Abstract
Background. The objectives of this study were to estimate the incidence and evaluate risk factors for development of minocycline-induced cutaneous hyperpigmentation in patients with orthopedic infections.
Methods. Patients with orthopedic infections evaluated at Mayo Clinic (Rochester, MN) and treated with minocycline from 1 January 2002 to 31 December 2011 were retrospectively identified. Long-term minocycline suppression was defined as daily minocycline use for at least 3 months. A proportional hazards model was used to evaluate potential risk factors.
Results. Of 291 patients receiving long-term minocycline suppression, 54% (156 of 291) developed hyperpigmentation after a mean follow-up of 4.8 years (range, 0.3–13.2 years); 88% involved blue-gray pigmentation of normal skin that appeared most commonly in the lower (75%) and upper extremities (44%). The mean duration of minocycline therapy before hyperpigmentation was 1.5 years (range, 0.1–9 years) with a mean cumulative dosage of 107.3 g (range, 8.6–657 g). Notable risk factors include a history of vitamin D deficiency (relative risk [RR], 6.29; 95% confidence interval [CI], 1.91–15.27; P = .0052), presence of a shoulder prosthesis (RR, 3.2; 95% CI, 1.23–6.56; P = .0062), noncirrhotic liver pathology (RR, 3.63; 95% CI, 1.11–8.75; P = .0359), and use of a concurrent medication also known to cause hyperpigmentation (RR, 4.75; 95% CI, 1.83–10.1; P = .0029).
Conclusions. Hyperpigmentation associated with the use of long-term minocycline suppression in patients with orthopedic infections is common.
Keywords: antimicrobial side effects, minocycline, orthopedic infections
Cutaneous hyperpigmentation is a recognized adverse effect of chronic minocycline therapy and was first reported in the 1970s in isolated cases [1]. Since then, minocycline-induced cutaneous hyperpigmentation has been reported with increasing frequency likely due to widespread use of minocycline for the treatment of acne [1, 2].
Studies on the use of minocycline in patients with acne and rosacea have reported a cumulative incidence of hyperpigmentation from 2.4% to 28%, respectively [3, 4]. In a cohort of patients with rheumatoid arthritis receiving minocycline for at least 3 months, the cumulative incidence of skin hyperpigmentation was 41% [5]. A subsequent study found a cumulative incidence of 36% associated with the use of at least 1 month of minocycline therapy [6].
Minocycline-induced cutaneous hyperpigmentation is not associated with adverse clinical effects, and it is mostly cosmetic in nature [7, 8]. Predisposing factors for hyperpigmentation in patients with long-term minocycline use remain unclear. Prior studies have suggested an association with higher doses in acne patients [4] and increased age in rheumatoid arthritis patients [6]. One small study found no correlation with skin fairness, eye color, or hair color in a rheumatoid arthritis cohort [5]. To our knowledge, there are no published reports on the incidence and risk factors for minocycline-induced cutaneous hyperpigmentation in patients with orthopedic infections.
The purpose of this large cohort study was to assess the incidence of minocycline-induced cutaneous hyperpigmentation in patients with orthopedics infections and to evaluate potential risk factors for its development. Determining the risk associated with chronic minocycline use will be useful to patients and healthcare providers alike and may influence the choice of chronic antimicrobial suppression therapy in patients with orthopedic infections.
METHODS
Patients seen at Mayo Clinic (Rochester, MN) between 1 January 2002 and 31 December 2011 with prosthetic joint infections or nonhardware-associated chronic musculoskeletal infections and receiving long-term minocycline therapy were included in this study. Eligible patients were identified through a search of the Mayo electronic medical records, medication prescribing records, and medication reconciliation records. Inclusion criteria included prescription of minocycline for an orthopedic infection, defined as a hardware or nonhardware-associated musculoskeletal infection diagnosed by an attending physician (typically an orthopedic infectious diseases specialist), and long-term minocycline suppression, defined as daily minocycline use for a minimum of 3 consecutive months. Exclusion criteria included patients younger than 18 years of age, minocycline prescription for a nonorthopedic indication, incomplete follow-up in the electronic medical record system, and lack of patient consent for review of records.
All relevant Mayo Clinic records were reviewed. Patients' demographics, medical and surgical comorbidities, type of orthopedic infection, and microbiologic data were recorded. Patient follow-up was recorded until the date last known to be alive as of December 15, 2013.
