic fibrosis (CF) lung disease and the mechanisms underlying these responses are not well-established. The unfolded protein response mediated by activation of the inositol-requiring enzyme 1 (IRE1)-α–dependent X-box–binding protein-1 (XBP-1) pathway has been implicated in CF airway epithelial inflammation. However, the functional role of IRE1α/XBP-1 in inflammatory responses of AMs from CF lungs is not known.
What This Study Adds to the Field
This study indicates that primary cultures of human CF AMs exhibit a robust production of
inflammatory mediators, and this response reflects an adaptation to the infectious/inflammatory environment of CF airways. The exaggerated response of CF AMs to LPS is mediated by activation of the IRE1α/XBP-1 arm of the unfolded protein response. Our findings offer the proof-of-concept that manipulation of the IRE1α/XBP-1 pathway in AMs may provide new therapeutic opportunities for CF airways disease.
Cystic fibrosis (CF) airway disease is characterized by a chronic and robust inflammatory state often termed hyperinflammatory. In CF airways, the functional absence of the CF transmembrane conductance regulator (CFTR) results in an abnormal airway surface liquid hydration, adherence of thickened mucus to airway surfaces, and persistent airway infection leading to chronic inflammation (1, 2). As evidence of the robust inflammation in CF airways, cytokines and neutrophil elastase are elevated in sputa from patients with CF versus without CF (3). In particular, levels of proinflammatory cytokines and inflammatory cells triggered by bacterial infection are higher in patients with CF versus without CF with acute lung infection (4–6), including the cytokines tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-8 (7–10).
Alveolar macrophages (AMs) represent a first line of defense against inhaled pathogens, including Pseudomonas aeruginosa (11), via phagocytosis of pathogens and secretion of inflammatory mediators. Like neutrophils, persistent activation of AMs without resolution of infection could lead to lung damage. In one scenario, chronic exposure of AMs to the products of persistent intraluminal infection could produce AM-mediated lung damage. A second scenario has suggested that CFTR is expressed in AMs and loss of functional CFTR results in specific defects in AM function (12–17), including impaired bacterial killing linked to CFTR endolysosomal dysfunction (16, 18, 19) and alterations in inflammatory responses of AMs. Evidence for this scenario is provided by studies demonstrating that AMs from CFTR−/− mice exhibit exaggerated inflammatory responses to bacterial LPS (14), and inhibition or mutation of CFTR enhances production of cytokines in murine AMs (20). Moreover, CFTR+/− mice release larger amounts of IL-6 in response to transforming growth factor-β, as compared with wild-type mice (21). In agreement with the murine data, silencing of CFTR in human AMs results in increased secretion of IL-8 (22). These data suggest that human CF AMs might have abnormal function and contribute to an increased inflammatory response.
Inflammation of CF airway epithelia induces endoplasmic reticulum (ER) stress, which triggers the unfolded protein response (UPR) (23–26). Eukaryotic cells exhibit three UPR pathways: (1) inositol-requiring enzyme 1 (IRE1), which exists in two isoforms, α (ubiquitous) and β (present in mucous cells of the gut and respiratory tracts); (2) activating transcription factor 6; and (3) PKR-like ER kinase/pancreatic eIF2α kinase (24, 27, 28). In normal airways, the UPR constitutes an adaptive response to ER stress that provides cellular protection and survival. For instance, activation of IRE1α induces splicing (activation) of the mRNA of X-box–binding protein-1 (XBP-1s) (29, 30). XBP-1s is a transcription factor that up-regulates the ER protein folding capacity and expands the secretory pathway, facilitating increased production of inflammatory mediators involved in innate defense (24–26). In contrast, in obstructed CF airways, persistent high levels of ER stress can have a detrimental effect for airway homeostasis, including high levels of cytokine production that may mediate inflammation-induced airway wall damage.
Activation of the IRE1α-dependent XBP-1s pathway has been linked to cytokine production in macrophages from murine bone marrow or peripheral human monocytes (31). We hypothesized that IRE1α-mediated XBP-1s is required for AM cytokine production and activation of IRE1α-dependent XBP-1s is increased in CF AMs. Hence, this study addressed whether inflammatory responses are larger in primary cultures of AMs from CF versus noninfected/inflamed human lungs, and the role of IRE1α-dependent XBP-1s in inflammatory responses of non-CF and CF AMs. Some results from this study have been reported in the form of an abstract at the 2014 North American Cystic Fibrosis Conference (32).
Methods
For further details on the applied methods, see the online supplement.
Isolation and Culture of Primary Human AMs
Lungs were lavaged with phosphate-buffered saline (PBS) and the retrieved fluid centrifuged (250 × g for 10 min; 4°C). The cell pellet was resuspended in macrophage medium. AM isolation was performed as previously described (33). Non-CF and CF AMs were seeded onto 12-well plates (1 × 105 AMs per well) and cultured in macrophage medium.
Macrophage-like Differentiated THP-1 Cells
THP-1 human monocytic cells were exposed to 50 ng/ml of phorbol 12-myristate 13-acetate in the culture medium for 72 hours.
THP-1 Cells Expressing shRNA Targeting XBP-1
THP-1 cells were infected with a control shRNA or an anti–XBP-1 shRNA lentiviral vector. Positive cells were selected with 1 μg/ml puromycin.
Stable Expression of Control, XBP-1s, and Dominant Negative XBP-1 Vectors
THP-1 cells were infected with a control retroviral vector or retroviral vectors containing XBP-1s or dominant-negative XBP-1 (DN-XBP-1). Positive cells were selected with 200 μg/ml neomycin G418.
Immunohistochemistry
Cytocentrifuge slides were fixed with 4% paraformaldehyde, blocked with 1% bovine serum albumin in PBS with 0.1% Tween-20, incubated overnight at 4°C with a HAM-56 antibody, followed by incubation with a secondary antibody for 1 hour at room temperature.
Flow Cytometry
One hundred thousand 3-day-old cells were incubated for 30 minutes at 4°C with anti–CD11c-APC and/or anti–CD163-PerCP/Cy5.5 conjugated antibody. Data were analyzed using FlowJo X.0.7 (Ashland, OR).
Treatment with Pharmacologic Agents
AMs were washed with PBS and treated with 100 ng/ml LPS from P. aeruginosa. CFTRinh-172 (3-[(3-trifluoromethyl)phenyl]-5-[(4-carboxyphenyl)methylene]-2-thioxo-4-thiazolidinone) was used at the concentration of 10 μM. 8-Formyl-7-hydroxy-4-methylcoumarin (4μ8C), an IRE1α inhibitor, was used at the concentration of 50 μM.
Treatment with Supernatant of Mucopurulent Material
Mucopurulent material was harvested from the airways of human CF lungs and processed as previously described (23–25, 34). Filtered supernatant from mucopurulent material (SMM) was pooled from five CF lungs. Non-CF AMs were exposed to 30 μl of PBS or SMM (1:3 dilution in culture medium) for 3 hours.
Quantitative Real-Time Polymerase Chain Reaction
RNA isolation, cDNA preparation, and real-time polymerase chain reaction were performed as previously described (27). mRNA values were normalized to 18S mRNA values.
ELISA Assays
TNF-α and IL-6 secretion was evaluated by ELISA.
Statistical Analysis
Data are reported as mean ± SD with a P value less than 0.05 considered statistically significant.
Results
Isolation and Characterization of AMs
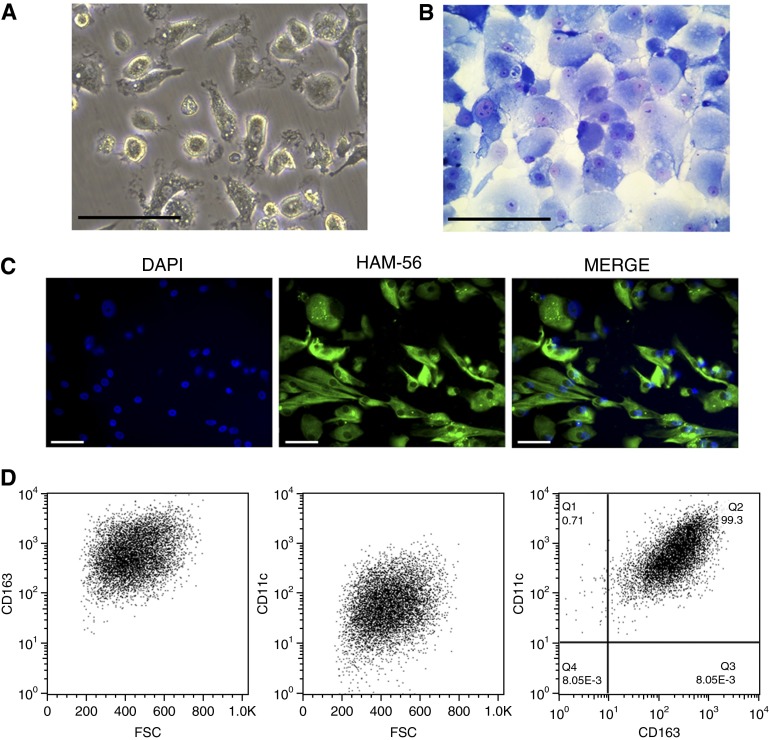
AM isolation and their attachment to plastic dishes was performed as previously described (33). Each lung tissue yielded 2–20 × 106 AMs, which consisted mostly of large, round cells heterogeneous in size, as observed by light microscopy (Figure 1A). AM evaluation using Diff-Quik (Polysciences, Inc., Warrington, PA) indicated that the preparation consisted of more than 99% AMs (Figure 1B). The purity of the AM samples was further indicated by the expression of the macrophage marker HAM-56 (35) in all isolated cells (Figure 1C).
