Abstract
Objective
α/β-Hydrolase domain-6 (ABHD6) is a newly identified monoacylglycerol (MAG) lipase. We recently reported that it negatively regulates glucose stimulated insulin secretion (GSIS) in the β cells by hydrolyzing lipolysis-derived MAG that acts as a metabolic coupling factor and signaling molecule via exocytotic regulator Munc13-1. Whether ABHD6 and MAG play a role in response to all classes of insulin secretagogues, in particular various fuel and non-fuel stimuli, is unknown.
Methods
Insulin secretion in response to various classes of secretagogues, exogenous MAG and pharmacological agents was measured in islets of mice deficient in ABHD6 specifically in the β cell (BKO). Islet perifusion experiments and determinations of glucose and fatty acid metabolism, cytosolic Ca2+ and MAG species levels were carried out.
Results
Deletion of ABHD6 potentiated insulin secretion in response to the fuels glutamine plus leucine and α-ketoisocaproate and to the non-fuel stimuli glucagon-like peptide 1, carbamylcholine and elevated KCl. Fatty acids amplified GSIS in control and BKO mice to the same extent. Exogenous 1-MAG amplified insulin secretion in response to fuel and non-fuel stimuli. MAG hydrolysis activity was greatly reduced in BKO islets without changes in total diacylglycerol and triacylglycerol lipase activity. ABHD6 deletion induced insulin secretion independently from KATP channels and did not alter the glucose induced rise in intracellular Ca2+. Perifusion studies showed elevated insulin secretion during second phase of GSIS in BKO islets that was not due to altered cytosolic Ca2+ signaling or because of changes in glucose and fatty acid metabolism. Glucose increased islet saturated long chain 1-MAG species and ABHD6 deletion caused accumulation of these 1-MAG species at both low and elevated glucose.
Conclusion
ABHD6 regulates insulin secretion in response to fuel stimuli at large and some non-fuel stimuli by controlling long chain saturated 1-MAG levels that synergize with other signaling pathways for secretion.
Keywords: α/β-Hydrolase domain-6, Monoacylglycerol, Insulin secretion, Pancreatic islets, Cytosolic Ca2+
Abbreviations: ABHD6, α/β-hydrolase domain-6; ATGL, adipose triglyceride lipase; BKO, β cell specific ABHD6-knockout; Carb, carbamylcholine; DAG, diacylglycerol; FFA, free fatty acid; Flox, flox/flox; GL/FFA, glycerolipid/ free fatty acid; GLP1, glucagon-like peptide 1; GPCR, G-protein coupled receptor; GSIS, glucose stimulated insulin secretion; HSL, hormone sensitive lipase; Kic, α-ketoisocaproate; KO, knockout; MAG, monoacylglycerol; OGTT, oral glucose tolerance test; ROS, reactive oxygen species; TG, triacylglycerol; WT, wild type; 1-OG, 1-oleoylglycerol; 1-PG, 1-palmitoylglycerol; 1-SG, 1-stearoylglycerol
Highlights
-
•
ABHD6 is the major monoacylglycerol (MAG) hydrolase in pancreatic β cells.
-
•
1-MAG level is elevated in islets from β cell specific ABHD6-KO mice (BKO).
-
•
BKO islets show enhanced fuel and non-fuel induced insulin secretion.
-
•
ABHD6 accessible 1-MAG synergizes with other signals for insulin secretion.
1. Introduction
Insulin secretion plays a central role in glucose homeostasis, and glucose is the most prominent secretagogue for the pancreatic islet β cell [1]. Yet, despite decades of research, we still do not fully understand how the intracellular metabolism of glucose and other fuel stimuli, such as fatty acids and some amino acids, is coupled to the exocytotic release of insulin containing granules. Glucose stimulated insulin secretion (GSIS) is a biphasic process with a rapid first phase followed by a sustained second phase [2]. Glucose stimulation of the β cell results in a rise in the ATP/ADP ratio with associated closure of KATP channels and a rise in intracellular Ca2+ that acts as a trigger for insulin release that can be amplified by additional metabolic coupling factors besides adenine nucleotides [1], [3]. Candidate metabolic coupling factors include NADPH [4], reactive oxygen species (ROS) [5], glutamate [6], acyl-CoA compounds [7], [8] and additional lipid signaling molecules [9]. Although there is a general consensus that some lipid signaling molecules play key role in the amplification pathway of GSIS, the nature of these signals has remained elusive [1].
Glucose and free fatty acid (FFA) both increase flux through the glycerolipid/free fatty acid (GL/FFA) cycle in β cells, with its lipogenesis and lipolysis arms [1]. We [10], [11], [12], [13] and others [14], [15] have proposed that the lipolysis segment of the GL/FFA cycle generates signal(s) involved in β cell activation for insulin release. Deletion in the β cell of adipose triglyceride lipase (ATGL) [10] and hormone sensitive lipase (HSL) [11], [13], [15], that respectively catalyze the hydrolysis of triacylglycerol (TG) to diacylglycerol (DAG) and DAG to monoacylglycerol (MAG), resulted in reduced GSIS. On the basis of these observations, we hypothesized that one of the molecules downstream of DAG in the lipolysis cascade (MAG, FFA or glycerol) acts as a metabolic coupling factor [16]. MAG hydrolysis provides FFA and glycerol. Since reduced GSIS in ATGL knockout (KO) islets was not rescued by exogenous FFA [10] and glycerol is not a secretagogue, we hypothesized that this long-searched metabolic coupling factor is MAG.
We recently demonstrated that in the β cell, MAG hydrolysis is conducted by α/β-hydrolase domain-6 (ABHD6), a newly identified MAG hydrolase and that in these cells the expression of the classical MAG lipase is very low [16]. We further showed that 1-MAG, which is produced in response to glucose stimulation of β cells, acts as a coupling factor for GSIS. Furthermore, the level of 1-MAG increases by the suppression of ABHD6 activity either pharmacologically or by its genetic deletion. MAG was found to mediate its effect via activation of the exocytosis regulator protein Munc13-1, thereby enhancing insulin granule exocytosis [16]. The bulk of the evidence for a role of MAG as a coupling factor was obtained using whole body ABHD6-KO mice and we also showed that GSIS is enhanced in vivo and ex vivo in islets from β cell specific ABHD6-KO (BKO) mice. However, several questions remained with respect to the role of ABHD6 and MAG in the regulation of insulin secretion that are addressed here using islets from BKO mice, where ABHD6 was deleted in β cells at adult stage by tamoxifen-induced Cre expression. Are ABHD6 and MAG involved more globally in β cell activation, that is in the regulation of insulin secretion in response to other fuels besides glucose and non-fuel stimuli? Are they implicated in: the KATP-dependent or independent pathway of secretion; the first or second phase of GSIS; the glucose or fatty acid metabolism or Ca2+ signaling?
Our results with BKO mice provide evidence that ABHD6 accessible MAG, which is produced during nutrient metabolism, synergizes with other cellular signals arising from fuel and some non-fuel stimuli to potentiate insulin secretion and that MAG activates the KATP-independent pathway and second phase of secretion. ABHD6 deletion in β cells does not alter glucose and fatty acid metabolism or Ca2+ influx. The data highlight the importance of ABHD6 and MAG in regulating insulin secretion in response to all stimuli to control glucose homeostasis, and thus ABHD6 is a potential candidate for developing drugs against type 2 diabetes.
2. Materials and methods
2.1. Animals
The use of animals for the experiments in this study was approved by the Institutional Committee for the Protection of Animals. C57BL/6N mice were housed at room temperature (22 °C) with 12-h light/dark cycling, with free access to water and standard chow diet (11% fat by energy).
2.2. Generation of BKO mice
The generation of inducible BKO mice where ABHD6 is deleted specifically in the β cell has been described before [16]. In our previous report, we made a detailed comparison among wild type (WT), MIP-cre/ERT, and ABHD6 Flox/Flox (Flox) mice for many parameters. We found that WT, MIP-cre/ERT and Flox mice behaved similarly in terms of body weight gain and food intake over 5 weeks following i.p. tamoxifen injection, fed and fasting glycemia, islet morphology and β cell mass, oral glucose tolerance test (OGTT) and ex vivo GSIS. Based on this observation, in the present, we employed only Flox mice as control mice [16]. Flox mice were bred with Flox/Flox; Mipcre+ (BKO) mice to obtain 50% littermates as Flox mice and 50% BKO mice. Flox and BKO mice at 8 weeks of age received daily tamoxifen injections (50 mg/kg BW), for 5 consecutive days. Two weeks later, the mice were used for islet isolation.
