Abstract
Objective
Metabolic inflammation is believed to promote insulin resistance and type 2 diabetes progression in obesity. TRAF3, a cytoplasmic signaling protein, has been known to mediate/modulate cytokine signaling in immune cells. The goal is to define the metabolic function of hepatic TRAF3 in the setting of obesity.
Methods
Hepatocyte-specific TRAF3 knockout mice were generated using the loxp/albumin-cre system. Liver TRAF3 was deleted in adult obese mice via Cre adenoviral infection. Both high fat diet-induced and genetic obesity were examined. TRAF3 levels and insulin signaling were measured by immunoblotting. Insulin sensitivity, hepatic glucose production, and glucose metabolism were examined by glucose, insulin, and pyruvate tolerance tests. Hepatic steatosis was examined by Oil red O staining of liver sections and measuring liver triacylglycerol levels.
Results
Liver TRAF3 levels were lower in the fasted states in normal mice, and were aberrantly higher in obese mice and in mice with streptozotocin-induced hyperglycemia. Glucose directly increased TRAF3 levels in primary hepatocytes. Hepatocyte-specific deletion of TRAF3 decreased hyperinsulinemia, insulin resistance, glucose intolerance, and hepatic steatosis in mice with either high fat diet-induced obesity or genetic obesity (ob/ob); conversely, in lean mice, adenovirus-mediated overexpression of TRAF3 in the liver induced hyperinsulinemia, insulin resistance, and glucose intolerance. Deletion of TRAF3 enhanced the ability of insulin to stimulate phosphorylation of Akt in hepatocytes, whereas overexpression of TRAF3 suppressed insulin signaling.
Conclusions
Glucose increases the levels of hepatic TRAF3. TRAF3 in turn promotes hyperglycemia through increasing hepatic glucose production, thus forming a glucose-TRAF3 reinforcement loop in the liver. This positive feedback loop may drive the progression of type 2 diabetes and nonalcoholic fatty liver disease in obesity.
Keywords: TRAF3, Obesity, Insulin, Liver, Inflammation, Diabetes, Gluconeogenesis
Abbreviations: TRAF3, TNF receptor-associated factor 3; HFD, high fat diet; NAFLD, nonalcoholic fatty liver disease; HKO, hepatocyte-specific TRAF3 knockout; GTT, glucose; ITT, insulin; PTT, pyruvate; LTT, lactate tolerance test; GFP, green fluorescent protein; DKO, hepatocyte TRAF3 and leptin double knockout; LD, lipid droplet
Highlights
-
•
Glucose increases TRAF3 levels in hepatocytes.
-
•
Liver TRAF3 levels are aberrantly higher in obese mice.
-
•
Deletion of hepatic TRAF3 attenuates insulin resistance, glucose intolerance, and NAFLD in obese mice.
-
•
Liver-specific overexpression of TRAF3 induces insulin resistance and glucose intolerance in lean mice.
1. Introduction
Obesity is a primary risk factor for the development of insulin resistance, type 2 diabetes, and cardiovascular diseases [1]. Prevalence rates of obesity and obesity-associated metabolic diseases are increasing rapidly, posing a huge challenge to the society. Obesity is associated with chronic, low grade inflammation in metabolically active tissues, particularly in adipose tissue and the liver [2], [3], [4], [5]. The liver produces glucose from glycerol, pyruvate, lactate, and amino acids through hepatic gluconeogenesis. During periods of fasting and starvation, glucose generated by the liver serves as a critical metabolic fuel for extrahepatic tissues, particularly the brain and red blood cells [6]. In the postprandial states, food-derived glucose stimulates pancreatic β cells to secret insulin which suppresses hepatic gluconeogenesis and hepatic glucose production, thus preventing hyperglycemia [6]. Obesity is associated with hepatic insulin resistance and nonalcoholic fatty liver disease (NAFLD) [7]. Liver insulin resistance contributes to increased hepatic glucose production, hyperglycemia, and glucose intolerance in obese states [6]. Numerous pro-inflammatory mediators, including various cytokines, damage-associated molecular patterns (DAMPs), and pathogen-associated molecular patterns (PAMPs), are believed to promote insulin resistance in obesity [2], [3], [4], [8], [9]; however, the intracellular signaling pathways, which link these factors to insulin resistance, remain largely unknown.
We recently identified TNF receptor-associated factor 3 (TRAF3) in myeloid cells as an important mediator of high fat diet (HFD)-induced insulin resistance in mice [10]. Myeloid cell-specific deletion of TRAF3 attenuates insulin resistance and hepatic steatosis in mice with either HFD-induced obesity or genetic obesity (ob/ob) [10]. TRAF3 is a ubiquitously expressed cytoplasmic adapter protein which mediates/modulates cytokine, DAMP, and PAMP signaling [11], [12], [13], [14], [15], [16]. In B lymphocytes and myeloid cells, TRAF3 inhibits NF-κB-inducing kinase (NIK) and the NIK-activated noncanonical NF-κB2 pathway by promoting ubiquitination and degradation of NIK [17]. A subset of cytokines stimulates ubiquitination and degradation of TRAF3, thus activating NIK and the noncanonical NF-κB2 pathway [11]. In myeloid cells, TRAF3 also binds to and activates TBK1 and IKKi in response to cytokines, DAMPs, and/or PAMPs; TBK1/IKKi in turn activate the IRF3/IRF7 pathways, thus stimulating the expression of type 1 interferons and IL-10 [12], [13], [14]. Furthermore, TRAF3 is able to inhibit the canonical NF-κB1 and the MAPK pathways under specific cellular contexts [18], [19], [20], [21], [22], [23]. In immune cells, TRAF3 is able to regulate the expression and secretion of either pro-inflammatory or anti-inflammatory cytokines, depending on body weight and adiposity [10]. However, the function of TRAF3 in metabolic tissues remains unclear.
In this study, we demonstrate that TRAF3 levels in the liver are aberrantly higher in obese mice, presumably due to hyperglycemia. We generated and metabolically characterized mice with hepatocyte-specific deletion of TRAF3. Hepatocyte-specific TRAF3 knockout mice are protected against obesity-associated insulin resistance, glucose intolerance, and hepatic steatosis. We defined a glucose-TRAF3 reinforcement loop in the liver and examined its contribution to the progression of type 2 diabetes and NAFLD in obesity.
2. Material and methods
2.1. Mice
Animal experiments were conducted following the protocols approved by the University Committee on the Use and Care of Animals (UCUCA). TRAF3f/f mice were provided by Dr. Robert Brink (Garvan Institute of Medical Research, Australia). TRAF3f/f mice were crossed with albumin-cre mice (Jackson laboratory, Bar Harbor, ME) to obtain HKO mice (in C57BL/6 genetic background). HKO mice were crossed with ob/+ mice (Jackson laboratory, Bar Harbor, ME) to obtain DKO mice. Mice were housed on a 12 h light–dark cycle in the Unit for Laboratory Animal Medicine at the University of Michigan, and fed ad libitum either a normal chow diet (9% fat in calories; TestDiet, St. Louis, MO) or a HFD (60% fat in calories; Research Diets, New Brunswick, NJ).