Figure 1.
Characterization of alveolar macrophages (AMs) isolated from resected human lungs. (A) Adherent cells were isolated as described in the Methods. Examination of cell morphology by phase-contrast inverted light microscopy. (B) Morphology of sedimented AMs. Cells were sedimented onto slides (cytospin), stained (Diff-Quik), and examined by light microscopy. (C) Immunofluorescence microscopy of AMs. Green = macrophage migration inhibitor factor HAM-56 (human macrophage marker). Blue = nuclei stain with 4’6-diamidino-2-phenylindole (DAPI). (D) Flow cytometric analysis of AMs. The preparations were analyzed according to their forward scatter characteristics (FSC), CD163 (macrophage marker), and CD11c (macrophage marker) expression in human AMs. Number in right panel indicates the percentage of cells positive for the macrophage markers. Scale bars = 100 μm.
Flow cytometry was next performed to establish the purity of the AMs. The preparations were analyzed according to their forward scatter characteristics, and for their expression of CD163+ and CD11c+ (Figure 1D, left and middle). CD163 is expressed exclusively on monocytes and macrophages (36), and CD11c is expressed on many monocytic-derived cells, including macrophages (37). The combination of CD11c+ and CD163+ expression, which is necessary and sufficient to accurately distinguish macrophages from other lung cells, indicates that our isolation procedure yielded a highly purified preparation of AMs (Figure 1D, right).
Primary Cultures of CF AMs Express a Robust Inflammatory Phenotype
We next evaluated the basal and LPS-induced cytokine production in non-CF versus CF AMs in primary cultures for 3 days. Treatment with vehicle (0.1% dimethyl sulfoxide) did not affect the expression of inflammatory markers, as compared with untreated AMs (data not shown). Therefore, cytokine values obtained under vehicle treatment were considered baseline values.
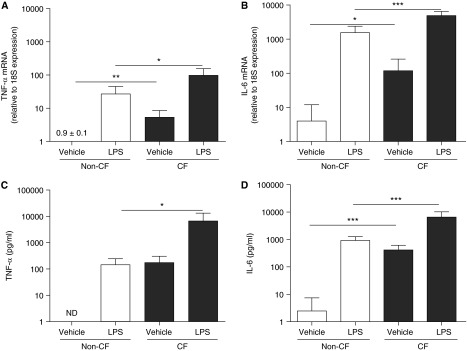
We first compared the baseline mRNA levels of TNF-α and IL-6 in primary cultures of CF versus non-CF AMs. These inflammatory mediators were evaluated because of their relevance to CF airways disease (7, 8). Under baseline conditions, CF AMs exhibited 25- and 24-fold higher mRNA levels of TNF-α and IL-6, as compared with non-CF AMs (Figures 2A and 2B). We then determined the effect of LPS from P. aeruginosa on the mRNA levels of TNF-α and IL-6 in CF and non-CF AMs. Pilot studies indicated that 100 ng/ml LPS was a maximal dose for inducing cytokine mRNA expression and secretion (see Figure E1 in the online supplement). Moreover, 6-hour LPS led to maximal cytokine mRNA up-regulation associated with increased cytokine secretion (see Figure E2). The 100 ng/ml (6-h treatment) LPS-up-regulated TNF-α and IL-6 mRNA levels were higher in CF versus non-CF AMs (Figures 2A and 2B).
Figure 2.
Primary cultures of cystic fibrosis (CF) alveolar macrophages (AMs) exhibit robust basal and LPS-induced tumor necrosis factor (TNF)-α and IL-6 production. Non-CF and CF AMs were stimulated for 6 hours with 0.1% dimethyl sulfoxide (vehicle) or 100 ng/ml LPS from Pseudomonas aeruginosa. Levels of TNF-α (A) and IL-6 (B) mRNA were determined by quantitative reverse transcriptase polymerase chain reaction and expressed as fold change relative to 18S mRNA. TNF-α (C) and IL-6 (D) protein secretion into the culture media were determined by ELISA. Open bars correspond to primary cultures of non-CF AMs. Solid bars correspond to primary cultures of CF AMs. The y-axis uses a logarithmic scale. Data are from six independent experiments and represent mean ± SD. Unpaired t test was used to compare non-CF with CF AMs and paired t test was used to compare vehicle with LPS treatment. *P < 0.05, **P < 0.01, ***P < 0.001. ND = not detected. The fold change of TNF-α mRNA from cells exposed to the vehicle is 0.9 ± 0.1.
To address whether the higher mRNA expression of TNF-α and IL-6 corresponded to higher TNF-α and IL-6 protein levels, the secretion of TNF-α and IL-6 was evaluated. Under basal conditions, CF AMs secreted 175- and 260-fold higher levels of TNF-α and IL-6 than non-CF AMs (Figures 2C and 2D). In addition, LPS-up-regulated TNF-α and IL-6 secretion was 49-fold and sevenfold higher in CF versus non-CF AMs (Figures 2C and 2D). These data indicate that CF AMs exhibit a greater inflammatory phenotype than non-CF AMs under basal condition and following LPS exposure.
CFTR Expression Levels in Human AMs
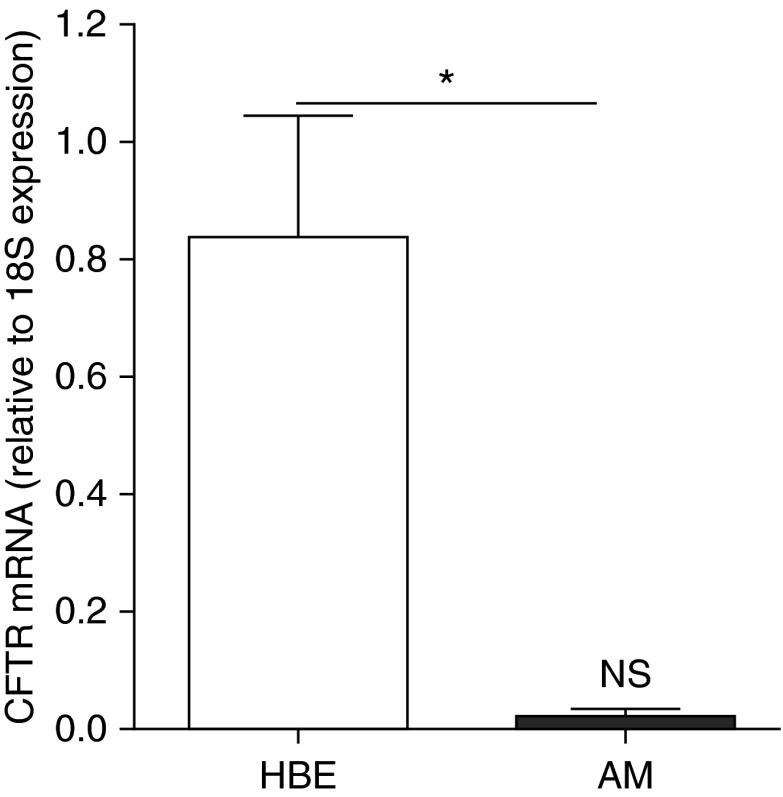
Because it has been suggested that macrophages express CFTR at a low, but functional level (12), we addressed whether the absence of CFTR function is coupled to the robust inflammatory response of CF AMs. We first evaluated CFTR expression in primary non-CF AMs from human lungs versus primary human bronchial epithelial (HBE) cells known to express CFTR. CFTR mRNA levels were very low in AMs as compared with HBE cells (Figure 3). Notably, the low CFTR expression levels in AMs did not differ from the hypothetical value zero, based on one-sample t test (Figure 3). These data suggest that primary cultures of human AMs either do not express CFTR or their CFTR expression is extremely low.
