Abstract
Chronic pain is thought to affect patients’ cognitive functioning, including attention. Loss of attention is likely to have an impact on the execution of daily tasks, and, therefore, to have negative effects. However, relationships between chronic pain and cognitive deficits are still debated. Pre-clinical studies using laboratory animals prove useful to model pain-related cognitive impairment, but animal models had to predict effects in the real world. This study investigates attentional engagement of domestic horses by comparing observations in a home setting and evaluations of vertebral disorders. We found that lower attentional engagement and the level of back disorders were correlated. Two different evaluation techniques of the state of horses’ spines gave similar results. We suggest that novel animal models would prove useful for identifying spontaneous behaviours indicative of chronic pain. We suggest that more ethological studies in human patients’ home environments would help to improve our understanding of the processes involved. Finally, these results yield interesting indications for evaluating animal welfare, as attentional engagement could become a reliable indicator of chronic pain and thus a useful tool for identification of suffering individuals.
Chronic pain is thought to affect patients’ cognitive functioning. As the neural systems involved in cognition and pain processing are closely linked, they may modulate one another1. Chronic pain has an impact on a subset of cognitive domains including attention, learning, memory, or speed of information processing2. Loss of function in these domains is likely to have an impact on the execution of daily tasks and to affect relationships, capacity for work, moods, quality of life and, therefore, affect individuals negatively3.
An increasing body of evidence from anatomical, neurochemical, and behavioural studies supports the concept of structural and functional plasticity of the brain of patients with chronic pain (i.e. pain persisting for at least 3 months) (e.g.4,5,6). The chronic pain literature repeatedly reports attention deficits and other cognitive impairments of patients with chronic low back pain (CLBP), fibromyalgia, migraine, rheumatoid arthritis, and musculoskeletal pains (e.g.7,8,9). Pain is an inherently attention-demanding sensory process and distraction of attention has been shown to reduce perception of acute pain10. Although some studies report a direct correlation between the levels of cognitive dysfunction and pain ratings by patients1,11, relationships between chronic pain, in particular chronic low back pain, and cognitive deficits remain debated11,12. The effects of chronic pain on neuropsychological functions could be cumulative and quantitatively different from acute/experimental pain6. Different procedures, medication of patients with chronic pain, subjective perception of pain and experimental clinical settings (i.e. different from daily living conditions) are aspects that can explain controversial findings1.
Although pre-clinical studies using laboratory animals as models prove useful to model pain-related cognitive impairments1, studies of animals in their environment are still scarce. Difficulties of these studies are related to the fact that they are based on locomotor activity and appetitive responds, which can decrease as a result of the pain induced1. A report showed that rats’ vigilance decreased with increased arthritic pain even under transient analgesia and concluded that fundamental areas for attention processing were affected by the development of chronic pain13. Animals can thus provide interesting models of attention deficits in chronic pain. However, a model is a tool for predicting effects in the real world; a link must be maintained between the model and the system of interest14. Therefore, observations of unconditioned behaviour provide a rapid, economical and justified means to quantify animals’ attention15. For example, rats’ head scans (i.e. a rat elevates its head, moves it side to side, and lowers it) signal general alertness and have been used to assess the effects of toxin-induced lesions in the forebrain16. Rats that did not perform this behaviour were considered to express ‘sensory inattention’.
Humans can also express sensory inattention: because chronic pain is an environment where the source of pain cannot be removed, it can induce a chronic interruption of current attentional engagement, associated with a widespread avoidance of pain and movement and a withdrawal from social contacts17. Patients with chronic pain ‘inhabit an impoverished and restricted environment’17. This daily loss of attention paid to their environment is not assessed by clinical studies on pain and cognition and thus appears only through self-reports. The extent pain chronicity or intensity influences spontaneous daily attentional engagement remains unknown. This is where spontaneous animal models could be of great help.
Domestic animals (dogs, cats and horses) share their environment with humans (e.g. daily working activities) and, in common with humans, frequently suffer from neck or back pains (more than 60% North American humans, more than 70% riding horses in Europe18,19,20,21,22). Diagnoses are difficult for these species and many dogs, cats and horses are treated inappropriately or not at all if they do not show overt clinical signs18,22,23,24. If sensory inattention (i.e. attention deficit) is a behavioural sign of chronic pain, it would constitute an interesting way to assess animals’ pain and be a tool for further studies of the links between human patients’ attention and chronic pain.