2.2. Adenoviral infection and streptozotocin (STZ) treatments
TRAF3f/f and ob/ob;TRAF3f/f male mice were infected with GFP or Cre adenoviruses (1 × 1011 viral particles/mouse) via tail vein injection. WT C57BL/6 male mice were infected with TRAF3 or GFP adenoviruses at 3.8 × 1011 particles/mouse. STZ was dissolved in 0.1 M citrate buffer (pH 4.5). WT C57BL/6 male mice (10 weeks) were intravenously injected with STZ at 100 mg/kg body weight, and then at 80 mg/kg one week later. The liver was harvested 4 weeks after STZ injection.
2.3. Blood chemistry and glucose (GTT), insulin (ITT), pyruvate (PTT), and lactate (LTT) tolerance tests
Blood samples were collected from tail veins. Plasma insulin was measured using mouse insulin ELISA kits (CRYSTAL CHEM, Downers Grove, IL). For GTTs, PTTs, and LTTs, mice were fasted overnight (Figure 2, Figure 3, Figure 4) or 6 h (Figure 7) and intraperitoneally injected with d-glucose, pyruvate, or lactate. For ITTs, mice were fasted for 6 h and intraperitoneally injected with human insulin. Blood glucose was measured 0, 15, 30, 60, and 120 min after injection.
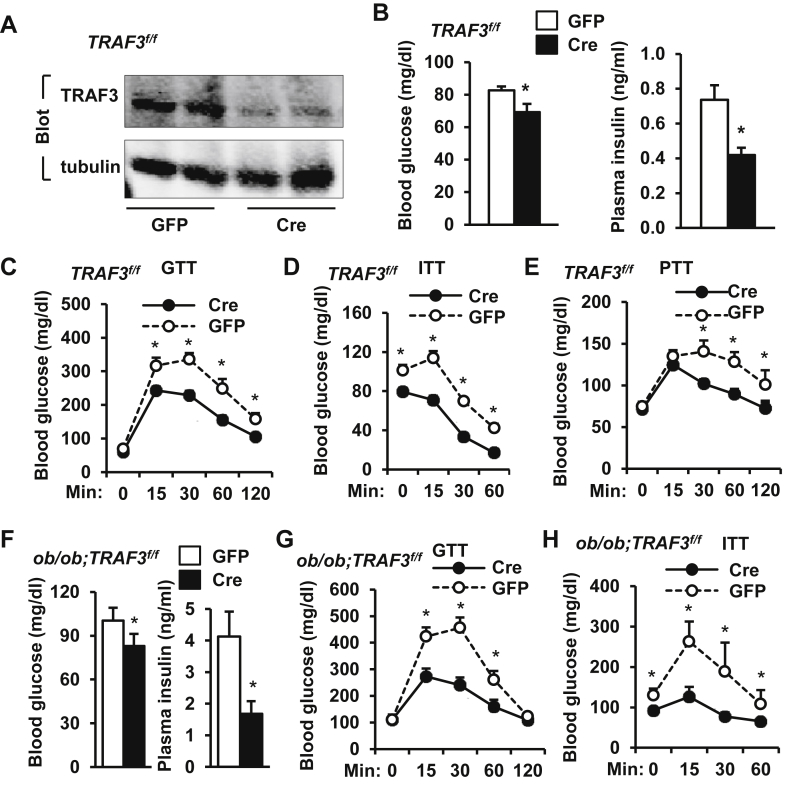
Figure 2.
Adult onset, liver-specific deletion of TRAF3 attenuates insulin resistance and glucose intolerance in obese mice. A–E.TRAF3f/f male mice (7 weeks) were fed a HFD for 8 weeks and infected with GFP (n = 12) or Cre (n = 11) adenoviruses via tail vein injection. A. Liver extracts were prepared 30 days after infection and immunoblotted with antibodies against TRAF3 or tubulin. B. Overnight-fasted blood glucose and insulin 25 days after infection. C. GTTs (glucose: 2 g/kg body weight) were performed 10 days after infection. D. ITTs (insulin: 1 unit/kg) 13 days after infection. E. PTTs (pyruvate: 2 g/kg) 21 days after infection. F–H.ob/ob;TRAF3f/f male mice (8–9 weeks) were infected with GFP (n = 7) or Cre (n = 8) adenoviruses via tail vein injection. F. Overnight-fasted blood glucose and insulin 8 days after infection. G. GTTs (glucose: 0.5 g/kg) were formed 16 days after infection. H. ITTs (insulin: 2 unit/kg) were performed 14 days after infection. *p < 0.05.
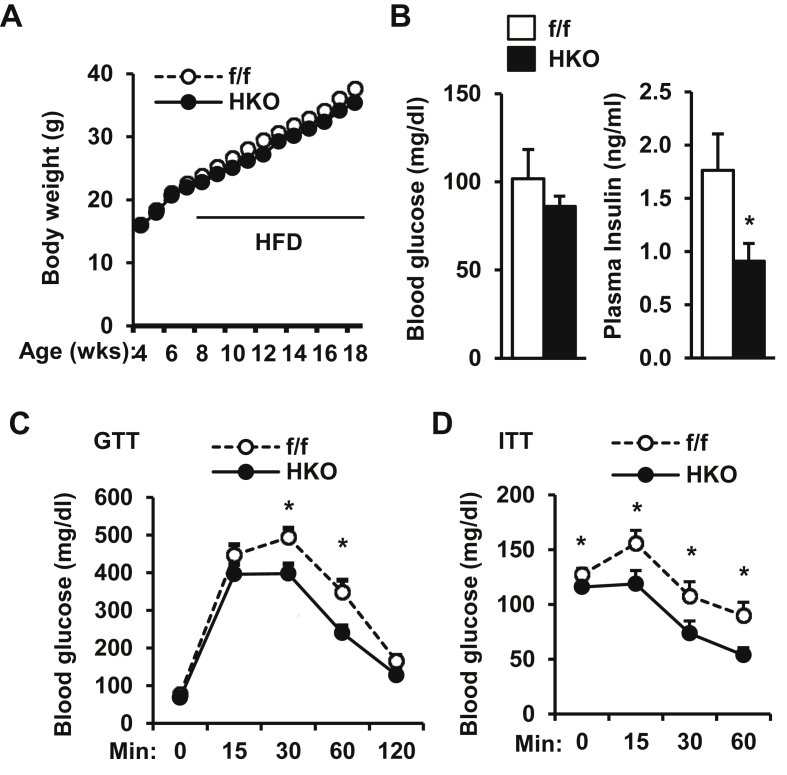
Figure 3.
Embryonic onset, hepatocyte-specific deletion of TRAF3 prevents HFD-induced insulin resistance and glucose intolerance. HKO (n = 11) and TRAF3f/f (n = 11–12) male mice (7–8 weeks) were fed a HFD. A. Growth curves. B. Overnight-fasted blood glucose and insulin. C. GTTs (glucose: 2 g/kg body weight). D. ITTs (insulin: 1 unit/kg). B–D: mice fed a HFD for 10–12 weeks. *p < 0.05.
