To the Editor:
Tuberculosis (TB) remains a major threat to health in developing countries and in HIV-1–infected persons (1). In sub-Saharan Africa, the most common etiology of pericardial effusions in HIV-1–infected persons is TB (2). Mortality in patients with tuberculous pericarditis coinfected with HIV-1 can reach 40% in the absence of antiretroviral treatment, and neither antimicrobial therapy of TB alone nor the addition of corticosteroids to chemotherapy resulted in a clinically satisfactory mortality reduction, although corticosteroids significantly reduced hospitalization and incidence of constrictive pericarditis, regardless of HIV status (3). Improved understanding of immunological mechanisms at the disease site is required for the development of more effective host-directed therapies. Because HIV-1 coinfection alters the memory phenotype of CD4+ T cells in the pericardium (4), we hypothesized that HIV infection would also affect transcript abundance of key immune mediators in pericardial TB at the disease site. Based on known transcriptional perturbation in extrapulmonary TB (5–9), we selected 42 analytes and performed differential transcriptomic analysis by quantitative reverse transcription polymerase chain reaction in paired blood and pericardial fluid from 27 patients (15 definite and 12 probable patients with tuberculous pericarditis, and 17 patients coinfected with HIV-1). We report a detailed immunopathological characterization of pericardial TB, with 21 analytes also confirmed at the protein level. Methods, patient characteristics (see Tables E1 and E2 in the online supplement), and raw data for all genes in blood and pericardial fluid (Tables E3 and E4) are detailed in the online supplement. Preliminary data have been reported in the form of an abstract (10).
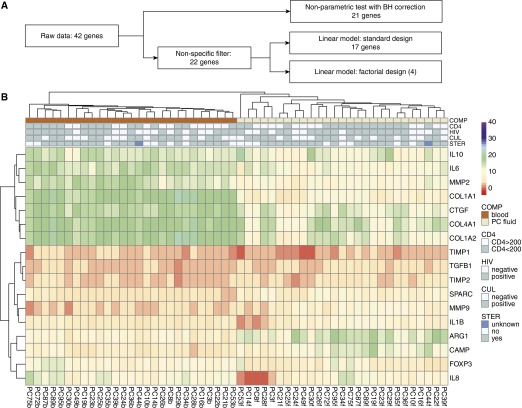
A rigorous data analysis was conducted (Figure 1A). Briefly, pairwise fluid versus blood comparisons of individual genes yielded 21 differentially expressed genes in pericardial fluid compared with blood after application of Benjamini-Hochberg multiple testing correction (Tables E4). Next, cluster analysis was employed, with the resulting heat map (Figure E1) showing that strikingly, most blood and pericardial fluid samples from individual patients clustered together. This appeared to be driven by highly correlated gene expression patterns between the two compartments, as further shown in the correlation matrices of blood and pericardial fluid samples (Figure E2). Gene coexpression patterns in blood differed from pericardial fluid, in which pronounced coexpression of fibrosis-associated and neutrophil-associated genes were evident, as well as coexpression of some pro- and anti-inflammatory genes. Given the very low levels of transcript for some genes, a nonspecific filter was applied, which removed transcripts with a delta cycle threshold value higher than 38 in 5% or more of the samples. This step left 22 genes that separated blood and pericardial fluid, with five samples not assigned to either of the main blood or pericardial fluid clusters (Figure E3). This result indicates that overall gene expression in the two compartments is very different and confirmed our previous impression that genes with low levels of expression drive the patient-specific clustering.
Figure 1.
(A) Outline showing the flow of data in the analytic pipeline. (B) Clustered heat map of 17 selected genes, showing blood and pericardial fluid samples grouped into two separate compartments with prominence of fibrosis-associated genes in pericardial fluid. Expression values expressed as delta cycle threshold relative to β-actin are shown on a continuous color scale. For each sample, sampling compartment (COMP), CD4 count category (CD4), HIV-1 infection status (HIV), tuberculosis culture status (CUL), and concurrent steroid (STER) use are shown in the column annotations. ARG1 = arginase 1; BH = Benjamini-Hochberg; CAMP = cathelicidin antimicrobial peptide; COL = collagen; CTGF = connective tissue growth factor; FOXP3 = forkhead box P3; MMP = matrix metalloproteinase; SPARC = secreted protein acidic and rich in cysteine; TGFB1 = transforming growth factor-β1; TIMP = tissue inhibitor of metalloproteinase.