The present study aims to evaluate domestic horses’ attentional engagement and compare it to estimates of vertebral disorders assumed to be linked to chronic pain20, using observations of unconditioned behaviour in their home setting. ‘Classical’ ethological observations were combined with evaluations of the state of their spine based on two approaches, one relying on a practitioner’s diagnosis, the other on static surface electromyography (sEMG), a technique considered highly reliable to evaluate human patients’ low back pains25. These approaches proved useful for earlier studies and led to similar evaluations for horses20,21, whose back disorders are difficult to evaluate objectively in the field by radiographic, ultrasonic or scintigraphic imaging26.
Alertness or attentional engagement can be affected by altered welfare27,28, but has never been measured precisely and never been related to chronic disorders such as back disorders. Here we focused on ‘observation or monitoring behaviour’ which is the most widely used indicator of attention in a variety of species and contexts (e.g. corvids29,30, primates31,32, dolphins33 and horses34). Monitoring includes head movements to scan the environment and gazing towards the environment while the body remains immobile33,30,34. It also corresponds to a readiness to react to any sensory stimulation in the environment27,28. Horses’ attention or monitoring behaviour is characterized by standing motionless at the stall door, with their neck horizontal or slightly elevated, ears and neck mobile, and slowly scanning their environment by moving their head laterally or occasionally gazing for a short moment at environmental stimuli35 (Fig. 1). For example, Waring (2003)35 describes as attentive horses, subjects that frequently exhibit visual investigation of their surroundings using monocular as well as binocular vision. Horses rotate the head and eyes looking around and often fix its gaze on nearby objects (p91) or expressions of forward attention are characterized by anteriorly directed orientation for reception of visual, auditory, olfactory, and sometimes tactile cues. The ears are up and rotated forward. The eyes are directed forward and appear to emphasize the binocular visual field. The neck and head angle adjusts to facilitate use of the sensory receptors (p274). This quiet state differs significantly from ‘vigilance’ postures, where neck and tail are raised, fixed neck, ear and head postures and that indicate strong emotional states36,37. More than 100 riding-stable horses were observed in this study. The results evidence that not only the presence/absence of back disorders is related to lower attention paid to the environment but also that attention and back disorders are correlated.
Figure 1.

Attention or monitoring behaviour in horses is characterized by standing motionless at the stall door, with their neck horizontal or slightly elevated, ears and neck mobile, and slowly scanning their environment by moving their head laterally or occasionally gazing for a short moment at environmental stimuli.
Results
Our two studies corresponded to two different types of spine evaluations. Study 1 included 59 riding-stable horses (from 3 facilities), and study 2 included 44 horses (from 5 facilities). Instantaneous scan samples (total of 90 scans per horse) of all horses were recorded during three sessions during calm moments in the stables (at least 1h30 after riding activity for each subject) and their spines were examined independently by either a practitioner (study 1) or by sEMG (study 2) on horses’ resting day, at least 10 h after work. Spine states were evaluated by the number of vertebral sites showing an elevated tension during manual palpation or a high muscular activity during sEMG examinations (>10 mV see21).
Study 1
All horses expressed monitoring behaviour at least once (Fig. 1), accounting for 21.04 ± 2.73% of their time budget. Inter-individual variations were large, ranging from 3 to 51% of the time budget spent monitoring. Time spent monitoring was explained neither by sex (Mann Whitney U test, Nfemale = 15, Nmale = 44, 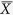 female ± s.e.m. = 22.30 ± 2.47%,
female ± s.e.m. = 22.30 ± 2.47%, 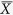 male ± s.e.m = 20.61 ± 1.42%, U = 309.5, P = 0.72), nor by age (Spearman correlation test, N = 59, rs = −0.16, P = 0.22).
male ± s.e.m = 20.61 ± 1.42%, U = 309.5, P = 0.72), nor by age (Spearman correlation test, N = 59, rs = −0.16, P = 0.22).
Spine evaluations presented large inter-individual variations ranging from 0% (for 15% of the horses) to 88% of the vertebral sites being estimated affected. This was not explained by sex (Mann Whitney U test, Nfemale = 15, Nmale = 44, 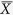 female ± s.e.m = 19.48 ± 4.67%,
female ± s.e.m = 19.48 ± 4.67%, 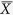 male ± s.e.m = 14.30 ± 2.82%, U = 522.5, P = 0.21) and age (Spearman correlation test, N = 59, rs = 0.21, P = 0.09).
male ± s.e.m = 14.30 ± 2.82%, U = 522.5, P = 0.21) and age (Spearman correlation test, N = 59, rs = 0.21, P = 0.09).
