Abstract
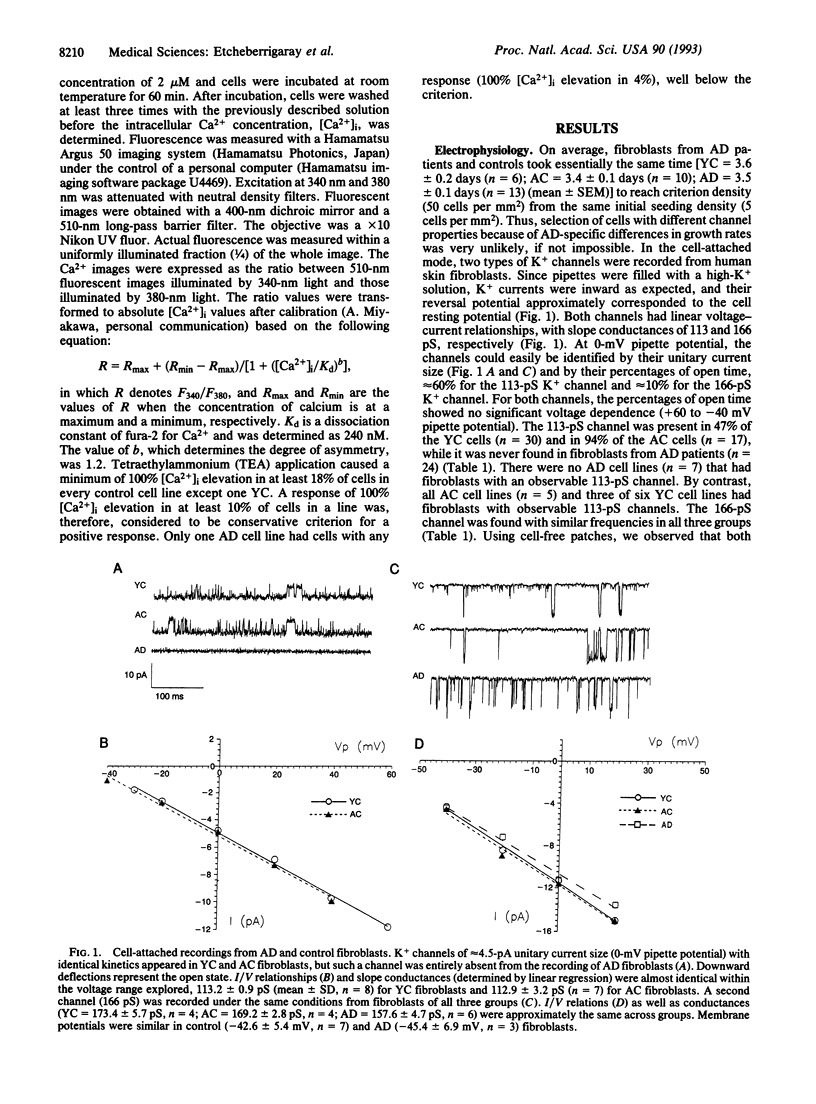
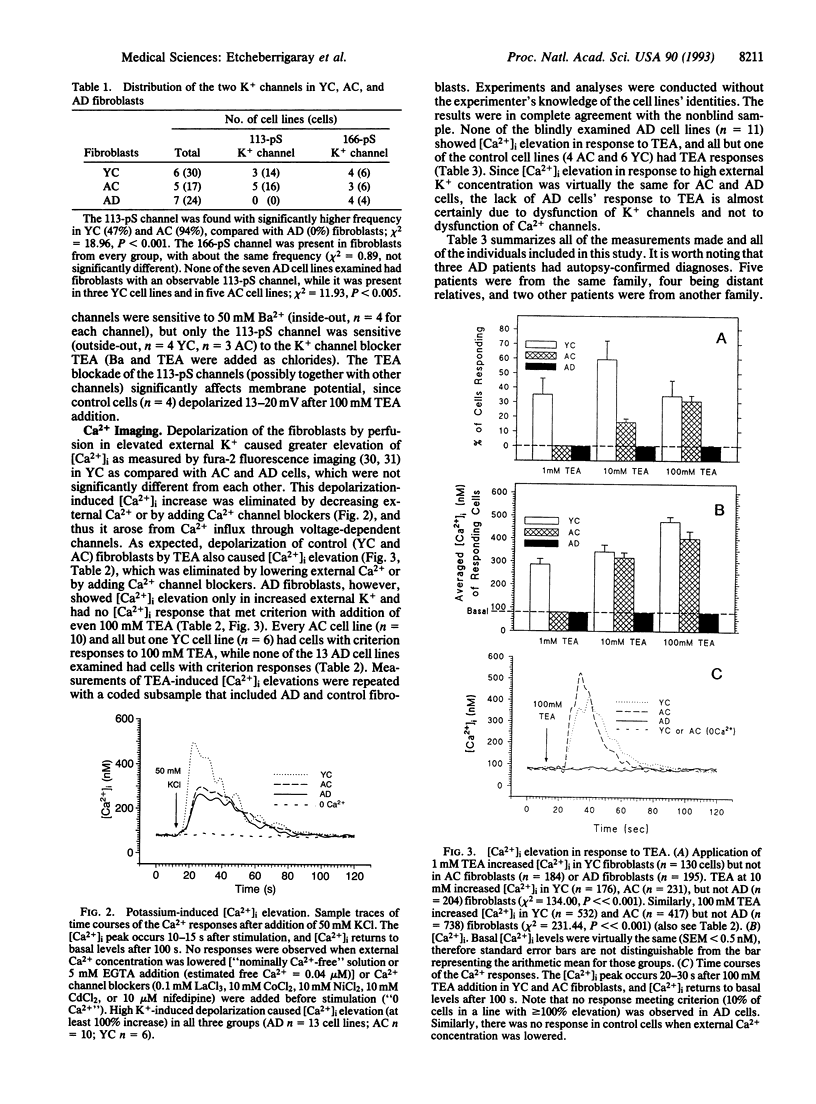
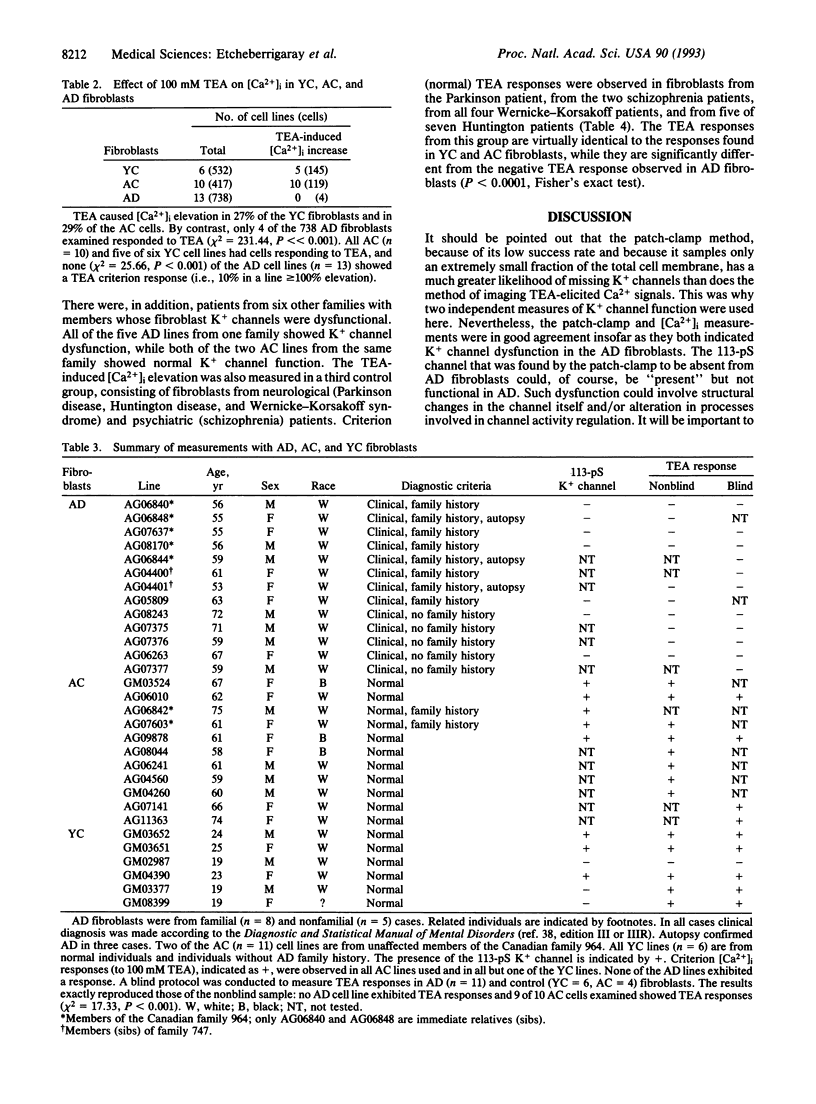
Since memory loss is characteristic of Alzheimer disease (AD), and since K+ channels change during acquisition of memory in both molluscs and mammals, we investigated K+ channel function as a possible site of AD pathology and, therefore, as a possible diagnostic index as well. A 113-pS tetraethylammonium (TEA)-sensitive K+ channel was consistently absent from AD fibroblasts, while it was often present in young and aged control fibroblasts. A second (166-pS) K+ channel was present in all three groups. Elevated external potassium raised intracellular Ca2+ in all cases. TEA depolarized and caused intracellular Ca2+ elevation in young and aged control fibroblasts but not AD fibroblasts. The invariable absence of a 113-pS TEA-sensitive K+ channel and TEA-induced Ca2+ signal indicate K+ channel dysfunction in AD fibroblasts. These results suggest the possibility of a laboratory method that would diagnostically distinguish AD patients, with or without a family history of AD, from normal age-matched controls and also from patients with non-AD neurological and psychiatric disorders.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L. Calcium-mediated reduction of ionic currents: a biophysical memory trace. Science. 1984 Nov 30;226(4678):1037–1045. doi: 10.1126/science.6093258. [DOI] [PubMed] [Google Scholar]
- Alkon D. L. Memory storage and neural systems. Sci Am. 1989 Jul;261(1):42–50. doi: 10.1038/scientificamerican0789-42. [DOI] [PubMed] [Google Scholar]
- Arispe N., Rojas E., Pollard H. B. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch A. B., Rieder H. L., Kelly G. D., Cauthen G. M., Hayden C. H., Snider D. E., Jr The epidemiology of tuberculosis in the United States. Implications for diagnosis and treatment. Clin Chest Med. 1989 Sep;10(3):297–313. [PubMed] [Google Scholar]
- Borden L. A., Maxfield F. R., Goldman J. E., Shelanski M. L. Resting [Ca2+]i and [Ca2+]i transients are similar in fibroblasts from normal and Alzheimer's donors. Neurobiol Aging. 1992 Jan-Feb;13(1):33–38. doi: 10.1016/0197-4580(92)90005-i. [DOI] [PubMed] [Google Scholar]