2.3. Islet isolation
Pancreatic islets from Flox and BKO mice were isolated as described previously [10]. The isolated islets were handpicked and let to recover by incubation overnight in RPMI 1640, supplemented with 10% FBS and 11 mM glucose (recovery medium).
2.4. MAG, DAG and TG hydrolysis activity
Procedures for measuring MAG, DAG and TG hydrolysis activities have been described earlier [38]. Briefly, immediately after isolation, islets were washed twice with cold PBS, and then were homogenized in cold PBS. Assays for MAG, DAG and TG hydrolysis using corresponding specific substrates were done with 0.5 μg, 0.5 μg or 10 μg protein, respectively, per assay. MAG hydrolysis was assayed in 96-well plates, with 1-S-arachidonoylthioglycerol (Cayman Chemical Co) as the substrate, and the released 1-thioglycerol was measured by reaction with ThioGlo-1 (Covalent Associates, Corvallis, OR) to form a fluorescent adduct, measured in a FLUOstar microplate reader instrument (BMG Labtech, Germany). DAG hydrolysis activity was assayed in 96-well plates using p-nitrophenylbutyrate (Sigma Aldrich) as the substrate by monitoring the reaction at 507 nm, in a plate reader. TG hydrolysis activity was measured using EnzChek lipase substrate (Life technologies) and fluorescence (excitation 485 nm; emission 510 nm) was monitored every 30 s for 90 min. Enzyme specific inhibitors were employed in the assays for ascertaining the activity of the enzyme that was being measured. Thus for ABHD6 catalyzed MAG hydrolysis assay, 1 μM WWL70 (Cayman Chemical), an ABHD6 inhibitor [16] was included. For total DAG hydrolysis assay, 1 μM orlistat as DAGL inhibitor since at this low concentration, orlistat inhibits mainly DAGL [38]. For the TG hydrolysis assay, we employed 1 μM Cay10499 as TG hydrolysis inhibitor [38].
2.5. Ex vivo insulin secretion
The procedure for insulin secretion using isolated islets has been described [16]. Briefly, after overnight culture in recovery medium, the islets were starved in RPMI 1640 with 10% FBS and 2 mM glucose for 2 h. After starvation, the islets were washed twice with ‘enriched’ KRBH, which contained 0.5% defatted BSA, 50 μM carnitine, 2 mM Gln and 4 mM glucose. This enriched KRBH, which better matches the physiological milieu [1], was used in order to prevent fuel-depleted conditions that may arise with the commonly used KRBH, which contained only 2.8 mM glucose. Islets were pre-incubated in this enriched KRBH for 45 min, followed by incubation with different concentrations of glucose and in the presence or absence of different fuel and non-fuel stimuli (0.15 mM oleate plus 0.15 mM palmitate, 10 mM α-ketoisocaproate (Kic), 5 mM glutamine plus leucine (Gln plus Leu), 20 nM glucagon-like peptide 1 (GLP1), 35 mM KCl, 200 μM carbamylcholine (Carb) for 1 h. For the experiment regarding the effect of exogenous MAG in the presence of various stimuli, dispersed Wistar rat islet cells were used because MAG is lipophilic and may not access all islet cells within an intact islet but only cells at the periphery. The islets were dispersed into single islet cells by trypsin digestion [17], and the cells were plated into 48-well plates at a density of 1 × 105 cells per well. After 2 days culture in RPMI medium, insulin secretion experiments were performed. Following similar starvation and pre-incubation conditions as described above for islets, the dispersed islet cells were incubated at 6 mM glucose with or without 20 nM GLP-1, 200 μM Carb and 10 mM Kic in the absence or presence of 100 μM 1-palmitolglycerol (1-PG) for 1 h. At the end of the experiments, incubation media were collected for insulin release analysis. Total insulin content was measured after islets or isolated islet cells extraction by a mixture of ethanol and HCl (75%: 1.5%). Insulin was measured using Alphalisa (Perkin Elmer, Waltham, Massachusetts) [16].
2.6. Glucose and fatty acid metabolism
Glucose oxidation and utilization and fatty acid oxidation were measured as described before [18] with minor modifications. Briefly, for glucose metabolism, the islets were cultured in recovery medium overnight. Then, the islets were starved in RPMI 1640 with 2 mM glucose for 2 h. Batches of 20 islets were pre-incubated in enriched KRBH for 45 min, and then incubated in KRBH containing 0.5 μCi of [5-3H] d-glucose (16 Ci/mmol) and 1 μCi/ml [U-14C] d-glucose (250 mCi/mmol) at 4, 10, and 16 mM glucose. The incubation was stopped by adding citrate/NaOH buffer (400 mM, pH 4.9) containing antimycin-A (10 μM), rotenone (10 μM), and KCN (5 mM). Glucose oxidation was followed by measuring the generated 14CO2 after 60 min in KOH trap. Glucose utilization was determined by measuring the 3H2O produced. For fatty acid oxidation, after a first starvation in RPMI 1640 with 2 mM glucose for 2 h, batches of 50 islets were pre-incubated in enriched KRBH for 45 min and then incubated for 2 h in KRBH containing 0.25% BSA, 0.1 mM palmitate and 0.2 μCi/ml [9,10(n)-3H]-palmitate (74 kBq/ml) at 4 and 16 mM glucose. The supernatant was collected to separate 3H2O from radioactive fatty acids and fatty acid oxidation was calculated by measuring 3H2O produced.
2.7. Intracellular Ca2+ measurement
After overnight recovery of islets as above, the islets were dispersed into single cells by trypsin digestion [17]. The dispersed cells were placed in 96-well black plates with clear bottom at a density of 80,000 cells per well. After overnight culture in RPMI 1640 plus 10% FBS, the cell were pre-loaded in enriched KRBH with 2.5 mM probenicid, 0.2 mM sulfinpyrazone, 0.1 mM 3-isobutyl-1-methylxanthine (IBMX), equal volume of Fura-2 AM (Life technologies) (6 μM) and Pluronic F-127 for 75 min. Then the cells were washed once with enriched KRBH, and incubated in enriched KRBH with 2.5 mM probenicid and 0.2 mM sulfinpyrazone for 30 min. Then the cells were monitored using a plate reader (FLUOstar) with two different excitation filters of 340 nm and 380 nm and with emission at 510 nm. High glucose was added manually to reach a final concentration of 16 mM, and KCl was injected by the machine to reach a final concentration of 35 mM. The intracellular Ca2+ was calculated as ratio of fluorescence outputs at 340 nm and 380 nm (F340/F380).
2.8. Perifusion experiments
Overnight recovered Flox and BKO islets were starved in RPMI 1640 medium at 2 mM glucose for 2 h. Then batches of 30 islets were placed in the perifusion chambers and perifused at a flow rate of 0.5 ml/min for 45 min in enriched KRBH, followed by perifusion with 16 mM glucose for 30 min, and then with 35 mM KCl for another 30 min. At different time points indicated in the figure legend, 0.5 ml samples were collected and centrifuged for insulin analysis. After the perifusion, the islets were removed from the chamber and extracted with a mixture of ethanol and HCl (75%: 1.5%) to release total insulin for normalization. Insulin was measured by Alphalisa analysis.
2.9. MAG species analysis
MAG species analysis was done as described before [16]. Briefly, overnight recovered islets were starved in RPMI 1640 with 2 mM glucose for 2 h, and then pre-incubated in enriched KRBH for 45 min, followed by incubation with low (4 mM) and high (16 mM) glucose in the absence and presence of different stimuli for 1 h. For each condition, 250 islets were used. After the treatment, the islets were collected for lipid extraction in Folch reagent. After lipid extraction, the dried lipids were dissolved in a small volume of chloroform and loaded on silica gel thin layer chromatography plates and developed in the solvent system (Chloroform: acetone: acetic acid = 60:40:1, v/v) to separate 1-MAG and 2-MAG. The bands corresponding to 1-MAG and 2-MAG were scraped and saponified and the released FFAs were extracted by Dole's procedure and measured by HPLC. The molar quantity of different FFA species corresponds to the amount of corresponding particular species of 1-MAG and 2-MAG and the total 1-MAG and 2-MAG were calculated by the sum of these individual MAG species.
