Abstract
Treatment response to methotrexate (MTX) for rheumatoid arthritis (RA) is not universal and non-adherence may partially explain this. The aims of this systematic review were to: (1) summarise existing rates of adherence to MTX, (2) identify predictors of adherence to MTX, and (3) assess the association between non-adherence and patient outcomes. The authors conducted a systematic search of papers published from January 1980 to February 2015 in PubMed, PsycINFO, EMBASE and CINAHL databases. Studies were eligible for inclusion if: (1) MTX was used as monotherapy or in combination with other therapies, (2) MTX was used in an RA or inflammatory polyarthritis population, (3) adherence was defined and measured as the extent to which patients followed their MTX regimen during the period of prescription, and (4) it was an original piece of research. In total, 10 studies met the inclusion criteria and 8 were evaluated as high quality. Rates of adherence ranged from 59% to 107%, and exposed differences in definitions of adherence, study methodologies and sample heterogeneity. A number of potential predictors of MTX adherence were identified; the strongest being related to beliefs in the necessity and efficacy of MTX, absence of low mood, mild disease and MTX monotherapy. Furthermore, 3 studies tested the association of adherence with disease activity as an outcome measure; all 3 found non-adherence associated with poor treatment response. This systematic review shows the importance of adherence to MTX treatment and summarises the associated modifiable factors.
Keywords: Rheumatoid Arthritis, Methotrexate, DMARDs (synthetic), Epidemiology
Introduction
Methotrexate (MTX) was recommended as the first-line therapy for the management of rheumatoid arthritis (RA) by EULAR in the 2013 guidelines and by the National Institute for Health and Care Excellence (NICE) clinical guidelines published in 2009 and updated again in 2013.1 2 The recommendation was based on the evidence that MTX has the best drug retention rate (persistence), and equivocal or superior efficacy, in comparison with other synthetic disease-modifying antirheumatic drugs (sDMARDs).3 However, response to MTX is not universal; only 28–45% of patients achieved disease activity score (DAS)-defined remission (DAS28<2.6) 1 year after starting MTX monotherapy.4 5 In an observational study, with a longer follow-up, remission was observed to drop to 6% and 14%, at 2 and 5 years, respectively.6 Response to MTX therapy is likely to be determined by a number of factors but adherence to the treatment regimen may be important.
Adherence, defined by the WHO as “the extent to which the patient's behaviour—taking medication, following a diet, and/or executing lifestyle changes, corresponds with agreed recommendations from a health-care provider” has long been recognised as an important factor in response to treatment.7 In today's society non-adherence to medication contributes to increasing healthcare costs with one study reporting a cost to the National Health Service (NHS) in the UK of £300 million every year due to medicines wastage.8 There are a range of behaviours that could constitute non-adherence, ranging from patients who do not take their medication at all (complete non-adherence), drug holidays (a period of time of taking no medication), and catch-up dosing (following a drug holiday, an increased dosing frequency to catch-up on missed doses). Adherence was reported to be highest for acute illnesses and reduced with long-term drug use, with substantial reductions seen beyond 6 months of treatment in chronic conditions such as RA.9–11
There have been a few systematic reviews of adherence to DMARDs.12–17 A review by Pasma et al12 identified that sDMARD use in the 6 months prior to antitumour necrosis factor initiation and the belief that taking the medication is necessary increased adherence. However, in the review, pharmacological therapies for RA were grouped together to estimate overall adherence rates and investigate predictors. Since MTX is the sDMARD of first choice, it is imperative to have accurate estimates of adherence rates to MTX in the RA population, the effect this has on clinical response, and to investigate potential modifiers of adherence which may be used as targets for intervention. Early interventions to improve adherence to MTX may reduce the need for more aggressive and expensive therapies in the future.
The aims of this systematic review were therefore to (1) obtain an overview of rates of adherence to MTX reported in the literature; (2) evaluate possible predictors of adherence; and (3) describe the strength of association between adherence to MTX and patient-reported and clinical outcomes in patients with RA.
Methods
Search strategy
EMBASE, MEDLINE, CINAHL (Cumulative Index to Nursing and Allied Health Literature) and PsycInfo databases were searched from January 1980, until February 2015, using Patient Intervention Comparison Outcome (PICO) search methodology to build the following strategy.18 (P) rheumatoid or arthritis patient population; (I) MTX as an intervention; and (O) adherence as a measured study predictor or outcome. The PICO comparison (C) category was not applicable and dropped from the search design. Synonyms for each PICO category were defined and the database search identified abstracts that included a synonym from each category in the title, original title, abstract, subject heading, name of substance or registry word fields (see online supplementary table S1).
Study inclusion
Studies obtained from the systematic search were eligible for inclusion if: (1) MTX was used as a monotherapy or in combination with other DMARDs, (2) MTX was used in a RA or inflammatory polyarthritis (IP) population, (3) adherence was defined and measured as the extent to which patients followed their MTX regimen during the period of prescription, and (4) it was an original piece of research.
Titles and abstracts obtained from the search were independently evaluated by two researchers (JB and HFH) for inclusion and, where there was a disagreement, adjudicated by a third reviewer (SMMV). In studies evaluating other therapies in addition to MTX therapy, abstracts were excluded where adherence to the overall regimen, rather than to MTX specifically, was assessed. Where original research published since 2013 met the other inclusion criteria but only existed as an abstract, thesis or conference proceedings, efforts were made to contact the authors to obtain a manuscript, and were excluded if the information required to evaluate the quality of these studies was unavailable. Relevant reviews and opinion articles were retrieved in order to cross-reference to ensure all relevant articles were included. The full papers were obtained for the resulting list and reviewed in a similar fashion to the abstracts of published papers. Papers were included where MTX was prescribed in combination with other drugs, provided adherence to MTX had been calculated separately, papers that provided overall adherence rates only were excluded. If included papers used multiple methods to measure adherence, we describe the methods and report the results specific to MTX adherence.
Quality assessment
The quality of the included studies was formally assessed using an adapted measure from the systematic review of Pasma et al.12 The quality assessment consisted of 16 items, premised on the recommendations from Sanderson et al19 that state observational studies should be evaluated on the use of appropriate methods to: (1) select participants, (2) measure exposure and outcome variables, (3) control confounding, (4) reduce bias, and (5) analyse data. See online supplementary table S2 to review all the items in tool. Papers that scored 7 or more out of 10, or 14 or more out of 17 were considered to be of high quality.
Evidence synthesis
We assessed the association between possible predictors of adherence and the effect size of the association. This evidence was evaluated with reference to the quality of the study, based on the definition of strong, moderate, weak and conflicting evidence of van Tulder et al.20
Results
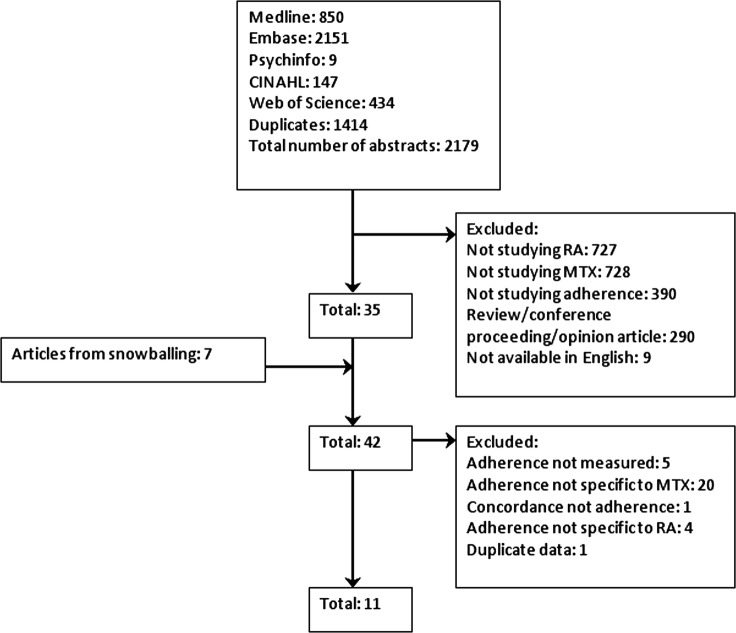
The systematic search generated 1778 abstracts and 27 articles were selected for full paper review, of which 1021–30 papers were selected for inclusion in this review (figure 1).