Figure 3.
Cystic fibrosis transmembrane conductance regulator (CFTR) expression levels in human alveolar macrophages (AMs). Bars represent mRNA levels, determined by quantitative reverse transcriptase polymerase chain reaction, of CFTR in primary cultures of human bronchial epithelial (HBE) cells and primary cultures of human AMs. Data are expressed as fold change relative to 18S mRNA and represent mean ± SD from three independent experiments. Unpaired t test was used for the statistical analysis. *P < 0.05, HBE versus AMs. NS = not significant, CFTR levels in AMs versus the hypothetical value 0, based on one-sample t test.
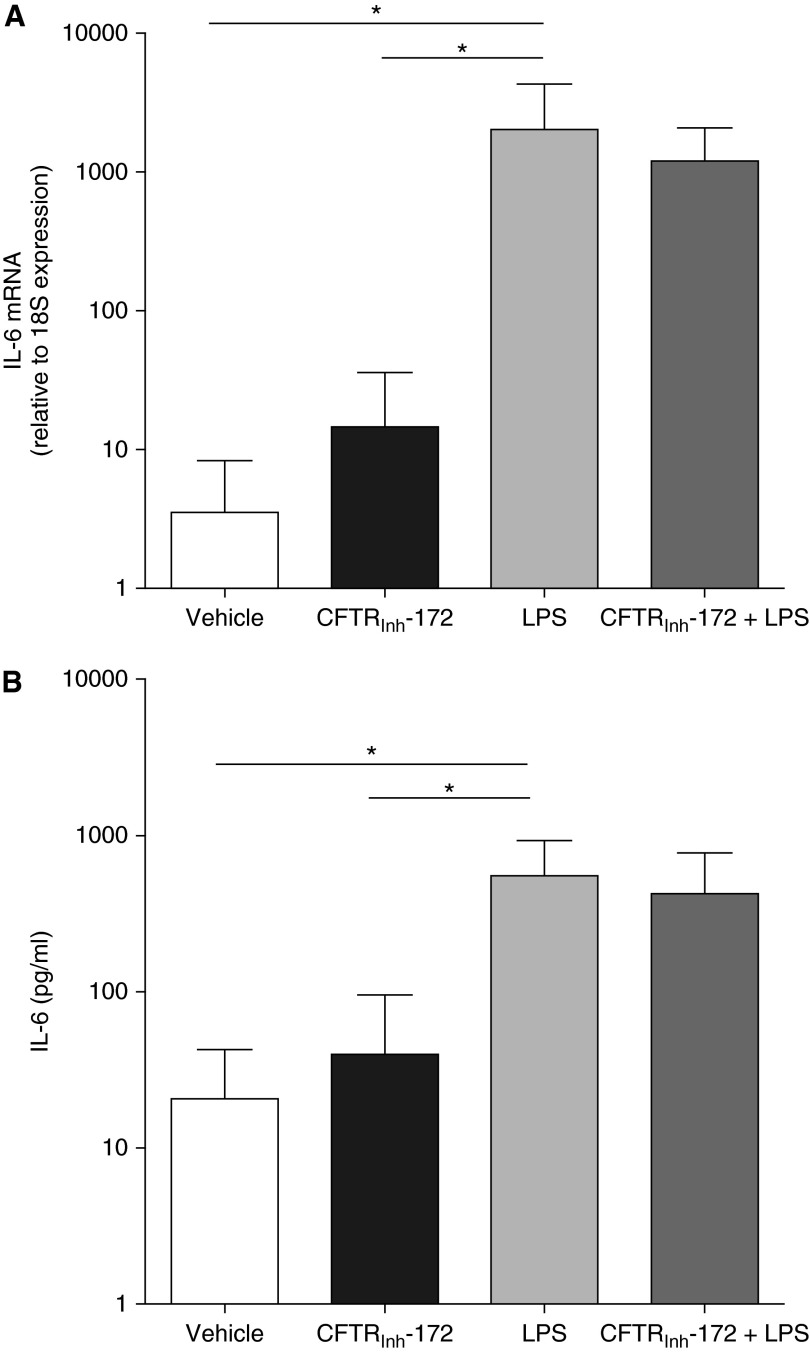
The putative very low level of CFTR expression in non-CF AMs could be, nevertheless, important for AM function. Hence, we considered that the loss of functional CFTR in CF AMs could be implicated in the exaggerated inflammatory response to LPS (Figure 2). To address this issue, we used CFTRinh-172 to pharmacologically inhibit putative CFTR function (12, 38) in non-CF AMs. Treatment of non-CF AMs for 72 hours with a maximal dose of CFTRinh-172 (10 μM) (39) had no effect on basal IL-6 mRNA levels and protein secretion (Figures 4A and 4B). We also determined the effect of 100 ng/ml LPS on AMs pretreated with CFTRinh-172 for 72 hours. Although LPS significantly increased IL-6 mRNA levels (Figure 4A) and IL-6 secretion (Figure 4B), pretreatment with CFTRinh-172 did not potentiate the LPS-induced inflammatory response (Figures 4A and 4B). These data suggest that the robust inflammatory phenotype with respect to these cytokines of CF AMs is not linked to a defective CFTR function.
Figure 4.
Cystic fibrosis transmembrane conductance regulator (CFTR) inhibition does not induce an inflammatory response in non-CF alveolar macrophages. Non-CF alveolar macrophages were pretreated with CFTRinh-172 (10 μM) for 72 hours. Where applicable, 100 ng/ml LPS from Pseudomonas aeruginosa was added during the last 6 hours. (A) Quantitative reverse transcriptase polymerase chain reaction was used to determine the levels of IL-6 mRNA, which are expressed as fold change relative to 18S mRNA. (B) IL-6 secretion into the culture media was evaluated by ELISA. The y-axis uses a logarithmic scale. Data are from six independent experiments and represent mean ± SD. Paired t test was used for the statistical analysis. *P < 0.05.
AM Inflammatory Responses Are Coupled to UPR Activation
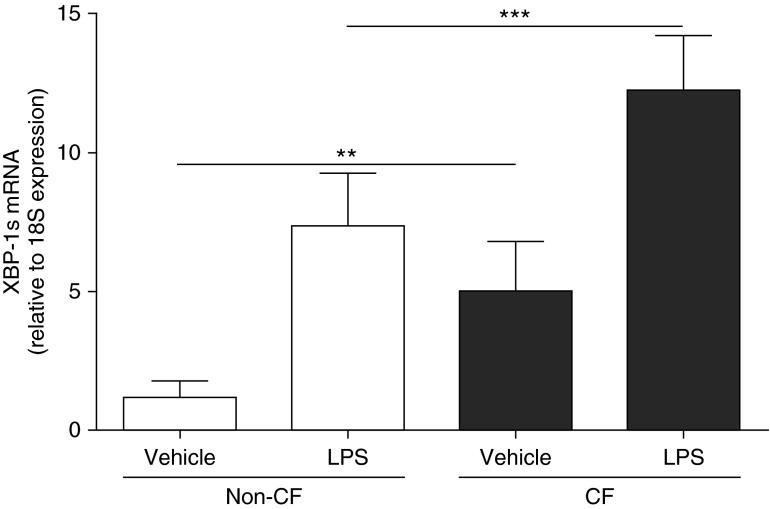
To investigate whether the inflammatory response of AMs is linked to activation of IRE1α-dependent XBP-1 mRNA splicing (XBP-1s), a UPR pathway implicated in inflammatory responses of peripheral macrophages (31), we compared the levels of XBP-1s in non-CF versus CF AMs. Under basal conditions, CF AMs exhibited fourfold higher mRNA levels of XBP-1s versus non-CF AMs. LPS up-regulated the levels of XBP-1s in non-CF and CF AMs. Notably, the absolute magnitude of response of CF AMs to LPS was higher than non-CF AMs (Figure 5).
Figure 5.
Primary cultures of cystic fibrosis (CF) alveolar macrophages (AMs) express high levels of X-box–binding protein-1 mRNA splicing (XBP-1s). Non-CF and CF AMs were stimulated for 6 hours with 100 ng/ml LPS from Pseudomonas aeruginosa. Quantitative reverse transcriptase polymerase chain reaction was used to determine the mRNA levels of XBP-1s. Data are expressed as fold change relative to 18S mRNA and represent mean ± SD from six independent experiments. Open bars correspond to primary cultures of non-CF AMs. Solid bars correspond to primary cultures of CF AMs. Unpaired t test was used to compare non-CF with CF AMs, and paired t test was used to compare vehicle with LPS treatment. **P < 0.01, ***P < 0.001.