- Bylund D. J., Ziegner U. H., Hooper D. G. Review of testing for human immunodeficiency virus. Clin Lab Med. 1992 Jun;12(2):305–333. [PubMed] [Google Scholar]
- Clark E. A., Leach K. L., Trojanowski J. Q., Lee V. M. Characterization and differential distribution of the three major human protein kinase C isozymes (PKC alpha, PKC beta, and PKC gamma) of the central nervous system in normal and Alzheimer's disease brains. Lab Invest. 1991 Jan;64(1):35–44. [PubMed] [Google Scholar]
- Collin C., Ikeno H., Harrigan J. F., Lederhendler I., Alkon D. L. Sequential modification of membrane currents with classical conditioning. Biophys J. 1988 Nov;54(5):955–960. doi: 10.1016/S0006-3495(88)83031-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A. Intracellular calcium mobilization by inositol 1,4,5-trisphosphate: intracellular movements and compartmentalization. Cell Calcium. 1993 Mar;14(3):185–200. doi: 10.1016/0143-4160(93)90066-f. [DOI] [PubMed] [Google Scholar]
- Etcheberrigaray R., Matzel L. D., Lederhendler I. I., Alkon D. L. Classical conditioning and protein kinase C activation regulate the same single potassium channel in Hermissenda crassicornis photoreceptors. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7184–7188. doi: 10.1073/pnas.89.15.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French A. S., Stockbridge L. L. Potassium channels in human and avian fibroblasts. Proc R Soc Lond B Biol Sci. 1988 Jan 22;232(1269):395–412. doi: 10.1098/rspb.1988.0003. [DOI] [PubMed] [Google Scholar]
- Gibson G. E., Nielsen P., Sherman K. A., Blass J. P. Diminished mitogen-induced calcium uptake by lymphocytes from Alzheimer patients. Biol Psychiatry. 1987 Sep;22(9):1079–1086. doi: 10.1016/0006-3223(87)90050-3. [DOI] [PubMed] [Google Scholar]
- Gold J. W. HIV-1 infection. Diagnosis and management. Med Clin North Am. 1992 Jan;76(1):1–18. doi: 10.1016/s0025-7125(16)30368-6. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer's disease. Trends Pharmacol Sci. 1991 Oct;12(10):383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Selkoe D. J. The seminal role of beta-amyloid in the pathogenesis of Alzheimer disease. Alzheimer Dis Assoc Disord. 1992 Spring;6(1):7–34. doi: 10.1097/00002093-199205000-00003. [DOI] [PubMed] [Google Scholar]