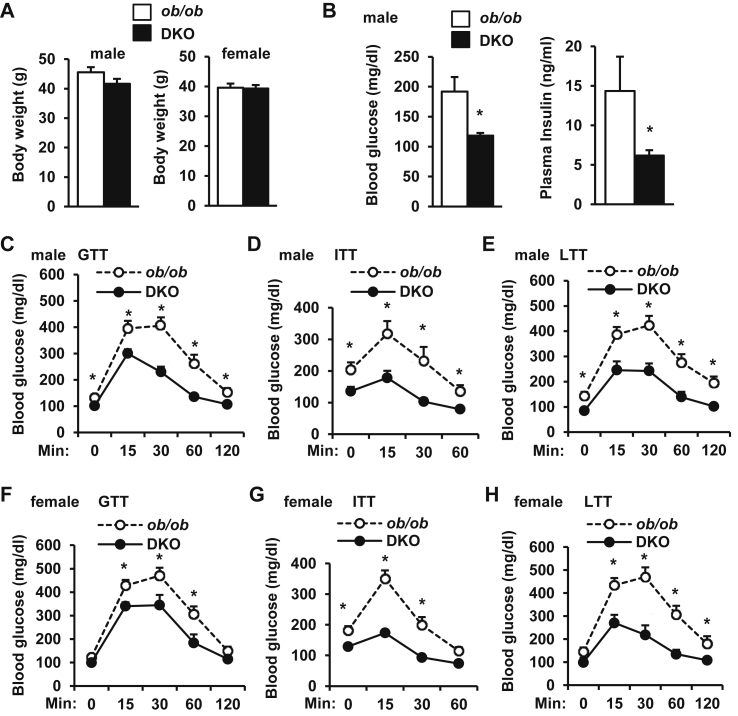
Figure 4.
Embryonic onset, hepatocyte-specific deletion of TRAF3 attenuates insulin resistance and glucose intolerance in ob/ob mice. A. Body weight at 10 weeks of age. B. Overnight-fasted blood glucose and insulin at 10 weeks of age. C and F. GTTs (glucose: 0.5 g/kg body weight) at 8 weeks of age. D and G. ITTs (insulin: 4 unit/kg) at 8 weeks of age. E and H. LTTs (lactate: 1 g/kg) at 10 weeks of age. DKO males: n = 10–13, DKO females: n = 8, ob/ob; TRAF3f/f males: n = 15–16, females: n = 14–16. *p < 0.05.
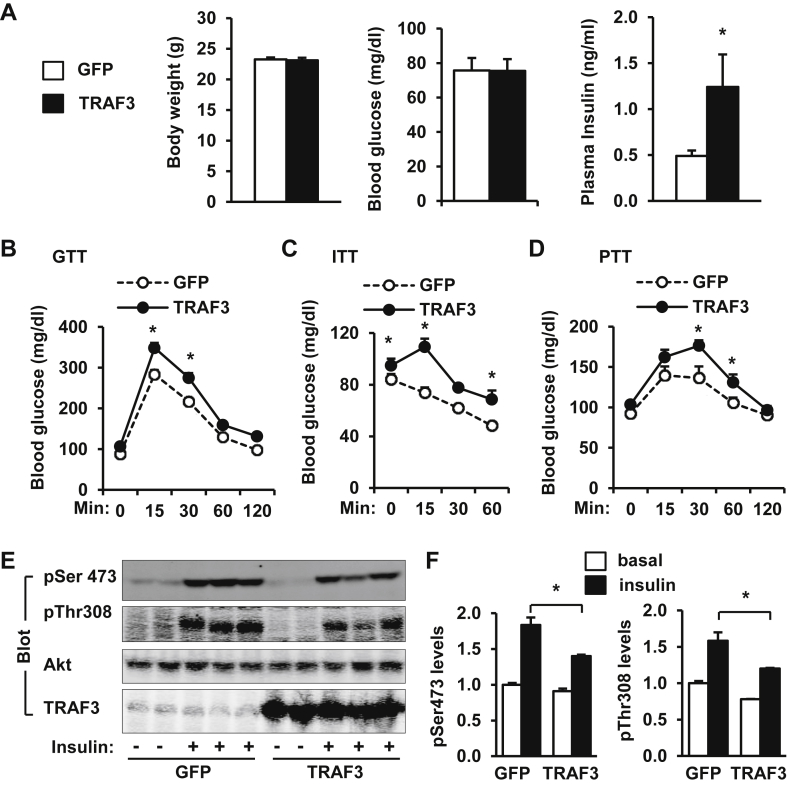
Figure 7.
Overexpression of recombinant TRAF3 in the liver induces insulin resistance and glucose intolerance in lean mice. C57BL/6 male mice (8–9 weeks) were infected with GFP or TRAF3 adenoviruses via tail vein injection. A. Body weight and overnight-fasted blood glucose and insulin levels 14 days after adenoviral infection. B–D. GTTs (glucose: 2 g/kg body weight), ITTs (insulin: 0.5 unit/kg), and PTTs (pyruvate: 2 g/kg) were performed 6–10 days after infection. E. Fourteen days after infection, mice were fasted for 20–24 h and stimulated with insulin (0.75 units/kg) for 5 min. Liver extracts were immunoblotted with antibodies against phospho-Akt (pThr308 and pSer473), Akt, or TRAF3. F. Phosphorylation of Akt was quantified and normalized to total Akt levels. *p < 0.05.
2.4. Immunoprecipitation, immunoblotting, and staining
Tissues or cells were homogenized in a lysis buffer (50 mM Tris HCl, pH 7.5, 1.0% NP-40, 150 mM NaCl, 2 mM EGTA, 1 mM Na3VO4, 100 mM NaF, 10 mM Na4P2O7, 1 mM PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin). Tissue/cell extracts were immunoblotting with antibodies against TRAF3 (Santa Cruz, sc-947; Santa Cruz, sc-949), tubulin (Santa Cruz, sc-5286), phospho-Akt pThr308 (Cell Signaling, #4056; Santa Cruz, sc-16646-R), phospho-Akt pSer473 (Cell Signaling, #4060), or Akt (Cell signaling, #4691). Liver paraffin sections were subjected to H&E staining. Liver frozen sections were stained with Oil red O (0.5% in propylene glycol) & Hematoxylin (0.6% weight/).
2.5. Liver triacylglycerol (TAG) levels
Liver samples were homogenized in 1% acetic acid, and lipids were extracted using 80% chloroform/Methanol (2:1). The organic fractions were dried in chemical hood, resuspended in 3 M KOH, incubated at 70 °C for 1 h, mixed with MgCl2 (0.75 M), and centrifuged. The aqueous fractions were used to measure TAG levels using Free Glycerol Reagent (Sigma).
2.6. Primary hepatocyte cultures
Primary hepatocyte cultures were prepared by liver-perfusion with type II collagenase (Worthington Biochem, Lakewood, NJ) and grown on collagen-coated plates as described previously [24]. For glucose stimulation experiments, hepatocytes were incubated overnight in serum-free DMEM supplemented with 0, 25, or 100 mM glucose.