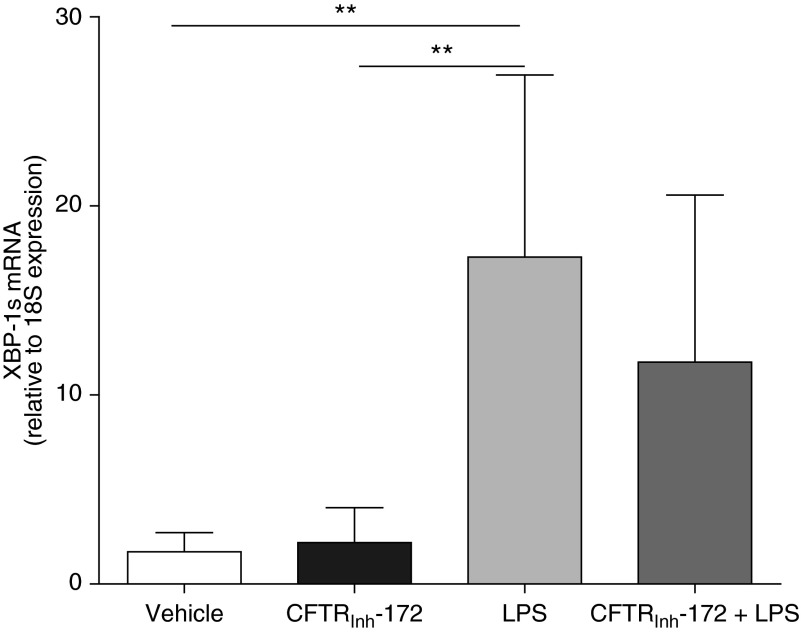
We also evaluated the effect of CFTRinh-172 on the mRNA levels of XBP-1s. Under basal and LPS-stimulated conditions, CFTRinh-172 did not affect XBP-1s levels (Figure 6), suggesting that loss of CFTR function per se was not associated with the higher levels of XBP-1s in CF AMs (Figure 5).
Figure 6.
Cystic fibrosis transmembrane conductance regulator (CFTR) inhibition does not induce X-box–binding protein-1 mRNA splicing (XBP-1s) in primary cultures of non-CF alveolar macrophages. Non-CF alveolar macrophages were pretreated with vehicle or CFTRinh-172 (10 μM) for 72 hours and, when applicable, stimulated during the last 6 hours with 100 ng/ml LPS from Pseudomonas aeruginosa. Quantitative reverse transcriptase polymerase chain reaction was used to determine the mRNA levels of XBP-1s. Data are expressed as fold change relative to 18S mRNA and represent mean ± SD from six independent experiments. Paired t test was used for the statistical analysis. **P < 0.01.
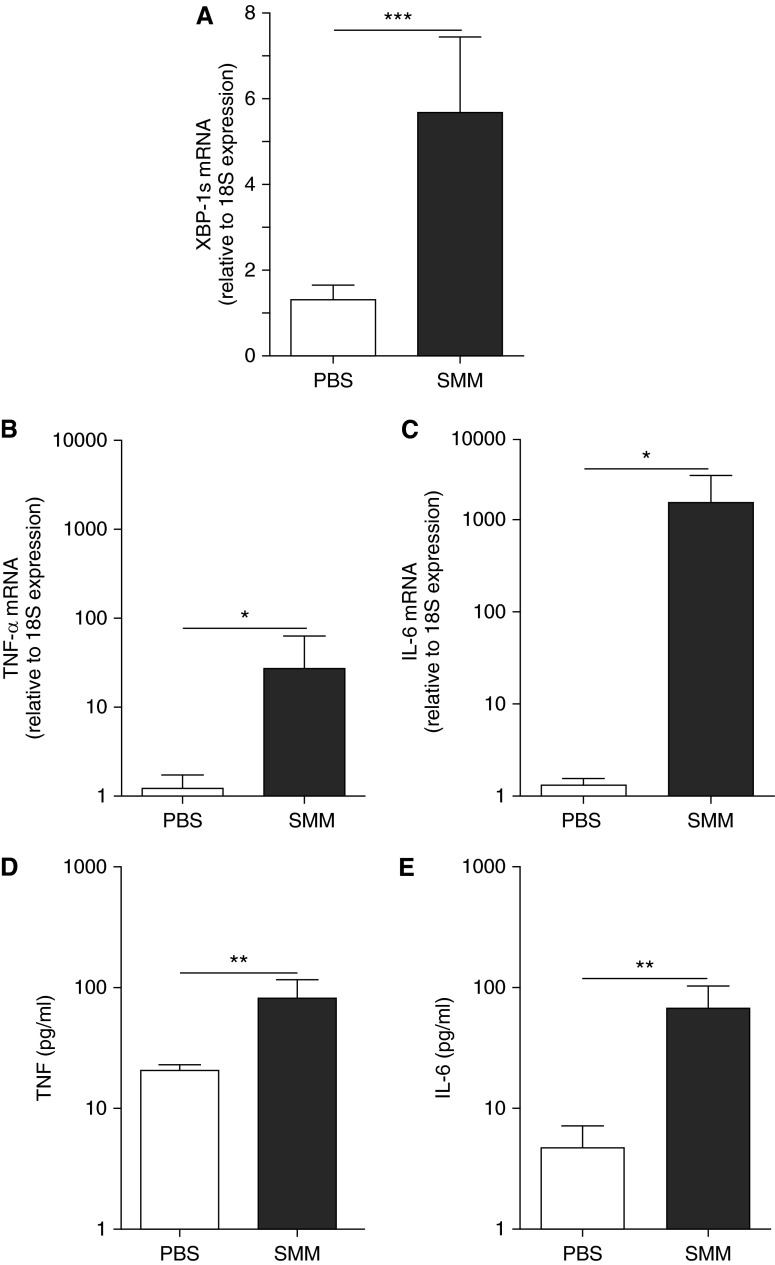
To address whether the robust inflammatory response coupled to higher XBP-1s levels in CF AMs resulted from an acquired response to the CF airway infectious/inflammatory milieu, non-CF AMs were exposed to SMM from human CF airways (23–25). SMM up-regulated XBP-1s, TNF-α, and IL-6 (Figures 7A–7C) mRNA levels. Moreover, SMM up-regulated TNF-α and IL-6 protein secretion (Figures 7D and 7E), reproducing the robust inflammatory phenotype of CF AMs (Figure 2) associated with higher XBP-1s levels (Figure 5).
Figure 7.
The increased X-box–binding protein-1 mRNA splicing (XBP-1s) and the robust inflammation of cystic fibrosis (CF) alveolar macrophages reflect an acquired response to the luminal infectious and inflammatory milieu of CF airways. Primary cultures of non-CF alveolar macrophages were exposed to phosphate-buffered saline (PBS) or supernatant from mucopurulent material (SMM; 1:3 dilution; pooled from the airways of five human CF lungs) for 3 hours. Quantitative reverse transcriptase polymerase chain reaction was used to evaluate the levels of XBP-1s (A), tumor necrosis factor (TNF)-α (B), and IL-6 (C) mRNA. Data are expressed as fold change relative to 18S mRNA. TNF-α (D) and IL-6 (E) protein secretion into the culture media was determined by ELISA. Open bars correspond to PBS exposure. Solid bars correspond to SMM exposure. The y-axis uses a logarithmic scale for TNF-α and IL-6 mRNA and protein secretion. Data are from six independent experiments and represent mean ± SD. Paired t test was used for the statistical analysis. *P < 0.05, **P < 0.01, and ***P < 0.001.
Together, these data suggest that (1) activation of IRE1α-dependent XBP-1s is coupled with inflammatory responses in both non-CF and CF AMs; (2) the higher levels of XBP-1s in CF AMs are proportionate to their robust inflammatory phenotype; and (3) the greater inflammatory response of CF AMs linked to increased levels of XBP-1s is not associated with the absence of CFTR function but, rather, reflects a response to persistent inflammatory stimulation.