The initial date of minocycline-induced cutaneous hyperpigmentation was considered the date when skin findings related to minocycline per provider's opinion first occurred, based on patient reported history or the date at which skin findings were first reported in the medical record. The time to development of minocycline-induced cutaneous hyperpigmentation was calculated from the date of minocycline initiation. Minocycline-induced cutaneous hyperpigmentation was classified according to the types as described in the literature [9] or marked as unknown if it was unspecified in the medical record. Specifically, Type I included blue-black pigmentation confined to sites of scarring or prior inflammation; Type II included blue-gray pigmentation of normal skin; and Type III included diffuse, muddy brown pigmentation of normal skin, accentuated in sun-exposed areas (Figure 1) [9]. Type II hyperpigmentation reactions were further characterized by the locations in which they appeared. The total time on minocycline and the total cumulative dosage before the initial date of hyperpigmentation were calculated, taking into account dosing adjustments and treatment discontinuities up until the last known dosage.
Figure 1.
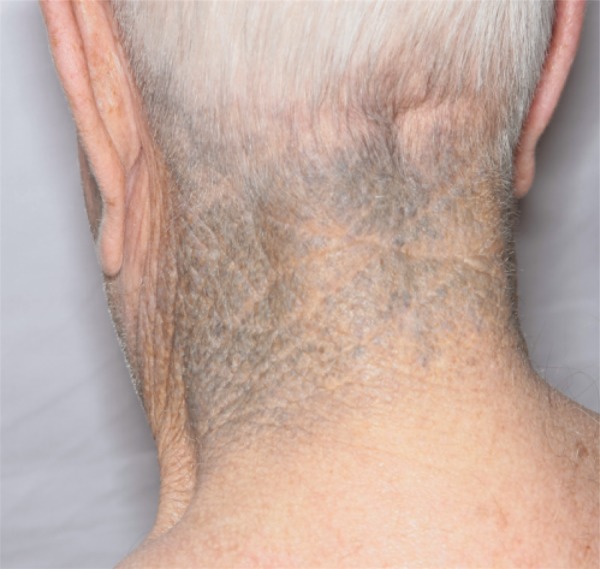
Minocycline-induced cutaneous hyperpigmentation. Blue-gray pigmentation of normal skin indicative of Type II hyperpigmentation. Type I hyperpigmentation is of similar appearance, but it is limited to sites of scarring or prior inflammation.
Dermatologic considerations, including previous drug-related hyperpigmentation, use of other medications that are known to cause hyperpigmentation, activities with significant ultraviolet light exposure during minocycline therapy or evidence of sun-damaged skin, occupational exposure to sun, and smoking history were specifically evaluated as risk factors, in addition to the orthopedic indication, associated microbiology, minocycline dosing scheme, and demographic characteristics. Clinical considerations such as comorbidities present before development of hyperpigmentation and medications in use before development of hyperpigmentation were also abstracted for risk analysis.
Statistical analyses were performed in JMP version 10.0.0, including Kaplan–Meier estimates for time to hyperpigmentation and Cox proportional hazards modeling for risk factor assessment. This study was approved by the Mayo Clinic Institutional Review Board.
RESULTS
Two hundred ninety-one patients (51% male, 96% Caucasian, mean age 65.7 years at minocycline initiation) were included in this cohort (Table 1). The most common underlying orthopedic infections for which minocycline was prescribed were knee (32%), hip (29%), or vertebral (20%) infection with retained hardware. The most common bacterial pathogens were methicillin-resistant Staphylococcus epidermidis (46%), methicillin-resistant Staphylococcus aureus (23%), and methicillin-sensitive S epidermidis (12%).
Table 1.