Figure 1.
Flow diagram to show the article selection process (MTX, methotrexate; RA, rheumatoid arthritis).
Study characteristics
Table 1 provides an overview of the study design and study population of all 10 studies. All the studies were observational studies of RA cohorts; none contained patients with IP. The majority of studies were set in the USA21 23 25 28–30 and were typical RA populations with respect to age and gender except for the study by Cannon et al,28 who utilised data from the Veterans Affairs Rheumatoid Arthritis (VARA) registry. Information about MTX dosage, either the starting dose or average dose, was present in five studies,21 26–28 30 and only four studies reported an average dose.21 26–28
Table 1.
Studies investigating adherence to MTX treatment
| Study | Place | N | Study design | RA definition | Age (years) | Percentage of females | Disease severity | Disease duration (years) | First time or established MTX user | Follow-up | Average MTX dose mg/week |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Harley et al21 | USA | 2662 | Retro | ICD9 | 53.3±14.7 | 73 | NP | NP | First time | 365 days | 10±NP |
| de Klerk et al22 | NED | 127 | Prosp cohort | Rheum | 60±14* | 66* | NP | NP | First time | 210 days | NP |
| Grijalva et al23 | USA | 14 932 | Retro cohort | ICD9 | 54 (44–63) | 78 | NP | NP | First time | NP | NP |
| Contreras-Yanez et al24 | MEX | 93 | Prosp cohort | Rheum | 40.8±13.9† | 80† | DAS28: 2.1±1.1† | <1† | Established | 6 months | NP |
| Grijalva et al25 | USA | 14 586 | Retro cohort | ICD9 | 55 (45–64)† | 76† | NP | NP | First time | NP | NP |
| de Thurah et al26 | DEN | 941 | Retro cohort | ICD10 | 60.5 | 69 | Erosions 71% | 52.9%<5 | First time | 384 (233–931) days | 15 |
| de Thurah et al27 | DEN | 103 | Prosp cohort | ICD10 | 63 (32–80) | 64 | Erosions 60% | 6.3 (0–27) | First time | 9 months | 13.8 (12.5–15.1) |
| Cannon et al28 | USA | 455 | Retro cohort | ACR 1987 | 64±11 | 8 | DAS: 3.9±1.6 | NP | First time | 42.7±3 1.2 months | 16±4 |
| Salt and Frazier29 | USA | 108 | Cross-sectional | ACR 1987 | 52±13† | 76† | NP | 10±10† | Established | NA | NP |
| Waimann et al30 | USA | 111 | Prosp cohort | ACR 1987 | NP | 87† | DAS28: 4.7±1.6† | 8±6† | Both | 24 months | NP |
Median (IQR), mean (95%CI) otherwise mean±SD.
†Values represent total RA sample and not specific to MTX users.
ACR1987, American College of Rheumatology Classification 1987 Criteria; DAS, Disease Activity Score; DEN, Denmark; ICD, International Statistical Classification of Diseases; MEX, Mexico; MTX, methotrexate; NED, Netherlands; NP, information not presented; Prosp, prospective; RA, rheumatoid arthritis; Retro, retrospective; Rheum, RA diagnosed by a rheumatologist.
Quality of the included studies
Overall, the quality of the studies was considered high in 7/10 studies;21 23 25–28 30 all 10 studies used reproducible methods of adherence measurement, 8/10 studies used sampling methods that reduced bias21 23–28 30 and 7/10 studies had a response rate of more than 80%.21 23–28 Where associations with other factors were tested, 7/8 studies presented statistics with CIs23 25–30 and 7/8 studies used analysis that accounted for the skewed adherence data.22–24 27–30 See online supplementary table S3 for a full description.
Rates of adherence to MTX
Rates of adherence differed in how they were calculated and presented between studies. Adherence was reported as a proportion or percentage in six,22–26 30 and participants were categorised as non-adherent and adherent in four.21 26 28 29 In general, estimates of adherence to MTX ranged from 59% to 107% (table 2).
Table 2.
Comparison of MTX rates of adherence across studies
| Study | QA score | Quality rating | Adherence definition | Adherence is primary outcome? | Data on predictors of adherence? | Data on adherence and outcomes? | N | MTX adherence rate | 95% CI/SD |
|---|---|---|---|---|---|---|---|---|---|
| MEMS | |||||||||
| de Klerk et al22 | 12 | Low | Per cent of adherence (ratio) | Yes | Yes | No | 23 | 107% | 98 to 117 |
| Waimann et al30 | 15 | High | Per cent of adherence (ratio) | Yes | Yes | Yes | 76 | 63% | 20% |
| Pharmacy refill | |||||||||
| Harley et al21 | 8 | High | Per cent of adherent (MPR ≥80%) | Yes | No | No | 1668 | 64% | 24 to 102 |
| Grijalva et al23 | 15 | High | Per cent of adherence (MPR) | Yes | Yes | No | 2933 | 80% | NP |
| Grijalva et al25 | 9 | High | Per cent of adherence (MPR) | No | No | No | NP | 59% | 31 to 82 |
| de Thurah et al26 | 14 | High | Per cent of non-adherence (CMG) | Yes | Yes | No | 941 | 12% | 1113 |
| Cannon et al28 | 15 | High | Per cent of adherent (MPR ≥80%) | Yes | No | Yes | 384 | 84% | NP |
| Self-report | |||||||||
| Contreras-Yanez et al24 | 11 | Low | Per cent of adherent (7-day DRR ≥80%) | Yes | Yes | Yes | 10 | 78% | NP |
| de Thurah et al27 | 14 | High | Per cent of non-adherent (CQ-R ≤25th centile) | Yes | Yes | No | 85 65 |
BL 23% 9 mo 23% |
NP NP |
| Salt and Frazier29 | 9 | Low | Per cent of adherent (MARS ≥39) | Yes | Yes | No | 77 | 92% | NP |
9 mo, 9 months; BL, baseline; CMG, continuous medication gap; CQ-R, Compliance Questionnaire-Rheumatology; DRR, Drug Record Registry; MARS, Medication Adherence Revised Scale; MEMS, Medication Event Monitoring System; MPR, medication possession ratio; MTX, methotrexate; NP, information not presented.
Across studies various methods were used to determine adherence. Two studies22 30 used Medication Electronic Monitoring Systems (MEMS), which captures details of pill bottle openings, and is considered an accurate indirect method to evaluate adherence.31 Waimann et al30 reported that the average percentage of correctly taken doses was 63% (SD 20%), with underdosing accounting for 22% (SD 18%) of non-adherence and overdosing 14% (SD 10%). A study judged to be of low quality used the same methodology22 and reported 107% (95% CI 98% to 117%) average adherence representing overdosage.
