Abstract
Rationale: Dysregulation of cellular metabolism has been shown to participate in several pathologic processes. However, the role of metabolic reprogramming is not well appreciated in the pathogenesis of organ fibrosis.
Objectives: To determine if glycolytic reprogramming participates in the pathogenesis of lung fibrosis and assess the therapeutic potential of glycolytic inhibition in treating lung fibrosis.
Methods: A cell metabolism assay was performed to determine glycolytic flux and mitochondrial respiration. Lactate levels were measured to assess glycolysis in fibroblasts and lungs. Glycolytic inhibition by genetic and pharmacologic approaches was used to demonstrate the critical role of glycolysis in lung fibrosis.
Measurements and Main Results: Augmentation of glycolysis is an early and sustained event during myofibroblast differentiation, which is dependent on the increased expression of critical glycolytic enzymes, in particular, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3). Augmented glycolysis contributes to the stabilization of hypoxia-inducible factor 1-α, a master regulator of glycolytic enzymes implicated in organ fibrosis, by increasing cellular levels of tricarboxylic acid cycle intermediate succinate in lung myofibroblasts. Inhibition of glycolysis by the PFKFB3 inhibitor 3PO or genomic disruption of the PFKFB3 gene blunted the differentiation of lung fibroblasts into myofibroblasts, and attenuated profibrotic phenotypes in myofibroblasts isolated from the lungs of patients with idiopathic pulmonary fibrosis. Inhibition of glycolysis by 3PO demonstrates therapeutic benefit in bleomycin-induced and transforming growth factor-β1–induced lung fibrosis in mice.
Conclusions: Our data support the novel concept of glycolytic reprogramming in the pathogenesis of lung fibrosis and provide proof-of-concept that targeting this pathway may be efficacious in treating fibrotic disorders, such as idiopathic pulmonary fibrosis.
Keywords: lung fibrosis, glycolysis, myofibroblast, PFKFB3, HIF-1α
At a Glance Commentary
Scientific Knowledge on the Subject
Although aberrant cellular metabolism has been shown to participate in many pathologic processes, there are few attempts to identify metabolic dysregulations associated with organ fibrosis, including idiopathic pulmonary fibrosis. Metabolic programs in fibrotic lungs remain uncharacterized. Metabolism-based therapeutics for treating fibrotic disorders is lacking.
What This Study Adds to the Field
In this study, we found that glycolytic reprogramming is critical to lung myofibroblast differentiation and pulmonary fibrosis. We identified glycolytic enzymes that mediate aerobic glycolysis in myofibroblasts. We demonstrated a unique mechanism of lung fibrosis in that augmented glycolysis leads to up-regulation of the tricarboxylic acid cycle intermediate succinate, which stabilizes hypoxia-inducible factor 1-α to directly promote myofibroblast differentiation. More importantly, we provided the first evidence of treating pulmonary fibrosis through glycolytic inhibition.
Glucose metabolism begins with its transportation via glucose transporters into cytosol, where it is converted into pyruvate through a sequence of 10 biochemical reactions termed glycolysis (1–6). Three reactions in the glycolytic pathway that are catalyzed by hexokinase (HK), phosphofructokinase (PFK), and pyruvate kinase are irreversible and often subject to regulation for the control of the glycolytic flux rate (1–6). With sufficient oxygen, most of pyruvate is carried into mitochondria to be converted to acetyl coenzyme A, which enters the tricarboxylic acid (TCA) cycle to produce nicotinamide adenine dinucleotide reduced that then flows through oxidative phosphorylation (1–3). Under oxygen deprivation, pyruvate is broken down to create lactate in the cytosol (1–3). Augmented glycolysis takes place not only in the absence of oxygen, but also under aerobic conditions, a phenomenon dubbed as “Warburg effect” (1–5, 7).
A defining feature of idiopathic pulmonary fibrosis (IPF) and other progressive fibrotic pathologies is the activation and persevering presence of tissue myofibroblasts (myo-Fbs) (8–13). Myo-Fbs are characterized by de novo expression of smooth muscle actin (SMA)-α; formation of stress fibers; and enhanced abilities to migrate, contract, and produce extracellular matrix (14–16). These features have led to the consensus that targeting myo-Fbs represents an effective therapeutic strategy in the treatment of these clinically refractory disorders, including IPF (12, 14–16).
Metabolic perturbation is implicated in the pathogenesis of several diseases, such as diabetes and cancer (2, 5, 17). However, there is a paucity of information regarding the role of cellular metabolism in tissue fibrosis. More pertinently, it is unknown what glycolytic program lung myo-Fbs use, whether glycolysis is required for lung myo-Fb differentiation and sustainment of their distinctive phenotypes, or whether modulation of glycolysis affects the progression of lung fibrosis in vivo.
In this study, we found that lung myo-Fbs adopt a unique glycolytic program (i.e., aerobic glycolysis). We found that key enzymes that catalyze glycolytic reactions are up-regulated in in vitro differentiated myo-Fbs and myo-Fbs from patients with IPF. Furthermore, we found that glycolysis is required for the establishment and sustainment of profibrotic phenotypes. Lastly, we demonstrated that inhibition of glycolysis is an effective approach to treat pulmonary fibrosis. Our study has thus established a solid foundation for developing glycolysis-based therapies in treating fibrotic disorders. Some of the results of this study have been previously reported in the form of an abstract (18).
Methods
Reagents
Rat tail collagen was obtained from Invitrogen (Carlsbad, CA). Human recombinant transforming growth factor (TGF)-β1 was obtained from Peprotech (Rocky Hill, NJ). Sircol collagen assay kit was obtained from Biocolor (Carrickfergus, UK). Bleomycin was obtained from Besse Medical (West Chester, OH). 3-(3-Pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) was obtained from Axon Medchem (Reston, VA).
Experimental Pulmonary Fibrosis Model
C57BL/6 mice were purchased from Charles River (Wilmington, MA). The bleomycin and TGF-β1–induced lung fibrosis models were described previously (19, 20) and detailed in the online supplement Methods. The animal protocol was approved by the University of Alabama Birmingham Institutional Animal Care and Use Committee.
Cell Lines
Human lung Fb line MRC-5 was purchased from American Type Culture Collection (Manassas, VA).
Human Lung Tissues
IPF and failed donor control tissues were obtained from the University of Alabama Birmingham Tissue Procurement and Cell Culture Core. The protocol was approved by the institutional review board at the University of Alabama at Birmingham.
Isolation of Primary Human Lung Fbs
Primary human lung Fbs were established as previously described (21) and detailed in the online supplement Methods.
Collagen Content Determination (Sircol Assay)
Sircol assay was performed as previously described (22).
Masson Trichrome Assay
The assay was performed using trichrome stain (Masson) kit from Sigma-Aldrich (St. Louis, MO).
Immunohistochemistry
Immunohistochemistry was performed as described in our previous studies (23).
Real-Time Polymerase Chain Reaction
RNA levels of SMA-α, hypoxia-inducible factor 1-α (HIF-1α), fibronectin (Fn), collagen 1A1 (Col1A1), and tubulin were determined by real-time polymerase chain reaction using SYBR Green Master Mix Kit (Roche, Indianapolis, IN). Primer sequences were provided in the online supplement Methods.
CRISPR/Cas9 Lentivirus Generation
The lentiviral vector LentiCRISPRv2 that expresses Cas9 and a guide RNA was developed by Dr. Feng Zhang at Massachusetts Institute of Technology and deposited to Addgene (Cambridge, MA) (24). Detailed information was provided in the online supplement Methods.
Western Blotting
Western blotting was performed as previously described (23). Mouse anti–SMA-α and -tubulin antibodies were from Sigma. Anti–p-Smad2, -Smad2, and –HIF-1α antibodies were from Cell Signaling (Danvers, MA). Anti–6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) antibody was from Proteintech (Chicago, IL) and Abcam (Cambridge, MA).
Chromatin Immunoprecipitation Assay
Chromatin immunoprecipitation assays were performed as previously described (25). Detailed information was provided in the online supplement Methods.
Real-Time Cell Metabolism Assay
XF-24 Extracellular Flux Analyzer (Seahorse Bioscience, North Billerica, MA) was used for real-time analysis of extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) (26). Detailed experimental protocol was provided in the online supplement Methods.
Intracellular and Extracellular Lactate Assays
Intracellular and extracellular levels of lactate were determined using lactate assay kit (BioVision, Milpitas, CA) according to the manufacturer's instructions.
Intracellular Succinate Assay
Intracellular levels of succinate were determined using succinate assay kit (BioVision) according to the manufacturer's instructions.
Statistical Analysis
One-way analysis of variance followed by the Bonferroni test was used for multiple group comparisons. Student’s t test was used for comparison between two groups. P less than 0.05 was considered statistically significant.