- Katzman R. Alzheimer's disease. N Engl J Med. 1986 Apr 10;314(15):964–973. doi: 10.1056/NEJM198604103141506. [DOI] [PubMed] [Google Scholar]
- Kosik K. S. Alzheimer's disease: a cell biological perspective. Science. 1992 May 8;256(5058):780–783. doi: 10.1126/science.1589757. [DOI] [PubMed] [Google Scholar]
- Kukuljan M., Goncalves A. A., Atwater I. Charybdotoxin-sensitive K(Ca) channel is not involved in glucose-induced electrical activity in pancreatic beta-cells. J Membr Biol. 1991 Jan;119(2):187–195. doi: 10.1007/BF01871418. [DOI] [PubMed] [Google Scholar]
- Li G., Silverman J. M., Mohs R. C. Clinical genetic studies of Alzheimer's disease. Psychiatr Clin North Am. 1991 Jun;14(2):267–286. [PubMed] [Google Scholar]
- Mattson M. P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R. E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992 Feb;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris J. C., McKeel D. W., Jr, Fulling K., Torack R. M., Berg L. Validation of clinical diagnostic criteria for Alzheimer's disease. Ann Neurol. 1988 Jul;24(1):17–22. doi: 10.1002/ana.410240105. [DOI] [PubMed] [Google Scholar]
- Perl D. P. Relationship of aluminum to Alzheimer's disease. Environ Health Perspect. 1985 Nov;63:149–153. doi: 10.1289/ehp.8563149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C., Ratan R. R., Shelanski M. L., Goldman J. E. Altered response of fibroblasts from aged and Alzheimer donors to drugs that elevate cytosolic free calcium. Neurobiol Aging. 1988 May-Jun;9(3):261–266. doi: 10.1016/s0197-4580(88)80063-0. [DOI] [PubMed] [Google Scholar]
- Peterson C., Ratan R. R., Shelanski M. L., Goldman J. E. Cytosolic free calcium and cell spreading decrease in fibroblasts from aged and Alzheimer donors. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7999–8001. doi: 10.1073/pnas.83.20.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C., Ratan R., Shelanski M., Goldman J. Changes in calcium homeostasis during aging and Alzheimer's disease. Ann N Y Acad Sci. 1989;568:262–270. doi: 10.1111/j.1749-6632.1989.tb12515.x. [DOI] [PubMed] [Google Scholar]
- Pulst S. M., Fain P., Cohn V., Nee L. E., Polinsky R. J., Korenberg J. R. Exclusion of linkage to the pericentromeric region of chromosome 21 in the Canadian pedigree with familial Alzheimer disease. Hum Genet. 1991 Jun;87(2):159–161. doi: 10.1007/BF00204173. [DOI] [PubMed] [Google Scholar]