Characteristics of Patients on Long-Term Minocycline Suppression (n = 291)
| Demographics | |
| Male (%) | 50.9 |
| Mean age at minocycline initiation (years) | 65.7 ± 14.5 |
| Race (%) | |
| Caucasian | 96.2 |
| African American | 1.0 |
| Other | 1.6 |
| Unknown | 1.0 |
| Orthopedic infection site (%) | |
| Hardware associated | |
| Knee | 32.0 |
| Hip | 28.5 |
| Vertebrae | 20.3 |
| Shoulder | 2.4 |
| Elbow | 2.4 |
| Internal fixation—lower extremity | 8.9 |
| Internal fixation—upper extremity | 3.1 |
| External fixation—lower extremity | 1.4 |
| External fixation—upper extremity | 0.7 |
| Spacer/graft—lower extremity | 2.1 |
| Spacer/graft—upper extremity | 0.3 |
| Nonhardware associated | |
| Vertebrae | 1.4 |
| Othera | 5.2 |
| Microbiology (%) | |
| S epidermidis—methicillin resistant | 46.0 |
| S epidermidis—methicillin sensitive | 11.7 |
| S aureus—methicillin resistant | 22.7 |
| S aureus—methicillin sensitive | 10.0 |
| Enterococcus—vancomycin sensitive | 6.9 |
| Enterococcus—vancomycin resistant | 2.7 |
| Propionibacterium | 8.2 |
| Corynebacterium | 6.2 |
| Streptococcus | 5.8 |
| Peptostreptococcus | 4.5 |
| Otherb | 15.5 |
| Mixed, NOS | 0.7 |
| Culture negativec | 7.2 |
| Candida coinfection | 1.0 |
Abbreviations: NOS, ; S,Staphylococcus.
a Infection of the native hip, ankle, shoulder, pelvis, toe, or sternum; chronic musculoskeletal abscesses, decubitus ulcers, or bursitis; chronic osteomyelitis due to retained shrapnel; clavicle internal fixation.
b Achromobacter, Acinetobacter, Actinomyces, Bacillus, Bacteroides, Campylobacter, Citrobacter, Dermabacter, Enterobacter, Escherichia coli, Fusobacterium, Gemella, Haemophilus parainfluenzae, Lactobacillus, Leclercia, Micrococcus, Mycobacterium fortuitum, Mycobacterium hemophilum, Periodonticum, Prevotella, Pseudomonas, Serratia, unspeciated coagulase-negative Staphylococcus, S lugdunensis, Stenotrophomonas, Veillonella.
c One patient with negative cultures did have a positive intraoperative Gram stain demonstrating Gram-positive cocci.
The majority of patients (278 of 291, 96%) were prescribed an initial dosing schedule of 100 mg twice daily. Ten patients were on an initial schedule of 50 mg twice daily, 2 on 25 mg twice daily, and 1 on 150 mg twice daily. Dosing was adjusted during the treatment course in <10% of patients. Patients who developed minocycline-induced cutaneous hyperpigmentation had a mean cumulative dosage of 107.3 g (range, 8.6–657 g) and mean suppression time of 1.5 years (range, 0.1–9 years) before development of hyperpigmentation. Patients who did not develop hyperpigmentation had a mean cumulative dosage of 174.8 g (range, 7.25–7185 g) over a total mean suppression time of 1.8 years (range, 0.25–11.5 years).
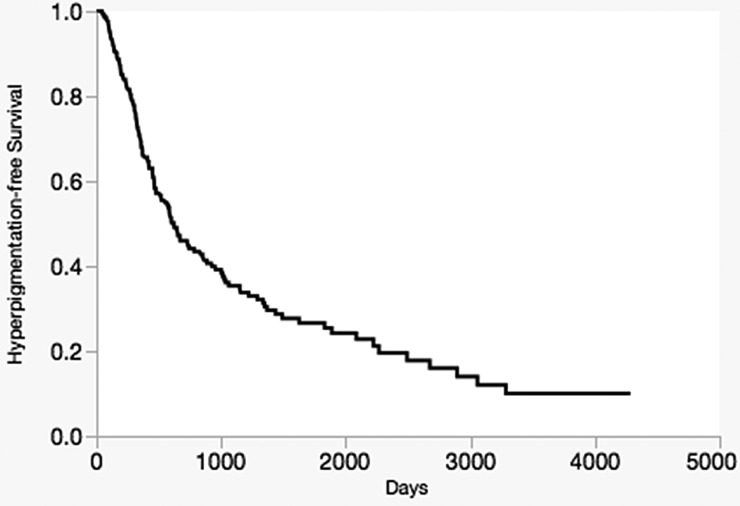
Overall, 54% of patients (49% male, 98% Caucasian, mean age 69.8 years at minocycline initiation) developed minocycline-induced cutaneous hyperpigmentation with suppressive therapy over a mean follow-up time of 4.8 years (range, 0.3–13.2 years). The highest rate of hyperpigmentation occurred within the first 500 days after initiating long-term minocycline therapy, during which 42.8% of patients developed hyperpigmentation (Figure 2).