Results
Lung myo-Fbs and Fibrotic Lungs Demonstrate Augmented Glycolysis
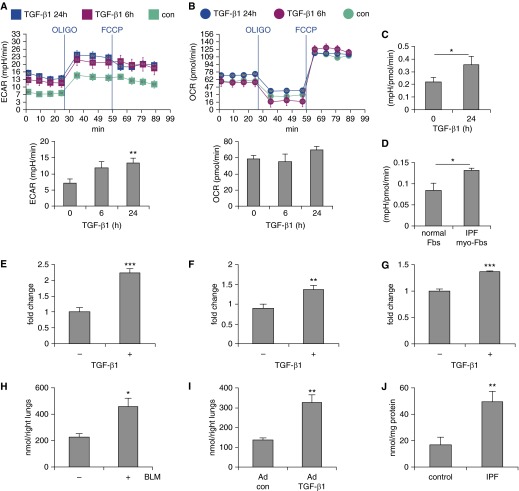
To determine if the pathogenesis of lung fibrosis involves glycolytic dysregulation, we assessed the glycolytic program initially in lung myo-Fbs that were differentiated with TGF-β1, a putative profibrotic growth factor (27, 28). We determined ECAR as a measure of lactate production (a surrogate for glycolytic rate) (29), using Seahorse X24 Extracellular Flux Analyzer. As shown in Figure 1A, TGF-β1 treatment led to an early and sustained increase in ECAR in lung Fbs, indicating the occurrence of enhanced glycolysis in these cells. OCR, a representation of mitochondrial respiratory activity, also had an increase after treatment with TGF-β1, but to a lesser extent than did ECAR (Figure 1B). As a result, the ratio of ECAR/OCR was increased in TGF-β1–differentiated myo-Fbs (Figure 1C).
Figure 1.
Lung myofibroblasts (myo-Fbs) and fibrotic lungs demonstrate augmented glycolysis. (A and B) Human normal lung Fbs MRC-5 were seeded in Seahorse XF-24 cell culture microplates. The cells were treated with 2 ng/ml transforming growth factor (TGF)-β1 for 0, 6, or 24 hours, followed by sequential treatments with oligomycin (OLIGO) and carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP). Real-time extracellular acidification rate (ECAR) and oxygen consumption rate (OCR) were recorded. Bottom rows show the basal levels of ECAR and OCR. n = 5. **P < 0.01 compared with time 0 by unpaired Student’s t test. (C) Three independent normal lung Fb lines were treated with 2 ng/ml TGF-β1 for 0 or 24 hours, followed by sequential treatments with OLIGO and FCCP. Real-time ECAR and OCR were recorded. Mean ratios of ECAR/OCR were calculated. *P < 0.05 compared with time 0 by unpaired Student’s t test. (D) Human normal lung Fbs and idiopathic pulmonary fibrosis (IPF) lung myo-Fbs were seeded in Seahorse microplates. Real-time ECAR and OCR were recorded. Mean ratios of ECAR/OCR were calculated. n = 4 individual lung Fb lines for each group. (E and F) Lung Fbs were treated without or with 2 ng/ml TGF-β1 for 24 hours. The cells were lysed and lactate contents in the cellular lysates (E) and culture media (F) determined. The data are presented as fold change relative to the levels of the untreated control group. (G) Experiments were performed as in F. Glucose contents in culture media were determined. (H) Mice were intratracheally instilled with saline or bleomycin (BLM; 1.5 U/kg). Lungs were harvested 3 weeks later, tissue extracts prepared, and lactate contents and protein concentrations determined. n = 3–4. (I) Mice were intratracheally instilled with control or TGF-β1–expressing adenoviruses (∼6 × 109 pfu/kg). Lungs were harvested 3 weeks later and lactate contents determined. n = 4. (J) Lactate contents in control normal and IPF lungs were determined. n = 7–10. A–C and G–I, mean ± SEM. D–F, mean ± SD. C–J, *P < 0.05, **P < 0.01, ***P < 0.001 by unpaired Student’s t test. Experiments in A–G were performed two to three times. con = control.
These data suggest that lung myo-Fbs demonstrate features of aerobic glycolysis (7). More importantly, we found that myo-Fbs from IPF lungs also adopt the aerobic glycolytic program, as reflected by greater ratios of ECAR/OCR in these cells than those in control normal lung Fbs (Figure 1D). Together, these data suggest that aerobic glycolysis may serve as a new defining phenotype for myo-Fbs. To confirm the glycolytic alterations in lung myo-Fbs, we directly measured the levels of lactate and found that both intracellular and extracellular lactate in lung myo-Fbs were significantly increased (Figures 1E and 1F). Consistent with the augmented glycolysis in lung myo-Fbs, these cells also demonstrated greater glucose consumption (Figure 1G). Altogether, the data suggest that enhanced glycolysis may participate in myo-Fb differentiation during pathologic fibrogenesis in the lungs.
To determine if a similarly augmented glycolytic program takes place in vivo in fibrotic lungs, we directly measured lactate contents in lung tissues and found that there is significantly more lactate in the lungs of mice that received intratracheal instillation of bleomycin, a protocol widely used to establish experimental pulmonary fibrosis (28), than that in the control mouse lungs (Figure 1H). To further confirm that the augmented glycolysis does occur during lung fibrosis, we used another well-recognized model in that mice were intratracheally administered adenovirus that express constitutively active TGF-β1 (Ad-TGF-β1223/225) (19). As shown in Figure 1I, there were significantly greater levels of lactate in the lungs of mice that received TGF-β1–expressing adenovirus. More importantly, lactate levels in IPF lungs were also markedly increased compared with those in normal human lungs (Figure 1J). Altogether, these data clearly demonstrate that glycolysis is reprogrammed to a higher level during the pathogenesis of lung fibrosis.
Key Glycolytic Enzymes Are Up-regulated in Lung myo-Fbs
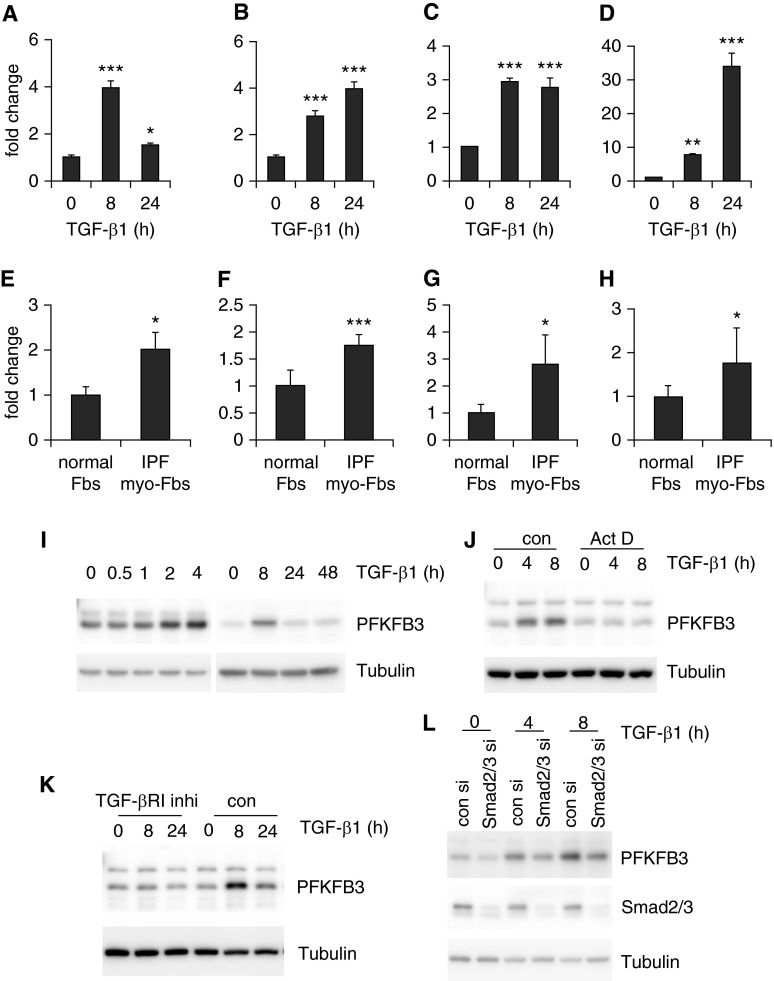
To delineate the mechanisms that contribute to the augmented glycolysis observed in lung myo-Fbs, we assessed the expression of key glycolytic enzymes in these cells. We found that PFKFB3 is markedly induced by TGF-β1 in lung Fbs (Figure 2A). PFKFB3 is not a rate-limiting glycolytic enzyme; instead it catalyzes the conversion of fructose-6-phosphate to fructose-2,6-bisphosphate, an allosteric activator of PFK1 and a potent stimulator of glycolysis (30–32). PFK1 and HK2, rate-limiting enzymes in the glycolytic pathway (33, 34), were also significantly up-regulated in TGF-β1–induced lung myo-Fbs (Figures 2B and 2C). These data suggest that the augmented glycolytic program in lung myo-Fbs is caused by increased expression of key enzymes that catalyze this process. As expected, the myo-Fb marker SMA-α was up-regulated in TGF-β1–induced lung myo-Fbs (Figure 2D).
Figure 2.