Next, computation of differential transcript abundance with multiple testing correction using a linear models approach identified 17 transcripts to be differentially abundant between the blood and pericardial fluid compartments (Table 1), which collectively clustered the samples into two separate compartments of blood and pericardial fluid (Figure 1B). A correlation matrix of the 17 selected genes pointed to the prominent coexpression of fibrosis-associated genes in pericardial fluid (Figure E4). Principal component analysis of the data using the three gene sets (“all genes,” showing no differential gene expression between the compartments; 22 “filtered genes,” which indicated a difference between the two compartments; and 17 “selected genes”) showed that the first principal component of the 17 differentially expressed genes explained 60% of the observed variance (Figure E5).
Table 1.
Differential Abundance of Gene Products by Disease Site, as Measured by Either Reverse Transcription Polymerase Chain Reaction (RNA) or ELISA/Luminex (Protein)
| Category and Gene Symbol | RNA |
Protein |
RNA/Protein Congruence | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DA† | ΔΔCT | Adj P Value* | DA‡ | PCF Median (pg/L) | Blood Median (pg/L) | Difference: Fluid − Blood | log2FC | Adj P Value* | ||
| TH1 |
||||||||||
| IL6 | Yes | −3.82 | 9.30 × 10−11 | Yes | 1437.7 | 21.7 | 1,416.1 | 6.05 | 8.36 × 10−8 | Yes |
| CXCL10 | No | −1.78 | 0.414 | Yes | 3138.2 | 556.6 | 2,581.6 | 2.50 | 7.23 × 10−5 | Yes |
| TNF | No | −0.82 | 0.113 | No | 17.0 | 13.0 | 4.0 | 0.39 | 0.497 | Yes |
| IL18 | No | −0.74 | 0.763 | |||||||
| IFNG | No | 1.66 | 0.293 | Yes | 1531.2 | 0.0 | 1,531.2 | High | 9.03 × 10−6 | No |
| IL1B | Yes | 1.72 | 0.007 | Yes | 10.1 | 0.0 | 10.1 | High | 0.034 | No |
| TH2 | ||||||||||
| IL13 | § | No | 7.1 | 0.0 | 7.1 | High | 0.103 | |||
| TH17 | ||||||||||
| IL17A | § | No | 0.0 | 0.0 | 0.0 | 1.00 | 1.000 | |||
| IL22 | § | Yes | 182.0 | 21.6 | 160.4 | 3.07 | 0.019 | |||
| IL23A | No | 0.05 | 0.934 | || | ||||||
| Immunoregulation | ||||||||||
| FOXP3 | Yes | −2.57 | 1.61 × 10−4 | ** | ||||||
| IL10 | Yes | −1.75 | 0.009 | No | 74.7 | 0.3 | 74.3 | 7.85 | 0.350 | Yes |
| TGFB1 | Yes | 0.82 | 7.48 × 10−4 | Yes | 0.0 | 131.5 | −131.5 | Low | 0.001 | Yes |
| MMPs/inhibitors | ||||||||||
| MMP1 | § | Yes | 10.4 | 2.5 | 7.9 | 2.07 | 0.002 | |||
| MMP2 | Yes | −4.54 | 9.46 × 10−12 | Yes | 332.0 | 156.9 | 175.1 | 1.08 | 0.001 | Yes |
| TIMP1 | Yes | −0.95 | 0.003 | Yes | 60.7 | 39.1 | 21.6 | 0.64 | 0.001 | Yes |
| MMP8 | § | No | 83.0 | 69.1 | 13.9 | 0.26 | 0.443 | |||
| TIMP2 | Yes | 0.76 | 0.008 | No | 24.5 | 25.1 | −0.7 | −0.04 | 0.634 | Yes |
| MMP3 | § | No | 23.1 | 36.2 | −13.0 | −0.64 | 0.240 | |||
| MMP7 | § | Yes | 16.9 | 35.9 | −19.1 | −1.09 | 0.011 | |||
| MMP9 | Yes | 4.50 | 1.32 × 10−10 | Yes | 54.5 | 580.6 | −526.1 | −3.41 | 1.93 × 10−5 | Yes |
| Neutrophil | ||||||||||
| IL8 | Yes | −2.82 | 0.003 | Yes | 1034.9 | 0.7 | 1034.3 | 10.60 | 2.09 × 10−11 | Yes |
| CAMP | Yes | 5.22 | 1.32 × 10−10 | ** | ||||||
| Growth factors | ||||||||||
| CTGF | Yes | −3.96 | 1.25 × 10−10 | ** | ||||||
| Fibrosis | ||||||||||
| COL1A1 | Yes | −9.51 | 1.43 × 10−21 | ** | ||||||
| COL1A2 | Yes | −5.99 | 3.75 × 10−13 | ** | ||||||
| COL4A1 | Yes | −4.17 | 1.15 × 10−8 | ** | ||||||
| SPARC | Yes | 3.15 | 1.17 × 10−8 | ** | ||||||
| ARG1 | Yes | 5.50 | 9.30 × 10−11 | ** | ||||||
Definition of abbreviations: Adj = adjusted; ARG1 = arginase 1; CAMP = cathelicidin antimicrobial peptide; COL = collagen; CT = cycle threshold; CTGF = connective tissue growth factor; CXCL10 = C-X-C motif chemokine 10; DA = differentially abundant; FOXP3 = forkhead box P3; IFNG = interferon-γ; log2FC = log2 fold change; MMP = matrix metalloproteinase; PCF = pericardial fluid; SPARC = secreted protein acidic and rich in cysteine; TGFB1 = transforming growth factor-β1; TIMP = tissue inhibitor of metalloproteinase; TNF = tumor necrosis factor.