Figure 2.
Time-dependent analysis showing the incidence of development of minocycline-induced cutaneous hyperpigmentation in 291 patients while on minocycline therapy between 2002 and 2011.
Type II hyperpigmentation was the most common type, occurring in 89% of patients with minocycline-induced cutaneous hyperpigmentation I (Table 2). The type of minocycline-induced cutaneous hyperpigmentation was not documented in 6.4% of patients, whereas 28% of patients presented with more than 1 type of hyperpigmentation, typically Types I and II combined. Of the 156 patients with Type II, minocycline-induced cutaneous hyperpigmentation appeared most commonly in the lower extremities (75%), upper extremities (44%), and face (38%). Patients also presented with noncutaneous staining of the teeth (4.4%), nails (3.6%), and sclera (2.9%). Fifty-six percent of patients with minocycline-induced cutaneous hyperpigmentation opted to continue minocycline, whereas 44% eventually discontinued therapy. Of these patients who discontinued minocycline due to hyperpigmentation, 24% had no improvement in the cutaneous discoloration over a mean follow-up of 1.3 years (range, 20 days–3.2 years), 15% had improvement, typically noted as “fading” or “faintly residual,” over a mean follow-up of 1.2 years (range, 28 days–2 years), whereas 60% had an unknown outcome due to incomplete reporting in the medical record. Complete resolution was reported in only 1 patient after 1.3 years of discontinuing minocycline. Forty percent of patients explicitly expressed concern or distress to their provider regarding the development of hyperpigmentation.
Table 2.
Type and Anatomic Distribution of Minocycline-Induced Cutaneous Hyperpigmentation (n = 156)
| Type (%) | |
| I | 32.1 |
| II | 87.8 |
| III | 1.9 |
| Unknown | 6.4 |
| >1 Type | 27.6 |
| Type II Distribution (%; n=137) | |
| Lower extremities | 74.5 |
| Upper extremities | 43.8 |
| Face | 38.0 |
| Trunk | 10.2 |
| Neck | 8.8 |
| Generalized hue | 11.7 |
| Noncutaneous sites | 16.7 |
| Oral mucosa | 5.8 |
| Teeth | 4.4 |
| Nails | 3.6 |
| Sclera | 2.9 |
Factors associated with developing minocycline-induced cutaneous hyperpigmentation are outlined in Table 3. In total, 113 medications or medication groupings by pharmacologic mechanism and 91 comorbidities were identified across the cohort and evaluated as potential risk factors in addition to the factors specifically mentioned in the Methods section. Notable risk factors include a history of vitamin D deficiency (relative risk [RR], 6.29; 95% confidence interval [CI] 1.91–15.27; P = .0052), presence of a shoulder prosthesis (RR, 3.2; 95% CI, 1.23–6.56; P = .0062), noncirrhotic liver pathology (RR, 3.63; 95% CI, 1.11–8.75; P = .0359), and use of a concurrent medication also known to cause hyperpigmentation (RR, 4.75; 95% CI, 1.83–10.1; P = .0029).
Table 3.
Factors Associated With Development of Minocycline-Induced Cutaneous Hyperpigmentation
| Increased Risk | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Minocycline Characteristics | |||
| Indication: Shoulder prosthesis | 3.2 | 1.23–6.56 | .0062 |
| Microbiology: Enterococcusa | 2.1 | 1.2–3.6 | .0114 |
| Concurrent Medications | |||
| Drug known to cause hyperpigmentation | 4.75 | 1.83–10.1 | .0029 |
| Calcium channel blocker | 1.45 | 1.00–2.07 | .0474 |
| Comorbidities | |||
| Vitamin D deficiency | 6.29 | 1.91–15.27 | .0052 |
| Noncirrhotic liver pathology | 3.63 | 1.11–8.75 | .0359 |
| COPD | 1.78 | 1.09–2.76 | .0220 |
| Atrial fibrillation | 1.74 | 1.15–2.56 | .0104 |
| Chronic lymphedema or edema | 1.69 | 1.02–2.65 | .0415 |
| Coronary artery disease | 1.53 | 1.08–2.14 | .0163 |
| Decreased Risk | |||
| Minocycline Characteristics | |||
| Dose: Any decrease | 0.21 | .03–.66 | .0038 |
| Indication: Upper extremity spacer or graft | 1.9E-9 | .83–.83 | .0319 |
| Patient Characteristics | |||
| African American | 0.14 | .05–.59 | .0011 |
| Concurrent Medications | |||
| Laxatives | 0.66 | .48–.9 | .0091 |
| Antifungal | 0.62 | .38–.98 | .0378 |