Inhibition of IRE1α‐Dependent XBP-1s Decreases LPS-induced Inflammation
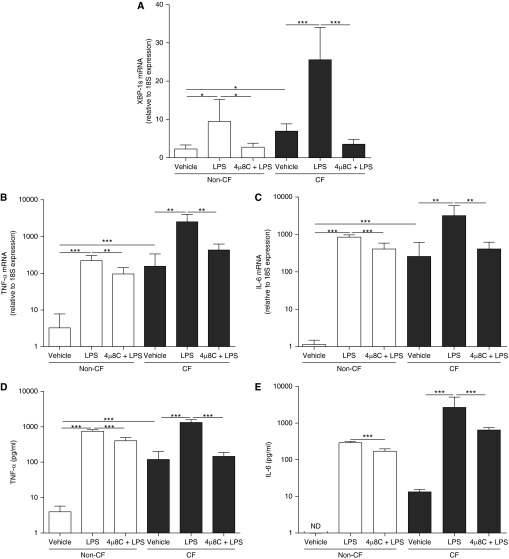
We next evaluated whether inhibition of IRE1α-dependent XBP-1s decreased AM cytokine secretion. The effect of the IRE1α inhibitor 4μ8C (40, 41) was tested on LPS-induced IRE1α activation–dependent XBP-1s and cytokine production in non-CF and CF AMs. AMs were pretreated for 1 hour with 50 μM 4 μ8C (40, 41) before induction of inflammation with 100 ng/ml LPS. Our pilot studies indicated that this dose promotes the highest inhibition of LPS-induced IL-6 mRNA associated with IL-6 secretion (see Figure E3). 4μ8C significantly decreased LPS-induced XBP-1s mRNA levels in non-CF and CF AM cultures, as compared with LPS-treated cultures that were not administered 4µ8C (Figure 8A). Pretreatment with 4μ8C significantly decreased LPS-increased TNF-α mRNA levels by 57% and 83%, and IL-6 mRNA levels by 52% and 87%, respectively, in non-CF and CF AMs (Figures 8B and 8C). In addition, 4μ8C significantly decreased LPS-up-regulated TNF-α secretion by 45% and 89%, and IL-6 secretion by 41% and 76%, respectively, in non-CF and CF AM cultures (Figures 8D and 8E). Notably, the decrease of TNF-α and IL-6 protein secretion resulting from 4μ8C pretreatment was more marked in CF versus non-CF AMs. These findings suggest that 4μ8C has antiinflammatory properties resulting from its inhibitory effect on IRE1α-mediated XBP-1s in AMs.
Figure 8.
Inhibition of inositol-requiring enzyme 1 α–dependent X-box–binding protein-1 mRNA splicing (XBP-1s) suppresses LPS-induced cytokine secretion in primary cultures of alveolar macrophages (AMs). Non–cystic fibrosis (CF) and CF AMs were stimulated for 6 hours with 100 ng/ml LPS from Pseudomonas aeruginosa in the absence or presence of the Inhibition of inositol-requiring enzyme 1 α inhibitor 8-formyl-7-hydroxy-4-methylcoumarin (4μ8C) (50 μM; 1 h pretreatment). Quantitative reverse transcriptase polymerase chain reaction was used to evaluate the levels of XBP-1s (A), tumor necrosis factor (TNF)-α (B), and IL-6 (C) mRNA. Data are expressed as fold change relative to 18S mRNA. TNF-α (D) and IL-6 (E) secretion into the culture media was determined by ELISA. Open bars correspond to primary cultures of non-CF AMs. Solid bars correspond to primary cultures of CF AMs. The y-axis uses a logarithmic scale for TNF-α and IL-6 mRNA and protein secretion. Data are from five independent experiments and represent mean ± SD. Unpaired t test was used to compare non-CF with CF AMs and paired t test was used to compare the different treatments. *P < 0.05, **P < 0.01, and ***P < 0.001. ND = not detected.
XBP-1 Is Required for LPS‐induced AM Cytokine Production
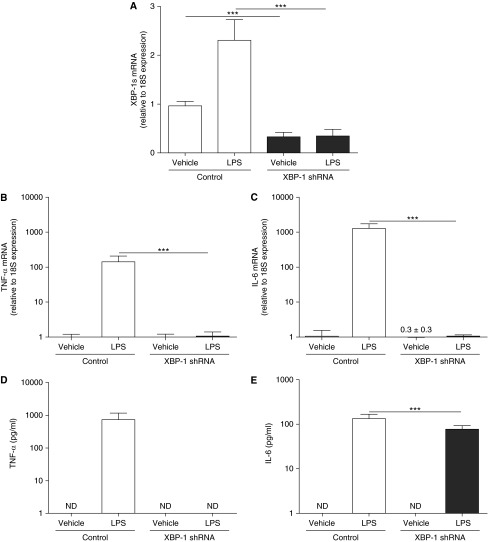
The previously mentioned data indicate that activation of IRE1α-dependent XBP-1s is required for AM inflammatory responses, and that XBP-1s mediates, at least in part, the robust inflammatory phenotype of CF AMs. To further evaluate the functional role of XBP-1 in AM inflammatory responses, a XBP-1-specific short hairpin RNA (shRNA) was used to knockdown XBP-1 in cultures of dTHP-1 cells. In the absence of LPS stimulation, the XBP-1 shRNA knocked down the baseline mRNA levels of XBP-1s by approximately 60% as compared with control shRNA (Figure 9A). LPS-up-regulated XBP-1s was significantly blunted in XBP-1 shRNA expressing cells as compared with control cells (Figure 9A). The XBP-1 shRNA also blunted LPS-increased TNF-α and IL-6 mRNA levels (Figures 9B and 9C) and LPS-up-regulated TNF-α and IL-6 protein secretion (Figures 9D and 9E), as compared with cells expressing the control shRNA. Notably, the inhibitory effect of the XBP-1 shRNA was stronger for LPS-stimulated TNF-α versus IL-6 secretion.
Figure 9.
Knockdown of X-box–binding protein-1 (XBP-1) suppresses LPS-induced XBP-1 mRNA splicing (XBP-1s) and cytokine production. Macrophage-like differentiated THP-1 cultures stably expressing a control vector or a XBP-1-specific short hairpin RNA (shRNA) were stimulated during 6 hours with 100 ng/ml LPS from Pseudomonas aeruginosa. Quantitative reverse transcriptase polymerase chain reaction was used to evaluate the levels of XBP-1s (A), tumor necrosis factor (TNF)-α (B), and IL-6 (C) mRNA. Data are expressed as fold change relative to 18S mRNA. TNF-α (D) and IL-6 (E) protein secretion into the culture media was determined by ELISA. The y-axis uses a logarithmic scale for TNF-α and IL-6 mRNA and protein secretion. Data are from six independent experiments and represent mean ± SD. One-way analysis of variance was used for the statistical analysis. ***P < 0.001. ND = not detected. The fold change of IL-6 mRNA from dTHP-1 cells expressing XBP-1 shRNA and exposed to vehicle is 0.3 ± 0.3.
These findings suggested that in dTHP-1 cells the temporal and quantitative regulation of TNF-α and IL-6 by XBP-1 is different. To investigate this disparity, we evaluated the mRNA levels and protein secretion of TNF-α and IL-6 in control and XBP-1 shRNA expressing dTHP-1 cells at an earlier time point, after 3 hours LPS stimulation. LPS up-regulated the mRNA and protein secretion levels of TNF-α (see Figure E4). The mRNA levels of TNF-α were greatly decreased and TNF-α protein secretion was not detectable after 3 hours LPS in cells expressing the XBP-1 shRNA (see Figure E4). At 3 hours postexposure to LPS the IL-6 mRNA levels and IL-6 secretion were decreased in cells expressing the XBP-1 shRNA, but these inhibitory responses were not as robust as those for LPS-induced TNF-α (see Figure E4). We speculate that, unlike the rapid inhibition of TNF-α transcription, there was a delay in inhibition of IL-6 mRNA levels after 3 hours LPS in XBP-1 shRNA expressing cells that produced a pool of IL-6 mRNAs to be translated into IL-6 protein at 3 hours and, then, at 6 hours LPS exposure.