The ΔΔCT column represents the difference of the mean pericardial fluid and blood delta CT values; negative values indicate higher levels of transcript abundance in pericardial fluid. For protein, the medians for the blood and pericardial fluid values are shown, as is their difference and log2FC of pericardial fluid relative to blood. log2FC values are coded as “high” where division by zero occurs, and as “low” where a zero value occurs in the numerator. The last column shows whether the direction of change for mRNA transcript abundance and protein concentration is congruent.
Corrected (Benjamini-Hochberg) P values <0.05.
Transcripts were regarded as significantly DA based on a corrected P value <0.05 as output by limma.
DA for proteins was defined as a significant difference between the medians of the two compartments (P < 0.05, Mann-Whitney U test).
Transcripts not detected (less than 5% of samples had a delta CT of 38 or lower).
Protein not detected (less than 5% of all samples had levels greater than 0 pg/L).
Protein assay not performed.
To assess whether differential transcript abundance between blood and pericardial fluid was modulated by factors that plausibly reflect differential immune status or modulation thereof, we implemented an analysis in limma (Linear Models for Microarray Data), based on a factorial design, in which we compared the transcript abundance levels between compartments, taking into account the following interaction terms: HIV-1 coinfection status, CD4 count below 200 cells/μl, concurrent corticosteroid use, and pericardial fluid Mycobacterium tuberculosis culture result. None of these factors had a significant effect on differential transcript abundance (Figure 1B and Figure E6).
The immunophenotype of the host response at the disease site was next assessed at the protein level (Table E4); results of fold changes between compartments are summarized in Table 1. In general, protein levels and patterns of over- or underabundance mirrored those of mRNA transcripts. Two exceptions were IFN-γ and IL-1β, both of which were significantly more abundant as proteins in pericardial fluid, but the corresponding mRNA transcript abundance was either no different or significantly lower than in blood (Table 1). We hypothesized that the difference could be a result of antigen-specific T cells that enter the pericardium, release IFN-γ, and die, potentially activating the inflammasome pathway, resulting in pyroptosis and release of IL-1β protein (11). Therefore, we assessed cell death in the pericardial compartment by comparing the cell death enrichment factors in pericardial fluid from cases of tuberculous pericarditis (n = 24) and those from asymptomatic controls undergoing cardiac surgery (n = 28) and found that cell death significantly increased in tuberculous pericarditis cases (P = 2.4 × 10−7) compared with controls (Figure E7), with no difference observed between HIV-infected and uninfected samples.
In summary, we report a strong profibrotic response of gene expression in pericardial fluid, with a differentially stronger pro-inflammatory response also confirmed at the protein level. We show a transcriptomic gene expression signature of 17 genes that differentiate blood from pericardial fluid in patients with pericardial TB, and these transcripts were associated with fibrosis and regulators of fibrosis, as well as matrix metalloproteinases and tissue inhibitors of metalloproteinases. Also, unexpectedly and contrary to the effect on T-cell phenotype, HIV-1 infection does not affect the expression profile of key immune mediators at the pericardial TB disease site. Although we recognize the bias and the limitation introduced by the selection of 42 very specific genes, our data serve to justify a full transcriptional analysis at the microarray level. Further studies including more patients and an unbiased selection of genes will provide more detailed insight into molecular mechanisms and immunopathology during TB infection of the pericardium, and thereby lead to improved host-directed therapy. Future therapeutic approaches could target regulators of fibrosis and apoptosis, with the aim of preventing fibrosis, morbidity, and mortality.