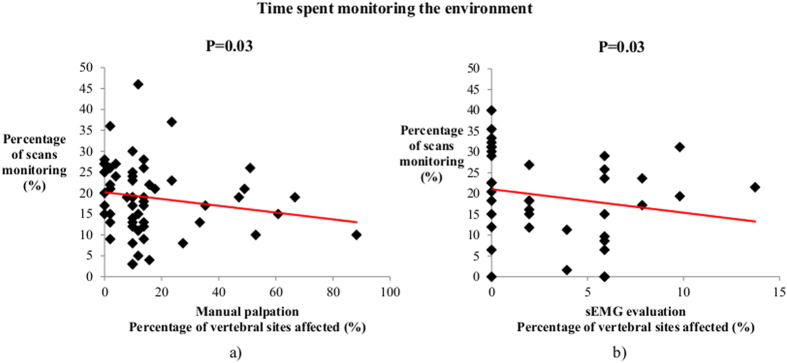
We evidenced a negative correlation between behaviour and spine evaluation: the more the horses monitored their environment, the less affected they were (Spearman correlation test, N = 59, rs = −0.26, P = 0.03) (Fig. 2a). In other words, healthy horses were more attentive to their environment. Not- or slightly-affected (one vertebral site) horses monitored their environment significantly more than did severely affected (2 or more vertebral sites) horses (Mann Whitney U test, N0−1 = 16, N2 = 43, 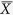 0−1 ± s.e.m = 17.77 ± 1.34%,
0−1 ± s.e.m = 17.77 ± 1.34%, 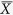 2 ± s.e.m = 22.06 ± 1.73%, U = 223, P = 0.03). This was not due to a restricted mobility as there was no correlation between spine evaluation and the time spent moving in the box (i.e. walk) (Spearman correlation test, N = 59, rs = −0.09, P = 0.49).
2 ± s.e.m = 22.06 ± 1.73%, U = 223, P = 0.03). This was not due to a restricted mobility as there was no correlation between spine evaluation and the time spent moving in the box (i.e. walk) (Spearman correlation test, N = 59, rs = −0.09, P = 0.49).
Figure 2. Relationship between vertebral states and monitoring behaviours.
Correlations between the percentage of vertebral sites affected as indicated by (a) manual palpations; (b) sEMG evaluations; and monitoring behaviours under everyday living conditions (Spearman correlation test, p < 0.05).
Study 2
In this study, 93% of horses (41/44) expressed monitoring behaviour at least once (Fig. 1), accounting for about 20.16 ± 2.71% of their time budget. Inter-individual variations were large, ranging from 1.61 to 40.00% of the time budget spent monitoring the environment. Time spent monitoring was explained neither by sex (Mann Whitney U test, Nfemale = 22, Nmale = 22, 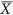 female ± s.e.m = 21.79 ± 2.30%,
female ± s.e.m = 21.79 ± 2.30%, 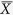 male ± s.e.m = 18.55 ± 2.39%, U = 208, P = 0.43) nor by age (Spearman correlation test, N = 44, rs = 0.16, P = 0.29).
male ± s.e.m = 18.55 ± 2.39%, U = 208, P = 0.43) nor by age (Spearman correlation test, N = 44, rs = 0.16, P = 0.29).
The sEMG evaluations indicated that 61.37% (N = 27) of the horses presented high muscular activity at the level of at least 1 vertebral site (from 0% to 13.72%‘ of the vertebral sites were affected per horse). As in study 1, neither sex (Mann Whitney U test, Nfemale = 22, Nmale = 22, 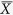 female ± s.e.m = 2.67 ± 0.71%,
female ± s.e.m = 2.67 ± 0.71%, 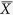 male ± s.e.m = 4.28 ± 0.81%, U = 480, P = 0.73) nor age (Spearman correlation test, N = 44, rs = 0.01, P = 0.93) had any significant effect on sEMG measures.
male ± s.e.m = 4.28 ± 0.81%, U = 480, P = 0.73) nor age (Spearman correlation test, N = 44, rs = 0.01, P = 0.93) had any significant effect on sEMG measures.
We evidenced a negative correlation between the time spent monitoring the environment and the number of vertebral sites affected according to the sEMG evaluations (Spearman correlation test, N = 44, rs = −0.35, P = 0.03) (Fig. 2b). This was confirmed by the fact that the subjects presenting mild mean muscular activity (<10 μV) expressed more monitoring behaviours than did subjects with high mean muscular activity (>10 μV) (Mann-Whitney U-test, Nlma = 16; Nhma = 22; 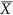 lma = ± s.e.m = 25.42 ± 1.60%,
lma = ± s.e.m = 25.42 ± 1.60%, 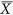 hma = ± s.e.m = 14.71 ± 1.63%, U = 72, P = 0.001). Correlation between cervical spine area evaluations and monitoring was still stronger (Spearman correlation test, N = 44, rs = −0.37, p = 0.01) (Fig. 3), suggesting that neck problems may impact more attentional engagement. No significant correlations could be evidenced between spine evaluation and the time spent moving in the box (i.e. walk) (Spearman correlation test, N = 44, rs = −0.21, P = 0.16).