2.7. Quantitative real time RT-PCR (qPCR)
Total RNAs were extracted using TRIzol reagent (Invitrogen life technologies, Carlsbad, CA). The first-strand cDNAs were synthesized using random primers and M-MLV reverse transcriptase (Promega, Madison, WI). qPCR was performed using Absolute QPCR SYBR Mix (Thermo Fisher Scientific, UK) and Mx3000P real time PCR system (Stratagene, LA Jolla, CA). qPCR primers were: TRAF3 forward: 5′-TGAGCTGGAGAGCGTAGACA-3′, reverse: 5′-AGATCAGCACCCCGTTGTAG-3′; IL-1β forward: 5′-GCCTTGGGCCTCAAAGGAAAGAATC-3′, reverse: 5′-GGAAGACACAGATTCCATGGTGAAG-3′; IL-6 forward: 5′-AGCCAGAGTCCTTCAGA-3′, reverse: 5′-GGTCCTTAGCCACTCCT-3′; IL-10 forward: 5′-CTGGACAACATACTGCTAACCG-3′, reverse: 5′-GGGCATCACTTCTACCAGGTAA-3′; TNFα forward: 5′-CATCTTCTCAAAATTCGAGTGACAA-3′, reverse: 5′-TGGGAGTAGACAAGGTACAACCC-3′; MCP1 forward: 5′-ACTGAAGCCAGCTCTCTCTTCCTC-3′, reverse: 5′-TTCCTTCTTGGGGTCAGCACAGAC-3′; 36B4 forward: 5′-AAGCGCGTCCTGGCATTGTCT-3′, reverse: 5′-CCGCAGGGGCAGCAGTGGT-3′; β-actin forward: 5′-AAATCGTGCGTGACATCAAA-3′, reverse: 5′-AAGGAAGGCTGGAAAAGAGC-3′.
2.8. Statistical analysis
Data were presented as means ± SEM. Differences between groups were analyzed by two-tailed Student's t tests. P < 0.05 was considered statistically significant.
3. Results
3.1. TRAF3 levels in the liver are regulated by the metabolic state and are aberrantly higher in obese mice
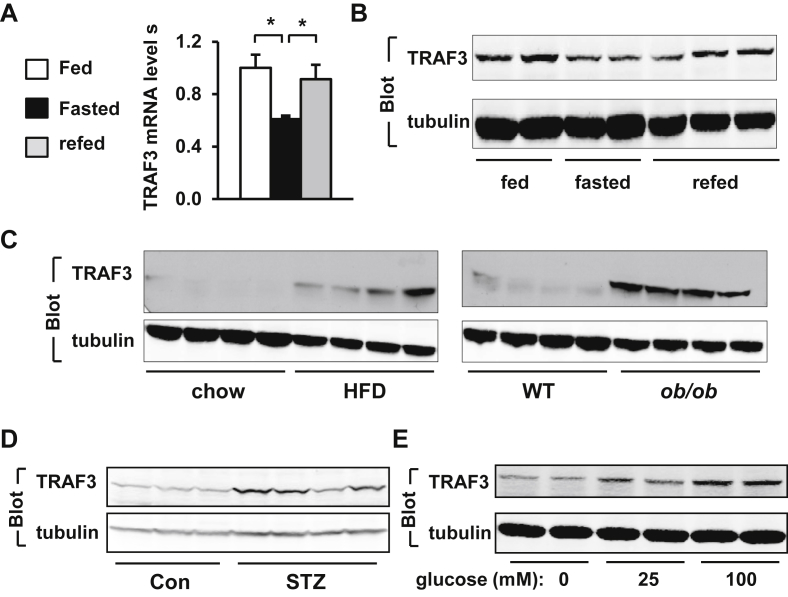
To determine whether the metabolic state affects the expression of TRAF3 in the liver, male mice were fasted for 24 h and refed a normal chow diet for 3 h. The levels of TRAF3 mRNA and protein in the liver were measured by qPCR and immunoblotting, respectively. Fasting (24 h) lowered the levels of both TRAF3 mRNA and protein, and refeeding largely reversed fasting-induced reduction of TRAF3 in the liver (Figure 1A–B). Liver TRAF3 levels appear to be regulated by nutritional signals.
Figure 1.
TRAF3 levels in the liver are aberrantly higher in obese mice. A. C57BL/6 male mice (8–9 weeks) were fasted for 24 h and then refed for 3 h. Liver TRAF3 mRNA abundance was measured by qPCR and normalized to β-actin expression. Randomly-fed: n = 8, fasted: n = 8, refed: n = 8. B. C57BL/6 male mice were fasted and refed as described above. Liver extracts were immunoblotted with antibodies against TRAF3 or tubulin. C. C57BL/6 male mice (7–8 weeks) were fed a chow diet or HFD for 10 weeks. WT and ob/ob mice (13 weeks) were fed a chow diet. Liver extracts were immunoblotted with antibodies against TRAF3 or tubulin. D. C57BL/6 male mice (10 weeks) were injected with STZ. Liver extracts were prepared 4 weeks after STZ treatments and immunoblotted with antibodies against TRAF3 or tubulin. E. Primary hepatocytes were prepared from C57BL/6 males (8–9 weeks) and grown overnight in serum-free DMEM supplemented with 0, 25 or 100 mM G-glucose. Cell extracts were immunoblotted with the indicated antibodies. *p < 0.05.
To determine whether overnutrition increases TRAF3 levels in the liver, C57BL/6 male mice were fed a HFD for 10 weeks, and liver TRAF3 protein levels were measured by immunoblotting. Mice fed a HFD developed obesity as revealed by heavier body weight (normal chow: 28.1 ± 0.6 g, n = 6; HFD: 43.3 ± 1.8 g, n = 6). Liver TRAF3 levels were higher in the HFD group than in the normal chow diet group (Figure 1C). To determine whether genetic obesity is associated with aberrant expression of hepatic TRAF3, liver extracts were prepared from leptin-deficient ob/ob (body weight: 56.5 ± 0.6 g, n = 6) and wild type (WT) male mice, and immunoblotted with antibody against TRAF3. TRAF3 protein levels were also markedly higher in ob/ob than in WT mice (Figure 1C).
Blood glucose levels are lower in the fasting states, and are higher in obese mice, raising the possibility that blood glucose may increase the levels of hepatic TRAF3 in vivo. To test this idea, male mice were treated with streptozotocin (STZ) to induce hyperglycemia. STZ is a β-cell toxin and induces hyperglycemia through destroying pancreatic β cells [25]. Four weeks after STZ injection, STZ-treated, but not citrate vehicle-treated mice, developed hyperglycemia (STZ: 523.3 ± 19.5 mg/dl, n = 4; citrate vehicle: 123.0 ± 7.5 mg/dl, n = 4). STZ treatments markedly increased TRAF3 protein levels in the liver (Figure 1D). To determine whether glucose is able to directly increase TRAF3 levels, primary hepatocytes were incubated with increasing concentrations of glucose. Glucose treatment dose-dependently increased TRAF3 protein levels in hepatocytes (Figure 1E). Glucose did not significantly change TRAF3 mRNA levels as measured by qPCR (0 mM glucose: 1.02 ± 0.12, 100 mM glucose: 1.01 ± 0.07, n = 3, p = 0.96), raising the possibility that glucose may increase TRAF3 protein stability. Palmitic acid (50 μM), TNFα (6 ng/ml), and H2O2 (10 μM) were unable to increase TRAF3 levels in hepatocytes (data not shown). Together, these results suggest that hyperglycemia increases the levels of hepatic TRAF3 in obese mice.