Five studies determined adherence according to pharmacy refill records,21 23 25 26 28 two of which categorised optimal adherence as ≥80% and the proportion of adherent patients was 63.7% and 84%.21 28 Two studies conceptualised adherence as a dimension and raw medication possession ratios (MPR) were reported, ranging from 59% to 80%.23 25 One study reported gaps in medication possession, using this method non-adherence was estimated to be 12%.26
Where studies used self-report methods, rates of adherence ranged from 78% to 92%.24 29 One study reported 23% of patients were non-adherent based on questionnaires scores.27 Two out of the three self-report studies did not define the recall period.27 29 Salt and Frazier29 used the validated Medication Adherence Revised Scale (MARS), which required patients to endorse the frequency from ‘not at all’ to ‘very often’ they engaged in specific non-adherent behaviours, for example, altering the dose, taking a drug holiday or forgetting to take their medication.32 de Thurah et al27 used the Compliance Questionnaire-Rheumatology (CQ-R), a validated self-report measure that consists of 19 items that do not ask directly about MTX use, instead patients endorsed the extent to which they held adherent attitudes.33 Contreras-Yanez et al24 used a 7-day diary to record the day, timing and dose of MTX over a 7-day period before a clinic appointment at three time points at two monthly intervals. Adherence over 6 months was calculated by dividing the reported MTX use at the three time points by the expected MTX use. Patients were categorised as adherent if they took 80% or more of MTX as prescribed.
None of the selected studies directly measured adherence to MTX but Contreras-Yanez et al24 measured MTX concentrations in serum to evaluate MTX persistence. Whereas 100% of participants reported MTX persistence using a ‘7-day diary’, serum-detected persistence was lower, indicated by the moderate agreement (κ=0.67, p<0.0001) between diary-recorded persistence and serum concentration of MTX, although this result is difficult to interpret in light of variable timing of blood tests in relation to MTX dosing.34
Factors associated with adherence
Seven studies investigated 38 factors and their association with adherence to MTX in RA.22–24 26 27 29 30
Demographic factors
Six studies investigated demographic factors (table 3).22 24 26 27 29 30 Overall there was weak evidence that demographic factors were associated with adherence. de Klerk et al22 reported being female improved adherence, but this was not replicated in four other studies.24 26 27 29 de Thurah et al26 reported being older than 67 years was associated with non-adherence, but four other studies found no association with age.24 27 29 30 Salt and Frazier29 revealed that ethnicity (white vs non-white) in the unadjusted analysis was strongly associated with adherence; however, this was not replicated by Waimann et al.30 The latter study found being married, and living with someone was associated with better adherence but this was a univariate association unadjusted for other factors.
Table 3.
Summary of evidence for demographic predictors of adherence to MTX
| Predictor | Study | N | Outcome | Unadjusted ES (95% CI)/univariate analyses | p Value | Adjusted ES (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Per cent of male | Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 8 (17%) vs 5 (11.9%) | 0.55 | NP | NP |
| Salt and Frazier29* | 108 | Adherent (MARS ≥39) vs non-adherent (MARS ≤38) | 23 (24%) vs 4 (32%) | 0.69 | NP | NP | |
| Male vs female | de Thurah et al26 | 941 | Non-adherence (CMG) | 12.0 (10.5 to 13.5) vs 12.5 (11.4 to 13.5) | NS | ||
| Being male | de Thurah et al27† | 85 | Non-adherence (CQR ≤25th centile) at BL | PR 0.7 (0.2 to 1.7) | NS | PR 0.8 (0.3 to 2.0) | NS |
| de Thurah et al27† | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 0.4 (0.1 to 1.3) | NS | PR 0.3 (0.1 to 1.3) | NS | |
| de Klerk et al22*,‡ | 127 | Per cent of adherence (MEMS) | β 13.5 (NP) | <0.05 | 0.38 (NP) | <0.05 | |
| Age | |||||||
| Age in years | Salt and Frazier29* | 108 | Adherent (MARS ≥39) vs non-adherent (MARS ≤38) | 52±14 vs 53±9 | 0.77 | OR 1.01 (0.94 to 1.08) | 0.8 |
| Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 42.7±14.1 vs 38.9±13.4 | 0.18 | NP | NP | |
| Waimann et al30 | 107 | Per cent of adherence (MEMS) | r −0.07 | >0.20 | NP | NP | |
| >67 vs <55 years old | de Thurah et al26 | 941 | Non-adherence (CMG) | 13.1 (11.6 to 14.6) vs 10.8 (9.3 to 12.3) | <0.01 | Not defined | Sig |
| >55 | de Thurah et al27† | 85 | Non-adherence (CQR ≤25th centile) at BL | 0.5 (0.2 to 1.1) | NS | PR 0.6 (0.2 to 1.6) | NS |
| >55 | de Thurah et al27† | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 0.9 (0.4 to 2.0) | NS | PR 0.7 (0.3 to 1.7) | NS |
| Ethnicity | |||||||
| White vs non-white | Salt and Frazier29* | 108 | Adherent (MARS ≥39) vs non-adherent (MARS ≤38) | 84 (86%) vs 5 (56%) | 0.04 | OR 10.1 (1.66 to 61.4) | 0.01 |
| Hispanic vs white vs African-American | Waimann et al30 | 107 | Per cent of adherence (MEMS) | 66±17 vs 64±20 vs 60±24 | NS | NP | NP |
| Being single/living alone | |||||||
| Single | Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 23 (49%) vs 25 (54%) | 0.68 | NP | NP |
| Salt and Frazier29* | 108 | Adherent (MARS ≥39) vs non-adherent (MARS ≤38) | 16 (16%) vs 2 (22%) | 0.74 | OR 1.44 (0.15 to 13.7) | 0.75 | |
| Widowed/separated vs married | Waimann et al30 | 107 | Per cent of adherence (MEMS) | 56±19 vs 72±16 | <0.01 | NP | NP |
| Widowed/separated vs single | Waimann et al30 | 107 | Per cent of adherence (MEMS) | 56±19 vs 69±18 | <0.01 | NP | NP |
| Living alone vs not living alone | Waimann et al30 | 107 | Per cent of adherence (MEMS) | 56±21 vs 66±19 | <0.05 | NP | NP |
| Education | |||||||
| < High school vs ≥ high school | Waimann et al30 | 107 | Per cent of adherence (MEMS) | 67±19 vs 62±20 | NS | NP | NP |
| Years of education | Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 10.1±4.2 vs 11.4±3.5 | 0.09 | NP | NP |
| Salt and Frazier29* | 108 | Non-adherence (MARS ≤38) | OR 1.09 (1.50 to 0.79) | 0.61 | NP | NP | |
| School >10 years | de Thurah et al27† | 85 | Non-adherence (CQR ≤25th centile) at BL | PR 1.7 (0.7 to 3.8) | NS | PR 1.5 (0.5 to 4.1) | NS |
| de Thurah et al27† | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 1.3 (0.6 to 3.1) | NS | PR 1.0 (0.3 to 2.8) | NS | |
| Residence (rural vs urban) | Salt and Frazier29* | 108 | Adherent (MARS ≥39) vs non-adherent (MARS ≤38) | 55 (51%) vs 48 (53%) | 0.18 | OR 7.52 (0.70 to 83.3) | 0.1 |
| Employment status | |||||||
| Employed vs unemployed | Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 16 (34%) vs 15 (33%) | 1 | NP | NP |
| Employed | Salt and Frazier29* | 108 | Adherent (MARS ≥39) vs non-adherent (MARS ≤38) | 27 (26%) vs 25 (26%) | 0.72 | OR 2.19 (21.3 to 0.21) | 0.52 |
| Employed vs unemployed | Waimann et al30 | 104 | Per cent of adherence (MEMS) | 64±15 vs 63±14 | NS | NP | NP |
| Income <$20 000/year vs ≥$20 000/year | Waimann et al30 | 90 | Per cent of adherence (MEMS) | 62±19 vs 69±20 | NS | NP | NP |
| Low socioeconomic status | Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 43 (92%) vs 40 (87%) | 0.52 | NP | NP |
| Uninsured vs private vs medicaid | Waimann et al30 | 103 | Per cent of adherence (MEMS) | 65±15 vs 66±13 vs 64±19 | NS | NP | NP |
| English vs Spanish language | Waimann et al30 | 107 | Per cent of adherence (MEMS) | 64±21 vs 65±19 | NS | NP | NP |
*Studies judged low quality.