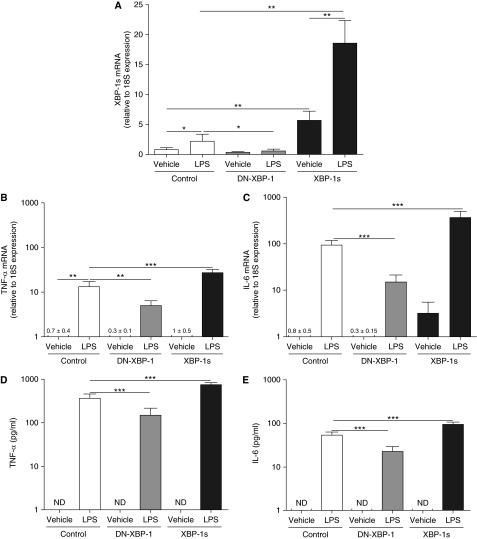
To directly test the role of XBP-1s in LPS-induced inflammatory responses relevant to CF AMs, dTHP-1 cells stably overexpressing a retroviral pQCXIN vector (control), a pQCXIN vector expressing a DN-XBP-1 construct, or a pQCXIN vector expressing a XBP-1s construct (24, 27, 42) were used. LPS up-regulated XBP-1s levels in control cultures, and this response was attenuated in cells expressing the DN-XBP-1 (Figure 10A). The inhibitory effect of the DN-XBP-1 was also observed for LPS-up-regulated TNF-α and IL-6 mRNA levels (Figures 10B and 10C) and protein secretion (Figures 10D and 10E). Assessment of XBP-1s mRNA confirmed that XBP-1s mRNA levels were increased in dTHP-1 cell overexpressing XBP-1s (Figure 10A). Importantly, LPS-increased XBP-1s, TNF-α, and IL-6 mRNA levels (Figures 10A–10C) and TNF-α and IL-6 protein secretion (Figures 10D and 10E) were potentiated in dTHP-1 cells overexpressing XBP-1s. These findings further establish the functional role of XBP-1s in AM inflammatory responses.
Figure 10.
LPS-dependent cytokine production is mediated by X-box–binding protein-1 mRNA splicing (XBP-1s). Macrophage-like differentiated THP-1 cultures stably expressing a control pQCXIN vector, a pQCXIN vector containing a dominant negative XBP-1 (DN-XBP-1), or a pQCXIN vector containing XBP-1s were stimulated for 6 hours with vehicle or 100 ng/ml LPS from Pseudomonas aeruginosa. The levels of XBP-1s (A), tumor necrosis factor (TNF)-α (B), and IL-6 (C) mRNA were analyzed by quantitative reverse transcriptase polymerase chain reaction and expressed as fold change relative to 18S mRNA. TNF-α (D) and IL-6 (E) protein secretion into the culture media was determined by ELISA. The y-axis uses a logarithmic scale for TNF-α and IL-6 mRNA and protein secretion. Data are from six independent experiments and represent mean ± SD. One-way analysis of variance was used for the statistical analysis. *P < 0.05, **P < 0.01, and ***P < 0.001 spliced XBP-1 or DN-XBP-1 expressing cells versus control cells. ND = not detected. The fold changes of TNF-α mRNA in dTHP-1 cells expressing control, DN-XBP, and XBP-1s and exposed to vehicle are 0.7 ± 0.5, 0.3 ± 0.1, and 1 ± 0.5, respectively. The fold changes of IL-6 mRNA in dTHP-1 cells expressing control and DN-XBP-1 and exposed to vehicle are 0.8 ± 0.5 and 0.3 ± 0.15, respectively.
Discussion
AMs are critical for the maintenance of airway homeostasis, but their role in CF lung disease has not been fully studied. AMs clear the air spaces of infectious, toxic, and allergic particles; regulate innate alveolar defense against infection by secreting cytokines (43); and initiate inflammatory responses by recruiting activated neutrophils into air spaces (44). However, AM dysregulation could impair resolution of inflammation via a failure to act as suppressor cells, leading to chronic pulmonary infection and inflammation (38). Furthermore, in chronically obstructed CF airways, the persistent exposure of AMs to the infectious/inflammatory milieu can lead to persistent and, possibly, inappropriate activation of their inflammatory responses, contributing to lung damage.
Activation of the UPR has been implicated in airway epithelial inflammatory responses characteristic of CF airways (25, 26). Freshly isolated airway epithelia from chronically infected/inflamed human CF lungs exhibit increased levels of XBP-1s, and XBP-1s is required for cytokine production by inflamed human airway epithelia (24). These findings suggested that the IRE1α–XBP-1 arm of the UPR plays a pivotal role in CF airway epithelial inflammation. Based on the role of XBP-1s in inflammatory responses of airway epithelia, we hypothesized that AMs from chronically infected/inflamed human CF lungs also exhibit robust inflammation (e.g., increased basal and LPS-stimulated cytokine production), and XBP-1s is required for these responses.
Our data demonstrate that primary cultures of human CF AMs indeed exhibit a robust inflammatory phenotype (Figure 2). The greater inflammatory response of CF AMs required IRE1α activation–dependent generation of XBP-1s based on these findings: (1) increased XBP-1s levels were associated with increased LPS-induced cytokine production (Figures 5, 8–10); (2) treatment with the IRE1α inhibitor 4µ8C reduced LPS-increased XBP-1s, TNF-α, and IL-6 mRNA levels and TNF-α and IL-6 protein secretion (Figure 8); (3) knockdown of XBP-1 inhibited LPS-increased XBP-1s, TNF-α, and IL-6 mRNA levels and TNF-α and IL-6 protein secretion (Figure 9); (4) overexpression of DN-XBP-1 decreased LPS-up-regulated XBP-1s and inhibited LPS-induced TNF-α and IL-6 production (Figure 10); and (5) macrophage cultures overexpressing XBP-1s exhibited increased LPS-induced TNF-α and IL-6 production (Figure 10).
The baseline levels of IL-6 and TNF-α mRNA and protein secretion in CF AMs were higher than non-CF AMs, and the absolute increase after LPS stimulation was greater in CF than non-CF AMs (Figure 2). The same effect was observed with the mRNA levels of XBP-1s (Figure 5). These data suggest that native CF AMs exhibit higher XBP-1s-mediated TNF-α and IL-6 production in response to acute and chronic exposure to bacterial infection (and LPS) in CF lungs. Note, the in vitro exposure to LPS resulted in a lower fold increase of these genes in CF versus non-CF AMs, perhaps reflecting the higher basal levels in CF AMs.
It is generally accepted that LPS promotes cytokine production via activation of the transcription factor nuclear factor (NF)-κB during innate immune responses to pathogens (45). Our study suggests that TLR activation by LPS triggers IRE1α activation–dependent XBP-1 mRNA splicing, which identifies a novel pathway required for LPS-induced cytokine production and secretion in human AMs.
These findings agree with a report describing the role of XBP-1 in innate immune responses of peripheral macrophages based on the observations that transgenic mice deficient for XBP-1 in peripheral macrophages exhibited decreased inflammatory responses (31). This study also suggested that LPS (via TLR-4) and IRE1α/XBP-1 are interconnected and cooperate to maximize innate immune responses to pathogens (31). Activation of TLRs can couple to activation of IRE1α via TRAF6- and NOX2-dependent pathways (46). The activated IRE1α interacts via TRAF2 with the IKK complex and with ASK and JNK protein kinases (47), thereby modulating activation of the transcription factors NF-κB and AP-1 (48). Deletion of the IRE1α gene reduces proinflammatory cytokine production because of impairment of JNK activation and the lack of functional XBP‐1s in bone marrow–derived macrophages from IRE1α knockout mice (41). In parallel, the XBP-1s resulting from IRE1α activation can directly promote transcriptional up-regulation of cytokine genes, contributing to maximal cytokine production (46). These findings suggest that the two mechanisms (e.g., IRE1α-dependent XBP-1s and IRE1α-mediated NF-κB and/or JNK activation) can coexist with canonical LPS–NF-κB signaling in AMs. Additional studies are necessary to define the pathways for IRE1α-regulated NF-κB and JNK activation in AM inflammatory responses.
The IRE1α inhibitor 4μ8C, a synthetic coumarin derivative, blocks substrate access to the active site of IRE1α and selectively inactivates IRE1α-dependent mRNA splicing of XBP-1 (40). Several studies have shown that coumarin compounds have strong antiinflammatory effects (49, 50), including findings suggesting that coumarin compounds can also target NF-κB (51) and mitogen-activated protein kinase (52). Our data demonstrated that 4μ8C decreased the levels of XBP-1s and suppressed LPS-induced cytokine production (Figure 8). Additional studies are needed to expand the understanding of the mechanism underlying the antiinflammatory action of 4μ8C in CF airways disease.
Previous studies have reported that CFTR is expressed in murine and human AMs (12) and CFTR malfunction in macrophages is directly linked with the robust inflammation in CF (14–16). It has been suggested that altered properties of murine CF AMs may contribute to uncontrolled lung inflammation (14, 15). For instance, it has been reported that functional CFTR is critical for regulation of phagosomal pH in murine AMs (12), and CFTR-deficient macrophages fail to acidify lysosomes and phagolysosomal compartments and display altered bactericidal activity (13, 16, 19, 53). Furthermore, malfunction of CFTR and excessive inflammation in human and murine macrophages have been associated with a higher proinflammatory cytokine secretion (14, 38).
