Abstract
Objective
Endurance exercise training reduces insulin resistance, adipose tissue inflammation and non-alcoholic fatty liver disease (NAFLD), an effect often associated with modest weight loss. Recent studies have indicated that high-intensity interval training (HIIT) lowers blood glucose in individuals with type 2 diabetes independently of weight loss; however, the organs affected and mechanisms mediating the glucose lowering effects are not known. Intense exercise increases phosphorylation and inhibition of acetyl-CoA carboxylase (ACC) by AMP-activated protein kinase (AMPK) in muscle, adipose tissue and liver. AMPK and ACC are key enzymes regulating fatty acid metabolism, liver fat content, adipose tissue inflammation and insulin sensitivity but the importance of this pathway in regulating insulin sensitivity with HIIT is unknown.
Methods
In the current study, the effects of 6 weeks of HIIT were examined using obese mice with serine–alanine knock-in mutations on the AMPK phosphorylation sites of ACC1 and ACC2 (AccDKI) or wild-type (WT) controls.
Results
HIIT lowered blood glucose and increased exercise capacity, food intake, basal activity levels, carbohydrate oxidation and liver and adipose tissue insulin sensitivity in HFD-fed WT and AccDKI mice. These changes occurred independently of weight loss or reductions in adiposity, inflammation and liver lipid content.
Conclusions
These data indicate that HIIT lowers blood glucose levels by improving adipose and liver insulin sensitivity independently of changes in adiposity, adipose tissue inflammation, liver lipid content or AMPK phosphorylation of ACC.
Keywords: HIIT, Exercise, Obesity-induced insulin resistance, Type 2 diabetes, NAFLD, AMPK
Highlights
-
•
High-intensity interval training (HIIT) improves exercise capacity and whole-body glucose homeostasis.
-
•
HIIT enhances liver and adipose tissue insulin sensitivity independent of body weight and adiposity.
-
•
HIIT does not change adipose tissue cell size, macrophage infiltration, inflammation and liver lipid content.
-
•
HIIT exercise training improves insulin sensitivity independently of the AMPK-ACC signaling pathway.
Abbreviations
- ACC
acetyl-CoA carboxylase
- AccDKI
serine–alanine knock-in mutations of ACC1 Ser79 and ACC2 Ser212
- ALT
alanine transaminase
- AMPK
AMP-activated protein kinase
- AST
aspartate transaminase
- AUC
area under the curve
- CPT-1
carnitine palmitoyl transportase-1
- CT
computed tomography
- DAG
diacylglycerol
- GDR
glucose disposal rate
- GIR
glucose infusion rate
- HFD
high-fat diet (45% kcal fat)
- HGP
hepatic glucose production
- HIIT
high-intensity interval training
- ITT
insulin tolerance test
- NEFA
non-esterified fatty acids
- RER
respiratory exchange ratio
- TAG
triacylglycerol
- WT
wildtype
1. Introduction
Endurance exercise training improves insulin sensitivity and delays the onset of type 2 diabetes through mechanisms which are not fully understood [1], [2], [3]. Despite the importance of endurance exercise training, less than 20% of individuals complete the recommended 150 min of endurance exercise per week, frequently citing a lack of time as a major deterrent [4]. Over the last decade, several studies in humans have found that high-intensity interval training (HIIT), an exercise training program involving brief bouts of intense exercise (90–100% of VO2 max) followed by periods of recovery, can elicit similar metabolic adaptations to classical endurance exercise training but with a much shorter time commitment. Importantly, recent studies have established that HIIT can lower blood glucose and markers of insulin resistance independently of alterations in adiposity/body mass in individuals with insulin resistance and type 2 diabetes [5], [6], [7], [8], [9]. Despite these beneficial metabolic effects, the tissues involved and mechanisms underlying the glucose lowering effects of HIIT have not yet been defined.
Insulin resistance is associated with the development of low grade inflammation caused by an increased accumulation of pro-inflammatory macrophages into adipose tissue and ectopic accumulation of lipid in the liver (also known as non-alcoholic fatty liver disease (NAFLD)) [10], [11], [12], [13], [14], [15]. Endurance exercise training can reduce liver lipid content [16], [17], [18], [19], [20], [21], [22], [23] and adipose tissue inflammation [10], [11], [24], [25], [26]; however, a caveat of these studies is that they are often accompanied by significant weight loss/reductions in adiposity [18], [19], [20], [21], [22], [23], [24], [25], thus making it difficult to conclude whether improvements were attributable to the exercise training per se or weight loss. HIIT improves insulin sensitivity without weight loss, but the molecular events and tissues involved in mediating these effects are largely unknown.
One mechanism by which HIIT may improve insulin sensitivity involves the activation of AMP-activated protein kinase (AMPK) which occurs in skeletal muscle [27], [28], [29], [30], liver [29], [31] and adipose tissue [29], [32], [33] during intense exercise. AMPK is vital for suppressing inflammation in adipose tissue macrophages [34], [35], [36], [37], an effect associated with increases in macrophage fatty acid oxidation and reductions in macrophage lipid content [34]. Similarly, the activation of AMPK in hepatocytes also increases fatty acid oxidation, while reducing fatty acid synthesis and liver lipid content [34], [38]. The effects of AMPK on fatty acid metabolism are mediated through the phosphorylation and inhibition of acetyl-CoA carboxylase 1 (ACC1) at Ser79 and ACC2 at Ser221 (Ser212 in mice) which inhibits the production of malonyl-CoA, a metabolic intermediate that provides acetyl groups that are incorporated into fatty acids during their synthesis and is also an allosteric inhibitor of carnitine palmitoyltransferase 1 (CPT-1) (for review see [39]). The mutation of AMPK phosphorylation sites on ACC1 (Ser79Ala) and ACC2 (Ser212Ala) (AccDKI mice) results in constitutively active ACC isozymes resulting in fatty and fibrotic liver and impaired insulin sensitivity when mice are fed a control chow diet [38]. Although feeding mice a high-fat diet (HFD) reduces the differences in metabolic profile between WT and AccDKI mice, metformin was shown to improve insulin sensitivity through ACC phosphorylation and subsequent reductions in de novo lipogenesis and liver lipid content [38]. Whether or not exercise training also regulates inflammation, liver lipid content and insulin sensitivity via an AMPK-ACC signaling pathway is currently unknown.
The primary aim of this study was to assess the mechanisms by which HIIT improves insulin sensitivity in obese mice. We hypothesized that this would involve improvements in adipose tissue and liver insulin sensitivity, effects that would be mediated through the phosphorylation and inhibition of ACC and subsequent reductions in liver lipid content and adipose tissue inflammation. We found that HIIT improved liver and adipose tissue insulin sensitivity but that these effects were independent of liver lipid content, adipose tissue inflammation and ACC phosphorylation.
2. Materials and methods
2.1. Mouse experiments
Male AccDKI (serine–alanine knock-in mutations of ACC1 Ser79 and ACC2 Ser212) mice generated on a C57Bl/6 background and wild-type (WT) littermates were first fed ad libitum with high-fat diet (HFD) (45 kcal% fat, D12451, Research Diets; New Brunswick, NJ). Mice were maintained on a 12 h light/dark cycle and fed a HFD starting at 6–8 weeks of age for 12 weeks. After the first 6 weeks of HFD, mice were either exercise trained or remained sedentary for the final 6 weeks. All experiments were approved by the McMaster University (Hamilton, Canada) Animal Ethics Committee.
2.2. Exercise capacity and HIIT
Mice assigned to the HIIT exercise training group were acclimatized to the treadmill over 3 days, running at 10–15 m/min for 15 min. To assess improvements in exercise performance with training, an exercise capacity test was performed before training and after 5 weeks of training. Mice began treadmill running at 8 m/min and treadmill speed was increased by 1 m/min every 2 min until exhaustion. Exhaustion was defined as the point at which instead of running on the treadmill, mice remained on the shockers that serve to encourage running for more than 10 s. At exhaustion, time and speed were recorded. Distance traversed was calculated by adding the distance covered during each 2 min interval at the different workloads/treadmill speed. The experimenter was blinded to the mouse genotypes.
HIIT involved treadmill running 3 days per week for the final 6 weeks of HFD. Exercise training entailed 2 min of running at 100% of maximal running speed from the initial exercise capacity test followed by 2 min of rest for a total 60 min. This meant that HIIT mice ran on the treadmill at 15 m/min for 2 min followed by 2 min of rest for a total of 60 min during the first week. The speed of running was increased by 1 m/min every week with a final speed of 22 m/min obtained during the final week of training. During the period that mice were trained, the sedentary group remained in their cages and ate HFD ad libitum.
2.3. Metabolic parameters
The Oxymax Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) uses indirect calorimetry to measure metabolic gas exchange, ambient activity, and food intake as described previously [34]. Measurements began ∼24 h after the latest exercise session to avoid any post-exercise effects. Mice were acclimatized to the cages for 12 h prior to measurements. Basal metabolic rate was the VO2 when mice were inactive (as determined by ≤ 100 beam breaks per minute) as we have described previously [40].