2.10. Statistical analysis
Statistical analyses were performed by Graphpad Prism software. Values were expressed as mean ± SEM. Student's t test and one way and two ANOVA were used for inter-group comparisons.
3. Results
3.1. BKO islets display reduced MAG hydrolysis activity
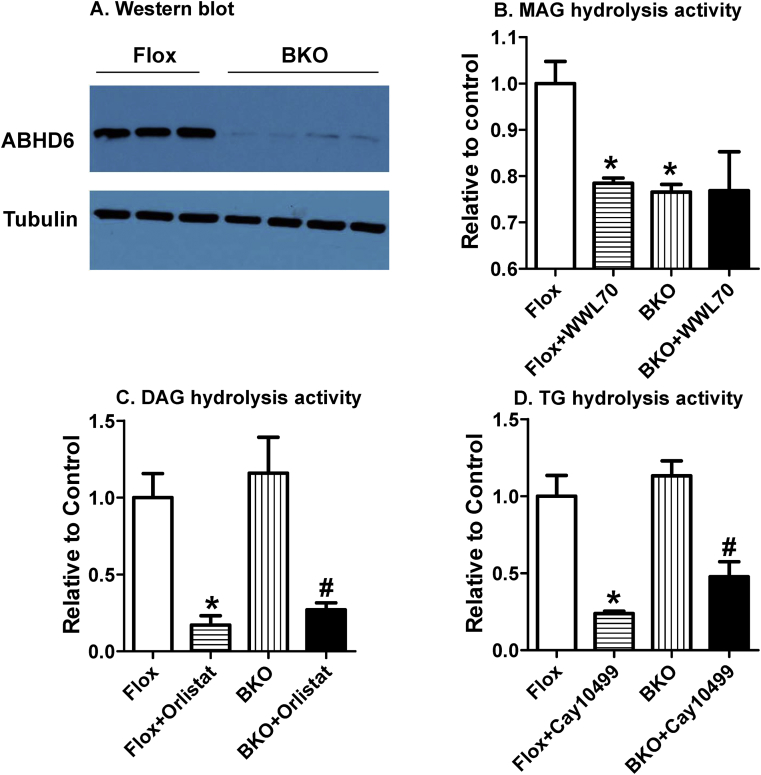
ABHD6 is known to hydrolyze MAG to glycerol and FFA in various tissues, such as brain, liver, adipocytes and islets [16], [19], [20]. We confirmed [16] that ABHD6 protein levels are reduced in BKO islets by >90% (Figure 1A). However, it is not known whether deletion of ABHD6 has any compensatory effects on DAG or TG hydrolysis. Since it has been shown that suppression of DAG hydrolyzing HSL or TG hydrolyzing ATGL hampered insulin secretion [10], [11], [15], it is necessary to ascertain that ABHD6 deletion in β cells has no secondary or compensatory effects on DAG or TG hydrolysis. In order to examine this, we measured the activities of total hydrolysis of MAG, 1,2-DAG and TG in extracts of Flox and BKO mouse islets. ABHD6-specific activity was assayed by measuring the hydrolysis of 1-S-thioarachidonylglycerol in the presence and absence of WWL70, an ABHD6-specific inhibitor. MAG hydrolysis was reduced by 20% in Flox mouse islets in the presence of WWL70 and was similarly reduced by 20% in BKO vs Flox islets. The addition of WWL70 to BKO islet extracts did not further reduce the residual MAG hydrolysis, indicating complete deletion of ABHD6 activity in islet β cells (Figure 1B). Orlistat has been reported to inhibit sn1-DAG lipases at low micromolar concentrations [21] while higher concentrations are needed to inhibit HSL, which also hydrolyzes DAG [14]. Total DAG hydrolysis activity was similar in Flox and BKO islet extracts as well as the orlistat sensitive and the residual activity (Figure 1C). Similarly, there were no differences in TG hydrolysis activity between Flox and BKO islets and, in both the cases, TG hydrolysis could be equally inhibited by the lipase inhibitor Cay10499 (Figure 1D). The results demonstrate that deletion of ABHD6 activity in islet β cells does not alter DAG and TG hydrolysis.
Figure 1.
BKO islets show reduced MAG hydrolysis activity. (A) Western blot analysis of ABHD6 protein level in Flox and BKO islets. (B) Total MAG hydrolysis activity was measured in the absence and presence of 1 μM WWL70. (C) DAG hydrolysis activity was measured in the absence and presence of 1 μM orlistat. (D) TG hydrolysis activity was measured in the absence and presence of 1 μM Cay10499. Results are Mean ± SEM from three different experiments with pooled islets from 6 to 9 mice in each group. *p ≤ 0.05 vs Flox mice; #p < 0.05 versus BKO mice.
3.2. Insulin secretion in response to various fuel and non-fuel stimuli in BKO islets
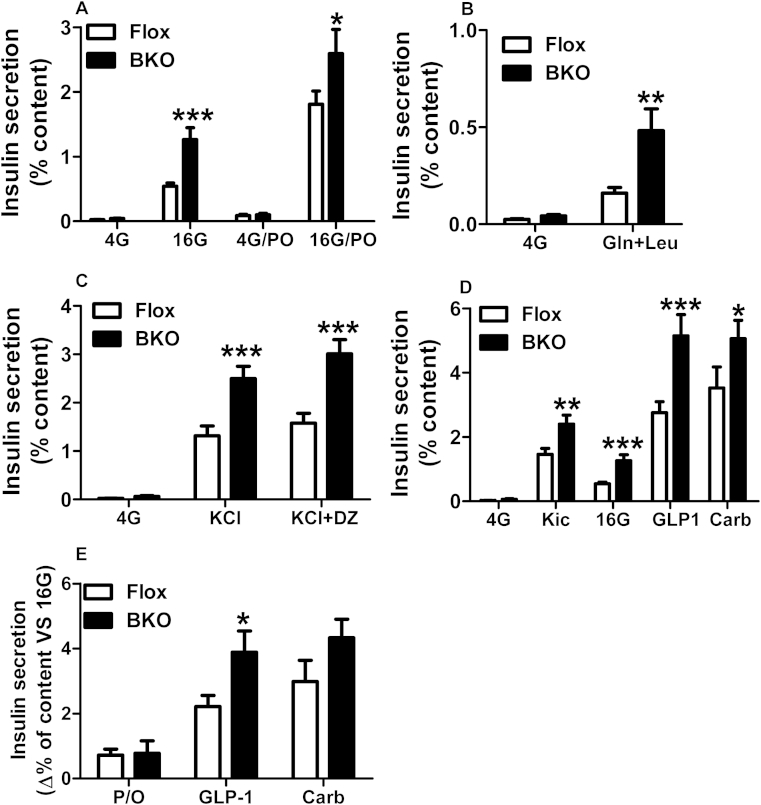
We have earlier shown that isolated islets from global or β cell specific ABHD6-KO mice show enhanced GSIS [16]. However, the effect of ABHD6 deletion on insulin secretion in response to other fuels and non-fuel stimuli is not known. As noticed earlier [16], deletion of ABHD6 in islets did not affect islet morphology or β cell mass, but increasing glucose concentration to 16 mM resulted in much higher level of insulin secretion in the BKO islets than that in Flox islets (Figure 2A). The presence of palmitate and oleate (0.15 mM each) elevated insulin secretion at both 4 and 16 mM glucose in control and BKO islets to the same extent (Figure 2A). ABHD6 deletion had no further enhancing effect on fatty acid-augmented GSIS, in addition to what was noticed at 16 mM glucose alone in the Flox control (Figure 2E). Besides glucose, a combination of glutamine and leucine is known to stimulate insulin secretion at low glucose concentration [22]. At 4 mM glucose, Gln plus Leu also stimulated insulin secretion in control and in BKO islets (Figure 2B). Interestingly, similar to what was seen with high glucose concentration, there was nearly 3-fold higher secretion with Gln plus Leu in BKO islets than in Flox islets, indicating that ABHD6 deletion also enhanced amino acid stimulated insulin secretion (Figure 2B).