hma = ± s.e.m = 14.71 ± 1.63%, U = 72, P = 0.001). Correlation between cervical spine area evaluations and monitoring was still stronger (Spearman correlation test, N = 44, rs = −0.37, p = 0.01) (Fig. 3), suggesting that neck problems may impact more attentional engagement. No significant correlations could be evidenced between spine evaluation and the time spent moving in the box (i.e. walk) (Spearman correlation test, N = 44, rs = −0.21, P = 0.16).
Figure 3. Relationship between cervical states and monitoring behaviours.
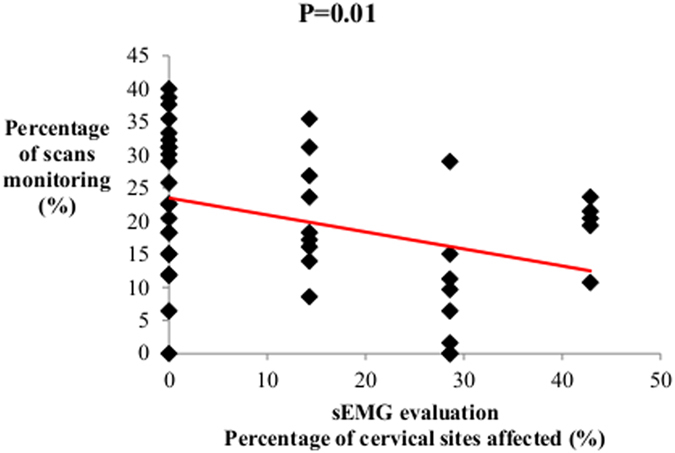
Correlations between the percentage of cervical vertebral sites affected as indicated by sEMG evaluations and monitoring behaviours under everyday living conditions (Spearman correlation test, p < 0.05).
Comparisons between studies
The rates of monitoring did not vary significantly between riding stables in both studies despite management differences (see methods) (Mann-Whitney U-test, Nstudy1 = 59; Nstudy2 = 44;  study1 = ± s.e.m = 21.04 ± 2.22%,
study1 = ± s.e.m = 21.04 ± 2.22%,  study2 = ± s.e.m = 20.16 ± 2.71%, U = 1056, P = 0.63). Prevalence of back disorders was higher in study 1; this could be due to management differences. Nevertheless, pooled data for the two studies revealed a still stronger negative relationship between times horses spent monitoring and the numbers of vertebral sites affected (Spearman correlation test, N = 103, rs = −0.29, P = 0.003). This correlation was confirmed when only the cervical area was considered (Spearman correlation test, N = 103, rs = −0.21, P = 0.03).
study2 = ± s.e.m = 20.16 ± 2.71%, U = 1056, P = 0.63). Prevalence of back disorders was higher in study 1; this could be due to management differences. Nevertheless, pooled data for the two studies revealed a still stronger negative relationship between times horses spent monitoring and the numbers of vertebral sites affected (Spearman correlation test, N = 103, rs = −0.29, P = 0.003). This correlation was confirmed when only the cervical area was considered (Spearman correlation test, N = 103, rs = −0.21, P = 0.03).
Discussion
This study of a large sample of domestic horses observed in their home environment indicated that lower attentional engagement is correlated with the level of back disorders. This is especially clear as two different evaluation techniques used to evaluate the state of the spines gave similar results. One study showed that data for the neck area in particular contributed to this correlation. This is to our knowledge the first ‘ecological’ estimate of attention deficits related to back disorders and hence to potential chronic back pain.
However, our evaluations may not be indicative of chronic pain, as they did not measure it directly. Although animals’ chronic back pain, unless intense enough to induce immobility or lameness, is difficult to detect, all practitioners consider that elevated tenseness at vertebral sites reflects chronic pain (e.g.18,23). Horses’ chronic back pain is known to be correlated with aggressiveness towards humans, a behaviour often associated with illness and acute as well as chronic pain20,28,38.
Our demonstration showing that an animal spontaneous attention to the environment can be impaired by chronic back pain opens new lines of research for humans. It suggests that novel animal models could prove useful for preclinical research on chronic pain related cognitive impairments. Similar studies could thus provide insights into the onset of these deficits and their development or help evaluate the effects of drugs or physical exercises in the home environment. These results also promote the idea that more ethological studies in the home environment of human patients would be a way to a better understanding of the processes involved: clinical settings may be a source of stimulation that prevents direct evaluation of daily attentional engagement.