Key glycolytic enzymes are up-regulated in lung myofibroblasts (myo-Fbs). (A–D) Normal lung Fbs were treated with 2 ng/ml transforming growth factor (TGF)-β1 for 0, 8, or 24 hours. RNA was purified and mRNA levels of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) (A), phosphofructokinase-1 (PFK-1) (B), hexokinase 2 (HK2) (C), and smooth muscle actin (SMA)-α (D) determined by real-time polymerase chain reaction. n = 3, mean ± SD, *P < 0.05, **P < 0.01, ***P < 0.001 compared with time 0 by one-way analysis of variance. (E–H) mRNA levels of PFKFB3 (E), PFK-1 (F), HK2 (G), and SMA-α (H) in control normal lung Fbs and idiopathic pulmonary fibrosis (IPF) lung myo-Fbs were determined by real-time polymerase chain reaction. n = 5–6, mean ± SEM, *P < 0.05, ***P < 0.001 by Student’s t test. (I) Normal lung Fbs were treated with 2 ng/ml TGF-β1 for the indicated periods of time and protein levels of PFKFB3 and tubulin determined by Western blotting. (J) Normal lung Fbs were pretreated with actinomycin D (Act D; 5 μg/ml) for 1 hour, followed by TGF-β1 treatment for 0, 4, or 8 hours. Protein levels of PFKFB3 and tubulin were determined. (K) Normal lung Fbs were pretreated with type I TGF-β1 receptor inhibitor SB-431542 (10 μM) for 1 hour, followed by TGF-β1 treatment for 0, 8, or 24 hours. Protein levels of PFKFB3 and tubulin were determined. (L) Normal lung Fbs were transfected with 20 μM control or Smad2/3 small interfering RNAs (si). Two days after transfection, cells were treated with TGF-β1 for 0, 4, or 8 hours. Protein levels of PFKFB3, Smad2/3, and tubulin were determined. Experiments in A–D and I–L were performed two to three times. con = control; inhi = inhibitor.
To determine if the similar mechanism that contributes to the augmented glycolysis in TGF-β1–differentiated lung myo-Fbs is also involved in IPF myo-Fbs, we examined the expression of these glycolytic enzymes in the cells. As shown in Figures 2E–2G, the expression of PFKFB3, PFK1, and HK2 was also significantly enhanced in IPF myo-Fbs, compared with that in human normal lung Fbs. Of note, there was increased expression of SMA-α in IPF myo-Fbs, consistent with the profibrotic phenotype of these cells (Figure 2H).
Although we identified several enzymes that are presumably responsible for the augmented glycolysis in lung myo-Fbs, we chose to primarily focus on PFKFB3. We reasoned that inhibition of enzymes mediating the key rate-limiting glycolytic steps, such as PFK1 and HK2, may result in drastic and irreversible plunge in glycolytic flux and potentially cause unwanted adverse effects, particularly during in vivo applications. However, inhibition of the non-rate-limiting PFKFB3 may have milder impact on glycolysis and thus avoid this situation (31).
During the detailed characterization of PFKFB3 expression, we found that the protein levels of this enzyme were increased rapidly and peaked at 8 hours in lung Fbs after TGF-β1 stimulation (Figure 2I), in accordance with the early increased glycolysis in these cells. The up-regulation of PFKFB3 took place at the transcriptional level because inhibition of cellular transcription by actinomycin blunted the elevated expression (Figure 2J). PFKFB3 induction was also mediated by Smad2/3-dependent TGF-β1 signaling events because either the inhibitor against TGF-β1 type I receptor or small interfering RNAs targeting Smad2/3 abolished TGF-β1–induced PFKFB3 in lung Fbs (Figures 2K and 2L).
IPF myo-Fbs are known to be heterogeneous, with cells being at different stages of differentiation (35). To determine the correlation of PFKFB3 expression and myo-Fb differentiation in single cells, we performed flow cytometry analysis and found that both TGF-β1–induced myo-Fbs (see Figure E1A in the online supplement) and IPF Fbs (see Figure E1B) with lower PFKFB3 expression demonstrate less expression of differentiation marker SMA-α than those with greater PFKFB3 expression. These data suggest that PFKFB3 promotes myo-Fb differentiation.
PFKFB3 Is Up-regulated in Fibrotic Lungs
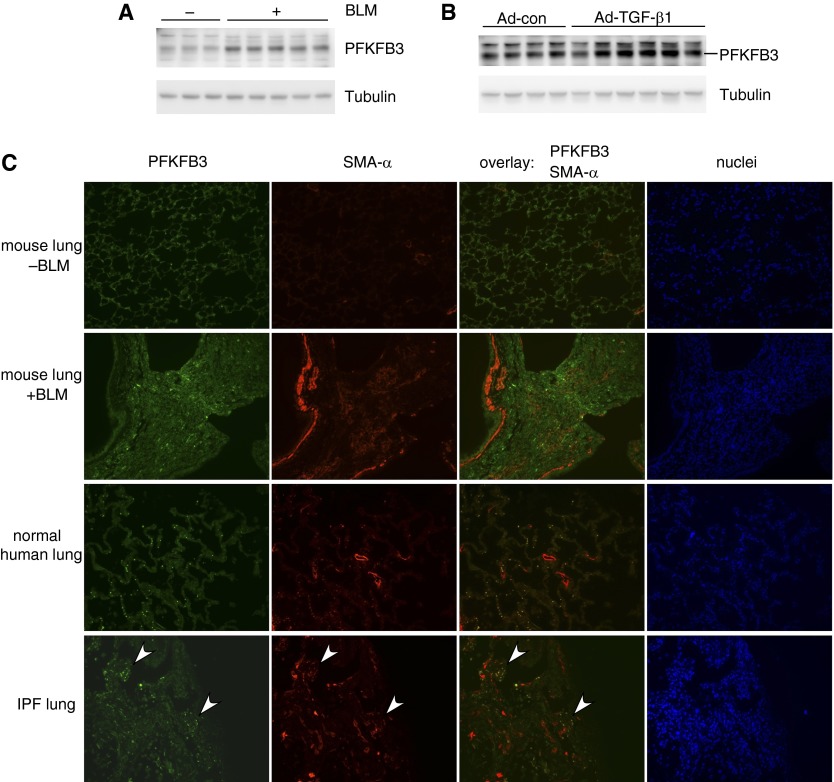
We have shown that PFKFB3 is significantly up-regulated in lung myo-Fbs. We next examined PFKFB3 expression in vivo in the lungs. As shown in Figures 3A and 3B, there was markedly increased expression of PFKFB3 in the lungs of mice that were treated with bleomycin or adenovirus that deliver active TGF-β1. To thoroughly characterize the localization of PFKFB3 expression in the lungs, we performed immunofluorescence microscopy and found that PFKFB3 is ubiquitously expressed in the lungs of normal mouse, including lung epithelial cells, stromal cells, and macrophages (Figure 3C, top row).
Figure 3.
6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) is up-regulated in fibrotic lungs. (A and B) Protein levels of PFKFB3 and tubulin in the lungs of mice that were treated intratracheally with saline (A), bleomycin (BLM) (A), control adenovirus (Ad-con) (B), or transforming growth factor (TGF)-β1–expressing adenovirus (Ad-TGF-β1) (B) for 3 weeks were determined by Western blotting. (C) Expression in situ of PFKFB3 and smooth muscle actin (SMA)-α in the lungs of mice that were treated intratracheally with saline or BLM (top two rows) and in human normal or idiopathic pulmonary fibrosis (IPF) lungs (bottom two rows) was determined by immunofluorescence microscopy. Arrowheads in the bottom row point to two myofibroblastic foci in the IPF lung. Original magnification, ×20.
The expression of myo-Fb marker SMA-α was observed along the walls of blood vascular and bronchial structures in normal and fibrotic mouse lungs and also present in the fibrotic areas of fibrotic mouse lungs (Figure 3C, top two rows). There was increased expression of PFKFB3 in the dense fibrotic areas of fibrotic mouse lungs, with some of the elevated PFKFB3 colocalized with SMA-α (Figure 3C, second row from top), suggesting that augmented glycolysis caused by PFKFB3 up-regulation may contribute to myo-Fb differentiation during lung fibrogenesis. There was also low and ubiquitous expression of PFKFB3 in normal human lungs (Figure 3C, third row from top). It needs to be specified that the small rounded bright green spots in the normal human lungs are autofluorescence derived from red blood cells. More importantly, PFKFB3 elevation was evident in typical myofibroblastic foci in IPF lungs that was colocalized with SMA-α (Figure 3C, bottom row). Together, these data suggest that PFKFB3 participates in myo-Fb differentiation in fibrotic human and mouse lungs. However, it should be noted that PFKFB3 elevation was also present in lung epithelial cells, macrophages, and so forth. Therefore, the role of the induced PFKFB3 in these cell populations in the pathogenesis of lung fibrosis warrants further investigations.