3.2. Deletion of liver TRAF3 in adult obese mice attenuates insulin resistance, hyperglycemia, and glucose intolerance
To determine whether aberrant levels of hepatic TRAF3 contribute to metabolic disorders in obesity, we generated and metabolically characterized mice with liver-specific deletion of TRAF3. TRAF3f/f mice, in which the TRAF3 gene is flanked by two loxp sites, have been described previously [10], [26]. TRAF3f/f male mice were fed a HFD for 8 weeks to induce obesity. To delete TRAF3 specifically in the liver, obese TRAF3f/f mice were infected with Cre adenoviruses via tail vein injection, and green fluorescent protein (GFP) adenoviruses were used as control. Cre adenoviral infection is commonly used to generate liver-specific deletion of target genes [27]. Liver TRAF3 protein levels were ∼81% lower in the Cre group than in the GFP group (Figure 2A). Body weight was similar between the GFP (30.3 ± 1.2 g, n = 12) and the Cre (29.8 ± 0.9 g, n = 11) groups. Both overnight fasting blood glucose and insulin levels were significantly lower in the Cre group (Figure 2B). To further analyze insulin sensitivity and glucose metabolism, we performed glucose tolerance (GTT), insulin tolerance (ITT), and pyruvate tolerance (PTT) tests on mice 10–21 days after infection. Liver-specific deletion of TRAF3 significantly improved glucose intolerance and insulin resistance (Figure 2C–D) and reduced hepatic gluconeogenesis in these obese mice (Figure 2E). Blood glucose increased 15 min after insulin injection (Figure 2D), presumably due to a combination of insulin resistance and stress response to injection.
To determine whether acute deletion of liver TRAF3 similarly improves insulin resistance and glucose intolerance in mice with genetic obesity, TRAF3f/f mice were crossed with ob/+ mice to generate ob/ob;TRAF3f/f double mutant mice. Adult ob/ob;TRAF3f/f male mice (8–9 weeks) were infected with Cre adenoviruses via tail vein injection to delete TRAF3 specifically in the liver. Deletion of liver TRAF3 significantly decreased overnight fasting blood glucose and insulin (Figure 2F), and markedly improved glucose intolerance and insulin resistance (Figure 2G–H) in ob/ob;TRAF3f/f mice. Together, these data indicate that inhibition of liver TRAF3 is sufficient to improve insulin resistance and glucose intolerance in both dietary and genetic obesity.
3.3. Embryonic onset, hepatocyte-specific deletion of TRAF3 protects against obesity-associated insulin resistance and glucose intolerance
To determine whether TRAF3 deficiency in hepatocytes is able to prevent obesity-induced insulin resistance, hepatocyte-specific TRAF3 knockout (HKO) mice were generated by crossing TRAF3f/f mice with albumin-Cre transgenic mice. TRAF3 was deleted specifically in the liver but not in skeletal muscle and adipose tissue (Supplemental Fig. 1). Blood glucose, plasma insulin, GTT, and ITT were similar between HKO and TRAF3f/f (control) mice fed a normal chow diet (data not shown), indicating that hepatocyte TRAF3 is dispensable under normal conditions. HKO and TRAF3f/f male mice (7–8 weeks) were fed a HFD, and body weight was similar between these two groups (Figure 3A). Overnight fasting insulin levels were significantly lower in HKO mice fed a HFD for 10 weeks (Figure 3B). Obese HKO mice displayed improvement in glucose intolerance and insulin resistance relative to TRAF3f/f mice (Figure 3C–D).
To determine whether embryonic onset, hepatocyte-specific deletion of TRAF3 also prevents insulin resistance and glucose intolerance in ob/ob mice, HKO mice were crossed with ob/+ mice to generate leptin/TRAF3 double mutant mice (DKO) (genotype: ob/ob;TRAF3f/f;albumin-Cre+/−). ob/ob;TRAF3f/f (designated as ob/ob) mice were used as control. Body weight was comparable between DKO and ob/ob mice (Figure 4A), but overnight fasting blood glucose and insulin levels were significantly lower in DKO male mice (Figure 4B). Deletion of hepatic TRAF3 markedly improved glucose intolerance and insulin resistance in DKO male mice (Figure 4C–D). Pyruvate treatment in ob/ob mice was lethal, so we performed lactate tolerance tests (LTT) instead to estimate hepatic gluconeogenesis. Hepatic gluconeogenesis was lower in DKO male mice (Figure 4E). Similar to male DKO mice, embryonic onset, hepatocyte-specific deletion of TRAF3 also markedly improved glucose intolerance and insulin resistance (Figure 4F–G) and decreased hepatic gluconeogenesis (Figure 4H) in female DKO mice. Therefore, early inactivation of hepatic TRAF3 has a protective effect against obesity-induced insulin resistance and glucose intolerance.
3.4. Deletion of hepatic TRAF3 ameliorates obesity-associated hepatic steatosis
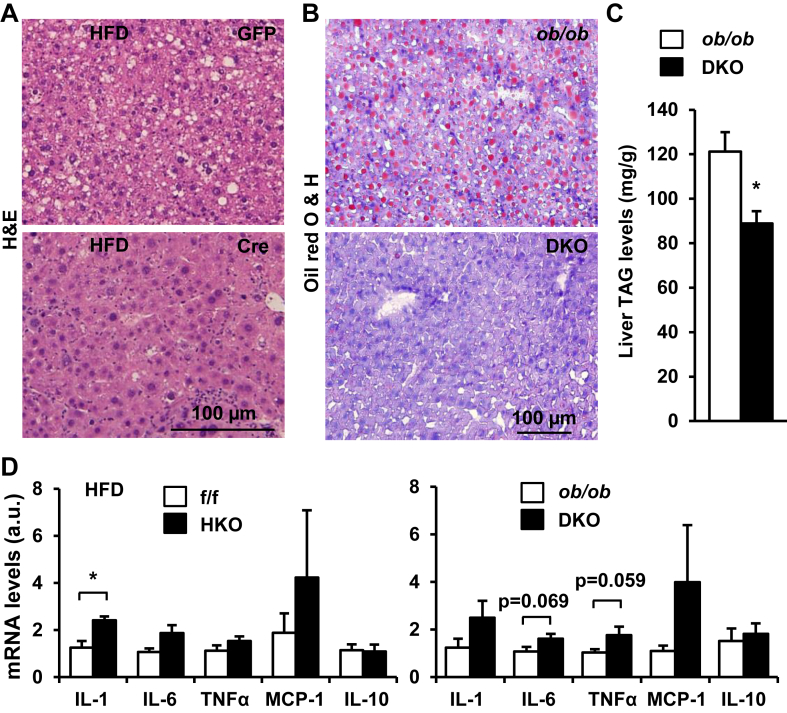
Obesity is a risk factor for NAFLD [7]. To determine whether hepatic TRAF3 is involved in NAFLD development, TRAF3f/f male mice (7 weeks) were fed a HFD for 8 weeks to induce obesity and NAFLD, and then infected with Cre adenoviruses for 30 days to delete TRAF3 in the liver. Lipid droplets (LDs) were visualized by H&E staining of liver sections, and liver triacylglycerol (TAG) content were measured chemically. Numerous LDs were detected in the livers of GFP adenovirus-infected control mice, and deletion of liver TRAF3 markedly decreased LD abundance in the livers of Cre adenovirus-infected mice (Figure 5A). Liver TAG levels were lower in the Cre than in the GFP groups (data not shown). To determine whether deletion of hepatocyte TRAF3 ameliorates hepatic steatosis in mice with genetic obesity, we measured liver lipid content in DKO and ob/ob;TRAF3f/f male mice (11 weeks). LDs were visualized by Oil red O staining of liver sections. A large number of LDs were detected in the livers of ob/ob;TRAF3f/f mice, and hepatocyte-specific deletion of TRAF3 markedly reduced LD content in the livers of DKO mice (Figure 5B). In agreement, liver TAG levels were significantly lower in DKO than in ob/ob;TRAF3f/f mice (Figure 5C). These data suggest that aberrant hepatic TRAF3 levels contribute to NAFLD development.