| Iron supplement | 0.62 | .39–.94 | .0218 |
| Gabapentin | 0.57 | .35–.88 | .0110 |
| Multivitamin | 0.57 | .41–.79 | .0006 |
| Amoxicillin | 0.49 | .27–.83 | .0061 |
| Pregabalin | 0.48 | .2–.95 | .0331 |
| Alternative supplement | 0.47 | .28–.75 | .0011 |
| H2 antagonist | 0.43 | .17–.90 | .0222 |
| Cephalosporin | 0.38 | .15–.79 | .007 |
| Fiber supplement | 0.36 | .13–.79 | .0077 |
| Topical anesthetic | 0.18 | .03–.55 | .0008 |
| Leflunomide | 0.16 | .01–.73 | .0115 |
| Triptan | 6.99E-10 | .73–.73 | .0220 |
| Comorbidities | |||
| Chronic pain syndrome | 0.49 | .26–.85 | .0089 |
| Paraplegia | 0.32 | .11–.71 | .0030 |
| Bowel/bladder incontinence | 0.25 | .06–.66 | .0025 |
| Decubitus ulcer(s) | 0.19 | .01–.84 | .0240 |
Abbreviations: CI, confidence interval; COPD,chronic obstructive pulmonary disease.
a Vancomycin susceptible.
There was no association with age, sex, region of residence, smoking status, occupational exposure to sun, evidence or history of significant recreational UV exposure, and dosing scheme.
The relapse rate while on long-term minocycline suppression was 12%, with 5.7% of these patients experiencing at least 1 subsequent relapse episode. The median time to first relapse was 0.9 years. Relapses were most common in patients with knee prostheses (46%), with methicillin-resistant S epidermidis as the most common organism involved (51%). The reinfection rate was 7.6%, with 13.6% of these patients experiencing at least 1 subsequent reinfection episode. The median time to first reinfection was 1.3 years. Reinfections were most common in patients with hip prostheses (36%), with methicillin-sensitive S epidermidis (18%), methicillin-resistant S epidermidis (14%), vancomycin-sensitive Enterococcus (14%), and Streptococcus (14%) as the most common organisms involved.
DISCUSSION
This is the first study to report on the incidence of minocycline-induced hyperpigmentation in patients with orthopedic infections receiving chronic long-term minocycline. In this study, more than half of patients developed minocycline-induced hyperpigmentation over a mean follow-up of 4.8 years.
The risk of minocycline-induced hyperpigmentation was not significantly increased with higher minocycline dosage. The lack of dose effect is discordant with an earlier study in patients with acne, in which patients on 200 mg daily (4%) were statistically more likely to develop hyperpigmentation compared with those on alternating 100/200 mg daily doses (1.1%) or 100 mg daily (0.4%) [4]. However, our study did find a significantly decreased risk of hyperpigmentation among patients who experienced a decrease in minocycline dosing during their treatment course, which supports the influence of dosing in developing hyperpigmentation. Hyperpigmentation appears to be relatively uncommon in younger patients with acne, where minocycline is routinely prescribed as 100 mg daily [4] compared with the typical 200 mg daily in this orthopedic infection population, and in a small study performed in patients with immunobullous cutaneous disease receiving mostly 200 mg daily of minocycline, the incidence of minocycline-induced hyperpigmentation was 78%; similar to our orthopedic cohort.
The duration of therapy may play a greater role in explaining the difference in incidence of hyperpigmentation between patients with acne versus orthopedic infections. The clinical effect of oral antibiotic therapy on acne typically takes 4–8 weeks [10]. Therefore, a 2-month trial is generally used to assess therapeutic response, with the average course lasting 6 months [11]. The need for chronic therapy with minocycline is dependent on retained infected orthopedic hardware or the nature of rheumatoid arthritis and therefore often requires longer duration of therapy or, at times, indefinite therapy. The median duration of minocycline therapy before the development of hyperpigmentation in 2 separate rheumatoid arthritis studies was 9.1 [6] and 12 months [5], which is comparable to our study.