- Rocca W. A., Amaducci L. A., Schoenberg B. S. Epidemiology of clinically diagnosed Alzheimer's disease. Ann Neurol. 1986 May;19(5):415–424. doi: 10.1002/ana.410190502. [DOI] [PubMed] [Google Scholar]
- Sanchez-Andres J. V., Alkon D. L. Voltage-clamp analysis of the effects of classical conditioning on the hippocampus. J Neurophysiol. 1991 Apr;65(4):796–807. doi: 10.1152/jn.1991.65.4.796. [DOI] [PubMed] [Google Scholar]
- Schellenberg G. D., Bird T. D., Wijsman E. M., Orr H. T., Anderson L., Nemens E., White J. A., Bonnycastle L., Weber J. L., Alonso M. E. Genetic linkage evidence for a familial Alzheimer's disease locus on chromosome 14. Science. 1992 Oct 23;258(5082):668–671. doi: 10.1126/science.1411576. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Shoji M., Golde T. E., Ghiso J., Cheung T. T., Estus S., Shaffer L. M., Cai X. D., McKay D. M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992 Oct 2;258(5079):126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P. H., Myers R. H., Haines J. L., Farrer L. A., Tanzi R. E., Abe K., James M. F., Conneally P. M., Polinsky R. J., Gusella J. F. Familial Alzheimer's disease: progress and problems. Neurobiol Aging. 1989 Sep-Oct;10(5):417–425. doi: 10.1016/0197-4580(89)90082-1. [DOI] [PubMed] [Google Scholar]
- Starke J. R. Modern approach to the diagnosis and treatment of tuberculosis in children. Pediatr Clin North Am. 1988 Jun;35(3):441–464. doi: 10.1016/s0031-3955(16)36465-3. [DOI] [PubMed] [Google Scholar]
- Steiner B., Mandelkow E. M., Biernat J., Gustke N., Meyer H. E., Schmidt B., Mieskes G., Söling H. D., Drechsel D., Kirschner M. W. Phosphorylation of microtubule-associated protein tau: identification of the site for Ca2(+)-calmodulin dependent kinase and relationship with tau phosphorylation in Alzheimer tangles. EMBO J. 1990 Nov;9(11):3539–3544. doi: 10.1002/j.1460-2075.1990.tb07563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesco G., Vergelli M., Amaducci L., Sorbi S. Growth properties of familial Alzheimer skin fibroblasts during in vitro aging. Exp Gerontol. 1993 Jan-Feb;28(1):51–58. doi: 10.1016/0531-5565(93)90019-a. [DOI] [PubMed] [Google Scholar]