Figure 2.
Enhanced insulin secretion in response to various fuel and non-fuel stimuli in ABHD6-BKO islets. (A) The effect of glucose and fatty acids (palmitate plus oleate (P/O)) on insulin secretion. Insulin secretion was measured in Flox and BKO islets at basal (4 mM), and high (16 mM) glucose in the absence or presence of 0.15 mM palmitate and 0.15 mM oleate. (B) Effect of glutamine plus leucine on insulin secretion. Insulin secretion was measured at 4 mM glucose in the absence or presence of 5 mM glutamine plus 5 mM leucine. (C) The effect of KCl and diazoxide (DZ) on insulin secretion. Insulin secretion was measured at 4 mM glucose in the absence or presence of 35 mM KCl and 0.1 mM diazoxide. (D) The effect of Kic, GLP1 and Carb on insulin secretion. Insulin secretion was measured at 4 and 16 mM glucose. The effect of Kic was tested at 4 mM glucose, whereas the effect of 20 nM GLP1 and 200 μM Carb were tested at 16 mM glucose. (E) The net calculated effect of fatty acids (P/O), GLP-1 and Kic on the top of 16 mM glucose on insulin secretion. The results show the calculated difference in panel A of insulin secretion at 16 mM glucose in the presence of P/O minus the secretion at 16 mM glucose only, and similar calculation for the true GLP1 and Carb effects on the top of 16 mM glucose in panel D. Mean ± SEM are from three different experiments with totally 9 mice in each groups. *p < 0.05; **p < 0.01; ***p < 0.001 versus Flox group.
Using islets from whole body ABHD6-KO mice, we had previously noticed that besides GSIS, even KCl-stimulated insulin secretion (at 2.8 mM glucose) is also slightly elevated [16]. We now further examined the involvement of KATP-independent amplification mechanism(s) [23] in the elevated insulin secretion seen due to ABHD6 deletion, using BKO mouse islets. The combined use of diazoxide plus an elevated concentration of KCl is a classical way to study the so-called amplifying KATP-independent pathways [23] when β cell [Ca2+] is elevated maximally and clamped by a depolarizing elevated concentration of KCl in the presence of diazoxide (KATP channels are held open to exclude an effect on these channels). The results indicated that KCl-induced insulin secretion (at 4 mM glucose) was strongly potentiated in BKO islets, compared to Flox islets. We made a similar observation when KATP channels were by-passed, in the presence of elevated KCl, with the use of diazoxide (Figure 2C) [24]. Thus, ABHD6 deletion caused augmentation of the KATP-independent amplification pathways of glucose signaling for secretion. Kic, which is actively metabolized in the β cell and promotes insulin secretion [25], was found to augment insulin release in both control and BKO islets at 4 mM glucose and this Kic augmented secretion was much higher in BKO islets than in control islets (Figure 2D). We then examined whether ABHD6 deletion influences GSIS stimulated by GLP1, which acts via Gs-coupled G-protein coupled receptor (GPCR) [26], and by the muscarinic receptor agonist carbamylcholine, which acts via Gq-coupled GPCR [27]. GLP1 considerably augmented GSIS in control and BKO islets and its effect was markedly amplified in BKO islets reaching a very elevated 6% of total insulin content being secreted (Figure 2D,E). Carb also amplified GSIS in control islets. There was a clear trend of enhancement of its effect in BKO islets (calculated as the difference of Carb effect on the top of 16 mM glucose in BKO vs control islets) but it did not reach statistical significance (p < 0.1) (Figure 2E). The results indicate that a signal generated by ABHD6 deletion synergizes with those produced by the non-fuel stimulus GLP1 and possibly with those produced by Carb as well.
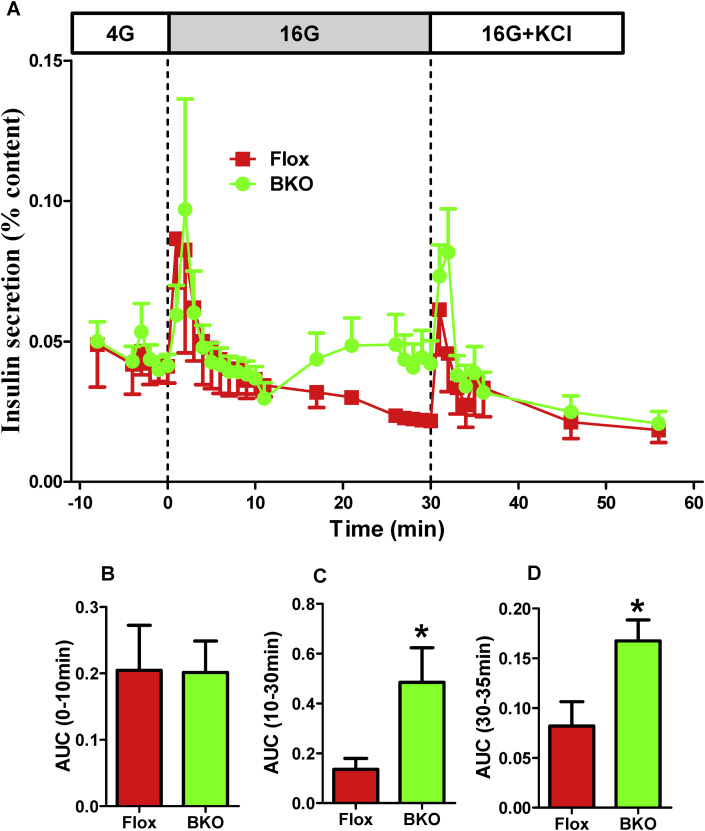
In response to glucose, insulin secretion by isolated islets or cultured β cells follows a biphasic process. In order to understand which of these phase(s) of insulin secretion is affected by ABHD6 deletion, we performed perifusion experiments using islets from Flox and BKO mice. Perifusion with 16 mM glucose induced a transient first phase insulin secretion in both control and BKO islets, and there were no differences between Flox and BKO islets in either the amplitude or the time course of first phase secretion peak (Figure 3A,B). The second phase insulin secretion was very minor in control islets, consistent with previous reports that used mouse islets [17], [28], while it was significantly elevated in BKO islets (Figure 3A,C), indicating that ABHD6 deletion enhances GSIS mainly by affecting the second phase. BKO islets also exhibited increased insulin secretion during perifusion with 35 mM KCl that followed 16 mM glucose, as compared to control islets, similar to what was observed in static incubations (Figure 3A,D).
Figure 3.
Enhanced second phase and KCl-induced insulin secretion in perifused ABHD6-BKO islets. Batches of 30 islets were perifused in enriched KRBH with 4 mM glucose for 45 min, and then followed by KRBH with 16 mM glucose for 30 min and then with 35 mM KCl for another 30 min. Results are Mean ± SEM from 4 different experiments with 8 control mice and 10 BKO mice. (A) The original trace of perifusion study. (B) First phase insulin secretion, calculated area under curve (AUC) between 0 and 10 min (AUC0–10min); (C) Second phase insulin secretion, calculated AUC between 10 and 30 min (AUC10–30min); (D) KCl-stimulated insulin secretion, calculated AUC between 30 and 35 min (AUC30–35min); *p ≤ 0.05 vs control islets.