†MTX adherence.
‡Includes RA, PMR and gout.
9 mo, 9 months; BL, baseline; CMG, continuous measure of medication gaps; CQ, Compliance Questionnaire; ES, effect size; MEMS, Medication Electronic Monitoring System; CQR, Compliance Questionnaire-Rheumatology; MARS, Medication Adherence Revised Scale; MTX, methotrexate; NP, information not presented; NS, non-significant; PR, prevalence ratio; r, Pearson correlation coefficient; RA, rheumatoid arthritis; Sig, significant; β, regression coefficient.
Psychological factors
Only three studies tested psychological factors (see table 4),22 27 30 but psychological factors consistently associated with adherence. de Thurah et al27 found higher levels of baseline adherence in patients with high beliefs about the necessity of MTX; however, this association only remained at 9 months in the unadjusted analysis. In comparison, low concerns about MTX were not associated with higher adherence at baseline or at 9 months, although there was a trend for MTX concerns to become more predictive over time. In unadjusted analyses, Waimann et al30 demonstrated that good mental health indicated by lower scores on the Center for Epidemiological Studies Depression Scale 10-item survey (CES-D10), and higher scores on the mental component summary of the Medical Outcomes Study Questionnaire (MOS SF-12 MCS), were significantly associated with lower adherence rates. de Klerk et al22 examined several psychological predictors; non-avoidant coping, passive reactive coping and self-efficacy with regard to taking medications significantly associated with higher adherence. Further, de Klerk et al found that patient reported lower quality of life as measured by the European Quality of Life measure (EuroQol) and the Nottingham Health Profile (NHP) were associated with lower adherence. This finding was not replicated by Waimann et al30 where health-related quality of life was measured using the physical component summary of the Medical Outcomes Study Questionnaire (MOS SF-12 PCS).
Table 4.
Summary of evidence for disease-related and psychological predictors of adherence to MTX
| Predictor | Study | N | Adherence outcome | Unadjusted effect size (95% CI)/univariate analyses | p Value | Adjusted effect size (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| RA duration | |||||||
| Years | Salt and Frazier29* | 108 | Non-adherent (MARS ≤38) | NP | NP | OR 1.00 (1.01 to 1.00) | 0.83 |
| Waimann et al30 | 107 | Adherence (MEMS) | r 0.08 | >0.20 | NP | NP | |
| 1–5 | de Thurah et al26† | 941 | Non-adherence (CMG) | β 0.03 (0.01 to 0.06) | Sig | β 0.01 (−0.01 to 0.04) | NS |
| >5 | de Thurah et al26† | 941 | Non-adherence (CMG) | β 0.02 (−0.01 to 0.04) | NS | β −0.04 (−0.07 to −0.02) | Sig |
| >5 | de Thurah et al27† | 85 | Non-adherence (CQR ≤25th centile) at BL | PR 1.7 (0.7 to 4.1) | NS | PR 1.5 (0.5 to 4.7) | NS |
| >5 | de Thurah et al27† | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 1.5 (0.6 to 3.6) | NS | PR 1.2 (0.4 to 3.1) | NS |
| Inflammatory biomarkers | |||||||
| CRP | Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 2.4±2.6 vs 2.6±2.4 | 0.68 | NP | NP |
| CRP 8–32 | de Thurah et al26† | 941 | Non-adherence (CMG) | β 0.00 (−0.02 to 0.02) | NS | β −0.02 (−0.04 to 0.01) | NS |
| CRP >32 | de Thurah et al26† | 941 | Non-adherence (CMG) | β −0.02 (−0.05 to 0.01) | NS | β −0.04 (−0.07 to −0.02) | Sig |
| Erythrocyte sedimentation rate | Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 24.1±17.4 37.5±23.8 | 0.003 | NP | NP |
| Disease Activity Score-28 | |||||||
| Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 3.6±1.3 vs 5.1±1.9 | ≤0.001 | NP | NP | |
| Waimann et al30 | 90 | Per cent of adherent (MEMS) | r −0.27 | 0.01 | NP | NP | |
| Sharp score | Waimann et al30 | 79 | Per cent of adherent (MEMS) | r −0.06 | >0.20 | NP | NP |
| Functional ability | |||||||
| HAQ | de Klerk et al22*,‡ | 127 | Per cent of adherence (MEMS) | ANOVA (no data) | NS | NP | NP |
| Contreras-Yanez et al24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 0.2±0.4 vs 0.4±0.5 | 0.04 | NP | NP | |
| HAQ >1.75 | de Thurah et al27† | 85 | Nonadherence (CQR ≤25th centile) | PR 1.2 (0.5 to 2.5) | NS | PR 1.4 (0.6 to 3.1) | NS |
| 0.75–1.75 | de Thurah et al27† | 65 | Non-adherence (CQR ≤25th centile) at BL | PR 1.5 (0.5 to 4.9) | NS | PR 0.8 (0.2 to 3.3) | NS |
| HAQ >1.75 | de Thurah et al27† | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 0.8 (0.3 to 2.5) | NS | PR 1.0 (0.2 to 3.4) | NS |
| Modified—HAQ | Waimann et al30 | 107 | Per cent of adherence (MEMS) | r −0.20 | 0.04 | NP | NP |
| Comorbidity | |||||||
| Number of comorbidities | Waimann et al30 | 107 | Per cent of adherence (MEMS) | r −0.06 | >0.20 | NP | NP |
| Per cent with comorbidity | Contreras-Yanez et al 24* | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 40% (85) vs 36% (78.3) | 0.43 | NP | NP |
| Any vs none | de Thurah et al27† | 85 | Non-adherence (CQR ≤25th centile) at BL | PR 1.3 (0.4 to 3.9) | NS | PR 1.1 (0.4 to 3.3) | NS |