2.4. Metabolic studies
To detect acute phosphorylation of AMPK and ACC in the liver of obese mice fed a HFD for 6 weeks, liver was collected and snap frozen immediately after an acute bout of HIIT exercise. For our chronic training, body mass of mice was monitored and recorded weekly. Computed tomography (CT) was used to assess the effects of exercise training on whole body adiposity and analyzed with the Amira Visage Imaging Software Program, as described previously [41]. Blood (∼100 μL) was collected by facial bleed in the fed and 12 h fasted state (with fasting beginning in the evening), after 5 and 6 weeks of exercise training, respectively. Blood glucose concentrations were recorded by hand-held glucometer. Serum analysis of 12 h fasting insulin (Millipore) was performed according to manufacturer instructions. Serum analysis of alanine transaminase (ALT) and aspartate transaminase (AST) after 12 h of fasting was conducted as per manufacturer instructions (Biooscientific). Serum adipokines (interleukin-6 (IL-6), leptin, tissue plasminogen activator inhibitor-1 (tPAI-1), resistin, tumor necrosis alpha (TNF-α), and monocyte chemoattractant protein-1/CCL2 (MCP-1)) were measured using the Mouse Serum Adipokine kit from Millipore (St. Charles, MO), according to manufacturer's instructions. In addition, intraperitoneal (ip) glucose (d-glucose (1 g/kg)) and insulin (human insulin (1 U/kg, NovoRapid)) tolerance tests were performed after a 6 h fast after 5 and 6 weeks of exercise training, respectively. Blood glucose was measured by glucometer from a small nick of the tail vein during a 2 h span after ip injection. After 12 weeks of the study with 6 weeks of exercise training and a cannulation of the jugular vein surgery (with <10% loss in body weight change), hyperinsulinemic-euglycemic clamps were performed as previously described [34], [38]. After a 5 h fast and 1 h of basal D- [3-3H]-glucose (7.5 μCi/h, 0.12 ml/h in 0.9% saline) infusion, a constant infusion of insulin (10 mU/kg/min insulin in 0.9% saline) (Novorapid), containing D- [3-3H]-glucose (7.5 μCi/h, 0.12 ml/h in 0.9% saline) was begun. This was followed by an infusion of 50% dextrose that was slowly increased until euglycemia–blood glucose between 5.8 and 7.0 mM for at least 30 min – was reached [34]. Steele's equation for steady state conditions was used to determine rates of hepatic glucose output and glucose disposal in the basal and clamped state [42]. Finally, clamped blood was collected and serum was used to measure non-esterified fatty acid (NEFA) concentration, as per manufacturer instructions (Wako). 2-deoxyglucose (DG) uptake into epididymal white adipose tissue (eWAT) and mixed gastrocnemius muscle was measured following an intravenous injection of 2-[14C]-DG (10 μCi) at the conclusion of the clamp as we have described previously [38]. Briefly, blood samples were taken at 10, 20, and 30 min. Mice were euthanized with an intravenous injection of ketamine–xylazine and tissues were collected, snap frozen in liquid nitrogen, and stored at minus 80 °C for later analysis [41]. eWAT and gastrocnemius muscle lysates were prepared and glucose uptake was quantified via scintillation counting in which the unphosphorylated 2-[14C] DG fraction was subtracted from the total fraction to give the quantity of 2-[14C] DG-P. 2-[14C] DG-P was then expressed relative to blood glucose and 2-[14C] DG infusion in the blood.
2.5. Analytical techniques
Liver tissue was powdered on dry ice and homogenized in cell lysis buffer using a Precellys 24 Homogenizer (Bertin Technologies; Paris, France). To quantify liver triacylglycerol (TAG) content, lipids were extracted with chloroform and methanol [43], were saponified and glycerol content measured using Glycerol Reagent (Sigma). Diacylglycerols (DAG) and ceramides were quantified using the DAG kinase assay, as previously described [44]. Briefly, lipids were extracted from freeze-dried liver and gastrocnemius muscle tissue sample that was incubated in chloroform:methanol:0.2% SDS (1:2:0.8) in PBS. Cardiolipin/octylglucoside was used to reconstitute the lipids and [γ-32P] ATP reaction mixture was added. The reaction was stopped with chloroform:methanol (2:1) and samples were separated by thin layer chromatography as described previously [41]. To measure glycogen content, liver tissue was incubated in 6N HCl at 80 °C and then neutralized with 6N NaOH. Glucose content was then measured using a Glucose Assay (Sigma).
2.6. Adipose tissue macrophage collection
Adipose tissue macrophages were isolated as previously described [34]. Briefly, adipose tissue was minced and digested with Type II Collagenase (Sigma). The resulting cell suspension was filtered (100 μm cell strainer) and centrifuged (500 g for 5 min). Pellet was re-suspended in recommended medium (2% FBS, 1 mM EDTA) and labeled with CD11b + PE Labeling Reagent, PE Selection Cocktail, and Magnetic Nanoparticles (StemCell Technologies; Vancouver, BC). Macrophages were separated using the EasySep® kit magnet. Macrophages were resuspended in 1 mL TRIzol® Reagent (Invitrogen; Carlsbad, CA) and stored at −80 °C.
2.7. Histology and immunohistochemistry
A small piece of liver and adipose tissue were dissected and formalin-fixed in 10% buffered formalin. Paraffin-embedding and slicing of 5 μm thick sections were completed by trained technicians in the histopathological lab in the Department of Medicine at McMaster University. Sections of liver and adipose tissue were stained with hematoxylin and eosin (H&E) to assess the overall degree of steatosis compared between groups. In addition, a section of liver was made for trichrome staining. Collagen content (area fibrosis) was quantified using ImageJ software. Sections of adipose tissue were also prepared for immunohistochemistry (IHC) to assess macrophage infiltration in the adipose tissue. Paraffin-embedded adipose tissue was dewaxed and rehydrated to perform antigen retrieval by boiling (15 min) the slides in 10 mM sodium citrate (pH 6.5). Endogenous peroxidase was quenched (1% fetal calf serum and 3% hydrogen peroxide in PBS) and tissue samples were then blocked (5% normal rabbit serum) for 40 min, incubated with Avidin D (Biotin/Avidin Blocking Kit, Vector Laboratories; Burlinghame, CA) for 15 min, and incubated with Biotin (Biotin/Avidin Blocking Kit, Vector Laboratories) for 15 min. Sections were then incubated with primary antibody rat anti-mouse F4/80 (1:100) (AbD Serotec, Oxford, UK) for 2 h, a biotin-conjugated secondary antibody (1:50) (Vector Laboratories) for 1 h, and Vectastain ABC solution (Vector Laboratories) for 30 min. The sections were developed using the DAB Substrate Kit (Vector Laboratories) and slides were counterstained with hematoxylin.
2.8. Real-time quantitative PCR
RNA from liver, adipose tissue, and macrophages were extracted using TRIzol reagent and cDNA was made using Superscript III Reverse Transcriptase (Invitrogen), according to manufacturer instructions. TaqMan® Gene Expression Assays (Applied Biosystems; Foster City, CA) were used for real-time quantification with Rotor-Gene 6000 (Corbett Research; Mortlake, Australia) of (1) liver glucose 6-phosphatase (G6pc, Mm00839363_m1), phosphoenolpyruvate carboxykinase (Pck1, Mm01247058_m1), interleukin 1 beta (Il1β, Mm00434228_m1), tumor necrosis factor alpha (Tnfa, Mm00443258_m1), F4/80 (Emr1, Mm00802529_m1), interleukin 10 (Il10, Mm00439616_m1), macrophage arginase (Arg, Mm01190441_g1), inducible nitric oxide synthase (Nos2, Mm01309902_m1), Cd68 (Mm00839636_g1), and (Kc, Mm00433859_m1) genes; and (2) adipose tissue interleukin-6 (Il6, Mm00446190_m1), Cd68 (Mm00839636_g1), and F4/80 (Emr1, Mm00802529_m1), uncoupling protein 1 (Ucp1, Mm01244861_m1), cell death activator (Cidea, Mm00432554_m1), PR domain containing 16 (Prdm16, Mm00712556_m1), pyruvate dehydrogenase kinase isoform 4 (Pdk4, Mm01166879_m1), peroxisome proliferator-activated receptor gamma coactivator-1-alpha (Ppargc1a, Mm00447183_m1), glucose transporter 4 (Glut4, Mm00436615_m1); adipose tissue macrophage arginase (Arg, Mm01190441_g1), inducible nitric oxide synthase (Nos2, Mm01309902_m1), interleukin 1 beta (Il1β); and adipose tissue and macrophage, chemokine ligand 1 (Kc, Mm00433859_m1), and tumor necrosis factor alpha (Tnfa, Mm00443258_m1). Gene expression was measured using the ddCT method [45], in which the expression of a gene of interest is compared to that of the housekeeping gene (CycA–Mm00839493_m1, Tbp–Mm00446973_m1 and Rplp0–Mm01974474_gH) and expressed relative to HFD WT.
2.9. Western blotting
Protein extracts were separated by SDS-PAGE, and immunoblotting was performed after transfer to nitrocellulose membranes. Membranes were blocked for 1 h at room temperature with 5% BSA in 1x TBST (Tris buffered saline-Tween-20, 25 mM Tris·HCl (pH 7.5), 1 mM NaCl, and 0.1% Tween-20) and then incubated overnight at 4 °C with primary antibodies (from Cell Signaling, unless indicated) phosphorylated (p)-acetyl-CoA carboxylase (ACC) Ser79/221 (1:1000), ACC total (1:1000), pAMPK Thr172 (1:1000), AMPK total (1:1000), pAkt Ser473 (1:1000), pAkt Thr308 (1:1000), Akt (1:1000), pAS160 (Akt substrate of 160 kDa) Thr642, (1:1000), OXPHOS (MitoSciences, 1:5000), anti-PGC-1 (Millipore, 1:1000) in liver and/or quadriceps muscle. After overnight incubation, membranes were washed (3 × 5 min) in TBST, incubated at room temperature for 1-hr with corresponding secondary antibody, washed (3 × 5 min) with TBST and developed with Clarity™ Western ECL Substrate (Biorad). Protein expression was shown relative to GAPDH (Cell Signaling, 1:5000). Densitometry was performed using ImageJ software.