3.3. Intracellular Ca2+ in flox and BKO mouse islets
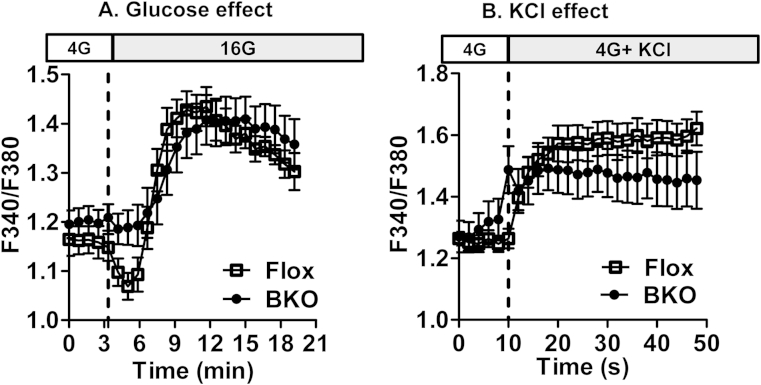
In order to determine whether ABHD6 deletion changes cytosolic Ca2+ levels and that such effect could contribute to the enhanced secretion in BKO islets, we measured intracellular Ca2+ in Flox and BKO islets in the presence of high glucose or 35 mM KCl. In both control and BKO islets, following glucose addition, cytosolic Ca2+ started to increase by 3 min and reached a peak by 10 min; thereafter, intracellular Ca2+ started to decrease slowly (Figure 4A). There were no differences between Flox and BKO islets in the glucose stimulated elevation in cytosolic Ca2+. Elevated glucose caused an initial small reduction of cytosolic Ca2+ in control but not BKO islets. The reason for the difference in this slight initial reduction in Ca2+ between control and BKO islets is not known. This initial reduction in cytosolic Ca2+ upon a rise in glucose concentration has been reported before in some islet studies [29] but the basis for this reduction in Ca2+ has not been elucidated. It may be related to an accelerated Ca2+ uptake by the endoplasmic reticulum due to a rise in the ATP/ADP ratio promoting activation of the Ca2+ ATPase transporter. As expected, KCl induced a rapid Ca2+ rise in both Flox and BKO islets, and there was no difference between these islets in their Ca2+ response (Figure 4B). These results indicate that ABHD6 deletion-mediated increase in GSIS response by islets is not due to altered Ca2+ influx or mobilization form endogenous stores or cytosolic Ca2+ concentrations.
Figure 4.
Cytosolic Ca2+ measurements in Flox and BKO islet β cells. Dispersed cells from islets from Flox and BKO mice were used for cytosolic free calcium measurement using a fluorescence plate reader. (A) The effect of high glucose in control and BKO islets. 4G, 4 mM glucose; 16G, 16 mM glucose. (B) Effect of 35 mM KCl on cytosolic Ca2+. Mean ± SEM are of 6 different measurements with 10–15 mice per group. Results are expressed as fluorescence ratios (F340/F380).
3.4. Glucose and fatty acid metabolism in β cells with ABHD6 deletion
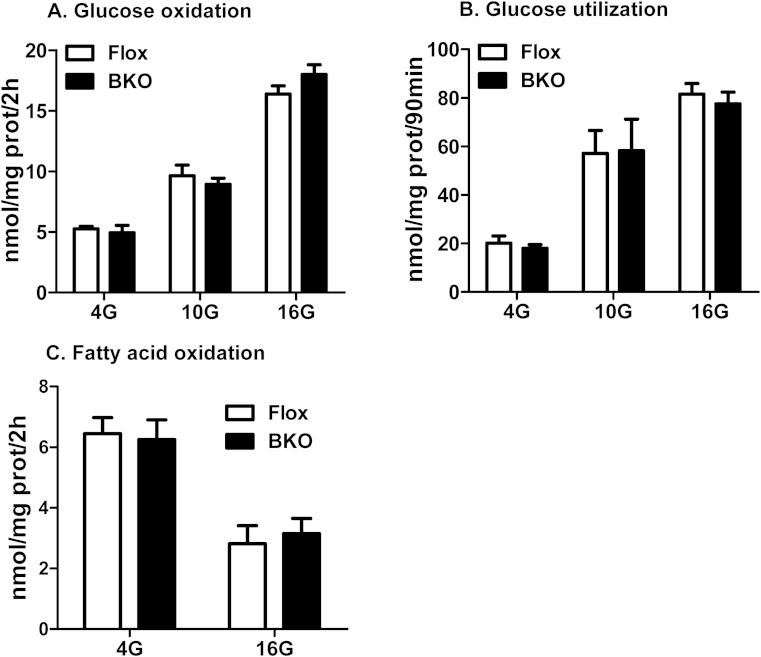
Inasmuch as ABHD6 is a metabolic enzyme and its deletion greatly enhances glucose responsiveness of pancreatic islets to secrete insulin, it is important to ascertain whether deletion of ABHD6, a MAG hydrolase, has any effect on glucose or fatty acid metabolism in β cells. In both control and BKO islets, there was significant level of glucose utilization and oxidation at 4 mM glucose, which increased further with increasing glucose concentration. However, there were no differences between Flox and BKO islets (Figure 5A,B). Fatty acid oxidation negatively correlates with insulin secretion [1]. In the present study, we also noticed that with increasing glucose concentration, fatty acid oxidation was significantly decreased; however, there were no differences between Flox and BKO islets (Figure 5C).
Figure 5.
Glucose and fatty acid metabolism in Flox and BKO islets. (A) Glucose oxidation. (B) Glucose utilization. (C) Fatty acid oxidation. Each experiment was performed with pooled islets from 8 mice per group. Mean ± SEM of 6–10 determinations.
3.5. MAG levels in BKO islets
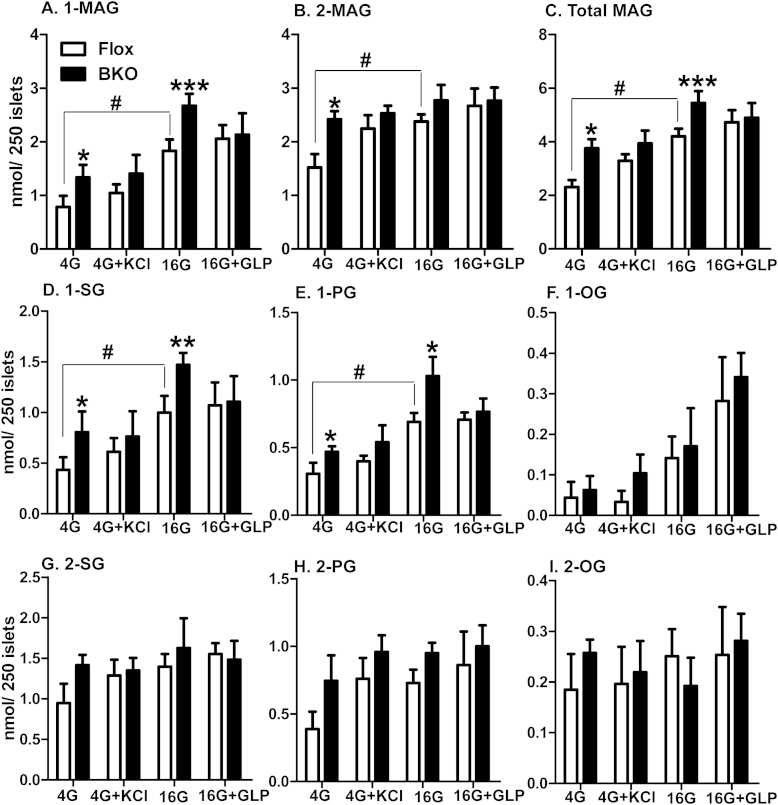
We previously reported that total MAG and 1-MAG levels are elevated in glucose concentration-dependent manner in whole body ABHD6-KO mouse islets [16]. However, in order to eliminate the problems and uncertainties associated with global deletion in which ABHD6 is absent right from embryo stage and might affect the expression and activities of other enzymes and factors, we analyzed MAG levels in BKO islets, where ABHD6 was deleted in β cells at adult stage. In order to directly correlate 1-MAG levels with insulin secretion, we measured both 1-MAG and 2-MAG levels in islets incubated with low (4 mM) and high glucose (16 mM) concentration in the presence or absence of 35 mM KCl and 20 nM GLP-1. Islets were incubated under conditions similar to those employed for insulin secretion measurement. The results indicated increased total MAG and 1-MAG levels in BKO islets at basal conditions, and at 16 mM glucose, compared to Flox islets (Figure 6A,C). 2-MAG levels were only increased at low glucose (Figure 6B). Addition of GLP1 or KCl had no additional effect on 1-MAG, 2-MAG and total MAG levels (Figure 6A–C). In agreement with our previous report in whole body ABHD6-KO islets [16], compared to low glucose, elevated glucose increased the levels of the long chain saturated 1-stearoylglycerol (1-SG) and 1-palmitoylglycerol (1-PG), and the levels of these MAG species were much higher in BKO islets (Figure 6D,E). The level of the monounsaturated 1-oleoylglycerol (1-OG) was not significantly changed in BKO islets in comparison to control islets under all tested conditions (Figure 6F). There were no significant changes in 2-SG, 2-PG and 2-OG levels in BKO islets except for a rise at low glucose in 2-SG and 2-PG (Figure 6G–I). Addition of KCl and GLP-1 did not significantly change either 1-MAG or 2-MAG levels under all tested conditions in Flox and BKO islets (Figure 6D–I). Most of the other 1-MAG and 2-MAG species were too low to be detected, and some unidentified MAG peaks in the HPLC analysis were not considered in the analysis. Thus, ABHD6 deletion that results in enhanced insulin secretion in response to various fuel and non-fuel stimuli is associated with increased levels of long chain saturated 1-MAG species, consistent with their postulated role as signaling molecules for insulin secretion [16].