Inhibition of Glycolysis Attenuates the Profibrotic Phenotypes of Lung myo-Fbs
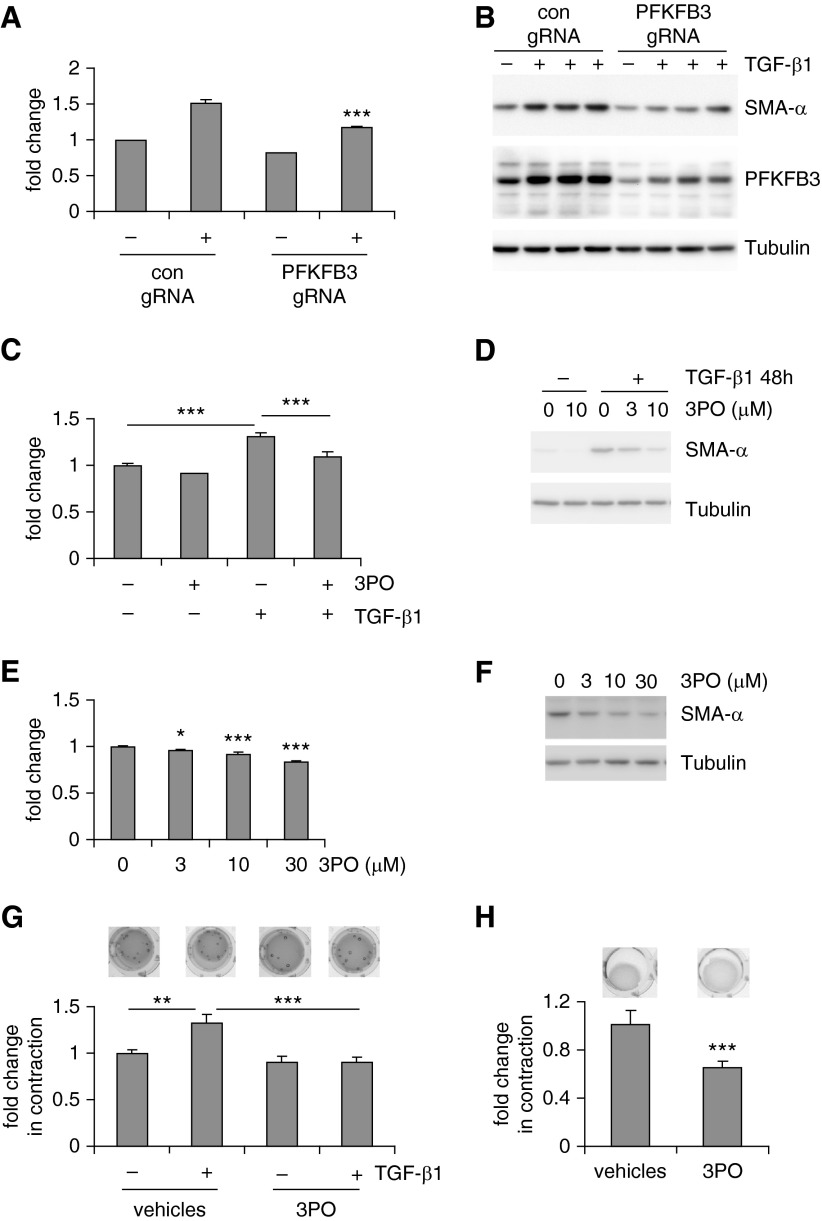
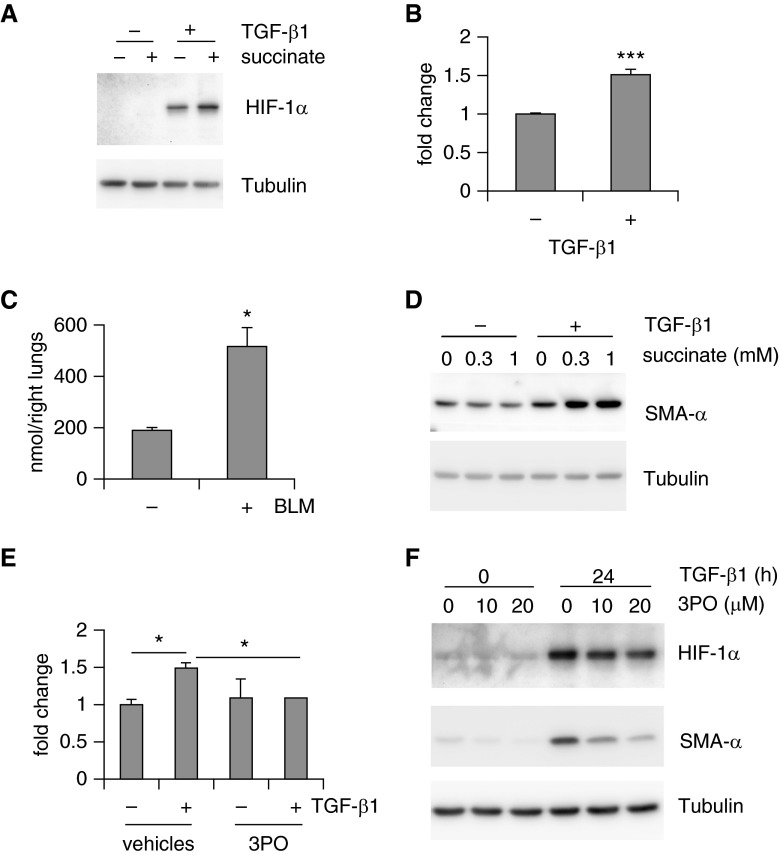
We demonstrated that lung myo-Fbs adopt enhanced glycolysis. We next asked if glycolysis is necessary for the differentiation of lung Fbs into myo-Fbs. To test this hypothesis, we used the powerful CRISPR-Cas9 system to disrupt the genomic locus of the PFKFB3 gene, which would lead to decreased protein expression in lung Fbs. We confirmed that PFKFB3 disruption down-regulates PFKFB3 protein levels and dampens the augmented glycolysis in TGF-β1–induced lung myo-Fbs (Figures 4A and 4B). More importantly, TGF-β1–induced myo-Fb differentiation was also inhibited when PFKFB3 was knocked down (Figure 4B). To further characterize the role of the augmented glycolysis in myo-Fb differentiation, we used a well-defined PFKFB3 inhibitor 3PO to suppress glycolysis (31, 36). As shown in Figure 4C, 3PO dampened glycolysis in TGF-β1–induced myo-Fbs. Consistently, 3PO also effectively inhibited TGF-β1–induced myo-Fb differentiation (Figure 4D). Furthermore, 3PO diminished the glycolytic program in IPF lung myo-Fbs and reversed the differentiated state of these cells (Figures 4E and 4F). We also examined if glycolysis is required for myo-Fb contraction, a property that has been recognized to significantly contribute to many fibrotic pathologies (37). As shown in Figure 4G, inhibition of glycolysis by 3PO remarkably decreased the contractile activity of TGF-β1–induced myo-Fbs. Furthermore, 3PO was able to attenuate the contractility of IPF myo-Fbs (Figure 4H). These data suggest that glycolysis is essential to the acquirement of the characteristic phenotypes of myo-Fbs.
Figure 4.
Inhibition of glycolysis attenuates the profibrotic phenotypes of lung myofibroblasts (myo-Fbs). (A) Normal lung Fbs were infected with control Cas9 lentivirus (con) or Cas9 lentivirus expressing 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) guide RNA (gRNA). Two days after infection, the cells were starved for 1 day, followed by transforming growth factor (TGF)-β1 treatment for 0 or 24 hours. Lactate contents in culture media were determined. n = 3, mean ± SD, ***P < 0.001 compared with con+ by Student’s t test. (B) The experiments were performed as in A and protein levels of PFKFB3, smooth muscle actin (SMA)-α, and tubulin determined. (C) Normal lung Fbs were pretreated with the PFKFB3 inhibitor 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) (10 μM) for 1 hour, followed by TGF-β1 treatment for 0 or 24 hours. Lactate contents in culture media were determined. n = 3, mean ± SD, ***P < 0.001 by one-way analysis of variance (ANOVA). (D) The experiments were performed as in C and protein levels of SMA-α and tubulin determined. (E) Idiopathic pulmonary fibrosis (IPF) lung myo-Fbs were treated with 3PO at the indicated concentrations for 1 day. Lactate contents in culture media were determined. n = 3, mean ± SD, *P < 0.05, ***P < 0.001 compared with 0 by one-way ANOVA. (F) The experiments were performed as in E and protein levels of SMA-α and tubulin determined. (G) Normal lung Fbs were resuspended in rat tail collagen gels. The cells were then pretreated without or with 3PO (10 μM) for 1 hour, followed or not by TGF-β1 treatment. Surface areas of the gels were calculated 2 days after gel release. n = 3, mean ± SD, **P < 0.01, ***P < 0.001 by one-way ANOVA. (H) IPF myo-Fbs were resuspended in collagen gels, followed or not by 3PO (10 μM) treatment for 48 hours. n = 3, mean ± SD, ***P < 0.001 by Student’s t test. Experiments in A–H were performed two to three times.
Although we were concerned that inhibition of key rate-limiting glycolytic enzymes may cause deleterious side effects in vivo, we remained intrigued to know if it would produce similar effects to those observed with suppression of PFKFB3 in vitro. To do this, we pretreated lung Fbs with 2-deoxyglucose (2-DG), a glucose analog that inhibits glycolysis via its actions on HK2 (38). We first confirmed that 2-DG leads to a remarkably decreased glycolysis in normal lung Fb MRC-5 cells and TGF-β1–induced lung myo-Fbs (see Figure E2A). Consistent with its inhibition of glycolysis, 2-DG was potent in attenuating TGF-β1–induced lung myo-Fb differentiation (see Figure E2B). Furthermore, we found that 2-DG diminishes glycolysis in IPF lung myo-Fbs and attenuates the differentiated phenotype of these cells (see Figures E2C and E2D). 2-DG also significantly decreased the contractility of IPF myo-Fbs and the contractile and migratory activities of TGF-β1–induced myo-Fbs (see Figures E2E–E2G). Another HK2 inhibitor, 3-bromopyruvate, was able to achieve effects similar to those by 2-DG (see Figures E3A–E3D) (39). Altogether, these data further confirm that glycolysis is required for lung myo-Fb differentiation.