2.10. Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM) and analyzed using a two-way ANOVA with Bonferroni post-hoc tests for group comparisons (unless otherwise denoted), using GraphPad Prism software. Repeated measures ANOVA was used to measure body mass, GTT, ITT, and GIR. Significance was accepted at p ≤ 0.05.
3. Results
3.1. Exercise capacity is increased with HIIT independent of body weight and adiposity.
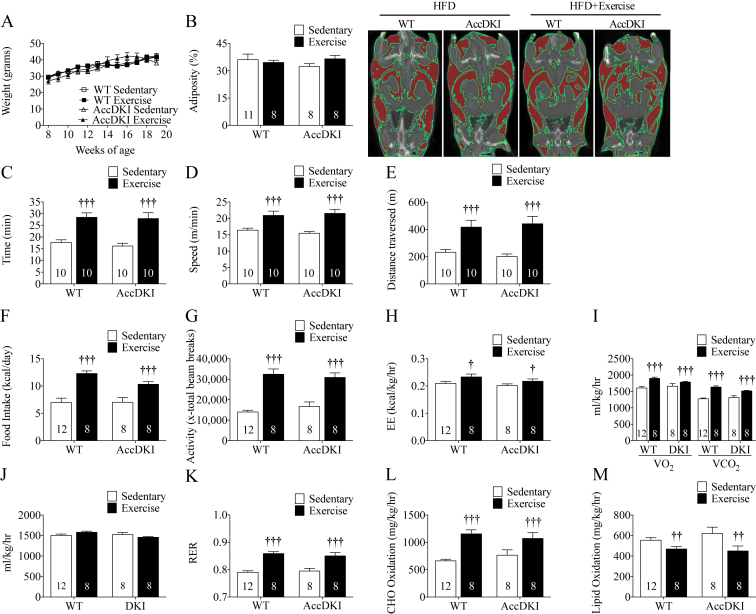
To first establish that the HIIT activated AMPK, we collected liver from WT mice following a single training session and found increased AMPK (Supplementary Figure 1A) and ACC (Supplementary Figure 1B) phosphorylation. All subsequent data were collected in AccDKI or WT littermates fed a HFD that were either sedentary or completed the HIIT protocol 3 times per week for 6 weeks. Consistent with our previous report [38], we found that WT and AccDKI mice had comparable weight gain and adiposity when fed HFD (Figure 1A,B). Exercise training did not alter these variables in either genotype (Figure 1A,B). There was no difference in liver, eWAT or heart tissue weights between any group with or without HIIT (Table 1). Furthermore, HFD-fed WT and AccDKI mice did not show differences in exercise capacity and both improved time to exhaustion, speed at exhaustion, and distance traversed to an equivalent degree following HIIT (Figure 1C,D,E). This improvement in exercise capacity is comparable to what other studies have observed using higher volume endurance exercise training programs [18], [46].
Figure 1.
HIIT increases exercise capacity and metabolic flexibility independent of body weight and adiposity. (A) Body mass over time of WT and AccDKI mice that were sedentary or trained using HIIT. (B) Whole-body percent adiposity, with representative CT scan images. Areas highlighted in red represent adipose region. (C) Time to exhaustion, (D) Speed at exhaustion, and (E) Distance traversed. (F) Average daily food intake. (G) Average daily ambient activity. (H) Average daily energy expenditure. (I) Average daily VO2 consumption and VO2 production. (J) Basal VO2 consumption (K) Daily average RER. (L) Daily average CHO oxidation. (M) Daily average lipid oxidation. Number of mice are denoted on the graph. Data are expressed as means ± SEM, †p < 0.05, ††p < 0.01, †††p < 0.001, for difference from sedentary vs exercise, as determined by two-way ANOVA and Bonferonni post hoc test.
Table 1.
Analysis of tissue weights.
| HFD |
HFD + Exercise |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT |
AccDKI |
WT |
AccDKI |
|||||||||
| Average | ±SEM | N | Average | ±SEM | N | Average | ±SEM | N | Average | ±SEM | N | |
| Liver | ||||||||||||
| Weight (mg) | 1275.3 | 52.8 | 6 | 1162.9 | 35.1 | 8 | 1237.9 | 79.2 | 8 | 1260.6 | 91.9 | 8 |
| Weight/gram bw (mg/g bw) | 33.4 | 1.7 | 6 | 30.6 | 1.0 | 8 | 32.2 | 1.3 | 8 | 32.1 | 1.5 | 8 |
| Epididymal adipose | ||||||||||||
| Weight (mg) | 2018.1 | 191.6 | 6 | 2375.4 | 112.7 | 8 | 2087.1 | 74.3 | 8 | 2213.0 | 82.0 | 8 |
| Weight/gram bw (mg/g bw) | 52.7 | 7.1 | 6 | 62.34 | 2.7 | 8 | 55.1 | 2.7 | 8 | 57.0 | 2.5 | 8 |
| Heart | ||||||||||||
| Weight (mg) | 129.7 | 3.4 | 6 | 119.7 | 4.1 | 8 | 132.5 | 4.1 | 8 | 133.1 | 3.1 | 8 |
| Weight/gram bw (mg/g bw) | 3.3 | 0.1 | 6 | 3.2 | 0.1 | 8 | 3.5 | 0.1 | 8 | 3.4 | 0.1 | 8 |
Data are expressed as means ± SEM.
3.2. HIIT improves metabolic flexibility independently of AMPK phosphorylation of ACC
Energy intake and expenditure, activity levels and substrate utilization were examined using metabolic cages. We found that HIIT increased food intake and ambient activity in both WT and AccDKI mice (Figure 1F,G). Consistent with increased ambient activity, exercise trained mice had increased energy expenditure (Figure 1H) in line with elevations in VO2 and VCO2 (Figure 1I). This increase in VO2 was not due to alterations in basal metabolic rate, which was comparable between sedentary and exercise trained mice irrespective of genotype (Figure 1J), suggesting that increases in VO2 were the result of an increase in activity levels. There were no differences in respiratory exchange ratio (RER) (Figure 1K), carbohydrate oxidation (Figure 1L), or lipid oxidation (Figure 1M) between sedentary HFD-fed WT and AccDKI mice. However, HIIT increased RER and calculated rates of carbohydrate oxidation while suppressing lipid oxidation in both WT and AccDKI mice (Figure 1K,L,M), findings suggestive of improved whole-body insulin sensitivity following exercise training [47], [48].
3.3. HIIT improves insulin sensitivity independently of AMPK phosphorylation of ACC
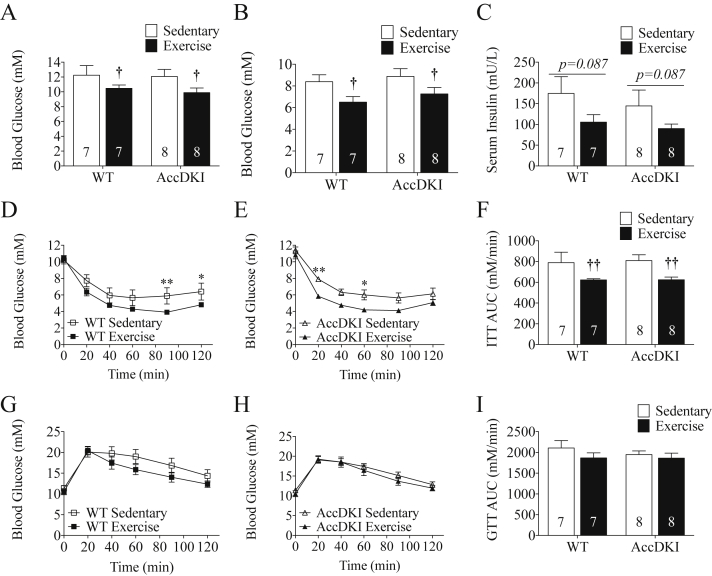
All of the assessments of glucose homeostasis were completed 48–72 h after treadmill running to minimize the acute insulin sensitizing actions of exercise. Consistent with our previous findings [38], sedentary HFD-fed WT and AccDKI mice had comparable fed blood glucose (Figure 2A), fasting blood glucose and serum insulin levels (Figure 2B,C). Insulin tolerance test (ITT), glucose tolerance test (GTT), and corresponding area under the curves (AUCs) were also comparable between WT and AccDKI mice (Figure 2D–I). While no chow group was examined in this study, these measures of glucose and insulin were all much higher than we have previously reported in chow fed WT and AccDKI mice [38] or other mice fed a chow diet in our laboratory [49], indicating the development of insulin resistance following the 12 weeks of HFD. Importantly, HIIT reduced fed (Figure 2A) and 12 h fasted (Figure 2B) blood glucose levels and tended to improve fasting serum insulin levels (Figure 2C). Insulin tolerance tests indicated improvements in whole-body insulin sensitivity by ∼20% in both WT and AccDKI mice following HIIT (Figure 2D,E,F); however, surprisingly, glucose tolerance was not significantly different (Figure 2G,H,I).
Figure 2.
HIIT improves insulin sensitivity independent of AMPK phosphorylation of ACC. (A) Fed blood glucose concentration. (B) 12 h fasting blood glucose concentration and (C) serum insulin concentration. (D) ITT (ip) comparison between WT sedentary and WT exercise trained mice. (E) ITT (ip) comparison between AccDKI sedentary and AccDKI exercise trained mice. (F) ITT (ip) AUC. (G) GTT (ip) comparison between WT sedentary and WT exercise trained mice. (H) GTT (ip) comparison between AccDKI sedentary and AccDKI exercise trained mice. (I) GTT (ip) AUC. Number of mice is denoted on the graph. Data are expressed as means ± SEM, †p < 0.05, ††p < 0.01, for difference from sedentary vs exercise, as determined by two-way ANOVA and Bonferonni post hoc test. *p < 0.05, and **p < 0.01 as a comparison between sedentary and exercise, as determined by two-way repeated measures ANOVA and Bonferonni post hoc test.