Figure 6.
MAG species levels in Flox and BKO islets. Islets were incubated for 1 h in enriched KRBH at 4 and 16 mM glucose in the presence or absence of 35 mM KCl and 20 nM GLP-1, and then the islets were extracted for lipid analysis. (A) Total 1-MAG levels; (B) Total 2-MAG; (C) Total MAG. (D). 1-stearoylglycerol (1-SG); (E) 1-palmitoylglycerol (1-PG); (F) 1-oleoylglycerol (1-OG); (G) 2-stearoylglycerol (2-SG); (H) 2-palmitoylglycerol (2-PG); (I) 2-oleoylglycerol (2-OG). Mean ± SEM of 5–6 different measurements with 14 mice per group. *p < 0.05; **p < 0.01; ***p < 0.001 versus corresponding Flox group. #p < 0.05 versus Flox at 4 mM glucose (4G).
3.6. 1-MAG amplifies fuel and non-fuel induced insulin secretion
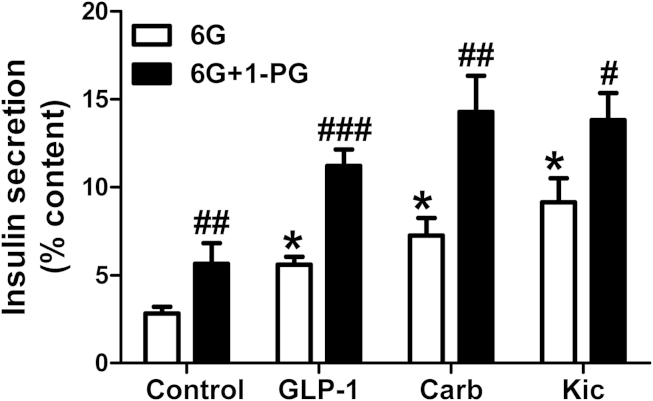
If the increase in 1-MAG levels in BKO islets is causally implicated in amplifying the secretory response of various insulinotropic agents, then exogenously added 1-MAG should mimic the effect of ABHD6 deletion. This prediction was verified as indicated in Figure 7. As the potentiating effects of GLP-1 and Carb on insulin secretion require a glucose concentration higher than basal levels, we performed this experiment using 6 mM instead of 4 mM glucose. Our results indicated that 1-PG amplified the secretion of insulin at 6 mM glucose and the effect of the nutrient stimulus Kic as well as those of the neurohormonal agonists GLP-1 and Carb.
Figure 7.
Synergic effect on insulin secretion of 1-MAG with other stimuli in dispersed rat islet cells. Insulin secretion was measured at 6 mM glucose (6G) with or without 20 nM GLP-1, 200 μM Carb and 10 mM Kic in the absence or presence of 100 μM 1-palmitolglycerol (1-PG). Mean ± SEM from 3 different experiments, using 13 rats in total. *p < 0.01 versus control-6G; #p < 0.05; ##p < 0.01; ###p < 0.001 versus corresponding 6G group.
4. Discussion
This study reveals that ABHD6 and MAG play a general role in insulin secretion as they regulate the process not only in response to glucose but also to amino acids (Gln plus Leu) and Kic acting as fuel stimuli, and in response to various non-fuel stimuli (GLP-1, carbamylcholine and a depolarizing concentration of KCl). It also gives information about the mechanisms implicated in this process. Thus, the data indicate that: a) the elevated insulin secretion response of β cells upon ABHD6 deletion is not related to altered DAG or TG hydrolysis; b) ABHD6 deletion enhances insulin secretion promoted by elevated glucose but does not potentiate fatty acid stimulated secretion; c) β cell specific deletion of ABHD6 increases total MAG and 1-MAG in islets at both low and high glucose, and this is specifically due to increased level of saturated long chain 1-MAG species; d) Enhanced GSIS caused by ABHD6 deletion is mediated by KATP/Ca2+ independent mechanisms of insulin secretion; e) Augmented insulin secretion in islet deficient in ABHD6 occurs independently of mitochondrial energy metabolism as glucose and fatty acid oxidation remained unchanged in BKO islets.
We have ruled out before [16] the possibility that endocannabinoid receptors that bind 2-arachidonoylglycerol (2-AG), but not saturated MAG, are involved in the MAG-mediated effects on insulin secretion. Thus, a specific antagonist of the CB1 receptor (AM251) and an inverse agonist (AM630) of the CB2 receptor did not alter GSIS. Also 2-AG levels even at high glucose were <1% of the total β cell MAG, whereas the saturated MAG that rose in the presence of glucose was 100-fold higher as compared to 2-AG and stimulated insulin secretion. Noteworthy, only 2-AG, but not saturated MAG, can bind CB receptors and act as their ligand.
In our earlier study, we showed that global ABHD6 suppression leads to elevated 1-MAG in islets and that 1-MAG activates the exocytosis facilitating protein Munc13-1, thus promoting enhanced insulin secretion [16]. We now show that the effect of ABHD6 deletion occurs on second but not first phase insulin secretion after stimulation by glucose. This is in accordance with a previous study in islets from Munc13-1 deficient mice, in which only second phase GSIS was reduced [30]. However, another study in haplodeficient Munc13-1 mice [31] observed alterations in both phases of secretion, and β cell capacitance determinations in these mice showed reduced exocytosis of both the ready releasable and refilling pools of secretory granules [16]. The reason for the discrepancy among these studies with respect to first phase only and not second phase GSIS is not known. It is possible that second phase GSIS is more sensitive to an elevation in 1-MAG than is first phase, that islet MAG levels were different in the two Munc13-1 deficient islets studies, and that exogenous 1-MAG at a high concentration stimulated both phases in the β cell capacitance study. The fact that ABHD6 deletion does not amplify the glucose induced Ca2+ rise and enhances KATP-independent pathways of insulin secretion that are thought to be implicated primarily in second phase of GSIS [23] is consistent with the observation that second phase is enhanced.
What is the possible mechanism whereby second phase GSIS is amplified by ABHD6 accessible MAG in the β cell? It has been suggested that Ca2+ binds to synaptotagmin-7, the major Ca2+ sensor in β cells for insulin exocytosis [32], [33], to facilitate loosening of cortical actin beneath the plasma membrane and fusion of insulin granule membrane with the plasma membrane. On the other hand, 1-MAG that accumulates in ABHD6 deleted β cells activates Munc13-1 [16], which is essential for forming the SNARE exocytosis complex, by direct interaction with syntaxin-2 [34] and with Rab3 interacting molecule 2 [35]. Thus, we propose that the elevated Ca2+ and 1-MAG signals in response to various stimuli synergize for insulin secretion because 1-MAG via its activation of Munc13-1 will promote the formation of novel granules associated to the plasma membrane (enhancement of the readily-releasable pool of granules) that can be fused when the Ca2+ signal occurs.
We observed that insulin secretion in response to all tested fuel and non-fuel agents, except for fatty acids, were enhanced in BKO islets. What is the reason for this observation that appears surprising at first sight? A likely explanation lies in the fact that glucose and fatty acid signaling for secretion share common pathways. Both glucose and FFA activate the GL/FFA cycle that generates MAG. FFA also acts via the receptor FFAR1 but a recent study documented that FFAR1 stimulation by FFA enhances the GL/FFA cycle in INS-1 β cells [36]. Thus, unlike the other stimuli (for example GLP1 that acts via cAMP and Ca2+ signaling), there may not be a possibility of synergy among glucose and FFA signaling pathways for secretion. Both glucose and FFA converge to signaling MAG that would reach maximal levels for secretion at elevated glucose plus FFA already in control Flox islets, such that secretion cannot be further enhanced in BKO islets.