| de Thurah et al27† | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 1.3 (0.4 to 3.8) | NS | PR 2.2 (0.5 to 9.7) | NS | |
| COPD | de Thurah et al26† | 941 | Non-adherence (CMG) | β 0.00 (−0.04 to 0.04) | NS | β 0.04 (0.00 to 0.07) | Sig |
| Diabetes | de Thurah et al26† | 941 | Non-adherence (CMG) | β −0.04 (−0.1 to 0.02) | NS | β 0.00 (−0.05 to 0.05) | NS |
| Liver disease | de Thurah et al26† | 941 | Non-adherence (CMG) | β 0.06 (0.02 to 0.10) | Sig | β 0.04 (0.00 to 0.08) | Sig |
| BMQ low concern about MTX | de Thurah et al27† | 85 | Non-adherence (CQR ≤25th centile) at BL | PR 0.8 (0.4 to 1.8) | NS | PR 0.7 (0.3 to 1.8) | NS |
| de Thurah et al27† | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 0.5 (0.2 to 1.2) | NS | PR 0.5 (0.2 to 1.3) | NS | |
| BMQ high perceptions of MTX necessity | de Thurah et al27† | 85 | Non-adherence (CQR ≤25th centile) at BL | PR 0.4 (0.1 to 0.8) | Sig | PR 0.3 (0.1 to 0.8) | Sig |
| de Thurah et al27† | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 0.2 (0.1 to 0.6) | Sig | PR 0.4 (0.1 to 1.1) | NS | |
| LTMBS (self-efficacy) | de Klerk et al22‡ | 127 | Per cent of adherence (MEMS) | F 5.9 | 0.02 | NP | NP |
| UCL avoidant coping | de Klerk et al22*,‡ | 127 | Per cent of adherence (MEMS) | NP | NS | β −0.41 | < 0.05 |
| Passive reactive coping | |||||||
| UCL passive coping | de Klerk et al22*,‡ | 127 | Per cent of adherence (MEMS) | NP | NS | β 0.79 | <0.05 |
| UCL reactive coping | de Klerk et al22*,‡ | 127 | Per cent of adherence (MEMS) | NP | NS | β 0.4 | <0.05 |
| UCL active coping | de Klerk et al22*,‡ | 127 | Per cent of adherence (MEMS) | NP | NS | NP | NP |
| UCL reassuring thoughts | de Klerk et al22*,‡ | 125 | Per cent of adherence (MEMS) | NP | NS | NP | NP |
| Mental health | |||||||
| CES-D10 | Waimann et al30 | 107 | Per cent of adherence (MEMS) | r −0.19 | 0.05 | NP | NP |
| MOS SF-12 MCS | Waimann et al30 | 107 | Per cent of adherence (MEMS) | r 0.34 | <0.01 | NP | NP |
| MOS social support | Waimann et al30 | 107 | Per cent of adherence (MEMS) | r 0.17 | 0.08 | NP | NP |
| Health-related quality of life | |||||||
| European Quality of Life Measure | de Klerk et al22*,‡ | 127 | Per cent of adherence (MEMS) | F 5.42 | <0.01 | NP | NP |
| RA Quality of Life Measure | de Klerk et al22*,‡ | 81 | Per cent of adherence (MEMS) | F 0.21 | 0.65 | NP | NP |
| Nottingham Health Profile | de Klerk et al22*,‡ | 127 | Per cent of adherence (MEMS) | NP | NS | β −0.62 | <0.05 |
| MOS SF-12 Physical Component Summary | Waimann et al30 | 107 | Per cent of adherence (MEMS) | r 0.07 | >0.20 | NP | NP |
*Studies judged low quality.
†MTX adherence.
‡Includes RA, PMR and gout.
9 mo, 9 months; ANOVA, analysis of variance; BL, baseline; BMQ, Beliefs in Medicines Questionnaire; CES-D10, Centre of Epidemiologic Studies Depression Scale; CMG, continuous medication gap; COPD, chronic obstructive pulmonary disease; CQ, Compliance Questionnaire; CQR, Compliance Questionnaire—Rheumatology; CRP, C reactive protein; F, ANOVA test statistic; HAQ, Health Assessment Questionnaire; MARS, Medication Adherence Revised Scale; MEMS, Medicine Event Monitoring System; MOS, Medical Outcomes Study; MTX, methotrexate; NP, not presented; NS, non-significant; PR, prevalence ratio; r, Pearson correlation coefficient; RA, rheumatoid arthritis; SF-12 MCS, Mood Component Summary of MOS 12-item Short Form Health Survey; Sig, significant; UCL, Utrecht Coping List; β, regression coefficient.
Disease-related factors
Six studies investigated disease-related factors (table 4).22 24 26 27 29 30 One study suggested adherence reduced with increasing disease duration,26 but this finding was not replicated in three other studies.27 29 30 Two studies measured disease activity using DAS28,24 30 and reported higher DAS28 score to be associated with lower adherence. In one study, there was no observed association between the inflammatory erythrocyte sedimentation rate and a negative association between C reactive protein (CRP) and adherence,24 whereas, in another study, high CRP was associated with increased adherence.26 In unadjusted analyses, two studies found that disability was associated with lower adherence rates,24 30 but two other studies did not replicate these findings.22
Treatment-related factors
Five studies investigated treatment-related factors (table 5).23 24 26 27 30 Grijalva et al23 found adherence to MTX monotherapy was higher compared with MTX in combination with another sDMARD or biological DMARD (bDMARD). Contreraz-Yanez et al24 reported a similar trend; however, only MTX in combination with three other DMARDs reached statistical significance. In contrast, Waimann et al30 found the addition of a bDMARD, or number of RA-related drugs did not affect adherence to MTX. One study found no effect of MTX dose or folic acid use on adherence,27 and one study reported no association between incidence of adverse events (AEs) and adherence.22
Table 5.