3.4. HIIT reduces muscle DAG and increases skeletal muscle Akt phosphorylation
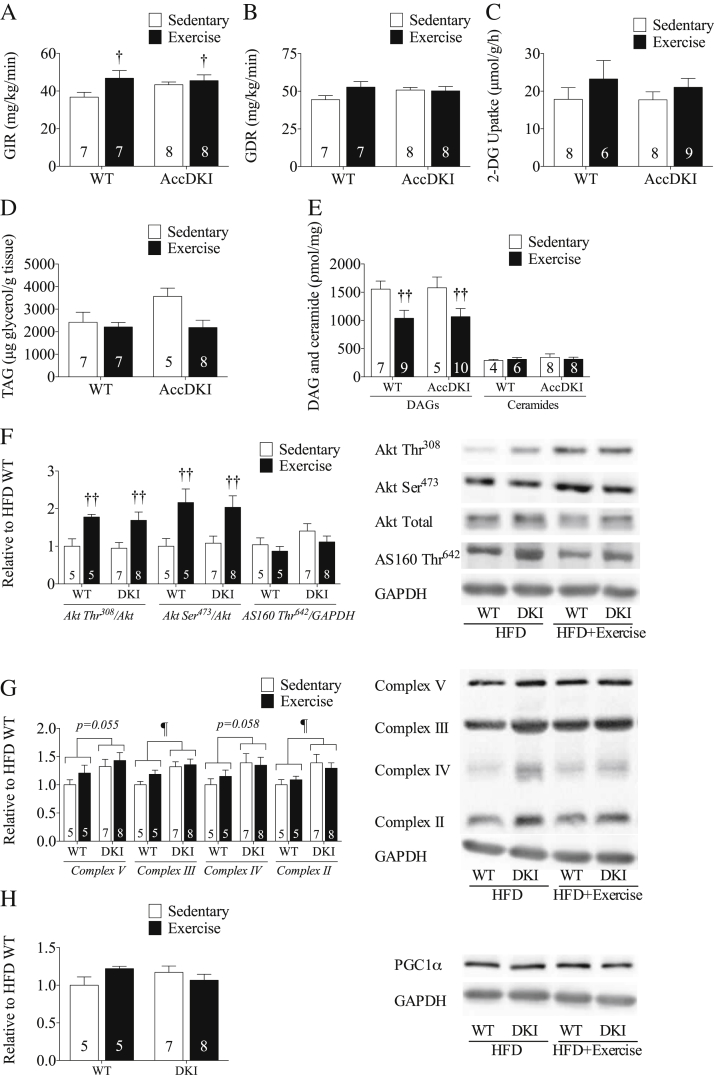
To examine the mechanisms mediating improvements in insulin sensitivity, we conducted hyperinsulinemic-euglycemic clamps. An important caveat of this experiment is that based on the time to catheterize the mice and allow recovery, the clamp was completed 5 days after the last exercise bout and, as such, significant detraining effects may have occurred, thus minimizing differences between the sedentary and HIIT intervention groups. In the clamped state, glucose infusion rates (GIR) were comparable between sedentary WT and AccDKI mice but, importantly, increased with HIIT, indicating improvements in whole-body insulin sensitivity (Figure 3A and Supplementary Figure 1C–F). Surprisingly, glucose disposal rates (GDR) were not different between WT and AccDKI mice and only tended to increase in WT mice that were exercise trained (Interaction p = 0.12) (Figure 3B). Consistent with a similar GDR, 2-DG uptake into mixed gastrocnemius muscle was comparable between genotypes (Figure 3C). Skeletal muscle insulin resistance has been associated with DAG and ceramide accumulation [50], [51]. Although there was no difference either in TAG or ceramide accumulation in skeletal muscle, HIIT training resulted in reductions in DAG content in both WT and AccDKI mice (Figure 3D,E). To further assess muscle insulin sensitivity, the phosphorylation of Akt Ser473, Akt Thr308, and AS160/TBC1D4 Thr642 was determined in clamped quadriceps muscle (Figure 3F). HIIT training increased Akt Ser473 and Thr308 phosphorylation; however, surprisingly, the phosphorylation of its downstream substrate AS160/TBC1D4 phosphorylation was not enhanced (Figure 3F). This suggests that while improving proximal components of the insulin signaling cascade, HIIT training was unable to fully rescue HFD-induced suppression of AS160/TBC1D4 phosphorylation, a finding consistent with the similar 2-DG uptake and GDR in the hyperinsulinemic-euglycemic clamp between sedentary and HIIT trained mice. Future studies investigating the mechanisms contributing to this differential response are warranted.
Figure 3.
HIIT improves insulin sensitivity independently of skeletal muscle insulin sensitivity. (A) Clamped glucose infusion rate (GIR) and (B) glucose disposal rate (GDR). (C) Skeletal muscle 2-DG uptake, (D) TAG, (E) DAG and ceramide accumulation. (F) Skeletal muscle pAkt Thr308/Akt total, pAkt Ser473/Akt total and pAS160 Thr642/GAPDH at the completion of the clamp (from separate gels). (G) Protein expression of Complex V, Complex IV, Complex III and Complex II/GAPDH of the electron transport chain and (H) PGC1α/GAPDH in skeletal muscle relative to HFD WT. Number of mice are denoted on the graph. Data are expressed as means ± SEM, ††p < 0.01 for difference from sedentary vs exercise, ¶p < 0.05 for difference between WT and AccDKI, as determined by two-way ANOVA and Bonferonni post hoc test.
As muscle mitochondrial content is a robust measure of the exercise training response, we measured protein expression of complexes of the electron transport chain (OXPHOS) in quadriceps muscle. We found that AccDKI mice tended to have higher OXPHOS expression (Figure 3G), a finding consistent with their modest fiber type shift [52]. However, HIIT training only had a tendency to increase OXPHOS protein expression (Figure 3G). Consistent with similar OXPHOS expression with exercise training, PGC1-α protein expression was also unchanged with HIIT training (Figure 3H). Given the robust increase in exercise capacity (which was measured within 72 h of the last exercise bout), these data suggest that detraining may have occurred between the last bout of exercise and the completion of the clamps. This rapid detraining effect is consistent with other studies, which have also shown rapid reductions in mitochondrial markers in skeletal muscle following the cessation of exercise training [53], [54], [55], [56].
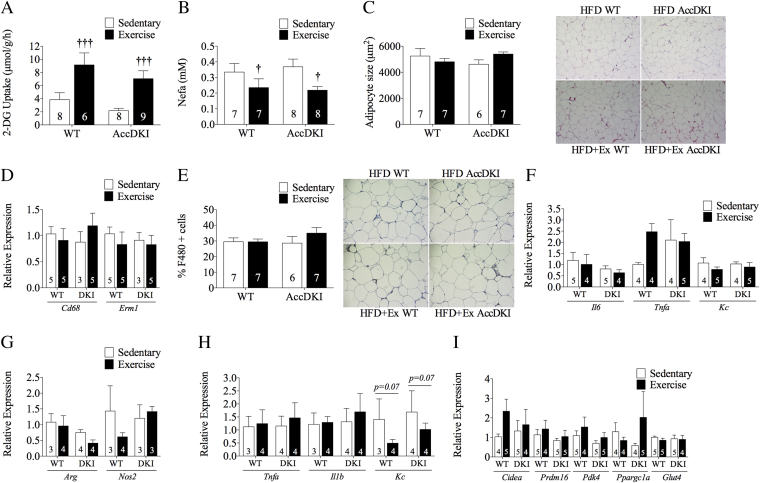
3.5. HIIT increases adipose tissue glucose uptake without altering inflammation, macrophage infiltration or browning markers
In contrast to the muscle, HIIT dramatically improved eWAT insulin sensitivity, as indicated by increased 2-DG uptake (Figure 4A) and lower circulating NEFA levels (Figure 4B) during the clamp. eWAT adipocyte size did not differ between WT and AccDKI mice with or without exercise training (Figure 4C). We hypothesized that exercise training improved adipose tissue insulin sensitivity by reducing the number and inflammatory status of adipose macrophages. However, we found that exercise training did not alter the mRNA expression of the macrophage markers CD68 and F4/80 (Emr1) (Figure 4D) or the protein expression of F4/80+ cells in eWAT (Figure 4E), suggesting that HIIT did not influence macrophage accumulation in adipose tissue. There were also no differences in the eWAT mRNA expression of Il6, Tnfa or the neutrophil chemoattractant Kc with HIIT (Figure 4F). Serum adipokine levels of IL-6, tPAI01, and resistin also did not differ between mice with or without training (Table 2). Leptin (Interaction p = 0.01) was lower in WT mice following HIIT (Table 2). These data indicate that HIIT improves adipose tissue insulin sensitivity independently of ACC phosphorylation and without altering macrophage infiltration or markers of adipose tissue inflammation.
Figure 4.
HIIT increases adipose tissue insulin sensitivity but does not alter inflammation, macrophage infiltration or browning markers. (A) Adipose tissue 2-DG uptake. (B) Clamped circulating non-esterified free fatty acid levels. (C) Adipocyte size. (D) Adipose tissue Cd68 and Emr1 mRNA expression. (E) F4/80+ cells in adipose tissue. (F) Adipose tissue inflammatory markers Il6, Tnfa, and Kc. (G) Adipose tissue macrophage arginase and Nos2 mRNA expression. (H) Adipose tissue macrophage Tnfa, Il1b, and Kc mRNA expression. (I) Adipose tissue Cidea, Prdm16, Pdk4, Pgc1a, and Glut4 mRNA expression.