Glycolysis has a net yield of two molecules of ATP (7). To determine if the attenuation of lung myo-Fb differentiation by glycolytic inhibition is not simply caused by a drop in the intracellular ATP levels, we treated lung Fbs with 3PO for 3 or 24 hours. As shown in Figure E4A, 3PO only had a marginal effect on the ATP levels in the cells. Furthermore, TGF-β1–induced phosphorylation of Smad2 was not affected by 3PO (see Figure E4B). 2-DG treatment drastically decreased intracellular ATP levels (see Figure E4C). However, it had no effect on TGF-β1–induced Smad2 phosphorylation (see Figure E4D). These data suggest that the diminished myo-Fb differentiation by inhibition of glycolysis is independent of ATP generated in this process. Additionally, glycolytic suppression by PFKFB3 inhibitor 3PO had no effect on cell proliferation or viability, in contrast to that by 2-DG (see Figure E4E; data not shown).
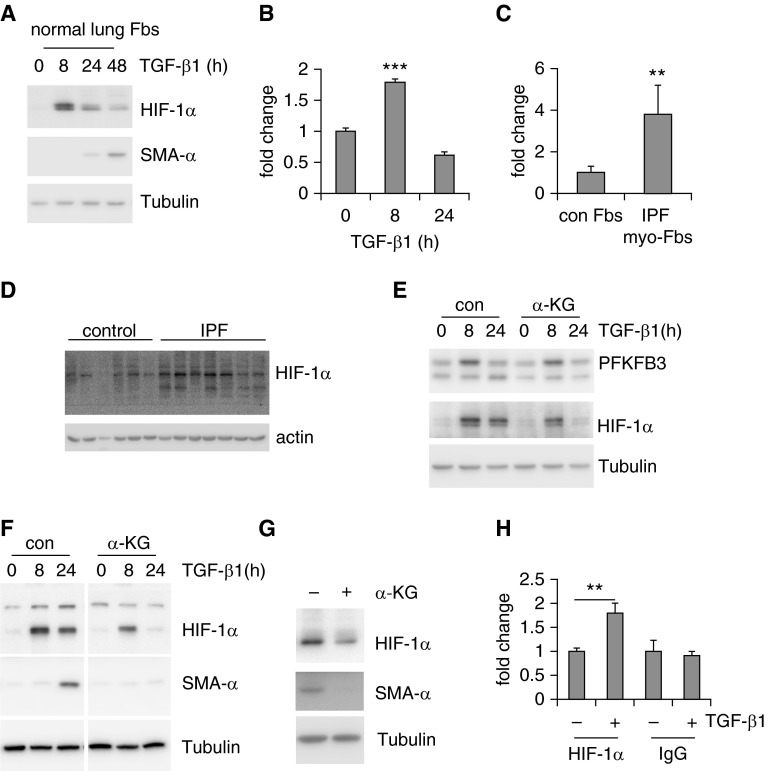
HIF-1α Is Up-regulated in Lung myo-Fbs and Required for myo-Fb Differentiation
HIF-1α has been shown to be the master regulator of the expression of glycolytic enzymes and key transcriptional factor that drives glycolysis under hypoxic conditions (40–42). It is also well recognized that HIF-1α plays an important role in tissue fibrosis (43–47). We here asked if HIF-1α is involved in the elevated expression of PFKFB3 and myo-Fb differentiation. First, we found that HIF-1α is rapidly up-regulated by TGF-β1 in lung Fbs at both protein and RNA levels (Figures 5A and 5B). HIF-1α was also up-regulated in IPF lung myo-Fbs and IPF lung tissues (Figures 5C and 5D). Surprisingly, we found that a cell-permeable α-ketoglutarate (α-KG) derivative, which stimulates prolyl hydroxylase activity and thus depleting HIF-1α (48), does not decrease PFKFB3 expression in lung myo-Fbs (Figure 5E). However, HIF-1α depletion by α-KG markedly diminished TGF-β1–induced myo-Fb differentiation and reversed the differentiated phenotype of IPF myo-Fbs (Figures 5F and 5G). To determine the mechanism by which HIF-1α regulates lung myo-Fb differentiation, we searched the promoter region of the SMA-α gene and identified a putative HIF-1α responsive element (A/GCGTG) at 184 bp upstream of the transcriptional starting site (49). We used chromatin immunoprecipitation assay to confirm that HIF-1α directly binds to this region after TGF-β1 treatment (Figure 5H). These data suggest that although HIF-1α does not participate in PFKFB3-mediated glycolytic augmentation, it is required for lung myo-Fb differentiation.
Figure 5.
Hypoxia-inducible factor 1-α (HIF-1α) is up-regulated in lung myofibroblasts (myo-Fbs) and required for myo-Fb differentiation. (A) Normal lung Fbs were treated with transforming growth factor (TGF)-β1 for 0, 8, 24, or 48 hours and protein levels of HIF-1α, smooth muscle actin (SMA)-α, and tubulin determined. (B) mRNA levels of HIF-1α were determined in TGF-β1–treated MRC-5 cells. n = 3, mean ± SD, ***P < 0.001 by one-way analysis of variance. (C) mRNA levels of HIF-1α were determined in control (con) normal lung Fbs and idiopathic pulmonary fibrosis (IPF) lung myo-Fbs. n = 5–6, mean ± SEM, **P < 0.01 by Student’s t test. (D) Protein levels of HIF-1α and actin were determined in control normal lungs and IPF lungs. (E) Normal lung Fbs were pretreated with α-ketoglutarate (α-KG) (0.5 mM) for 1 hour, followed by TGF-β1 treatment for 0, 8, or 24 hours. Protein levels of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), HIF-1α, and tubulin were determined. (F) Normal lung Fbs were pretreated with α-KG (0.5 mM) for 1 hour, followed by TGF-β1 treatment for 0, 8, or 24 hours. Protein levels of HIF-1α, SMA-α, and tubulin were determined. The images separated by vertical white lines were derived from the same blots, with unrelated lanes removed. (G) IPF lung myo-Fbs were treated with α-KG (0.5 mM) for 0 or 24 hours. Protein levels of HIF-1α, SMA-α, and tubulin were determined. (H) Normal lung Fbs were treated with TGF-β1 for 0 or 8 hours. The cells were then fixed and lysed. Immunoprecipitations with anti–HIF-1α or -rabbit IgG were performed and DNA in the immunocomplexes purified. The binding of HIF-1α to the HIF-1α responsive element within the SMA-α promoter was quantified by real-time polymerase chain reaction and presented as fold change relative to the untreated control group. n = 3, mean ± SD, **P < 0.01 by Student’s t test. Experiments in A–C and E–H were performed two to three times.
Augmented Glycolysis Leads to Increased Succinate That Stabilizes HIF-1α and Promotes Lung myo-Fb Differentiation
Prolyl hydroxylase mediates HIF-1α hydroxylation, a process that uses α-KG as a cosubstrate and converts it into succinate (40–42). Buildup of succinate in turn inhibits HIF-1α hydroxylation, leading to HIF-1α stabilization (48, 50). Because we were aware of that, succinate is also an intermediate of the TCA cycle and dysregulated glycolysis has been shown to alter succinate levels (48). To determine if succinate plays a role in the up-regulation of HIF-1α in lung myo-Fbs, we treated lung Fbs with succinate. As shown in Figure 6A, succinate alone did not induce HIF-1α, indicating the necessity of the up-regulation of HIF-1α messenger RNAs in lung myo-Fbs. Nevertheless, succinate markedly enhanced TGF-β1–induced HIF-1α expression (Figure 6A). These data suggest that succinate stabilizes HIF-1α independently of hypoxic conditions. Next, we found that succinate is significantly up-regulated in lung myo-Fbs (Figure 6B). Succinate levels were also increased in fibrotic lungs (Figure 6C). More importantly, succinate promoted TGF-β1–induced myo-Fb differentiation (Figure 6D). These findings were consistent with the elevated expression of HIF-1α in lung myo-Fbs. The rise of succinate was dependent on the augmented glycolysis in lung myo-Fbs because inhibition of glycolysis by 3PO nearly abolished such an increase in these cells (Figure 6E). Likely as the result of attenuation of succinate elevation, HIF-1α was destabilized by 3PO in TGF-β1–induced lung myo-Fbs (Figure 6F). HIF-1α in TGF-β1 differentiated lung myo-Fbs and IPF lung myo-Fbs was also decreased by treatment with 2-DG (see Figure E5). Taken together, these data suggest that augmented glycolysis promotes myo-Fb differentiation by boosting succinate-dependent HIF-1α stabilization.
Figure 6.
Augmented glycolysis leads to increased succinate that stabilizes hypoxia-inducible factor 1-α (HIF-1α) and promotes lung myofibroblasts (myo-Fbs) differentiation. (A) Normal lung Fbs were starved for 1 day, followed by pretreatment with succinate (0.5 mM) for 1 hour and transforming growth factor (TGF)-β1 treatment for 0 or 8 hours. Protein levels of HIF-1α and tubulin were determined. (B) Cellular succinate levels were determined in TGF-β1 untreated or treated MRC-5 cells. n = 3, mean ± SD, ***P < 0.001 by Student’s t test. (C) Succinate levels were determined in the lungs of mice that were intratracheally treated with saline or bleomycin (BLM) for 3 weeks. n = 3–4, mean ± SEM, *P < 0.05 by Student’s t test. (D) Normal lung Fbs were starved for 1 day, followed by pretreatment with succinate (0, 0.3, or 1 mM) for 1 hour and TGF-β1 treatment for 0 or 24 hours. Protein levels of smooth muscle actin (SMA)-α and tubulin were determined. (E) Normal lung Fbs were starved for 1 day, followed by pretreatment with 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) (0 or 10 μM) for 1 hour and TGF-β1 treatment for 0 or 24 hours. Cellular succinate levels were determined. n = 3, mean ± SD, *P < 0.05 by one-way analysis of variance. (F) Normal lung Fbs were starved for 1 day, followed by pretreatment with 3PO (0, 10, 20 μM) for 1 hour and TGF-β1 treatment for 0 or 24 hours. Protein levels of HIF-1α, SMA-α, and tubulin were determined. Experiments in A and B and D–F were performed two to three times.