Summary of evidence for treatment-related predictors of adherence to MTX
| Predictor | Study | N | Outcome | Unadjusted ES (95% CI)/univariate analyses | p Value | Adjusted ES (95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| MTX dose | |||||||
| MTX 12.5–17.5 mg | de Thurah et al27* | 85 | Non-adherence (CQR ≤25th centile) at BL | PR 0.6 (0.2 to 1.5) | NS | PR 0.7 (0.3 to 1.7) | NS |
| >17.5 mg/week | de Thurah et al27* | 85 | Non-adherence (CQR ≤25th centile) at BL | PR 0.5 (0.2 to 1.7) | NS | PR 0.6 (0.1 to 2.4) | NS |
| MTX 12.5–17.5 mg | de Thurah et al27* | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 0.2 (0.0 to 1.7) | NS | PR 0.4 (0.0 to 3.9) | NS |
| >17.5 mg/week | de Thurah et al27* | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 1.0 (0.4 to 2.4) | NS | PR 1.1 (0.4 to 3.1) | NS |
| Other sDMARDs | |||||||
| MTX + HCQ vs MTX | Grijalva et al23 | NP | Adherence (MPR) | β 0.13 (0.14 to 0.11) | NP | β 0.11 (0.13 to 0.09) | <0.001 |
| MTX + HCQ vs MTX | Contreras-Yanez et al24† | 93 | ≥80% adherent (7-day DRR) | OR 3.9 (0.64 to 23.05) | 0.14 | NP | NP |
| MTX + HCQ + SSZ + LEF vs MTX | Contreras-Yanez et al24† | 70 | ≥80% adherent (7-day DRR) | OR 21 (1.5 to 293) | 0.02 | NP | NP |
| MTX + HCQ + SSZ vs MTX | Contreras-Yanez et al24† | 70 | ≥80% adherent (7-day DRR) | OR 3.7 (0.68 to 20.2) | 0.13 | NP | NP |
| MTX + SSZ vs MTX | Contreras-Yanez et al24† | 70 | ≥80% adherent (7-day DRR) | OR 5.3 (0.49 to 56.8) | 0.17 | NP | NP |
| Other bDMARDs | |||||||
| MTX + INF vs MTX | Grijalva et al23 | NP | Adherence (MPR) | β 0.12 (0.07 to 0.18) | NP | β 0.12 (0.07 to 0.17) | <0.001 |
| MTX + ETA vs MTX | Grijalva et al23 | NP | Adherence (MPR) | β 0.12 (0.09 to 0.15) | NP | β 0.11 (0.08 to 0.14) | <0.001 |
| MTX + ADA vs MTX | Grijalva et al23 | NP | Adherence (MPR) | β 0.06 (0.02 to 0.10) | NP | β 0.07 (0.03 to 0.11) | 0.001 |
| Biological yes vs no | Waimann et al30 | 107 | Per cent of adherence (MEMS) | 63±21 vs 66±17 | NS | NP | NP |
| Salt and Frazier29† | 108 | Non-adherent (MARS ≤38) | NP | NP | OR 1.26 (0.63 to 2.53) | 0.51 | |
| Drugs related to RA | |||||||
| Number of RA drugs | Contreras-Yanez et al24† | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 4.8±1.5 vs 4.7±1.4 | 0.65 | NP | NP |
| Waimann et al30 | 107 | Per cent of adherence (MEMS) | r 0.05 | >0.20 | NP | NP | |
| Pills/day of RA drugs | Waimann et al30 | 107 | Per cent of adherence (MEMS) | r 0.08 | >0.20 | NP | NP |
| Drugs unrelated to RA | |||||||
| Number of drugs for comorbidity | Contreras-Yanez et al24† | 93 | Adherent (CQ ≥9) vs non-adherent (CQ ≤8) | 3.2±1.7 vs 3.2±1.9 | 0.94 | NP | NP |
| Number of non-RA drugs | Waimann et al30 | 107 | Per cent of adherence (MEMS) | r −0.17 | 0.07 | NP | NP |
| Pills/day of non-RA drugs | Waimann et al30 | 107 | Per cent of adherence (MEMS) | r −0.15 | 0.12 | NP | NP |
| Adverse events | de Klerk et al22†,‡ | 127 | Per cent of adherence (MEMS) | NP | NS | NP | NP |
| Folic acid use | de Thurah et al27* | 85 | Non-adherence (CQR ≤25th centile) at BL | PR 0.2 (0.0 to 1.6) | NS | PR 0.3 (0.0 to 2.7) | NS |
| de Thurah et al27* | 65 | Non-adherence (CQR ≤25th centile) at 9 mo | PR 0.6 (0.2 to 2.4) | NS | PR 0.4 (0.1 to 2.6) | NS | |
*MTX adherence.
†Studies judged low quality.
‡Includes RA, PMR and gout.
9 mo, 9 months; ADA, adalimumab; bDMARD, biological disease-modifying antirheumatic drug; BL, baseline; CQ, Compliance Questionnaire; CQR, Compliance Questionnaire—Rheumatology; DRR, drug record registry; ES, effect size; ETA, etanercept; HCQ, hydroxychloroquine; INF, infliximab; LEF, leflunomide; MARS, Medication Adherence Revised Scale; MEMS, Medicine Event Monitoring System; MPR, medication possession ratio; MTX, methotrexate; NP, not presented; NS, non-significant; PR, prevalence ratio; r, Pearson correlation coefficient; RA, rheumatoid arthritis; sDMARD, synthetic disease-modifying antirheumatic drug; Sig, significant; SSZ, sulfasalazine; β, regression coefficient.
Associations with patient-reported and clinical outcomes
Only a few studies investigated the association between adherence and clinical outcomes (n=3),24 28 30 patient-reported outcomes (n=2),28 30 and radiographic damage (n=1).30 Despite study heterogeneity, all three studies observed a negative association between adherence and treatment response. One study investigated adherence to MTX alone28 with the other two studies including other DMARDs within the analysis.
Contreras-Yanez et al24 reported that self-reported non-adherent patients who were in remission at baseline were more at risk of a disease flare than adherent patients during follow-up (48.41 per 100 person/years vs 13.31 per 100 person/years, p<0.002), the relative risk of non-adherence was borderline significant when adjusted for other factors (RR=4.8 (0.8 to 27.6), p=0.08).
The main finding of Cannon et al28 was that being adherent (MPR ≥80%) negatively associated with change in DAS28 over follow-up in unadjusted and adjusted analyses for the entire cohort (β=−0.34 (−0.68 to −0.06), p<0.05), adjusted (β=−0.37 (−0.67 to −0.07), p<0.05). A subanalysis compared the effect of adherence on outcomes for established and first-time users of MTX. There was a significant negative association between being adherent and DAS28 response in the established user cohort (β=−0.38 (−0.67 to −0.05) p<0.05, βadj=−0.37 (−0.72 to −0.02), p<0.05), but this negative association did not reach significance in the first-time user cohort (β=−0.54 (−1.18 to 0.11), p>0.05, βadj=−0.40 (−1.11 to 0.30), p>0.05).28
Waimann et al30 reported a small negative association between adherence (MEMS) and disease activity when adjusted for other factors (β=−0.2 p=0.03). Non-adherent patients (MEMS ≤80%) had consistently greater radiographic damage than adherent patients did at baseline (58 vs 80, p=0.01) and by 12 months (61 vs 86, p=0.02), but this difference lost significance at 24 months (69 vs 87, p=0.12).30 See online supplementary table S4 for a description of all the associations between adherence to MTX and patient outcomes.
Discussion
This systematic review found some evidence that adherence to MTX is suboptimal. In this review, mean adherence could be summarised as suboptimal (59–63%) in two studies,25 30 optimal in two studies (80–88%),23 26 and in one study mean adherence was 107% indicating MTX non-adherence through overuse.22 Three studies dichotomised patients into adherent and non-adherent groups based on indirect measurement of MTX doses taken and the percentage of patients who had optimal adherence ranged from 64% to 85%.21 24 28 Two studies defined patients as adherent based on questionnaire scores, and the proportion classed adherent ranged from 77% to 92%.27 29 The variation observed in this review probably reflects differences in definition and measurement of adherence, sample characteristics and size, study design and statistical models. This heterogeneity meant that it was not possible to perform a combined meta-analysis to produce an overall estimate of adherence or the factors influencing it.
Evidence synthesis revealed a high prevalence of psychological factors that impacted MTX adherence. Higher baseline DAS28 was associated with reduced adherence in two studies suggesting that patients with more severe baseline disease activity have reduced adherence to MTX.24 30 All three studies that examined the impact of MTX non-adherence on clinical outcomes reported that suboptimal adherence was significantly associated with reduced response to treatment.24 28 30
All indirect measures of adherence have limited validity due to the assumptions one has to make. One has to assume the self-reported behaviour on a questionnaire, or affiliated behaviour of bottle opening, or prescription collection, is equivalent to actually taking the medication.31 The generalisability of findings in this review obtained using these methods were constrained by the well-understood issues of small sample sizes22 24 and using cohorts obtained from US Medical insurance companies21 23 25 and the US Veteran registry.28
Two studies27 29 used questionnaires to assess adherence to MTX that have been validated for use with RA populations.32 33 However, the psychometric properties of the MARS and the CQ-R have been tested in a RA population and shown to be multidimensional,29 which suggests they may be measures of important correlates of adherence, rather than adherence per se.