Figure 5.
Aberrant hepatic TRAF3 promotes liver steatosis in obesity. A. H&E staining of liver sections. B. Oil red O & hematoxylin staining of liver sections. C. Liver TAG levels were measured chemically in DKO (n = 5) and ob/ob;TRAF3f/f (n = 4) mice (11 weeks) and normalized to liver weight. D. The expression of liver cytokines were measured by qPCR and normalized to 36B4 levels. TRAF3f/f: n = 6, HKO: n = 5, ob/ob; TRAF3f/f: n = 5, DKO: n = 5. *p < 0.05.
To determine whether inflammation is involved in mediating TRAF3 action in obesity, we examined the effect of hepatocyte-specific deletion of TRAF3 on the expression of various cytokines in the liver. HKO and TRAF3f/f (control) male mice were fed a HFD for 12 weeks, and the mRNA abundance of the cytokines were quantified by qPCR. Expression of pro-inflammatory cytokine interleukin (IL)-1β was significantly higher in HKO than in TRAF3f/f mice (Figure 5D). The expression of IL-6 and TNFα was also higher in DKO than in ob/ob;TRAF3f/f mice (Figure 5D). These data suggest that hepatic TRAF3 has anti-inflammation activity in the setting of obesity.
3.5. Hepatic TRAF3 negatively regulates insulin signaling in the liver
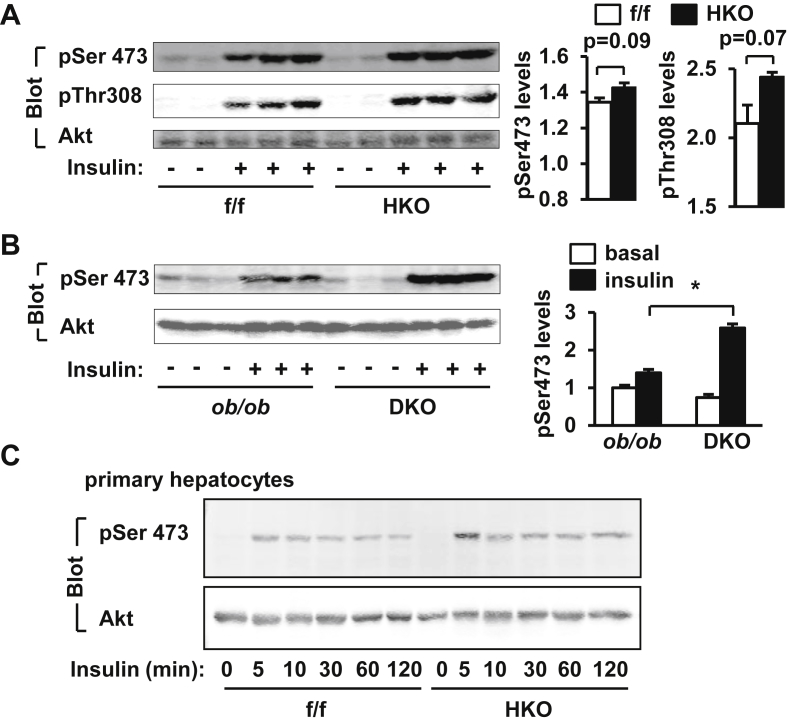
To examine the effect of hepatocyte-specific deletion of TRAF3 on insulin signal transduction, HKO and TRAF3f/f mice were fed a HFD for 10–12 weeks, fasted for 20–24 h, and stimulated with insulin (2 units/kg body weight) for 5 min. Insulin-stimulated phosphorylation of Akt (both pThr308 and pSer473) in the liver, and hepatocyte-specific deletion of TRAF3 further increased insulin-stimulated phosphorylation of hepatic Akt in HKO mice (Figure 6A). To determine whether hepatocyte-specific deletion of TRAF3 improves insulin signaling in ob/ob mice, DKO and ob/ob;TRAF3f/f mice were fasted for 20–24 h and injected with insulin (4 units/kg body weight), and insulin-stimulated phosphorylation of Akt was examined in the liver 5 min later. Insulin also stimulated phosphorylation of hepatic Akt in ob/ob;TRAF3f/f mice, and hepatocyte-specific deletion of TRAF3 further increased insulin-stimulated phosphorylation of hepatic Akt in DKO mice (pSer473) (Figure 6B). To further verify inhibition of insulin signaling by endogenous TRAF3, primary hepatocytes were prepared from HKO and TRAF3f/f mice and stimulated with insulin. Deletion of TRAF3 increased the ability of insulin to stimulate phosphorylation of Akt in cultured hepatocytes (Figure 6C). These results suggest that TRAF3 cell-autonomously inhibits insulin signal transduction in hepatocytes.
Figure 6.
TRAF3 negatively regulates insulin signaling in the liver. A.TRAF3f/f and HKO male mice (7–8 weeks) were fed a HFD for 10–12 weeks, fasted for 20–24 h, and injected with insulin (2 units/kg body weight) via inferior vena. Liver extracts were prepared 5 min after insulin stimulation and immunoblotted with antibodies against phospho-Akt (pThr308 and pSer473) or Akt. Insulin-stimulated phosphorylation of Akt was quantified and normalized to total Akt levels. B. DKO and ob/ob;TRAF3f/f male mice (10 weeks) were fasted overnighted and stimulated with insulin (4 units/kg) for 5 min. Liver extracts were immunoblotted with antibodies against phospho-Akt (pSer473) or Akt. Phosphorylation of Akt was quantified and normalized to total Akt levels. C. Primary hepatocytes were prepared from TRAF3f/f and HKO male mice (8–9 weeks) and stimulated with 100 nM insulin for the indicated time. Cell extracts were immunoblotted with antibodies against phospho-Akt (pSer473) or Akt. *p < 0.05.