Table 2.
Serum cytokine levels.
| HFD |
HFD + Exercise |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT |
AccDKI |
WT |
AccDKI |
|||||||||
| Average | ±SEM | N | Average | ±SEM | N | Average | ±SEM | N | Average | ±SEM | N | |
| Leptin (ρg/mL) | 10916.3 | 798.7 | 10 | 9783.7 | 776.9 | 7 | 6466.9∗∗∗ | 852.8 | 9 | 9570.4 | 920.7 | 10 |
| IL-6 (ρg/mL) | 9.9 | 0.6 | 10 | 7.9 | 1.0 | 6 | 8.0 | 0.5 | 10 | 10.9 | 1.1 | 10 |
| tPAI-1 (ρg/mL) | 2130.4 | 307.5 | 10 | 2624.3 | 855.2 | 7 | 2045.7 | 295.2 | 10 | 1950.1 | 263.5 | 10 |
| Resistin (ρg/mL) | 4156.0 | 300 | 10 | 4153.8 | 466.7 | 8 | 4080.1 | 450.6 | 9 | 4502.5 | 483.6 | 10 |
| TNF-α (ρg/mL) | Not detectable | 10 | Not detectable | 7 | Not detectable | 9 | Not detectable | 10 | ||||
| MCP-1 (ρg/mL) | Not detectable | 10 | Not detectable | 7 | Not detectable | 9 | Not detectable | 10 | ||||
Data are expressed as means ± SEM, ∗∗∗p < 0.001, for difference between WT sedentary vs WT exercise; §§p < 0.01, for difference from WT vs AccDKI, as determined by two-way ANOVA and Bonferonni post hoc test.
Since adipose tissue macrophages are the primary source of inflammation in eWAT, we next isolated this cell type (using a CD11b positive selection kit) and examined markers of polarization by assessing arginase and iNOS. Arginase is an anti-inflammatory marker expressed with alternatively activated (M2) macrophages, and iNOS is a pro-inflammatory marker expressed with classically activated (M1) macrophages. Exercise training did not change Arg1 or iNos expression in adipose tissue macrophages (Figure 4G) nor did it affect the expression of Tnfa and Il1b (Figure 4H). There was a strong tendency for the chemoattractant Kc to be reduced with exercise training (p = 0.07) (Figure 4H). Collectively, these data suggest that HIIT does not alter adipose tissue macrophage inflammation.
Although eWAT is resistant to stimuli that induce “browning” it was possible that increased 2-DG uptake into this fat pad following exercise training could potentially be mediated by an increased population of metabolically active beige/brite adipocytes [57], [58]. However, we were not able to detect Ucp1 (data not shown) and there was no difference in the mRNA expression of Cidea, Prdm16, Pdk4 and Ppargc1a irrespective of genotype or training status (Figure 4I). HIIT training also did not result in changes in Glut4 expression (Figure 4I).
3.6. HIIT improves liver insulin sensitivity independently of alterations in liver fat content or inflammation
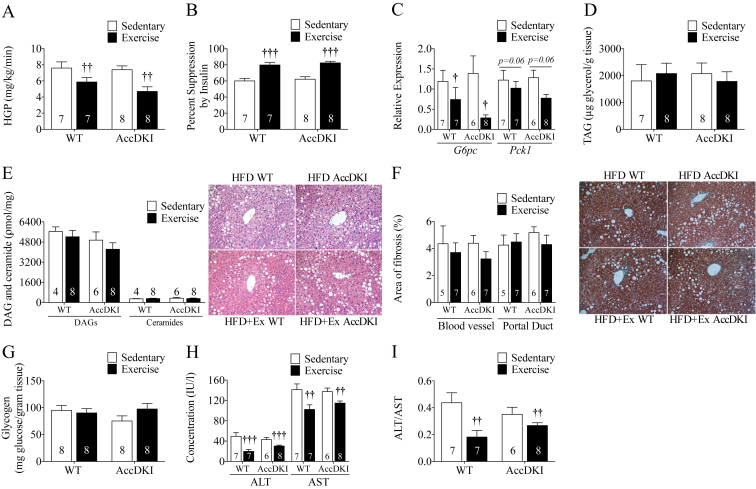
Hepatic glucose production (HGP) and percent suppression of HGP by insulin did not differ between sedentary WT and AccDKI mice fed a HFD, consistent with our previous findings [38]. Importantly, exercise training resulted in reduced HGP and greater percent suppression of HGP by insulin in both WT and AccDKI mice (Figure 5A,B). Consistent with greater reductions in HGP, the mRNA expression of the gluconeogenic enzyme G6pase was reduced and there was a strong tendency (p = 0.06) for reduced Pepck expression in the livers of clamped WT and AccDKI mice following exercise training (Figure 5C).
Figure 5.
HIIT improves hepatic insulin sensitivity independent of ACC phosphorylation and liver triglycerides. (A) HGP and (B) percent suppression of insulin. (C) Relative expression of liver gluconeogenic gene expression of G6pase and Pck1. (D) Liver TAG content. (E) Liver DAG, and ceramide content and representative liver H&E images. (F) Liver area of fibrosis and representative liver trichrome stain images. Fibrosis represented by collagen is stained in blue. (G) Liver glycogen content. (H) Serum ALT and AST concentrations. (I) ALT/AST ratio. Number of mice is denoted on the graphs. Data are expressed as means ± SEM, †p < 0.05, ††p < 0.01, †††p < 0.001 for difference from sedentary vs exercise, as determined by two-way ANOVA and Bonferonni post hoc test.
We hypothesized that improvements in liver insulin sensitivity would be accompanied by reductions in liver lipid content but found that surprisingly TAG, DAG, and ceramide content in the liver were comparable (Figure 5D,E). Histological examination of the livers supported similar amounts of lipid deposition and there were no changes in liver fibrosis with HIIT or between genotypes (Figure 5F). No differences were observed in liver glycogen content (Figure 5G). Consistent with similar degrees of liver lipid content and fibrosis, HIIT did not reduce the expression of a number of markers of liver inflammatory status (I11b, Il10, iNos, and Arg – Table 3), and surprisingly, increased the expression of Tnfa and F4/80 (Emr1) (Table 3). However, the monocyte/macrophage markers Cd68 and Kc were lower in both WT and AccDKI mice that were exercise trained (Table 3). Despite similar lipid content and fibrosis, there was a reduction in serum ALT and AST levels with HIIT in both WT and AccDKI mice (Figure 5H) and a reduction in the ALT/AST ratio (Figure 5I).
Table 3.
Analysis of liver inflammatory markers.
| HFD |
HFD + Exercise |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WT |
AccDKI |
WT |
AccDKI |
|||||||||
| Average | ±SEM | N | Average | ±SEM | N | Average | ±SEM | N | Average | ±SEM | N | |
| Il1b | 1.16 | 0.33 | 7 | 0.95 | 0.15 | 6 | 1.13 | 0.26 | 7 | 1.19 | 0.20 | 8 |
| Tnfa | 1.11 | 0.22 | 7 | 1.56 | 0.31 | 6 | 2.93††† | 0.74 | 7 | 3.12††† | 0.51 | 8 |
| Erm1 | 1.03 | 0.14 | 7 | 1.43 | 0.14 | 6 | 2.24††† | 0.19 | 7 | 2.13††† | 0.27 | 8 |
| Il10 | 1.13 | 0.28 | 7 | 1.52 | 0.37 | 6 | 1.60 | 0.33 | 7 | 1.61 | 0.30 | 8 |
| Nos2 | 1.08 | 0.21 | 7 | 1.64 | 0.54 | 6 | 0.73 | 0.29 | 7 | 0.94 | 0.28 | 8 |
| Arg | 1.10 | 0.24 | 7 | 0.94 | 0.11 | 6 | 0.87 | 0.09 | 7 | 0.69 | 0.10 | 8 |
| Cd68 | 1.06 | 0.18 | 7 | 0.97 | 0.47 | 6 | 0.69† | 0.07 | 7 | 0.76† | 0.04 | 8 |
| Kc | 1.26 | 0.40 | 7 | 1.53 | 0.36 | 6 | 0.61††† | 0.22 | 7 | 0.64††† | 0.15 | 8 |
Data are expressed as means ± SEM, †p < 0.05, †††p < 0.001, for difference from sedentary vs exercise, as determined by two-way ANOVA and Bonferonni post hoc test.
4. Discussion
Consistent with studies in humans, we found that HIIT led to a substantial improvement in exercise capacity without altering body mass or adiposity and that this was accompanied by significant reductions in fasting and fed blood glucose levels. HIIT improved adipose tissue insulin sensitivity independently of adipocyte cell size, macrophage accumulation in adipose tissue, adipose tissue inflammation or markers of adipose tissue browning. HIIT also improved liver insulin sensitivity independently of reductions in liver TAG/DAG/ceramide levels. Importantly, improvements in adipose and liver insulin sensitivity occurred independently of ACC phosphorylation, which we have previously shown is required for the insulin sensitizing effects of metformin [38]. These findings indicate that HIIT can uncouple inflammation and lipid metabolism from insulin sensitivity and does not depend on AMPK phosphorylation and inhibition of ACC.