A limitation that applied to all the existing studies was a failure to detect medically advised missed doses. Patients can be advised to lower or miss doses when they experience AEs; therefore, adherence rates may be underestimated. de Thurah et al26 performed a sensitivity analysis that excluded weeks where antibiotics were co-prescribed from the calculation of MPR, and reported MPR increased slightly; however, there are several other valid reasons for a person with RA to temporarily halt MTX. For example, in the same study, ulcer/mild liver disease was negatively associated with MTX adherence.26 It is feasible that this was due to the association of MTX with abnormalities of liver function and thus did not represent true non-adherence.
Taking the above limitations into account we concluded the strongest evidence was for psychological predictors of adherence, such as treatment beliefs, coping styles and mood.22 27 30 Unfortunately, there was little cross-over between studies with respect to the measures used to assess beliefs, coping and mood to make strong recommendations for specific predictors. Some studies were cross-sectional,27 limiting the establishment of a causal relationship between psychological factors and adherence. In studies of other diseases patients with higher perceived necessity of MTX assessed the long-term benefits of MTX use more positively, and placed a higher emphasis on good adherence;35 this supports the findings of de Thurah et al.27 A high perception of need for medication is influenced by previous experiences, expectations of the disease and therapy, and current symptoms.36 Therefore, perceived need can be expected to change over time and patients with early RA may perceive less necessity compared with patients with established RA.37 Increasing perceived necessity has previously been suggested as an intervention to improve adherence in other diseases, as has psychological therapy such as cognitive behaviour therapy and motivational psychology.38
The finding that more severe disease was associated with reduced adherence contradicts a recent systematic review that synthesised data for chronic and acute diseases. The authors reported that the severity of chronic diseases, which included RA, correlated with improved adherence.39 One possible explanation for the finding from the current review is that prior non-adherence before the start of study might have contributed to more severe disease at baseline. However, the association between disease severity and non-adherence may also indicate the mediating role of particular illness beliefs that are triggered by disease events and lead to decisions to non-adhere. Alternatively, patients with more active disease may have received higher doses of MTX, and experienced more AEs, which led to reduced adherence due to increased concerns about the medication.40
Limitations of review
This was a thorough review of 10 papers pertaining to MTX adherence in a RA population but there were some limitations. First, the review was limited to English articles; however, this was unlikely to change the overall findings or recommendations for future research as we only excluded nine abstracts on that basis. Second, the QA tool used was bespoke to the current review and not a validated measure; however, the domains of the tool were based on a systematic review of quality measures for observational studies.19 Further, the QA tool is presented to guide the reader, and not to exclude articles deemed low quality.
We searched for articles which studied inflammatory arthritis and RA populations in order to reflect current clinical practice; sometimes patients start MTX who clinically look like RA but do not strictly fulfil classification criteria for RA. Our search did not retrieve any early disease cohorts where the classification criteria for RA had not been fulfilled, but we did include two papers22 24 where the classification criteria had not been applied. In these studies, we do not know if all the patients would have been classified as having RA, but we considered it important to include these papers in the final results.
Research recommendations
Measuring adherence: This review has highlighted gaps in knowledge with respect to MTX adherence; first, research is needed that addresses the extent to which patients are genuine non-adherers or are adhering to medical advice and not taking MTX; second, the reasons for MTX non-adherence are not known and the predictors of intentional non-adherence are likely to be different to those who unintentionally forget to take MTX; third, studies should be designed to include multiple measures of adherence to compensate for the inherent limitations of each methodology.
Sample selection
An important unobserved confounder of any association between a potential predictor and MTX non-adherence is prior non-adherent behaviour. Therefore, samples need to exclude patients who have used MTX before. Retrospective studies have to make assumptions when defining cohorts as first-time users; therefore, prospective inception cohort studies are needed to overcome this problem.
Investigation of psychological predictors
The causal role of psychological factors in determining adherence to MTX needs to be addressed urgently. The extent to which patient beliefs, coping styles and mood can be said to predict adherence can only be addressed in specifically designed prospective cohort studies that rigorously assess modifiable illness and treatment beliefs over time. The reviewed studies tended to examine psychological, disease or treatment-related predictors in isolation, and further studies are required to investigate the possible interplay between psychology, treatment and illness in determining non-adherence.
Conclusion
In conclusion, this systematic review shows adherence to MTX does impact patient clinical outcomes, and therefore it is important to address. Estimates of adherence vary widely; currently, there is no direct test for MTX adherence; further research is therefore required to develop a direct reliable test of adherence. This review highlights a number of modifiable patient factors including treatment beliefs, self efficacy around medicine taking and coping styles that were shown to associate with MTX adherence; these factors require further research and may lead to interventions that will improve MTX adherence.
Footnotes
Twitter: Follow Holly Hope at @hfhope
Funding: This research was funded by The National Institute of Health Research Manchester Musculoskeletal Biomedical Research Unit and Arthritis Research UK (grant ref 20385). JB is a MRC Clinical Training Fellow supported by the North West England Medical Research Council Fellowship Scheme in Clinical Pharmacology and Therapeutics, which is funded by the Medical Research Council (grant number G1000417/94909), ICON, GlaxoSmithKline, AstraZeneca and the Medical Evaluation Unit.
Disclaimer: The views expressed in this paper are those of the authors and not necessarily those of the NHS, NIHR Department of Health or the University of Manchester.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Maetzel A, Wong A, Strand VS et al. . Meta-analysis of treatment termination rates among rheumatoid arthritis patients receiving disease-modifying anti-rheumatic drugs. Rheumatology (Oxford) 2000;39:975–81. 10.1093/rheumatology/39.9.975 [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewe R, Breedveld FC et al. . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis 2014;73:492–509. 10.1136/annrheumdis-2013-204573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence. Rheumatoid arthritis: the management of rheumatoid arthritis in adults. CG79. Manchester: NICE, 2009. [Google Scholar]
- 4.Emery P, Breedveld FC, Hall S et al. . Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 2008;372:375–82. 10.1016/S0140-6736(08)61000-4 [DOI] [PubMed] [Google Scholar]
- 5.Emery P, Burmester GR, Bykerk VP et al. . Evaluating drug-free remission with abatacept in early rheumatoid arthritis: results from the phase 3b, multicentre, randomised, active-controlled AVERT study of 24 months, with a 12-month, double-blind treatment period. Ann Rheum Dis 2015;74:19–26. 10.1136/annrheumdis-2014-206106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hider SL, Silman A, Bunn D et al. . Comparing the long-term clinical outcome of treatment with methotrexate or sulfasalazine prescribed as the first disease-modifying antirheumatic drug in patients with inflammatory polyarthritis. Ann Rheum Dis 2006;65:1449–55. 10.1136/ard.2005.049775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The World Health Organisation. Adherence to long-term therapies: evidence for action. 2003. http://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1 (accessed March 2013).