3.6. Liver-specific overexpression of recombinant TRAF3 induces insulin resistance and glucose intolerance in lean mice
To further determine whether aberrant overexpression of TRAF3 in the liver is a causal factor for insulin resistance, C57BL/6 male mice (8–9 weeks) were infected with TRAF3 or GFP (control) adenoviruses via tail vein injection. Liver-specific overexpression of TRAF3 did not alter body weight and blood glucose levels, but significantly increased plasma insulin levels (Figure 7A). To further analyze insulin sensitivity and glucose metabolism, we performed GTT, ITT, and PTT. Liver-specific overexpression of TRAF3 induced glucose intolerance and insulin resistance (Figure 7B–C) and increased hepatic gluconeogenesis (Figure 7D). Fourteen days after infection, mice were fasted overnight and injected with insulin (0.75 U/kg body weight). Liver extracts were prepared 5 min after insulin stimulation and immunoblotted with antibodies against phospho-Akt, Akt, or TRAF3. Insulin robustly stimulated phosphorylation of Akt (pThr308 and pSer473), and overexpression of TRAF3 suppressed insulin-stimulated phosphorylation of Akt (Figure 7E–F). TRAF3 was overexpressed specifically in the liver but not in the skeletal muscle, adipose tissue and brain (Figure 7E and data not shown). These data further support the concept that elevated levels of hepatic TRAF3 is a causal factor for insulin resistance and glucose intolerance in obesity.
4. Discussion
Metabolic inflammation has been extensively examined in adipose tissue, but liver inflammation and its contribution to glucose metabolism are not fully understood. In the current study, we identified hepatic TRAF3 as a new linker between inflammation, insulin resistance, and glucose intolerance in obesity. In addition to mediating immune responses, TRAF3 may also act as a metabolic regulator in the setting of obesity and is involved in type 2 diabetes and NAFLD progression.
We have provided several lines of evidence that support a critical metabolic function of hepatic TRAF3. First, the levels of hepatic TRAF3 were regulated by the metabolic state. Liver TRAF3 levels were lower in the fasted state, and increased after refeeding. Second, glucose, the principal metabolic fuel, directly increased TRAF3 levels in hepatocytes, so hepatic TRAF3 may be a component of the nutrient sensing system. Third, liver TRAF3 levels were aberrantly higher in mice with either HFD-induced obesity or genetic (ob/ob) obesity. Hyperglycemia is likely to be an important stimulator which increases liver TRAF3 levels in obesity. Forth, deletion of hepatocyte TRAF3 ameliorated hyperinsulinemia, glucose intolerance, hepatic steatosis, and insulin resistance in mice with either HFD-induced obesity or genetic obesity. Fifth, overexpression of recombinant TRAF3 in the liver impaired insulin signaling and the ability of insulin to reduce blood glucose, leading to hyperinsulinemia and glucose intolerance in lean mice. Together, these data provide powerful evidence supporting that aberrant expression of hepatic TRAF3 is an important causal factor for insulin resistance, type 2 diabetes, and NAFLD in obesity.
We used two approaches to delete hepatocyte TRAF3 in mice. In the first approach, TRAF3f/f mice were fed a HFD to induce obesity and insulin resistance, and then infected with Cre adenoviruses to conditionally delete liver TRAF3 in obese mice. This approach addresses a concern about potential developmental compensations as observed in other animal models. The results demonstrate that inhibition of liver TRAF3 has a curative effect against insulin resistance, glucose intolerance, and hepatic steatosis in obese mice. Cre adenoviral infection may cause liver inflammation, thus complicating data interpretation; therefore, GFP adenoviral infection was used as control to address this concern. In the second approach, HKO mice were generated by crossing TRAF3f/f mice with albumin-Cre drivers. The results demonstrate that early inactivation of liver TRAF3 has a preventive effect against the development of insulin resistance and type 2 diabetes. These two approaches complemented each other, and provided convincing evidence to support the concept that aberrantly-high levels of hepatic TRAF3 is a risk factor for insulin resistance, type 2 diabetes, and NAFLD. We also examined both dietary and genetic obesity models in which liver TRAF3 levels were abnormally higher. Deletion of hepatic TRAF3 attenuated insulin resistance, glucose intolerance, and hepatic steatosis in both models, suggesting that aberrantly higher levels of hepatic TRAF3 serve as a common pathway to induce insulin resistance, glucose intolerance, and NAFLD in various forms of obesity with different etiologies. However, our data do not exclude the possibility that TRAF3 may regulate hepatic glucose production and lipid metabolism through an insulin-independent mechanism.
The molecular details of TRAF3 action are currently unknown. TRAF3 is a negative regulator of NIK. We previously reported that inhibition of NIK in the liver attenuates hepatic glucose production and protects against obesity-associated hyperglycemia and glucose intolerance [28]. Mechanistically, NIK enhances the ability of glucagon to stimulate gluconeogenesis in hepatocytes, thus coupling inflammation to glucose counterregulation in obesity [28]. NIK activation does not affect insulin signaling [28], so NIK is unlikely to mediate TRAF3 action in the liver. TRAF3 binds to TRAF2 [29], [30], and deletion of TRAF2 in hepatocytes attenuates HFD-induced hyperglycemia [31]. TRAF2 is able to enhance glucagon response, but it does not affect insulin action in the liver [31]; thus, TRAF2 is also unlikely to mediate the metabolic action of hepatic TRAF3. Myeloid cell TRAF3 suppresses inflammation in lean mice, but it switches its mode of action from anti-inflammation to pro-inflammation in obesity [10]. In contrast, hepatic TRAF3 appears to retain its anti-inflammatory effect in obesity. In agreement with this, deletion of hepatocyte TRAF3 decreased the expression of pro-inflammatory cytokines in mice with either HFD-induced or genetic obesity. Thus, hepatic TRAF3 action on glucose and lipid metabolism cannot be explained by its regulation of liver inflammation in obesity.
We observed that glucose stimulation directly increased TRAF3 levels in primary hepatocytes. Hyperglycemia was associated with an increase in hepatic TRAF3 levels. It will be interesting to determine whether ChREBP, a glucose sensor, mediates the effect of glucose on TRAF3 levels. Elevated levels of hepatic TRAF3 in turn promoted hyperglycemia by increasing hepatic gluconeogenesis, thus forming a glucose-TRAF3 reinforcement loop in the liver. The glucose-TRAF3 positive feedback loop may be further activated and/or amplified by additional obesity-associated factors, and may play an important role in driving type 2 diabetes and NAFLD progression.
5. Conclusions
We have identified hepatic TRAF3 as a new metabolic regulator in obese mice. Liver TRAF3 levels are abnormally higher in obesity, presumably due to hyperglycemia. Hepatocyte-specific deletion of TRAF3 attenuates insulin resistance, glucose intolerance, and hepatic steatosis in obese mice. Obesity-associated factors trigger and/or amplify a glucose-TRAF3 reinforcement loop in the liver, which is likely to drive type 2 diabetes and NAFLD progression. Therefore, disrupting this glucose-TRAF3 positive feedback loop may serve as a new strategy to treat type 2 diabetes and NAFLD.