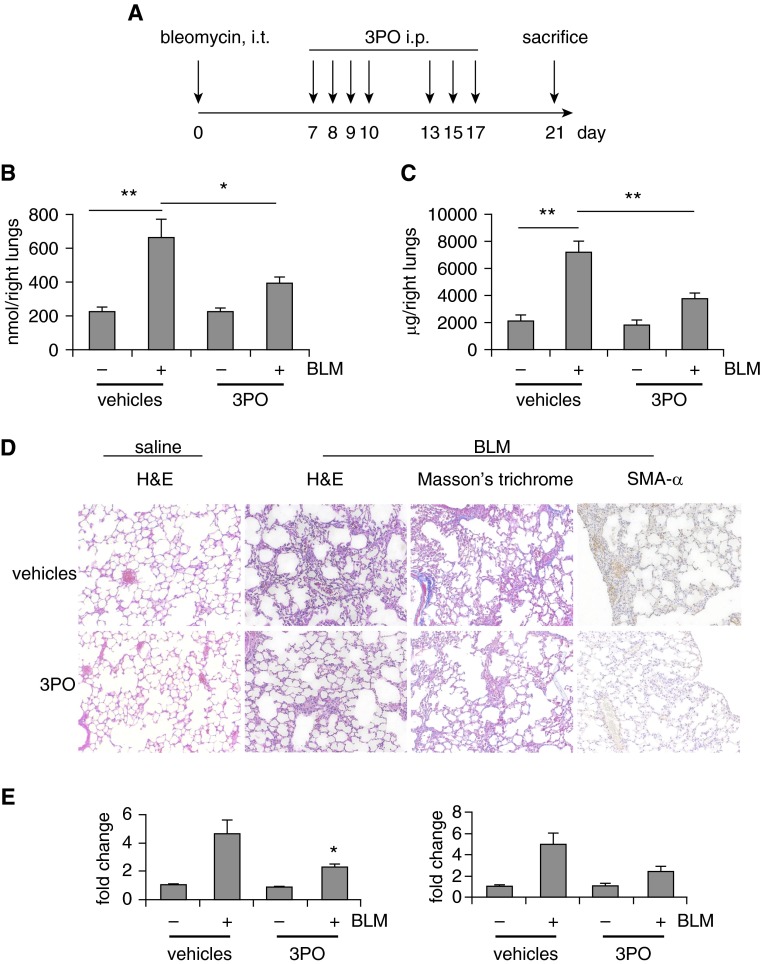
Inhibition of Glycolysis Therapeutically Attenuates Bleomycin-induced Pulmonary Fibrosis In Vivo
The compelling evidence that glycolysis is required for lung myo-Fb differentiation in vitro intrigued us to ask if glycolytic inhibition has the potential to become a novel therapeutic to treat lung fibrosis. We chose 3PO over 2-DG for this purpose because 2-DG inhibits glycolysis high up within the glycolytic flux and invokes more complete and permanent glycolytic suppression (31). Furthermore, 2-DG needs to compete with mM levels of glucose in the blood, necessitating administration of high or toxic dosages of 2-DG (31). To determine the therapeutic value of glycolytic inhibition, we started 3PO injection 1 week after intratracheal instillation of bleomycin (Figure 7A), a time point when prominent lung inflammation recedes and active fibrogenetic process takes place (28). We first confirmed that 3PO diminishes the augmented glycolysis in fibrotic lungs, whereas it does not affect the basal glycolysis in normal mouse lungs (Figure 7B). More importantly and consistent with it inhibiting glycolysis, 3PO treatment significantly attenuated collagen deposition in the lungs of bleomycin-treated mice, compared with that in the vehicle control animals (Figure 7C). Histologic examination of the lung sections, Masson trichrome staining of the interstitial collagen, immunohistochemical assays of SMA-α, and examination of the expression of Fn and Col1A1 all confirmed that 3PO effectively attenuates bleomycin-induced lung fibrosis (Figures 7D and 7E).
Figure 7.
Inhibition of glycolysis therapeutically attenuates bleomycin (BLM)-induced pulmonary fibrosis in vivo. (A) Schematic illustration of the experiment design. C57BL/6 mice were intratracheally (i.t.) instilled with saline or BLM (1.5 U/kg in 50 μl saline). Seven days after the administration, the mice were intraperitoneally (i.p.) injected with 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) (50 mg/kg dissolved in 50 μl dimethyl sulfoxide) or dimethyl sulfoxide for seven times within 2 weeks. At the end of the treatment, mice were killed and lungs harvested. (B) Lactate contents in the lungs from the experiments were determined. n = 3, 8, 5, 10, respectively. mean ± SEM, *P < 0.05, **P < 0.01 by one-way analysis of variance (ANOVA) and Bonferroni test. (C) Collagen contents in right lungs were determined by Sircol assay. n = 3, 8, 5, 10, respectively. mean ± SEM, **P < 0.01 by one-way ANOVA and Bonferroni test. (D) Lung tissue sections were prepared from the above experiments. Representative images of hematoxylin–eosin (H&E) staining, Masson trichrome staining for collagen deposition, and immunohistochemistry staining for smooth muscle actin (SMA)-α are shown. Original magnification, ×20. (E) Messenger RNA levels of fibronectin (left) and collagen 1A1 (right) in the lungs in this experiment were determined by real-time polymerase chain reaction. n = 3–10, mean ± SEM, *P < 0.05 by one-way ANOVA and Bonferroni test.
In all, these data suggest that glycolytic inhibition is a promising therapeutic strategy to treat organ fibrotic disorders, such as IPF. Of note, at 10 days after bleomycin injection, there were no significant amounts of proinflammatory cytokines in the lungs (see Figure E6B). Although there were still increased leukocytes and protein leaks in the bronchoalveolar lavage fluids from fibrotic lungs at this time point, 3PO did not affect these injury-associated parameters (see Figures E6C and E6D), suggesting that inflammatory regulation does not play a significant role in the antifibrotic effect of 3PO. Additionally, 3PO did not cause significant weight loss (see Figure E6A).
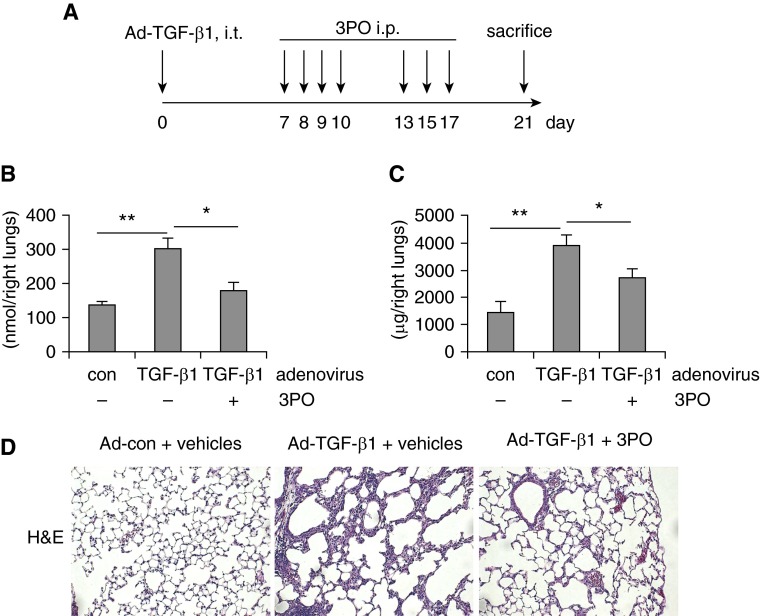
Inhibition of Glycolysis Diminishes TGF-β1–induced Pulmonary Fibrosis In Vivo
Although bleomycin-induced lung fibrosis is the most widely used mouse model in the field, it is incompletely representative of the pathologic progression of human IPF (28). To further test the therapeutic value of glycolytic suppression by the PFKFB3 inhibitor 3PO in treating this disease, we performed a similar experiment with the TGF-β1–induced pulmonary fibrosis model (19). 3PO was given 1 week after the administration of TGF-β1–expressing adenovirus for a total of seven times (Figure 8A). As shown in Figures 8B and 8C, 3PO effectively inhibited glycolysis and fibrosis in the lungs of mice that received TGF-β1–expressing adenovirus. Taken together, we have convincingly demonstrated the therapeutic efficacy of 3PO in two well-established pulmonary fibrosis models.
Figure 8.