Our results are of great interest for evaluations of animal welfare and suggest that this approach should be developed for other domestic or captive species. If attentional engagement (or sensory inattention) proves a reliable indicator of chronic pain or discomfort for a variety of species, it would be a useful tool for a rapid and economical identification of potentially suffering individuals, leading to appropriate treatments or remediation. Depressed-like animals and anaemic horses tend to isolate themselves from environmental stimuli28,39,40, suggesting that, like human patients suffering from chronic pain, ‘they live in an impoverished and restricted environment’17. Chronic pain, being inescapable, is often associated with depression also in humans (e.g.1). Animal models should help disentangle the processes involved. The finding that the neck area of the spine is particularly involved in cognitive impairments related to both horses’ (this study) and humans’11 chronic pain deepens the parallels.
Methods
Ethical note
This study was approved by the University of Rennes Animal Care Committee and complied with the French laws related to animal experimentation and the European directive 86/609/CEE. Horse husbandry and care were under management of the riding stable: the horses used in this experiment were not research animals.
Behavioural observations
Observations of horses were recorded by a single trained observer for each studies (study 1: CF, study 2: CL, trained together until they reached 98% agreement) in their individual home stall, using scan sampling41 every 2 minutes for 1 hour periods, repeated 3 times: first, early in the morning before the horses’ meal and riding activity; second, during the morning at least one hour after riding activity; and lastly, at the beginning of the afternoon, after having been fed and before riding activity, at least 1h30 after the morning riding activity. This yielded 90 scans per subject.
The observer walked silently and regularly along the corridor and observations were made 3 m from the horses. All behaviours were recorded (see35 for a detailed ethogram). Particular interest was given to their monitoring behaviours that is characterized by the horse standing motionless at the stall door, with its neck held horizontally or slightly elevated, ears and neck mobile, and slowly scanning with lateral head movements or occasional short gazes at environmental stimuli35. This quiet state differs highly from ‘vigilance’ postures, where neck and tail are raised and neck, ears and head in a fixed position, that indicate strong emotional states36,37.
Study 1
The first study included 59 horses (44 geldings, 15 mares; 5–20 years old (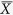 ± s.e.m = 12.81 ± 0.46); mostly French saddlebreds (68%). They were in three riding stables belonging to agricultural colleges presenting similar activities and housing conditions. These horses worked from Monday to Saturday (±4 h/day) and were free on Sundays, with maximal activity during school time (Monday to Friday). Horses were kept singly in straw-bedded individual boxes with windows facing indoors (i.e. onto the barn corridor) or outdoors. The boxes were cleaned once a day; horses were fed industrial pellets three times a day, hay once a day and had water ad libitum.
± s.e.m = 12.81 ± 0.46); mostly French saddlebreds (68%). They were in three riding stables belonging to agricultural colleges presenting similar activities and housing conditions. These horses worked from Monday to Saturday (±4 h/day) and were free on Sundays, with maximal activity during school time (Monday to Friday). Horses were kept singly in straw-bedded individual boxes with windows facing indoors (i.e. onto the barn corridor) or outdoors. The boxes were cleaned once a day; horses were fed industrial pellets three times a day, hay once a day and had water ad libitum.
The horses’ backs were evaluated via manual palpation known to be efficient in detecting back pains42,43, by a licensed chiropractor who was totally blind to the results of the observations recorded before and did not know the horses beforehand. A second practitioner double checked with 94.28 ± 3.69% % agreement. Manual palpation was performed from head to tail and the mobility of each vertebral site was evaluated (N = 51 vertebral sites: 7 cervical, 18 thoracic, 6 lumbar, 5 sacral and 15 coccygeal). Examination was based on bony and soft tissue manual palpation to localise regions of vertebral stiffness based on spinal mobilisation and palpable areas of muscle hypertonicity44, leading to an overview of the percentage of affected vertebral sites. Examinations were performed outside the horses’ working times (horses’ backs were evaluated at least 10 h after work), in their box. The examined horse was restrained slightly by one unknown (to the horses) experimenter (MH) also blind to the behavioural observations.