Our HIIT training protocol is similar to that originally proposed by the Gibala laboratory, which involved 60 s of intense exercise (at 100% of VO2 max) followed by 75 s of rest, repeated for 8–12 cycles, 3 times per week [59]. Importantly, this study demonstrated that the adaptations achieved in regards to muscle mitochondrial biogenesis were similar to those obtained with more than 5 times the volume of endurance (50–70% of VO2 max) exercise training [59]. While there are higher intensity protocols that utilize less time, these protocols may not be appropriate for obese individuals with cardiometabolic disease. And while we acknowledge that the protocol we have used is still longer than that used in many human studies, it constitutes only 90 min of treadmill running per week, which – to the best of our knowledge – is the lowest volume of exercise shown to have a positive effect on insulin sensitivity in obese rodents. Importantly, this volume of exercise is approximately 75% less than the majority of studies conducted in rodents which typically complete around 60 min of treadmill exercise per day 5 days per week [20], [21], [22], [25], [26], [60]. In future studies, it will be interesting to titer down the work volume to determine the minimal exercise volume necessary to elicit improvements in insulin sensitivity in rodents.
Research indicates that improvements in insulin sensitivity with HIIT are comparable to [61], [62], if not greater than [61], [63], improvements elicited by moderate intensity continuous endurance exercise. Importantly, a number of studies have demonstrated that HIIT can improve insulin sensitivity, independent of weight loss and adiposity, in adults who are sedentary [6], overweight/obese [9] or have type 2 diabetes [7], [8]. However, these studies provide little information regarding the tissue-specific mechanisms by which HIIT may improve insulin sensitivity; although it was generally assumed that the primary effects resulted from improvements in muscle insulin sensitivity. Therefore, our findings from hyperinsulinemic-euglycemic clamps indicating that HIIT improves adipose tissue and liver insulin sensitivity provide important information regarding the mechanisms by which HIIT improves glycemic control. Future studies using clamps are warranted to investigate whether adipose tissue and liver insulin sensitivity are also improved in humans performing HIIT.
Consistent with our previous findings [38], weight gain/adiposity between sedentary WT and AccDKI mice on the HFD was comparable. As intended by our selection of the exercise training program, there were no changes in body mass or adiposity compared to sedentary mice. This allowed for examination of the metabolic effects of exercise without the confounding influence of differences in adiposity. We found that despite similar adiposity and body weight, HIIT increased daily food intake in both WT and AccDKI mice, which appeared to be offset by elevations in spontaneous physical activity and subsequent increases in oxygen consumption and energy expenditure compared to sedentary controls. This is especially important since many animal studies examining effects of exercise training on insulin sensitivity [21], [64], [65], liver lipids [18], [19], [20], [21], [22], [23], [66], [67], [68] and white adipose tissue inflammation [24], [25] all showed reductions in body mass, making it difficult to directly assess the effects of exercise training.
We found that our exercise training program in obese mice induced similar improvements in exercise capacity and insulin sensitivity compared to previous studies examining effects of endurance training [24], [25]. In contrast to the ip ITT, the ip GTT did not show significant improvements with HIIT in either genotype, although a tendency was present in the WT mice. The reason for the lack of clear effect on glucose tolerance is likely related to the fact that we conducted an ip rather than oral GTT. Previous studies have demonstrated that exercise enhances glucose tolerance due to IL-6 stimulation of GLP-1 [69], an effect that would not be expected to be enhanced due to the ip delivery of glucose. As we did not collect blood during the ip GTT, it is not possible to measure serum insulin or GLP-1 but we would hypothesize that insulin may have been lower in the exercise-trained mice. Future studies completing oral glucose tolerance tests and investigating the effects on this response are warranted.
Improvements in whole body insulin sensitivity following HIIT were associated with marked increases in eWAT 2-DG uptake and reductions in circulating NEFA during the hyperinsulinemic-euglycemic clamp, indicating improvements in white adipose tissue insulin sensitivity. However, in contrast to previous exercise training studies, we did not detect reductions in markers of eWAT macrophage inflammation that have been linked to insulin resistance in obesity [11], [70], [71]. These data suggest that reductions in inflammation with exercise training reported by previous studies are most likely due to a loss of adiposity. Our studies also suggest that improvements in adipose tissue insulin sensitivity with HIIT can occur independently of reductions in inflammation. These results are in agreement with several recent studies, which have also detected a dissociation between adipose tissue inflammatory status and insulin resistance [72], [73]. Due to limited quantity of tissue, we were unable to assess adipose insulin signaling in our study. In future studies, it will be important to examine the mechanisms by which exercise improves adipose tissue insulin sensitivity.
In addition to improving adipose tissue insulin sensitivity, we also found that HIIT improved hepatic insulin sensitivity, an effect that occurred independently of reductions in liver lipid content. Many studies in rodents [18], [23], [66], [67], [68] and humans [16], [17], [74] have indicated that exercise training can lower liver fat content. As small reductions in adiposity can have a large impact on liver fat content [75], interpreting the relative importance of exercise training on reductions in liver fat content has been difficult given that there are also reductions in adiposity in most studies. Our findings indicating that HIIT does not alter adiposity or liver fat content suggest that exercise alone is likely insufficient to lower liver lipid content. Surprisingly, despite similar lipid content, the ALT:AST ratio was lower, suggesting that liver damage induced by HFD was blunted following HIIT training. Future studies examining liver fat content and markers of liver function following HIIT training in humans are warranted.
A limitation of this study is that only one type of exercise training was used and does not speak to the effects of other training types. HIIT is generally associated with greater use of carbohydrates rather than lipids in comparison to high-volume endurance exercise training [76]. Future studies directly comparing HIIT to classical endurance exercise training in mice would help delineate potential differences between these two types of training. Another limitation of our study was that we did not detect improvements in skeletal muscle insulin sensitivity, which may have been due to the hyperinsulinemic-euglycemic clamps being conducted 5 days after the last bout of exercise. Future studies in which mice are clamped 72 h after the last exercise bout may be important to avoid detraining effects in muscle.
In summary, HIIT improved exercise capacity and whole-body glucose homeostasis by enhancing liver and adipose tissue insulin sensitivity. These effects were independent of reductions in adiposity/adipose tissue cell size indicating the therapeutic potential of exercise independent of weight loss. Improved insulin sensitivity was also independent of adipose tissue inflammation, macrophage infiltration/inflammation or reductions in liver lipid content indicating dissociation between these parameters and insulin resistance. Lastly, we demonstrated that in contrast to metformin, HIIT exercise training improves insulin sensitivity independently of the AMPK-ACC signaling pathway. Future studies identifying the mechanism by which HIIT improves insulin sensitivity may reveal novel strategies to improve glucose homeostasis in individuals with type 2 diabetes.
Author contributions
KM, SRS, MF and GRS conception and design of research; KM, SS, MCS, and MDF performed experiments; KM and GRS analyzed data; KM and GRS interpreted results of experiments; KM and GRS prepared figures; KM and GRS drafted manuscript; KM, BEK, MDF and GRS edited and revised manuscript; KM, SRS, BEK, MCS, MDF and GRS approved final version of manuscript. KM and GRS are responsible for the integrity of the work as a whole.
Acknowledgments
We thank R. Rhem and C. Saab from the McMaster Centre for Translational Imaging for completing the computed tomography scans. These studies were supported by the Natural Sciences and Engineering Research Council (GRS) (RGPIN-371870-2009), the Canadian Institutes of Health Research (CIHR) (GRS) (MOP-114880), the National Health and Medical Research Council (BEK, GRS) (APP1085460) and the Australian Research Council (BEK) (DP130104548). Supported in part by the Victorian Government's OIS Program (BEK) and Canadian Foundation for Innovation (GRS). MDF was supported by a CIHR Banting Fellowship. MCS was supported by New Investigator Fund Grant from Hamilton Health Sciences (11-272). GRS is a Canada Research Chair in metabolism and obesity and is the J Bruce Duncan Chair in Metabolic Diseases.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2015.09.006.
Conflict of interest
Authors do not have any conflict of interest to declare.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
(A) Acute pAMPK Thr172 to AMPK total and (B) pACC Ser79/212 to ACC total in liver after an acute bout of exercise. (C) Clamped GIR curve comparison between WT sedentary and WT exercise trained mice during clamp. (D) Clamped GIR curve comparison between AccDKI sedentary and AccDKI exercise trained mice during clamp. (E) Blood glucose comparison between WT sedentary and WT exercise trained mice during clamp. (F) Blood glucose comparison between AccDKI sedentary and AccDKI exercise trained mice during clamp. Number of mice is denoted on the graph. Data are expressed as means ± SEM, *p < 0.05 for difference between sedentary vs exercise as determined by t-test.