Inhibition of glycolysis diminishes transforming growth factor (TGF)-β1–induced pulmonary fibrosis in vivo. (A) Schematic illustration of the experiment design. C57BL/6 mice were intratracheally (i.t.) instilled with control adenovirus or adenovirus expressing active TGF-β1 (Ad-TGF-β1) (6 × 109 pfu/kg). Seven days after the administration, the mice were intraperitoneally (i.p.) injected with 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) (50 mg/kg dissolved in 50 μl dimethyl sulfoxide) or dimethyl sulfoxide for seven times within 2 weeks. At the end of the treatment, mice were killed and lungs harvested. (B) Lactate contents in the lungs from the experiments were determined. n = 4, 7, 6, respectively; mean ± SEM, *P < 0.05, **P < 0.01 by one-way analysis of variance and Bonferroni test. (C) Collagen contents in right lungs were determined by Sircol assay. n = 4, 7, 6, respectively. mean ± SEM, *P < 0.05, **P < 0.01 by one-way analysis of variance and Bonferroni test. (D) Lung tissue sections were prepared from the above experiments. Representative images of the hematoxylin–eosin (H&E) staining are shown. Original magnification, ×20. con = control.
Discussion
In this study, we found that glycolytic augmentation is an early and lasting event during lung myo-Fb differentiation and an essential step for the development of lung fibrosis. More importantly, we demonstrated that glycolytic suppression using the PFKFB3 inhibitor has clear therapeutic values in two experimental pulmonary fibrosis models. Thus, we have firmly established a new concept that pulmonary fibrosis, probably other organ fibrotic pathologies as well, is a disorder of glycolytic dysregulation. This has significant implications in terms of finding new therapies for the treatment of fibrotic diseases because practical and feasible approaches to inhibit glycolysis in vivo have been identified and are being tested in some other metabolic disorders, such as cancer and inflammatory diseases (6, 48, 51). These new therapeutics can be readily adapted to the application of treating organ fibrotic disorders, including IPF.
We demonstrated that glycolysis is required for the initiation and sustainment of myo-Fb differentiation, which apparently accounts for the essentiality of such a metabolic reprogramming in the development of pulmonary fibrosis. However, it should be noted that other pathologic phenotypes of myo-Fbs, such as augmented proliferation and resistance to apoptosis, could be also dependent on the elevated glycolysis. In addition, highly glycolytic myo-Fbs may be capable of creating a metabolically favorable microenvironment through secreting lactate and other glycolytic intermediates to cause pathologic changes in adjacent pulmonary cells, such as cellular dysplasia, which is frequently found in the alveolar epithelia surrounding the myofibroblastic foci in IPF lungs (52). Although these hypotheses remain to be tested, there is abundant evidence alluding that such events could take place during pulmonary fibrosis. For example, ramping up of glycolysis is known to be an essential step to ensure rapid proliferation of cancer cells by supplying their synthetic demands (2). Additionally, augmented glycolysis in cancer stromal cells, including Fbs, has been shown to promote malignancy of cancer cells (53).
Although TGF-β1–induced myo-Fbs shares many profibrotic features with IPF myo-Fbs, they may not be identical in terms of mechanisms of establishment. It has been shown that myo-Fbs differentiation in IPF lungs involves several transcriptional and epigenetic mechanisms and many profibrotic mediators, including TGF-β1 (54). Therefore, it is likely that factors other than TGF-β1 could also contribute to the sustained high PFKFB3 expression in IPF myo-Fbs. This definitely remains our focus of future interests. It is also worth noting that IPF fibroblasts are known to be heterogeneous, with cells being at different stages of differentiation (35). We found that IPF fibroblasts with lower PFKFB3 expression demonstrate less differentiation than those with greater PFKFB3 expression (see Figure E1B), suggesting that PFKFB3 promotes myo-Fbs differentiation. More importantly, PFKFB3 inhibition was able to reverse the profibrotic phenotype of IPF myo-Fbs (Figures 4F and 4H). Together, these data suggest that PFKFB3 is likely required for the initiation and sustainment of myo-Fbs differentiation in IPF lungs.
We found that the TCA cycle intermediate succinate is up-regulated in lung myo-Fbs. Succinate is known to originate from several sources, including from glycolytic flux via pyruvate and glutamine (55). Although it remains to be identified which source is responsible for the succinate elevation in myo-Fbs, our data that inhibition of glycolysis decreased intracellular succinate in myo-Fbs suggest the amplified glycolytic flux as a possible explanation. Consistent with the activity of succinate in stabilizing HIF-1α, glycolytic suppression led to decreased HIF-1α in myo-Fbs.
Although HIF-1α is a master regulator that controls hypoxic induction of many glycolytic enzymes (40–42), it is unlikely to be responsible for the increased expression of PFKFB3 in lung myo-Fbs. This conclusion is based on the finding that destabilization of HIF-1α has no effect on TGF-β1–induced PFKFB3 expression in lung Fbs. Nevertheless, we found that PFKFB3 up-regulation is dependent on TGF-β1–activated Smad2/3 because either blocking TGF-β1 type I receptor or knocking down Smad2/3 abrogates the induction of PFKFB3. These findings place HIF-1α downstream of the augmented glycolysis in lung myo-Fbs. We have found that HIF-1α is required for lung myo-Fb differentiation and that binds to the HIF-1α responsive element site within the SMA-α promoter. Therefore, the regulation of HIF-1α by glycolysis-modulated succinate suggests the promotion of lung myo-Fb differentiation by HIF-1α as one of the direct mechanisms by which glycolysis participates in pathologic fibrogenesis.
Our data, along with previous studies from others, have identified HIF-1α as a central player in the pathogenesis of lung fibrosis (43–47). However, it should be noted that the role of the augmented glycolysis in myo-Fb differentiation involves mechanisms other than HIF-1α. A reasonable possibility involves the increased succinate in the glycolytic lung myo-Fbs. Succinate has been recently found to be capable of generating a new type of post-translational modification, named succinylation, which is able to alter protein localizations and functions (56). Therefore, identification of succinylated proteins in lung myo-Fbs could shed new light into the mechanisms by which glycolysis regulates pulmonary fibrosis.
We found that although 3PO is not as potent as 2-DG to inhibit glycolysis, it is as effective to suppress myo-Fb differentiation. These data suggest that a mild ramping down of glycolysis is sufficient to change the course of myo-Fb differentiation. Such findings are of particular importance when to harness glycolytic inhibition for treating pulmonary fibrosis because a drastic reduction of glycolysis in vivo, such as that presumably caused by 2-DG, could bring serious side effects and thus becoming intolerable to patients (31). Such an implication is likely to place 3PO above 2-DG for being tested in future clinical trials for the treatment of organ fibrosis, including IPF.
Although we have found that lung myo-Fbs adopt augmented glycolysis, attenuation of bleomycin and TGF-β1–induced pulmonary fibrosis by the PFKFB3 inhibitor 3PO may not be solely attributable to the diminished glycolytic program in these cells. It is possible that the interruption of the glycolysis in other lung cell populations, such as alveolar epithelial cells and macrophages, contributes to the in vivo efficacy of 3PO. Although this is not critical from the therapeutic standpoint, it is mechanistically worthwhile to consider these alternative explanations, which require targeted deletion of PFKFB3 in the various types of lung cells.
In conclusion, we demonstrated that augmentation of aerobic glycolysis is an essential step during myo-Fb differentiation. We found that PFKFB3 is up-regulated and mediates the glycolytic reprogramming in myo-Fbs. Finally, we showed that ramping down glycolysis by inhibiting PFKFB3 is effective in diminishing myo-Fb differentiation and limiting lung fibrosis. Our findings identify a novel target for developing new therapeutic strategies to treat organ fibrosis, including IPF.
Acknowledgments
Acknowledgment
The authors thank Danni Zhou for editorial assistance.
Footnotes
Supported by National Institutes of Health grants HL105473, HL076206, and HL114470.