Study 2
The second study included 44 horses (22 mares, 22 geldings), of varied ages (6–23 years old; 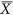 ± s.e.m. = 13.34 ± 1.79), and breeds (N = 13, mostly unregistered horses: 28.57%, French ponies: 25.00%, French Saddlebreds: 17.85% and smaller proportions of Connemaras, Anglo-Arabians, Haflingers, Merens, French trotters, Throughbreds, Welsh ponies and Pottoks) at five riding stables all over France. These horses worked two days a week, on Wednesdays and Saturdays (±4 h/day), and one to two hours a day during the rest of the week. All had at least one free day (usually Sunday). These horses were under the management of the riding stables, lived in straw-bedded individual boxes in large barns with windows facing indoors and outdoors. Most of them were fed industrial pellets (87.3%), two (43.8%) or three times (48.3%) a day. Most of them also had hay for 1 to 5 meals a day. All horses had water ad libitum.
± s.e.m. = 13.34 ± 1.79), and breeds (N = 13, mostly unregistered horses: 28.57%, French ponies: 25.00%, French Saddlebreds: 17.85% and smaller proportions of Connemaras, Anglo-Arabians, Haflingers, Merens, French trotters, Throughbreds, Welsh ponies and Pottoks) at five riding stables all over France. These horses worked two days a week, on Wednesdays and Saturdays (±4 h/day), and one to two hours a day during the rest of the week. All had at least one free day (usually Sunday). These horses were under the management of the riding stables, lived in straw-bedded individual boxes in large barns with windows facing indoors and outdoors. Most of them were fed industrial pellets (87.3%), two (43.8%) or three times (48.3%) a day. Most of them also had hay for 1 to 5 meals a day. All horses had water ad libitum.
These horses’ back problems were evaluated by a new approach, by sEMG, which is easily transportable, adapted to horses and has proved efficient in detecting humans’ back pain25. The sEMG examinations were conducted by the same experimenter (CL), using a wire free device (Myovision®). The device was composed of 2 joysticks with 5 electrodes on each, designed to record muscle activities at the level of the vertebrae at the front and at the back of the joystick location. Muscular activities recorded were sent to a receptor connected to a computer. The joysticks were placed at different levels of the vertebrae on both sides of the spine and the muscular activities at these levels were recorded (see21,24). Raw sEMG values were used (μV). As sEMG values are known to correlate with vertebral disorders21, a vertebral site was considered as ‘affected’ when muscular activity was above 10 μV on both sides of the spine. This 10 μV threshold is known to be related to chronic vertebral disorders as evaluated by the practitioner (Lesimple et al., 2012)21. Back examinations were performed on the resting day, at least 10 h after work. Examinations were performed on flat ground, in the stable corridor in front of each horse’s box, without any noise or disturbance (e.g. working activity, people around) to avoid any intrusive muscular mobilization. The experimenter paid attention to the positions of the horses’ feet: anterior and posterior feet were aligned. Horses were kept motionless, slightly restrained.
Statistical analyses
The numbers of subjects for each factor (site, sex, age) were unbalanced because of the availability of the different categories in the riding stables. As data were not normally distributed, we used non-parametric statistical tests45. Mann-Whithney U-tests were used to assess the effects of sex and vertebral state on monitoring behaviour. Spearman correlation tests investigated the relationships between horses’ ages and behavioural and vertebral variables, and the relationship between horses’ monitoring and vertebral states. Furthermore, in one riding stable where horses were kept in stalls with and without windows facing outwards, a negative correlation was found between the percentage of scans expressing monitoring behaviours and the numbers of vertebral sites affected as indicated by the sEMG evaluation (Spearman correlation test, N = 6, rs = −0.84, P = 0.03), suggesting that facing indoors or outdoors did not influence significantly this relation between back disorders and monitoring rates. All statistics were computed using R software (using an alpha of 0.05, and two-tailed tests). Descriptive statistics are means (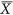 ) followed by standard error (s.e.m.).
) followed by standard error (s.e.m.).
Additional Information
How to cite this article: Rochais, C. et al. Lower attention to daily environment: a novel cue for detecting chronic horses’ back pain? Sci. Rep. 6, 20117; doi: 10.1038/srep20117 (2016).
Acknowledgments
We would like to thank the managers of the riding stables for allowing us to work with their horses and all staff members for their help and cooperation. The authors thank Hervé Menguy and Gérard Bohn for performing manual palpations and Dr Ann Cloarec for improving the English. This study was supported by the Caisse Centrale de la Mutualité Sociale Agricole, the French Research Ministry and the CNRS. The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. We thank the anonymous referees for their comments on the manuscript.
Footnotes
Author Contributions M.H., C.F. and C.L. designed the experiments. C.F. and C.L. performed the experiments. C.L., M.H. and C.R. analyzed the data. C.R. and M.H. wrote the manuscript; all authors have read and commented on the manuscript.