References
- 1.Tuomilehto J., Lindström J., Eriksson J.G., Valle T.T., Hämäläinen H., Ilanne-Parikka P. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. The New England Journal of Medicine. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 2.Knowler W.C., Barrett-Connor E., Fowler S.E., Hamman R.F., Lachin J.M., Walker E.A. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan X.R., Li G.W., Hu Y.H., Wang J.X., Yang W.Y., An Z.X. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT and diabetes study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 4.Godin G., Desharnais R., Valois P., Lepage L., Jobin J., Bradet R. Differences in perceived barriers to exercise between high and low intenders: observations among different populations. American Journal of Health Promotion. 1994;8(4):279–285. [Google Scholar]
- 5.Marquis-Gravela G., Hayamia D., Juneaua M., Nigama A., Guilbeaulta V., Latoura E. Intensive lifestyle intervention including high-intensity interval training program improves insulin resistance and fasting plasma glucose in obese patients. Preventive Medicine Reports. 2015;2:314–318. doi: 10.1016/j.pmedr.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocks M., Shepherd S., Tipton K., Wagenmakers A., Shaw C. High-intensity interval training improves microvascular and macrovascular function and insulin sensitivity. British Journal of Sports Medicine. 2010;44(i9) [Google Scholar]
- 7.Shaban N., Kenno K.A., Milne K.J. The effects of a 2 week modified high intensity interval training program on the homeostatic model of insulin resistance (HOMA-IR) in adults with type 2 diabetes. The Journal of Sports Medicine and Physical Fitness. 2014;54(2):203–209. [PubMed] [Google Scholar]
- 8.Little J.P., Gillen J.B., Percival M.E., Safdar A., Tarnopolsky M. a, Punthakee Z. Low-volume high-intensity interval training reduces hyperglycemia and increases muscle mitochondrial capacity in patients with type 2 diabetes. Journal of Applied Physiology (Bethesda, Md. : 1985) 2011;111(6):1554–1560. doi: 10.1152/japplphysiol.00921.2011. [DOI] [PubMed] [Google Scholar]
- 9.Cocks M., Shaw C., Shepherd S., Fisher J., Ranasinghe A., Barker T. Sprint interval and moderate-intensity continuous training have equal benefits on aerobic capacity, insulin sensitivity, muscle capillarisation and endothelial eNOS/NAD(P)Hoxidase. Journal of Physiology. 2015 doi: 10.1113/jphysiol.2014.285254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lumeng C.N., Delproposto J.B., Westcott D.J., Saltiel A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lumeng C.N., DeYoung S.M., Bodzin J.L., Saltiel A.R. Increased inflammatory properties of adipose tissue macrophages recruited during diet-induced obesity. Diabetes. 2007;56(1):16–23. doi: 10.2337/db06-1076. [DOI] [PubMed] [Google Scholar]
- 12.Fabbrini E., Sullivan S., Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51(2):679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pagadala M.R., McCullough A.J. Non-alcoholic fatty liver disease and obesity: not all about body mass index. The American Journal of Gastroenterology. 2012;107(12):1859–1861. doi: 10.1038/ajg.2012.320. [DOI] [PubMed] [Google Scholar]
- 14.Heilbronn L.K., Campbell L.V. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. Current Pharmaceutical Design. 2008;14(12):1225–1230. doi: 10.2174/138161208784246153. [DOI] [PubMed] [Google Scholar]
- 15.Lee J. Adipose tissue macrophages in the development of obesity-induced inflammation, insulin resistance and type 2 diabetes. Archives of Pharmacal Research. 2013:208–222. doi: 10.1007/s12272-013-0023-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelber-Sagi S., Nitzan-Kaluski D., Goldsmith R., Webb M., Zvibel I., Goldiner I. Role of leisure-time physical activity in nonalcoholic fatty liver disease: a population-based study. Hepatology (Baltimore, Md.) 2008;48(6):1791–1798. doi: 10.1002/hep.22525. [DOI] [PubMed] [Google Scholar]
- 17.Johnson N.A., Sachinwalla T., Walton D.W., Smith K., Armstrong A., Thompson M.W. Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology. 2009;50(4):1105–1112. doi: 10.1002/hep.23129. [DOI] [PubMed] [Google Scholar]
- 18.Jordy A.B., Kraakman M.J., Gardner T., Estevez E., Kammoun H.L., Weir J.M. Analysis of the liver lipidome reveals insights into the protective effect of exercise on high-fat diet-induced hepatosteatosis in mice. American Journal of Physiology. Endocrinology and Metabolism. 2015;308(9):E778–E791. doi: 10.1152/ajpendo.00547.2014. [DOI] [PubMed] [Google Scholar]
- 19.Cintra D.E., Ropelle E.R., Vitto M.F., Luciano T.F., Souza D.R., Engelmann J. Reversion of hepatic steatosis by exercise training in obese mice: the role of sterol regulatory element-binding protein-1c. Life Sciences. 2012;91(11–12):395–401. doi: 10.1016/j.lfs.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Cho J., Lee I., Kim D., Koh Y., Kong J., Lee S. Effect of aerobic exercise training on non-alcoholic fatty liver disease induced by a high fat diet in C57BL/6 mice. Journal of Exercise Nutrition & Biochemistry. 2014;18(4):339–346. doi: 10.5717/jenb.2014.18.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marques C.M.M., Motta V.F., Torres T.S., Aguila M.B., Mandarim-de-Lacerda C.A. Beneficial effects of exercise training (treadmill) on insulin resistance and nonalcoholic fatty liver disease in high-fat fed C57BL/6 mice. Brazilian Journal of Medical and Biological Research. 2010;43(5):467–475. doi: 10.1590/s0100-879x2010007500030. [DOI] [PubMed] [Google Scholar]
- 22.Berglund E.D., Lustig D.G., Baheza R.A., Hasenour C.M., Lee-Young R.S., Donahue E.P. Hepatic glucagon action is essential for exercise-induced reversal of mouse fatty liver. Diabetes. 2011;60(11):2720–2729. doi: 10.2337/db11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Haram P.M., Kemi O.J., Lee S.J., Bendheim M., Al-Share Q.Y., Waldum H.L. Aerobic interval training vs. continuous moderate exercise in the metabolic syndrome of rats artificially selected for low aerobic capacity. Cardiovascular Research. 2009;81(4):723–732. doi: 10.1093/cvr/cvn332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bradley R.L., Jeon J.Y., Liu F.-F., Maratos-Flier E. Voluntary exercise improves insulin sensitivity and adipose tissue inflammation in diet-induced obese mice. American Journal of Physiology. Endocrinology and Metabolism. 2008;295(3):E586–E594. doi: 10.1152/ajpendo.00309.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vieira V.J., Valentine R.J., Wilund K.R., Antao N., Baynard T., Woods J.A. Effects of exercise and low-fat diet on adipose tissue inflammation and metabolic complications in obese mice. American Journal of Physiology. Endocrinology and Metabolism. 2009;296(5):E1164–E1171. doi: 10.1152/ajpendo.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawanishi N., Yano H., Yokogawa Y., Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exercise Immunology Review. 2010;16:105–118. [PubMed] [Google Scholar]
- 27.Gibala M.J., McGee S.L., Garnham A.P., Howlett K.F., Snow R.J., Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. Journal of Applied Physiology (Bethesda, Md. : 1985) 2009;106(3):929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- 28.Stephens T.J., Chen Z.-P., Canny B.J., Michell B.J., Kemp B.E., McConell G.K. Progressive increase in human skeletal muscle AMPKalpha2 activity and ACC phosphorylation during exercise. American Journal of Physiology. Endocrinology and Metabolism. 2002;282(3):E688–E694. doi: 10.1152/ajpendo.00101.2001. [DOI] [PubMed] [Google Scholar]
- 29.Park H., Kaushik V.K., Constant S., Prentki M., Przybytkowski E., Ruderman N. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. Journal of Biological Chemistry. 2002;277(36):32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- 30.Wojtaszewski J.F., Nielsen P., Hansen B.F., Richter E.A., Kiens B. Isoform-specific and exercise intensity-dependent activation of 5’-AMP-activated protein kinase in human skeletal muscle. The Journal of Physiology. 2000;528(Pt 1):221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Camacho R.C., Donahue E.P., James F.D., Berglund E.D., Wasserman D.H. Energy state of the liver during short term and exhaustive exercise in C57BL/6J mice. American Journal of Physiology. Endocrinology Metabolism. 2005;290(3):E405–E408. doi: 10.1152/ajpendo.00385.2005. [DOI] [PubMed] [Google Scholar]
- 32.Watt M.J., Holmes A.G., Pinnamaneni S.K., Garnham A.P., Steinberg G.R., Kemp B.E. Regulation of HSL serine phosphorylation in skeletal muscle and adipose tissue. American Journal of Physiology. Endocrinology and Metabolism. 2006;290(3):E500–E508. doi: 10.1152/ajpendo.00361.2005. [DOI] [PubMed] [Google Scholar]
- 33.Koh H.-J., Hirshman M.F., He H., Li Y., Manabe Y., Balschi J.A. Adrenaline is a critical mediator of acute exercise-induced AMP-activated protein kinase activation in adipocytes. The Biochemical Journal. 2007;403(3):473–481. doi: 10.1042/BJ20061479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galic S., Fullerton M.D., Schertzer J.D., Sikkema S., Marcinko K., Walkley C.R. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. Journal of Clinical Investigation. 2011;121(12):4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sag D., Carling D., Stout R.D., Suttles J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. Journal of Immunology (Baltimore, Md. : 1950) 2008;181(12):8633–8641. doi: 10.4049/jimmunol.181.12.8633. Doi: 181/12/8633 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinberg G.R., Schertzer J.D. AMPK promotes macrophage fatty acid oxidative metabolism to mitigate inflammation: implications for diabetes and cardiovascular disease. Immunology and Cell Biology. 2014;92(4):340–345. doi: 10.1038/icb.2014.11. [DOI] [PubMed] [Google Scholar]