Of note, in a cohort of rheumatoid arthritis patients receiving chronic minocycline [5], patients were started on 100 mg of minocycline daily, and of those who developed hyperpigmentation, 45% had had their dose increased to 200 mg daily after the first month. However, of those who did not develop hyperpigmentation, 75% had also increased their dose to 200 mg daily. This supports the notion that (1) the dose of minocycline is not the only factor to consider and that (2) underlying pathophysiology and patient comorbidities may also play a role in the development of minocycline-induced cutaneous hyperpigmentation.
Although the retrospective nature of this study prevented complete and accurate analysis of the resolution of minocycline-induced cutaneous hyperpigmentation, the results reported here support the concept that after cessation of therapy, hyperpigmentation may take a long time to resolve [7, 8]. In addition, aside from discontinuing minocycline therapy, there are limited options for the management of hyperpigmentation. Selective photothermolysis therapy with a variety of Q-switched lasers appears to be a promising therapeutic intervention, with at least 90% resolution achieved after multiple treatment sessions, as described in small case series or reports [12–15]. However, insurance coverage for these procedures may be variable due to the cosmetic nature of the indication. The issues in reversing minocycline hyperpigmentation highlight the importance of appropriate counseling before initiating therapy.
This study identified a number of interesting risk factors, a number of which are most likely confounded or interrelated. For example, cardiovascular comorbidities and their related medications such as calcium channel blockers, both of which are associated with increased risk, may be interdependent. In addition, the presence of any of these risk factors may have influenced the approach to prescribing long-term minocycline suppression, with risk factors themselves as markers of increased cumulative doses or treatment duration. Conversely, factors associated with decreased risk may in fact be surrogates for decreased cumulative doses, decreased treatment duration, or medication noncompliance. There also may be an inherent pathophysiologic explanation for the propensity of patients with these risk factors to develop hyperpigmentation that this study cannot elucidate. Multivariate analysis and further investigation into the possible pathophysiologic bases of risk factors identified in this study would assist in clarifying the clinical relevance of these factors in the development or prevention of hyperpigmentation.
Some risk factors appear driven by pharmacokinetics. In particular, iron, associated with a decreased risk of hyperpigmentation in this study, is known to reduce the absorption of minocycline [16]. Multivitamin supplements were also associated with a decreased risk—it is interesting to note that a small animal study suggested that ascorbic acid may afford protection against hyperpigmentation via antioxidative effects [17]. In addition, the increased risk of hyperpigmentation associated with the presence of hepatic pathology may correlate with the inability to effectively metabolize minocycline.
Also of interest is the increased risk of hyperpigmentation in patients with vitamin D deficiency. The importance of vitamin D in systemic diseases and infections has been increasingly recognized, and there is evidence that vitamin D plays a therapeutic role in certain skin diseases [18]. Vitamin D has been shown to effect dermal cell proliferation, differentiation, collagen synthesis, and immune regulation [18], but the exact mechanism by which its deficiency would contribute to minocycline-induced cutaneous hyperpigmentation is unknown. Likewise, whereas the deleterious effects of smoking on skin [19] and wound healing [20] are well studied, there is no straightforward hypothesis as to why the act of quitting smoking actually appears to increase the risk of developing hyperpigmentation. Additional in vivo studies would be needed to evaluate the compounded effects of minocycline and smoking, and the subsequent cessation of smoking, on skin.
This study has a number of limitations, most of which are inherent to its retrospective nature. Risk factors assessed were extracted from a retrospective review of the medical records. This may create a recall bias of risk factors in patients with minocycline-induced cutaneous hyperpigmentation. The true onset of hyperpigmentation is likely shorter than the 1.5 years reported here, because hyperpigmentation was often an incidental patient- or physician-reported finding at the time of scheduled follow-up appointment. This variability in when hyperpigmentation actually appeared versus when it was reported in the medical record may have also masked a seasonal effect. It is also possible that Type III hyperpigmentation was underreported, because this may be a less distinctive and recognized entity. More recent studies have suggested that hyperpigmentation may be duration- or dose-dependent based on the hyperpigmentation type. Localized pigmentation, as with Type I, appears to occur with no direct relation to duration or total cumulative dosage, whereas diffuse pigmentation, as with Types II and III, tends to occur in patients with prolonged duration of approximately 3 years and high total cumulative doses ranging from 130 to 144 g [1, 5]. Lastly, the demographics of the presented cohort, in particular the lack of racial diversity, may limit generalizability.