- 8.Trueman P, Taylor DG, Lowson K et al. . Evaluation of the scale, causes and costs of waste medicines. Report of DH funded national project YHEC/The School of Pharmacy, University of London; 2010. [Google Scholar]
- 9.Cramer J, Rosenheck R, Kirk G et al. . VA Naltrexone Study Group. Medication compliance feedback and monitoring in a clinical trial: predictors and outcomes. Value Health 2003;6:566–73. 10.1046/j.1524-4733.2003.65269.x [DOI] [PubMed] [Google Scholar]
- 10.Haynes RB, McDonald HP, Garg AX. Helping patients follow prescribed treatment: clinical applications. JAMA 2002;288:2880–3. 10.1001/jama.288.22.2880 [DOI] [PubMed] [Google Scholar]
- 11.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA 2002;288:462–7. 10.1001/jama.288.4.462 [DOI] [PubMed] [Google Scholar]
- 12.Pasma A, van't Spijker A, Hazes JM et al. . Factors associated with adherence to pharmaceutical treatment for rheumatoid arthritis patients: a systematic review. Semin Arthritis Rheum 2013;43:18–28. 10.1016/j.semarthrit.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 13.Fidder HH, Singendonk MM, van der Have M et al. . Low rates of adherence for tumor necrosis factor-alpha inhibitors in Crohn's disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol 2013;19:4344–50. 10.3748/wjg.v19.i27.4344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blum MA, Koo D, Doshi JA. Measurement and rates of persistence with and adherence to biologics for rheumatoid arthritis: a systematic review. Clin Ther 2011;33:901–13. 10.1016/j.clinthera.2011.06.001 [DOI] [PubMed] [Google Scholar]
- 15.de Achaval SD, Suarez-Almazor ME. Treatment adherence to disease-modifying antirheumatic drugs in patients with rheumatoid arthritis and systemic lupus erythematosus. Int J Clin Rheumtol 2010;5:313–26. 10.2217/ijr.10.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koncz T, Pentek M, Brodszky VS et al. . Adherence to biologic DMARD therapies in rheumatoid arthritis. Expert Opin Biol Ther 2010;10:1367–78. 10.1517/14712598.2010.510508 [DOI] [PubMed] [Google Scholar]
- 17.Harrold LR, Andrade SE. Medication adherence of patients with selected rheumatic conditions: a systematic review of the literature. Semin Arthritis Rheum 2009;38:396–402. 10.1016/j.semarthrit.2008.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Costa Santos CM, de Mattos Pimenta CA, Nobre MR. The PICO strategy for the research question construction and evidence search. Rev Lat Am Enfermagem 2007;15:508–11. 10.1590/S0104-11692007000300023 [DOI] [PubMed] [Google Scholar]
- 19.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol 2007;36:666–76. 10.1093/ije/dym018 [DOI] [PubMed] [Google Scholar]
- 20.van Tulder M, Furlan A, Bombardier C et al. . Updated method guidelines for systematic reviews in the Cochrane collaboration back review group. Spine 2003;28:1290–9. 10.1097/01.BRS.0000065484.95996.AF [DOI] [PubMed] [Google Scholar]
- 21.Harley CR, Frytak JR, Tandon N. Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. Am J Manag Care 2003;9(6 Suppl):S136–43. [PubMed] [Google Scholar]
- 22.de Klerk E, van der Heijde D, Landewe R et al. . Patient compliance in rheumatoid arthritis, polymyalgia rheumatica, and gout. J Rheumatol 2003;30:44–54. [PubMed] [Google Scholar]
- 23.Grijalva CG, Chung CP, Arbogast PG et al. . Assessment of adherence to and persistence on disease-modifying antirheumatic drugs (DMARDs) in patients with rheumatoid arthritis. Med Care 2007;45(Suppl 2):S66–76. 10.1097/MLR.0b013e318041384c [DOI] [PubMed] [Google Scholar]
- 24.Contreras-Yanez I, Ponce de Leon S, Cabiedes J et al. . Inadequate therapy behavior is associated to disease flares in patients with rheumatoid arthritis who have achieved remission with disease-modifying antirheumatic drugs. Am J Med Sci 2010;340:282–90. 10.1097/MAJ.0b013e3181e8bcb0 [DOI] [PubMed] [Google Scholar]
- 25.Grijalva CG, Kaltenbach L, Arbogast PG et al. . Adherence to disease-modifying antirheumatic drugs and the effects of exposure misclassification on the risk of hospital admission. Arthritis Care Res (Hoboken) 2010;62:730–4. 10.1002/acr.20087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Thurah A, Norgaard M, Johansen MB et al. . Methotrexate compliance among patients with rheumatoid arthritis: the influence of disease activity, disease duration, and co-morbidity in a 10-year longitudinal study. Scand J Rheumatol 2010;39:197–205. 10.3109/03009740903251318 [DOI] [PubMed] [Google Scholar]
- 27.de Thurah A, Norgaard M, Harder I et al. . Compliance with methotrexate treatment in patients with rheumatoid arthritis: influence of patients’ beliefs about the medicine. A prospective cohort study. Rheumatol Int 2010;30:1441–8. 10.1007/s00296-009-1160-8 [DOI] [PubMed] [Google Scholar]
- 28.Cannon GW, Mikuls TR, Hayden CL et al. . Merging Veterans Affairs rheumatoid arthritis registry and pharmacy data to assess methotrexate adherence and disease activity in clinical practice. Arthritis Care Res (Hoboken) 2011;63:1680–90. 10.1002/acr.20629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salt E, Frazier SK. Predictors of medication adherence in patients with rheumatoid arthritis. Drug Dev Res 2011;72:756–63. 10.1002/ddr.20484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waimann CA, Marengo MF, de Achaval S et al. . Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis: consequences of low adherence. Arthritis Rheum 2013;65:1421–9. 10.1002/art.37917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quittner AL, Modi AC, Lemanek KL et al. . Evidence-based assessment of adherence to medical treatments in pediatric psychology. J Pediatr Psychol 2008;33:916–36. 10.1093/jpepsy/jsm064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999;47:555–67. 10.1016/S0022-3999(99)00057-4 [DOI] [PubMed] [Google Scholar]
- 33.de Klerk E, van der Heijde D, Landewe R et al. . The compliance-questionnaire-rheumatology compared with electronic medication event monitoring: a validation study. J Rheumatol 2003;30:2469–75. [PubMed] [Google Scholar]
- 34.Godfrey C, Sweeney K, Miller K et al. . The population pharmacokinetics of long-term methotrexate in rheumatoid arthritis. Br J Clin Pharmacol 1998;46:369–76. 10.1046/j.1365-2125.1998.t01-1-00790.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller W, Rollnick S. Motivational interviewing. New York: Guilford Press, 1999. [Google Scholar]
- 36.Horne R. Patients’ beliefs about treatment: the hidden determinant of treatment outcome? J Psychosom Res 1999;47:491–5. 10.1016/S0022-3999(99)00058-6 [DOI] [PubMed] [Google Scholar]
- 37.Neame R, Hammond A. Beliefs about medications: a questionnaire survey of people with rheumatoid arthritis. Rheumatology (Oxford) 2005;44:762–7. 10.1093/rheumatology/keh587 [DOI] [PubMed] [Google Scholar]
- 38.Haynes RB, Ackloo E, Sahota N et al. . Interventions for enhancing medication adherence. Cochrane Database Syst Rev 2008;(2):CD000011 10.1002/14651858.CD000011.pub3 [DOI] [PubMed] [Google Scholar]
- 39.DiMatteo MR, Haskard KB, Williams SL. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med Care 2007;45:521–8. 10.1097/MLR.0b013e318032937e [DOI] [PubMed] [Google Scholar]
- 40.Hoekstra M, van Ede AE, Haagsma CJ et al. . Factors associated with toxicity, final dose, and efficacy of methotrexate in patients with rheumatoid arthritis. Ann Rheum Dis 2003;62:423–6. 10.1136/ard.62.5.423 [DOI] [PMC free article] [PubMed] [Google Scholar]