- 37.Mounier R., Théret M., Arnold L., Cuvellier S., Bultot L., Göransson O. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metabolism. 2013;18(2):251–264. doi: 10.1016/j.cmet.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Fullerton M.D., Galic S., Marcinko K., Sikkema S., Pulinilkunnil T., Chen Z.-P. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nature Medicine. 2013;19(12):1649–1654. doi: 10.1038/nm.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinberg G.R., Jorgensen S.B. The AMP-activated protein kinase: role in regulation of skeletal muscle metabolism and insulin sensitivity. Mini Reviews in Medical Chemistry. 2007;7(5):519–526. doi: 10.2174/138955707780619662. [DOI] [PubMed] [Google Scholar]
- 40.Crane J.D., Palanivel R., Mottillo E.P., Bujak A.L., Wang H., Ford R.J. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nature Medicine. 2015;21:166–172. doi: 10.1038/nm.3766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watt M.J., Dzamko N., Thomas W.G., Rose-John S., Ernst M., Carling D. CNTF reverses obesity-induced insulin resistance by activating skeletal muscle AMPK. Nature Medicine. 2006;12(5):541–548. doi: 10.1038/nm1383. [DOI] [PubMed] [Google Scholar]
- 42.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 43.Bligh E.G., Dyer W.J. A rapid method of total lipid Extraction and purification. Canadian Journal of Biochemistry and Physiology. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 44.Preiss J., Loomis C.R., Bishop W.R., Stein R., Niedel J.E., Bell R.M. Quantitative measurement of sn-1,2-diacylglycerols present in platelets, hepatocytes, and ras- and sis-transformed normal rat kidney cells. Journal of Biological Chemistry. 1986;261(19):8597–8600. [PubMed] [Google Scholar]
- 45.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods (San Diego, Calif.) 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Lund J., Hafstad A.D., Boardman N.T., Rossvoll L., Rolim N.P., Ahmed M.S. Exercise training promotes cardioprotection through oxygen-sparing action in high fat-fed mice. American Journal of Physiology. Heart and Circulatory Physiology. 2015;308(8):H823–H829. doi: 10.1152/ajpheart.00734.2014. [DOI] [PubMed] [Google Scholar]
- 47.Apontes P., Liu Z., Su K., Benard O., Youn D.Y., Li X. Mangiferin stimulates carbohydrate oxidation and protects against high fat diet induced metabolic disorders. Diabetes. 2014;63(11):3626–3636. doi: 10.2337/db14-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corpeleijn E., Saris W.H.M., Blaak E.E. Metabolic flexibility in the development of insulin resistance and type 2 diabetes: effects of lifestyle: etiology and pathophysiology. Obesity Reviews. 2009;10(2):178–193. doi: 10.1111/j.1467-789X.2008.00544.x. [DOI] [PubMed] [Google Scholar]
- 49.Jorgensen S.B., O'Neill H.M., Sylow L., Honeyman J., Hewitt K.A., Palanivel R. Deletion of skeletal muscle SOCS3 prevents insulin resistance in obesity. Diabetes. 2013;62(1):56–64. doi: 10.2337/db12-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shulman G.I. Cellular mechanisms of insulin resistance in humans. The American Journal of Cardiology. 1999;84(1A):3J–10J. doi: 10.1016/s0002-9149(99)00350-1. [DOI] [PubMed] [Google Scholar]
- 51.Yu C., Chen Y., Cline G.W., Zhang D., Zong H., Wang Y. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. Journal of Biological Chemistry. 2002;277(52):50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 52.O'Neill H.M., Lally J.S., Galic S., Thomas M., Azizi P.D., Fullerton M.D. AMPK phosphorylation of ACC2 is required for skeletal muscle fatty acid oxidation and insulin sensitivity in mice. Diabetologia. 2014;57(8):1693–1702. doi: 10.1007/s00125-014-3273-1. [DOI] [PubMed] [Google Scholar]
- 53.Nagasawa J., Sato Y., Ishiko T. Effect of training and detraining on in vivo insulin sensitivity. International Journal of Sports Medicine. 1990;11(2):107–110. doi: 10.1055/s-2007-1024772. [DOI] [PubMed] [Google Scholar]
- 54.Chi M.M., Hintz C.S., Coyle E.F., Martin W.H., Ivy J.L., Nemeth P.M. Effects of detraining on enzymes of energy metabolism in individual human muscle fibers. The American Journal of Physiology. 1983;244(3):C276–C287. doi: 10.1152/ajpcell.1983.244.3.C276. [DOI] [PubMed] [Google Scholar]
- 55.Mazzucatto F., Higa T., Fonseca-Alaniz M., Evangelista F. Reversal of metabolic adaptations induced by physical training after two weeks of physical detraining. International Journal of Clinical and Experimental Medicine. 2014;7(8):2000–2008. [PMC free article] [PubMed] [Google Scholar]
- 56.Nogueira L., Ramirez-Sanchez I., Perkins G.A., Murphy A., Taub P.R., Ceballos G. (-)-Epicatechin enhances fatigue resistance and oxidative capacity in mouse muscle. The Journal of Physiology. 2011;589(Pt 18):4615–4631. doi: 10.1113/jphysiol.2011.209924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peirce V., Vidal-Puig A. Regulation of glucose homoeostasis by brown adipose tissue. The Lancet Diabetes & Endocrinology. 2013;1(4):353–360. doi: 10.1016/S2213-8587(13)70055-X. [DOI] [PubMed] [Google Scholar]
- 58.Boström P., Wu J., Jedrychowski M.P., Korde A., Ye L., Lo J.C. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Little J.P., Safdar A., Wilkin G.P., Tarnopolsky M.A., Gibala M.J. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: potential mechanisms. The Journal of Physiology. 2010;588(Pt 6):1011–1022. doi: 10.1113/jphysiol.2009.181743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Motta V.F., de Lacerda C.A. Beneficial effects of exercise training (Treadmill) on body mass and skeletal muscle capillaries/myocyte ratio in C57BL/6 mice fed high-fat diet. International Journal of Morphology. 2012;30(1):205–210. [Google Scholar]
- 61.Mitranun W., Deerochanawong C., Tanaka H., Suksom D. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scandinavian Journal of Medicine & Science in Sports. 2014;24:e69–76. doi: 10.1111/sms.12112. [DOI] [PubMed] [Google Scholar]
- 62.Nybo L., Sundstrup E., Jakobsen M.D., Mohr M., Hornstrup T., Simonsen L. High-intensity training versus traditional exercise interventions for promoting health. Medicine and Science in Sports and Exercise. 2010;42(10):1951–1958. doi: 10.1249/MSS.0b013e3181d99203. [DOI] [PubMed] [Google Scholar]
- 63.Tjønna A.E., Lee S.J., Rognmo Ø., Stølen T.O., Bye A., Haram P.M. Aerobic interval training versus continuous moderate exercise as a treatment for the metabolic syndrome: a pilot study. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rao X., Zhong J., Xu X., Jordan B., Maurya S., Braunstein Z. Exercise protects against diet-induced insulin resistance through downregulation of protein kinase Cβ in mice. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarvas J., Otis J., Khaper N., Lees S. Voluntary physical activity prevents insulin resistance in a tissue specific manner. Physiological Reports. 2015;3(2):e12277. doi: 10.14814/phy2.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rector R.S., Thyfault J.P., Morris R.T., Laye M.J., Borengasser S.J., Booth F.W. Daily exercise increases hepatic fatty acid oxidation and prevents steatosis in Otsuka Long-Evans Tokushima Fatty rats. American Journal of Physiology. Gastrointestinal and Liver Physiology. 2008;294(3):G619–G626. doi: 10.1152/ajpgi.00428.2007. [DOI] [PubMed] [Google Scholar]
- 67.Rector R.S., Thyfault J.P., Laye M.J., Morris R.T., Borengasser S.J., Uptergrove G.M. Cessation of daily exercise dramatically alters precursors of hepatic steatosis in Otsuka Long-Evans Tokushima Fatty (OLETF) rats. The Journal of Physiology. 2008;586(Pt 17):4241–4249. doi: 10.1113/jphysiol.2008.156745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thyfault J.P., Rector R.S., Uptergrove G.M., Borengasser S.J., Morris E.M., Wei Y. Rats selectively bred for low aerobic capacity have reduced hepatic mitochondrial oxidative capacity and susceptibility to hepatic steatosis and injury. The Journal of Physiology. 2009;587(Pt 8):1805–1816. doi: 10.1113/jphysiol.2009.169060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ellingsgaard H., Hauselmann I., Schuler B., Habib A.M., Baggio L.L., Meier D.T. Interleukin-6 enhances insulin secretion by increasing glucagon-like peptide-1 secretion from L cells and alpha cells. Nature Medicine. 2011:1481–1489. doi: 10.1038/nm.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pedersen B.K. The anti-inflammatory effect of exercise: its role in diabetes and cardiovascular disease control. Essays in Biochemistry. 2006;42:105–117. doi: 10.1042/bse0420105. [DOI] [PubMed] [Google Scholar]
- 72.Turner N., Kowalski G.M., Leslie S.J., Risis S., Yang C., Lee-Young R.S. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia. 2013;56(7):1638–1648. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 73.Aparicio-Vergara M., Hommelberg P.P.H., Schreurs M., Gruben N., Stienstra R., Shiri-Sverdlov R. Tumor necrosis factor receptor 1 gain-of-function mutation aggravates nonalcoholic fatty liver disease but does not cause insulin resistance in a murine model. Hepatology (Baltimore, Md.) 2013;57(2):566–576. doi: 10.1002/hep.26046. [DOI] [PubMed] [Google Scholar]
- 74.Hallsworth K., Fattakhova G., Hollingsworth K.G., Thoma C., Moore S., Taylor R. Resistance exercise reduces liver fat and its mediators in non-alcoholic fatty liver disease independent of weight loss. Gut. 2011;60(9):1278–1283. doi: 10.1136/gut.2011.242073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Petersen K.F., Dufour S., Befroy D., Lehrke M., Hendler R.E., Shulman G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54(3):603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romijn J.A., Gastaldelli A., Horowitz J.F., Endert E., Wolfe R.R. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. The American Journal of Physiology. 1993;265(3p1):380–391. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Acute pAMPK Thr172 to AMPK total and (B) pACC Ser79/212 to ACC total in liver after an acute bout of exercise. (C) Clamped GIR curve comparison between WT sedentary and WT exercise trained mice during clamp. (D) Clamped GIR curve comparison between AccDKI sedentary and AccDKI exercise trained mice during clamp. (E) Blood glucose comparison between WT sedentary and WT exercise trained mice during clamp. (F) Blood glucose comparison between AccDKI sedentary and AccDKI exercise trained mice during clamp. Number of mice is denoted on the graph. Data are expressed as means ± SEM, *p < 0.05 for difference between sedentary vs exercise as determined by t-test.