Author Contributions: N.X. and G.L. designed the study. N.X., Z.T., S.B., H.C., J.G., R.-M.L., and K.B. performed the experiments, contributed materials, and/or analyzed the data. V.J.T. contributed intellectual inputs. N.X., V.J.T., and G.L. wrote the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201504-0780OC on August 18, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Koppenol WH, Bounds PL, Dang CV. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11:325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 2.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 3.DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell. 2012;148:1132–1144. doi: 10.1016/j.cell.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Lunt SY, Dayton TL, Fiske BP, Israelsen WJ, Mattaini KR, Vokes NI, Stephanopoulos G, Cantley LC, Metallo CM, et al. Metabolic pathway alterations that support cell proliferation. Cold Spring Harb Symp Quant Biol. 2011;76:325–334. doi: 10.1101/sqb.2012.76.010900. [DOI] [PubMed] [Google Scholar]
- 5.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123:3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 8.Thannickal VJ, Toews GB, White ES, Lynch JP, III, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 9.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 11.Kis K, Liu X, Hagood JS. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011;13:e27. doi: 10.1017/S1462399411001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noble PW, Barkauskas CE, Jiang D. Pulmonary fibrosis: patterns and perpetrators. J Clin Invest. 2012;122:2756–2762. doi: 10.1172/JCI60323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eickelberg O, Laurent GJ. The quest for the initial lesion in idiopathic pulmonary fibrosis: gene expression differences in IPF fibroblasts. Am J Respir Cell Mol Biol. 2010;42:1–2. doi: 10.1165/rcmb.2009-0245ED. [DOI] [PubMed] [Google Scholar]
- 14.Hinz B. Mechanical aspects of lung fibrosis: a spotlight on the myofibroblast. Proc Am Thorac Soc. 2012;9:137–147. doi: 10.1513/pats.201202-017AW. [DOI] [PubMed] [Google Scholar]
- 15.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmoulière A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wen H, Ting JP, O’Neill LA. A role for the NLRP3 inflammasome in metabolic diseases: did Warburg miss inflammation? Nat Immunol. 2012;13:352–357. doi: 10.1038/ni.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie N, Tan Z, Cui H, Thannickal VJ, Liu G. Glycolytic reprogramming is required for myofibroblast differentiation and lung fibrosis [abstract] Am J Respir Crit Care Med. 2015;191:A4922. doi: 10.1164/rccm.201504-0780OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu RM, Vayalil PK, Ballinger C, Dickinson DA, Huang WT, Wang S, Kavanagh TJ, Matthews QL, Postlethwait EM. Transforming growth factor β suppresses glutamate-cysteine ligase gene expression and induces oxidative stress in a lung fibrosis model. Free Radic Biol Med. 2012;53:554–563. doi: 10.1016/j.freeradbiomed.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang S, Cui H, Xie N, Icyuz M, Banerjee S, Antony VB, Abraham E, Thannickal VJ, Liu G. miR-145 regulates myofibroblast differentiation and lung fibrosis. FASEB J. 2013;27:2382–2391. doi: 10.1096/fj.12-219493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Königshoff M, Kramer M, Balsara N, Wilhelm J, Amarie OV, Jahn A, Rose F, Fink L, Seeger W, Schaefer L, et al. WNT1-inducible signaling protein-1 mediates pulmonary fibrosis in mice and is upregulated in humans with idiopathic pulmonary fibrosis. J Clin Invest. 2009;119:772–787. doi: 10.1172/JCI33950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–1597. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang S, Banerjee S, de Freitas A, Sanders YY, Ding Q, Matalon S, Thannickal VJ, Abraham E, Liu G. Participation of miR-200 in pulmonary fibrosis. Am J Pathol. 2012;180:484–493. doi: 10.1016/j.ajpath.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–784. doi: 10.1038/nmeth.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu G, Park YJ, Abraham E. Interleukin-1 receptor-associated kinase (IRAK)-1-mediated NF-kappaB activation requires cytosolic and nuclear activity. FASEB J. 2008;22:2285–2296. doi: 10.1096/fj.07-101816. [DOI] [PubMed] [Google Scholar]
- 26.Tan Z, Xie N, Banerjee S, Cui H, Fu M, Thannickal VJ, Liu G. The monocarboxylate transporter 4 is required for glycolytic reprogramming and inflammatory response in macrophages. J Biol Chem. 2015;290:46–55. doi: 10.1074/jbc.M114.603589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cutroneo KR, White SL, Phan SH, Ehrlich HP. Therapies for bleomycin induced lung fibrosis through regulation of TGF-beta1 induced collagen gene expression. J Cell Physiol. 2007;211:585–589. doi: 10.1002/jcp.20972. [DOI] [PubMed] [Google Scholar]
- 28.Moore BB, Hogaboam CM. Murine models of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2008;294:L152–L160. doi: 10.1152/ajplung.00313.2007. [DOI] [PubMed] [Google Scholar]
- 29.Kaplon J, Zheng L, Meissl K, Chaneton B, Selivanov VA, Mackay G, van der Burg SH, Verdegaal EM, Cascante M, Shlomi T, et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature. 2013;498:109–112. doi: 10.1038/nature12154. [DOI] [PubMed] [Google Scholar]
- 30.Van Schaftingen E, Lederer B, Bartrons R, Hers HG. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982;129:191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- 31.Schoors S, De Bock K, Cantelmo AR, Georgiadou M, Ghesquière B, Cauwenberghs S, Kuchnio A, Wong BW, Quaegebeur A, Goveia J, et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 32.De Bock K, Georgiadou M, Schoors S, Kuchnio A, Wong BW, Cantelmo AR, Quaegebeur A, Ghesquière B, Cauwenberghs S, Eelen G, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 33.Wang L, Xiong H, Wu F, Zhang Y, Wang J, Zhao L, Guo X, Chang LJ, Zhang Y, You MJ, et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Reports. 2014;8:1461–1474. doi: 10.1016/j.celrep.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yi W, Clark PM, Mason DE, Keenan MC, Hill C, Goddard WA, III, Peters EC, Driggers EM, Hsieh-Wilson LC. Phosphofructokinase 1 glycosylation regulates cell growth and metabolism. Science. 2012;337:975–980. doi: 10.1126/science.1222278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akamatsu T, Arai Y, Kosugi I, Kawasaki H, Meguro S, Sakao M, Shibata K, Suda T, Chida K, Iwashita T. Direct isolation of myofibroblasts and fibroblasts from bleomycin-injured lungs reveals their functional similarities and differences. Fibrogenesis Tissue Repair. 2013;6:15. doi: 10.1186/1755-1536-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, An X, Guo X, Habtetsion TG, Wang Y, Xu X, Kandala S, Li Q, Li H, Zhang C, et al. Endothelial PFKFB3 plays a critical role in angiogenesis. Arterioscler Thromb Vasc Biol. 2014;34:1231–1239. doi: 10.1161/ATVBAHA.113.303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y, Huang X, Hecker L, Kurundkar D, Kurundkar A, Liu H, Jin TH, Desai L, Bernard K, Thannickal VJ. Inhibition of mechanosensitive signaling in myofibroblasts ameliorates experimental pulmonary fibrosis. J Clin Invest. 2013;123:1096–1108. doi: 10.1172/JCI66700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker-Samuel S, Ramasawmy R, Torrealdea F, Rega M, Rajkumar V, Johnson SP, Richardson S, Gonçalves M, Parkes HG, Arstad E, et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat Med. 2013;19:1067–1072. doi: 10.1038/nm.3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nilsson H, Lindgren D, Mandahl Forsberg A, Mulder H, Axelson H, Johansson ME. Primary clear cell renal carcinoma cells display minimal mitochondrial respiratory capacity resulting in pronounced sensitivity to glycolytic inhibition by 3-bromopyruvate. Cell Death Dis. 2015;6:e1585. doi: 10.1038/cddis.2014.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 41.Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277:23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- 42.Yeung SJ, Pan J, Lee MH. Roles of p53, MYC and HIF-1 in regulating glycolysis: the seventh hallmark of cancer. Cell Mol Life Sci. 2008;65:3981–3999. doi: 10.1007/s00018-008-8224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozen-Zvi B, Hayashida T, Hubchak SC, Hanna C, Platanias LC, Schnaper HW. TGF-β/Smad3 activates mammalian target of rapamycin complex-1 to ‘e collagen production by increasing HIF-1α expression. Am J Physiol Renal Physiol. 2013;305:F485–F494. doi: 10.1152/ajprenal.00215.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzouvelekis A, Harokopos V, Paparountas T, Oikonomou N, Chatziioannou A, Vilaras G, Tsiambas E, Karameris A, Bouros D, Aidinis V. Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis. Am J Respir Crit Care Med. 2007;176:1108–1119. doi: 10.1164/rccm.200705-683OC. [DOI] [PubMed] [Google Scholar]
- 45.Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, et al. Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest. 2007;117:3810–3820. doi: 10.1172/JCI30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moon JO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G582–G592. doi: 10.1152/ajpgi.90368.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kottmann RM, Kulkarni AA, Smolnycki KA, Lyda E, Dahanayake T, Salibi R, Honnons S, Jones C, Isern NG, Hu JZ, et al. Lactic acid is elevated in idiopathic pulmonary fibrosis and induces myofibroblast differentiation via pH-dependent activation of transforming growth factor-β. Am J Respir Crit Care Med. 2012;186:740–751. doi: 10.1164/rccm.201201-0084OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, Frezza C, Bernard NJ, Kelly B, Foley NH, et al. Succinate is an inflammatory signal that induces IL-1β through HIF-1α. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sciacovelli M, Guzzo G, Morello V, Frezza C, Zheng L, Nannini N, Calabrese F, Laudiero G, Esposito F, Landriscina M, et al. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase. Cell Metab. 2013;17:988–999. doi: 10.1016/j.cmet.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galluzzi L, Kepp O, Vander Heiden MG, Kroemer G. Metabolic targets for cancer therapy. Nat Rev Drug Discov. 2013;12:829–846. doi: 10.1038/nrd4145. [DOI] [PubMed] [Google Scholar]
- 52.Weng T, Poth JM, Karmouty-Quintana H, Garcia-Morales LJ, Melicoff E, Luo F, Chen NY, Evans CM, Bunge RR, Bruckner BA, et al. Hypoxia-induced deoxycytidine kinase contributes to epithelial proliferation in pulmonary fibrosis. Am J Respir Crit Care Med. 2014;190:1402–1412. doi: 10.1164/rccm.201404-0744OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lisanti MP, Martinez-Outschoorn UE, Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle. 2013;12:2723–2732. doi: 10.4161/cc.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thannickal VJ, Zhou Y, Gaggar A, Duncan SR. Fibrosis: ultimate and proximate causes. J Clin Invest. 2014;124:4673–4677. doi: 10.1172/JCI74368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mills E, O’Neill LA. Succinate: a metabolic signal in inflammation. Trends Cell Biol. 2014;24:313–320. doi: 10.1016/j.tcb.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Zhang Z, Tan M, Xie Z, Dai L, Chen Y, Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat Chem Biol. 2011;7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]