References
- Moriarty O., McGuire B. E. & Finn D. P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 93, 385–404 (2011). [DOI] [PubMed] [Google Scholar]
- Eccleston C. Chronic pain and attention: A cognitive approach. Br. J. Clin. Psychol. 33, 535–547 (1994). [DOI] [PubMed] [Google Scholar]
- Hart R. P., Martelli M. F. & Zasler N. D. Chronic pain and neuropsychological functioning. Neuropsychol. Rev. 10, 131–149 (2000). [DOI] [PubMed] [Google Scholar]
- Apkarian A. V., Baliki M. N. & Geha P. Y. Towards a theory of chronic pain. Prog. Neurobiol. 87, 81–97 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian A. V., Hashmi J. A. & Baliki M. N. Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152, S49–64 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- May A. Chronic pain may change the structure of the brain. Pain 137, 7–15 (2008). [DOI] [PubMed] [Google Scholar]
- Dick B., Eccleston C. & Crombez G. Attentional functioning in fibromyalgia, rheumatoid arthritis, and musculoskeletal pain patients. Arthritis Rheum. 47, 639–644 (2002). [DOI] [PubMed] [Google Scholar]
- Grisart J. M. & Plaghki L. H. Impaired selective attention in chronic pain patients. Eur. J. Pain 3, 325–333 (1999). [DOI] [PubMed] [Google Scholar]
- Weiner D. K., Rudy T. E., Morrow L., Slaboda J. & Lieber S. The relationship between pain, neuropsychological performance, and physical function in community-dwelling older adults with chronic low back pain. Pain Med. Malden Mass 7, 60–70 (2006). [DOI] [PubMed] [Google Scholar]
- Bantick S. J. et al. Imaging how attention modulates pain in humans using functional MRI. Brain 125, 310–319 (2002). [DOI] [PubMed] [Google Scholar]
- Mao C. P. et al. Decreased activation of cingulo-frontal-parietal cognitive/attention network during an attention-demanding task in patients with chronic low back pain. Neuroradiology 56, 903–912 (2014). [DOI] [PubMed] [Google Scholar]
- Tamburin S. et al. Cognition and emotional decision-making in chronic low back pain: an ERPs study during Iowa gambling task. Front. Psychol. 5, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pais-Vieira M., Lima D. & Galhardo V. Sustained attention deficits in rats with chronic inflammatory pain. Neurosci. Lett. 463, 98–102 (2009). [DOI] [PubMed] [Google Scholar]
- Warburton D. M. From mice to men and vice versa: comparative cognition and behavioural pharmacology. Commentary on Olton et al. ‘Comparative cognition and assessment of cognitive processes in animals’. Behav. Pharmacol. 3, 319–322 (1992). [PubMed] [Google Scholar]
- Bushnell P. J. Behavioral approaches to the assessment of attention in animals. Psychopharmacology (Berl.) 138, 231–259 (1998). [DOI] [PubMed] [Google Scholar]
- Dunnet S. B., Whishaw I. Q., Jones G. H. & Bunch S. T. Behavioural, biochemical and histochemical effects of different neurotoxic amino acids injected into nucleus basalis magnocellularis of rats. Neuroscience 20, 653–669 (1987). [DOI] [PubMed] [Google Scholar]
- Eccleston C. & Crombez G. Pain demands attention: a cognitive-affective model of the interruptive function of pain. Psychol. Bull. 125, 356–366 (1999). [DOI] [PubMed] [Google Scholar]
- Webb A. A. Potential Sources of Neck and Back Pain in Clinical Conditions of Dogs and Cats: A Review. Vet. J. 165, 193–213 (2003). [DOI] [PubMed] [Google Scholar]
- Jeffcott L. B. Disorders of the thoracolumbar spine of the horse — a survey of 443 cases. Equine Vet. J. 12, 197–210 (1980). [DOI] [PubMed] [Google Scholar]
- Fureix C., Menguy H. & Hausberger M. Partners with Bad Temper: Reject or Cure? A Study of Chronic Pain and Aggression in Horses. PLoS ONE 5, e12434 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesimple C. et al. Towards a Postural Indicator of Back Pain in Horses (Equus caballus). Plos One 7, e44604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesimple C., Fureix C. & Hausberger M. Detecting horses’ sickness: The example of back disorders. Appl. Anim. Behav. Sci. (in press). [Google Scholar]
- Haussler K. K. Equine rehabilitation. Chiropractic treatment for athletic horses. Equine Sports Med. Surg. 10.1016/B978-0-7020-4771-8.00063-6 1225–1229 (2014). [DOI] [Google Scholar]