Acknowledgments
Acknowledgment
The authors are grateful to Dr. Okechukwu Usim, Sister Naomi Hare, Sister Joanne Hartnick, Sister Veronica Francis, Sister Unita September, Sister Melanie Stahl, Sister Maitele Tshifularo, Mrs. Carolina Lemmer, Ms. Lerato Motete, Ms. Sharon Mosie, Ms. Margaret van den Berg, Ms. Connie Talliard, Ms. Lowena van Wyk, Mr. Simphiwe Nkepu, and Mr. Jimmy Williams for assistance with this research. They further thank Prof. Nicola J. Mulder for helpful reviews of the manuscript.
Footnotes
This work was supported by the Wellcome Trust (grants 084323 and 104803 to R.J.W.; grant 083226 to K.M.), the Medical Research Council (grant U1175.02.002.00014 to R.J.W.), and the European Union (grants FP7-PEOPLE-2011-IRSES and FP7-HEALTH-F3-2013-305578 to R.J.W.). Additional funding was obtained from the South African Medical Research Council and the Lily and Ernst Hausmann Research Trust.
This letter has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Mayosi BM, Benatar SR. Health and health care in South Africa: 20 years after Mandela. N Engl J Med. 2014;371:1344–1353. doi: 10.1056/NEJMsr1405012. [DOI] [PubMed] [Google Scholar]
- 2.Ntsekhe M, Mayosi BM. Tuberculous pericarditis with and without HIV. Heart Fail Rev. 2013;18:367–373. doi: 10.1007/s10741-012-9310-6. [DOI] [PubMed] [Google Scholar]
- 3.Mayosi BM, Ntsekhe M, Bosch J, Pandie S, Jung H, Gumedze F, Pogue J, Thabane L, Smieja M, Francis V, et al. IMPI Trial Investigators. Prednisolone and Mycobacterium indicus pranii in tuberculous pericarditis. N Engl J Med. 2014;371:1121–1130. doi: 10.1056/NEJMoa1407380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews K, Ntsekhe M, Syed F, Scriba T, Russell J, Tibazarwa K, Deffur A, Hanekom W, Mayosi BM, Wilkinson RJ, et al. HIV-1 infection alters CD4+ memory T-cell phenotype at the site of disease in extrapulmonary tuberculosis. Eur J Immunol. 2012;42:147–157. doi: 10.1002/eji.201141927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess LJ, Reuter H, Carstens ME, Taljaard JJF, Doubell AF. Cytokine production in patients with tuberculous pericarditis. Int J Tuberc Lung Dis. 2002;6:439–446. [PubMed] [Google Scholar]
- 6.Ntsekhe M, Matthews K, Syed FF, Deffur A, Badri M, Commerford PJ, Gersh BJ, Wilkinson KA, Wilkinson RJ, Mayosi BM. Prevalence, hemodynamics, and cytokine profile of effusive-constrictive pericarditis in patients with tuberculous pericardial effusion. PLoS One. 2013;8:e77532. doi: 10.1371/journal.pone.0077532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes PF, Lu S, Abrams JS, Wang E, Yamamura M, Modlin RL. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthews K, Wilkinson KA, Kalsdorf B, Roberts T, Diacon A, Walzl G, Wolske J, Ntsekhe M, Syed F, Russell J, et al. Predominance of interleukin-22 over interleukin-17 at the site of disease in human tuberculosis. Tuberculosis (Edinb) 2011;91:587–593. doi: 10.1016/j.tube.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marquis J-F, Nantel A, LaCourse R, Ryan L, North RJ, Gros P. Fibrotic response as a distinguishing feature of resistance and susceptibility to pulmonary infection with Mycobacterium tuberculosis in mice. Infect Immun. 2008;76:78–88. doi: 10.1128/IAI.00369-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matthews K, Ntsekhe M, Syed F, Russell J, Mayosi BM, Wilkinson RJ, Wilkinson KA.mRNA transcript profiling of pericardial tuberculosis [abstract]. In: 14th International Immunology Meeting Abstracts Int Immunol Suppl 201022(Suppl 1 Pt 3):iii111–iii121. Abstract PP-061-21, p. iii113 [Google Scholar]
- 11.Blander JM. A long-awaited merger of the pathways mediating host defence and programmed cell death. Nat Rev Immunol. 2014;14:601–618. doi: 10.1038/nri3720. [DOI] [PubMed] [Google Scholar]