Funding
This study was supported by grants DK091591 and DK094014 (to L.R.) from the National Institutes of Health. ZC is currently supported by National Natural Science Foundation of China Grant 31500957. This work utilized the cores supported by the Michigan Diabetes Research and Training Center (NIH DK020572), Michigan Metabolomics and Obesity Center (DK089503), the University of Michigan's Cancer Center (NIH CA46592), the University of Michigan Nathan Shock Center (NIH P30AG013283), and the University of Michigan Gut Peptide Research Center (NIH DK34933).
Acknowledgments
We thank Drs. Hong Shen, Yan Liu, and Chengxin Sun for assistance and discussion. We thank Dr. Robert Brink for providing us with TRAF3f/f mice.
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.molmet.2015.09.013.
Conflict of interest
None declared.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Rui L. Brain regulation of energy balance and body weight. Reviews in Endocrine & Metabolic Disorders. 2013;14:387–407. doi: 10.1007/s11154-013-9261-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 3.Shoelson S.E., Goldfine A.B. Getting away from glucose: fanning the flames of obesity-induced inflammation. Nature Medicine. 2009;15:373–374. doi: 10.1038/nm0409-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lumeng C.N., Saltiel A.R. Inflammatory links between obesity and metabolic disease. Journal of Clinical Investigation. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuan M., Konstantopoulos N., Lee J., Hansen L., Li Z.W., Karin M. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 6.Rui L. Energy metabolism in the liver. Comprehensive Physiology. 2014;4:177–197. doi: 10.1002/cphy.c130024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chawla A., Nguyen K.D., Goh Y.P. Macrophage-mediated inflammation in metabolic disease. Nature Reviews Immunology. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glass C.K., Olefsky J.M. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metabolism. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z., Shen H., Sun C., Yin L., Tang F., Zheng P. Myeloid cell TRAF3 promotes metabolic inflammation, insulin resistance, and hepatic steatosis in obesity. American Journal of Physiology Endocrinology and Metabolism. 2015;308:E460–E469. doi: 10.1152/ajpendo.00470.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun S.C. The noncanonical NF-kappaB pathway. Immunological Reviews. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hacker H., Redecke V., Blagoev B., Kratchmarova I., Hsu L.C., Wang G.G. Specificity in toll-like receptor signalling through distinct effector functions of TRAF3 and TRAF6. Nature. 2006;439:204–207. doi: 10.1038/nature04369. [DOI] [PubMed] [Google Scholar]
- 13.Oganesyan G., Saha S.K., Guo B., He J.Q., Shahangian A., Zarnegar B. Critical role of TRAF3 in the toll-like receptor-dependent and -independent antiviral response. Nature. 2006;439:208–211. doi: 10.1038/nature04374. [DOI] [PubMed] [Google Scholar]
- 14.Hacker H., Tseng P.H., Karin M. Expanding TRAF function: TRAF3 as a tri-faced immune regulator. Nature Reviews Immunology. 2011;11:457–468. doi: 10.1038/nri2998. [DOI] [PubMed] [Google Scholar]
- 15.Saha S.K., Pietras E.M., He J.Q., Kang J.R., Liu S.Y., Oganesyan G. Regulation of antiviral responses by a direct and specific interaction between TRAF3 and Cardif. EMBO Journal. 2006;25:3257–3263. doi: 10.1038/sj.emboj.7601220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Watanabe T., Asano N., Fichtner-Feigl S., Gorelick P.L., Tsuji Y., Matsumoto Y. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. Journal of Clinical Investigation. 2010;120:1645–1662. doi: 10.1172/JCI39481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liao G., Zhang M., Harhaj E.W., Sun S.C. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. Journal of Biological Chemistry. 2004;279:26243–26250. doi: 10.1074/jbc.M403286200. [DOI] [PubMed] [Google Scholar]
- 18.Bista P., Zeng W., Ryan S., Bailly V., Browning J.L., Lukashev M.E. TRAF3 controls activation of the canonical and alternative NFkappaB by the lymphotoxin beta receptor. Journal of Biological Chemistry. 2010;285:12971–12978. doi: 10.1074/jbc.M109.076091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zarnegar B., Yamazaki S., He J.Q., Cheng G. Control of canonical NF-kappaB activation through the NIK-IKK complex pathway. Proceedings of the National Academy of Sciences U. S. A. 2008;105:3503–3508. doi: 10.1073/pnas.0707959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He L., Grammer A.C., Wu X., Lipsky P.E. TRAF3 forms heterotrimers with TRAF2 and modulates its ability to mediate NF-{kappa}B activation. Journal of Biological Chemistry. 2004;279:55855–55865. doi: 10.1074/jbc.M407284200. [DOI] [PubMed] [Google Scholar]
- 21.Zhu S., Pan W., Shi P., Gao H., Zhao F., Song X. Modulation of experimental autoimmune encephalomyelitis through TRAF3-mediated suppression of interleukin 17 receptor signaling. The Journal of Experimental Medicine. 2010;207:2647–2662. doi: 10.1084/jem.20100703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuzawa A., Tseng P.H., Vallabhapurapu S., Luo J.L., Zhang W., Wang H. Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science. 2008;321:663–668. doi: 10.1126/science.1157340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng P.H., Matsuzawa A., Zhang W., Mino T., Vignali D.A., Karin M. Different modes of ubiquitination of the adaptor TRAF3 selectively activate the expression of type I interferons and proinflammatory cytokines. Nature Immunology. 2010;11:70–75. doi: 10.1038/ni.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Y., Jiang L., Rui L. Identification of MUP1 as a regulator for glucose and lipid metabolism in mice. Journal of Biological Chemistry. 2009;284:11152–11159. doi: 10.1074/jbc.M900754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Z., Morris D.L., Jiang L., Liu Y., Rui L. SH2B1 in beta-cells regulates glucose metabolism by promoting beta-cell survival and islet expansion. Diabetes. 2014;63:585–595. doi: 10.2337/db13-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gardam S., Sierro F., Basten A., Mackay F., Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 27.Sheng L., Liu Y., Jiang L., Chen Z., Zhou Y., Cho K.W. Hepatic SH2B1 and SH2B2 regulate liver lipid metabolism and VLDL secretion in mice. PLoS One. 2013;8:e83269. doi: 10.1371/journal.pone.0083269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheng L., Zhou Y., Chen Z., Ren D., Cho K.W., Jiang L. NF-kappaB-inducing kinase (NIK) promotes hyperglycemia and glucose intolerance in obesity by augmenting glucagon action. Nature Medicine. 2012;18:943–949. doi: 10.1038/nm.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zarnegar B.J., Wang Y., Mahoney D.J., Dempsey P.W., Cheung H.H., He J. Noncanonical NF-kappaB activation requires coordinated assembly of a regulatory complex of the adaptors cIAP1, cIAP2, TRAF2 and TRAF3 and the kinase NIK. Nature Immunology. 2008;9:1371–1378. doi: 10.1038/ni.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vallabhapurapu S., Matsuzawa A., Zhang W., Tseng P.H., Keats J.J., Wang H. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nature Immunology. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Z., Sheng L., Shen H., Zhao Y., Wang S., Brink R. Hepatic TRAF2 regulates glucose metabolism through enhancing glucagon responses. Diabetes. 2012;61:566–573. doi: 10.2337/db11-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.