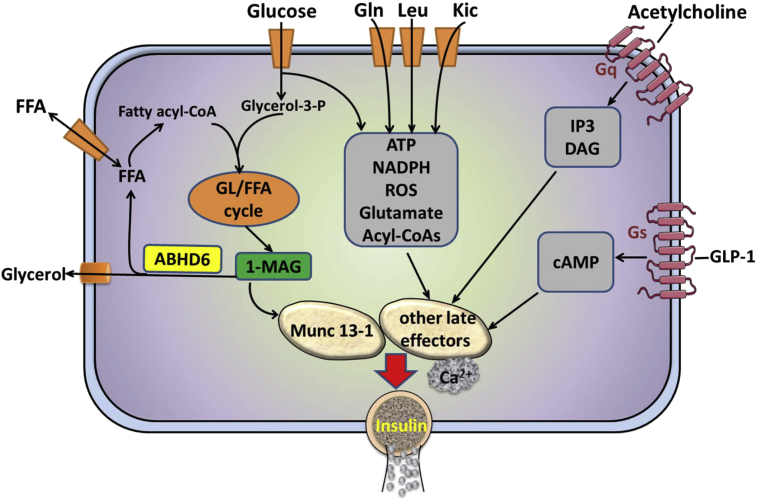
ABHD6 deletion further augmented GSIS enhanced by both GLP1 and elevated KCl. GLP1 enhances secretion only at high glucose concentration and we observed earlier that GLP1 does not alter lipolysis in islet β cells [37]. Consistent with this we now show that GLP1 does not change MAG levels in islets. How does deletion of ABHD6, which causes MAG buildup, further amplify GLP1 action on insulin secretion? Recent studies showed that GLP1 signaling leads to protein kinase A mediated activation of the Ca2+ sensor synaptotagmin-7 in β cells, leading to more efficient GSIS [32]. It is possible that the signals generated by GLP1 receptor via protein kinase-A converge with 1-MAG signaling and have synergistic effect on insulin exocytosis. Depolarizing concentrations of KCl markedly increase Ca2+ in β cells and likely the amplification of its effect on secretion in BKO islets is due to a synergy between Ca2+ and MAG signaling pathways. Similarly, 1-MAG signaling may amplify the inositol trisphosphate/Ca2+ and DAG signaling cascades generated by the Gq receptor agonist carbamylcholine. Figure 8 shows a model proposing how the ABHD6/1-MAG/Munc13-1 network regulates insulin secretion in response to various classes of insulin secretagogues.
Figure 8.
Model illustrating how the ABHD6/1-MAG/Munc13-1 network regulates insulin secretion in response to various classes of insulin secretagogues. Glucose and fatty acids enter the glycerolipid/fatty acid (GL/FA) cycle in its lipogenesis arm via the esterification of glucose-derived glycerol-3-phosphate with fatty acyl-CoA. Subsequent lipolysis produces long chain saturated 1-monoacylglycerol that act as a metabolic coupling factor causing insulin secretion via binding and activation of the exocytosis coordinator Munc13-1. Glucose and other fuel stimuli, including Gln, Leu and 2-ketoisocaproate (Kic), produce additional coupling factors (eg ATP, NADPH, ROS, Glutamate, short chain acyl-CoAs) that activate insulin secretion via other mechanisms that synergize, together with an elevation in cytosolic Ca2+, with the 1-MAG signal. Non-fuel neurohormonal stimuli, such as glucagon-like peptide 1 and acetylcholine, activate Gs and Gq protein coupled receptors that signal via cAMP and inositol-1,4,5 trisphosphate and diacylglycerol, respectively. These second messengers also synergize with the 1-MAG signal for insulin secretion.
5. Conclusion
MAG-mediated GSIS enhancement is a second phase event during insulin secretion and is a KATP and Ca2+ influx independent process. The importance of ABHD6 accessible MAG as a signal for insulin secretion is recognized under conditions of fuel stimuli at large and also when secretion is stimulated by various neurohormonal GPCR agonists. The data highlight the importance of ABHD6 and MAG as candidates for developing antidiabetic drugs.
Acknowledgments
This study was supported by grants from the Canadian Institutes of Health Research to MP and SRMM. MP holds the Canada Research Chair in Diabetes and Metabolism. SZ is supported by the fellowship from Université de Montréal and the Montreal Diabetes Research Center; YM and KV are supported by a fellowship from Fond de Recherche Santé Québec (FRQS); CA is supported by a fellowship from the Canadian Diabetes Association.
Contributor Information
S.R. Murthy Madiraju, Email: murthy.madiraju@crchum.qc.ca.
Marc Prentki, Email: marc.prentki@umontreal.ca.
Conflict of interest
None declared.
References
- 1.Prentki M., Matschinsky F.M., Madiraju S.R. Metabolic signaling in fuel-induced insulin secretion. Cell Metabolism. 2013;18:162–185. doi: 10.1016/j.cmet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 2.Bratanova-Tochkova T.K., Cheng H., Daniel S., Gunawardana S., Liu Y.J., Mulvaney-Musa J. Triggering and augmentation mechanisms, granule pools, and biphasic insulin secretion. Diabetes. 2002;51(Suppl. 1):S83–S90. doi: 10.2337/diabetes.51.2007.s83. [DOI] [PubMed] [Google Scholar]
- 3.Ashcroft F.M., Rorsman P. K(ATP) channels and islet hormone secretion: new insights and controversies. Nature Reviews Endocrinology. 2013;9:660–669. doi: 10.1038/nrendo.2013.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivarsson R., Quintens R., Dejonghe S., Tsukamoto K., in 't Veld P., Renstrom E. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 5.Pi J., Bai Y., Zhang Q., Wong V., Floering L.M., Daniel K. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes. 2007;56:1783–1791. doi: 10.2337/db06-1601. [DOI] [PubMed] [Google Scholar]
- 6.Maechler P., Wollheim C.B. Mitochondrial glutamate acts as a messenger in glucose-induced insulin exocytosis. Nature. 1999;402:685–689. doi: 10.1038/45280. [DOI] [PubMed] [Google Scholar]
- 7.Prentki M., Vischer S., Glennon M.C., Regazzi R., Deeney J.T., Corkey B.E. Malonyl-CoA and long chain acyl-CoA esters as metabolic coupling factors in nutrient-induced insulin secretion. The Journal of Biological Chemistry. 1992;267:5802–5810. [PubMed] [Google Scholar]
- 8.Brun T., Roche E., Assimacopoulos-Jeannet F., Corkey B.E., Kim K.H., Prentki M. Evidence for an anaplerotic/malonyl-CoA pathway in pancreatic beta-cell nutrient signaling. Diabetes. 1996;45:190–198. doi: 10.2337/diab.45.2.190. [DOI] [PubMed] [Google Scholar]
- 9.Kaneko Y.K., Ishikawa T. Diacylglycerol signaling pathway in pancreatic beta-cells: an essential role of diacylglycerol kinase in the regulation of insulin secretion. Biological & Pharmaceutical Bulletin. 2015;38:669–673. doi: 10.1248/bpb.b15-00060. [DOI] [PubMed] [Google Scholar]
- 10.Peyot M.L., Guay C., Latour M.G., Lamontagne J., Lussier R., Pineda M. Adipose triglyceride lipase is implicated in fuel- and non-fuel-stimulated insulin secretion. The Journal of Biological Chemistry. 2009;284:16848–16859. doi: 10.1074/jbc.M109.006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peyot M.L., Nolan C.J., Soni K., Joly E., Lussier R., Corkey B.E. Hormone-sensitive lipase has a role in lipid signaling for insulin secretion but is nonessential for the incretin action of glucagon-like peptide 1. Diabetes. 2004;53:1733–1742. doi: 10.2337/diabetes.53.7.1733. [DOI] [PubMed] [Google Scholar]
- 12.Nolan C.J., Leahy J.L., Delghingaro-Augusto V., Moibi J., Soni K., Peyot M.L. Beta cell compensation for insulin resistance in Zucker fatty rats: increased lipolysis and fatty acid signalling. Diabetologia. 2006;49:2120–2130. doi: 10.1007/s00125-006-0305-5. [DOI] [PubMed] [Google Scholar]
- 13.Roduit R., Masiello P., Wang S.P., Li H., Mitchell G.A., Prentki M. A role for hormone-sensitive lipase in glucose-stimulated insulin secretion: a study in hormone-sensitive lipase-deficient mice. Diabetes. 2001;50:1970–1975. doi: 10.2337/diabetes.50.9.1970. [DOI] [PubMed] [Google Scholar]
- 14.Mulder H., Yang S., Winzell M.S., Holm C., Ahren B. Inhibition of lipase activity and lipolysis in rat islets reduces insulin secretion. Diabetes. 2004;53:122–128. doi: 10.2337/diabetes.53.1.122. [DOI] [PubMed] [Google Scholar]