Our findings suggest that the robust inflammatory response of human CF AMs reflects an adaptive response to the chronic infectious/inflammatory milieu of CF airways in vivo and is independent of CFTR function, based on the following observations. First, as compared with the levels of CFTR expression in HBE cultures, the levels of CFTR expression in non-CF AMs are close to zero (Figure 3). Second, pretreatment of non-CF AMs with CFTRinh-172 neither increased basal PS-induced cytokine production (Figure 4). Third, exposure of non-CF AMs to SMM, the infectious/inflammatory milieu of native CF airways, reproduced the robust inflammatory phenotype of CF AMs coupled to larger XBP-1s levels (Figure 7). Hence, although very low levels of CFTR expression may be important for regulation of other AM functions (12, 13), the present studies indicate that the exaggerated inflammation of primary cultures of human CF AMs is not linked to defective CFTR function.
In summary, our study revealed that AMs harvested from chronically infected/inflamed human CF lungs exhibit increased cytokine production and secretion. These data indicate that this response reflects a cellular adaptation to the infectious/inflammatory milieu of CF airways and requires activation of IRE1α/XBP-1. The observation that CF AMs exhibit a larger response to LPS-induced inflammation in an XBP-1s–dependent manner supports the notion that XBP-1s is a positive regulator of genes resulting from TLR-4 activation in AMs. These findings offer the proof-of-principle that targeting the IRE1α/XBP-1 pathway may be a therapeutic strategy to decrease the robust inflammatory response of AMs in chronically infected/inflamed CF lungs. This view is supported by recent studies suggesting that targeting the IRE1α/XBP-1 pathway may improve clinical outcomes for patients with drug-resistant pre-B-cell acute lymphoblastic leukemia (54) and patients with multiple myeloma (55).
Acknowledgments
Acknowledgment
The authors thank Dr. Scott Randell and the personnel of the University of North Carolina (UNC) CF Center Tissue Procurement and Cell Culture Core (supported by CF Foundation grant R026-CR11 and by National Institutes of Health grant P30DK065988) for providing the lungs, Dr. Hong Dang for statistical advice, Mary B. E. Martino and Dr. Gang Chen for useful discussions, the UNC Flow Cytometry Core Facility for assistance (supported by NCI Center Core Support Grant P30CA016086 to the UNC Lineberger Comprehensive Cancer Center), Kimberly Burns from the UNC CF Histology Core for histologic samples, Dr. Michael Chua from the Michael Hooker Microscopy Core for assistance with cell imaging, the UNC CGIBD Advanced Analytics Core (supported by National Institutes of Health grant (P30 DK34987) for assistance with cytokine measurements, Dr. Laurie Glimcher for the DN-XBP-1, Dr. David Ron for providing 4μ8C, and Alexandra Infanzon for editorial assistance.
Footnotes
Supported by grants from the National Heart, Lung, and Blood Institute (5 P01 HL 108808-02S1), the National Institute of Diabetes and Digestive and Kidney Diseases (P30DK065988), the Cystic Fibrosis Foundation (CFF R026), and a Scholarship for Excellence grant from Wallonia-Brussels International.
Author Contributions: B.A.L. and L.C.J. have performed the experiments. B.A.L., R.C.B., W.K.O’N., and C.M.P.R. designed the study. B.A.L. and C.M.P.R. analyzed the data. B.A.L., W.K.O’N., R.C.B., and C.M.P.R. drafted and finalized the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201504-0657OC on September 2, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Saiman L, Siegel J. Infection control in cystic fibrosis. Clin Microbiol Rev. 2004;17:57–71. doi: 10.1128/CMR.17.1.57-71.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boucher RC. Evidence for airway surface dehydration as the initiating event in CF airway disease. J Intern Med. 2007;261:5–16. doi: 10.1111/j.1365-2796.2006.01744.x. [DOI] [PubMed] [Google Scholar]
- 3.Koller DY, Nething I, Otto J, Urbanek R, Eichler I. Cytokine concentrations in sputum from patients with cystic fibrosis and their relation to eosinophil activity. Am J Respir Crit Care Med. 1997;155:1050–1054. doi: 10.1164/ajrccm.155.3.9116985. [DOI] [PubMed] [Google Scholar]
- 4.Muhlebach MS, Noah TL. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. Am J Respir Crit Care Med. 2002;165:911–915. doi: 10.1164/ajrccm.165.7.2107114. [DOI] [PubMed] [Google Scholar]
- 5.Elizur A, Cannon CL, Ferkol TW. Airway inflammation in cystic fibrosis. Chest. 2008;133:489–495. doi: 10.1378/chest.07-1631. [DOI] [PubMed] [Google Scholar]
- 6.Downey DG, Bell SC, Elborn JS. Neutrophils in cystic fibrosis. Thorax. 2009;64:81–88. doi: 10.1136/thx.2007.082388. [DOI] [PubMed] [Google Scholar]
- 7.Bonfield TL, Panuska JR, Konstan MW, Hilliard KA, Hilliard JB, Ghnaim H, Berger M. Inflammatory cytokines in cystic fibrosis lungs. Am J Respir Crit Care Med. 1995;152:2111–2118. doi: 10.1164/ajrccm.152.6.8520783. [DOI] [PubMed] [Google Scholar]
- 8.Nichols D, Chmiel J, Berger M. Chronic inflammation in the cystic fibrosis lung: alterations in inter- and intracellular signaling. Clin Rev Allergy Immunol. 2008;34:146–162. doi: 10.1007/s12016-007-8039-9. [DOI] [PubMed] [Google Scholar]
- 9.Cohen-Cymberknoh M, Kerem E, Ferkol T, Elizur A. Airway inflammation in cystic fibrosis: molecular mechanisms and clinical implications. Thorax. 2013;68:1157–1162. doi: 10.1136/thoraxjnl-2013-203204. [DOI] [PubMed] [Google Scholar]
- 10.Cantin AM, Hartl D, Konstan MW, Chmiel JF. Inflammation in cystic fibrosis lung disease: pathogenesis and therapy. J Cyst Fibros. 2015;14:419–430. doi: 10.1016/j.jcf.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Gordon SB, Read RC. Macrophage defenses against respiratory tract infections. Br Med Bull. 2002;61:45–61. doi: 10.1093/bmb/61.1.45. [DOI] [PubMed] [Google Scholar]
- 12.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 13.Deriy LV, Gomez EA, Zhang G, Beacham DW, Hopson JA, Gallan AJ, Shevchenko PD, Bindokas VP, Nelson DJ. Disease-causing mutations in the cystic fibrosis transmembrane conductance regulator determine the functional responses of alveolar macrophages. J Biol Chem. 2009;284:35926–35938. doi: 10.1074/jbc.M109.057372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, Krause DS, Egan ME. Macrophages directly contribute to the exaggerated inflammatory response in cystic fibrosis transmembrane conductance regulator-/- mice. Am J Respir Cell Mol Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruscia EM, Zhang PX, Satoh A, Caputo C, Medzhitov R, Shenoy A, Egan ME, Krause DS. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J Immunol. 2011;186:6990–6998. doi: 10.4049/jimmunol.1100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Del Porto P, Cifani N, Guarnieri S, Di Domenico EG, Mariggiò MA, Spadaro F, Guglietta S, Anile M, Venuta F, Quattrucci S, et al. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One. 2011;6:e19970. doi: 10.1371/journal.pone.0019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kopp BT, Abdulrahman BA, Khweek AA, Kumar SB, Akhter A, Montione R, Tazi MF, Caution K, McCoy K, Amer AO. Exaggerated inflammatory responses mediated by Burkholderia cenocepacia in human macrophages derived from cystic fibrosis patients. Biochem Biophys Res Commun. 2012;424:221–227. doi: 10.1016/j.bbrc.2012.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vandivier RW, Richens TR, Horstmann SA, deCathelineau AM, Ghosh M, Reynolds SD, Xiao YQ, Riches DW, Plumb J, Vachon E, et al. Dysfunctional cystic fibrosis transmembrane conductance regulator inhibits phagocytosis of apoptotic cells with proinflammatory consequences. Am J Physiol Lung Cell Mol Physiol. 2009;297:L677–L686. doi: 10.1152/ajplung.00030.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bessich JL, Nymon AB, Moulton LA, Dorman D, Ashare A. Low levels of insulin-like growth factor-1 contribute to alveolar macrophage dysfunction in cystic fibrosis. J Immunol. 2013;191:378–385. doi: 10.4049/jimmunol.1300221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Z, Su X. CFTR regulates acute inflammatory responses in macrophages. QJM. doi: 10.1093/qjmed/hcv067. [online ahead of print] 15 Mar 2015; pii: hcv067. [DOI] [PubMed] [Google Scholar]