CONCLUSIONS
In conclusion, minocycline-induced hyperpigmentation in patients with orthopedic infections occurs frequently. Patients should be appropriately counseled and monitored throughout their treatment course.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Patterson JW, Wilson B, Wick MR, Heath C. Hyperpigmented scar due to minocycline therapy. Cutis 2004; 74:293–8. [PubMed] [Google Scholar]
- 2.Bowen AR, McCalmont TH. The histopathology of subcutaneous minocycline pigmentation. J Am Acad Dermatol 2007; 57:836–9. [DOI] [PubMed] [Google Scholar]
- 3.Dwyer CM, Cuddihy AM, Kerr RE et al. Skin pigmentation due to minocycline treatment of facial dermatoses. Br J Dermatol 1993; 129:158–62. [DOI] [PubMed] [Google Scholar]
- 4.Goulden V, Glass D, Cunliffe WJ. Safety of long-term high-dose minocycline in the treatment of acne. Br J Dermatol 1996; 134:693–5. [DOI] [PubMed] [Google Scholar]
- 5.Roberts G, Capell HA. The frequency and distribution of minocycline induced hyperpigmentation in a rheumatoid arthritis population. J Rheumatol 2006; 33:1254–7. [PubMed] [Google Scholar]
- 6.Fay BT, Whiddon AP, Puumala S et al. Minocycline-induced hyperpigmentation in rheumatoid arthritis. J Clin Rheumatol 2008; 14:17–20. [DOI] [PubMed] [Google Scholar]
- 7.Fenske NA, Millns JL, Greer KE. Minocycline-induced pigmentation at sites of cutaneous inflammation. JAMA 1980; 244:1103–6. [PubMed] [Google Scholar]
- 8.McGrae JD Jr, Zelickson AS. Skin pigmentation secondary to minocycline therapy. Arch Dermatol 1980; 116:1262–5. [PubMed] [Google Scholar]
- 9.Mouton RW, Jordaan HF, Schneider JW. A new type of minocycline-induced cutaneous hyperpigmentation. Clin Exp Dermatol 2004; 29:8–14. [DOI] [PubMed] [Google Scholar]
- 10.Gollnick H, Cunliffe W, Berson D et al. Management of acne: a report from a global alliance to improve outcomes in acne. J Am Acad Dermatol 2003; 49(1 Suppl):S1–37. [DOI] [PubMed] [Google Scholar]
- 11.Tan HH. Antibacterial therapy for acne: a guide to selection and use of systemic agents. Am J Clin Dermatol 2003; 4:307–14. [DOI] [PubMed] [Google Scholar]
- 12.Knoell KA, Milgraum SS, Kutenplon M. Q-switched ruby laser treatment of minocycline-induced cutaneous hyperpigmentation. Arch Dermatol 1996; 132:1251–3. [PubMed] [Google Scholar]
- 13.Greve B, Schonermark MP, Raulin C. Minocycline-induced hyperpigmentation: treatment with the Q-switched Nd:YAG laser. Lasers Surg Med 1998; 22:223–7. [DOI] [PubMed] [Google Scholar]
- 14.Alster TS, Gupta SN. Minocycline-induced hyperpigmentation treated with a 755-nm Q-switched alexandrite laser. Dermatol Surg 2004; 30:1201–4. [DOI] [PubMed] [Google Scholar]
- 15.Nisar MS, Iyer K, Brodell RT et al. Minocycline-induced hyperpigmentation: comparison of 3 Q-switched lasers to reverse its effects. Clin Cosmet Investig Dermatol 2013; 6:159–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agwuh KN, MacGowan A. Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 2006; 58:256–65. [DOI] [PubMed] [Google Scholar]
- 17.Bowles WH. Protection against minocycline pigment formation by ascorbid acid (vitamin C). J Esthet Dent 1998; 10:182–6. [DOI] [PubMed] [Google Scholar]
- 18.Shahriari M, Kerr PE, Slade K, Grant-Kels JE. Vitamin D and the skin. Clin Dermatol 2010; 28:663–8. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz A, Grando SA. Smoking and the skin. Int J Dermatol 2012; 51:250–62. [DOI] [PubMed] [Google Scholar]
- 20.Sorensen LT. Wound healing and infection in surgery. The clinical impact of smoking and smoking cessation: a systematic review and meta-analysis. Arch Surg 2012; 147:373–83. [DOI] [PubMed] [Google Scholar]