- 15.Fex M., Haemmerle G., Wierup N., Dekker-Nitert M., Rehn M., Ristow M. A beta cell-specific knockout of hormone-sensitive lipase in mice results in hyperglycaemia and disruption of exocytosis. Diabetologia. 2009;52:271–280. doi: 10.1007/s00125-008-1191-9. [DOI] [PubMed] [Google Scholar]
- 16.Zhao S., Mugabo Y., Iglesias J., Xie L., Delghingaro-Augusto V., Lussier R. alpha/beta-Hydrolase domain-6-accessible monoacylglycerol controls glucose-stimulated insulin secretion. Cell Metabolism. 2014;19:993–1007. doi: 10.1016/j.cmet.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 17.Peyot M.L., Pepin E., Lamontagne J., Latour M.G., Zarrouki B., Lussier R. Beta-cell failure in diet-induced obese mice stratified according to body weight gain: secretory dysfunction and altered islet lipid metabolism without steatosis or reduced beta-cell mass. Diabetes. 2010;59:2178–2187. doi: 10.2337/db09-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim-Muller J.Y., Zhao S., Srivastava S., Mugabo Y., Noh H.L., Kim Y.R. Metabolic inflexibility impairs insulin secretion and results in MODY-like diabetes in triple FoxO-deficient mice. Cell Metabolism. 2014;20:593–602. doi: 10.1016/j.cmet.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blankman J.L., Simon G.M., Cravatt B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chemistry & Biology. 2007;14:1347–1356. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas G., Betters J.L., Lord C.C., Brown A.L., Marshall S., Ferguson D. The serine hydrolase ABHD6 is a critical regulator of the metabolic syndrome. Cell Reports. 2013;5:508–520. doi: 10.1016/j.celrep.2013.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bisogno T., Howell F., Williams G., Minassi A., Cascio M.G., Ligresti A. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. The Journal of Cell Biology. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li C., Najafi H., Daikhin Y., Nissim I.B., Collins H.W., Yudkoff M. Regulation of leucine-stimulated insulin secretion and glutamine metabolism in isolated rat islets. The Journal of Biological Chemistry. 2003;278:2853–2858. doi: 10.1074/jbc.M210577200. [DOI] [PubMed] [Google Scholar]
- 23.Gembal M., Gilon P., Henquin J.C. Evidence that glucose can control insulin release independently from its action on ATP-sensitive K+ channels in mouse B cells. The Journal of Clinical Investigation. 1992;89:1288–1295. doi: 10.1172/JCI115714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yajima H., Komatsu M., Schermerhorn T., Aizawa T., Kaneko T., Nagai M. cAMP enhances insulin secretion by an action on the ATP-sensitive K+ channel-independent pathway of glucose signaling in rat pancreatic islets. Diabetes. 1999;48:1006–1012. doi: 10.2337/diabetes.48.5.1006. [DOI] [PubMed] [Google Scholar]
- 25.Heissig H., Urban K.A., Hastedt K., Zunkler B.J., Panten U. Mechanism of the insulin-releasing action of alpha-ketoisocaproate and related alpha-keto acid anions. Molecular Pharmacology. 2005;68:1097–1105. doi: 10.1124/mol.105.015388. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald P.E., El-Kholy W., Riedel M.J., Salapatek A.M., Light P.E., Wheeler M.B. The multiple actions of GLP-1 on the process of glucose-stimulated insulin secretion. Diabetes. 2002;51(Suppl. 3):S434–S442. doi: 10.2337/diabetes.51.2007.s434. [DOI] [PubMed] [Google Scholar]
- 27.Peter-Riesch B., Fathi M., Schlegel W., Wollheim C.B. Glucose and carbachol generate 1,2-diacylglycerols by different mechanisms in pancreatic islets. The Journal of Clinical Investigation. 1988;81:1154–1161. doi: 10.1172/JCI113430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferdaoussi M., Bergeron V., Zarrouki B., Kolic J., Cantley J., Fielitz J. G protein-coupled receptor (GPR)40-dependent potentiation of insulin secretion in mouse islets is mediated by protein kinase D1. Diabetologia. 2012;55:2682–2692. doi: 10.1007/s00125-012-2650-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lund P.E., Gylfe E., Hellman B. Leucine induces initial lowering of cytoplasmic Ca2+ in pancreatic beta-cells without concomitant inhibition of insulin release. Biochemistry International. 1989;19:83–87. [PubMed] [Google Scholar]
- 30.Kang L., He Z., Xu P., Fan J., Betz A., Brose N. Munc13-1 is required for the sustained release of insulin from pancreatic beta cells. Cell Metabolism. 2006;3:463–468. doi: 10.1016/j.cmet.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Kwan E.P., Xie L., Sheu L., Nolan C.J., Prentki M., Betz A. Munc13-1 deficiency reduces insulin secretion and causes abnormal glucose tolerance. Diabetes. 2006;55:1421–1429. doi: 10.2337/db05-1263. [DOI] [PubMed] [Google Scholar]
- 32.Wu B., Wei S., Petersen N., Ali Y., Wang X., Bacaj T. Synaptotagmin-7 phosphorylation mediates GLP-1-dependent potentiation of insulin secretion from beta-cells. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:9996–10001. doi: 10.1073/pnas.1513004112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gauthier B.R., Wollheim C.B. Synaptotagmins bind calcium to release insulin. American Journal of Physiology Endocrinology and Metabolism. 2008;295:E1279–E1286. doi: 10.1152/ajpendo.90568.2008. [DOI] [PubMed] [Google Scholar]
- 34.Xie L., Zhu D., Gaisano H.Y. Role of mammalian homologue of Caenorhabditis elegans unc-13-1 (Munc13-1) in the recruitment of newcomer insulin granules in both first and second phases of glucose-stimulated insulin secretion in mouse islets. Diabetologia. 2012;55:2693–2702. doi: 10.1007/s00125-012-2640-z. [DOI] [PubMed] [Google Scholar]
- 35.Kwan E.P., Xie L., Sheu L., Ohtsuka T., Gaisano H.Y. Interaction between Munc13-1 and RIM is critical for glucagon-like peptide-1 mediated rescue of exocytotic defects in Munc13-1 deficient pancreatic beta-cells. Diabetes. 2007;56:2579–2588. doi: 10.2337/db06-1207. [DOI] [PubMed] [Google Scholar]
- 36.El-Azzouny M., Evans C.R., Treutelaar M.K., Kennedy R.T., Burant C.F. Increased glucose metabolism and glycerolipid formation by fatty acids and GPR40 receptor signaling underlies the fatty acid potentiation of insulin secretion. The Journal of Biological Chemistry. 2014;289:13575–13588. doi: 10.1074/jbc.M113.531970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peyot M.L., Gray J.P., Lamontagne J., Smith P.J., Holz G.G., Madiraju S.R. Glucagon-like peptide-1 induced signaling and insulin secretion do not drive fuel and energy metabolism in primary rodent pancreatic beta-cells. PLoS One. 2009;4:e6221. doi: 10.1371/journal.pone.0006221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Iglesias J., Lamontagne J., Elb H., Gazzar S., Zhao S., Joly E. Simplified assays of lipolysis enzymes for drug discovery and specificity assessment of known inhibitors. Journal of Lipid Research. 2015 Sept 30 doi: 10.1194/jlr.D058438. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]