- 21.Woods PS, Tazi MF, Chesarino NM, Amer AO, Davis IC. TGF-β-induced IL-6 prevents development of acute lung injury in influenza A virus-infected F508del CFTR-heterozygous mice. Am J Physiol Lung Cell Mol Physiol. 2015;308:L1136–L1144. doi: 10.1152/ajplung.00078.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Krause A, Hamai H, Harvey BG, Worgall TS, Worgall S. Proinflammatory phenotype and increased caveolin-1 in alveolar macrophages with silenced CFTR mRNA. PLoS One. 2010;5:e11004. doi: 10.1371/journal.pone.0011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O’Neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280:17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- 24.Martino ME, Olsen JC, Fulcher NB, Wolfgang MC, O’Neal WK, Ribeiro CM. Airway epithelial inflammation-induced endoplasmic reticulum Ca2+ store expansion is mediated by X-box binding protein-1. J Biol Chem. 2009;284:14904–14913. doi: 10.1074/jbc.M809180200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ribeiro CM, Boucher RC. Role of endoplasmic reticulum stress in cystic fibrosis-related airway inflammatory responses. Proc Am Thorac Soc. 2010;7:387–394. doi: 10.1513/pats.201001-017AW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeiro CM, O’Neal WK. Endoplasmic reticulum stress in chronic obstructive lung diseases. Curr Mol Med. 2012;12:872–882. doi: 10.2174/156652412801318791. [DOI] [PubMed] [Google Scholar]
- 27.Martino MB, Jones L, Brighton B, Ehre C, Abdulah L, Davis CW, Ron D, O’Neal WK, Ribeiro CM. The ER stress transducer IRE1β is required for airway epithelial mucin production. Mucosal Immunol. 2013;6:639–654. doi: 10.1038/mi.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 29.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 30.Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature. 2002;415:92–96. doi: 10.1038/415092a. [DOI] [PubMed] [Google Scholar]
- 31.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lubamba BA, Martino MB, Boucher RC, Ribeiro CM. XBP-1 mediates cytokine production by CF human alveolar macrophages [abstract] Pediatr Pulmonol. 2014;49:S254. [Google Scholar]
- 33.Davies JQ, Gordon S. Isolation and culture of human macrophages. Methods Mol Biol. 2005;290:105–116. doi: 10.1385/1-59259-838-2:105. [DOI] [PubMed] [Google Scholar]
- 34.Ribeiro CM, Hurd H, Wu Y, Martino ME, Jones L, Brighton B, Boucher RC, O’Neal WK. Azithromycin treatment alters gene expression in inflammatory, lipid metabolism, and cell cycle pathways in well-differentiated human airway epithelia. PLoS One. 2009;4:e5806. doi: 10.1371/journal.pone.0005806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abreu Velez AM, Dejoseph LM, Howard MS. HAM56 and CD68 antigen presenting cells surrounding a sarcoidal granulomatous tattoo. N Am J Med Sci. 2011;3:475–477. doi: 10.4297/najms.2011.3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122:794–801. doi: 10.1309/QHD6-YFN8-1KQX-UUH6. [DOI] [PubMed] [Google Scholar]
- 37.Manicassamy B, Manicassamy S, Belicha-Villanueva A, Pisanelli G, Pulendran B, García-Sastre A. Analysis of in vivo dynamics of influenza virus infection in mice using a GFP reporter virus. Proc Natl Acad Sci USA. 2010;107:11531–11536. doi: 10.1073/pnas.0914994107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simonin-Le Jeune K, Le Jeune A, Jouneau S, Belleguic C, Roux PF, Jaguin M, Dimanche-Boitre MT, Lecureur V, Leclercq C, Desrues B, et al. Impaired functions of macrophage from cystic fibrosis patients: CD11b, TLR-5 decrease and sCD14, inflammatory cytokines increase. PLoS One. 2013;8:e75667. doi: 10.1371/journal.pone.0075667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma T, Thiagarajah JR, Yang H, Sonawane ND, Folli C, Galietta LJ, Verkman AS. Thiazolidinone CFTR inhibitor identified by high-throughput screening blocks cholera toxin-induced intestinal fluid secretion. J Clin Invest. 2002;110:1651–1658. doi: 10.1172/JCI16112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cross BC, Bond PJ, Sadowski PG, Jha BK, Zak J, Goodman JM, Silverman RH, Neubert TA, Baxendale IR, Ron D, et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proc Natl Acad Sci USA. 2012;109:E869–E878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu Q, Zheng Z, Chang L, Zhao YS, Tan C, Dandekar A, Zhang Z, Lin Z, Gui M, Li X, et al. Toll-like receptor-mediated IRE1α activation as a therapeutic target for inflammatory arthritis. EMBO J. 2013;32:2477–2490. doi: 10.1038/emboj.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23:7448–7459. doi: 10.1128/MCB.23.21.7448-7459.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haslett C. Granulocyte apoptosis and its role in the resolution and control of lung inflammation. Am J Respir Crit Care Med. 1999;160:S5–S11. doi: 10.1164/ajrccm.160.supplement_1.4. [DOI] [PubMed] [Google Scholar]
- 44.Rubins JB. Alveolar macrophages: wielding the double-edged sword of inflammation. Am J Respir Crit Care Med. 2003;167:103–104. doi: 10.1164/rccm.2210007. [DOI] [PubMed] [Google Scholar]
- 45.Mills KH. TLR-dependent T cell activation in autoimmunity. Nat Rev Immunol. 2011;11:807–822. doi: 10.1038/nri3095. [DOI] [PubMed] [Google Scholar]
- 46.Janssens S, Pulendran B, Lambrecht BN. Emerging functions of the unfolded protein response in immunity. Nat Immunol. 2014;15:910–919. doi: 10.1038/ni.2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science. 2000;287:664–666. doi: 10.1126/science.287.5453.664. [DOI] [PubMed] [Google Scholar]
- 48.Brenner DA, O’Hara M, Angel P, Chojkier M, Karin M. Prolonged activation of jun and collagenase genes by tumour necrosis factor-alpha. Nature. 1989;337:661–663. doi: 10.1038/337661a0. [DOI] [PubMed] [Google Scholar]
- 49.Pochet L, Frédérick R, Masereel B. Coumarin and isocoumarin as serine protease inhibitors. Curr Pharm Des. 2004;10:3781–3796. doi: 10.2174/1381612043382684. [DOI] [PubMed] [Google Scholar]
- 50.Kontogiorgis CA, Savvoglou K, Hadjipavlou-Litina DJ. Antiinflammatory and antioxidant evaluation of novel coumarin derivatives. J Enzyme Inhib Med Chem. 2006;21:21–29. doi: 10.1080/14756360500323022. [DOI] [PubMed] [Google Scholar]
- 51.Neelgundmath M, Dinesh KR, Mohan CD, Li F, Dai X, Siveen KS, Paricharak S, Mason DJ, Fuchs JE, Sethi G, et al. Basappa. Novel synthetic coumarins that targets NF-kappaB in hepatocellular carcinoma. Bioorg Med Chem Lett. 2015;25:893–897. doi: 10.1016/j.bmcl.2014.12.065. [DOI] [PubMed] [Google Scholar]
- 52.Niu X, Xing W, Li W, Fan T, Hu H, Li Y. Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited TNF-α production in LPS-induced mouse peritoneal macrophages in vitro via the MAPK pathway. Int Immunopharmacol. 2012;14:164–171. doi: 10.1016/j.intimp.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 53.Swanson J. CFTR: helping to acidify macrophage lysosomes. Nat Cell Biol. 2006;8:908–909. doi: 10.1038/ncb0906-908. [DOI] [PubMed] [Google Scholar]
- 54.Kharabi Masouleh B, Geng H, Hurtz C, Chan LN, Logan AC, Chang MS, Huang C, Swaminathan S, Sun H, Paietta E, et al. Mechanistic rationale for targeting the unfolded protein response in pre-B acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2014;111:E2219–E2228. doi: 10.1073/pnas.1400958111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vincenz L, Jäger R, O’Dwyer M, Samali A. Endoplasmic reticulum stress and the unfolded protein response: targeting the Achilles heel of multiple myeloma. Mol Cancer Ther. 2013;12:831–843. doi: 10.1158/1535-7163.MCT-12-0782. [DOI] [PubMed] [Google Scholar]