- Lesimple C., Fureix C., Biquand V. & Hausberger M. Comparison of clinical examinations of back disorders and humans’ evaluation of back pain in riding school horses. BMC Vet. Res. 9, 209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson S., Donaldson M. & Snelling L. SEMG Evaluations: An Overview. Appl. Psychophysiol. Biofeedback 28, 121–127 (2003). [DOI] [PubMed] [Google Scholar]
- Cauin E. Assessment of back pain in horses. In Pract. 19, 522–533 (1997). [Google Scholar]
- Burn C. C., Dennison T. L. & Whay H. R. Relationships between behaviour and health in working horses, donkeys, and mules in developing countries. Appl. Anim. Behav. Sci. 126, 109–118 (2010). [Google Scholar]
- Hausberger M., Fureix C. & Lesimple C. Detecting horses’ sickness: in search of visible signs. Appl. Anim. Behav. Sci. (in press). [Google Scholar]
- Scheid C., Range F. & Bugnyar T. When, what, and whom to watch? Quantifying attention in ravens (Corvus corax) and jackdaws (Corvus monedula). J. Comp. Psychol. 121, 380–386 (2007). [DOI] [PubMed] [Google Scholar]
- Schloegl C., Kotrschal K. & Bugnyar T. Gaze following in common ravens, Corvus corax: ontogeny and habituation. Anim. Behav. 74, 769–778 (2007). [Google Scholar]
- McNelis N. L. & Boatright-Horowitz S. L. Social monitoring in a primate group: the relationship between visual attention and hierarchical ranks. Anim. Cogn. 1, 65–69 (1998). [Google Scholar]
- Hattori Y., Kuroshima H. & Fujita K. I know you are not looking at me: capuchin monkeys’ (Cebus apella) sensitivity to human attentional states. Anim. Cogn. 10, 141–148 (2007). [DOI] [PubMed] [Google Scholar]
- Xitco, Gory J. D. & Ii S. A. K. Dolphin pointing is linked to the attentional behavior of a receiver. Anim. Cogn. 7, 231–238 (2004). [DOI] [PubMed] [Google Scholar]
- Sankey C., Henry S., André N., Richard-Yris M.-A. & Hausberger M. Do Horses Have a Concept of Person? PLoS ONE 6, e18331 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring G. H. Horse Behavior. (Noyes Publications/William Andrew Pub., 2003). [Google Scholar]
- Kiley-Worthington M. The tail movements of ungulates, canids and felids with particular references to their causation and function as displays. Behaviour (1976). [Google Scholar]
- Paul E. S., Harding E. J. & Mendl M. Measuring emotional processes in animals: the utility of a cognitive approach. Neurosci. Biobehav. Rev. 29, 469–491 (2005). [DOI] [PubMed] [Google Scholar]
- Ashley F. H., Waterman-Pearson A. E. & Whay H. R. Behavioural assessment of pain in horses and donkeys: application to clinical practice and future studies. Equine Vet. J. 37, 565–575 (2005). [DOI] [PubMed] [Google Scholar]
- Fureix C., Jego P., Henry S., Lansade L. & Hausberger M. Towards an Ethological Animal Model of Depression? A Study on Horses. PLoS ONE 7, e39280 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fureix C. et al. Investigating anhedonia in a non-conventional species: Do some riding horses Equus caballus display symptoms of depression? Appl. Anim. Behav. Sci. 162, 26–36 (2015). [Google Scholar]
- Altmann J. Observational Study of Behavior: Sampling Methods. Behaviour 49, 227–266 (1974). [DOI] [PubMed] [Google Scholar]
- Wood T. G., Colloca C. J. & Matthews R. A pilot randomized clinical trial on the relative effect of instrumental (MFMA) versus manual (HVLA) manipulation in the treatment of cervical spine dysfunction. J. Manipulative Physiol. Ther. 24, 260–271 (2001). [DOI] [PubMed] [Google Scholar]
- Shearar K. A., Colloca C. J. & White H. L. A Randomized Clinical Trial of Manual Versus Mechanical Force Manipulation in the Treatment of Sacroiliac Joint Syndrome. J. Manipulative Physiol. Ther. 28, 493–501 (2005). [DOI] [PubMed] [Google Scholar]
- Sullivan K. A., Hill A. E. & Haussler K. K. The effects of chiropractic, massage and phenylbutazone on spinal mechanical nociceptive thresholds in horses without clinical signs. Equine Vet. J. 40, 14–20 (2008). [DOI] [PubMed] [Google Scholar]
- Siegel S. & Castellan N. J. Nonparametric statistics for the behavioral sciences. (McGraw-Hill, 1988). [Google Scholar]